Abstract
A series of symmetric and unsymmetrical benzimidazolium-based N-heterocyclic carbene (NHC) precursors (1a-i) and their silver complexes (2a-i) have been synthesized. The Ag(I)–NHC complexes were characterized by 1H, 13C{1H} NMR, FTIR, LC/MS-QTOF, and elemental analysis. Anticancer and cytotoxic activity of all Ag(I)–NHC complexes were tested against healthy fibroblast cell line (L929), breast cancer cell line (MCF-7), and neuroblastoma cell line (SH-SY5Y) by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4sulfophenyl)-2H-tetrazolium] assay. The 2b, 2c, 2e, 2g, 2h, and 2i complexes showed higher cytotoxicity than cisplatin against SH-SY5Y and MCF-7 and lower cytotoxic activity against L929 cell lines. Because of their high cytotoxic activity against cancer cells and low cytotoxicity against healthy fibroblast cell lines, the 2b, 2c, 2e, 2g, 2h, and 2i are expected to be new lead compounds. In addition, molecular docking studies were performed to explore the binding interactions of silver complexes with the enzyme to explore new anticancer compounds. Furthermore, ADME properties of all complexes were predicted to explore lead-like characteristics and may be a potential drug candidate for cancer treatment.
Keywords: Healthy fibroblast cell line (L929), Breast cancer cell line (MCF-7), Neuroblastoma cell line (SH-SY5Y), Anticancer activity, Silver-N-heterocyclic carbene
Healthy fibroblast cell line (L929); Breast cancer cell line (MCF-7); Neuroblastoma cell line (SH-SY5Y); Anticancer activity; Silver-N-heterocyclic carbene.
1. Introduction
Metallopharmaceutics such as cisplatin and its analogues are used in clinical practices; however, they have unavoidable neurotoxic side effects [1]. Cisplatin is one of the most known drugs to cure many cancers. Although cisplatin is effective in treating a variety of cancer, its adverse effects, such as toxicity, immunization of cancer cells, and low solubility, limit its use. Cisplatin use in cancerous cell treatment is a milestone in using metal-derived compounds in chemotherapy. However, platinum-based drugs treat both types of cancer, though hematological, and gastrointestinal side effects should be avoided [2, 3, 4, 5]. Synthesis of metal-derived compounds that do not show platinum-based drugs' side effects and give better cytotoxicity than cisplatin in chemotherapy remains a challenge [6]. Even though non-platinum metal anticancer drugs have not been approved for use in chemotherapy until now, various transition-metal complexes are now being under-investigated as drug candidates for future development [7, 8, 9, 10, 11]. NAMI-A, KP1019, Budotitane, and gallium-based compounds are some drug candidates [7, 8, 9, 10, 11].
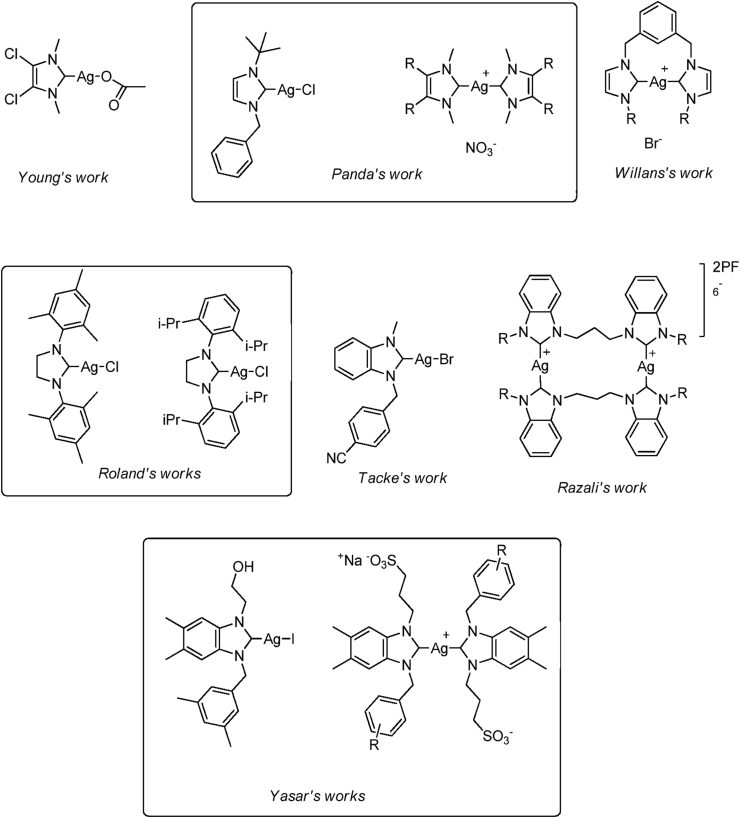
N-heterocyclic carbenes (NHCs) are easily synthesized, chemically modified and have donor properties similar to phosphine ligands. NHC can form a stronger bond with the metal canters, leading to more stable complexes against moisture, heat, and air. NHC complexes are primarily used in catalysis as catalysts [12, 13, 14, 15, 16, 17]. Recently, NHCs have been beginning to be excellent cytotoxic metal carriers due to their ready prerequisites for an efficient drug design and fast optimizations [2, 13, 18, 19, 20, 21, 22]. The lipophilic terminal is essential in drug molecules. It needs to be chemically modified to serve this lipophilic terminal on NHC. Thus, easy chemical modification of NHCs to serve lipophilic terminals in NHC-based drug molecules is very important. Last two decades, an increasing number of publications have focused on the biological application of Ag(I), Au(I), Ru(II), Rh(II), Pt(II), and Cu(I)–NHC complexes as metallopharmaceutic [23, 24, 25, 26, 27, 28, 29]. Silver complexes showed considerable anticancer activity, some of them in vitro and in vivo [19, 30, 31, 32]. The mechanism of action of silver complexes is based on the release of the Ag+ ions that enter the cell membranes and disrupt the cell function. Additionally, silver complexes lose their activity very quickly because of the rapid release of the Ag+ ions. There is a need to synthesize a stable complex that can control or slow Ag+ ions release. Slow release of Ag+ ions is only possible when firmly coordinated between the silver and the ligand. Due to the firm σ-donor properties of NHC, Ag–NHC complexes remain therapeutically active for a longer time than AgNO3, which may be rationalized by a slower release of Ag+ ions from stable Ag(I)–NHC complex [20]. Ag(I)–NHC complexes gained increased stability via strong σ-donor properties of NHC. They could easily overcome the fast loss of activity problems and sulphonamide resistance of the pathogens against conventional silver-based antibiotics [21]. Different groups reported that Ag–NHC complexes could act as antimicrobial agents since the release of Ag+ ions from Ag–NHC is behind the usual schedule [13, 22, 33, 34]. After these encouraging results, Youngs et al. investigated the cytotoxic activity of Ag–NHC complexes against OVCAR-3, MB157 cancer cells (Scheme 1). These Ag–NHC complexes showed cytotoxic activity but did not affect HeLa cancer cells [34]. Panda et al. reported a similar cytotoxic effect of Ag–NHC complex against HeLa cancer cells [35] (Scheme 1). However, Youngs et al. reported that the same complex had good activity against ovarian cancer in mice. After these results, activity of Ag–NHC complex can differ according to the type of cancer cell [34]. The Ag-Bis(NHC) complexes showed similar efficiency against H460 lung cancer cells [36]. The anticancer potential of Ag–NHC complexes and their synthesis have been the attention of various research teams. Ag–NHC complexes have been reported to show good to excellent anticancer potential against many cell lines [23, 36, 37, 38, 39, 40, 41, 42].
Scheme 1.
Structure of cytotoxic properties studied Ag–NHC complexes against different cancer cells.
Herein, molecular docking and investigation of the cytotoxic activity of benzimidazole-based Ag–NHC complexes were reported. The MCF-7 and SH-SY5Y cell lines were significantly inhibited by these complexes, and the L929 cell lines showed moderate cytotoxic activity.
2. Experimental
2.1. General considerations
All these experiments were performed using normal Schlenk techniques in freshly dried solvents under in air or nitrogen atmosphere.
Synthesis of Ag(I)–NHC complex reactions were performed in the absence of light. Ag2O and solvents used in this work were purchased from Sigma-Aldrich. The compounds' elements were examined using the ElementarVario EL III Carlo Erba 1108 instrument. The melting points of the compounds were calculated using a Stuart automated melting point apparatus (SMP-40). The 1H and 13C{1H} NMR spectra were obtained using Bruker Avance III HD 300 and 400 MHz NMR spectrometers. The J values for coupling constants are displayed in hertz. For the HRMS study, an Agilent 6530 Accurate-Mass spectrometer was used. A PerkinElmer Spectrum 100 GladiATR FT/IR spectrometer was used to record FTIR spectrum.
