Abstract
Background
Endoscopic dissection (ED) shows relatively high clinical value in early esophageal cancer (cT1N0) such as lower incidence of postoperative complications and hospitalization costs and enhanced recovery. However, whether ED still has certain advantages over esophagectomy in terms of long-term survival remains unclear.
Purpose
The aim of this meta-analysis was to compare the long-term outcomes of ED and surgery in the treatment of cT1N0 esophageal cancer.
Methods
Several electronic databases including the PubMed, EMBASE, Web of Science and Cochrane Library databases were searched up to April 7, 2022 for studies which compared the overall survival (OS) and disease-specific survival (DSS) of cT1N0 esophageal cancer patients receiving the ED or esophagectomy. The hazard ratios (HRs) and 95% confidence intervals (CIs) were combined and all statistical analysis was conducted through STATA 15.0 software.
Results
A total of 12 studies involving 3,732 patients were enrolled. No significant difference in the OS between ED and surgery groups was observed (HR = 0.78, 95% CI, 0.59–1.04, p = 0.089). However, the DSS of the ED group was significantly longer than that of the surgery group (HR = 0.56, 95% CI, 0.39–0.82, p = 0.003).
Conclusion
In overall, the current evidence manifested that the long-term survival of cT1N0 esophageal cancer patients undergoing ED was not worse than that of patients undergoing esophagectomy. ED may be considered as the primary treatment for cT1N0 esophageal carcinoma patients.
Keywords: endoscopic dissection, esophagectomy, cT1N0, esophageal carcinoma, meta-analysis
Introduction
Esophageal cancer is one of the most common tumors with high mortality rate worldwide (1–3). Esophagectomy remains the primary treatment option for most cases (4, 5). However, the incidence of surgery-related complications is still relatively high and patient’s quality of life declines significantly postoperatively despite of the great advances in the surgical techniques in recent years (6, 7). With the improvement of the general public’s health awareness, the early screening of esophageal carcinoma is becoming more and more popular (8, 9). As a result, the proportion of superficial esophageal carcinoma which is confined to the mucosa among all esophageal cancer cases is also continuing increasing (10, 11). In addition to curing the disease, patients are increasingly demanding in terms of therapeutic risk, cost and quality of life.
Esophagoscope was originally designed to examine esophageal lesions. However, its clinical value in the treatment of superficial esophageal disease is gradually manifested. Besides, previous literatures have demonstrated that endoscopic dissection (ED) is obviously related to lower risk of adverse events and procedure-related mortality rate in early stage esophageal cancer patients (12). Furthermore, ED has significant advantages in terms of economic benefits over the surgery (13, 14). Thus, ED is believed to show high clinical value in treatment early stage (T1N0) esophageal cancer. However, due to the highly malignant and aggressive nature of esopahgeal cancer, long-term prognosis of patients receiving ED should be of great concern. Whether ED still has certain advantages in terms of long-term survival is unclear in cT1N0 esophageal cancer patients.
Therefore, the aim of this meta-analysis was to compare the long-term outcomes of ED and esophagectomy for cT1N0 esophageal cancer patients, which might contribute to the clinical treatment and management for this group of patients.
Materials and Methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (15).
Literature Search
The PubMed, EMBASE, Web of Science and Cochrane Library databases electronic databases were searched up to April 7, 2022 for relevant studies. The following terms were used during the literature search: endoscopic submucosal dissection, endoscopic mucosal dissection, esophagus, esophageal, tumor, cancer, carcinoma, neoplasm and esophagectomy. In detail, the specific search strategy was as follows: (endoscopic submucosal dissection OR endoscopic mucosal dissection) AND (esophagus OR esophageal) AND (tumor OR cancer OR neoplasm OR carcinoma) AND esophagectomy. Besides, the references cited in included papers were also reviewed for availability.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) patients were diagnosed with primary esophageal cancer; (2) the clinical stage was with cT1N0 which indicated that tumor confined to mucosa or submucosa; (3) patients received the ED including the endoscopic mucosal dissection (EMD) and endoscopic submucosal dissection (ESD) or esophagectomy and patients receiving the ED and then esophagectomy were divided into the surgery group; (4) the long-term survival representing as overall survival (OS) and disease-specific survival (DSS) including the disease-free survival (DFS), recurrence-free survival (RFS) and cancer-specific survival (CSS) was compared between ED and surgery groups; (5) HRs with 95% CIs were reported or enough data were provided to calculate them.
