Summary
Sodium-glucose co-transporter 2 (SGLT2) inhibitors, initially developed as a novel class of anti-hyperglycaemic drugs, have been shown to significantly improve metabolic indicators and protect the kidneys and heart of patients with or without type 2 diabetes mellitus. The possible mechanisms mediating these unexpected cardiorenal benefits are being extensively investigated because they cannot solely be attributed to improvements in glycaemic control. Notably, emerging data indicate that metabolic reprogramming is involved in the progression of cardiorenal metabolic diseases. SGLT2 inhibitors reprogram systemic metabolism to a fasting-like metabolic paradigm, involving the metabolic switch from carbohydrates to other energetic substrates and regulation of the related nutrient-sensing pathways, which might explain some of their cardiorenal protective effects. In this review, we will focus on the current understanding of cardiorenal protection by SGLT2 inhibitors, specifically its relevance to metabolic reprogramming.
Keywords: SGLT2 inhibitor, Metabolism, Organ protection, Kidney, Heart
Introduction
Diabetes mellitus (DM) is a global health burden that has reached alarming levels worldwide. The estimated prevalence of DM in 2019 was 9·3% (463 million people) worldwide and is expected to rise to 10·2% (578 million) by 2030 and 10·9% (700 million) by 2045.1 Type 2 diabetes mellitus (T2DM) is the most common age-related metabolic disorder, affecting approximately 25% of people over the age of 65 years worldwide.2 Patients with T2DM are likely to be accompanied by one or more of the other manifestations of metabolic syndrome, such as overweight, obesity, hypertension, and dyslipidemia.3 These metabolic abnormalities occurring with T2DM are associated with a high prevalence of chronic kidney disease (CKD), cardiovascular disease (CVD), and heart failure (HF), leading to high mortality and increased healthcare costs.3,4
T2DM is characterized by a progressive deterioration of β-cell function frequently on the basis of insulin resistance.5 Traditional anti-diabetic drugs, either enhance or directly replace the role of insulin, could reduce blood glucose by promoting glucose into the cell, which then transfers to glycogen, fat, and protein.6,7 Recently, a new generation of anti-hyperglycaemic agents, sodium-glucose co-transporter 2 (SGLT2) inhibitors, on the other hand, reduce blood glucose by promoting urinary glucose excretion, have been proven to exert striking effects on improving cardiorenal outcomes in patients with or without T2DM and in patients with HF with preserved or reduced ejection fraction in numerous large-scale clinical trials.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
A number of mechanistic hypotheses have been proposed to explain the benefits of SGLT2 inhibitors, such as the tubular hypothesis,19 the sodium hypothesis,20 and the ‘thrifty substrate’ hypothesis.21 Recently, the organ protection mechanism by SGLT2 inhibitors was proposed as aestivation-like water-conserving responses enabling physiological adaptation to energy and water shortage, which improves the lifespan of vital organs.22 It is becoming increasingly clear that the mechanisms underlining the cardiorenal benefits of SGLT2 inhibitors cannot be attributed solely to glycaemic control.
Metabolic reprogramming referred to a phenomenon of cancer cells metabolically adapting to changes in unfavourable environments to meet the requirements for survival and proliferation, which has been described as one of the hallmarks of cancer.23 However, emerging evidence suggests that metabolic reprogramming is also involved in CVD and kidney diseases, which may contribute to disease progression and affect the outcome and prognosis of patients.24, 25, 26 Notably, SGLT2 inhibitors were reported to induce a fasting-like metabolic paradigm involving the metabolic switch from carbohydrates to other energetic substrates and regulate the nutrient-sensing pathways, which may partially explain the unexpected cardiorenal protective effects of SGLT2 inhibitors.27
In this review, we will summarise the normal energy metabolism in the heart and the kidney and the metabolic reprogramming involved in HF and diabetic kidney disease (DKD). Different from previous reviews, we will focus on the beneficial effects of SGLT2 inhibitors on metabolic reprogramming in cardiorenal diseases, involving the induction of a fasting-like metabolic paradigm and a nutrient deprivation transcriptional paradigm. We integrated recent advances in clinical trials and experimental studies, and linked the cardiorenal protective effects of SGLT2 inhibitors to metabolic reprogramming and nutrient sensing pathways, hoping to provide a broader range of ideas and perspectives for future research on these promising drugs.
SGLT2 inhibitors—mechanisms of action
SGLTs belong to the mammalian solute carrier family SLC5, which encompasses 12 members expressed in different tissues, responsible for the active, sodium-driven transport process of sugars, anions, vitamins, and short-chain fatty acids (FAs).28 SGLT2 is a high-capacity, low-affinity co-transporter located in the renal proximal tubule S1 and S2 segments, responsible for the reabsorption of >90% of the filtered glucose. SGLT1, on the other hand, is a low-capacity and high-affinity glucose transporter located in the S3 segment, which contributes to the reabsorption of the remaining glucose. Glucose reabsorption via SGLTs on the apical membrane of the proximal tubule is a secondary active transport process that depends on the driving force generated by basolateral Na+/K+-ATPase. Glucose is then transported via the GLUTs on the basolateral membrane to enter the bloodstream.29,30
SGLT2 inhibitors significantly inhibit the reabsorption of glucose and sodium in the proximal tubule, resulting in increased urinary glucose excretion and a mild osmotic diuresis.31 The potential mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors are multifaceted, involving (1) promoting diuresis/natriuresis, reducing blood pressure, and ameliorating cardiac load, which may occur partially through inhibiting sodium-hydrogen exchanger 3 and sympathetic tone;32,33 (2) reducing the proximal reabsorption of sodium and glucose, normalizing tubuloglomerular feedback and lowering hyperfiltration;19 (3) mimicking systemic hypoxia and stimulating erythropoiesis, which may ameliorate organ oxygen delivery;34 (4) inhibiting SGLT1;35 (5) anti-inflammation, reducing oxidative stress and apoptosis, and increasing autophagy;36 (6) improving cardiac energy metabolism and reducing pathological remodeling;21 (7) increasing circulating pro-angiogenic progenitor cells, which may contribute to enhanced vascular health.37
Clinical evidence of SGLT2 inhibitors on metabolism and cardiorenal protection
A network meta-analysis of 38 clinical trials showed that, compared with placebo, SGLT2 inhibitors reduced glycated haemoglobin levels by 0·6–0·9 %, fasting plasma glucose levels by 1·1–1·9 mmol/L, body weight by 1·6–2·5 kg, systolic blood pressure levels by 2·8–4·9 mmHg, and diastolic blood pressure levels by 1·5–2·0 mm Hg, and slightly increased high-density lipoprotein cholesterol levels by 0·05–0·07 mmol/L in adult patients with T2DM.38
In terms of improving cardiovascular outcomes, a meta-analysis including three large cardiovascular outcome trials and 34,322 patients with T2DM demonstrated that SGLT2 inhibitors reduced the risk of major adverse cardiovascular events by 11%, with benefits only observed in patients with atherosclerotic CVD (ASCVD). In addition, SGLT2 inhibitors robustly reduced cardiovascular death or hospitalisation for HF by 23%, with similar benefits in patients with and without ASCVD.39 Even in patients without T2DM, SGLT2 inhibitors were reported to significantly reduce the combined risk of worsening HF or cardiovascular death in patients with HF and a reduced ejection fraction (HFrEF).12,13 More encouragingly, in patients with T2DM and recent worsening HF, initiation of SGLT2 inhibitor soon after an episode of HF was also found to markedly reduce the total number of deaths from cardiovascular causes and hospitalisations for HF.14,15 Recently, the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-PRESERVED) demonstrated that empagliflozin reduced the combined risk of cardiovascular death or hospitalisation for HF in patients with HF and a preserved ejection fraction (HFpEF), regardless of the presence or absence of diabetes.16 Another randomised controlled trial (RCT), the Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure (DELIVER, NCT03619213) trial, is currently ongoing, further confirming the cardiovascular benefits of SGLT2 inhibitors in patients with HFpEF.40
In terms of renal outcome trials, SGLT2 inhibitors were demonstrated to reduce the relative risk of kidney-related outcomes in patients with T2DM.17 Moreover, the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial was terminated early because of its clear ‘overwhelming effect’ on reducing the risk of kidney-related outcomes, regardless of the presence or absence of T2DM.18 The Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY, NCT03594110) is ongoing to test whether empagliflozin can improve cardiorenal outcomes in a broader range of patients with CKD, including overt albuminuria and eGFRs as low as 20 mL per min per 1·73 m2.41 The cardiorenal benefits of SGLT2 inhibitors in major clinical trials are summarised in Table 1.
Table 1.
