Abstract

Small-conductance Ca2+-activated potassium (KCa2.x) channels are gated exclusively by intracellular Ca2+. The activation of KCa2.3 channels induces hyperpolarization, which augments Ca2+ signaling in endothelial cells. Cilia are specialized Ca2+ signaling compartments. Here, we identified compound 4 that potentiates human KCa2.3 channels selectively. The subtype selectivity of compound 4 for human KCa2.3 over rat KCa2.2a channels relies on an isoleucine residue in the HA/HB helices. Positive modulation of KCa2.3 channels by compound 4 increased flow-induced Ca2+ signaling and cilia length, while negative modulation by AP14145 reduced flow-induced Ca2+ signaling and cilia length. These findings were corroborated by the increased cilia length due to the expression of Ca2+-hypersensitive KCa2.3_G351D mutant channels and the reduced cilia length resulting from the expression of Ca2+-hyposensitive KCa2.3_I438N channels. Collectively, we were able to associate functions of KCa2.3 channels and cilia, two crucial components in the flow-induced Ca2+ signaling of endothelial cells, with potential implications in vasodilation and ciliopathic hypertension.
1. Introduction
Small- and intermediate-conductance Ca2+-activated K+ (KCa2.x/KCa3.1 or SK/IK) channels are activated exclusively by intracellular Ca2+.1,2 Four subtypes in the KCa2.x/KCa3.1 channel family are encoded by the KCNN mammalian genes: including KCNN1 for KCa2.1 (SK1), KCNN2 for KCa2.2 (SK2), KCNN3 for KCa2.3 (SK3), and KCNN4 for KCa3.1 (IK or SK4) channels.
In blood vessels, KCa2.3 and KCa3.1 channel subtypes are often detected on the plasma membrane of endothelial (ET) cells,3−5 whereas KCa2.1 and KCa2.2 channel currents are rarely identifiable on the ET cell surface.6 KCa2.3 and KCa3.1 channel subtypes seem to have a distinctive distribution and function in ET cells. KCa3.1 channels are often found on the ET cell membrane close to the endoplasmic reticulum (ER) Ca2+ store.7−9 Ca2+ release from the ER triggered by acetylcholine or bradykinin receptors may lead to the opening of KCa3.1 channels nearby.10 In contrast, KCa2.3 channels seem to co-localize with mechanosensitive or receptor-operated transient receptor potential (TRP) cation channels.10,11 Ca2+ influx through these cation channels may activate KCa2.3 channels. The outflow of K+ can hyperpolarize ET cells, increase the inward electrochemical gradient for Ca2+, and augment the Ca2+ influx, which in turn enhances nitric oxide (NO) releases.12,13
Non-motile primary cilia are sensory organelles that sense fluid shear stress on the apical membrane of the cells.14−16 Fluid flow that produces enough drag force on the top of the cells will bend and activate sensory cilia. Transgenic mouse models with cilia mutations do not survive at birth, confirming the importance of primary cilia in the physiological processes.17−20 Primary cilia in vasculatures were once thought to be vestigial organelles and nonfunctional remnants. It has since been shown by different laboratories that cilia are mechanosensory organelles.21−25 Cilia in ET cells sense changes in the fluid shear stress and trigger Ca2+ signaling and NO releases.26,27
Primary cilia have been known as specialized Ca2+ signaling compartments.28,29 Ca2+ influx through TRPM4, TRPV4, TRPC1, polycystic kidney disease 2 (PKD2), and L-type voltage-gated Ca2+ (Cav) channels has been considered the main Ca2+ source for cilia.28,29 Ca2+ influx in response to fluid shear stress activates ET KCa2.3 channels.30 In ET cells, KCa2.3 channels functionally couple with Ca2+-permeable PKD211 and TRPV431 channels and exert a positive feedback influence on intracellular Ca2+ signaling.12,32 However, it is not clear whether this positive feedback mechanism extends back to the cilia, that is, whether the activation of KCa2.3 channels increases cilia length.
KCa2.3 and KCa2.2a channels have similar amino acid sequences in their cytoplasmic gates, which makes it difficult to develop subtype-selective positive modulators discriminating these two subtypes. We recently identified the binding site of a prototype KCa2.2a/KCa2.3 channel modulator, CyPPA.33 We have synthesized a new series of CyPPA analogues.34 Here, we report the identification of a subtype-selective KCa2.3 channel modulator, compound 4, that is ∼21-fold more potent on potentiating human KCa2.3 than rat KCa2.2a channels. The subtype selectivity of compound 4 relies on an I-to-V amino acid residue difference between KCa2.3 and KCa2.2a channels. The pharmacological activation of KCa2.3 channels by compound 4 increased cilia length, whereas the pharmacological inhibition of KCa2.3 channels by AP14145 decreased cilia length in a cultured ET cell line, suggesting the critical role of KCa2.3 channels in the regulation of cilia.
2. Results
2.1. Compound 4 Subtype Selectively Modulates KCa2.3 Channels
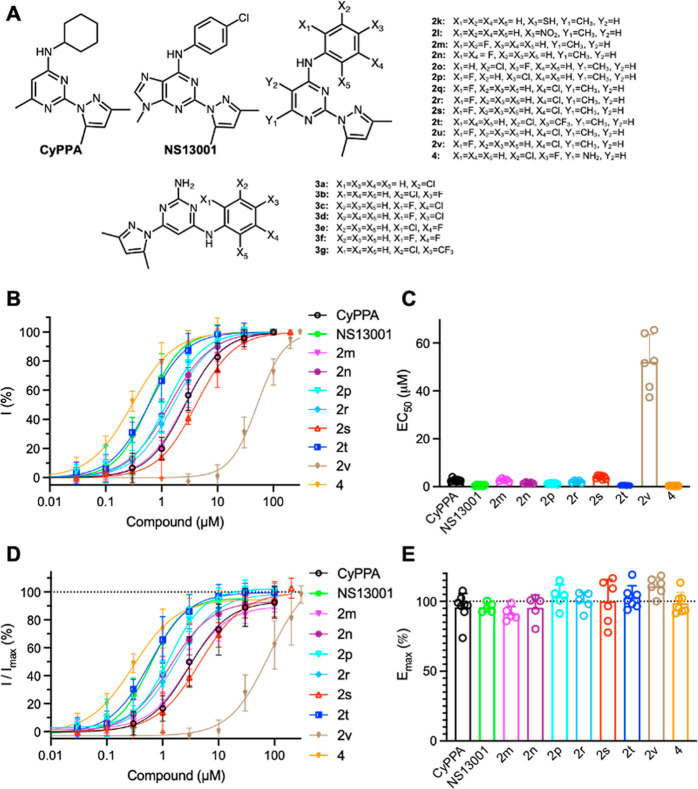
A series of CyPPA analogues (Figure 1A) were synthesized as described in our previous report.34 The potency of these compounds was measured using inside-out patch clamp electrophysiology recordings with human KCa2.3 channels heterologously expressed in HEK293 cells. Positive modulators of KCa2 channels require minimal concentration of Ca2+ to be effective.35 Therefore, we measured the concentration-dependent responses of the channels to compounds in the presence of 0.15 μM Ca2+ (Figure S1). To construct the concentration–response curves, the current amplitudes at −90 mV in response to various concentrations of the compound were normalized to that obtained at the maximal concentration of the compound. The normalized currents were plotted as a function of the compound concentrations. CyPPA, NS13001, and our compounds 2m–2n, 2p, 2r–2t, 2v, and 4 concentration-dependently potentiated the activity of KCa2.3 channels (Figure 1B). Among them, NS13001 and compounds 2t and 4 exhibited submicromolar EC50 values (Figure 1C).