The exclusion criteria were as follows: (1) duplicated or overlapped data; (2) other anti-tumor interventions were involved during the comparison between ED and esophagectomy; (3) meeting abstracts, editorials, letters, reviews or case reports; (4) low quality studies with the Newcastle Ottawa Scale (NOS) score of 5 or lower (16).
Data Extraction
The following data were collected form each included studies: the name of author, publication year, country, sample size, number of patients receiving ED, stage (T1a or T1b), tumor type, endpoint and hazard ratios (HRs) with corresponding 95% confidence intervals (CIs).
Methodological Quality Assessment
The quality of included studies were evaluated according to the NOS, and studies with a NOS score of 6 or higher were defined as high-quality studies and could be included (16).
The literature search, selection, data extraction and methodological quality evaluation were all performed by two authors independently. Any disagreement was resolved by team discussion.
Statistical Analysis
All statistical analysis was conducted by STATA 15.0 software. The HR with 95%CI were combined to assess the association between the treatment method and long-term survival of cT1N0 stage esophageal cancer patients. If the HRs with 95% CIs were not reported directly, then they would be calculated from Kaplan-Meier curves with the method described by Tierney et al. (17). The heterogeneity was evaluated by Cochran’s Q test and Higgins I2 statistic; Pheterogeneity < 0.10 and/or I2 > 50% was defined as significant heterogeneity among studies, and the random-effects model was applied for the pooled effect estimates, otherwise the fixed-effects model was used (18). Subgroup analyses stratified by the tumor stage (T1a vs. T1) and tumor type were further conducted. Begg’s funnel plot and Egger’s test were conducted to evaluate publication bias and significant publication bias was defined as p < 0.05 (19).
Results
Literature Search and Selection
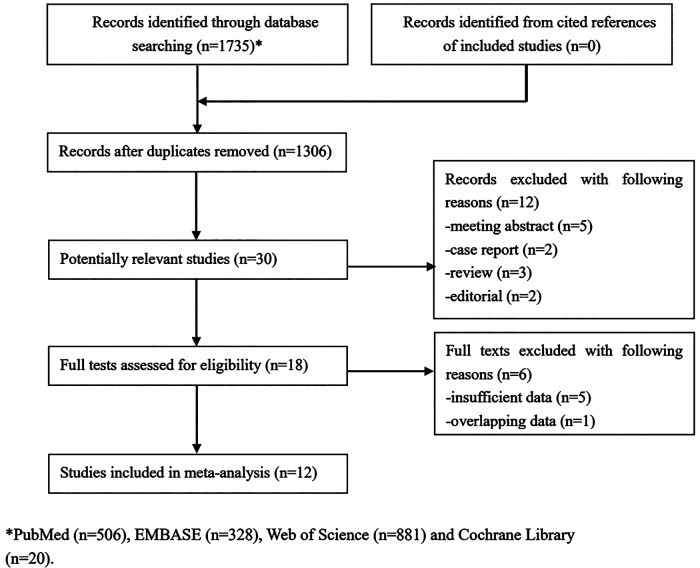
Initially, 1,735 records were searched from databases and 429 duplicated records were removed. Then after scanning the titles, 1,276 irrelevant publications were excluded. After reviewing the abstracts of 30 potentially related publications, 18 studies were then excluded. Finally, a total of 12 studies were included in this meta-analysis (20–31). The detailed selection process was presented in Figure 1.
Figure 1.
The flow diagram of this meta-analysis.
Basic Characteristics of Included Studies
Among the 12 studies, 3,732 patients were involved (20–31) with the sample size ranged from 100 to 954 and 1,671 patients received ED. Three studies focused on T1a stage patients whose tumor was confined to the mucosa (21, 22, 30). Besides, six and three studies focused on squamous cell carcinoma (SCC) (23, 25–28, 30) and adenocarcinoma (AC) (20–22, 29) cases. All studies were regarded as high-quality studies with the NOS score of 6 or higher. The other detailed information was shown in Table 1.
Table 1.