Large-scale clinical trials of SGLT2 inhibitors for the incidence of adverse cardiorenal outcomes.
| Clinical Trials | EMPA-REG OUTCOME8 | CANVAS Program9 | DECLAR-TIMI 5810 | VERTIS CV11 | DAPA-HF12 | EMPEROR-Reduced13 | SOLOIST-WHF14 | EMPEROR-Preserved16 | SCORED15 | CREDENCE17 | DAPA-CKD18 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Empagliflozin 10 or 25 mg vs. placebo | Canagliflozin 300 or 100 mg vs. placebo | Dapagliflozin 10 mg vs. placebo | Ertugliflozin 5 or 15 mg vs. placebo | Dapagliflozin 10 mg vs. placebo | Empagliflozin 10 mg vs. placebo | Sotagliflozin 200–400mg vs. placebo | Empagliflozin 10 mg vs. placebo | Sotagliflozin 200–400mg vs. placebo | Canagliflozin 100 mg vs. placebo | Dapagliflozin 10 mg vs. placebo |
| Population (n) | 7020 | 10,142 | 17,160 | 8246 | 4744 | 3730 | 1222 | 5988 | 10,584 | 4401 | 4304 |
| Follow-up (year) | 3·1 | 3·6 | 4·2 | 3·5 | 2 | 0·7 | 0·75 | 2·18 | 1·33 | 2·6 | 2·4 |
| T2DM (%) | 100 | 100 | 100 | 100 | 42 | 49·8 | 100 | 49 | 100 | 100 | 67·5 |
| ASCVD* or HFrEF† or HFpEF‡ | > 99% with ASCVD* | 72·7% with ASCVD* | 40·6% with ASCVD* | 100% with ASCVD* | 100% with HFrEF† | 100% with HFrEF† | 100% with recent worsening HF | 100% with HFpEF‡ | 19·9% with HFrEF† | 50·4% with ASCVD* | 37·4% with ASCVD* |
| eGFR (mL per min per 1·73m2) | ≥30 | ≥30 | ≥60 | ≥30 | ≥30 | ≥20 | ≥30 | ≥20 | 25–60 | 30–89 | 25–75 |
| Primary outcome (HR, 95%CI) | MACE§: 0·86 (0·74–0·99) | MACE§: 0·86 (0·75–0·97) | MACE§: 0·93 (0·84–1·03) | MACE§: 0·97 (0·85–1·11) | Worsening HF or cardiovascular death: 0·74 (0·65–0·85) | Cardiovascular death or hospitalization for worsening HF: 0·75 (0·65–0·86) | The total number of cardiovascular deaths and hospitalizations and urgent visits for HF: 0·67 (0·52–0·85) | A composite of cardiovascular death or hospitalization for HF: 0·79 (0·69–0·90) | The total number of cardiovascular deaths and hospitalizations and urgent visits for HF: 0·74 (0·63–0·88) | ESRD, a doubling of the Scr level, or renal or cardiovascular death: 0·70 (0·59–0·82) | A sustained decline in the eGFR≥50%, ESRD, or renal or cardiovascular death: 0·61 (0·51– 0·72) |
| Cardiovascular death (HR, 95%CI) | 0·62 (0·49–0·77) | 0·87 (0·72–1·06) | 0·98 (0·82–1·17) | 0·92 (0·77–1·11) | 0·82 (0·69–0·98) | 0·92 (0·75–1·12) | 0·84 (0·58–1·22) | 0·91 (0·76–1·09) | 0·90 (0·73–1·12) | 0·78 (0·61–1·00) | 0·81 (0·58–1·12) |
| All-cause mortality (HR, 95%CI) | 0·68 (0·57–0·82) | 0·87 (0·74–1·01) | 0·93 (0·82–1·04) | 0·93 (0·80–1·08) | 0·83 (0·71–0·97) | 0·92 (0·77–1·10) | 0·82 (0·59–1·14) | 1·00 (0·87–1·15) | 0·99 (0·83–1·18) | 0·83 (0·68–1·02) | 0·69 (0·53–0·88) |
| Hospitalization for HF (HR, 95%CI) | 0·65 (0·50–0·85) | 0·67 (0·52–0·87) | 0·73 (0·61–0·88) | 0·70 (0·54–0·90) | 0·70 (0·59–0·83) | 0·69 (0·59–0·81) | / | 0·71 (0·60– 0·83) | 0·67 (0·55–0·82) | 0·61 (0·47–0·80) | / |
| Renal outcomes (HR, 95%CI) | / | Progression of albuminuria: 0·73 (0·67–0·79); Sustained 40% reduction in eGFR, RRT, or renal death: 0·60 (0·47–0·77) | ≥ 40% decrease in eGFR to < 60 mL per min per 1·73m2, ESRD, or renal death: 0·53 (0·43–0·66) | Death from renal causes, RRT, or doubling of the Scr level: 0·81 (0·63–1·04) | A sustained decline in the eGFR ≥ 50% or ESRD, or renal death: 0·71 (0·44–1·16) | Mean slope of change in eGFR per year: –0·55 vs –2·28 mL per min per 1·73m2 (P<0·01) | Mean change in eGFR: −0·16 (–1·30– 0·98) | Mean slope of change in eGFR per year: –1·25 vs –2·62 mL per min per 1·73m2 (P<0·01) | A sustained decline in the eGFR≥50%, from baseline or sustained eGFR<15 mL per min per 1·73m2 for ≥30 days, RRT | ESRD, a doubling of the Scr level, or renal death: 0·66 (0·53–0·81) | Decline in eGFR≥50%, ESRD, or renal death: 0·56 (0·45–0·68) |
ASCVD*: atherosclerotic cardiovascular disease, involving the coronary, cerebrovascular, or peripheral arterial systems; HFrEF†: heart failure with reduced ejection fraction (New York Heart Association class II–IV and an LVEF≤40%); HFpEF‡: heart failure with a preserved ejection fraction (New York Heart Association class II–IV and an LVEF>40%); MACE§: major adverse cardiovascular events, representing a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. Abbreviations: EMPA-REG OUTCOME: the Empagliflozin Cardiovascular Outcome Event Trial in T2DM Patients; CANVAS Program: the Canagliflozin Cardiovascular Assessment Study Program; DECLARE-TIMI 58: the Dapagliflozin Effect on Cardiovascular Events-Thrombosis in Myocardial Infarction 58; VERTIS CV: the Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial; DAPA-HF: the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure Trial; EMPEROR-Reduced: the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction; SOLOIST-WHF: the Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure Trial; EMPEROR-PRESERVED: the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction; SCORED: the Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk Trial; CREDENCE: the Canagliflozin and Renal Events in Diabetes and Established Nephropathy Clinical Evaluation Trial; DAPA-CKD: the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease Trial; SGLT2: sodium-glucose co-transporter 2; T2DM: type 2 diabetes mellitus; HF: heart failure; eGFR: estimated glomerular filtration rate; HR: hazard ration; CI: confidence interval; RRT: renal-replacement therapy; ESRD: end-stage renal disease; CKD: chronic kidney disease; Scr: serum creatinine.
Energy metabolism of the normal heart and metabolism reprogramming in the failing heart
Energy metabolism of the normal heart
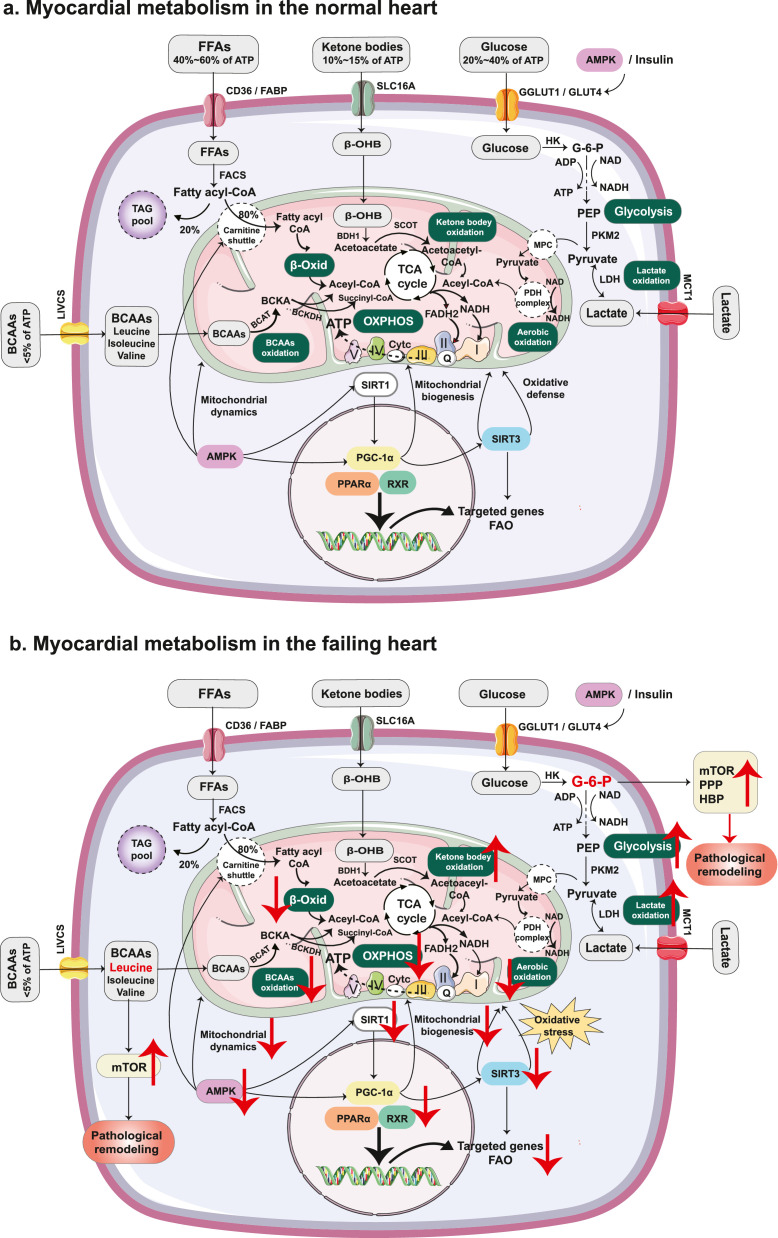
The heart is termed as a ‘metabolic omnivore’, which is capable of metabolising FAs, carbohydrates (glucose and lactate), ketone bodies, and amino acids in a supply-dependent manner in order to ensure the replenishment of ATP for the contractile demand.42 In the adult heart, mitochondria occupy over one-third of the cardiomyocyte volume and mitochondrial oxidative phosphorylation contributes to over 95% of ATP production, with glycolysis providing the remaining 5%.43 Under normoxic conditions, FA oxidation (FAO) serves as the chief energy source, providing approximately 40% to 60% of the total ATP, followed by carbohydrates metabolism (20–40%), with only modest utilisation (10%–15%) of ketone bodies or branched-chain amino acids (BCAAs).44, 45, 46
FAs are transported into the cardiomyocytes partly via FA translocase cluster of differentiation 36 (CD36) and plasma membrane FA binding protein (FABP), and are then esterified to fatty acyl-CoA by fatty acyl-CoA synthetase (FACS).43 Over 80% of fatty acyl-CoA is then transferred to mitochondria with the aid of carnitine, and the unused FAs are mainly stored as triglycerides. Carnitine palmitoyl transferase 1 (CPT-1) is the rate-limiting enzyme in this process.47 After that, fatty acyl-CoA undergoes β-oxidation, generating acetyl-CoA, which enters the tricarboxylic acid (TCA) cycle and produces nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) for oxidative phosphorylation (OXPHOS) and ATP production.44 FAO is highly regulated via different mechanisms, such as the substrate availability, intermediate metabolites (e.g. malonyl CoA can induce CPT-1 inhibition), posttranslational modification of key enzymes, and transcriptional regulation of FAO-related genes, which contributes to ensuring the high energy demand for cardiac work.24,44
Glucose is another important fuel in the heart, mainly taken up by the cardiomyocyte GLUT4, followed by GLUT1. After entering myocardial cells, glucose is phosphorylated by hexokinase to form glucose-6-phosphate (G-6-P) and is then converted to pyruvate, producing ATP from glycolysis in the cytoplasm and aerobic oxidation in the mitochondria.48 The pyruvate generated from glycolysis can be converted to lactate via lactate dehydrogenase (LDH). Lactate can also be taken up by cardiomyocytes via the lactate exporter monocarboxylate transporter 1 (MCT1) and then converted into pyruvate to enter the mitochondria via the mitochondrial pyruvate carrier (MPC), which serves as an energy substrate for the heart.49