Figure 1.
Positive modulation of human KCa2.3 channels by compounds. (A) Chemical structures of compounds 2k–2v, 3a–3g, and 4, compared with those of CyPPA and NS13001. (B) Concentration-dependent potentiation of KCa2.3 channels by compounds. (C) EC50 values to compounds of KCa2.3 channels. (D) Responses to compounds of KCa2.3 channels were normalized to the maximal currents induced by 10 μM Ca2+. (E) Emax to compounds of KCa2.3 channels. The numbers of independent recordings are shown in parentheses for CyPPA (8), NS13001 (5), 2m (5), 2n (5), 2p (5), 2r (5), 2s (6), 2t (7), 2v (6), and 4 (7). Data are presented as mean ± SD.
The responses induced by 10 μM Ca2+ are considered the maximal currents of the KCa2.x channels.35 To evaluate the efficacy (Emax) of the compounds on KCa2.3 channels, the current amplitudes at −90 mV in response to the compounds were normalized to that obtained at 10 μM Ca2+ [I/Imax(%), Figure 1D]. Non-linear regression curve fitting yielded Emax values for compounds on KCa2.3 channels that are comparable to the Emax of CyPPA (96 ± 10%, n = 8, Figure 1E).
The potency of these compounds on potentiating human KCa2.3 channels is summarized in Table 1 and compared with their previously determined EC50 values on rat KCa2.2a channels.34 CyPPA and NS13001 exhibited ∼2.7- and ∼4.3-fold selectivity for human KCa2.3 channels over rat KCa2.2a channels (Table 1). Compounds 2t and 4 are ∼6.3 and ∼21 times more potent, respectively, on potentiating the activity of human KCa2.3 channels than that of rat KCa2.2a channels (Table 1). Among these compounds, compound 4 caught our attention with its ∼21-fold selectivity for human KCa2.3 channels over that of rat KCa2.2a channels (Table 1). We further evaluated the effects of compound 4 on KCa2.1 and KCa3.1 channel subtypes. Compound 4 did not potentiate human KCa2.1 and human KCa3.1 channel subtypes substantively (Figure S2).
Table 1. Potency of Compounds on Human KCa2.3 Channels Compared with That on Rat KCa2.2a Channelsa.
| compound | EC50 on rat KCa2.2a (mean ± SD, μM) | EC50 on human KCa2.3 (mean ± SD, μM) |
|---|---|---|
| CyPPA | 7.5 ± 1.634 | 2.7 ± 0.6 |
| NS13001 | 2.2 ± 0.534 | 0.50 ± 0.18 |
| 2k | >10034 | >100 |
| 2l | >10034 | >100 |
| 2m | 5.0 ± 1.134 | 2.7 ± 0.6 |
| 2n | 1.9 ± 0.434 | 1.5 ± 0.3 |
| 2o | 1.0 ± 0.234 | 0.20 ± 0.0734 |
| 2p | 2.0 ± 0.334 | 1.2 ± 0.2 |
| 2q | 0.64 ± 0.1234 | 0.60 ± 0.1034 |
| 2r | 3.0 ± 0.734 | 2.1 ± 0.4 |
| 2s | 3.5 ± 1.034 | 3.9 ± 0.7 |
| 2t | 3.3 ± 0.834 | 0.52 ± 0.09 |
| 2u | >10034 | >100 |
| 2v | >3034 | 52 ± 11 |
| 3a | >10034 | >100 |
| 3b | >10034 | >100 |
| 3c | >10034 | >100 |
| 3d | >10034 | >100 |
| 3e | >10034 | >100 |
| 3f | >10034 | >00 |
| 3g | >10034 | >100 |
| 4 | 6.7 ± 1.634 | 0.31 ± 0.07 |
Some EC50 values are reported in ref (34).
2.2. Subtype Selectivity of Compound 4 Relies on the HA/HB Helices
Our recent study has revealed that the subtype selectivity of CyPPA for KCa2.2a and KCa2.3 over KCa3.1 channels relies on the HA/HB helices.33 We aligned the amino acid sequences of the rat KCa2.2a, human KCa2.3, and human KCa3.1 channel subtypes in the proximal C terminus (Figure 2A). Rat KCa2.2a has a valine residue (V420) equivalent to a methionine residue (M311) of the human KCa3.1 channel in the HA helix. In the HB helix, rat KCa2.2a has a lysine residue (K467), corresponding to an arginine residue (R355) of the human KCa3.1 channel. The V-to-M and K-to-R discrepancies between the amino acid sequences of rat KCa2.2a and human KCa3.1 channels provide an explanation for the subtype selectivity of CyPPA.33
Figure 2.
Subtype selectivity of compound 4 relies on the HA/HB helices. (A) Amino acid sequence alignment of rat KCa2.2a [GenBank: NP_062187.1], human KCa2.3 [GenBank: NP_002240.3], and human KCa3.1 [GenBank: NP_002241.1] channels at the proximal C terminus. HA and HB helices are highlighted in green. I568 in KCa2.3 channels and their equivalent residues are shown in bold. (B) Potentiation by compound 4 of the WT and mutant human KCa2.3 channels. (C) EC50 values for potentiation by compound 4. ***P < 0.001 compared with human KCa2.3_WT. (D) Responses to compound 4 were normalized to the maximal currents induced by 10 μM Ca2+. (E) Emax to compound 4 of the WT and mutant KCa2.3 channels. The numbers of independent recordings are shown in parentheses for KCa2.3_WT (7) and KCa2.3_I568V (6). Data are presented as mean ± SD.
We then set out to explore the structural determinants for the ∼21-fold subtype selectivity of compound 4 for human KCa2.3 over rat KCa2.2 channels. Human KCa2.3 has an isoleucine (I568) equivalent to V420 in the HA helix of rat KCa2.2a channels (Figure 2A). The side chain of KCa2.3_I568 would be bulkier than that of KCa2.2a_V420. Thus, the different sizes of a valine (rat KCa2.2a_V420) and an isoleucine (human KCa2.3_I568) may constitute the structural determinants for the subtype selectivity of compound 4. We tested this hypothesis by mutating KCa2.3_I568 to its corresponding amino acid residue in KCa2.2a, a valine (Figure 2B). The KCa2.3_I568V mutant channel exhibited an EC50 value of 6.2 ± 1.3 μM (n = 6), which is ∼20-fold less sensitive to compound 4 than the KCa2.3_WT with an EC50 value of 0.31 ± 0.07 μM (n = 7, Figure 2C). The KCa2.3_I568V mutation did not affect the Emax values to compound 4, compared with the KCa2.3_WT channel (Figure 2D,E). The KCa2.3_I568V mutation did not influence the apparent Ca2+ sensitivity of KCa2.3 channels (Figure S3A,B).