Basic characteristics of included studies.
| Author | Year | Country | Sample size | Number of ED | Stage | Tumor type | Endpoint | Source of HR | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Pech (20) | 2011 | Germany | 114 | 76 | T1 | AC | OS | E | 7 |
| Zehetner (21) | 2011 | USA | 101 | 40 | T1a | AC | OS | E | 7 |
| Schmidt (22) | 2016 | USA | 100 | 36 | T1a | AC | OS | E | 7 |
| Min (23) | 2018 | Korea | 240 | 120 | T1 | SCC | OS, DSS | R | 7 |
| Qin (24) | 2019 | USA | 954 | 224 | T1 | Mixed | OS, DSS | R | 8 |
| Yuan (25) | 2019 | China | 116 | 69 | T1 | SCC | OS | E | 7 |
| Zhang (26) | 2019 | China | 596 | 322 | T1 | SCC | OS, DSS | R/E | 7 |
| An (27) | 2020 | China | 406 | 222 | T1 | SCC | OS | R | 8 |
| Lee (28) | 2020 | Republic Korea | 184 | 70 | T1 | SCC | OS, DSS | R | 8 |
| Saunders (29) | 2020 | UK | 189 | 93 | T1 | AC | DSS | R | 7 |
| Kim (30) | 2021 | Korea | 463 | 263 | T1a | SCC | OS | R | 7 |
| Dunn (31) | 2022 | UK | 269 | 136 | T1 | Mixed | OS, DSS | R | 8 |
ESD, endoscopic dissection; HR, hazard ratio; NOS, Newcastle-Ottawa Scale; AC, adenocarcinoma; SCC, squamous cell carcinoma; OS, overall survival; DSS, disease-specific survival; E, estimated; R, reported.
Comparison of OS between Patients Receiving ED and Esophagectomy
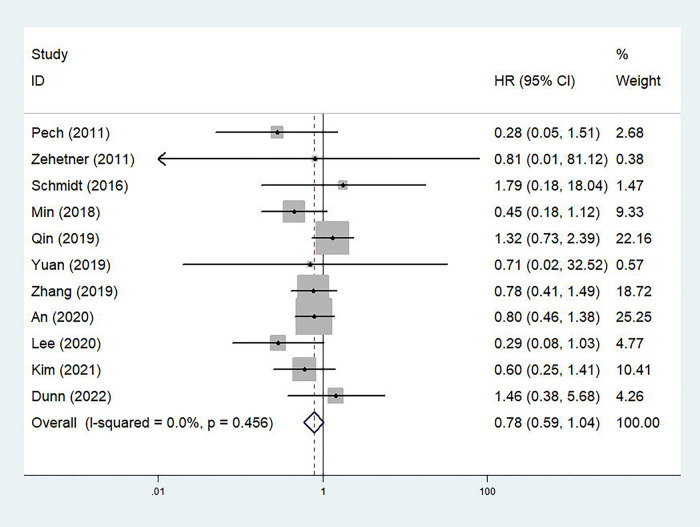
A total of 11 studies explored the association between the treatment option and OS of cT1N0 stage esophageal cancer patients (20–28, 30, 31). The pooled results indicated that no significant difference between the ED and surgery groups were observed (HR = 0.78, 95% CI: 0.59–1.04, p = 0.089; I2 = 0.0%, Pheterogeneity = 0.456) (Figure 2). Subgroup analysis based on the stage showed similar results. However, subgroup analysis stratified by the tumor type manifested that ED was superior to esophagectomy in terms of OS for cT1N0 stage esophageal SCC patients (HR = 0.65, 95% CI, 0.47–0.91, p = 0.013; I2 = 0.0%, Pheterogeneity = 0.696) (Table 2).
Figure 2.
Comparison of overall survival between patients receiving endoscopic dissection and esophagectomy.
Table 2.
Results of meta-analysis.
| No. of studies | HR | 95% CI | p value | I2 (%) | P heterogeneity | |
|---|---|---|---|---|---|---|
| Overall survival | 11 | 0.78 | 0.59–1.04 | 0.089 | 0.0 | 0.456 |
| Stage | ||||||
| T1 | 8 | 0.80 | 0.59–1.08 | 0.140 | 21.7 | 0.257 |
| T1a | 3 | 0.69 | 0.31–1.53 | 0.362 | 0.0 | 0.683 |
| Tumor type | ||||||
| Adenocarcinoma | 3 | 0.56 | 0.15–2.07 | 0.384 | 0.0 | 0.441 |
| Squamous cell carcinoma | 6 | 0.65 | 0.47–0.91 | 0.013 | 0.0 | 0.696 |
| Disease-specific survival | 6 | 0.56 | 0.39–0.82 | 0.003 | 19.5 | 0.287 |
| Tumor-type | ||||||
| Squamous cell carcinoma | 3 | 0.60 | 0.35–1.02 | 0.057 | 0.0 | 0.872 |
| Adenocarcinoma | 1 | 0.29 | 0.14–0.61 | 0.001 | - | - |
HR, hazard ratio; CI, confidence interval.
