Abstract
During present decade, targeted drug therapy has been the epitome for treatment of cancer. Drugs like Imatinib, a tyrosine kinase receptor inhibitor and Trastuzumab, an human epidermal growth factor receptor-2/neu inhibitor, has been developed and accepted widely for management of chronic myeloid leukaemia and breast cancer respectively. Recent development among the various immunotherapies is adoptive cell transfer (ACT). Research on development of various types of ACT immunotherapy is going on, but so far, Chimeric antigen receptors T cell therapy (CAR-T) has achieved the maximum advancement in terms of clinical development. CARs are the modified receptors that integrates specificity and responsiveness onto immune cells to enhance the recognition of cancer cells. For the CAR-T, the T cells are sequestered from a blood of a participant via apheresis. DNA of particular antigen is injected into harvested T cells to generate CARs on cell surface. Following surface manifestation of receptors, multiplication is carried out in enriched media followed by infusion into patient. After infusion, CAR-T cells targeted and exterminate the cancer cells. Initially, only two drugs targeting CD19 as genetically modified autologous immunotherapy has been approved in CAR-T therapy i.e., Tisagenlecleucel and Axicabtagene Ciloleucel, which are discussed in detail in current review. Recently two more drugs got approval i.e., brexucabtagene ciloleucel and lisocabtagene maraleucel, both are directed against CD19, similar to tisagenlecleucel. CAR-T cell therapy is approved for management of Acute Lymphoblastic Leukaemia, Chronic Lymphocytic Leukaemia and lymphoma. CAR-T cell persistence responsible for effectiveness and safety concerns are barriers for their wide application among patients. Growth factor receptors and cluster of differentiation are new drugs targets that are being explored as effective immunotherapy against cancers.
Keywords: Chimeric antigen receptor, chimeric antigen receptors T-cell, immunotherapy
Introduction
Three main modalities for the treatment of majority of cancers are chemotherapy, radiation and surgery. Since last two decades, targeted therapies like Imatinib, a tyrosine kinase receptor inhibitor and Trastuzumab, a human epidermal growth factor receptor-2 inhibitor/neu that specifically targets the cancer cells by acting on these receptors has been developed and gain popularity due to their enhanced efficacy. Recently the approach of therapy has been shifted to immunotherapies, which engages and strengthens the patient's immune system to attack tumours and have appeared as a powerful tool against cancer.
Adoptive cell transfer (ACT) is very swiftly emerging immunotherapy approach against cancer. In this therapy, the patients’ harvested immune cells are genetically modified to target an antigen, multiplied and then reinfused to treat their cancer. Easy and effective approaches of ACT are being researched. However, recently, Chimeric antigen receptors T cell (CAR T) treatment has showed utmost advancement in clinical development.[1]
The immune system which constitutes of humoral, cell mediated and innate immunity is body's ultimate defence against infections and malignancy. Immune system is composed of millions and millions of cells which are broadly categorised into different types. Lymphocytes, a type of white blood cells (WBCs), cover a significant segment of the immune system. Lymphocytes are of three types namely, the B cells which helps in combating infection by generating polyclonal antibodies, the T cells being multifunctional has the capacity to directly kills infected or cancer cells in the body. In addition, generate cytokines to promote the generation and persistence of B cells and also help in generation of plasma cells. Another important cell of innate immunity are the Natural killer cells that attacks bacteria, parasites, viruses and can eliminate cancerous cells.
Immunotherapy is therapeutic modality that enhances body's immune system ability to fight cancer. It enriches immune cells capacity to enhanced identification and killing of the cancer cells. Basic concept that immune cells or the antibodies generated by immune cells can recognize and kill the cancer cells has been explored for improvement in cure rates of cancers. With the current advance technology, the autologous or allogenic immune cells are being reengineered for increasing their capacity to recognise and kill cancer cells. Numerous types of immunotherapy like immune checkpoint inhibitors like program cell death inhibitors and cancer vaccines are approved or explored for applicability in cancer. Efficacy and safety is being explored in various clinical trials. One among them that has gained importance is CAR T cell modality.
Amid generation of new evidence from small clinical studies of effectiveness of CAR-T cell treatment in management of blood cancer, their role has been explored further with new indications. It also led to increased awareness among physicians and cancer specialists all over the world.