The corresponding mutation in rat KCa2.2a channels (KCa2.2a_V420I) did not change either the apparent Ca2+ sensitivity of KCa2.2a channels (Figure S4A,B) or the Emax to compound 4 (Figure S4C,D). The KCa2.2a_V420I increased the sensitivity of the channel to compound 4 (Figure S4E,F), corroborating the results acquired from the corresponding KCa2.3_I568V mutation (Figure 2B,C).
2.3. Pharmacological Modulation of KCa2.3 Channels Affected Cilia Length
Recently, we identified KCa2.3 channels as the predominant subtype expressed in a mouse ET cell line, whereas the expression of KCa2.1, KCa2.2, and KCa3.1 channel subtypes was not detected by immunoblots.36 Thus, we examined whether negative modulation by AP14145 of KCa2.3 channels affected the cilia length of the ET cells. AP14145 inhibited KCa2.3 channels with an IC50 value of 0.97 ± 0.39 μM (n = 5, Figure S5).
ET cells were incubated with AP14145 (20 μM) for 2 days before cells reached confluency, and the cilia length was evaluated using immunostaining with the antibody of the ciliary marker acetylated α-tubulin (green) and the nuclear marker DAPI (blue, Figure S6A). AP14145 shortened cilia to 2.8 ± 0.1 μm, compared with 6.3 ± 0.3 μm of the solvent control group (Figure S6B,C), suggesting a regulatory role of KCa2.3 channels in the cilia length of ET cells.
Compound 4 potentiated KCa2.3 channels with an EC50 value of 0.31 ± 0.07 μM (n = 7) (Table 1 and Figure 1C). ET cells were incubated with compound 4 (20 μM) for 2 days before cells reached confluency, and the cilia length was evaluated using immunostaining with the antibody of the ciliary marker acetylated α-tubulin (green) and the nuclear marker DAPI (blue, Figure 3A). Compound 4 increased the cilia length to 6.1 ± 0.6 μm compared with 4.3 ± 0.3 μm of the solvent control group (Figure 3B,C), suggesting potential therapeutic usefulness of KCa2.3 channel positive modulators (e.g., compound 4) in ciliopathy disease states with abnormal cilia.
Figure 3.
The effects of KCa2.3 channel potentiation by compound 4 on cilia length in ET cells. (A) Cells were stained with the antibody of the ciliary marker acetylated α-tubulin (green) and the nuclear marker (DAPI; blue). (B) Cilia length was grouped in a discreet range, and percent distribution was tabulated. (C) Cilia length is significantly longer in cells treated with the positive modulator, compound 4 (20 μM). N = 50–70 for each slide preparation, and a total of four independent slides were used in each group. Data are presented as mean ± SD. *p < 0.05 compared to the control.
To confirm the elongating effect of compound 4 on cilia (Figure 3), an additional ciliary marker Arl13b was used to measure the cilia length (Figure S7A). Also, the γ-tubulin was used as a marker for the basal body (base of a cilium), which cannot be used for the measurement of cilia length. Consistent with the measurements with acetylated α-tubulin (Figure 3), compound 4 (20 μM) increased cilia length (Figure S7B,C). A bee venom toxin, apamin (50 nM),37 that selectively blocks KCa2 channels, completely abolished the elongating effect of compound 4 on cilia (Figure S7B,C). The ET cells do not express KCa2.1, KCa2.2, and KCa3.1 channels.36 Therefore, the effect of apamin on the ET cells is mediated by the KCa2.3 channel blockade.
2.4. Expression of Mutant KCa2.3 Channels Affects Cilia Length
Positive modulators of KCa2.3 channels potentiate channel activity by increasing the apparent Ca2+ sensitivity of the channels,38 whereas negative modulators decrease the apparent Ca2+ sensitivity of the channels.39 To rule out the possibility that compound 4 and AP14145 affected cilia length through their off-target effects other than KCa2.3 channels, we heterologously expressed mutant KCa2.3 channels with altered apparent Ca2+ sensitivity (Figure 4). When expressed in ET cells, the KCa2.3 channels exhibited an apparent Ca2+ sensitivity of 0.67 ± 0.11 μM (n = 6). The G351D mutation significantly increased the apparent Ca2+ sensitivity to 0.16 ± 0.04 μM (n = 7), while the I438N mutation significantly reduced the apparent Ca2+ sensitivity to 1.8 ± 0.3 μM (n = 5, Figure 4). Immunoblots (Figures S8A–C) and immunostaining studies (Figure S8D) showed no evidence for different expression levels or localizations of the mutant channels.
Figure 4.
Mutant mouse KCa2.3 channels with altered Ca2+ sensitivity. Mutations channels were expressed in ET cells and their apparent Ca2+ sensitivity was evaluated using inside-out patch clamp recordings. (A) Representative KCa2.3_WT channel currents in response to Ca2+. (B) Concentration-dependent activation by Ca2+ of the mutant and WT KCa2.3 channels. (C) EC50 values to Ca2+ of the mutant and WT KCa2.3 channels. The numbers of independent recordings are shown in parentheses for KCa2.3_WT (6), KCa2.3_G351D (7), and KCa2.3_I438N (5). Data are presented as mean ± SD. ***P < 0.001 compared with KCa2.3_WT.
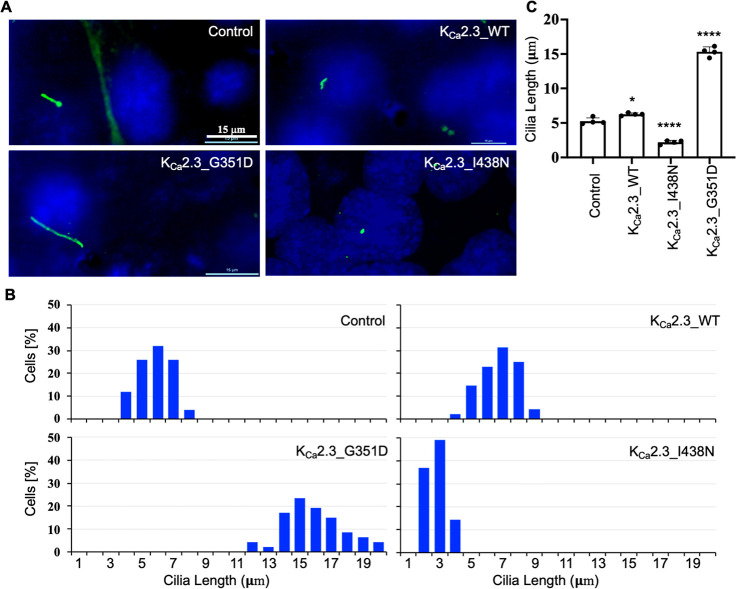
The higher the apparent Ca2+ sensitivity of the mutant channel, the more likely the KCa2.3 channel is opening and then augmenting the Ca2+ influx in a positive feedback mechanism. The overexpression of KCa2.3_WT led to a slightly increased cilia length (6.3 ± 0.2 μm) compared with the control (5.3 ± 0.5 μm, Figure 5). KCa2.3_G351D mutant channels with hypersensitivity to Ca2+ increased the cilia length even more drastically (15.3 ± 0.7 μm), while the KCa2.3_I438N mutant channels with hyposensitivity to Ca2+ reduced the cilia length (2.2 ± 0.3 μm, Figure 5), confirming a role of the KCa2.3 channel in the regulation of cilia length.