Bold values indicate significant statistical difference.
Comparison of DSS between Patients Receiving ED and Esophagectomy
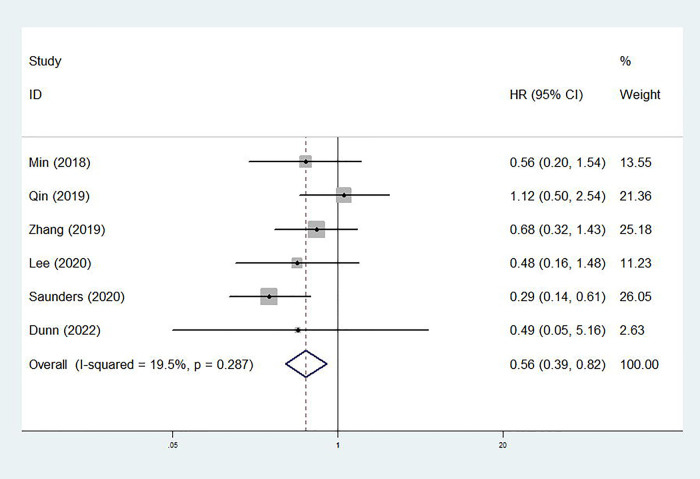
Only six studies explored the association between the treatment option and DSS of cT1N0 stage esophageal cancer patients (23, 24, 26, 28, 29, 31). The pooled results demonstrated that the DSS of patients in the ED group was significantly longer than that in the surgery group (HR = 0.56, 95% CI, 0.39–0.82, p = 0.003; I2 = 19.5%, Pheterogeneity = 0.287) (Figure 3). For AC patients, similar results were observed (HR = 0.29, 95% CI, 0.14–0.61, p = 0.001). However, no significant difference between the ED and surgery groups in SCC patients was observed (HR = 0.60, 95% CI, 0.35–1.02, p = 0.057; I2 = 0.0%, Pheterogeneity = 0.872) (Table 2).
Figure 3.
Comparison of disease-specific survival between patients receiving endoscopic dissection and esophagectomy.
Publication Bias
Due to the symmetric Begg’s funnel plot (Figure 4) and p = 0.483 for Egger’s test, no significant publication bias was detected in this meta-analysis.
Figure 4.
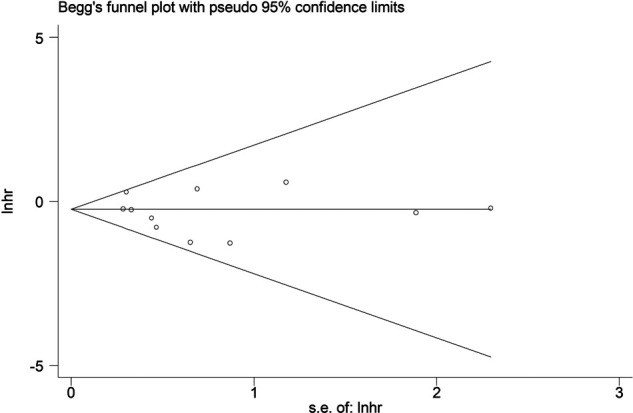
Begg’s funnel plot.
Discussion
The current meta-analysis demonstrated that the long-term prognosis of cT1N0 esophageal cancer patients undergoing ED was not worse than that of patients undergoing esophagectomy after including 12 studies and 3,732 patients. Meanwhile, ED was even superior to esophagectomy in some cases. Thus, based on the evidence provided by our study and previous literatures, ED could be considered as the primary treatment for cT1N0 stage esophageal cancer. However, more prospective high quality clinical trials are still needed to verify above findings.