CARs (called as chimeric T cell receptors (TCRs), chimeric immunoreceptors or artificial TCRs) are the modified receptors enhances the specificity and integrates specificity and responsiveness onto immune cells for recognition of targeted cancer cells. These receptors graft the definiteness of a monoclonal antibody on the T cell. CAR-T receptors are termed chimeric are as their parts like variable region and antigen recognition parts are being derived from different sources. CAR-T cell modality is unconventional type in nature as living T cells of a patient are reengineered for enhanced effectiveness. This modality is like “giving patients a living drug.”
The fundamental concept in designing of CAR-T cell therapy encompasses incorporation of recombinant receptors (known as CARs), that endorse target specific antigen-binding as well as T-cell stimulating function onto T-cells surface. In general, CAR-T treatment modality is basically artificially producing modified T-cells to target cancer cells, in which T-cells are harvested from a person, genetically modified with insertion of DNA using an inactive lentivirus or other techniques like sleeping beauty transposon (SBT) system or mRNA transfection into T-cells to generate CARs on cell surfaces. This CARs expression enhances recognition of cancer specific antigens present on cancer cells by modified CAR T-cells. After clonal expansion and activation, enriched CAR-T cells are reintroduced back into cancer patients for generation of immune response against cancer.[2]
Synthesis of Reengineered Chimeric Antigen Receptors T Cells and Mechanism of Effectiveness
Artificial genomic alteration of autologous T-cells by introduction of DNA of CARs with help of retrovirus, to manifest CAR on T-cells surfaces, which helps in enhanced recognition of tumour specific antigens (TSAs) on tumour cells. Reengineered T cells are called as “CAR-T cells.” First of all, leucocytes are furnished from a patient via leukapheresis, a process of withdrawing the blood from donor's body and components of blood like WBCs or platelets are separated through a mechanised process. Secondly, T-cells are separated from leucocytes by enrichment and washing. Enrichment is achieved by process of magnetic separation with the use of monoclonal antibody coupled metallic beads.
CARs coupled with viral vectors such as lentivirus, helps in reverse transcription into DNA and insertion into genome of T cells or APC. Lentivirus are preferred over gammaretroviral vectors as they have safest integration profile. During culture, viral vector is washed out with dilution or exchange of medium. Insertion of RNA can be achieved with mRNA transfection, SBT and Clustered regularly interspaced short palindromic repeats (CRISPR) associated nuclease9 (CRISPR/Cas9) technology. mRNA transfection needs repeated infusion of CAR-Tcells and insertional mutagenesis and remobilisation of transposons are drawbacks of SBT.[3]
After separation and transduction, CD4/CD8 T-cells subset are separated and activated by culturing them with growth factors. T-cells (CD4/CD8) subsets are separated using metallic beads coupled with monoclonal antibodies (anti-CD3/anti-CD28) specific for T-cells, or anti CD3 antibodies along with growth factors like Interleukin (IL-2).
Reengineered CAR T-cells are cultured (activation) using bio reactive culture systems, namely G-Rex, WAVE bioreactor and CliniMACS Prodigy. The last one doesn’t is preferred as it can run the whole process of enrichment, activation, transduction and amplification of CAR T-cells without opening the flask. The cells are amplified to achieve numbers effective as clinical dose and are stored in frozen form [Figure 1]. Before infusion of CAR-T cells, patients undergo chemotherapy with one or more chemotherapy agents, called “lymphodepletion,” so as to increase the chances of expansion of CAR-T cells by two to three-fold. Before infusion, proper thawing process is performed. Once CAR-T cells are perfused back, the cells amplify and attack cancer cells as their purpose is to identify and assault cells bearing targeted antigen on the surface.
Figure 1.