Figure 5.
Expression of mouse KCa2.3 channels changes the primary cilia length in ET cells. (A) Cells were stained with the antibody of the ciliary marker acetylated α-tubulin (green) and the nuclear marker DAPI (blue). (B) Cilia length was grouped in a discreet range, and percent distribution was tabulated. (C) Cilia length is significantly longer in cells expressing KCa2.3_WT and KCa2.3_G351D but shorter in cells expressing KCa2.3_I438N channels. N = 50–70 for each slide preparation, and a total of four independent slides were used in each group. Data are presented as mean ± SD. *p < 0.05 and ****p < 0.0001 compared to the control.
2.5. Pharmacological Intervention of KCa2.3 Channels Affected Ca2+ Signaling
The opening of KCa2.3 channels induces hyperpolarization, which may increase the inward electrochemical gradient for Ca2+ and thus augment the Ca2+ influx. Next, we investigated whether the positive modulation or negative modulation of KCa2.3 channels affected the Ca2+ signaling, using fluorescence Ca2+ imaging (Figure 6). Flow-induced cytosolic Ca2+ transients were measured using a ratiometric, high-affinity intracellular Ca2+ indicator Fura-2AM. Compared with the control ET cells (Figure 6A), the AP14145-treated ET cells exhibited much weaker Ca2+ transients (Figure 6B). In contrast, the compound 4-treated ET cells exhibited more prominent Ca2+ transients (Figure 6C) than the control cells. The significant effects of a negative modulator AP14145 and a positive modulator compound 4 on the flow-induced peak Ca2+ values (Figure 6D) suggest a link between the KCa2.3 channel opening and Ca2+ signaling, triggered by the shear stress. We have previously generated the non-ciliated IFT88 knockout (KO) mouse ET cells.40 Using these cells, we further validate that flow-induced cytosolic Ca2+ transients were largely abolished in IFT88 KO ET cells, suggesting the essential role of cilia in flow-induced Ca2+ signaling (Figure S9).
Figure 6.
Positive and negative modulation of KCa2.3 channels affected flow-induced cytosolic Ca2+ signaling. Fluorescence Ca2+ measurements of ET cells treated with (A) solvent control, (B) negative modulator AP14145 (20 μM), and (C) positive modulator compound 4 (20 μM). (D) Peak Ca2+ values are significantly increased by compound 4 but reduced by AP14145. The numbers of independent measurements are shown in parentheses for the control (5), AP14145 (5), and compound 4 (5). Data are presented as mean ± SD. *p < 0.05 and ****p < 0.0001 compared with the control.
3. Discussion
Among the four channel subtypes encoded by the mammalian KCNN genes, KCa2.3 closely resembles the KCa2.2 channel subtype in pharmacology.41 The human KCa2.2a channel does not express as well as the rat KCa2.2a channel, which prevented us from performing inside-out patch clamp experiments. Human and rat KCa2.2a channels are highly homologous, with differences only in the distal cytoplasmic N- and C- termini. In the transmembrane domains and in the cytoplasmic gate including the HA/HB helices (highlighted in green), which CyPPA interacts with, the similarity is 100% (Figure S10). The prototype subtype-selective positive modulator, CyPPA achieved selectivity for KCa2.2 and KCa2.3 channels over KCa2.1 and KCa3.1 subtypes.35 CyPPA is also ∼2.7 times more potent on human KCa2.3 than on rat KCa2.2a channels (Table 1). In this study, we identified a positive modulator, compound 4, that is ∼21-fold selective for human KCa2.3 over rat KCa2.2a channels (Table 1). Compound 4 is largely inactive on human KCa2.1 and human KCa3.1 channels (Figure S2). The significance of this study is not limited to compound 4 itself with an EC50 of ∼0.3 μM and a modest subtype-selectivity for human KCa2.3 over rat KCa2.2a channels. The subtype selectivity of compound 4 for human KCa2.3 over rat KCa2.2a channels relies on an I-to-V discrepancy in the HA/HB helices between the two subtypes (Figures 2 and S4), which may offer an opportunity for the development of even more subtype-selective modulators.
The expression of KCa2.3 together with KCa3.1 channels on the plasma membrane of ET cells is well-documented.3−5 KCa2.3 channels functionally couple with mechanosensitive and TRP Ca2+-entry channels (e.g. PKD211 and TRPV431). We observed a positive feedback effect of KCa2.3 channels on the flow-induced intracellular Ca2+ signaling through cilia (Figure 6). Most importantly, the positive feedback extends back to cilia themselves as the positive modulator compound 4 increased the cilia length (Figure 3), while the negative modulator AP14145 reduced the cilia length (Figure S6). These observations allow us to connect KCa2.3 channels and cilia, two crucial components in the flow-induced Ca2+ signaling in ET cells, with implications in vasodilation and blood pressure regulation.
The regulation of cilia length by KCa2.3 channel positive and negative modulators (Figures 3 and S6) has been corroborated by the effects on cilial length of the mutant KCa2.3 channels with altered apparent Ca2+ sensitivity (Figures 4 and 5). Expression of the Ca2+-hypersensitive KCa2.3_G351D mutant channel increased the cilia length, while the Ca2+-hyposensitive KCa2.3_I438N mutant channel reduced the cilia length (Figure 5). It is noteworthy that the mouse KCa2.3_G351D mutation used in our study is equivalent to the human KCa2.3_G350D mutation, which causes Zimmermann-Laband syndrome (ZLS).42 It has been speculated that during human embryonic development, excessive hyperpolarization due to hypersensitivity to Ca2+ of the ZLS-related mutant KCa2.3 channels might result in exaggerated vasodilation in response to shear stress. This in turn might cause edema and vascular ruptures in critical phases of embryonic development, leading to distal digital hypoplasia with aplastic or hypoplastic nails and terminal phalanges.42 Our results showed that the equivalent mouse KCa2.3_G351D mutation caused hypersensitivity to Ca2+ (Figure 4), which may contribute to vasodilation mediated by the endothelium-derived hyperpolarization.8,43,44 Our finding here that the expression of KCa2.3_G351D mutant channels increased cilia length in ET cells (Figure 5) could also be translated into increased sensitivity and vasodilation in response to blood flow. Both of these two mechanisms might underlie the vasodilation and vascular rupture speculated in the embryonic development of ZLS patients, although further studies will be needed to elucidate the developmental biology.
