Abstract
Background
The estimated glomerular filtration rate (eGFR) and the albumin-to-creatinine ratio (ACR) are risk factors for diabetes-related outcomes. A composite that captures information from both may provide a simpler way of assessing risk.
Methods
9115 of 9901 Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) participants with both an ACR and eGFR at baseline were included in this post hoc epidemiologic analysis. The hazard of higher baseline levels of 1/eGFR and natural log transformed ACR (calculated as ln [ACR × 100] to eliminate negative values) and their interaction for incident major adverse cardiovascular events (MACE), kidney outcomes, and deaths was estimated. The hazard of the geometric mean of these two baseline measures (the kidney disease index or KDI) was also assessed.
Results
A non-linear relationship was observed between 1/eGFR and all three outcomes, and between ln [ACR × 100] and the kidney outcome. There was also a negative interaction between these two risk factors with respect to MACE and death. Conversely, a linear relationship was noted between the KDI and all three outcomes. People in the highest KDI fifth experienced the highest incidence of MACE, death, and the kidney outcome (4.43, 4.56, and 5.55/100 person-years respectively). C statistics for the KDI were similar to those for eGFR and albuminuria.
Conclusions
The KDI combines the baseline eGFR and ACR into a novel composite risk factor that has a simple linear relationship with incident serious outcomes in people with diabetes and additional CV risk factors.
Trial Registration clinicaltrials.gov NCT01394952.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01594-6.
Keywords: Risk Factor, Kidney, Albuminuria, Cardiovascular Outcomes, Kidney Outcomes
Background
The estimated glomerular filtration rate (eGFR) and the degree of albuminuria measured by the albumin-to-creatinine ratio (ACR) are routinely measured in people with diabetes and are recognised as prognostic indices for kidney outcomes, cardiovascular outcomes, and death. Indeed, large meta-analyses and current guidelines support the combined use of both measures to identify people at highest risk of these outcomes [1, 2]. These guidelines are supported by later large prospective studies [3] that assess these risk factors as either continuous variables or as categorical variables focused on the stage of kidney disease (defined using various eGFR thresholds) and the presence of either microalbuminuria (defined as an ACR of 30–300 mg/g) or macroalbuminuria (defined as an ACR > 300 mg/g) [2]. These reports generally assessed these 2 risk factors independently and usually assumed a linear relationship between them and incident outcomes.
The Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial recruited 9901 individuals of mean age 66 years (46% women) who had type 2 diabetes and additional cardiovascular risk factors, and randomly assigned them to the glucagon-like peptide 1 receptor agonist dulaglutide or placebo. Participants were followed for a median of 5.4 years during which the hazard of major adverse cardiovascular events (MACE) was significantly reduced by 12% with dulaglutide versus placebo. The availability of baseline albuminuria and an eGFR in 9115 REWIND participants, the long-term follow-up and the high number of kidney outcomes, cardiovascular outcomes and deaths provide an opportunity to assess the nature of the relationship of these 2 baseline measures to the incidence of these outcomes. It also presents an opportunity to determine whether combining these two measures into one index has a similar or stronger relationship to these outcomes than the components.
Methods
As previously published [4, 5], the REWIND trial recruited people aged 50 or older with either newly diagnosed or established type 2 diabetes whose body mass index was ≥ 23 kg/m2, and whose HbA1c was 9.5% or less (with no lower limit) on stable doses of up to 2 oral glucose-lowering drugs with or without basal insulin between August 2011 and 2013. Exclusion criteria included an estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2. Participants were randomly assigned to weekly subcutaneous injections of either dulaglutide 1.5 mg or identical placebo and followed for a median period of 5.4 years for the occurrence of clinical outcomes. Annual laboratory assessments (measured at local laboratories) included serum creatinine and a urine albumin-to-creatinine ratio (ACR). The protocol was reviewed by Research Ethics Boards for 371 sites in 24 countries and all participants provided signed written informed consent.