2.2. In vitro cytotoxic activity using MTS assay
The DMEM (Dulbecco's Modified Eagles Medium) containing penicillin-streptomycin (1%) and fetal bovine serum (10%) was used to maintain the MCF-7, SH-SY5Y and L929 cell lines. They were then kept in 5% CO2 at 37 °C in a CO2 incubator. The cells' medium was replaced twice a week. When the cells were 85% confluent, the cells were removed from flasks using a trypsin-EDTA solution. Each 96-wells contained 5 × 103 healthy cells and was analyzed by MTS assay. The DMEM-containing plates were incubated in an incubator at 37 °C for 24 h. Following 24 h incubation at 37 °C in the CO2 incubator, the silver compounds were dissolved in dimethyl sulfoxide (DMSO). All cells were serially diluted (1, 10, 25, 50, and 100 μM) for 24 and 48 h. After incubation, the MTS solution (10 μL) was added to it and put in incubation for next 2 h.
Using an ELISA reader (BioTek Instruments, Inc. Winooski, Vermont, USA), the absorbance at 450 nm was measured to determine the viability of the cells. The cell viability percentage of each group was obtained focused on the control cells (cells not treated with silver compounds) as 100%. The MTS analyses were repeated for each concentration at 24 and 48 h (n: 4).
2.3. Molecular docking
Molecular docking was enforced for the 2a-i complexes to compare their theoretical and experimental binding affinity with MCF-7 and SH-SY5Y Cell Line. The 3D structure of MCF-7 (PDB ID: 1E31) at 2.71 Å resolution and SH-SY5Y cell line (PDB ID: 2H9V) at 3.10 Å resolution were downloaded from PDB (Protein Data Bank). The 2D structures of all nine complexes and cisplatin were drawn using Chem3Draw Ultra 16.0.1.2 software and were converted into a 3D structure using a discovery studio visualizer [43]. These two protein structures were the best in the PDB with their low-resolution values and thus were chosen for docking studies. The PyRx was use to investigate the rmsd (root-mean-square deviation) of the ligands and the amino acid residue surrounded to it. Ten conformations for each ligand were generated; the top 5 docking poses were retained for each compound to analyze the interactions of the docked conformations within the active site.
2.4. ADME prediction
An in-silico ADME study was conducted to predict the pharmacokinetic parameters of all the complexes using SwissADME prediction software [44]. The Structures were drawn by ChemDraw 16.0.1.4 and converted to smile format. These SMILE formats were loaded into the SwissADME database and evaluated their ADME profile.
2.5. Synthesis
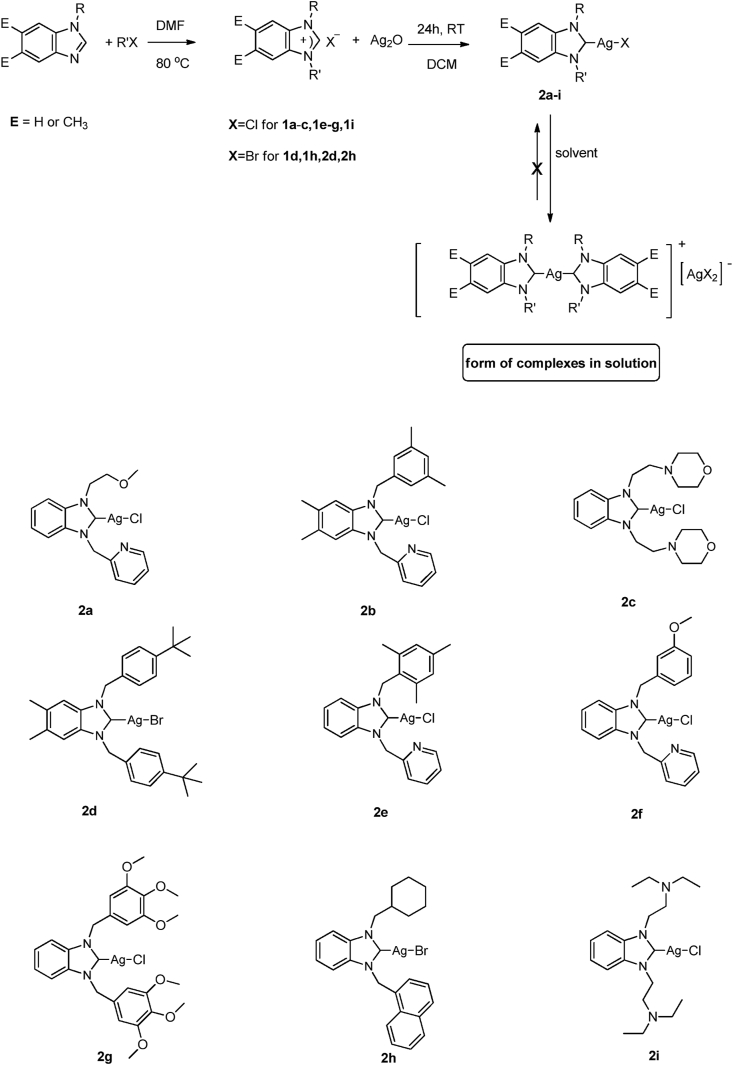
2.5.1. Synthesis pathway for benzimidazole-based NHCs (1a–i)
NHC precursors 1a-i were prepared based on the relevant literature (Scheme 2) [45]. The spectroscopic data (NMR and FTIR) of 1a-i are shown in the supporting information (Figures S1–S9 and S19–S24, respectively).
Scheme 2.
Synthesis of NHC salts (1a-i) and their Ag–NHC complexes (2a-i).
1-(methylpyridine)-3-(2-methoxyethyl)benzimidazolium chloride,1a: The 1a is produced in an excellent yield when 1-(methylpyridine)benzimidazole (10 mmol) and 2-methoxyethyl chloride (12 mmol) are reacted using DMF (5 mL) at 80 °C under Ar atmosphere. Thereafter, the precipitates were subsequently filtered out, repeatedly washed with diethyl ether, and dried in vacuo. Methanol/diethyl ether (1:1) solvent was used to crystallize the resultant brown solid (2.17 g, 75 percent).
1-(methylpyridine)-3-(3,5-dimethylbenzyl)-5,6-dimethylbenzimidazolium chloride,1b: 1b was synthesized with the synthesis pathway of 1a by the reaction of 1-(methylpyridine)-5,6-dimethylbenzimidazole (10 mmol) and 3,5-dimethylbenzyl chloride (11 mmol) to give 1b as brown powder (3.09 g, 85%).
1,3-Bis(ethylmorpholine)benzimidazolium chloride,1c:1c was synthesized using synthetic pathway of 1a by the reaction of 1-(ethylmorpholine)benzimidazole (10 mmol) and ethylmorpholine chloride (11 mmol) to give 1c as white powder (2.67 g, 70%).
1,3-bis(4-tert-butylbenzyl)-5,6-dimethylbenzimidazolium bromide,1d: 1d was synthesized following the 1a synthesis pathway by reacting 1-(4-tert-butylbenzyl)-5,6-dimethylbenzimidazole (10 mmol) and 4-tert-butylbenzyl chloride (11 mmol) to give 1d as white powder (4,00 g, 77%).
1-(methylpyridine)-3-(2,4,6-trimethylbenzyl)benzimidazolium chloride,1e: 1e was synthesized with the synthesis pathway of 1a by the reaction of 1-(methylpyridine)benzimidazole (10 mmol) and 2,4,6-trimethylbenzyl chloride (11 mmol) to give 1e as white powder (2.95 g, 78%).
1-(methylpyridine)-3-(3-methoxybenzyl)benzimidazolium chloride,1f: 1f was synthesized with the synthesis pathway of 1a by the reaction of 1-(methylpyridine)benzimidazole (10 mmol) and 3-methoxybenzyl chloride (11 mmol) to give 1f as white powder (4.43 g, 94%).
1,3-bis-(3,4,5-trimethoxybenzyl)benzimidazolium chloride1g: 1g was synthesized with the synthesis pathway of 1a by the reaction of 1-(3,4,5-trimethoxybenzyl)benzimidazole (10 mmol) and 3,4,5-trimethoxybenzyl chloride (11 mmol) to give 1g as white powder (4.22 g, 82%).
1-(methylcyclohexyl)-3-(2-methylnaphthalene)-benzimidazolium Bromide1h: 1h was synthesized with the synthesis pathway of 1a by the reaction of 1-(methylcyclohexyl)benzimidazole (10 mmol) and 2-methylnaphthalene bromide (11 mmol) to give 1h as white powder (2.96 g, 68%).
1,3-(bis-(2-diethylamino)ethyl)benzimidazolium chloride1i: 1i was synthesized with the synthesis pathway of 1a by the reaction of 1-(2-diethylaminoethyl)benzimidazole (10 mmol) and 2-diethylaminoethyl chloride (11 mmol) to give 1i as white powder (2.51 g, 71%).