We and other laboratories have previously reported that rapamycin increases cilia length in epithelial cells, resulting in the inhibition of cell proliferation.45,46 On the other hand, rapamycin-induced cilia length increase correlates to an elevated response to fluid shear stress in ET cells.47 The function of primary cilia as mechanosensory organelles depends on the length of cilia; lengthening primary cilia enhance cellular mechanosensitivity.48,49 Dopamine, for example, also increases cilia length and function, resulting in enhanced cellular mechanosensitivity.50 While dopamine specificity was a concern, drugs that improve sensory cilia function by elongating cilia length have been coined “ciliotherapy”.51 A more specific cilia-targeted therapy in ET cells has also been proposed to remedy hypertension.52,53 We therefore are hopeful that subtype-selective positive modulators of KCa2.3 channels (e.g., compound 4) would have a great potential to be a potential ciliotherapy.
4. Experimental Section
4.1. Materials
Materials are listed in Table 2.
Table 2.
| reagent or resources | source | identifier |
|---|---|---|
| Chemicals | ||
| CyPPA | Alomone Labs | C-110 |
| NS13001 | ChemShuttle | 104258 |
| compound 2k | in-house synthesis34 | N/A |
| compound 2l | in-house synthesis34 | N/A |
| compound 2m | in-house synthesis34 | N/A |
| compound 2n | in-house synthesis34 | N/A |
| compound 2o | in-house synthesis34 | N/A |
| compound 2p | in-house synthesis34 | N/A |
| compound 2q | in-house synthesis34 | N/A |
| compound 2r | in-house synthesis34 | N/A |
| compound 2s | in-house synthesis34 | N/A |
| compound 2t | in-house synthesis34 | N/A |
| compound 2u | in-house synthesis34 | N/A |
| compound 2v | in-house synthesis34 | N/A |
| compound 3a | in-house synthesis34 | N/A |
| compound 3b | in-house synthesis34 | N/A |
| compound 3c | in-house synthesis34 | N/A |
| compound 3d | in-house synthesis34 | N/A |
| compound 3e | in-house synthesis34 | N/A |
| compound 3f | in-house synthesis34 | N/A |
| compound 3g | in-house synthesis34 | N/A |
| compound 4 | in-house synthesis34 | N/A |
| Fura2-AM | Thermo Fisher Scientific | F-1221 |
| DAPI | Southern Biotech | 0100-20 |
| Antibodies | ||
| fluorescein secondary antibody | Vector Labs Burlingame | FI-2000 |
| anti-acetylated α-tubulin | Sigma-Aldrich | T-7451 |
| anti-GFP | Novus Biological | NB600-308SS |
| anti-GAPDH | Abcam | ab181602 |
| anti-Arl13b | Proteintech | 17711-1-AP |
| anti-γ-tubulin | Proteintech | 15176-1-AP |
| Experimental Models: Cell Lines | ||
| Human: HEK293 | ATCC | CRL-11268 |
| Mouse: ET | in-house26,27 | N/A |
| Mouse: IFT88 KO | in-house40 | N/A |
| Recombinant DNA | ||
| pcDNA3.1(+) | Thermo Fisher Scientific | V79020 |
| pIRES2-AcGFP1 | Takara Bio | 632435 |
| Software and Algorithms | ||
| GraphPad Prism 9.0.2 | GraphPad Software Inc. | RRID: SCR_002798 |
| Clampfit 10.5 | Molecular Devices | RRID: SCR_011323 |
| pClamp 10.5 | Molecular Devices | RRID: SCR_011323 |
| Clustal Omega server | https://www.ebi.ac.uk/Tools/msa/clustalo/ | RRID: SCR_001591 |
4.2. Electrophysiology
The effect of compounds on the KCa2.x/KCa3.1 channels was investigated as previously described.54,55 Briefly, the rat KCa2.2a, human KCa2.1, human KCa2.3, or human KCa3.1 channel cDNA constructs were either generated in-house or through molecular cloning services (Genscript, Piscataway, NJ, USA). These channel cDNAs in the pIRES2-AcGFP1 vector, along with calmodulin cDNA in the pcDNA3.1 + vector, at a ratio of 10:1 (ORF ratios), were transfected into cells using the calcium–phosphate method. KCa currents were recorded 1–2 days after transfection using an Axon200B amplifier (Molecular Devices, San Jose, CA) at room temperature. The resistance of the patch electrodes ranged from 3 to 5 MΩ. The pipette solution contained the following (in mM): 140 KCl, 10 Hepes (pH 7.4), and 1 MgSO4. The bath solution containing (in mM) 140 KCl, 10 Hepes (pH 7.2), 1 EGTA, 0.1 Dibromo-BAPTA, and 1 HEDTA was mixed with Ca2+ to obtain the desired free Ca2+ concentrations, calculated using the software written by Chris Patton (https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/webmaxc/webmaxcS.htm). The Ca2+ concentrations were verified using a Ca2+ calibration buffer kit (Thermo Fisher Scientific). Briefly, a standard curve was generated using the Ca2+ buffers from the kit and a fluorescence Ca2+ indicator. Then, the Ca2+ concentrations of the bath solution were determined through interpolation on the standard curve.
High-resistance seals (>1 GΩ) were formed before inside-out patches were obtained. The seal resistance of inside-out patches was >1 GΩ, when the intracellular face was initially exposed to a zero-Ca2+ bath solution. Currents were recorded by repetitive 1-s-voltage ramps from −100 to +100 mV from a holding potential of 0 mV. The currents were filtered at 2 kHz and digitized at a sampling frequency of 10 kHz. At the end of the experiment, the integrity of the patch was examined by switching the bath solution back to the zero-Ca2+ buffer. Data from patches, which maintained the seal resistance (>1 GΩ) after solution changes, were used for further analysis.
To measure the effect of the positive modulators, the intracellular face was exposed to bath solutions with 0.15 μM Ca2+. One minute after the switching of bath solutions, 10 sweeps with a 1 s interval were recorded at a series of concentrations of the compound in the presence of 0.15 μM Ca2+. The maximal KCa2.x/KCa3.1 current in response to 10 μM Ca2+ was then recorded.
To measure the effect of the negative modulator Ap14145, the intracellular face was exposed to bath solutions with 0.5 μM Ca2+. One minute after the switching of bath solutions, 10 sweeps with a 1 s interval were recorded at a series of concentrations of AP14145 in the presence of 0.5 μM Ca2+.