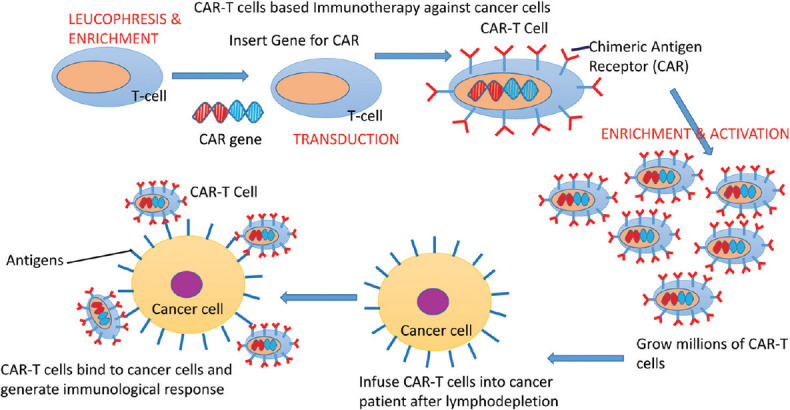
Steps involved in generation of reengineered CAR T-cells. CAR T-cells: Chimeric antigen receptors T cells
In addition to elimination of cancer cells from body, they also prevent against re-emergence of cancer cells due to their ability to persist in the circulation for months after their injection into body. Thus have the propensity of resulting in the long-term reductions or remissions in various types of blood cancers.
Structure and Generations of Chimeric Antigen Receptors
CAR-T cells receptors (CARs) are made up of three domain, ectodomain, endodomain and transmembrane, in similar fashion to TCRs with difference that it has been modified to identify the target cell with the help of target recognition and binding receptor. Ectodomain comprises of signal peptide, antigen recognition part and spacer. scFv, i.e., variable region of immunoglobulin (IG) act as signal peptide. Signal peptide along with complex recognition components serves as antigen recognition area. IgG1 hinge area can be used as spacer. Transmembrane domain act as stabilising complex for receptor and can also function as co-stimulatory domain for T-cells like CD-28. Endodomain part comprises of CD3z cytoplasmic component which includes three immunoreceptor tyrosine based activation motifs.[3]
This CAR is specific in recognising the cells like CD-19 or CD-22 or CD-33 positive cells. This antigen binding domain is coupled to intracellular signalling domain CD3z (signal-transduction constituent of CAR) with the help of spacer or with the help of co-stimulatory domain of T-cells (CD-28-Axicabtagene Ciloleucel, brexucabtagene autoleucel; 4-1BB– Tisagenlecleucel as well as lisocabtagene maraleucel).
CAR of the model scFv-spacer-CD3z layout are well known as “first-generation CARs.” In order to improve in vivo persistence, proliferation, their ability to secrete cytokines and resistance to apoptosis, second and third generation CARs are synthesized. Higher generations bear co-stimulatory domain like CD28 and/or 4-1BB.[4]
Third-generation has expanded effector utility such as tumour lysis and in vivo perseverance with regard to earlier generations of CARs. The most advanced fourth-generation, referred to as TRUCKs or armoured CARs. TRUCKs involves integration of domains within the endodomain which boosts the anti-tumour activity. These includes costimulatory ligands, cytokines or enzymes which helps in dissolving extracellular matrix of tumours[4] [Figure 2]. The CRISPR associated nuclease9 (CRISPR/Cas9) technology, which uses Cas9 as a nuclease protein in combination with two RNA (CRISPR) sequence for editing the genome. CRISPR/Cas9 by abscission of fas receptor helps in synthesis of CAR-T cells, capable of resilience to apoptosis by Fas/FasL-dependent activation induced cell death.[5] CRISPR/Cas9 can help in insertion of CAR into TCR alpha constant locus, increasing the efficiency of therapy by placement of CAR expression under the TCR promoter.[5]
Figure 2.