Ketone bodies are produced in the liver and are utilised in extrahepatic tissues. β-Hydroxybutyrate (β-OHB) is the predominant ketone body utilised in the heart and is taken up by cardiomyocytes via SLC16A1. β-OHB is then converted to acetoacetate via β-hydroxybutyrate dehydrogenase 1 (BDH1) and activated by the succinyl-CoA:3-oxoacid CoA transferase (SCOT) to form acetoacetyl CoA. Acetoacetyl-CoA undergoes a thiolysis process to form acetyl-CoA and then enters the TCA cycle to produce ATP for cardiac contraction.45
BCAAs (leucine, valine, and isoleucine) are also readily used as fuel in non-hepatocytes. The initial step in BCAAs metabolism is the transamination to form branched-chain α-ketoacids (BCKAs) via branched-chain aminotransferases (BCATs). Then, BCKAs undergo irreversible oxidative decarboxylation via the branched-chain α-ketoacid dehydrogenase (BCKDH) complex to form acetyl-CoA or succinyl-CoA, which then enter the TCA cycle to produce ATP.46 The energy metabolism in the normal heart is shown in Figure 1a.
Figure 1.
Energy metabolism in the normal heart and failing heart. (a). In the normal heart, FAs are the main substrate for cardiac ATP production, whereas carbohydrates (glucose and lactate), ketone bodies, and BCAAs play a lesser role. (b). In the condition of HF, myocardial substrate utilisation shifts from FAs towards carbohydrates (especially glycolysis). The uncoupling between glycolysis and glucose oxidation, and the diminished cardiac BCAA catabolism, lead to the accumulation of glycolytic intermediates and BCAAs (leucine), activating the mTOR signalling pathway, which contributes to insulin resistance and pathological remodelling. In addition, the myocardium increasingly relies on ketone bodies as an alternative fuel. Nutrient deprivation sensors, including SIRT1 and AMPK, are suppressed in the condition of HF, leading to reduced FAO, impaired mitochondria biogenesis and dynamics, and increased oxidative stress through the effects on downstream transcriptional regulators (mainly PCG-1α). Abbreviations: FFA:free fatty acid; ATP: adenosine triphosphate; ADP: adenosine diphosphate; CD36: cluster of differentiation 36; FABP: fatty acid binding protein; CoA: coenzyme A; FACS: fatty acyl-CoA synthetase; TAG: triglyceride; β-Oxid: β-oxidation; β-OHB: β-hydroxybutyrate; BDH1: β- hydroxybutyrate dehydrogenase-1; SCOT: succinyl-CoA: 3-oxoacid CoA transferase; TCA: tricarboxylic acid; AMPK: adenosine monophosphate-activated protein kinase; GLUT1: glucose transporter 1; GLUT4: glucose transporter 4; HK: hexokinase; G-6-P: glucose-6-phosphate; PEP: phosphoenolpyruvate; PKM2: pyruvate kinase; LDH: lactate dehydrogenase; MCT1 monocarboxylic acid transporter 1; MPC: mitochondrial pyruvate carrier; PDH: pyruvate dehydrogenase; FADH2: flavin adenine dinucleotide; NADH: nicotinamide adenine dinucleotide; OXPHOS: oxidative phosphorylation; Q: coenzyme Q; Cytc: cytochrome c; BCAA: branched-chain amino acid; LIVCS: branched chain amino acid:cation symporter; BCAT: branched-chain amino acid aminotransferase; BCKA: branched-chain ketoacid; BCKDH: branched chain alpha-keto acid dehydrogenase; SIRT1: silent information regulator 1; PGC-1α: peroxisome proliferator-activated receptor-gamma co-activator 1α; PPARα: peroxisome proliferator-activated receptor α; RXR: retinoid X receptors; FAO: fatty acid oxidation; SIRT3: silent information regulator 3; mTOR: mechanistic target of rapamycin; PPP: pentose phosphate pathway; HBP: hexosamine biosynthetic pathway.
Metabolism reprogramming in heart failure
In the failing heart, energy metabolism is perturbed, and metabolic flexibility is impaired, along with the impairment in mitochondrial function and oxidative metabolism, leading to an ‘energy-starved’ state.44,45,50 Myocardial energy substrate shifts from FAs towards carbohydrates (especially glycolysis) to adapt to reduced oxygen and energy demands, which is consistent with the re-induction of a fetal-like metabolic mode.51 Increased glycolysis is consistent with the increased expression of GLUT1 to accelerate glucose uptake, but it is insufficient to compensate for the energy deficit.44 In addition, the uncoupling between glycolysis and glucose oxidation leads to the accumulation of glycolytic intermediates. Among these, G-6-P, which was recently found to be controlled by phosphoglucose isomerase activity, could serve as an activator of the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) and also feed into accessory pathways, such as the hexosamine biosynthetic pathway and pentose phosphate pathway, exacerbating myocardial pathological remodelling.52,53
Moreover, recent studies indicate that the myocardium increasingly relies on ketone bodies as an alternative fuel in advanced HF.54,55 Using a method of metabolomics, Murashige et al.56 found that patients with HFrEF had nearly tripled consumption of ketone bodies (16·4% versus 6·4%) and doubled consumption of lactate (5·0% versus 2·8%), with reduced FAO from 85·9% to 71·4%. Given that the phosphorus/oxygen (P/O) ratio of ketones (2·50) is higher than palmitate (2·33), this shift in substrate preference may represent an adaptive mechanism in HFrEF. In contrast to HFrEF, a recent study developed a mouse model of HFpEF and found a reduction in the oxidation of β-OHB, indicating that the utilisation of ketone bodies may not contribute to its benefits for HFpEF. Interestingly, they found that mitochondrial hyperacetylation and inflammation are key drivers in the pathogenesis of HFpEF, which could be improved by increasing β-OHB level.57 Future studies are needed to explore the exact role of ketone bodies in HFpEF and their potential mechanisms.
Furthermore, elevated circulating and cardiac BCAAs were also found in HF, along with diminished cardiac BCAA catabolism.58 Utilising coronary artery ligation-induced murine myocardial infarction (MI) models, Wang et al.59 found that impaired cardiac BCAA catabolism acted as a direct contributor to post-MI cardiac dysfunction and remodelling, which might partially be through the activation of the mTOR signalling. Moreover, enhancing BCAA catabolism using BT2 (3,6-dichlorobenzo[b] thiophene-2-carboxylic acid), an inhibitor of BCKDH kinase, was demonstrated to improve cardiac systolic contractility and diastolic mechanics in a mouse model of HF, indicating that targeting BCAA catabolism could be a novel and potentially efficacious treatment for HF.60 In humans with established HF, impaired BCAA oxidation was also found to be associated with cardiac insulin resistance.61 How changes in BCAA metabolism alter HF remains incompletely understood, and the significance of BCAAs in the pathogenesis of HF requires further study.
Additionally, in the failing heart, the oxidative metabolism in mitochondria is hindered, leading to a state of intracellular nutrient surplus, which is reported to suppress the nutrient deprivation sensors, including silent information regulator 1 (SIRT1) and adenosine monophosphate-activated protein kinase (AMPK).62,63 SIRT1, a nicotinamide adenine dinucleotide-dependent deacetylase, acts as a master switch that controls the gluconeogenic/glycolysis pathways and lipid metabolism, as well as mitochondria quality maintenance through its action on downstream substrates.64 Activation of AMPK increases the rate of catabolic metabolism and decreases the rate of anabolic metabolism to promote ATP production, partly by inhibiting mTORC1.65 Peroxisome proliferator-activated receptor-gamma co-activator 1α (PGC-1α), a master transcriptional regulator of lipid metabolism and mitochondria function, could be activated by upstream SIRT1 through deacetylation or AMPK through phosphorylation.66 The effects of PGC-1α contain many aspects,67 such as (1) promoting mitochondria biogenesis and mitochondrial DNA replication, (2) optimising mitochondrial dynamics, (3) defending against oxidative stress by activating SIRT3, and (4) stimulating FAO through peroxisome proliferator-activated receptors (PPARs) and retinoid X receptors (RXRs). Under HF conditions, PGC-1α expression is repressed, which correlates with mitochondrial dysfunction, increased oxidative stress, and reduced FAO. Energy metabolism in the HF is shown in Figure 1b.