2.5.2. Synthesis pathway for Ag–NHC complexes (2a-i)
The synthesis of 2a-i complexes were done according to the literature [24]. The spectroscopic data (NMR and FTIR) of Ag(I)-NHC complexes are shown in the supporting information (Figures S10–S18 and S25–S33, respectively).
Chloro[1-(methylpyridine)-3-(2-methoxyethyl)benzimidazol-2-yliden]silver(I),2a: 2a was synthesized in goodyield by the reaction of 1a (1 mmol) and Ag2O (1 mmol) in 10 ml dichloromethane (DCM) at rt for overnight in the absence of light leading to give Ag–NHC complex. After the reaction, reaction solution was filtered by passing over a small layer of Celite and the amount of filtrate was reduced by half and diethylether (15 mL) was added to the solution to obtain a brown solid. This brown solid was then washed several times with diethylether, dried under vacuum and stored in the dark (0.34 g, 82%).
Chloro[1-(methylpyridine)-3-(3,5-dimethylbenzyl)-5,6-dimethylbenzimidazol-2-yliden]silver(I),2b: 2b was synthesized using the synthesis method of complex 2a by the reaction of compound 1b (1 mmol) and Ag2O (1 mmol) in 10 ml DCM at rt for overnight to give 2b as brown powder (0.38 g, 77%).
Chloro[1,3-Bis(ethylmorpholine)benzimidazol-2-yliden]silver(I),2c, (C19H28N4O2ClAg): 2c was synthesized using the synthesis method of complex 2a by the reaction of compound by the reaction of 1c (1 mmol) and Ag2O (1 mmol) to give 2c as white powder (0.39 g, 75%).
Bromo[1,3-bis(4-tert-butylbenzyl)-5,6-dimethylbenzimidazol-2-yliden]silver(I),2d: 2d was synthesized using the synthesis method of complex 2a by the reaction of compound 1d (0.34 g, 1 mmol) and Ag2O (1 mmol) to give 2d as white powder (0.43 g, 70%).
Chloro [1-(methylpyridine)-3-(2,4,6-trimethylbenzyl)benzimidazol-2-yliden] silver(I),2e: 2e was synthesized using the synthesis method of complex 2a by the reaction of 1e (1 mmol) and Ag2O (1 mmol) to give 2e as white powder (0.39 g, 78%).
Chloro [1-(methylpyridine)-3-(3-methoxybenzyl)benzimidazol-2-yliden]silver(I),2f: 2f was synthesized using the synthesis method of complex 2a by the reaction of 1f (1 mmol) and Ag2O (1 mmol) to give 2f as white powder (0.35 g, 76%).
Chloro [1,3-bis(3,4,5-trimethoxybenzyl)benzimidazol-2-yliden]silver(I),2g: 2g was synthesized using the synthesis method of complex 2a by the reaction of 1g (1 mmol) and Ag2O (1 mmol) to give 2g as white powder (0.32 g, 65%).
Bromo [1-(methylcyclohexyl)-3-(2-methylnaphthalene)benzimidazol-2-yliden]silver(I),2h: 2h was synthesized using the synthesis method of complex 2a by the reaction of 1h (1 mmol) and Ag2O (1 mmol) to give 2h as white powder (0.30 g, 69%).
Chloro[1,3-bis-(2-(diethylamino)ethyl)benzimidazol-2-yliden]silver(I),2i: 2i was synthesized using the synthesis method of complex 2a by the reaction of 1h (1 mmol) and Ag2O (1 mmol) to give 2i as white powder (0.3 g, 70%).
3. Results and discussion
3.1. Synthesis and characterization
The NHC salts and Ag(I)–NHC complexes, which are given in Scheme 2, were synthesized following reported procedures [24]. Both air-stable 1a-i and 2a-i (82%, 77%, 75%, 70%, 78%, 76%, 65%, 69%, and %70, respectively) were prepared with good yields using the methods in which wide range Ag(I)–NHC complexes prepared from the corresponding NHC salts and Ag2O in DCM under the absence of light at room temperature. All the synthesized compounds were fully characterized by spectroscopic techniques (1H NMR, 13C{1H} NMR, HRMS, FTIR, and Elemental analysis) and melting point determination.
The resonance of C2 protons and C2 carbons of 1a-i in the 1H NMR and 13C{1H} NMR appears at 11.23, 11.64, 11.21, 11.84, 9.44, 11.71, 12.21, 10.03, 11.08, 152.5, 152.8, 144.3, 162.6, 153.1, 152.5, 153.9, 144.1, and 144.1 ppm in CDCl3, respectively. The loss of the C2 proton of 1a-i in 1H NMR and the downfield shift of the C2 carbon to a new area in 13C{1H} NMR spectra of 2a-i complexes indicate the formation of 2a-i complexes. The C2 carbon of 1a and 1f downfield shifted to 189.2 and 189.4 ppm, respectively, indicating the formation of 2a and 2f complexes. However, the C2 carbon of 2b, 2c, 2d, 2e, 2g, 2h, and 2i have not been observed in 13C{1H} NMR spectra. According to the literature [46, 47, 48, 49, 50, 51, 52], the fluxional behaviour of the Ag–NHC complexes may cause not to be observed the resonance of the C2 carbene carbon in the 13C{1H} NMR spectrum. However, based on these data, C2 carbon of 2a and 2f complexes was observed in 13C{1H} NMR spectra; it can be said that fluxional movements are effective in 2b, 2c, 2d, 2e, 2g, 2h, and 2i complexes.
The HRMS analyses were performed to elucidate the 2a-i complexes structures, and obtained spectra are shown in the supporting information as Figures S34–S42 showed that the proposed structures for the 2a-i complexes are in bis-carbene ([Ag(NHC)2]+ [AgX2]−) form in solution. According to LC/MS-Q-TOF spectra, it was understood that 2a-i complexes were in the form of bis-carbene in the solution and did not transform into mono-carbene form. Although we do not have clear information about the fluxional behaviour of 2a-i complexes in solid form, it has been observed that 2a-i complexes in solution have a [Ag(NHC)2]+ [AgX2]- structure. We made every attempt to obtain a suitable crystal by single crystal X-ray diffraction analysis to ascertain the structure of silver-NHC complexes in solid form, but we were unable to do so. According to the literature [53], the Ag–NHC complexes' structure was characterized by X-ray analysis found in the form of mono-carbon. Due to the structural similarity of Ag(I)–NHC complexes with literature results [53], we think that 2a-i complexes have the same structure in solid form.
3.2. Biological evaluation
3.2.1. Antitumor activity
The inhibitory effect of 2a-i complexes and cisplatin on the growth of L929, MCF-7 and SH-SY5Y cell lines were measured using the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4sulfophenyl)-2H-tetrazolium] assay (Table 1). Antiproliferative effects of the Ag(I)–NHC complexes were assessed for 24 and 48 h at various concentrations (1, 10, 25, 50, and 100 μM). The concentration corresponding to 50% growth inhibition (IC50) of 2a-i on MCF-7, SH-SY5Y, and L929 is shown in Figures 1, 2, and 3 and Table 1. The results show that all Ag(I)–NHC complexes had more significant inhibitory effects than cisplatin on MCF-7 and SH-SY5Y cells at all concentrations.
Table 1.
The IC50 (μM) ± S.E.[a] for Ag–NHC complexes 2a-i on L929, MCF-7, and SH-SY5Y cell lines and relative activity of Ag(I)–NHC complexes to cisplatin.
| Compound | L929 Cell Line | MCF-7 Cell Line | SH-SY5Y Cell Line |
|---|---|---|---|
| Cisplatin | 11.6 ± 0.5 | 7.2 ± 0.4 | 10.2 ± 0.4 |
| 2a | 63.48 ± 0.4 | 98.29 ± 1 | 23.60 ± 0.7 |
| 2b | 37.11 ± 0.9 | 20.67 ± 0.6 | 2.55 ± 0.7 |
| 2c | 53.53 ± 0.9 | 76.02 ± 0.4 | 8.86 ± 0.4 |
| 2d | 1.18 ± 0.3 | 2.66 ± 0.6 | 0.25 ± 0.8 |
| 2e | 155.9 ± 0.5 | 21.32 ± 1 | 71.17 ± 1 |
| 2f | 404.3 ± 0.7 | 35.63 ± 0.5 | 82.46 ± 0.6 |
| 2g | 18.71 ± 0.5 | 6.56 ± 0.6 | 13.74 ± 0.5 |
| 2h | 45.41 ± 1 | 16.33 ± 0.8 | 24.73 ± 0.9 |
| 2i | 140.7 ± 0.5 | 53.64 ± 0.6 | 88.89 ± 0.5 |
∗Values are mean value ±standard error of three different replicates.