Outcomes
The primary outcome of the REWIND trial was the first occurrence of MACE comprising blindly adjudicated nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular or unknown causes. This post-hoc epidemiologic analysis focused on this outcome, total mortality, and a kidney composite outcome comprising new macroalbuminuria (i.e., a ACR > 33.9 mg/mmol), a sustained decline in eGFR of ≥ 40%, or chronic kidney replacement therapy.
Model validation
Validation was done using data from 12,187 Outcomes Reduction with and Initial Glargine Intervention (ORIGIN) trial participants (mean age 64 years, 35% women) who had a baseline eGFR and ACR measurement and who were followed for a median of 6.2 years [6]. MACE and death from cardiovascular causes were assessed in ORIGIN using the same definition as the REWIND trial. The kidney composite outcome in that trial (based on measurements at baseline, 2-years and study end) was defined as the first occurrence of a doubling of serum creatinine, worsening of albuminuria category (from normal to microalbuminuria or clinical proteinuria, or from microalbuminuria to clinical proteinuria), chronic kidney replacement therapy or death due to kidney failure [7].
Statistical analyses
This report describes epidemiologic analyses focused on the prognostic relevance of the eGFR and ACR, regardless of random assignment, and is restricted to participants who had both measurements recorded at baseline. Continuous variables were summarized using either the arithmetic mean and standard deviation or the median for which either interquartile or inter-decile (i.e., between the 10th and 90th percentile) ranges were reported. Categorical variables were summarized as counts with percentages and compared across categories using the chi-square statistic.
The eGFR was calculated at each visit using the Modification of Diet in Renal Disease equation [8] and was transformed prior to analyses by calculating the inverse of its value (i.e., 1/eGFR) so that higher levels would reflect worse kidney function. The skewed distribution of ACR was normalized by calculating the natural logarithm (ln) of each measurement. ACR values below the lower limit of detection of 0.1 mg/g were assigned this lower limit. Because the ACR could be less than 1 (for which the ln would be negative) the ACR values were multiplied by 100 prior to this transformation. These two indices of kidney status were analyzed separately and combined into a novel kidney disease index (KDI) by calculating the geometric mean of these two values for each participant [9]. Baseline characteristics and the incidence of the reported outcomes were presented overall and after dividing participants into 5 groups that were defined using quintiles of KDI.
Cox proportional hazards models were used to analyze the relationship between age and sex-adjusted levels of these three kidney measures (i.e., 1/eGFR, ln (ACR × 100), and the KDI) and the first occurrence of the MACE outcome, death, and the kidney composite outcome. The shape of the relationship between these three kidney measures and the hazard of the three outcomes was assessed using Martingale residuals and spline plots. For these analyses the 3 kidney measures were expressed as Z-scores (calculated as an individual’s level minus the mean divided by the standard deviation). If a variable appeared to have a nonlinear relationship with an outcome, the corresponding model was re-estimated after including both the linear and squared variable in the model. The possibility of an interaction between 1/eGFR and ln (ACR × 100) with respect to these three outcomes was assessed by adding an interaction term to the models. Proportionality was assessed by plotting the log of negative log of the survival function against the log of time. Model performance was assessed using the C-statistic. Participants were censored at either the date of the final follow-up visit, the date of death, or the date of discontinuation from the trial.
All analyses were done using SAS software (version 9.4). This trial is registered with ClinicalTrials.gov, number NCT01394952.
Results
The baseline clinical and biochemical characteristics of the 9,115 individuals, including 4222 (46.3%) women, who had a baseline ACR and a baseline eGFR are noted in Additional file 1: Table S1. Their mean (SD) age, diabetes duration and HbA1c levels were 66.2 (6.5) years, 10.5 (7.2) years and 7.3 (1.1%), respectively. The mean (SD) eGFR was 76.9 (22.8) ml/min/1.73 m2, and the mean (SD) inverse of eGFR (i.e., 1/eGFR) was 0.014 (0.007). The median ACR was 1.8 (IQR 0.7, 7.4), and the mean ln (ACR X 100) was 5.5 (SD 1.7) mg/mmol. The arithmetic mean (SD) of participants’ KDI values calculated as the geometric mean of their 1/eGFR and ln (100 X ACR) was 0.27 (0.08).