4.3. Cilia Measurements
Cilia length was measured by direct immunofluorescence for the cilia marker with anti-acetylated α-tubulin or Arl13b staining. The cells were fixed for 10 min (4% paraformaldehyde/2% sucrose in PBS) and permeabilized for 5 min (10% Triton X-100). Acetylated α-tubulin (1:10,000 dilution, Sigma-Aldrich, St. Louis, MO) or Arl13b (1:50 dilution, Proteintech, Rosemont, IL) and fluorescein isothiocyanate-conjugated (1:1000 dilution, Vector Labs Burlingame, CA) antibodies were each incubated with the cells for 1 h at 37 °C. Microscope slides were then mounted with DAPI (Southern Biotech, Birmingham, AL) hard set mounting media. A Nikon Eclipse Ti-E inverted microscope with NIS-Elements imaging software (version 4.30) was used to capture the images of primary cilia. Automated image acquisition was conducted in 100× magnification fields. Cilia length analysis followed a standard calculation as previously described.56
4.4. Flow-Induced Ca2+ Measurements
Cells were loaded with 5 μM Fura2-AM (Thermo Fisher Scientific, Waltham, MA) at 37 °C for 30 min. Cells were then washed with Dulbecco’s phosphate-buffered saline and observed under a 40× objective lens using a Nikon Eclipse Ti-E microscope controlled by Elements software. Cytosolic calcium was observed by recording Ca2+-bound Fura excitation fluorescence at 340/380 nm and emission at 510 nm. Baseline Ca2+ was observed for 5 min prior to data acquisition. Fluid shear stress was then applied to cells utilizing an Instech P720 peristaltic pump with an inlet and outlet setup. The fluid was perfused on the glass-bottom plates at a shear stress of 5 dyn/cm2. After each experiment, the maximum calcium signal was obtained with ATP (10 μM) to confirm cell viability. Conditions for all experiments were maintained at 37 °C and 5% CO2 in a stage top cage incubator (okoLab, Burlingame, CA). Ca2+ analysis followed a standard calculation as previously described.56
4.5. Immunoblots
The protein concentrations in ET cell lysates were determined using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA). Equal amounts of protein (15 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel (Bio-Rad Laboratories, Hercules, CA). The proteins were transferred to polyvinylidene fluoride (PVDF) membranes and incubated overnight at 4 °C with the primary GFP antibody (1:2000; Novus Biological, Centennial, CO) or GAPDH antibody (1:5000; Abcam, Waltham, MA). The PVDF membranes were washed with Tris-buffered saline (0.1% Tween 20) and incubated with the anti-rabbit antibody (1:3000; cell signaling technology, Danvers, MA) as the secondary antibody for 1 h at room temperature and then washed with Tris-buffered saline (0.1% Tween 20). The chemiluminescence signals were detected on a ChemiDoc XRS system (Bio-Rad Laboratories, Hercules, CA) after incubation with Luminol/enhancer solution (Thermo Fisher Scientific, Waltham, MA). Densitometry analyses were performed using the ImageJ computer program.
4.6. Data and Statistical Analysis
Patch clamp recordings were analyzed using Clampfit 10.5 (Molecular Devices LLC, San Jose, CA), and concentration–response curves were analyzed in GraphPad Prism 9.0.2 (GraphPad Software Inc., La Jolla, CA). To construct the concentration-dependent potentiation of channel activities by the compound, the current amplitudes at −90 mV in response to various concentrations of the compound were normalized to that obtained at a maximal concentration of the compound. The normalized currents were plotted as a function of the concentrations of the compound. EC50 values and Hill coefficients were determined by fitting the data points to a standard concentration–response curve [Y = 100/(1 + (X/EC50)^ – Hill)]. To assess the efficacy of the compound, the current amplitudes obtained at the maximal concentration of the compound were normalized to the maximal KCa2.x/KCa3.1 current in response to 10 μM Ca2+. Concentration–response curves were acquired from multiple patches for each data set. Each curve was fitted individually, which yielded the EC50 value for that curve. EC50 values are shown as mean ± SD obtained from multiple patches, and the number of patches is indicated by n.
The Student’s t-test was used for data comparison if there were only two groups. One-way ANOVA and Tukey’s post hoc tests were used for data comparison of three or more groups. Post hoc tests were carried out only if F was significant and there was no variance in homogeneity.
Acknowledgments
We are grateful to L.Basilio, Y.Hur, and M.Nguyen for technical assistance.
Glossary
Abbreviations
- KCa2.1 channels
small-conductance Ca2+-activated potassium subtype 1 channels
- KCa2.2 channels
small-conductance Ca2+-activated potassium subtype 2 channels
- KCa2.3 channels
small-conductance Ca2+-activated potassium subtype 3 channels
- KCa3.1 channels
intermediate-conductance Ca2+-activated potassium channels
- Cav
voltage-gated Ca2+ channels
- ER
endoplasmic reticulum
- TRP
transient receptor potential
- NO
nitric oxide
- PKD2
polycystic kidney disease
- ZLS
Zimmermann-Laband syndrome
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.2c00469.
Electrophysiological recordings, cilia length measurements, intracellular Ca2+ signaling data, and amino acid sequence alignments (PDF)
Author Present Address
# Cellulose and Paper Department, National Research Center, 33 El-Bouhos St. (former Tahrir St.), Dokki, Giza, Egypt, P.O.X 12622
Author Contributions
Y.-W.N. and P.R. contributed equally to this work. Y.W.N., G.Y., R.O., M.A.R, M.D., and M.Z. undertook molecular biology and in vitro electrophysiology studies. P.R., D.L-H., F.A., and S.M.N. undertook cilia and Ca2+ imaging studies. N.S.E. and K.P. undertook chemical synthesis. S.M.N. and M.Z. wrote the manuscript. All authors contributed to the figures. All authors have read and agreed to the published version of the manuscript.
M.Z. was supported by an Internal Faculty Opportunity Fund of Chapman University Office of Research, a Scientist Development grant 13SDG16150007 from American Heart Association, and a National Institutes of Health grant 4R33NS101182. S.M.N. was supported by a National Institutes of Health grant R01HL147311.
The authors declare no competing financial interest.