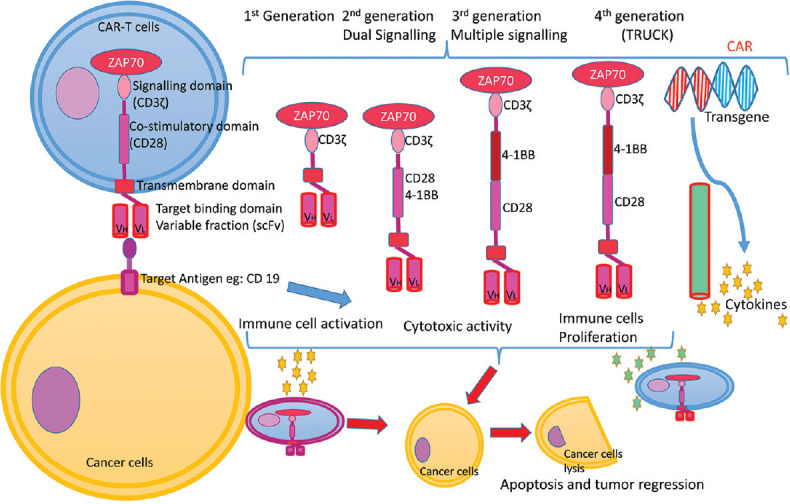
Four chimeric antigen receptors generations and mechanism of immunotherapy against cancer cells by CAR-T cells. CAR T-cells: Chimeric antigen receptors T cells
Advantages of Chimeric Antigen Receptors-T Cells
The adaptive immune cells are nonspecific, while CAR-T cells are highly specific and targets the cancer cells compromising of corresponding tumour associated antigens or TSAs. This mechanism prevents the undue assault of healthy human tissues.[6]
CAR-T cells may identify the target surface antigens without involvement of human leukocyte antigen (HLA) expressed on cells. Tumours usually escapes the cell mediated immune scrutiny by concealing HLA and other potent antigens which are modified and exhibited by the antigen-presenting cells to adaptive immune cells. Thus effectively camouflage in representing as normal cells of body.[7] As HLA expressed on cancer cells are not essential for identification of cancer cells by CAR-T cells, effective immune response could be provoked on non HLA expressed cancer cells.
The adaptability of intracellular signalling domains achieved with modifications of CARs along the generations help in combating the down-regulation invoked by cancer cells of co-stimulatory molecules. In addition to protein molecules, carbohydrate and lipid molecules can become potential antigens and can be effectively targeted with the CAR expressed upon CAR-T cell.[7]
Monoclonal antibodies used for targeting cancer cells such as trastuzumab have similar specificity for tumour cells as CAR-T cell treatment modality. However, CAR-T treatment strategy have been proposed to have enhanced effectiveness as compared to monoclonal bodies, because of their ability to be active in inflammation in addition to their capacity of clonal expansion along with generation of effective memory cells.[7]
Potential Therapeutic Application of Chimeric Antigen Receptors T Cell Therapy
Acute lymphoblastic leukaemia
The most adequate response to the CAR-T cell treatment is exhibited by B-cell Acute Lymphoblastic Leukaemia (ALL). The most effective CAR in the treatment of ALL is the important biomarker of B-cell lineage, anti-CD-19 which shows higher expression in B-cell ALL. Other potential targets are IG light chains and anti CD20.[8,9]
Chronic lymphocytic leukaemia
Leukaemia which has extremely varying clinical course and chemotherapeutic prognosis.[10] Allogeneic stem-cell transplantation is current treatment for chronic lymphocytic leukaemia (CLL).[10] CD19 CAR-T treatment was lately applied to manage relapsed CLL.[11] Besides, CD19, other treatment targets being explored included tyrosine-protein kinase transmembrane receptors and the CAR-T.[12]
Multiple myeloma
It is bone marrow derived refractory cancer causing anaemia, hypercalcemia, bone lesions, immunosuppression with repetitive infections and renal failure.[12] The treatment modalities being applied includes immunomodulatory agents, autologous hematopoietic stem cell transplantation and chemotherapy but the disease remains incurable due to heterogeneous molecular and cytogenetic aberrations of myeloma.[13] Evidence that the CAR-T cell therapy may prove to be beneficial in myeloma is because of known effect that the myeloma subsides due to the graft verses myeloma effect in patients who underwent allogenic stem cell transplantation.[14]
Drugs Used in Chimeric Antigen Receptors T Cell
A total of four drugs have been approved. Initially there were two drugs approved, first being tisagenlecleucel and second being Axicabtagene Ciloleucel, both are directed against CD-19 positive cancer cells, which are discussed in detail in current review. Recently two more drugs got approval i.e., brexucabtagene ciloleucel and lisocabtagene maraleucel, both are directed against CD19 similar to tisagenlecleucel and axicabtagene.
Difference in Structure and Mechanism of Immunotherapy of Two Drugs-Tisagenlecleucel and Axicabtagene Ciloleucel
Tisagenlecleucel and Axicabtagene, both are autologous immune treatment directed against CD19. Both of them has specific affinity to bind to CD19-positive cancer cells along with healthy B cells. Autologous T cells are encoded with transgene, encoding CAR specifically for binding to CD19 expressed cancer cells. Antigen recognition area of CAR of Tisagenlecleucel has a murine single-chain antibody variable fragment that helps in targeting CD19 ligand. This ectodomain is amalgamated to signal domain CD3ζ located intracellularly, with the help of co-stimulatory domain i.e., 4–1BB (CD137), for enhancing the effector response. The CD3 helps in activation of CAR-T cell in addition to anticancer action. The CD137 molecule promotes growth and perseverance of tisagenlecleucel cells.[15] Cells using 4–1BB as co-stimulatory domain are less potent but have greater CAR-T cell persistence in blood.