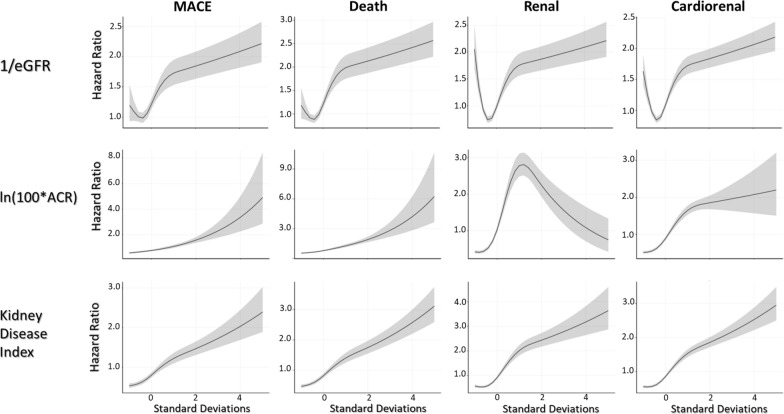
The incidence of MACE, death, and the kidney composite outcome during follow-up was 2.51, 2.19 and 2.71 per 100 person-years respectively (Table 1). As noted in the Fig. 1 (spline) there was a non-linear relationship between 1/eGFR and MACE, death, and the kidney composite outcome. To account for this, hazard ratios for these 3 outcomes were estimated with a Cox model that included both a linear and a quadratic term for 1/eGFR. Using this model (Table 2), one standard deviation above the mean was associated with a MACE HR of 1.31 (95% CI 1.22, 1.40), a death HR of 1.52 (95% CI 1.42, 1.61), and a kidney composite HR of 1.34 (95% CI 1.25, 1.44). A significant interaction between 1/eGFR and sex suggested a higher hazard for women versus men (Additional file 1: Table S2) for MACE (interaction P = 0.012).
Table 1.
Proportion of events and their incidence by fifths of the geometric mean of each person’s kidney disease index
| Overall N = 9115 |
Quintile 1 N = 1823 |
Quintile 2 N = 1823 |
Quintile 3 N = 1823 |
Quintile 4 N = 1823 |
Quintile 5 N = 1823 |
P | |
|---|---|---|---|---|---|---|---|
| Median KDI (10—90) | 0.26 (0.20–0.37) | 0.20 (0.16–0.21) | 0.23 (0.22–0.24) | 0.26 (0.25–0.27) | 0.30 (0.28–0.32) | 0.37 (0.33–0.47) | |
| Median eGFR (10—90) | 74.9 (48.8–106.8) | 97.3 (73.0–126.2) | 85.2 (67.2–109.9) | 76.2 (59.6–98.2) | 67.6 (51.7–86.9) | 51.8 (33.5–69.9) | |
| Median ACR (10—90) | 1.8 (0.3–29.8) | 0.4 (0.1–1.3) | 1.1 (0.4–3.7) | 1.8 (0.6–9.0) | 4.0 (1.0–27.0) | 18.1 (2.2–109.4) | |
| MACE | |||||||
| N (%) | 1161 (12.7) | 149 (8.2) | 175 (9.6) | 194 (10.6) | 264 (14.5) | 379 (20.8) | < 0.001 |
| N/100py | 2.51 | 1.54 | 1.84 | 2.08 | 2.90 | 4.43 | |
| Death | |||||||
| N (%) | 1045 (11.5) | 108 (5.9) | 140 (7.7) | 167 (9.2) | 223 (12.2) | 407 (22.3) | < 0.001 |
| N/100py | 2.19 | 1.09 | 1.43 | 1.74 | 2.36 | 4.56 | |
| Kidney composite | |||||||
| N (%) | 1226 (13.5) | 161 (8.8) | 136 (7.5) | 213 (11.7) | 274 (15.0) | 442 (24.2) | < 0.001 |
| N/100py | 2.71 | 1.68 | 1.43 | 2.31 | 3.08 | 5.55 | |
The kidney composite includes new macroalbuminuria, a sustained decline in eGFR ≥ 40%, or chronic kidney replacement therapy; the P value is from the chi-square test for trend
GM geometric mean; 10–90 the tenth and 90th percentile boundaries; ACR albumin-to-creatinine ratio; eGFR estimated glomerular filtration rate
Fig. 1.