Energy metabolism of the kidney and metabolism reprogramming in diabetic kidney disease
Energy metabolism of the normal kidney
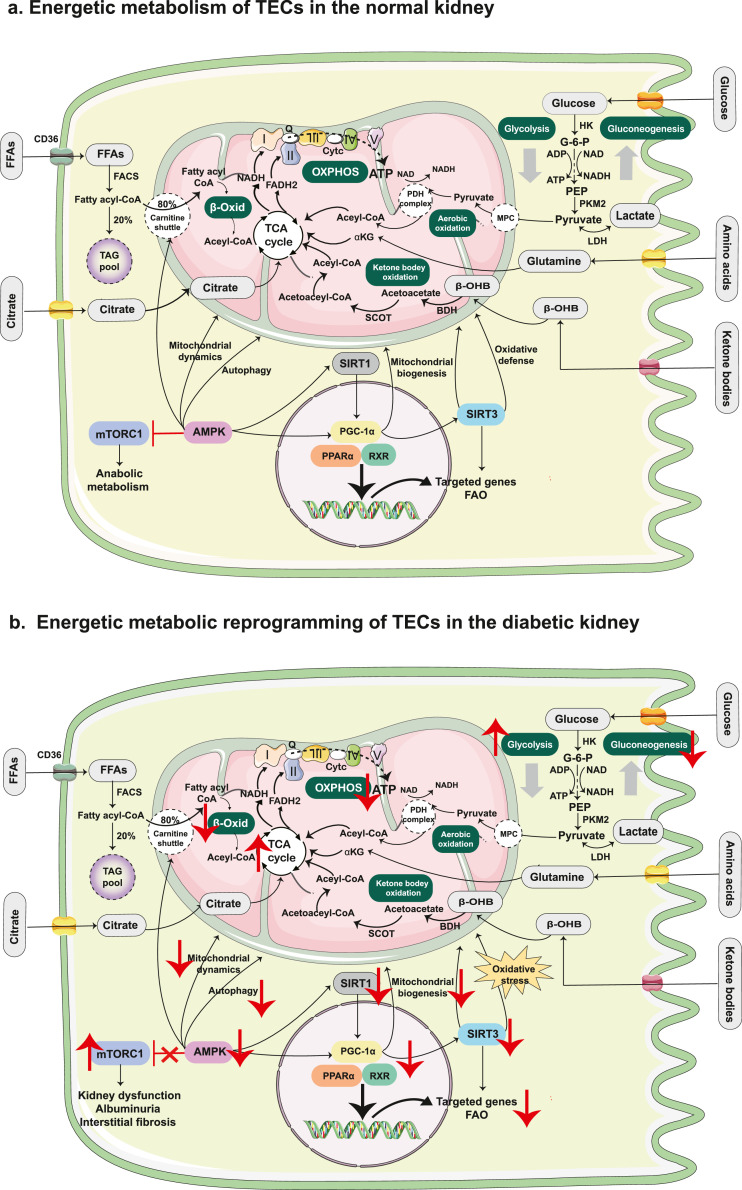
After the heart, the kidney is the second-most metabolically active organ, which requires a tremendous amount of ATP to remove waste products and reabsorb most filtered water and solutes.68 The kidney displays different metabolic programs in various regions. The renal medulla mainly exhibits high rates of glycolysis due to its low oxygen tension and low levels of oxidative enzymes, whereas the renal cortex, composed of a large population of proximal tubules, relies more heavily on FAO and gluconeogenesis.69 More specifically, the proximal tubule, responsible for the reabsorption of approximately 70% of the glomerular filtered fluid and solutes, generates ATP primarily via the aerobic metabolism of various substrates, including FAs, glutamine, pyruvate, citrate, and lactate.70 In contrast, the distal tubules rely substantially on anaerobic glycolysis to generate ATP under the low oxygen tension of the renal medulla.71 The energy metabolism in the renal tubular epithelial cells (TECs) is shown in Figure 2a.
Figure 2.
Energy metabolism in TECs of the normal kidney and the diabetic kidney. (a). TECs generate ATP primarily via aerobic metabolism of various substrates, including FAs, glutamine, pyruvate, citrate and lactate, which are metabolised to acetyl-CoA before entering the TCA cycle. Proximal TECs produces ATP mainly through FAO and also participate in the process of gluconeogenesis. In contrast, the distal tubules rely substantially on anaerobic glycolysis to generate ATP. (b). In DKD, the metabolic program shifts from FAO to glycolysis. The levels of transcriptional regulators and key enzymes related to FAO were significantly down-regulated in proximal TECs. Lipid accumulation due to decreased FAO or increased FA synthesis contributes to the development of DKD. The mTOR and AMPK signalling pathways play important roles in the progression of DKD, involving the regulation of energy homeostasis and mitochondrial function. mTORC1 was found to be activated in proximal TECs of the diabetic kidney, leading to kidney dysfunction, albuminuria, and interstitial fibrosis. AMPK can participate in the regulation of mitochondrial biogenesis by stimulating PGC-1α. In addition, AMPK stimulates the catabolic metabolism and activates autophagy by inhibiting mTORC1 under conditions of nutrient deprivation. Abbreviations: TEC: tubular epithelial cell; ATP: adenosine triphosphate; CoA: coenzyme A; TCA: tricarboxylic acid; FAO: fatty acid oxidation; FFA: free fatty acid; CD36: cluster of differentiation 36; ADP: adenosine diphosphate; FACS: fatty acyl-CoA synthetase; TAG: triglyceride; β-Oxid: β-oxidation; HK: hexokinase; G-6-P: glucose-6-phosphate; PEP: phosphoenolpyruvate; PKM2: pyruvate kinase; LDH: lactate dehydrogenase; αKG: α-ketoglutarate; β-OHB: β-hydroxybutyrate; BDH1: β- hydroxybutyrate dehydrogenase-1; SCOT: succinyl-CoA: 3-oxoacid CoA transferase; MPC: mitochondrial pyruvate carrier; PDH: pyruvate dehydrogenase; FADH2: flavin adenine dinucleotide; NADH: nicotinamide adenine dinucleotide; OXPHOS: oxidative phosphorylation; Q: coenzyme Q; Cytc: cytochrome c; mTOR: mechanistic target of rapamycin; SIRT1: silent information regulator 1; PGC-1α: peroxisome proliferator-activated receptor-gamma co-activator 1α; PPARα: peroxisome proliferator-activated receptor α; RXR: retinoid X receptors; SIRT3: silent information regulator 3.
Metabolism reprogramming in diabetic kidney disease
DKD is a chronic microvascular complication of DM and one of the leading causes of end-stage renal disease (ESRD).72 The pathophysiology of DKD is complex, multifactorial, and heterogeneous. Metabolic network alterations in the kidney induced by hyperglycaemia and dyslipidaemia play crucial roles in DKD progression.73 Sas et al.74 used metabolic flux analysis and found that the TCA cycle and glycolysis fluxes in the diabetic kidney cortex were higher in db/db mice than in control mice. Moreover, increased metabolism in the early stages of DKD was found to be associated with mitochondrial electron transport chain dysfunction, leading to less efficient ATP production and the progression of DKD.
With respect to renal glucose reabsorption, the proximal tubule plays a central role in the sodium-glucose linked transport, which accounts for 60% of the kidney's energy consumption. Notably, proximal TECs consume almost two-thirds of the energy used for reabsorption.70 In the context of diabetes, glucose reabsorption via proximal TECs is increased by hyperglycaemia due to the up-regulation of SGLT2.75 In DKD mice, the increased glucose uptake of proximal TECs was reported to lead to a hyper-nutrient state and thereby activated nutrient-sensing pathways in these cells, which contribute to kidney dysfunction, albuminuria, and interstitial fibrosis.76
In the diabetic kidney, the metabolic program shifts from FAO to glycolysis.74,77 In DKD, Cai et al.77 found that lipid accumulation was markedly elevated in the tubulointerstitial space of microdissected human kidney samples. In addition, the levels of the transcriptional regulator PPARα and key enzyme CPT-1 related to FAO were significantly lower in patients with DKD than in those without diabetes. Lipid accumulation due to decreased FAO or increased FA synthesis also contributes to the development of nephropathy in the context of metabolic diseases.78 DKD progression to renal fibrosis was reported to be characterised by a metabolic switch from FAO to glycolysis and lipid accumulation, which was associated with the increased expression of HIF-1α, a contributor to renal tubulointerstitial inflammation.77,79
In particular, two nutrient-sensing pathways have been extensively investigated in the kidney: the mTOR and AMPK signalling pathways. Both signalling pathways play a role in regulating energy homeostasis and mitochondrial function.80,81 mTORC1, activated by the availability of nutrients (mainly glucose and amino acids), was found to be activated in proximal TECs of the diabetic kidney, leading to kidney dysfunction, albuminuria, and interstitial fibrosis.76 In addition, hyperactivation of mTORC1 has also been found to be strongly related to podocyte damage in DKD by increasing cytotoxicity and inhibiting autophagy, indicating that inhibition of overactive mTORC1 could be a promising therapy for both non-proteinuric and proteinuric DKD.82 AMPK participates in the regulation of mitochondrial biogenesis by stimulating PGC-1α. Additionally, AMPK stimulates the catabolic metabolism and activates autophagy by inhibiting mTORC1 under conditions of nutrient deprivation, which is another therapeutic target for metabolic dysfunction in DKD.66 Energetic metabolic reprogramming of TECs in the diabetic kidney is shown in Figure 2b.