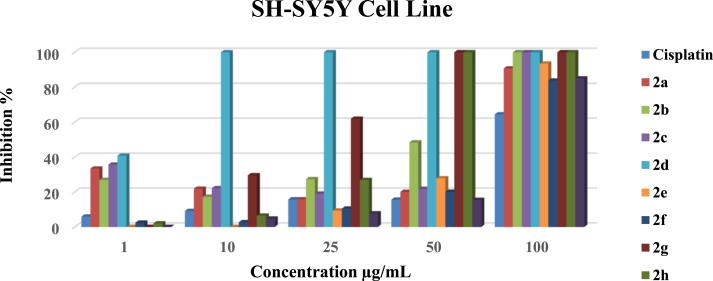
Figure 1.
The antiproliferative effects of 2a-i complexes on SH-SY5Y cells were analyzed by MTS assay.
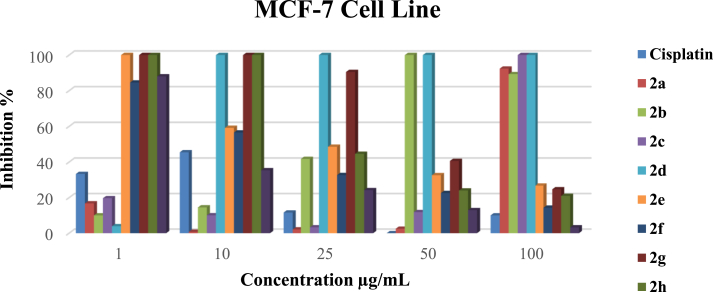
Figure 2.
The antiproliferative effects of 2a-i complexes on MCF-7 cells were analyzed by MTS assay.
Figure 3.
The antiproliferative effects of 2a-i complexes on L929 cells were evaluated using MTS assay.
3.2.1.1. The antitumor activity comparison of the complexes on the SH-SY5Y cell line
2a, 2b, 2c and 2d complexes showed moderate inhibitory effects on SH-SY5Y cell growth at a 1 μg/mL concentration (Figure 1). However, complex 2d, 2g and 2h showed excellent inhibitory effects on SH-SY5Y cell growth at concentrations of 10 μg/mL, 50 μg/mL, and 50 μg/mL, respectively (Figure 1). The growth inhibition effects were more significant than 99%, and the cell viability of the SH-SY5Y cells was <0.5%. The rest of the complexes had excellent inhibitory effects on SH-SY5Y cell growth at a 100 μg/mL concentration. The cell viability of complex 2c against SH-SY5Y cells decreased dose-dependently.
3.2.1.2. The antitumor activity comparison of the complexes on the MCF-7 cell line
2d, 2e, 2g, and 2h complexes showed excellent inhibitory effects on MCF-7 cell growth at a concentration of 1 μg/mL (Figure 2). However, complex 2f and 2i showed inhibitory effects on MCF-7 cell growth at the same concentration with very low cell viability. The growth inhibition effects were greater than 99%, and the cell viability of the MCF-7 cells was 85% and 88%, respectively. The rest of the complexes reached fewer inhibitory effects than the cisplatin on MCF-7 cell growth at a concentration of 1 μg/mL. When Ag(I)–NHC complex concentration increased to 10 μg/mL, inhibition of the complex 2d reached 100% inhibition. Some of Ag(I)–NHC complexes (2e, 2f, 2h, 2i) exhibit increase proliferative activity instead of antiproliferative activity in the 10, 20, 25, 50, and 100 μg/mL concentrations (Figure 2).
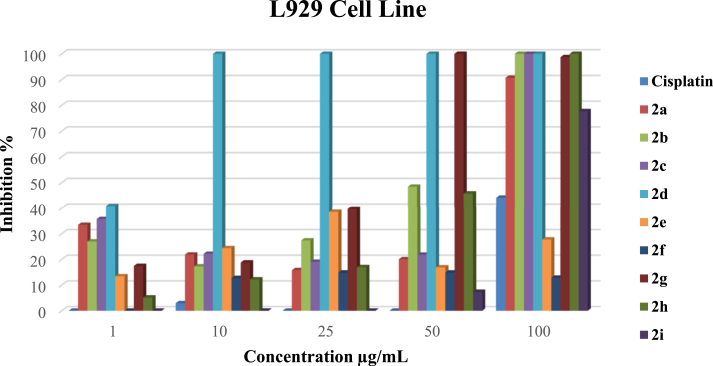
3.2.1.3. The proliferative activity comparison of the complexes on the L929 cell line
Against the L929 cell line, complex 2f and 2i complexes showed no cytotoxicity at a concentration of 1 μg/mL, which is very important in terms of the less harmful effect on healthy cells. However, all other complexes exhibited low cytotoxicity at the same concentration (Figure 3). Except for 2d and 2i complexes, all complexes showed low and similar cytotoxicity effects on L929 cell growth at the 10, 25, and 50 μg/mL concentrations with low cell inhibitions (Figure 3).
As a result, all 2a-i complexes except 2d have a low cytotoxic effect against L929 cells at 1–25 μg/mL. Complexes 2a, 2b, 2c, 2d, 2g, and 2h can be anticancer drug candidates at 1–50 μg/mL concentrations on the SH-SY5Y cell lines. However, Complexes 2e-i can be considered an anticancer drug candidate at 1–25 μg/mL concentrations and 2d complex 10–25 μg/mL on the MCF-7 cell lines.
From this, it seems silver complexes with different structures are quite effective in destroying different cancerous cells. When examining the complexes structures, it was seen that structural differences cause antiproliferative activity differences in different cancer cells. O- or N- heteroatom containing NHCs led to a difference in the antiproliferative activity of Ag–NHC complexes against both cancer cell lines and have low cytotoxicity against L929. However, complex 2d showed superior cytotoxic activity for all types of cancer cell lines at all concentrations. Thus, 2d has not been considered a drug candidate due to its high mortality in L929 cell lines. We think that the antiproliferative activity of the 2d complex is also affected by the 4-tert-butyl group or Br anion. These differences may lead to a sensitive balance on the release of Ag+ ions from the stable Ag–NHC complex. This result may indicate the importance of the anions or NHCs on silver(I)–NHC complexes in anticancer activity.
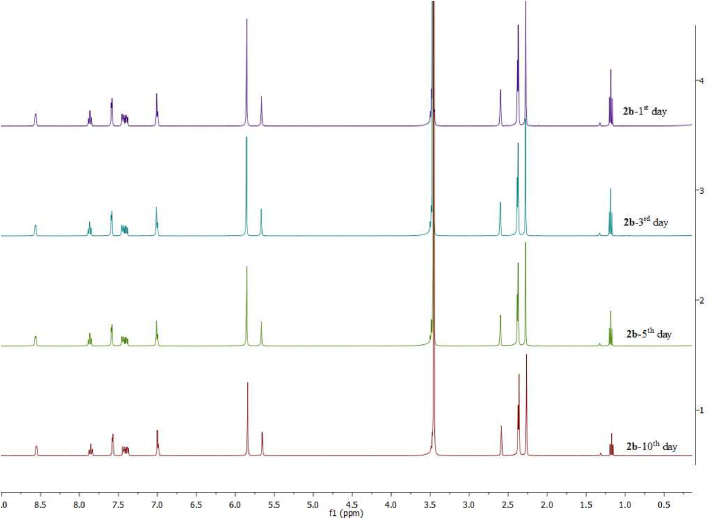
All the 2a-i complexes were soluble in DMSO and remained stable in the biological medium over the testing period (Figure 4 and please see supporting info Figures S43-S45). The degradation of the 2a-d complexes was examined by NMR spectroscopy dissolved in DMSO-d6 for ten days. According to the measurements, 2a-d complexes can remain stable in DMSO-d6 in the open air without decomposition. This good stability is essential for the slow release of Ag+ ions as required.
Figure 4.
Stability test of the 2b complex by NMR spectroscopy.