Spline curves illustrating the relationship between standard deviation-level increments in 1/eGFR, ln (100 × ACR), and the kidney disease index (i.e., the geometric mean of these 2 measures) that are displayed on the x axes, and hazards of the incident outcomes that are displayed on the y axis
Table 2.
Age and sex-adjusted hazard with a one standard deviation higher level of markers of kidney disease
| Ln (100*ACR) | 1/eGFRa | Interaction (P)b | KDI | |
|---|---|---|---|---|
| Mean | 5.47 | 0.014 | N/A | 0.27 |
| Standard deviation | 1.74 | 0.007 | N/A | 0.08 |
| MACE (per 1 SD) | 1.40 (1.32, 1.47) | 1.31 (1.22, 1.40) | Negative (0.031) | 1.27 (1.23, 1.31) |
| Death (per 1 SD) | 1.53 (1.44, 1.62) | 1.52 (1.42, 1.61) | Negative (0.018) | 1.30 (1.27, 1.31) |
| Kidney composite (per 1 SD) | 1.79 (1.68, 1.90) | 1.34 (1.25, 1.44) | No Interaction | 1.31 (1.28, 1.34) |
Hazard rates are per 1 SD (standard deviation) higher level of the risk factor and are from the age and sex adjusted models for each variable
ACR urine albumin-to-creatinine ratio; eGFR estimated glomerular filtration rate; KDI kidney disease index
aAs the relationship between 1/eGFR and the outcomes is not linear, the models include both 1/eGFR and the square of 1/eGFR as independent variables
bThe interaction column notes whether there was an interaction between Ln(100*ACR) and 1/eGFR with respect to each of the 4 outcomes and is based on an age and sex adjusted Cox model that includes each term, an interaction term between the two, and a term that includes the square of 1/eGFR. The kidney composite includes new macroalbuminuria, a sustained decline in eGFR ≥ 40%, or chronic kidney replacement therapy
A non-linear relationship was also noted between Ln (100 × ACR) and the composite kidney outcome, whereas a linear relationship was noted between this variable and MACE, and death (Fig. 1). Thus, the hazard ratio for the composite kidney outcome was estimated with a Cox model that included both a linear and a quadratic term, whereas only a linear term was needed for the other outcomes. Using these models (Table 2) one standard deviation above the mean was associated with a MACE HR of 1.40 (95%CI 1.32, 1.47), a death HR of 1.53 (95%CI 1.44, 1.62), and a kidney outcome HR of 1.79 (95%CI 1.68, 1.90).