Cardiorenal protection of SGLT2 inhibitor based on metabolic reprogramming
SGLT2 inhibitors induce a fasting-like metabolic paradigm
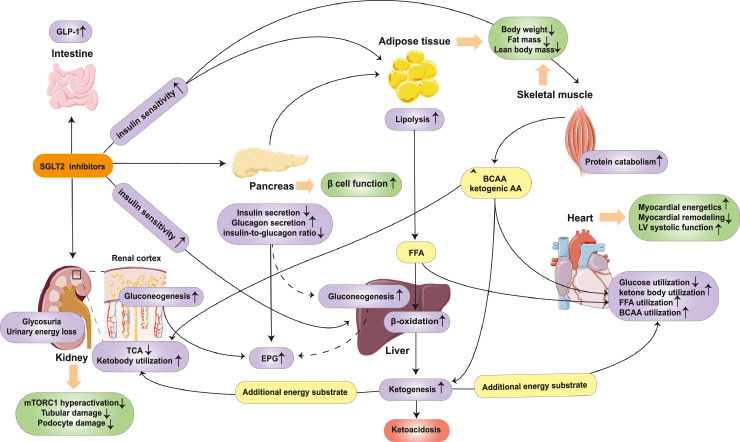
Given that SGLT2 is the most important mediator of filtered glucose reabsorption, selective inhibition of SGLT2 induces glycosuria and increases the net urinary caloric loss to approximately 200–300 kcal per day, despite the compensatory up-regulation of SGLT1. Calorie loss mediated by SGLT2 inhibition mimics a fasting-like metabolic paradigm, which further elicits adaptive responses of the whole-body energy metabolism involving glucose homeostasis, hormone release, fuel selection, and energy expenditure.83
The direct metabolic effect of SGLT2 inhibitors was shown to induce a decrease in blood glucose levels, which elicited a decrease in insulin secretion and a rise in glucagon, as well as a paradoxical increase in endogenous glucose production (EGP).84,85 The mechanisms underlying the increase in EPG are not completely understood, which may be attributed to (1) the increased glucagon levels and the glucagon-to-insulin ratio,86 (2) the direct inhibition of SGLT2 in pancreatic α-cells to promote glucagon secretion,87 (3) an increase in gluconeogenesis,88,89 and (4) a neuronal loop between the kidney and liver to stimulate hepatic glucose production.90,91 The increase in EPG potentially counteracts the glucose-lowering potency of SGLT2 inhibitors, thereby reducing the risk of hypoglycaemia. Thus, the cardiorenal protection of SGLT2 inhibitors may be derived from the dual benefits of lowering blood sugar levels and preventing hypoglycaemia. Along with the reduction in insulin secretion, β-cell function and whole-body/peripheral insulin sensitivity were significantly improved, which may be partially due to the rapidly reduced glucotoxicity and chronic decrease in body weight.84,92,93
SGLT2 inhibitiors were also found to exert indirect kidney and heart protecting effects independent of the direct effects of SGLT2 inhibition, at least in part. A metabolome analysis of diabetic mice showed that ipragliflozin reversed the accumulation of TCA cycle intermediates and increased oxidative stress in renal cortex, which was consistent with improvements in glomerular damage. They found that ipragliflozin ameliorated the accumulation of TCA cycle intermediates and oxidative stress in the cortex, which was consistent with improvements in glomerular damage. However, similar effects induced by ipragliflozin were not found in the inner medulla, future studies are needed to clarify the differential metabolic effects of SGLT2 inhibitors in different regions of the kidney.94 In addition, after chronic treatment with SGLT2 inhibitors, increased lipid responses, involving lipolysis, lipid oxidation, and ketogenesis, became more marked in patients with and without diabetes, suggesting a progressive shift in fuel utilisation from carbohydrates to FAs and ketone bodies.95,96 Elevated levels of FAs are used for the hepatic formation of ketone bodies. Subsequently, liver-derived ketone bodies, as energy-efficient substrates, are taken up by the heart and kidney, thereby improving the performance of the myocardium and kidney epithelia.97, 98, 99 In a study conducted by Verma et al.,97 although increased ketones did not enhance cardiac efficiency (cardiac work/oxygen consumed) in db/db mice, empagliflozin increased cardiac energy production rates owing to the additional contribution of ketone oxidation. Even in a non-diabetic porcine model with HF, the empagliflozin-treated group showed a reduction in myocardial glucose utilisation and switched towards utilisation of ketone bodies, FAs, and BCAAs, which improved myocardial energetics.98 In terms of the diabetic kidney, Tomita and his colleagues also demonstrated that empagliflozin raised endogenous ketone body levels and restored ATP levels in non-proteinuric DKD mice and diabetic db/db mice.99 Notably, β-OHB was found to enhance respiratory efficiency in isolated heart mitochondria of C57BL/6N mice,55 and was also reported to attenuate the formation of nucleotide-binding oligomerisation domain-like receptor P3 inflammasome, which may potentiate the effects of cardiorenal protection.57 So far, the detailed molecular mechanisms by which SGLT2 inhibitors contribute to an increase in ketone body levels, and mechanisms underlying the elevated ketone body utilisation in damaged kidneys and myocardia remains unclear. Further research is required to address this issue.
Furthermore, SGLT2 inhibition was reported to increase the utilisation of BCAAs in the failing myocardia of non-diabetic pigs, along with the increased activity of BCKDH and decreased activity of phosphorylation of the BCKD-E1a subunit (which is inverse to BCKDH activity), leading to a markedly improved myocardial energetics.98 In addition, Kappel et al.100 performed an untargeted metabolomics approach and found that empagliflozin increased the degradation products of BCAAs in patients with T2DM and CVD. However, the role of BCAAs in cardiorenal protection remains unclear, and further experimental studies are needed to investigate the effect of SGLT2 inhibitors on BCAA metabolism and explore the exact cardiorenal protective mechanisms induced by BCAA catabolism.
Taken together, SGLT2 inhibitor therapy leads to glycosuria and a net urinary energy loss, thereby eliciting a fasting-like metabolic paradigm involving a reduction in glucose utilisation and a switch towards utilisation of FAs, ketone bodies and BCAAs. The effects of SGLT2 inhibitors on energy metabolism are summarized in Figure 3.
Figure 3.
Effects of SGLT2 inhibitors on energy metabolism. SGLT2 inhibitor therapy leads to glycosuria and a net urinary energy loss, along with a decreased insulin level and increased glucagon level, as well as GLP-1. Despite the reduction in insulin secretion, β-cell function and the whole body/peripheral tissue insulin sensitivity improved. Increased glucagon levels and decreased insulin-to-glucagon ratio lead to the elevation of EPG, which may be partly derived from the increased gluconeogenesis in the liver and the kidney. Glucose utilisation is downregulated, while increased lipid responses, involving lipolysis, lipid oxidation, and ketogenesis, markedly increased after SGLT2 inhibitor treatment. The elevated levels of ketone bodies can serve as an additional energy substrate, thus improving the performance of the heart and the kidney. Catabolism of protein might also be involved in the metabolic adaptation process when treated with SGLT2 inhibitors. BCAAs comes from skeletal muscle or diet protein and will be further utilised in myocardial and renal cells to meet the needs of energy metabolism. Increased protein catabolism, as well as increased lipolysis, finally lead to the loss of body weight, including lean body mass and fat mass, contributing to the improvement of β-cell function and insulin sensitivity. Abbreviations: SGLT2: sodium-glucose co-transporter 2; GLP-1: glucagon-like peptide-1; TCA: tricarboxylic acid cycle; mTORC1: mechanistic target of rapamycin complex 1; FFA: free fatty acid; EPG: endogenous glucose production; LV: left ventricular; BCAA: branched-chain amino acid.