Axicabtagene Ciloleucel, interaction with CD19-positive cancer cells activates the co-stimulatory domains CD28, fused to intracellular signalling domain CD3-zeta to stimulate downstream pathways. Downstream pathway activation cause T-cell stimulation and propagation, acquisition of effector response and releasing cytokines causing effective killing of the CD19-expressing cells.[16] Axicabtagene ciloleucel, with CD-28 as co-stimulatory domain are more potent but have less CAR-T cell persistence in blood.
Indications and Dosage
Tisagenlecleucel
Tisagenlecleucel is CD19-targeted CAR T-cell therapy. Current and only approved indication is for children as well as adults with ALL. Participants of ELINA trial.[19] had complete and long-lasting remissions, evidence that nod US Food and Drug Administration (FDA) approval for tisagenlecleucel in year 2017.
Drug is approved for paediatric and young adult relapsed or refractory (R/R) B-cell ALL. The recommended dose for participants with weight ≤50 kg is 0.2–5.0 × 106 CAR-positive viable T cells per kg body weight while 0.1–2.5 × 108 CAR-positive viable T cells in participants weighing above 50 kg.[15]
Besides this, it is used for adult relapsed or refractory (R/R) diffuse large B-Cell Lymphoma (DLBCL). The recommended dose for Adult DLBCL is 0.6–6.0 × 108 CAR-positive viable T cells.[15]
Axicabtagene ciloleucel
Similar to tisagenlecleucel, it is CD19-directed CAR-T cell immunotherapy.
Axicabtagene is administered by parenteral route for management of relapsed and refractory large B-cell lymphoma. Must be resistant to two or more types of chemotherapy. Also approved for DLBCL arising from follicular lymphoma, primary mediastinal large as well as high-grade B-cell lymphomas.[16]
Volume of each single infusion bag includes approximately is 68 ml of suspension of CAR T-cell. The targeted dose for this indication is 2 × 106 CAR-positive viable T cells per kg body weight, maximum dose is upto 2 × 108 CAR-positive viable T cells. Safety checks similar to blood transfusion must be performed. The identity of patient should tally with patient identifiers present on infusion bag and the drug cassette. No administration should be done if there is any discrepancy in matching the patients identity.[16]
Evidence of Efficacy
Clinical trials done for tisagenlecleucel
The effectiveness of Tisagenlecleucel was assessed in multicentric, open-label, Phase II, single-arm study (ELIANA trial). Sixty eight paediatric patients of R/R B-cell ALL, were assessed on basis of overall response rate, for complete response (CR) within 3 months following the infusion and minimal residual disease (MRD) <0.01% by flow cytometry (MRD-negative). The CR of 83% was achieved at 3 months.[15] Event free survival was 50% at 1 year. The overall survival (OS) was 70% at 18 months.
In the multicentric, open label, single arm JULIET study of Tisagenlecleucel in adult R/R DLBCL patients. When the single dose of Tisagenlecleucel was given the objective response rate (ORR), CR and partial response (PR) was recorded as 54%, 40% and 12% respectively. The estimated 12-month OS among CR patients was 90%.[15]
Clinical studies for axicabtagene ciloleucel
The ZUMA-1 trial, designed as single-arm, nonblinded, multicentre study, analysed effectiveness of Axicabtagene ciloleucel in the 101 adults having R/R aggressive B-cell nonHodgkin lymphoma. The ORR was 82%. The CR, PR was achieved in 58% and 25% patients respectively. The median duration of response was 11.1 months but median OS was not achieved.[16] Evidence form real world corroborates with the results of ZUMA-1 study as remission has been observed in 40%–50% of patients at 1 year.