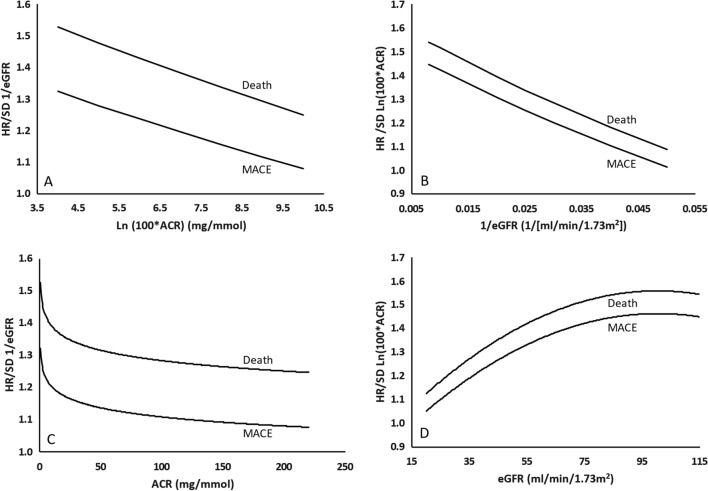
When both 1/eGFR and Ln (100 × ACR) were included in the same models, statistically significant, negative interactions for MACE (interaction P = 0.031) and death (interaction P = 0.018) were identified such that for these 2 outcomes (Table 2), the eGFR was a poor predictor in people with a high degree of albuminuria (Fig. 2a and c) and albuminuria was a poor predictor in people with a low eGFR (Fig. 2b and d). These interactions are illustrated in Fig. 2 which displays the predicted hazard ratio of these 2 outcomes at different levels of albuminuria and eGFR.
Fig. 2.
The figure illustrates the hazard of MACE, death, and the cardiorenal composite with a one standard deviation higher 1/eGFR at different levels of ln (100 × ACR) in A and at different levels of ACR in C. It also illustrates the hazard of these outcomes per standard deviation higher ln (100 × ACR) at different levels of 1/eGFR in B, and at different levels of eGFR in D. The figures were based on Cox models that included age, sex, 1/eGFR, ln(100 × ACR), the interaction of these two variables, and squared terms for 1/eGFR and ln (100 × ACR) where appropriate. MACE major adverse cardiovascular events; eGFR estimated glomerular filtration rate; ACR albumin-to-creatinine ratio
In contrast to both 1/eGFR and Ln (100 × ACR), a linear relationship was noted between the geometric mean of these 2 variables (the KDI) and all three outcomes (Fig. 1). Thus, every standard deviation higher KDI was associated with a 27% to 31% higher hazard of these outcomes (Table 2). A significant interaction between KDI and sex suggested a higher hazard for women versus men (Additional file 1: Table S2) for MACE (interaction P = 0.013).
As noted in Additional file 1: Table S1, those with higher KDI values were more likely to be women, and were older, had a longer duration of diabetes, and a higher systolic blood pressure, HbA1c and LDL cholesterol. They also had a higher prevalence of hypertension, cardiovascular disease, retinopathy and use of renin angiotensin system drugs, and a lower prevalence of tobacco and statin use. Higher fifths of KDI were also associated with a higher incidence of these outcomes (P for trend < 0.001) during follow-up (Table 1), such that participants in the highest fifth had an annual incidence of MACE, death, and the kidney composite outcome of 4.43, 4.56, and 5.55 per 100 person years respectively (Table 1).
The addition of the KDI to age and sex yielded areas under the receiver operating characteristics curves (i.e., C-statistics) that were greater than those for age and sex alone for MACE (0.63 versus 0.60), death (0.68 versus 0.63) and the renal composite (0.65 versus 0.52), and similar to those for the age and sex-adjusted eGFR and albuminuria components of the KDI when included in a multivariable model (Additional file 1: Table S3). The same pattern was noted when the KDI was assessed using data from the ORIGIN trial (Additional file 1: Table S4).
Discussion
Diabetes guidelines recommend the annual assessment of the eGFR and the ACR to identify patients at high risk for serious outcomes [10]. In this epidemiologic analysis of 9,115 middle-aged men and women with type 2 diabetes and additional cardiovascular risk factors, both kidney function and albuminuria expressed as 1/eGFR and ln (100 × ACR) respectively predicted MACE, death, and a kidney composite outcome. Notably, there was a non-linear relationship between 1/eGFR and all these outcomes, and between albuminuria and the kidney composite outcome. Moreover, for MACE and death the prognostic value of each measure was diminished in the presence of higher levels of the other (Fig. 2). When both albuminuria and the eGFR were combined into a kidney disease index, calculated as a geometric mean of 1/eGFR and ln (100 × ACR), higher values were linearly related to all 3 outcomes. Moreover, the age and sex- adjusted prognostic value of the KDI was similar to the full complex model that accounted for both variables, their non-linear relationships, and their interactions with each other.