3.2.2. Molecular docking studies
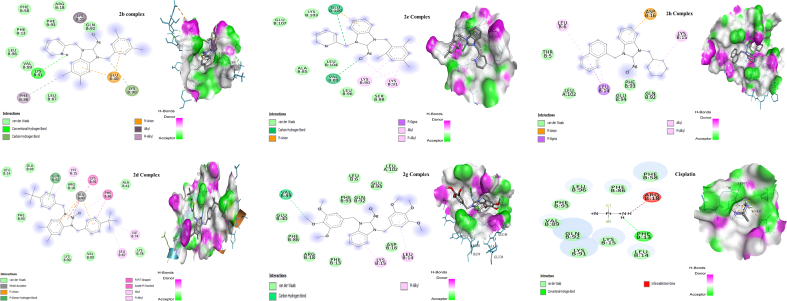
A molecular docking study is an alternate tool for analyzing the biologically active drug candidate. In this study, the complexes exhibiting the significant anticancer potential towards MCF-7 and SH-SY5Y cell lines docked with the protein PDBs: 1E31 & 2H9V. The results obtained via docking was compared in vitro results. In this study, the highest binding energy of silver complexes with MCF-7 cell line was calculated for 2b is -7.50 kcal/mol, while −6.30 kcal/mol is the lowest binding energy calculated for the complex 2e. Hydrogen bonding with LYS91, carbon-hydrogen bonding with LYS90, pi-anion with GLU40, alkyl with LYS15, pi-alkyl with PHE86 and van der Waals interaction have been identified. The detailed binding interaction of the most active complexes within the protein pockets is shown in Table 2 and Figure 5. The complexes docking results were compared standard cisplatin (−4.93 kcal/mol) as shown in Table 2 and Figure 5. The less binding energy indicates the formation of stable ligand-complex. According to the molecular modelling and in vitro results, all complexes are more effective than cisplatin.
Table 2.
Report of predicated interactions of MCF-7 cell line docking confirmations and docking scores.
| S. No | Bonding interaction | Interacting amino acid residue | Bond type with | Bond distance (Aͦ) | Binding affinity (Kcal/mol) |
|---|---|---|---|---|---|
| 2b | Hydrogen bonding C–H bonding Pi-Anion Alkyl pi-alkyl |
LYS:91 LYS:90 GLU:40 LYS:15 PHE:86 |
NH CH2N C6H6 Cl CH3 |
2.40 3.48 3.90, 4.23 3.81 4.26 |
-7.5 |
| 2d | Pi-Anion pi-sigma, Alkyl pi-alkyl |
GLU:94 LEU:102, LEU:14 ARG:106, LEU:102, LEU:14 TRP: 10 |
C6H6 C6H6 CH3, C(CH3)3 CH3 |
3.69 3.77, 3.83 3.54, 4.69, 4.84 5.29 |
-7.1 |
| 2e | C–H bonding Pi-Anion Pi-sigma Alkyl pi-alkyl |
GLU:100, VAL:89 GLU:100 GLU:100 VAL:89, LYS:90, LYS:91 LYS:90 |
CH2N, Cl C6H6 C6H6 C6H6, CH3, Cl C6H3 |
3.64, 3.14 3.81 3.86 3.42, 4.02, 3.99, 5.39 |
-6.3 |
| 2g | C–H bond pi-alkyl | VAL:89 LEU:14, LYS:15 |
OCH3 C6H6 |
3.48 5.08, 3.92 |
-6.4 |
| 2h | Pi-Anion pi-sigma, Alkyl pi-alkyl |
ASP:16 LEU:14 LYS:15 LEU:6, LEU:14 |
C6H6 C6H6 C6H12 C6H6 |
3.70 3.79 4.66 5.27, 5.25 |
-6.8 |
| Cisplatin | C–H bonding | PHE:13 | NH | 1.98 | -3.1 |
Figure 5.
2D and 3D diagram of the binding interactions of 2b, 2d, 2e, 2g, 2h silver complexes and Cisplatin with the active site residues of the MCF-7 cell line receptor.
The mechanism of action of silver complexes against cancer has been studies and revealed that the NHC ligand increased the lipophilicity of silver cations through the cell membrane, where it can easily penetrate the cell membrane resulting in the inhibition of the metabolic mechanism of the cancer cells [39]. Furthermore, the cancer cell has a higher level of reactive oxygen species (ROS) that involve DNA damage. It can induce or inhibit transcription, replication, signal transduction pathways, genomic instability and activation of oncogenes [54]. The silver complexes also contain amine groups with electron-donating properties, thereby donating the lone pair of the electron to neutralize the ROS. The electron density increases on the ring due to inductive effect produced by the presence of alkyl substituents to the benzene ring donates electrons. So, it is proposed that the electron-donating group of the complexes also play a role in breast cancer treatment.
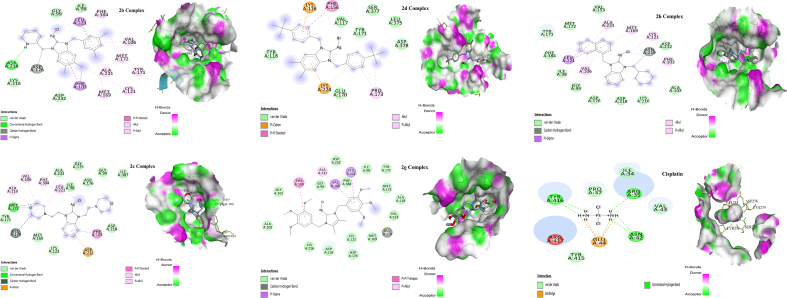
A docking study was also carried out for 2b, 2c, 2d, 2g and 2h complexes to determine the its binding interaction with protein pockets. The calculated binding energies of 2b, 2c, 2d, 2g and 2h were −10.0, −7.9, −8.00, −7.1 and −9.3 kcal/mol, respectively. Additionally, H-bonds and C–H bonds were observed between ASP218 and 2b; MET172 and 2c; GLU170 and 2g; ASN and 2h. H-bond with ASP218, C–H bond with ASP176, π-σ interaction with PHE103, LEU221, π-π stacked with PHE103, alkyl interaction with MET:172, MET:169, LYS:121, TYR:171, and pi-alkyl interaction with PHE:384, VAL:106, ALA:231, PHE:103, TYR:171 were noticed for 2b. In 2c, 2d, 2g and 2h, the Hydrogen bonding, C–H bonding, Pi-Anion, Pi-Pi Stacked, Alkyl and Pi-alkyl with amino acid residues are remarkable. The complexes docking results were compared to standard drug (cisplatin). The interaction of cisplatin and the amino acid residue was evaluated, and their binding affinity was calculated as −4.1 kcal/mol, as shown in the table. According to the result of molecular docking, all the complexes showed more activity than the cisplatin. Significant van der Waals interactions of complexes with several amino acids exist. Among the silver complexes, complex 2b is noteworthy. The detailed of silver complexes interaction with amino acid residue are shown in Table 3, Figure 6. The bond types with NH, CH2N, C6H6, CH3, etc., were observed in the docking studies of SH-SY5Y cell lines. The complexes have electron-donating groups that may inhibit oxidative damage, mitochondria dysfunction and cell apoptosis. The Binding of amino acid residue with complexes can stabilize the active pocket and cause lowering bind energy [55]. It is interesting that the silver complexes showed excellent anticancer activity and more negative binding energies than the positive control (cisplatin) in molecular docking. Thus, the docking result showed a resemblance to in vitro activities.
Table 3.
Report of predicated interactions of SH-SY5Y cell line docking confirmations and docking scores.
| S. No | Bonding interaction | Interacting amino acid residue | Bond type with | Bond distance (Aͦ) | Binding affinity (Kcal/mol) |
|---|---|---|---|---|---|
| 2b | Hydrogen bonding C–H bonding Pi-Sigma Pi-Pi Stacked Alkyl pi-alkyl |
ASP:218 ASP:176 PHE:103, LEU:221 PHE: 103 MET:172, MET:169, LYS:121, TYR:171, PHE:384, VAL:106, ALA:231, PHE:103, TYR:171 |
NH CH2N C6H6, CH3 C6H6 C6H6, CH3 CH3 |
2.44 3.47 3.61, 3.91 4.34 5.03, 4.77, 5.01, 4.37 4.60, 4.35, 4.14, 3.61, 4.43, 5.49, |
−10.0 |
| 2c | Hydrogen bonding C–H bonding Pi-Anion Pi-Pi Stacked Alkyl pi-alkyl |
MET:172 MET:172, GLU:170 ASP:232 PHE: 103 VAL:106, ALA:119, LEU:221, MET:172, PHE:384, |
CH2O CH2O C6H6 C6H6 C4H8NO Cl |
1.97 3.61, 3.25 4.13 4.13 5.16, 4.03, 54.67, 5,29 4.73 |
−7.9 |
| 2d | Pi-Cation Pi-Pi Stacked Alkyl pi-alkyl |
LYS:116, LYS:224 PHE: 156 PRO:173 PRO:173, LYS:224, PHE:156 |
C6H6 C6H6 (CH3)3 (CH3)3, C6H6 |
3.01, 3.19 4.56 4.51 5.15, 4.73, 4.59 |
−8.0 |
| 2g | C–H bonding Pi-Sigma Pi-Pi T shaped Pi-Alkyl |
GLU:170 VAL:106, LEU:221 PHE:103 VAL:89, LYS:90, LYS:91 |
OCH3 C6H6 C6H6 C6H6, Cl |
3.63 3.99, 3.90 4.71 3.17, 5.35 |
−7.1 |
| 2h | C–H bonding Pi-Sigma Alkyl Pi-Alkyl |
ASN:219 LEU:221 LYS:121 VAL:106, ALA:231, MET:169, PHE:103 |
CH2N C6H6 Cl C6H6, Cl |
3.46 3.80, 3.88, 3.91 5.01, 4.56, 5.39, 5.26, 4.50, 4.67 |
−9.3 |
| Cisplatin | C–H bonding Salt bridge |
TYR:256, ARG:35, ASN:42 GLU:44 |
NH NH+ |
2.03, 2.22, 2.53 2.60, 2.99, 5.31 |
−4.1 |
Figure 6.