SGLT2 inhibitors induce a nutrient deprivation transcriptional paradigm
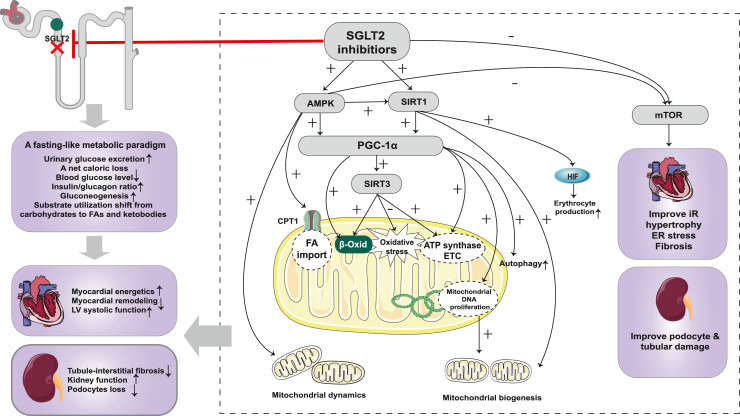
Activation of AMPK/SIRT1/PGC-1α signalling pathway
As mentioned above, SGLT2 inhibition leads to a net caloric loss, which mimics a fasting-like metabolic paradigm and thereby induces the nutrient deprivation pathway. In a series of experimental studies, SGLT2 inhibitors were shown to activate the AMPK/SIRT1/PGC-1α signalling pathway, thereby enhancing FAO, gluconeogenesis, and ketogenesis, which mimics a fasting-like metabolic state and remarkably improves the state of energy deficiency.101, 102, 103, 104, 105 More importantly, the activation of AMPK/SIRT1/PGC-1α induced by SGLT2 inhibition was also found to improve mitochondrial dysfunction, thereby attenuating oxidative stress, endoplasmic reticulum (ER) stress, inflammation and apoptosis.103, 104, 105, 106, 107, 108, 109, 110
Additionally, the AMPK/SIRT1/PGC-1α pathway activated by SGLT2 inhibition may exert other pleiotropic cardioprotective effects. The nutrient-sensing pathway induced by SGLT2 inhibition enhances autophagy in both the heart and kidneys. Autophagy-mediated recycling of cellular components may explain the restored ATP production and reduced oxidative stress and ER stress.109,111,112 Additionally, activation of the AMPK/SIRT1/PGC-1α pathway was reported to modulate the closely linked hypoxia-inducible factor (HIF) pathway in DM-related renal injury.113 However, the effect of SGLT2 inhibitors on the HIF pathway in the heart remains unclear. Given that the increase in haematocrit after SGLT2 inhibitor treatment has been postulated to account for ∼50% of the cardiovascular benefit of major adverse cardiovascular events in the EMPA-REG OUTCOME trial, the cardioprotection induced by SGLT2 inhibitors may be partially due to their erythropoietic effects.114 Further studies are needed to elucidate the effects and molecular mechanisms of SGLT2 inhibition on the HIF pathway, in order to better clarify the cardiorenal protective mechanisms of SGLT2 inhibitors.
Inhibition of the mTOR signalling pathway
mTOR is a serine/threonine-kinase complex. There are two distinct mTOR complexes: mTORC1 and mTORC2. mTORC1 is considered a nutrient sensor responsible for stimulating anabolic metabolism for cell growth and proliferation and inhibits autophagy.80 Overactivation of mTORC1 was reported to lead to LV dysfunction and adverse cardiac remodeling,115,116 as well as kidney dysfunction, albuminuria, and interstitial fibrosis.76 In a series of experimental studies, SGLT2 inhibitors were reported to inhibit the mTOR signalling pathway and thereby maintain redox homeostasis, upregulate autophagy, and suppress inflammation, which may partially explain the cardiorenal protective mechanisms induced by SGLT2 inhibition.76,117, 118, 119, 120, 121
Moreover, using a DKD mouse model, Tomita et al.99 reported that ATP production in proximal TECs shifted from lipolysis to ketolysis, and empagliflozin treatment restored renal ATP levels and improved kidney injury. Elevated ketone bodies were further demonstrated to attenuate mTORC1-associated tubular epithelial cell damage in non-proteinuric DKD mice and podocyte damage in diabetic mice, which provided a novel link between SGLT2 inhibition, ketone bodies, mTOR signalling, and renoprotection. So far, the detailed molecular mechanisms by which SGLT2 inhibitors contribute to the increase in ketone body levels, as well as the mechanisms underlying the elevated ketone body utilisation in damaged kidneys remain unclear. Further research is required to answer these questions.
In summary, metabolic reprogramming plays an important role in disease progression of HF and DKD. SGLT2 inhibitors trigger metabolic readjustments in the context of HF and DKD, which may partially explain the mechanisms of their cardiorenal protective effects. SGLT2 inhibition leads to marked glycosuria and a net caloric loss, which mimics a fasting-like metabolic response. The energy substrates shift from carbohydrates to FAs and ketone bodies, which restore ATP levels. In addition, SGLT2 inhibitors induce a nutrient deprivation transcriptional paradigm via acting on nutrient-sensing pathways, involving the AMPK/SIRT1/PGC-1α pathway and the mTOR pathway, leading to the improvements of mitochondrial function, insulin resistance, inflammation, oxidative stress, and the enhancement of autophagy, contributing to cardiorenal protection. The mechanisms of action of SGLT2 inhibitors in cardiorenal protection based on metabolic reprogramming are shown in Figure 4.
Figure 4.
Cardiorenal protection of SGLT2 inhibitor based on metabolic reprogramming. SGLT2 inhibition leads to marked glycosuria and a net caloric loss, which mimics a fasting-like metabolic response. The energy substrates shift from carbohydrates to FAs and ketone bodies, which restore ATP levels to exert cardiorenal protective effects. SGLT2 inhibitors induce a nutrient deprivation transcriptional paradigm via acting on nutrient-sensing pathways. SGLT2 inhibitors activate the AMPK/SIRT1/PGC-1α pathway, enhancing FAO and ketogenesis, which improve the energy state of the heart and kidney. In addition, the activated AMPK/SIRT1/PGC-1α pathway exerts other protective effects, including improving mitochondrial biogenesis and dynamics, enhancing autophagy, and attenuating oxidative stress. SGLT2 inhibitors inhibit the mTOR pathway and thereby improve insulin resistance, cardiac hypertrophy, fibrosis, and oxidative stress. In addition, inhibition of mTORC1 prevents tubular and podocyte damage, partially via the effects of increased ketone bodies on mTORC1. Abbreviations: SGLT2: sodium-glucose co-transporter 2; FA: fatty acids; LV: left ventricular; mTOR: mechanistic target of rapamycin; mTORC1: mechanistic target of rapamycin complex 1; SIRT1: silent information regulator 1; PGC-1α: peroxisome proliferator-activated receptor-gamma co-activator 1α; SIRT3: silent information regulator 3; HIF: hypoxia-inducible factor; CPT-1: carnitine palmitoyl transferase-1; β-Oxid: β-oxidation; ATP: adenosine triphosphate; ETC: electron transfer chain; DNA: deoxyribonucleic acid; IR: insulin resistance; ER: endoplasmic reticulum.
Conclusion
The striking cardiorenal protective effects of SGLT2 inhibitors have encouraged us to elucidate their potential mechanisms beyond hyperglycaemia control. Recent studies on their broad influence on glucose, lipid, and protein metabolism have provided a novel vision for understanding the beneficial cardiorenal effects of this drug class. Interestingly, SGLT2 inhibitors could induce a fasting-like metabolic paradigm involving the metabolic switch from carbohydrates to lipid utilisation and ketogenesis, which activates nutrient deprivation pathways and contributes to maintaining energy homeostasis. These metabolic improvements may partially explain the cardiorenal protective effects of SGLT2 inhibitors. Further studies are needed to elucidate the ambiguous questions regarding metabolic changes and obtain a complete view of the effects of SGLT2 inhibition on metabolism and its mechanisms.
Outstanding questiones
-
1.
The cardiorenal benefits of SGLT2 inhibitors in patients with HFpEF or more advanced CKD (eGFRs as low as 20 per min per 1·73 m2) are still incompletely understood and should be investigated in future large-scale RCTs.
-
2.
What are the potential benefits of shifting fuel utilisation pathways, especially the exact role of ketone bodies in patients with HF?
-
3.
The mechanisms that lead to an increase in EGP following SGLT2 inhibition remain poorly understood. Future studies elucidating the exact mechanisms will contribute to our understanding of glucose homeostasis after SGLT2 inhibition.
-
4.
The role of BCAA remains unclear. Further experimental studies are needed to investigate the effect of SGLT2 inhibitors on BCAA catabolism and explore the exact cardiorenal protective mechanisms induced by BCAA catabolism.
Search strategy and selection criteria
Data for this review were identified by searches of PubMed and references from relevant articles using the search terms ‘SGLT2 inhibitor’, ‘metabolism’, ‘metabolic reprogramming’, ‘heart failure’, ‘diabetes mellitus’, ‘DKD’, and ‘diabetic kidney disease’. Only articles published in English up to 31 March 2022 were included.
Contributors
Bi-Cheng Liu generated the initial concepts for this review, provided insightful comments, and approved the final version of the manuscript. Yue-Ming Gao and Song-Tao Feng performed the literature search, wrote the original draft, and designed the figures and the table. Yi Wen, Tao-Tao Tang and Bin Wang revised the manuscript. All authors have read and approved the final version of the manuscript and ensured that this was the case.
Declaration of interests
The authors confirm there are no conflicts of interest.
Acknowledgments
This study was supported by grants from the National Key Research Programme of the Ministry of Science and Technology (2018YFC130046, 2018YFC1314000) and the National Natural Science Foundation of China (82030024, 81720108007, 82070735) to Prof. Bi-Cheng Liu, a grant from the National Natural Science Foundation of China (82070735) to Bin Wang, and a grant from the National Natural Science Foundation of China (81900623) to Yi Wen. The funding source had no involvement in paper design, data collection, data analysis, interpretation and writing of the paper.
Contributor Information
Bin Wang, Email: wangbinhewei@126.com.
Bi-Cheng Liu, Email: liubc64@163.com.