Adverse drug reactions
The CAR-T treatment in haematological malignancies may lead to vigorous extensive cytokine-driven effects such as macrophage activation syndrome, cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis. The CRS caused due to elevated cytokine levels leads to symptoms such as fever, hypotension, hypoxia and neurological alterations.[17,18,19] Higher the dose of CAR-T cells and baseline disease burden, higher is the incidence of severe CRS.[20]
The management includes the supportive care i.e., oxygen supplementation for hypoxia, intravenous fluids for hypotension and acetaminophen for fever). Systemic corticosteroids are main stay of treatment in these patients. Steroids not only act by reducing the response of hyper-proliferative activated CAR-T cells but they also reduce their antitumor efficacy, resulting in relapse or progression of disease.[21,22] The chief cytokine induced by CAR-T is IL-6. Tocilizumab with systemic corticosteroids is used in management of CRS. Tocilizumab, by binding with the soluble as well as membrane attached IL-6 receptors (mIL-6R, sIL-6R), impede the spread of CRS.[23] The frequency of Adverse Drug Reaction reported in clinical trials with Tisagenlecleucel and Axicabtagene Ciloleucel are reported in Tables 1 and 2 respectively.[15,16]
Table 1.
Tisagenlecleucel adverse drug reactions narrated in clinical studies
| Trial | CRS | Neurological toxicity | Hypogamm-aglobunemia | Persistent cytopenias | Infections | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Encephalopathy | Headache | Delirium | Other ADR | |||||
| ELIANA study (n=68) | 54 | 23 | 25 | 13 | 15 | 29 | 25 | 28 |
| JULIET TRIAL (n=106) | 78 | 17 | 22 | 12 | 15 | 18 | 44 | |
| Maude et al. (n=75) | 35 | 4 | - | 30 | 7 | |||
| Schuster et al. (n=111) | 64 | 23 | - | 49 | 38 | |||
| Bishop et al. (n=7) | 4 | 1 | - | - | - | |||
CRS=Cytokine release syndrome, ADR=Adverse drug reaction
Table 2.
Axicabtagene ciloleucel adverse drug reactions narrated in clinical studies
| Trial | CRS | Neurological toxicity | Prolonged cytopenia | Serious infections | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Encephalopathy | Headache | Delirium | Other ADR | ||||
| ZUMA-1 study (n=108) | 12 | 40 | 50 | 23 | 62 | 48 | 30 |
| Neelapu et al. (n=101) | 94 | 34 | 5 | 29 | 77 | 85 | |
| ZUMA-2 trial (n=28) | 5 | 7 | 4 | 3 | 15 | ||
| ZUMA-3 trial (n=45) | 16 | 18 | 31 | ||||
| Bouchkouj et al. (n=108) | 94 | 57 | 45 | 17 | 70 | 71 | 26 |
CRS=Cytokine release syndrome, ADR=Adverse drug reaction
Neurological toxicity
The common ones are delirium, anxiety, sleep disorder, headache, dizziness tremors, seizures, mutism, aphasia, peripheral neuropathy and CRES (CAR-T cell related encephalopathy). CRES is manifested within 5 days of drug administration and the initial symptoms are diminished attention, impaired handwriting and language disturbances and with progression there may be aphasia, tremors, disorientation, agitation, confusion and seizures. Management of CRES and CRS is similar and is based on the neurological assessment score by the (CARTOX-10) CAR-T cell therapy toxicity 10 point neurological assessment.[23]
During the initial treatment it is advisable to abstain patients from operating heavy or dangerous machinery as the capability to work on machines and drive is impaired because of neurological side effects like encephalopathy, seizures and altered mental status. The altered level of consciousness or inability to coordinate was observed till 8 weeks after drug administration.
Infections (serious)
CAR-T cell therapy directed towards B cells leads to impaired humoral immunity. Hence, activation of Hepatitis B viruses leading to hepatic failure and death. It is therefore suggested that the testing for viral marker like HCV, HBV and HIV should be done before collecting T-cells for genetic reengineering.[15,16]
Type I hypersensitivity
The Type I hypersensitivity may develop after Axicabtagene and Tisagenlecleucel infusion. The dextran 40 or dimethyl sulfoxide in the Tisagenelecleucel may results in serious adverse reactions such as anaphylaxis and with axicabtagene may cause serious hypersensitivity reaction because of presence of dimethyl sulfoxide or the remaining gentamicin in drug.