2D and 3D diagram of the binding interactions of 2b, 2c, 2d, 2g, 2h silver complexes and Cisplatin with the active site residues of the SH-SY5Y cell line receptor.
Although the exact MOA of silver complexes is not clear, according to the literature [56]. The metabolism, transport and cell respiration can also be effected by the interaction of silver with cell surface. The proposed mechanism of action of silver complexes demonstrated in the literature that silver cations bind to surfaces of cell and interact with proteins and, enzymes which are essential for the cell wall synthesis, may cause apoptosis by internal/external stress interaction [57, 58, 59]. Silver cations are more bioavailable and active when they are bound to target cell [57]. Furthermore, it also described that silver cations inhibit the functionalities of cancer cells by getting deposited in the cytosol and inhibit enzymes and proteins. The NHCs cytotoxic effect could probably be due to high lipophilicity, transporting Ag–NHC complexes into the cell. As a result, Ag–NHCs may be hazardous by hindering biomolecule metabolism and cellular respiration [57]. In line with this information, we think that the antiproliferative effect of Ag(I)–NHCs might be the lipophilic nature of the complexes that transport NHCs to the cells and organelles where silver can causes toxicity by preventing metabolism and respiration of cells.
When the antitumor activities of Ag(I)–NHC complexes presented here were compared with the antitumor activities of similar complexes available in the literature [39, 40, 41, 42, 58, 60, 61, 62, 63, 64], it was seen that the complexes presented here had similar activity to the Ag(I)–NHC complexes presented in the literature. However, 2a-i complexes have been observed to exhibit better anticancer activity than both cisplatin and many other complexes reported in the literature, even at low concentrations in the MCF-7 and SH-SY5Y cell lines.
These observations point out that (a) the modification or fine-tune of the steric and electronic properties of NHCs through the N-substituents (contains hetero atoms) is crucial and that (b) the anions type and electron-donating methyl groups on benzimidazole ring have a significant influence on the antiproliferative activity of Ag(I)–NHC complexes and that (c) having properties that facilitate the cellular uptake of complexes into cells. This study clearly showed the metallodrugs potential of this structurally different Ag (I)–NHC complexes. Thus, the outcome of our results is according to the previously reported work in cancer cell lines which is a proof that silver ions deposition in the cell contributes to the cytotoxic activity of the tested compounds.
3.2.2.1. ADME and pharmacokinetic profile
ADME (Absorption, Distribution, Metabolism and Excretion) prediction was calculated to evaluate the druglikeness behaviour of all complexes. ADME is a vital process in discovery and development of drug. An ideal drug candidate, after administration, rapidly absorbs into the systemic circulation and eliminates without affecting the biological activity. The in silico studies for lead optimization plays the leading role in determining physicochemical properties. Because of these reasons, all complexes were screened for its ADME study. A drug consider to be an ideal that must be non-toxic, easily absorbed in the systemic circulation, and show specific biological responses without affecting other organs. SwissADME database was used for the prediction of drug-like characters for all complexes. Except for 2d, 2g and 2h, all the complexes showed drug-like characteristics according to the Lipinski rule, as shown in Table 4. According to Lipinski rule, also known as Pfizer's rule of five, should follow the following rule, i.e. five (MW < 500, LogP <5, Hydrogen bond donor ≤5, Hydrogen bond acceptor ≤10) [65]. As shown in Table 4, some of the complexes violate the Lipinski rule but generally, it is acceptable because the drug shows that not more than one violation is considered to be a good orally absorbed [66]. Some drugs, such as sorafenib, atorvastatin and several natural products [67], violate the rule of five but are given orally.
Table 4.
Lipinski’s rule of five (RO5) for the drug-likeness of all NHC complexes.
| Compound | M.Wt | HBA | HBD | LogP | TPSA | GI absorption | BBB permeant | P-gp substrate | Lipinski violation |
|---|---|---|---|---|---|---|---|---|---|
| 2a | 410.64 g/mol | 2 | 0 | 1.77 | 28.60 Å2 | High | Yes | Yes | No |
| 2b | 498.79 g/mol | 1 | 0 | 4.04 | 19.37 Å2 | High | Yes | Yes | No |
| 2c | 487.77 g/mol | 4 | 0 | 0.83 | 31.42 Å2 | High | No | Yes | No |
| 2d | 626.41 g/mol | 0 | 0 | 6.29 | 6.48 Å2 | Low | No | Yes | No; 2 violations: MW > 500, LogP>5 |
| 2e | 484.77 g/mol | 1 | 0 | 3.75 | 19.37 Å2 | High | Yes | Yes | No |
| 2f | 472.71 g/mol | 2 | 0 | 2.84 | 28.60 Å2 | High | Yes | Yes | No |
| 2g | 621.85 g/mol | 6 | 0 | 3.21 | 61.86 Å2 | High | Yes | Yes | Yes; 1 violation: MW > 500 |
| 2h | 542.25 g/mol | 0 | 0 | 4.83 | 6.48 Å2 | Low | No | Yes | Yes; 1 violation: MW > 500 |
| 2i | 459.80 g/mol | 2 | 0 | 2.36 | 12.96 Å2 | High | Yes | No | No |
M.Wt = Molecular weight, HBA = Hydrogen bond acceptors, HBD = Hydrogen bond donors, LogP = octanol–water partition co-efficient, TPSA = Topological Polar Surface Area, GI = Gastrointestinal, BBB = Blood Brain Barrier, P-gb = P-glycoprotein.
SwissADME also provides several parameters to predict whether the compound will be absorbed, distributed, metabolized and excreted. All the complexes except 2d, 2g and 2h showed low GI absorption, and these complexes also showed Lipinski rule violation. All the complexes that accept 2i showed to be the substrate for p-glycoprotein. So that it can be easily entered into the systemic circulation and the brain, it is also essential that the compound be quickly metabolized and excreted from the body-this help in minimizing the possible side effect [68].
Almost all the complexes showed well in silico ADME profiles that can be further investigated by in vivo and molecular studies.
4. Conclusions
Herein, a series of Ag–NHC complexes were reported, and anticancer activity was investigated against MCF-7, SH-SY5Y, and L929 cell lines. The cytotoxic activities of complex 2a-d against SH-SY5Y and complex 2d-i against MCF-7 cell lines showed excellent and better than the positive control cisplatin. Special attention needs for 2b, 2c, 2e, 2g, 2h and 2i complexes because these showed excellent anticancer activity and low toxicity against SH-SY5Y and MCF-7 cell lines. This finding indicates that the type of N-substituents, which contains heteroatoms such as O- and N- on NHC, may improve the high cytotoxic activity against SH-SY5Y, MCF-7, and low cytotoxic activity against L929 cells. The docking results also revealed that all complexes showed similar potential for in vitro anticancer activities. Except for 2d, 2g and 2h, all the complexes showed drug-like characteristics and low GI absorption. However, all the complexes that accept 2i showed to be the substrate for p-glycoprotein.
Declarations
Author contribution statement
Mitat Akkoç: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Siraj Khan: Analyzed and interpreted the data; Wrote the paper.