Many epidemiologic analyses evaluated multivariable prediction models for diabetes-related kidney outcomes and reported excellent performance for models that include many variables in addition to eGFR and/or ACR [11]. Some have identified both the eGFR and ACR as important indices for kidney outcomes [12–15], cardiovascular outcomes [16–18], and death [14–16]. However, few have formally interrogated the shape of the relationship between these 2 variables and kidney outcomes as well as cardiovascular outcomes and death. The observation of a non-linear relationship between the eGFR variable and all four outcomes in the REWIND data is consistent with the findings of a large meta-analysis published in 2012 [15].
Both the eGFR and albuminuria are generally viewed as independent determinants of these outcomes. Indeed, the 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines graphic which displays albuminuria and eGFR categories on 2 axes [2] (Additional file 1: Figure S1) implies an orthogonal or independent relationship between these 2 variables and suggests that highly abnormal levels of both confer a worse prognosis for various outcomes than abnormal levels of only one alone. These analyses show that this is indeed the case for the kidney outcome. However, the observed negative interactions illustrated in Fig. 2 in which high degrees of either of these risk factors (albuminuria or kidney dysfunction) were poor predictors of MACE and death in the presence of high degrees of the other, suggests that they are not independent risk factors for these other outcomes. Most epidemiologic analyses have not tested for such interactions. When they were assessed, a similar negative interaction for cardiovascular events was reported in one publication [17], but not another [16].
The non-linear relationships and interactions noted above plus the fact that both the eGFR and albuminuria are prognostically relevant for future serious outcomes highlight the potential value of a metric that combines the information contained in both the eGFR and the ACR. The KDI is such a metric. Its linear relationship to the 3 outcomes illustrated in Fig. 1, its straightforward calculation, and the fact that its area under the receiver operating characteristics curve is similar to that of complicated models that include interactions and squared terms, highlight its possible utility as a simple prognostic marker for kidney outcomes as well as cardiovascular outcomes and death.
Strengths of these exploratory, post hoc analyses include the international nature of these data, the fact that the participants are similar to people with diabetes in the general population [19], the long-term follow-up, the high number of outcomes, the fact that all clinical outcomes were adjudicated, and consistent findings when the new index was applied to a different cohort from the ORIGIN trial. The absence of a central laboratory for the urine and serum measurements is a limitation common to many epidemiologic studies of kidney function [20] that is mainly mitigated by the large sample size. These analyses are also limited by the fact that they were not prespecified, and the fact that they were conducted in middle-aged and older people with diabetes and additional cardiovascular risk factors who consented to participate in a long-term randomized controlled trial. They therefore may not be applicable other populations.
Conclusion
The eGFR and ACR are routinely measured risk factors for kidney and cardiovascular outcomes. The observation that these risk factors were not linearly related to these outcomes and were not independent of each other for predicting MACE and death highlights their complex relationship to each other and to these outcomes. The KDI is a composite measure of these two risk factors. It combines information from both risk factors and has a simple linear relationship to all three outcomes. Moreover, its ability to predict these three outcomes was similar to the ability of complex models that included the eGFR and ACR, nonlinear terms and interaction terms to predict these outcomes. The KDI could therefore simplify the identification of the highest risk individuals who are most likely to benefit from preventive therapies. Its utility as a risk stratification tool should be assessed and confirmed in future epidemiologic studies and clinical trials.