Actually, in addition to the long-term survival, a number of studies have explored the clinical role of ED in terms of other fields. In the meta-analysis conducted by Zheng et al., 2,467 patients undergoing ED and 2,264 patients undergoing surgery were enrolled (12). Their results demonstrated that patients receiving ED showed significantly lower incidence of major adverse events [relative risk (RR) = 0.46, 95% CI, 0.33–0.64, p < 0.001] and procedure-related mortality (RR = 0.27, 95% CI, 0.10–0.73, p < 0.001) than those receiving esophagectomy (12). Besides, several researches compared the hospitalization cost of patients between the ED and surgery groups and their results manifested that the hospitalization costs in the surgery group was significantly higher than that in the ED group (13, 14, 25, 26). Meanwhile, the length of stage in the ED group was also obviously shorter than that in the esophagectomy group (13, 14, 25, 26, 32). Considering the above mentioned aspects, ED is obviously better than esophagectomy in treatment of early stage esophageal cancer.
However, some scholars suggested that ED might show a lower R0 resection rate due to the operational limitations (13, 20, 22, 25, 26, 32). Thus, patients undergoing ED may be at higher risk of relapse compared to patients undergoing esophagectomy. After comprehensively reviewing previous relevant literatures focusing on T1N0 stage esophageal cancer patients, the recurrence rates in the esophagectomy group and ED group ranged from 0% to 28.6% and from 1.79% to 13.0%. In overall, there was no obvious difference in the recurrence rate between the two groups [odds ratio (OR) = 1.24, 95% CI, 0.73–2.10, p = 0.420] (20–22, 25, 26, 32–39). Identifying the long-term efficacy of ED for superficial esophageal cancer has been an issue we need to address. Therefore, we designed the current meta-analysis and demonstrated that ED remained non-inferior to esophagectomy in terms of long-term survival for cT1N0 stage esophageal carcinoma patients.
Actually, we deem that there are still some valuable fields worthy of more depth investigations about the ED for early stage esophageal cancer. For example, some scholars indicated that T1b stage patients who undergoing ESD should receive adjuvant therapies like the chemotherapy, but others had different opinions (40). Furthermore, it is also necessary to compare the therapeutic effects of ED combined chemotherapy and esophagectomy for cT1N0 stage esophageal cancer patients. Besides, our meta-analysis revealed that for specific population ED might show higher prognostic value than esophagectomy, which should be verified by more high-quality studies with big sample sizes. The operative skills of endoscopists might be closely related to the therapeutic effects of ED and future studies should take this into account.
There are several limitations in this meta-analysis. First, all included studies are retrospective with relatively small sample sizes, which might cause some bias. Second, stage is believed to be an essential factor affecting the treatment option. However, we were unable to conduct more detailed subgroup stratified by the stage (T1a vs. T1b) due to the lack of original data and subgroup analysis based on other important parameters such as the location of tumor and endoscopic techniques were also impracticable.
Conclusion
The current meta-analysis demonstrated that the long-term survival of cT1N0 esophageal cancer patients undergoing ED was not worse than that of patients undergoing esophagectomy. ED may be considered as the primary treatment for cT1N0 esophageal carcinoma patients. However, more prospective high-quality studies are still needed to verify above findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Author Contributions
YJ and WW designed this meta-analysis. WL and PL performed the literature search and selection, collected data, performed statistical analyses and wrote the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/Black People 2022. CA Cancer J Clin. (2022). 10.3322/caac.21718. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. (2022) 54(2):330–44. 10.4143/crt.2022.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). (2022) 135:584–90. 10.1097/cm9.0000000000002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalhammar K, Malmström M, Schelin M, Falkenback D, Kristensson J. The impact of initial treatment strategy and survival time on quality of end-of-life care among patients with oesophageal and gastric cancer: a population-based cohort study. PLoS One. (2020) 15:e0235045. 10.1371/journal.pone.0235045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda T. Future treatment strategy for esophageal cancer based on prediction of systemic recurrence: significance of pathologic nodal status after neoadjuvant chemotherapy. Ann Surg Oncol. (2018) 25:2127–8. 10.1245/s10434-018-6544-0 [DOI] [PubMed] [Google Scholar]