Prolonged cytopenias
Due to prolonged lymphodepleting chemotherapy and drug infusion, they patient may exhibit cytopenias. Risk of infection is increased due to prolonged neutropenia. The administration of myeloid growth factors particulary the Granulocyte-macrophage colony-stimulating factor is contraindicated for the first 3 weeks after the drug infusion or until the CRS is resolved.
Hypogammaglobulinemia
It may occur in patients after complete remission due to B cell aplasia caused by Tisagenlecleucel and Axicabtagene infusion. Hypogammaglobulinemia is reported in pregnant women and it is advisable to monitor the IG levels in new-borns of mother's receiving CAR-T therapy.
Secondary malignancies
It is advisable to do life-long monitoring as secondary malignancies or reoccurrence of cancers in reported in many patients.
Black box warnings
All four drugs, tisagenlecleucel, Axicabtagene Ciloleucel, brexucabtagene ciloleucel and lisocabtagene maraleucel have been approved by US FDA with black box warning of CRS and neurotoxicity.
Axicabtagene risk evaluation, and mitigation strategy
Because of increased incidence of CRS and neurologic adverse effects like encephalopathy associated with use of Axicabtagene, the drug has restricted availability through a program called the Axicabtagene Risk Evaluation, and Mitigation Strategy. For immediate management of CRS, a minimum of two doses of tocilizumab should be administered within 2 h of Axicabtagene administration. Healthcare providers competent for handling emergency should prescribe Axicabtagene.
New drug targets for chimeric antigen receptors T cell immunotherapy
Similar to CD-19 directed CAR-T cell therapy, there are many new targets[9,24] which are being explored for the treatment of cancers, as shown in Table 3.
Table 3.
Drug targets being currently explored for chimeric antigen receptors T cell immunotherapy
| New antigen target (molecule) | Mechanism | Application |
|---|---|---|
| LCAR-B38M | T lymphocyte replacement | Multiple myelomas (phase-1 and 2) |
| CAR-GPC3 T cells | Suppress tumour growth | Hepatocellular carcinoma |
| JWCAR029 | CD19 target CAR | B-cell NHL (phase-1) |
| PD-1 knocked down CD19 CAR-T | Acts on CD-19 | CNS B-cell acute lymphocytic leukaemia |
| CD22 | ` | B-cell leukaemia/lymphoma |
| CD123 | B-cell leukaemia | |
| BCMA proteins | Action on Plasma cells, B-cell subtype | Multiple myelomas |
| CD44v6 | T-cell, monocytes, myeloma, Keratinocyte | Multiple myelomas |
| Mesothelin | Solid tumours (lung and pancreatic cancer) | |
| EGFRv 3 | Aggressive brain tumor (Glioblastoma) | |
| CD30 | B-cell NHL | |
| CD33 | AML | |
| CD138 | Multiple myelomas | |
| Ep CAM | Liver neoplasm | |
| Her-2 | Breast and ovarian tumour, lung malignancy | |
| EGFR | Colorectal malignancy | |
| MUC1 | Advanced solid malignancy | |
| PSMA, Fra | Bladder malignancy, urothelial cancer | |
| Claudin 18.2 | Gastric adenocarcinoma | |
| CD70 | Activated lymphoid cells. Myeloma cells | Multiple myelomas |
| CD56 | NK and T cells, CD56 expression in seventy percent of myelomas, Neurons | Multiple myelomas |
| CD38 | PrecursorB, NK, plasma and T cells, myeloid progenitor, prostate cells, neurons, bone and muscle | Multiple myelomas |
| Uniformly expressed on myeloma cells | ||
| SLAMF7 | B, Plasma and NK cells, dendritic cells | Multiple myelomas |
| Immunoglobulin κ light chain | Multiple myelomas |
NHL=Non-Hodgkin’s lymphoma, AML=Acute myeloid leukaemia, CAR=Chimeric antigen receptor, NK=Natural killer, PD-1=Programmed death-1, BCMA=B cell maturation antigen, EGFR=Epidermal growth factor receptor, MUC1=Mucin 1, cell surface associated, PCMA=Prostrate specific membrane antigen
Conclusion
Clinical studies have generated enough evidence in favour of CAR-T cell therapy for management of haematological malignancies. Currently the only drugs approved are directed against CD-19 molecule. Many new targets are being explored. CAR-T treatment modality is unique in inciting T-cell immune response against the targeted cancer cells. Evidence suggests that CAR-T cell therapy in effective in the majority of haematological malignancies such as ALL, CLL and lymphoma; but safety concerns and cell persistence are the main roadblocks in its extensive application. Further elucidated research for invention of high quality CAR-T therapy and targeting of wide range of antigens will help in generating the evidence of effectiveness and its application in other malignancies also.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.CAR T. Cells: Engineering Patients’ Immune Cells to Treat Their Cancers; December 14, 2017. [Last accessed on 2019 Mar 15]. Available from: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells.