Supplementary Material
References
- Adelman J. P.; Maylie J.; Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu. Rev. Physiol. 2012, 74, 245–269. 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- Brown B. M.; Shim H.; Christophersen P.; Wulff H. Pharmacology of small- and intermediate-conductance calcium-activated potassium channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 219–240. 10.1146/annurev-pharmtox-010919-023420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Xie A.; Singh A. K.; Ehsan A.; Choudhary G.; Dudley S.; Sellke F. W.; Feng J. Inactivation of Endothelial Small/Intermediate Conductance of Calcium-Activated Potassium Channels Contributes to Coronary Arteriolar Dysfunction in Diabetic Patients. J. Am. Heart Assoc. 2015, 4, e002062 10.1161/JAHA.115.002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler R.; Brakemeier S.; Kühn M.; Behrens C.; Real R.; Degenhardt C.; Orzechowski H. D.; Pries A. R.; Paul M.; Hoyer J. Impaired hyperpolarization in regenerated endothelium after balloon catheter injury. Circ. Res. 2001, 89, 174. 10.1161/hh1401.093460. [DOI] [PubMed] [Google Scholar]
- Köhler R.; Degenhardt C.; Kühn M.; Runkel N.; Paul M.; Hoyer J. Expression and function of endothelial Ca(2+)-activated K(+) channels in human mesenteric artery: A single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ. Res. 2000, 87, 496–503. 10.1161/01.res.87.6.496. [DOI] [PubMed] [Google Scholar]
- Wulff H.; Köhler R. Endothelial small-conductance and intermediate-conductance KCa channels: an update on their pharmacology and usefulness as cardiovascular targets. J. Cardiovasc. Pharmacol. 2013, 61, 102–112. 10.1097/fjc.0b013e318279ba20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J.; Taylor M. S.; Bonev A. D.; Hannah R. M.; Solodushko V.; Shui B.; Tallini Y.; Kotlikoff M. I.; Nelson M. T. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 9627–9632. 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora K. A.; Gallagher N. T.; McNeish A.; Garland C. J. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ. Res. 2008, 102, 1247–1255. 10.1161/circresaha.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow S. L.; Neylon C. B.; Chen M. X.; Garland C. J. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function?. J. Anat. 2006, 209, 689–698. 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brähler S.; Kaistha A.; Schmidt V. J.; Wölfle S. E.; Busch C.; Kaistha B. P.; Kacik M.; Hasenau A. L.; Grgic I.; Si H.; et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 2009, 119, 2323. 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- MacKay C. E.; Leo M. D.; Fernandez-Pena C.; Hasan R.; Yin W.; Mata-Daboin A.; Bulley S.; Gammons J.; Mancarella S.; Jaggar J. H. Intravascular flow stimulates PKD2 (polycystin-2) channels in endothelial cells to reduce blood pressure. Elife 2020, 9, e60401 10.7554/elife.60401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J. Z.; Ella S.; Davis M. J.; Hill M. A.; Braun A. P. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J. 2009, 23, 1138–1145. 10.1096/fj.08-120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankevicius E.; Lopez-Valverde V.; Rivera L.; Hughes A. D.; Mulvany M. J.; Simonsen U. Combination of Ca2+ -activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery. Br. J. Pharmacol. 2006, 149, 560–72. 10.1038/sj.bjp.0706886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saternos H. C.; AbouAlaiwi W. A.. Implications of Dysfunction of Mechanosensory Cilia in Polycystic Kidney Disease. In Polycystic Kidney Disease; Li X., Ed.; Exon Publications: Brisbane (AU), 2015. [PubMed] [Google Scholar]
- Phua S. C.; Lin Y. C.; Inoue T. An intelligent nano-antenna: Primary cilium harnesses TRP channels to decode polymodal stimuli. Cell Calcium 2015, 58, 415–422. 10.1016/j.ceca.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando J.; Yamamoto K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc. Res. 2013, 99, 260–268. 10.1093/cvr/cvt084. [DOI] [PubMed] [Google Scholar]
- Cortellino S.; Wang C.; Wang B.; Bassi M. R.; Caretti E.; Champeval D.; Calmont A.; Jarnik M.; Burch J.; Zaret K. S.; et al. Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev. Biol. 2009, 325, 225–237. 10.1016/j.ydbio.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.; Markowitz G. S.; Li L.; D’Agati V. D.; Factor S. M.; Geng L.; Tibara S.; Tuchman J.; Cai Y.; Hoon Park J. H.; et al. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat. Genet. 2000, 24, 75–78. 10.1038/71724. [DOI] [PubMed] [Google Scholar]
- Lu W.; Peissel B.; Babakhanlou H.; Pavlova A.; Geng L.; Fan X.; Larson C.; Brent G.; Zhou J. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat. Genet. 1997, 17, 179–181. 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- Yoder B. K.; Richards W. G.; Sweeney W. E.; Wilkinson J. E.; Avener E. D.; Woychik R. P. Insertional mutagenesis and molecular analysis of a new gene associated with polycystic kidney disease. Proc. Assoc. Am. Physicians 1995, 107, 314. [PubMed] [Google Scholar]
- Mohieldin A. M.; Zubayer H. S.; Al Omran A. J.; Saternos H. C.; Zarban A. A.; Nauli S. M.; AbouAlaiwi W. A. Vascular Endothelial Primary Cilia: Mechanosensation and Hypertension. Curr. Hypertens. Rev. 2016, 12, 57–67. 10.2174/1573402111666150630140615. [DOI] [PubMed] [Google Scholar]
- Luo N.; Conwell M. D.; Chen X.; Kettenhofen C. I.; Westlake C. J.; Cantor L. B.; Wells C. D.; Weinreb R. N.; Corson T. W.; Spandau D. F.; et al. Primary cilia signaling mediates intraocular pressure sensation. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 12871–12876. 10.1073/pnas.1323292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A. M.; Jacobs C. R. Emerging role of primary cilia as mechanosensors in osteocytes. Bone 2013, 54, 196–204. 10.1016/j.bone.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius H. A.; Spring K. R. Removal of the MDCK cell primary cilium abolishes flow sensing. J. Membr. Biol. 2003, 191, 69–76. 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A.; Leonard M. L.; Bizios R.; Bowser S. S. Analysis and modeling of the primary cilium bending response to fluid shear. Am. J. Physiol. 1997, 272, F132. 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- AbouAlaiwi W. A.; Takahashi M.; Mell B. R.; Jones T. J.; Ratnam S.; Kolb R. J.; Nauli S. M. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ. Res. 2009, 104, 860–869. 10.1161/circresaha.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli S. M.; Kawanabe Y.; Kaminski J. J.; Pearce W. J.; Ingber D. E.; Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 2008, 117, 1161–1171. 10.1161/circulationaha.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablo J. L.; DeCaen P. G.; Clapham D. E. Progress in ciliary ion channel physiology. J. Gen. Physiol. 2017, 149, 37–47. 10.1085/jgp.201611696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli S. M.; Pala R.; Kleene S. J. Calcium channels in primary cilia. Curr. Opin. Nephrol. Hypertens. 2016, 25, 452–458. 10.1097/mnh.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold K. A.; Schwartz M. A. Ion Channels in Endothelial Responses to Fluid Shear Stress. Physiol. 2016, 31, 359–369. 10.1152/physiol.00007.