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association 12. Older adults: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl 1):S139–S147. doi: 10.2337/dc19-S012. [DOI] [PubMed] [Google Scholar]
- 3.Zerga AA, Bezabih AM. Metabolic syndrome and lifestyle factors among type 2 diabetes mellitus patients in Dessie referral hospital, Amhara region, Ethiopia. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22:1607–1618. doi: 10.1111/dom.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yong J, Johnson JD, Arvan P, Han J, Kaufman RJ. Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus. Nat Rev Endocrinol. 2021;17:455–467. doi: 10.1038/s41574-021-00510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: a review of multi-target drugs. Molecules. 2020;25:1987. doi: 10.3390/molecules25081987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeghate EA, Kalász H, Al Jaberi S, Adeghate J, Tekes K. Tackling type 2 diabetes-associated cardiovascular and renal comorbidities: a key challenge for drug development. Expert Opin Investig Drugs. 2021;30:85–93. doi: 10.1080/13543784.2021.1865914. [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 10.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 16.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 17.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 18.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 19.Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16:317–336. doi: 10.1038/s41581-020-0256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertero E, Prates Roma L, Ameri P, Maack C. Cardiac effects of SGLT2 inhibitors: the sodium hypothesis. Cardiovasc Res. 2018;114:12–18. doi: 10.1093/cvr/cvx149. [DOI] [PubMed] [Google Scholar]
- 21.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 22.Marton A, Kaneko T, Kovalik JP, et al. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat Rev Nephrol. 2021;17:65–77. doi: 10.1038/s41581-020-00350-x. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Cheng CF, Ku HC, Lin H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. 2018;19:3447. doi: 10.3390/ijms19113447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Sha Z, Peng H. Metabolic reprogramming in kidney diseases: evidence and therapeutic opportunities. Int J Nephrol. 2021;2021 doi: 10.1155/2021/5497346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Lu S, Li X. The role of metabolic reprogramming in tubular epithelial cells during the progression of acute kidney injury. Cell Mol Life Sci. 2021;78:5731–5741. doi: 10.1007/s00018-021-03892-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packer M. Cardioprotective effects of sirtuin-1 and its downstream effectors: potential role in mediating the heart failure benefits of SGLT2 (sodium-glucose cotransporter 2) inhibitors. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007197. [DOI] [PubMed] [Google Scholar]
- 28.Gyimesi G, Pujol-Giménez J, Kanai Y, Hediger MA. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application. Pflugers Arch. 2020;472:1177–1206. doi: 10.1007/s00424-020-02433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17:761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 30.Ghezzi C, Loo DDF, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61:2087–2097. doi: 10.1007/s00125-018-4656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol. 2021;83:503–528. doi: 10.1146/annurev-physiol-031620-095920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi T, Dohi K, Omori T, et al. Diuretic effects of sodium-glucose cotransporter 2 inhibitor in patients with type 2 diabetes mellitus and heart failure. Int J Cardiol. 2015;201:1–3. doi: 10.1016/j.ijcard.2015.07.072. Erratum in: Int J Cardiol 2016; 206: 173. [DOI] [PubMed] [Google Scholar]
- 33.Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–476. doi: 10.1016/j.jjcc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Packer M. Mechanisms leading to differential hypoxia-inducible factor signaling in the diabetic kidney: modulation by SGLT2 inhibitors and hypoxia mimetics. Am J Kidney Dis. 2021;77:280–286. doi: 10.1053/j.ajkd.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Bell RM, Yellon DM. SGLT2 inhibitors: hypotheses on the mechanism of cardiovascular protection. Lancet Diabetes Endocrinol. 2018;6:435–437. doi: 10.1016/S2213-8587(17)30314-5. [DOI] [PubMed] [Google Scholar]
- 36.Yu YW, Que JQ, Liu S, et al. Sodium-glucose co-transporter-2 inhibitor of dapagliflozin attenuates myocardial ischemia/reperfusion injury by limiting NLRP3 inflammasome activation and modulating autophagy. Front Cardiovasc Med. 2022;8 doi: 10.3389/fcvm.2021.768214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hess DA, Terenzi DC, Trac JZ, et al. SGLT2 inhibition with empagliflozin increases circulating provascular progenitor cells in people with type 2 diabetes mellitus. Cell Metab. 2019;30:609–613. doi: 10.1016/j.cmet.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18:783–794. doi: 10.1111/dom.12670. [DOI] [PubMed] [Google Scholar]
- 39.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. Erratum in: Lancet 2019; 393: 30. [DOI] [PubMed] [Google Scholar]
- 40.Williams DM, Evans M. Dapagliflozin for heart failure with preserved ejection fraction: will the DELIVER Study deliver? Diabetes Ther. 2020;11:2207–2219. doi: 10.1007/s13300-020-00911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almaimani M, Sridhar VS, Cherney DZI. Sodium-glucose cotransporter 2 inhibition in non-diabetic kidney disease. Curr Opin Nephrol Hypertens. 2021;30:474–481. doi: 10.1097/MNH.0000000000000724. [DOI] [PubMed] [Google Scholar]
- 42.Wende AR, Brahma MK, McGinnis GR, Young ME. Metabolic origins of heart failure. JACC Basic Transl Sci. 2017;2:297–310. doi: 10.1016/j.jacbts.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honka H, Solis-Herrera C, Triplitt C, Norton L, Butler J, DeFronzo RA. Therapeutic manipulation of myocardial metabolism: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:2022–2039. doi: 10.1016/S0735-1097(21)03378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–1513. doi: 10.1161/CIRCRESAHA.121.318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulze PC, Wu JMF. Ketone bodies for the starving heart. Nat Metab. 2020;2:1183–1185. doi: 10.1038/s42255-020-00310-6. [DOI] [PubMed] [Google Scholar]
- 46.Neinast MD, Jang C, Hui S, et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 2019;29:417–429.e4. doi: 10.1016/j.cmet.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laudette M, Sainte-Marie Y, Cousin G, et al. Cyclic AMP-binding protein Epac1 acts as a metabolic sensor to promote cardiomyocyte lipotoxicity. Cell Death Dis. 2021;12:824. doi: 10.1038/s41419-021-04113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran DH, Wang ZV. Glucose metabolism in cardiac hypertrophy and heart failure. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glancy B, Kane DA, Kavazis AN, Goodwin ML, Willis WT, Gladden LB. Mitochondrial lactate metabolism: history and implications for exercise and disease. J Physiol. 2021;599:863–888. doi: 10.1113/JP278930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He Y, Huang W, Zhang C, et al. Energy metabolism disorders and potential therapeutic drugs in heart failure. Acta Pharm Sin B. 2021;11:1098–1116. doi: 10.1016/j.apsb.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol. 2018;15:457–470. doi: 10.1038/s41569-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 52.Davogustto GE, Salazar RL, Vasquez HG, et al. Metabolic remodeling precedes mTORC1-mediated cardiac hypertrophy. J Mol Cell Cardiol. 2021;158:115–127. doi: 10.1016/j.yjmcc.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karlstaedt A, Khanna R, Thangam M, Taegtmeyer H. Glucose 6-phosphate accumulates via phosphoglucose isomerase inhibition in heart muscle. Circ Res. 2020;126:60–74. doi: 10.1161/CIRCRESAHA.119.315180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bedi KC, Jr, Snyder NW, Brandimarto J, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horton JL, Davidson MT, Kurishima C, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4 doi: 10.1172/jci.insight.124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murashige D, Jang C, Neinast M, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364–368. doi: 10.1126/science.abc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng Y, Xie M, Li Q, et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates hFpEF. Circ Res. 2021;128:232–245. doi: 10.1161/CIRCRESAHA.120.317933. [DOI] [PubMed] [Google Scholar]
- 58.Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol. 2019;81:139–164. doi: 10.1146/annurev-physiol-020518-114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W, Zhang F, Xia Y, et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2016;311:H1160–H1169. doi: 10.1152/ajpheart.00114.2016. [DOI] [PubMed] [Google Scholar]
- 60.Chen M, Gao C, Yu J, et al. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uddin GM, Zhang L, Shah S, et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol. 2019;18:86. doi: 10.1186/s12933-019-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wan X, Wen JJ, Koo SJ, Liang LY, Garg NJ. SIRT1-PGC1α-NFκB pathway of oxidative and inflammatory stress during trypanosoma cruzi infection: benefits of SIRT1-targeted therapy in improving heart function in Chagas disease. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu M, Xue RQ, Lu Y, et al. Choline ameliorates cardiac hypertrophy by regulating metabolic remodelling and UPRmt through SIRT3-AMPK pathway. Cardiovasc Res. 2019;115:530–545. doi: 10.1093/cvr/cvy217. [DOI] [PubMed] [Google Scholar]
- 64.D'Onofrio N, Servillo L, Balestrieri ML. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal. 2018;28:711–732. doi: 10.1089/ars.2017.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fontecha-Barriuso M, Martin-Sanchez D, Martinez-Moreno JM, et al. The role of PGC-1α and mitochondrial biogenesis in kidney diseases. Biomolecules. 2020;10:347. doi: 10.3390/biom10020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clark AJ, Parikh SM. Targeting energy pathways in kidney disease: the roles of sirtuins, AMPK, and PGC1α. Kidney Int. 2021;99:828–840. doi: 10.1016/j.kint.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cargill K, Sims-Lucas S. Metabolic requirements of the nephron. Pediatr Nephrol. 2020;35:1–8. doi: 10.1007/s00467-018-4157-2. [DOI] [PubMed] [Google Scholar]