- 2.Franceschetti AT, Burian HM, Linder A. Statistical analysis of accomodative convergence (AC-A ratio) Bull Mem Soc Fr Ophtalmol. 1970;83:189–94. [PubMed] [Google Scholar]
- 3.Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5:22. doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chmielewski M, Abken H. TRUCKs: The fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–54. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 5.Mollanoori H, Shahraki H, Rahmati Y, Teimourian S. CRISPR/Cas9 and CAR-T cell, collaboration of two revolutionary technologies in cancer immunotherapy, an instruction for successful cancer treatment. Hum Immunol. 2018;79:876–82. doi: 10.1016/j.humimm.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Bonini C, Mondino A. Adoptive T-cell therapy for cancer: The era of engineered T cells. Eur J Immunol. 2015;45:2457–69. doi: 10.1002/eji.201545552. [DOI] [PubMed] [Google Scholar]
- 7.Catalán E, Charni S, Jaime P, Aguiló JI, Enríquez JA, Naval J, et al. MHC-I modulation due to changes in tumor cell metabolism regulates tumor sensitivity to CTL and NK cells. Oncoimmunology. 2015;4:e985924. doi: 10.4161/2162402X.2014.985924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev. 2016;30:157–67. doi: 10.1016/j.blre.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Zhang T, Cao L, Zhang Y. Chimeric antigen receptor T cell based immunotherapy for cancer. Curr Stem Cell Res Ther. 2018;13:327–35. doi: 10.2174/1574888X13666180420110239. [DOI] [PubMed] [Google Scholar]
- 10.Mewawalla P, Nathan S. Role of allogeneic transplantation in patients with chronic lymphocytic leukemia in the era of novel therapies: A review. Ther Adv Hematol. 2014;5:139–52. doi: 10.1177/2040620714550773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang K, Desai A, Zeng D, Gong T, Lu P, Wang M. Magic year for multiple myeloma therapeutics: Key takeaways from the ASH 2015 annual meeting. Oncotarget. 2017;8:10748–59. doi: 10.18632/oncotarget.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdonck LF, Lokhorst HM, Dekker AW, Nieuwenhuis HK, Petersen EJ. Graft-versus-myeloma effect in two cases. Lancet. 1996;347:800–1. doi: 10.1016/s0140-6736(96)90871-5. [DOI] [PubMed] [Google Scholar]
- 14.Ramos CA, Heslop HE, Brenner MK. CAR-T cell therapy for lymphoma. Annu Rev Med. 2016;67:165–83. doi: 10.1146/annurev-med-051914-021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US, Food, and, Drug, Administration. KYMRIAH (tisagenlecleucel) Label. [Last accessed on 2019 May 20]. Available from: https://www.fda.gov/media/107296/download.
- 16.US, Food, and, Drug, Administration. YESCARTA (Axicabtagene Ciloleucel) Label. [Last accessed on 2019 May 20]. Available from: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert---YESCARTA.pdf.
- 17.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM, et al. Optimizing chimeric antigen receptor T-cell therapy for adults with acute lymphoblastic leukemia. J Clin Oncol. 2020;38:415–22. doi: 10.1200/JCO.19.01892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–22. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorentzen CL, Straten PT. CD19-chimeric antigen receptor T cells for treatment of chronic lymphocytic leukaemia and acute lymphoblastic leukaemia. Scand J Immunol. 2015;82:307–19. doi: 10.1111/sji.12331. [DOI] [PubMed] [Google Scholar]
- 23.Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–19. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagley SJ, O’Rourke DM. Clinical investigation of CAR T cells for solid tumors: Lessons learned and future directions. Pharmacol Ther. 2020;205:107419. doi: 10.1016/j.pharmthera.2019.107419. [DOI] [PubMed] [Google Scholar]