Supplementary Information
Additional file 1: Table S1. Distribution of Baseline Characteristics Across Fifths of the Kidney Disease Index*. Table S2. Age-adjusted Hazard of Different Outcomes According to Sex. Table S3. C-statistics for the Age and Sex-Adjusted Models. Table S4. C-statistics for the Age and Sex-Adjusted Models Using ORIGIN Data. Figure S1. The prognosis of CKD by GFR and Albuminuria Categories (KDIGO 2012) is indicated by the risk categories in the cells of the figure.
Acknowledgements
Not applicable.
Abbreviations
- REWIND
Researching Cardiovascular Events with a Weekly Incretin in Diabetes
- eGFR
Estimated glomerular filtration rate
- ACR
Albumin-to-creatine ratio
- KDI
Kidney disease index
- MACE
Major adverse cardiovascular events
Author contributions
HCG wrote the first draft of the paper and CR did the statistical analyses. All the authors researched the data and critically revised the paper. HCG is the guarantor of the study and made the final decision to submit and publish the manuscript.
Funding
The REWIND trial was funded by Eli Lilly.
Availability of data and materials
The REWIND data sharing policy is described in the Supplement.
Declarations
Ethics approval and consent to participate
The protocol was reviewed by Research Ethics Boards for 371 sites in 24 countries and all participants provided signed written informed consent.
Consent for publication
Not applicable.
Competing interests
HCG holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. He reports research grants from Eli Lilly, AstraZeneca, Merck, Novo Nordisk, and Sanofi; honoraria for speaking from Eli Lilly, Novo Nordisk, Sanofi, DKSH, Roche and Zuellig; and consulting fees from Abbott, Covance, Eli Lilly, Novo Nordisk, Sanofi, Pfizer, Kowa and Hanmi. AA reports honoraria for speaking from Eli Lilly, Novo Nordisk, and Boehringer Ingelheim; JB reports research grants from Recor and Ablative Solutions; consulting fees from Eli Lilly, Recor, and Up-to-Date. IC reports honoraria for speaking from Medtronic, Eli Lilly, Novo Nordisk, Sanofi Aventis, Astra Zeneca, Boehringer Ingelheim, and Merck Sharp and Dohme; and consulting fees from Medtronic, Eli Lilly, Novo Nordisk and Sanofi Aventis. WCC reports research grants from Eli Lilly, ReCor, and George Medicines. ML owns Eli-Lilly Stock. LAL has received research funding from AstraZeneca, Bayer, Lexicon, and Sanofi; honoraria for speaking from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, and Servier, and has acted as an adviser to AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, Pfizer, and Sanofi. JES reports a research grant from Astra Zeneca and honoraria for speaking and advisory boards from Astra Zeneca, Sanofi, Eli Lilly, MSD, Mylan, Pfizer, Roche, Abbott and Zuellig. PJR has received speaker honoraria from Aspen and Sanofi. WHHS reported as Advisor and/or Speaker for AstraZeneca, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals., Daiichi-Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Sanofi-Aventis, and Takeda Pharmaceutical Company.TT-K reports consulting fees from Bayer, AstraZeneca, and Hamilton Health Sciences. IT is employed by Eli Lilly and Company and owns Eli Lilly stock. DX reports grants from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Coca Cola India, the Indian Council of Medical Research, Pfizer, United Kingdom Medical Research Council, and Wellcome Trust, and honoraria from Eli Lilly and Sanofi. GRD, FL, NP, JP have nothing to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, Chronic Kidney Disease Prognosis C. van der Velde M, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 2.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 3.Collaboration GBDCKD Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Ryden L, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 5.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riddle MC, Ryden L, Xavier D, et al. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) Trial of Dulaglutide’s Cardiovascular Effects. Diabetes Obes Metab. 