Hande Yüce; Neşe Başak Türkmen: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Şeyma Yaşar: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sedat Yaşar: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
İsmail Özdemir: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at https://doi.org/10.1016/j.heliyon.2022.e10133.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Osella B.D., Bottone M.G. New platinum-based prodrug Pt (IV) Ac-POA: antitumour effects in rat C6 glioblastoma cells. Neurotox. Res. 2020;37:183. doi: 10.1007/s12640-019-00076-0. [DOI] [PubMed] [Google Scholar]
- 2.Wong E., Giandomenico C.M. Current status of platinum-based antitumor drugs. Chem. Rev. 1999;99:2451–2466. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 3.Monneret C. Platinum anticancer drugs. From serendipity to rational design. Ann. Pharm. Fr. 2011;69:286–295. doi: 10.1016/j.pharma.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Alberts D.S., Noel J.K. Cisplatin-associated neurotoxicity: can it be prevented? Anti Cancer Drugs. 1995;6:369–383. doi: 10.1097/00001813-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Rabik C.A., Dolan M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Günther K., Weber G. Vol. 20. Springer; Berlin, Heidelberg, New York: 1999. Analytiker-Taschenbuch; p. 71. [Google Scholar]
- 7.Gasser G., Ott I., Metzler-Nolte N. Organometallic anticancer compounds. J. Med. Chem. 2010;54:3–25. doi: 10.1021/jm100020w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rijt S.H., Sadler P.J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov. 2009;14:1089–1097. doi: 10.1016/j.drudis.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruijnincx P.C., Sadler P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008;12:197–206. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Ruiz S., Maksimovic-Ivanic D., Mijatovic S., Kaluderovic G.N. On the discovery, biological effects, and use of cisplatin and metallocenes in anticancer chemotherapy. Bioinorgan. Chem. Appl. 2012;14 doi: 10.1155/2012/140284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peacock A.F., Sadler P.J. Medicinal organometallic chemistry: designing metal arene complexes as anticancer agents. Chem. Asian J. 2008;3:1890–1899. doi: 10.1002/asia.200800149. [DOI] [PubMed] [Google Scholar]
- 12.Nolan S.P. The development and catalytic uses of N-heterocyclic carbene gold complexes. Acc. Chem. Res. 2011;44:91–100. doi: 10.1021/ar1000764. [DOI] [PubMed] [Google Scholar]
- 13.Mercs L., Albrecht M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 2010;39:1903–1912. doi: 10.1039/b902238b. [DOI] [PubMed] [Google Scholar]
- 14.Akkoc M., Oz E., Demirel S., Dorcet V., Roisnel T., Bayri A., Bruneau C., Altin S., Yasar S., Ozdemir I. Investigation of potential hybrid capacitor property of chelated N-Heterocyclic carbene Ruthenium (II) complex. J. Organomet. Chem. 2018;866:214–222. [Google Scholar]
- 15.Bugday N., Khan S., Yasar S., Ozdemir I. C-H Bond activation of 2-isobutylthiazole at C5 position catalysed by Pd-N-heterocyclic carbene complexes. J. Organomet. Chem. 2021;937 [Google Scholar]
- 16.Altın S., Oz E., Altundag S., Bayri A., Roisnel T., Dorcet V., Bruneau C., Ozdemir I., Yasar S. Investigation of hybrid-capacitor properties of ruthenium complexes. Int. J. Energy Res. 2019;43:6840–6851. [Google Scholar]
- 17.Imık F., Yasar S., Ozdemir I. Synthesis of bridged palladium-PEPPSI complexes and catalytic studies in C-C cross-coupling reactions. Inorg. Chim. Acta. 2019;495 [Google Scholar]
- 18.Ernesto R.F., Raúl C.P., Viviana R.M., Hugo V., David M.M. Fluorinated-NHC transition metal complexes: leading characters as potential anticancer metallodrugs. Anti Cancer Agents Med. Chem. 2021;21:938–948. doi: 10.2174/1871520620666200908103452. [DOI] [PubMed] [Google Scholar]
- 19.Etaiw Sel D., Sultan A.S., Badr El-Din A.S. A novel hydrogen bonded bimetallic supramolecular coordination polymer {[SnMe3(bpe)][Ag(CN)2]·2H2O} as anticancer drug. Eur. J. Med. Chem. 2011;46:5370–5378. doi: 10.1016/j.ejmech.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 20.Hopkinson M.N., Richhter C., Schedler M., Glarious F. An overview of N-heterocyclic carbenes. Nature. 2014;510:485–495. doi: 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]
- 21.Hindi K.M., Panzner M.J., Tessier C.A., Cannon C.L., Youngs W.J. The Medicinal applications of imidazolium carbene-metal complexes. Chem. Rev. 2009;109:3859–3884. doi: 10.1021/cr800500u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartinger C.G., Dyson P.J. Bioorganometallic chemistry-from teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009;38:391–401. doi: 10.1039/b707077m. [DOI] [PubMed] [Google Scholar]
- 23.Yasar S., Koprulu T.K., Tekin Ş., Yasar S. Sulfonated N-heterocyclic carbine-silver (I) complexes: Synthesis, characterisation and biological evaluation. Appl. Organomet. Chem. 2018;32 [Google Scholar]
- 24.Akkoc M., Balcıoglu S., Canbolat G., Tugba T.T., Ates B., Yasar S. Protonated water-soluble N-heterocyclic carbene ruthenium (II) complexes: Synthesis, cytotoxic and DNA binding properties and molecular docking study. J. Organomet. Chem. 2018;869:67–74. [Google Scholar]
- 25.Kunz P.C., Wetzel C., Kogel S., Kassack M.U., Spingler B. [(C3H4N2)2Au]Cl-a bis protic gold (I)-NHC. Dalton Trans. 2011;40:35–37. doi: 10.1039/c0dt01089h. [DOI] [PubMed] [Google Scholar]
- 26.Marzano C., Pellei M., Tisato F., Santini C. Copper complexes as anticancer agents. Anti Cancer Agents Med. Chem. 2009;9:185–211. doi: 10.2174/187152009787313837. [DOI] [PubMed] [Google Scholar]
- 27.Chardon E., Puleo G.L., Dahm G., Guichard G., Laponnaz S.B. Direct functionalisation of group 10 N-heterocyclic carbene complexes for diversity enhancement. Chem. Commun. 2011;47:5864–5866. doi: 10.1039/c1cc11391g. [DOI] [PubMed] [Google Scholar]
- 28.Lemke J., Metzler-Nolte N. Organometallic peptide NHC complexes of Cp∗ Rh (III) and arene Ru (II) moieties from l-thiazolylalanine. J. Organomet. Chem. 2011;696:1018–1022. [Google Scholar]
- 29.Liu W.K., Gust R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013;42:755–773. doi: 10.1039/c2cs35314h. [DOI] [PubMed] [Google Scholar]
- 30.Thati B., Noble A., Creaven B.S., Walsh M., McCann M., Kavanagh K., Devereux M., Egan D.A. In vitro anti-tumour and cyto-selective effects of coumarin-3-carboxylic acid and three of its hydroxylated derivatives, along with their silver-based complexes, using human epithelial carcinoma cell lines. Cancer Lett. 2007;248:321–331. doi: 10.1016/j.canlet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H.L., Zhang X.M., Liu X.Y., Wang X.J., Liu G.F., Usman A., Fun H.K. Clear Ag-Ag bonds in three silver (I) carboxylate complexes with high cytotoxicity properties. Inorg. Chem. Commun. 2003;6:1113–1116. [Google Scholar]
- 32.Liu J.J., Galettis P., Farr A., Maharaj L., Samarasinha H., McGechan A.C., Baguley B.C., Bowen R.J., Berners-Price S.J., McKeage M.J. In vitro antitumour and hepatotoxicity profiles of Au (I) and Ag (I) bidentate pyridyl phosphine complexes and relationships to cellular uptake. J. Inorg. Biochem. 2008;102:303–310. doi: 10.1016/j.jinorgbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Garrison J.C., Youngs W.J. Ag (I) N-heterocyclic carbene complexes: synthesis, structure, and application. Chem. Rev. 2005;105:3978–4008. doi: 10.1021/cr050004s. [DOI] [PubMed] [Google Scholar]
- 34.Medvetz D.A., Hindi K.M., Panzner M.J., Ditto A.J., Yun Y.H., Youngs W.J. Anticancer activity of Ag (I) N-heterocyclic carbene complexes derived from 4, 5-dichloro-1H-imidazole. Met. Base. Drugs. 2008;2008 doi: 10.1155/2008/384010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray S., Mohan R., Singh J.K., Samantaray M.K., Shaikh M.M., Panda D., Ghosh P. Anticancer and antimicrobial metallopharmaceutical agents based on palladium, gold, and silver N-heterocyclic carbene complexes. J. Am. Chem. Soc. 2007;129:15042–15053. doi: 10.1021/ja075889z. [DOI] [PubMed] [Google Scholar]
- 36.Siciliano T.J., Deblock M.C., Hindi K.M., Durmus S., Panzner M.J., Tessier C.A., Youngs W.J. Synthesis and anticancer properties of gold (I) and silver (I) N-heterocyclic carbene complexes. J. Organomet. Chem. 2011;696:1066–1071. [Google Scholar]
- 37.Yasar S., Kul Koprulu T., Tekin Ş., Yasar S. Synthesis, characterisation and cytotoxic properties of N-heterocyclic carbene silver (I) complexes. Inorg. Chim. Acta. 2018;479:17–23. [Google Scholar]
- 38.Gandina V., Pellei M., Marinellib M., Marzanoa C., Dolmellaa A., Giorgettic M., Santinib C. Synthesis and in vitro antitumor activity of water soluble sulfonate- and ester-functionalized silver(I) N-heterocyclic carbene complexes. J. Inorg. Biochem. 2013;129:135–144. doi: 10.1016/j.jinorgbio.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Luo W.-Q., Du X.-G., Chen L.-Y., Jin C.-M. Synthesis, structure, and anticancer activity of four silver (I)-N-heterocyclic carbene complexes and one polymer containing quinolin-8-yl groups. J. Organomet. Chem. 2021;952 [Google Scholar]
- 40.Gajare S.P., Patil P.V., Patil A.D., Pore D.M., Sonawane K.D., Dhanavade M.J., Khot V.M., Rashinkar G.S. Anticancer, Antibacterial and Hyperthermia Studies of a Caffeine-Based N-Heterocyclic Carbene Silver Complex Anchored on Magnetic Nanoparticles. ChemistrySelect. 2021;6:1958–1968. [Google Scholar]
- 41.Jakob C.H.G., Muñoz A.W., Schlagintweit J.F., Wei V., Reich R.M., Sieber S.A., Correia J.D.G., Kühn F.E. Anticancer and antibacterial properties of trinuclear Cu (I), Ag (I) and Au (I) macrocyclic NHC/urea complexes. J. Organomet. Chem. 2021;932 [Google Scholar]
- 42.Fatima T., Haque R.A., Ahmad A., Hassan L.E.A., Ahamed M.B.K., Majid A.M.S.A., Razali M.R. Tri N-heterocyclic carbene trinuclear silver (I) complexes: Synthesis and in vitro cytotoxicity studies. J. Mol. Struct. 2020;1222 [Google Scholar]
- 43.Discovery Studio Visualizer, San Diego, CA, USA 936.