- 70.Scholz H, Boivin FJ, Schmidt-Ott KM, et al. Kidney physiology and susceptibility to acute kidney injury: implications for renoprotection. Nat Rev Nephrol. 2021;17:335–349. doi: 10.1038/s41581-021-00394-7. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Fry BC, Layton AT. Modeling glucose metabolism and lactate production in the kidney. Math Biosci. 2017;289:116–129. doi: 10.1016/j.mbs.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117:662–675. doi: 10.1016/j.jfma.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Darshi M, Van Espen B, Sharma K. Metabolomics in diabetic kidney disease: unraveling the biochemistry of a silent killer. Am J Nephrol. 2016;44:92–103. doi: 10.1159/000447954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sas KM, Kayampilly P, Byun J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight. 2016;1:e86976. doi: 10.1172/jci.insight.86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang XX, Levi J, Luo Y, et al. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J Biol Chem. 2017;292:5335–5348. doi: 10.1074/jbc.M117.779520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kogot-Levin A, Hinden L, Riahi Y, et al. Proximal tubule mTORC1 is a central player in the pathophysiology of diabetic nephropathy and its correction by SGLT2 Inhibitors. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.107954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai T, Ke Q, Fang Y, et al. Sodium-glucose cotransporter 2 inhibition suppresses HIF-1α-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice. Cell Death Dis. 2020;11:390. doi: 10.1038/s41419-020-2544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lieben L. Diabetic nephropathy: lipid toxicity drives renal disease. Nat Rev Nephrol. 2017;13:194. doi: 10.1038/nrneph.2017.22. [DOI] [PubMed] [Google Scholar]
- 79.Li ZL, Lv LL, Tang TT, et al. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95:388–404. doi: 10.1016/j.kint.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 80.Fantus D, Rogers NM, Grahammer F, Huber TB, Thomson AW. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat Rev Nephrol. 2016;12:587–609. doi: 10.1038/nrneph.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim Y, Park CW. Adenosine monophosphate-activated protein kinase in diabetic nephropathy. Kidney Res Clin Pract. 2016;35:69–77. doi: 10.1016/j.krcp.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yasuda-Yamahara M, Kume S, Maegawa H. Roles of mTOR in diabetic kidney disease. Antioxidants (Basel) 2021;10:321. doi: 10.3390/antiox10020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730–1735. doi: 10.2337/dc15-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Al Jobori H, Daniele G, Adams J, et al. Empagliflozin treatment is associated with improved β-cell function in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2018;103:1402–1407. doi: 10.1210/jc.2017-01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muscelli E, Astiarraga B, Barsotti E, et al. Metabolic consequences of acute and chronic empagliflozin administration in treatment-naive and metformin pretreated patients with type 2 diabetes. Diabetologia. 2016;59:700–708. doi: 10.1007/s00125-015-3845-8. [DOI] [PubMed] [Google Scholar]
- 86.Kalra S, Gupta Y. The insulin:glucagon ratio and the choice of glucose-lowering drugs. Diabetes Ther. 2016;7:1–9. doi: 10.1007/s13300-016-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–517. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 88.Wolf P, Fellinger P, Pfleger L, et al. Gluconeogenesis, but not glycogenolysis, contributes to the increase in endogenous glucose production by SGLT-2 inhibition. Diabetes Care. 2021;44:541–548. doi: 10.2337/dc20-1983. [DOI] [PubMed] [Google Scholar]
- 89.Kim JH, Ko HY, Wang HJ, Lee H, Yun M, Kang ES. Effect of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on gluconeogenesis in proximal renal tubules. Diabetes Obes Metab. 2020;22:373–382. doi: 10.1111/dom.13905. [DOI] [PubMed] [Google Scholar]
- 90.Solis-Herrera C, Daniele G, Alatrach M, et al. Increase in endogenous glucose production with SGLT2 inhibition is unchanged by renal denervation and correlates strongly with the increase in urinary glucose excretion. Diabetes Care. 2020;43:1065–1069. doi: 10.2337/dc19-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Daniele G, Solis-Herrera C, Dardano A, et al. Increase in endogenous glucose production with SGLT2 inhibition is attenuated in individuals who underwent kidney transplantation and bilateral native nephrectomy. Diabetologia. 2020;63:2423–2433. doi: 10.1007/s00125-020-05254-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsuba R, Matsuba I, Shimokawa M, Nagai Y, Tanaka Y. Tofogliflozin decreases body fat mass and improves peripheral insulin resistance. Diabetes Obes Metab. 2018;20:1311–1315. doi: 10.1111/dom.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kullmann S, Hummel J, Wagner R, et al. Empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: a randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Care. 2022;45:398–406. doi: 10.2337/dc21-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanaka S, Sugiura Y, Saito H, et al. Sodium-glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018;94:912–925. doi: 10.1016/j.kint.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 95.Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 96.Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin on free fatty acids and ketone bodies in Japanese patients with type 2 diabetes mellitus: a randomized controlled trial. Adv Ther. 2019;36:2769–2782. doi: 10.1007/s12325-019-01045-x. [DOI] [PubMed] [Google Scholar]
- 97.Verma S, Rawat S, Ho KL, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3:575–587. doi: 10.1016/j.jacbts.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944. doi: 10.1016/j.jacc.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 99.Tomita I, Kume S, Sugahara S, et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab. 2020;32:404–419. doi: 10.1016/j.cmet.2020.06.020. .e6. [DOI] [PubMed] [Google Scholar]
- 100.Kappel BA, Lehrke M, Schütt K, et al. Effect of empagliflozin on the metabolic signature of patients with type 2 diabetes mellitus and cardiovascular disease. Circulation. 2017;136:969–972. doi: 10.1161/CIRCULATIONAHA.117.029166. [DOI] [PubMed] [Google Scholar]
- 101.Koyani CN, Plastira I, Sourij H, et al. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104870. [DOI] [PubMed] [Google Scholar]
- 102.Xu L, Nagata N, Nagashimada M, et al. SGLT2 Inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–149. doi: 10.1016/j.ebiom.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Swe MT, Thongnak L, Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Lungkaphin A. Dapagliflozin not only improves hepatic injury and pancreatic endoplasmic reticulum stress, but also induces hepatic gluconeogenic enzymes expression in obese rats. Clin Sci (Lond) 2019;133:2415–2430. doi: 10.1042/CS20190863. [DOI] [PubMed] [Google Scholar]
- 104.Wei D, Liao L, Wang H, Zhang W, Wang T, Xu Z. Canagliflozin ameliorates obesity by improving mitochondrial function and fatty acid oxidation via PPARα in vivo and in vitro. Life Sci. 2020;247 doi: 10.1016/j.lfs.2020.117414. [DOI] [PubMed] [Google Scholar]
- 105.He L, Ma S, Zuo Q, et al. An effective sodium-dependent glucose transporter 2 inhibition, canagliflozin, prevents development of hypertensive heart failure in Dahl salt-sensitive rats. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.856386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu Q, Liu J, Li X, et al. Empagliflozin attenuates ischemia and reperfusion injury through LKB1/AMPK signaling pathway. Mol Cell Endocrinol. 2020;501 doi: 10.1016/j.mce.2019.110642. [DOI] [PubMed] [Google Scholar]
- 107.Liu X, Xu C, Xu L, et al. Empagliflozin improves diabetic renal tubular injury by alleviating mitochondrial fission via AMPK/SP1/PGAM5 pathway. Metabolism. 2020;111 doi: 10.1016/j.metabol.2020.154334. [DOI] [PubMed] [Google Scholar]
- 108.Yang X, Liu Q, Li Y, et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte. 2020;9:484–494. doi: 10.1080/21623945.2020.1807850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mizuno M, Kuno A, Yano T, et al. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep. 2018;6:e13741. doi: 10.14814/phy2.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mancini SJ, Boyd D, Katwan OJ, et al. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep. 2018;8:5276. doi: 10.1038/s41598-018-23420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aragón-Herrera A, Feijóo-Bandín S, Otero Santiago M, et al. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem Pharmacol. 2019;170 doi: 10.1016/j.bcp.2019.113677. [DOI] [PubMed] [Google Scholar]
- 112.Lee YH, Kim SH, Kang JM, et al. Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy. Am J Physiol Renal Physiol. 2019;317:F767–F780. doi: 10.1152/ajprenal.00565.2018. [DOI] [PubMed] [Google Scholar]
- 113.Bessho R, Takiyama Y, Takiyama T, et al. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep. 2019;9:14754. doi: 10.1038/s41598-019-51343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41:356–363. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 115.Blackwood EA, Hofmann C, Santo Domingo M, et al. ATF6 regulates cardiac hypertrophy by transcriptional induction of the mTORC1 activator, Rheb. Circ Res. 2019;124:79–93. doi: 10.1161/CIRCRESAHA.118.313854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kubra KT, Akhter MS, Uddin MA, Barabutis N. Unfolded protein response in cardiovascular disease. Cell Signal. 2020;73 doi: 10.1016/j.cellsig.2020.109699. [DOI] [PubMed] [Google Scholar]
- 117.Sun X, Han F, Lu Q, et al. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating sestrin2-mediated AMPK-mTOR Signaling and redox homeostasis in high-fat diet-induced obese mice. Diabetes. 2020;69:1292–1305. doi: 10.2337/db19-0991. [DOI] [PubMed] [Google Scholar]
- 118.Yang L, Liang B, Li J, et al. Dapagliflozin alleviates advanced glycation end product induced podocyte injury through AMPK/mTOR mediated autophagy pathway. Cell Signal. 2022;90 doi: 10.1016/j.cellsig.2021.110206. [DOI] [PubMed] [Google Scholar]
- 119.Jaikumkao K, Promsan S, Thongnak L, et al. Dapagliflozin ameliorates pancreatic injury and activates kidney autophagy by modulating the AMPK/mTOR signaling pathway in obese rats. J Cell Physiol. 2021;236:6424–6440. doi: 10.1002/jcp.30316. [DOI] [PubMed] [Google Scholar]
- 120.Ren C, Sun K, Zhang Y, et al. Sodium-glucose cotransporter-2 inhibitor empagliflozin ameliorates sunitinib-induced cardiac dysfunction via regulation of AMPK-mTOR signaling pathway-mediated autophagy. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.664181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu J, Kitada M, Ogura Y, Liu H, Koya D. Dapagliflozin restores impaired autophagy and suppresses inflammation in high glucose-treated HK-2 cells. Cells. 2021;10:1457. doi: 10.3390/cells10061457. [DOI] [PMC free article] [PubMed] [Google Scholar]