- 6.Schembre D, Arai A, Levy S, Farrell-Ross M, Low D. Quality of life after esophagectomy and endoscopic therapy for Barrett’s esophagus with dysplasia. Dis Esophagus. (2010) 23:458–64. 10.1111/j.1442-2050.2009.01042.x [DOI] [PubMed] [Google Scholar]
- 7.Schwameis K, Zehetner J, Green KM, DeMeester SR. Workload, recurrence, quality of life and long-term efficacy of endoscopic therapy for high-grade Dysplasia and intramucosal esophageal adenocarcinoma. Ann Surg. (2020) 271:701–8. 10.1097/sla.0000000000003038 [DOI] [PubMed] [Google Scholar]
- 8.Maity AK, Stone TC, Ward V, Webster AP, Yang Z, Hogan A, et al. Novel epigenetic network biomarkers for early detection of esophageal cancer. Clin Epigenet. (2022) 14:23. 10.1186/s13148-022-01243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waters JK, Reznik SI. Update on management of squamous cell esophageal cancer. Curr Oncol Rep. (2022) 24:375–85. 10.1007/s11912-021-01153-4 [DOI] [PubMed] [Google Scholar]
- 10.Miller KD, Ortiz AP, Pinheiro PS, Bandi P, Minihan A, Fuchs HE, et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin. (2021) 71:466–87. 10.3322/caac.21695 [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Kang N, Huang Y, Zhao Y, Zhang R. Endoscopic resection versus esophagectomy for early esophageal cancer: a meta-analysis. Transl Cancer Res. (2021) 10:2653–62. 10.21037/tcr-21-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong L, Yue J, Duan X, Jiang H, Zhang H, Zhang X, et al. Comparison of the therapeutic effects of endoscopic submucosal dissection and minimally invasive esophagectomy for T1 stage esophageal carcinoma. Thoracic Cancer. (2019) 10(11):2161–7. 10.1111/1759-7714.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wirsching A, Boshier PR, Krishnamoorthi R, Larsen MC, Irani S, Ross AS, et al. Endoscopic therapy and surveillance versus esophagectomy for early esophageal adenocarcinoma: a review of early outcomes and cost analysis. Am J Surg. (2019) 218:164–9. 10.1016/j.amjsurg.2018.12.058 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Tan R, Lam WC, Yao L, Wang X, Cheng CW, et al. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Extension for Chinese Herbal Medicines 2020 (PRISMA-CHM 2020). Am J Chin Med. (2020) 48:1279–313. 10.1142/s0192415X20500639 [DOI] [PubMed] [Google Scholar]
- 16.Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal. (2017) 5:80–4. 10.13105/wjma.v5.i4.80 [DOI] [Google Scholar]
- 17.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Li J, Chang S, Dong Y, Che G. Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: a systematic review and two meta-analyses based on four million cases. J Thorac Oncol. (2021) 16:1893–908. 10.1016/j.jtho.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 20.Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Holscher A. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg. (2011) 254:67–72. 10.1097/SLA.0b013e31821d4bf6 [DOI] [PubMed] [Google Scholar]
- 21.Zehetner J, DeMeester SR, Hagen JA, Ayazi S, Augustin F, Lipham JC, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg. (2011) 141:39–47. 10.1016/j.jtcvs.2010.08.058 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt HM, Mohiuddin K, Bodnar AM, El Lakis M, Kaplan S, Irani S, et al. Multidisciplinary treatment of T1a adenocarcinoma in Barrett’s esophagus: contemporary comparison of endoscopic and surgical treatment in physiologically fit patients. Surg Endosc. (2016) 30:3391–401. 10.1007/s00464-015-4621-z [DOI] [PubMed] [Google Scholar]
- 23.Min YW, Lee H, Song BG, Min BH, Kim HK, Choi YS, et al. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: a propensity score-matched analysis. Gastrointestinal Endosc. (2018) 88(4):624–33. 10.1016/j.gie.2018.04.2360 [DOI] [PubMed] [Google Scholar]