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare S. K.; Bonev A. D.; Ledoux J.; Liedtke W.; Kotlikoff M. I.; Heppner T. J.; Hill-Eubanks D. C.; Nelson M. T. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 2012, 336, 597–601. 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X.; Francis M.; Köhler R.; Solodushko V.; Lin M.; Taylor M. S. Positive feedback regulation of agonist-stimulated endothelial Ca2+ dynamics by KCa3.1 channels in mouse mesenteric arteries. Arterioscler., Thromb., Vasc. Biol. 2014, 34, 127–135. 10.1161/atvbaha.113.302506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Y. W.; Cui M.; Salem N.; Orfali R.; Nguyen M.; Yang G.; Rahman M. A.; Lee J.; Zhang M. Subtype-selective positive modulation of KCa 2 channels depends on the HA/HB helices. Br. J. Pharmacol. 2021, 179, 460. 10.1111/bph.15676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed N. S.; Nam Y. W.; Egorova P. A.; Nguyen H. M.; Orfali R.; Rahman M. A.; Yang G.; Wulff H.; Bezprozvanny I.; Parang K.; et al. Structure-Activity Relationship Study of Subtype-Selective Positive Modulators of KCa2 Channels. J. Med. Chem. 2021, 65, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard C.; Eriksen B. L.; Jørgensen S.; Johansen T. H.; Dyhring T.; Madsen L. S.; Strøbaek D.; Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br. J. Pharmacol. 2007, 151, 655–665. 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Y. W.; Kong D.; Wang D.; Orfali R.; Sherpa R. T.; Totonchy J.; Nauli S. M.; Zhang M. Differential modulation of SK channel subtypes by phosphorylation. Cell Calcium 2021, 94, 102346. 10.1016/j.ceca.2020.102346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy C.; Goodchild S. J.; Weatherall K. L.; Jane D. E.; Liégeois J. F.; Seutin V.; Marrion N. V. Allosteric block of KCa2 channels by apamin. J. Biol. Chem. 2010, 285, 27067–27077. 10.1074/jbc.m110.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard C.; Eriksen B. L.; Jørgensen S.; Johansen T. H.; Dyhring T.; Madsen L. S.; Strøbaek D.; Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br. J. Pharmacol. 2007, 151, 655–665. 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simó-Vicens R.; Kirchhoff J. E.; Dolce B.; Abildgaard L.; Speerschneider T.; Sørensen U. S.; Grunnet M.; Diness J. G.; Bentzen B. H. A new negative allosteric modulator, AP14145, for the study of small conductance calcium-activated potassium (KCa 2) channels. Br. J. Pharmacol. 2017, 174, 4396–4408. 10.1111/bph.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohieldin A. M.; Pala R.; Beuttler R.; Moresco J. J.; Yates J. R. 3rd; Nauli S. M. Ciliary extracellular vesicles are distinct from the cytosolic extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12086 10.1002/jev2.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich R. W.; Chandy K. G.; Grissmer S.; Gutman G. A.; Kaczmarek L. K.; Wei A. D.; Wulff H.. Calcium- and Sodium-Activated Potassium Channels (Version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database; IUPHAR/BPS Guide to Pharmacology CITE, 2019. [Google Scholar]
- Bauer C. K.; Schneeberger P. E.; Kortüm F.; Altmüller J.; Santos-Simarro F.; Baker L.; Keller-Ramey J.; White S. M.; Campeau P. M.; Gripp K. W.; et al. Gain-of-Function Mutations in KCNN3 Encoding the Small-Conductance Ca(2+)-Activated K(+) Channel SK3 Cause Zimmermann-Laband Syndrome. Am. J. Hum. Genet. 2019, 104, 1139–1157. 10.1016/j.ajhg.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli S. P.; Eckmann M. S.; Hunte M. S. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am. J. Physiol. 2003, 285, H1590–H1599. 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- Edwards G.; Félétou M.; Weston A. H. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pfluegers Arch. Eur. J. Physiol. 2010, 459, 863–879. 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Jamal M. H.; Nunes A. C. F.; Vaziri N. D.; Ramchandran R.; Bacallao R. L.; Nauli A. M.; Nauli S. M. Rapamycin treatment correlates changes in primary cilia expression with cell cycle regulation in epithelial cells. Biochem. Pharmacol. 2020, 178, 114056. 10.1016/j.bcp.2020.114056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. A.; Willemarck N.; Talebi A.; Marchand A.; Binda M. M.; Dehairs J.; Rueda-Rincon N.; Daniels V. W.; Bagadi M.; Raj D. B.; et al. Identification of drugs that restore primary cilium expression in cancer cells. Oncotarget 2016, 7, 9975–9992. 10.18632/oncotarget.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherpa R. T.; Atkinson K. F.; Ferreira V. P.; Nauli S. M. Rapamycin Increases Length and Mechanosensory Function of Primary Cilia in Renal Epithelial and Vascular Endothelial Cells. Int. J. Educ. Res. 2016, 2, 91–97. [PMC free article] [PubMed] [Google Scholar]
- Upadhyay V. S.; Muntean B. S.; Kathem S. H.; Hwang J. J.; AbouAlaiwi W. A.; Nauli S. M. Roles of dopamine receptor on chemosensory and mechanosensory primary cilia in renal epithelial cells. Front. Physiol. 2014, 5, 72. 10.3389/fphys.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic M.; Jacobs C. R.; Jacobs C. Lengthening primary cilia enhances cellular mechanosensitivity. Eur. Cells Mater. 2017, 33, 158–168. 10.22203/ecm.v033a12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Majeed S.; Nauli S. M. Dopamine receptor type 5 in the primary cilia has dual chemo- and mechano-sensory roles. Hypertension 2011, 58, 325–331. 10.1161/hypertensionaha.111.172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathem S. H.; Mohieldin A. M.; Abdul-Majeed S.; Ismail S. H.; Altaei Q. H.; Alshimmari I. K.; Alsaidi M. M.; Khammas H.; Nauli A. M.; Joe B.; et al. Ciliotherapy: a novel intervention in polycystic kidney disease. J. Geriatr. Cardiol. 2014, 11, 63–73. 10.3969/j.issn.1671-5411.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala R.; Mohieldin A. M.; Sherpa R. T.; Kathem S. H.; Shamloo K.; Luan Z.; Zhou J.; Zheng J. G.; Ahsan A.; Nauli S. M. Ciliotherapy: Remote Control of Primary Cilia Movement and Function by Magnetic Nanoparticles. ACS Nano 2019, 13, 3555–3572. 10.1021/acsnano.9b00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala R.; Mohieldin A. M.; Shamloo K.; Sherpa R. T.; Kathem S. H.; Zhou J.; Luan Z.; Zheng J. G.; Ahsan A.; Nauli S. M. Personalized Nanotherapy by Specifically Targeting Cell Organelles To Improve Vascular Hypertension. Nano Lett. 2019, 19, 904–914. 10.1021/acs.nanolett.8b04138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Y. W.; Baskoylu S. N.; Gazgalis D.; Orfali R.; Cui M.; Hart A. C.; Zhang M. A V-to-F substitution in SK2 channels causes Ca(2+) hypersensitivity and improves locomotion in a C. elegans ALS model. Sci. Rep. 2018, 8, 10749. 10.1038/s41598-018-28783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Y. W.; Cui M.; Orfali R.; Viegas A.; Nguyen M.; Mohammed E. H. M.; Zoghebi K. A.; Rahighi S.; Parang K.; Zhang M. Hydrophobic interactions between the HA helix and S4-S5 linker modulate apparent Ca(2+) sensitivity of SK2 channels. Acta Physiol. 2021, 231, e13552 10.1111/apha.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli S. M.; Jin X.; AbouAlaiwi W. A.; El-Jouni W.; Su X.; Zhou J. Non-motile primary cilia as fluid shear stress mechanosensors. Methods Enzymol. 2013, 525, 1–20. 10.1016/b978-0-12-397944-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.