- 44.Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7(1):1–13. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasar S., Karaca E.O., Sahin C., Ozdemir I., Sahin O., Buyukgungor O. Novel ruthenium(II)–N-heterocyclic carbene complexes; synthesis, characterization and catalytic application. J. Organomet. Chem. 2015;789:1–7. [Google Scholar]
- 46.Kılınçarslan R., Sadıc N., Çetinkaya B. Ag (I) complexes of benzimidazol-2-ylidene ligands: a study of catalytic efficiency towards three-component coupling reactions. Turk. J. Chem. 2016;40:681–687. [Google Scholar]
- 47.Özdemir I., Demir S., Günal S., Özdemir I., Arıcı C., Ülkü D. Synthesis, characterization and antimicrobial activity of new silver complexes with N-heterocyclic carbene ligands. Inorg. Chim. Acta. 2010;363:3803–3808. [Google Scholar]
- 48.Nielsen D.J., Cavell K.J., Skelton B.W., White A.H. Tetrafluoroborate anion B-F bond activation-unusual formation of a nucleophilic heterocyclic carbene: BF3 adduct. Inorg. Chim. Acta. 2003;352:143–150. [Google Scholar]
- 49.Hu X., Castro-Rodriguez I., Olsen K., Meyer K. Group 11 metal complexes of N-heterocyclic carbene ligands: nature of the metal carbene bond. Organometallics. 2004;23:755–764. [Google Scholar]
- 50.Pytkowicz J., Roland S., Mangeney P.J. Synthesis of chiral silver (I) diaminocarbene complexes from (R, R)-4, 5-di-tert-butylimidazoline. Organomet. Chem. 2001;631:157–163. [Google Scholar]
- 51.Coleman K.S., Chamberlayne H.T., Turberville S., Gren M.L.H., Cowley A.R. Silver (I) complex of a new imino-N-heterocyclic carbene and ligand transfer to palladium (II) and rhodium (I) Dalton Trans. 2003:2917–2922. [Google Scholar]
- 52.Lee H.M., Chiu P.L., Hu C.H., Lai C.L., Chou Y.C. Synthesis and structural characterization of metal complexes based on pyrazole/imidazolium chlorides. J. Organomet. Chem. 2005;690:403–414. [Google Scholar]
- 53.Serdaroğlu G., Bölüknbaşı S.Ş., Celepci D.B., Sevinçek R., Şahin N., Gürbüz N., Özdemir İ. Synthesis, in vitro anticancer activities, and quantum chemical investigations on 1,3-bis-(2-methyl-2-propenyl)benzimidazolium chloride and its Ag(I) complex. J. Chem. Res. 2020;45:596–607. [Google Scholar]
- 54.Griñan-Lison C., Blaya-Cánovas J.L., López-Tejada A., Ávalos-Moreno M., Navarro-Ocón A., Cara F.E., González-González A., Lorente J.A., Marchal Juan A., Granados-Principal S. Antioxidants for the treatment of breast cancer: Are we there yet? Antioxidants. 2021;10:205. doi: 10.3390/antiox10020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamal A., Nazari M.V., Yaseen M., Iqbal M.A., Mohamed B., Ahamed K., Majid A.S.A., Bhattia H.N. Green synthesis of selenium-N-heterocyclic carbene compounds: Evaluation of antimicrobial and anticancer potential. Bioorg. Chem. 2019;90 doi: 10.1016/j.bioorg.2019.103042. [DOI] [PubMed] [Google Scholar]
- 56.Graham C. The role of silver in wound healing. Br. J. Nurs. 2005;14:S22. doi: 10.12968/bjon.2005.14.Sup5.19954. [DOI] [PubMed] [Google Scholar]
- 57.Iqbal M.A., Haque R.A., Ahamed M.B.K., Majid A.M.S.A., Al-Rawi S.S. Synthesis and anticancer activity of para-xylyl linked bis-benzimidazolium salts and respective Ag (I) N-heterocyclic carbene complexes. Med. Chem. Res. 2013;22:2455–2466. [Google Scholar]
- 58.Habib A., Nazari M.V., Iqbal M.A., Bhatti H.N., Ahmed M.B.K., Majid A.M.S.A. Unsymmetrically substituted benzimidazolium based Silver(I)-N-heterocyclic carbene complexes: Synthesis, characterization and in vitro anticancer study against human breast cancer and colon cancer. J. Saudi Chem. Soc. 2019;23:795–808. [Google Scholar]
- 59.Nebioğlu A.K., Panzner M.J., Tessier C.A., Cannon C.L., Youngs W.J. N-Heterocyclic carbene–silver complexes: A new class of antibiotics. Coord. Chem. Rev. 2007;251:884–895. [Google Scholar]
- 60.Ozdemir I., Ciftci O., Evren E., Gürbüz N., Kaloglu N., Turkmen N.B., Yasar Ş., Ustun E., Hamdi N., Mansour L., Ozdemir I. Synthesis, characterization and antitumor properties of novel silver (I) and gold (I) N-heterocyclic carbene complexes. Inorg. Chim. Acta. 2020;506 [Google Scholar]
- 61.Sharhan O., Heidelberg T., Hashim N.M., Al-Madhagi W.M., Ali H.M. Benzimidazolium-acridine-based silver N-heterocyclic carbene complexes as potential anti-bacterial and anti-cancer drug. Inorg. Chim. Acta. 2020;504 [Google Scholar]
- 62.Kızrak U., Ciftci O., Ozdemir I., Gurbuz N., Düşünceli S.D., Kaloğlu M., Mansour L., Zaghrouba F., Hamdi N., Ozdemir I. Amine-fnctionalized silver and gold N-heterocyclic carbene complexes: Synthesis, characterization and antitumor properties. J. Organomet. Chem. 2019;882:26–32. [Google Scholar]
- 63.Fabbrini M.G., Cirri D., Pratesi A., Ciofi L., Marzo T., Guerri A., Nistri S., Dell’Accio A., Gamberi T., Severi M., Bencini A., Messori L. A fluorescent silver (I) carbene complex with anticancer properties: synthesis, characterization, and biological studies. ChemMedChem. 2019;14:182–188. doi: 10.1002/cmdc.201800672. [DOI] [PubMed] [Google Scholar]
- 64.Fatima T., Haque R.A., Iqbal M.A., Ahmad A., Hassan L.E.A., Taleb-Agha M., Ahamed M.B.K., Majid A.M.S.A., Razali M.R. Tetra N-heterocyclic carbene dinuclear silver (I) complexes as potential anticancer agents: Synthesis and in vitro anticancer studies. J. Organomet. Chem. 2017;853:122–135. [Google Scholar]
- 65.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 66.Yehye W.A., Rahman N.A., Alhadi A.A., Khaledi H., Ng S.W., Ariffin A. Butylated hydroxytoluene analogs: Synthesis and evaluation of their multipotent antioxidant activities. Molecules. 2012;17(7):7645–7665. doi: 10.3390/molecules17077645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilhelm S., Carter C., Lynch M., Lowinger T., Dumas J., Smith R.A., Schwartz B., Simantov R., Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006;5(10):835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 68.Waseem D., Butt A.F., Ul Hakq İ., Bhatti M.H., Khan G.M. Carboxylate derivatives of tributyltin (IV) complexes as anticancer and antileishmanial agents. DARU J. Pharm. Sci. 2017;25(1):1–14. doi: 10.1186/s40199-017-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.