- 24.Qin J, Peng Y, Chen W, Ma H, Zheng Y, Li Y, et al. Comparative study of esophagectomy, endoscopic therapy, and radiotherapy for cT1N0M0 esophageal cancer in elderly patients: a SEER database analysis. Thorac Cancer. (2019) 10:1511–20. 10.1111/1759-7714.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan B, Liu L, Huang H, Li D, Shen Y, Wu B, et al. Comparison of the short-term and long-term outcomes of surgical treatment versus endoscopic treatment for early esophageal squamous cell neoplasia larger than 2cm: a retrospective study. Surg Endosc Other Interv Tech. (2019) 33:2304–12. 10.1007/s00464-018-6524-2 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Ding H, Chen T, Zhang X, Chen WF, Li Q, et al. Outcomes of endoscopic submucosal dissection vs esophagectomy for T1 esophageal squamous cell carcinoma in a real-world cohort. Clin Gastroenterol Hepatol. (2019) 17:73–81.e3. 10.1016/j.cgh.2018.04.038 [DOI] [PubMed] [Google Scholar]
- 27.An W, Liu MY, Zhang J, Cui YP, Gao J, Wang LP, et al. Endoscopic submucosal dissection versus esophagectomy for early esophageal squamous cell carcinoma with tumor invasion to different depths. Am J Cancer Res. (2020) 10:2977–92. [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HD, Chung H, Kwak Y, Choi J, Lee A, Kim JL, et al. Endoscopic submucosal dissection versus surgery for superficial esophageal squamous cell carcinoma: a propensity score-matched survival analysis. Clin Transl Gastroenterol. (2020) 11:e00193. 10.14309/ctg.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders JH, Al-Zubaidi S, Waller RC, Ortiz-Fernandez-Sordo J, Parsons SL, Ragunath K, et al. The management and long-term outcomes of endoscopic and surgical treatment of early esophageal adenocarcinoma. Dis Esophagus. (2020) 33:doz097. 10.1093/dote/doz097 [DOI] [PubMed] [Google Scholar]
- 30.Kim GH, Na HK, Ahn JY, Lee JH, Jung KW, Kim DH, et al. Long-term outcomes and factors affecting the survival of patients with mucosal esophageal squamous cell carcinoma. Gut Liver. (2021) 15:705–12. 10.5009/gnl20254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn JM, Reyhani A, Santaolalla A, Zylstra J, Gimson E, Pennington M, et al. Transition from esophagectomy to endoscopic therapy for early esophageal cancer. Dis Esophagus. (2022) 35:doab047. 10.1093/dote/doab047 [DOI] [PubMed] [Google Scholar]
- 32.Jin XF, Gai W, Chai TH, Li L, Guo JQ. Comparison of endoscopic resection and minimally invasive esophagectomy in patients with early esophageal cancer. J Clin Gastroenterol. (2017) 51:223–7. 10.1097/mcg.0000000000000560 [DOI] [PubMed] [Google Scholar]
- 33.Le Page PA, Velu PP, Penman ID, Couper GW, Paterson-Brown S, et al. Surgical and endoscopic management of high grade dysplasia and early oesophageal adenocarcinoma. Surgeon. (2016) 14:315–21. 10.1016/j.surge.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 34.Li C, Yamashita DT, Hawel JD, Bethune D, Henteleff H, Lamb PJ. Endoscopic mucosal resection versus esophagectomy for intramucosal adenocarcinoma in the setting of barrett’s esophagus. Surg Endosc. (2017) 31:4211–6. 10.1007/s00464-017-5479-z [DOI] [PubMed] [Google Scholar]
- 35.Watanabe M, Suehara N, Koga K, Tamae K, Namoto M, et al. Outcomes of endoscopic submucosal dissection and esophagectomy for early and superficial carcinoma of the esophagus. Esophagus. (2010) 7(4):215–7. 10.1007/s10388-010-0249-1 [DOI] [Google Scholar]
- 36.Cummings LC, Kou TD, Schluchter MD, Chak A, Cooper GS. Outcomes after endoscopic versus surgical therapy for early esophageal cancers in an older population. Gastrointestinal Endosc. (2016) 84:232–240.e1. 10.1016/j.gie.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson DB, Dhupar R, Katkhuda R, Correa A, Goltsov A, Maru D, et al. Outcomes after endoscopic mucosal resection or esophagectomy for submucosal esophageal adenocarcinoma. J Thoracic Cardiovasc Surg. (2018) 156:406–413.e3. 10.1016/j.jtcvs.2018.02.093 [DOI] [PubMed] [Google Scholar]
- 38.Yang AJ, Choi SH, Byun HK, Kim HJ, Choi J, Lee YC, et al. Management of clinical T1N0M0 esophageal cancer. Gut and Liver. (2019) 13:315–24. 10.5009/gnl18254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballard DD, Choksi N, Lin J, Choi EY, Elmunzer BJ, Appelman H, et al. Outcomes of submucosal (T1b) esophageal adenocarcinomas removed by endoscopic mucosal resection. World J Gastrointest Endosc. (2016) 8:763–9. 10.4253/wjge.v8.i20.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C-Y, Chen B-H, Lee C-H, Le P-H, Tsou Y-K, Lin C-H. cT1N0M0 esophageal squamous cell carcinoma invades the muscularis mucosa or submucosa: comparison of the results of endoscopic submucosal dissection and esophagectomy. Cancers. (2022) 14(2):424. 10.3390/cancers14020424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.