2017;20(1):42–49. doi: 10.1111/dom.13028. [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert RE, Mann JF, Hanefeld M, Spinas G, Bosch J, Yusuf S, Gerstein HC. Basal insulin glargine and microvascular outcomes in dysglycaemic individuals: results of the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial. Diabetologia. 2014;57(7):1325–1331. doi: 10.1007/s00125-014-3238-4. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerstein HC, Ramasundarahettige C, Bangdiwala SI. Creating composite indices from continuous variables for research: the geometric mean. Diabetes Care. 2021;44(5):e85–e86. doi: 10.2337/dc20-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Professional Practice C. American Diabetes Association Professional Practice C. Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, et al. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Supplement_1):S46–S59. doi: 10.2337/dc22-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slieker RC, van der Heijden A, Siddiqui MK, Langendoen-Gort M, Nijpels G, Herings R, Feenstra TL, Moons KGM, Bell S, Elders PJ, et al. Performance of prediction models for nephropathy in people with type 2 diabetes: systematic review and external validation study. BMJ. 2021;374:n2134. doi: 10.1136/bmj.n2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murton M, Goff-Leggett D, Bobrowska A, Garcia Sanchez JJ, James G, Wittbrodt E, Nolan S, Sorstadius E, Pecoits-Filho R, Tuttle K. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: a systematic review. Adv Ther. 2021;38(1):180–200. doi: 10.1007/s12325-020-01568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson RG, Grams ME, Ballew SH, Sang Y, Azizi F, Chadban SJ, Chaker L, Dunning SC, Fox C, Hirakawa Y, et al. Development of risk prediction equations for incident chronic kidney disease. JAMA. 2019;322(21):2104–2114. doi: 10.1001/jama.2019.17379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308(22):2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim CC, Teo BW, Ong PG, Cheung CY, Lim SC, Chow KY, Meng CC, Lee J, Tai ES, Wong TY, et al. Chronic kidney disease, cardiovascular disease and mortality: a prospective cohort study in a multi-ethnic Asian population. Eur J Prev Cardiol. 2015;22(8):1018–1026. doi: 10.1177/2047487314536873. [DOI] [PubMed] [Google Scholar]
- 17.Sasso FC, Chiodini P, Carbonara O, De Nicola L, Conte G, Salvatore T, Nasti R, Marfella R, Gallo C, Signoriello S, et al. High cardiovascular risk in patients with Type 2 diabetic nephropathy: the predictive role of albuminuria and glomerular filtration rate. The NID-2 Prospective Cohort Study. Nephrol Dial Transplant. 2012;27(6):2269–2274. doi: 10.1093/ndt/gfr644. [DOI] [PubMed] [Google Scholar]
- 18.Alexander N, Matsushita K, Sang Y, Ballew S, Mahmoodi BK, Astor BC, Coresh J. Kidney measures with diabetes and hypertension on cardiovascular disease: the Atherosclerosis Risk in Communities Study. Am J Nephrol. 2015;41(4–5):409–417. doi: 10.1159/000433450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boye KS, Riddle MC, Gerstein HC, Mody R, Garcia-Perez LE, Karanikas CA, Lage MJ, Riesmeyer JS, Lakshmanan MC. Generalizability of glucagon-like peptide-1 receptor agonist cardiovascular outcome trials to the overall type 2 diabetes population in the United States. Diabetes Obes Metab. 2019;21:1299–1304. doi: 10.1111/dom.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coresh J, Heerspink HJL, Sang Y, Matsushita K, Arnlov J, Astor BC, Black C, Brunskill NJ, Carrero JJ, Feldman HI, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–127. doi: 10.1016/S2213-8587(18)30313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Distribution of Baseline Characteristics Across Fifths of the Kidney Disease Index*. Table S2. Age-adjusted Hazard of Different Outcomes According to Sex. Table S3. C-statistics for the Age and Sex-Adjusted Models. Table S4. C-statistics for the Age and Sex-Adjusted Models Using ORIGIN Data. Figure S1. The prognosis of CKD by GFR and Albuminuria Categories (KDIGO 2012) is indicated by the risk categories in the cells of the figure.
Data Availability Statement
The REWIND data sharing policy is described in the Supplement.