Abstract
• PURPOSE:
To report the therapeutic efficacy of integrating neoadjuvant chemotherapy with conventional bimodal therapies for lacrimal gland adenoid cystic carcinoma by providing an additional 8 years of follow-up data on the same cohort of patients whose cumulative 10-year disease-free survival outcomes were reported in 2013.
• DESIGN:
Non-randomized, retrospective, interventional case series.
• METHODS:
Nineteen consecutive patients treated with neoadjuvant intra-arterial cytoreductive chemotherapy (IACC), orbital exenteration, chemoradiotherapy, and adjuvant intravenous chemotherapy at a single institution were included. Analyses were undertaken of locoregional recurrences and distant metastases, disease-free survival time, TNM tumor stage at presentation, response to IACC, and prognostic impact of positive resection margins. The main outcome measures were overall survival, disease-free survival, disease relapse, positive tumor resection margins, and tumor stage at presentation.
• RESULTS:
Eight patients with an intact lacrimal artery (group 1), 7 with AJCC stage T4a-c, had significantly better overall survival (87.5% versus 14.3% at 15 years), disease-specific mortality, and recurrences (all < .001, log-rank test) than prior conventionally treated patients from the Bascom Palmer Eye Institute. Group 1 was superior to group 2, patients lacking an intact lacrimal artery, concerning overall survival (P = .042) and recurrence (P = .017), but with no significant difference in disease-specific mortality (P = .23). Group 2 was associated with a significantly lower cause-specific mortality than the institutional comparator group (P = .039). Prior tumor resection with lateral wall osteotomy and failure to adhere to all protocol elements were adverse prognostic factors for suboptimal outcomes. Positive tumor margins increased the risk of all-cause mortality 4.1 times (P = .036, stratified Cox proportional hazards regression) and disease-specific mortality 8.0 times (P = .043, stratified Cox proportional hazards regression) than a patient with negative margins.
• CONCLUSIONS:
Extended follow-up supplemented with AJCC staging data supports neoadjuvant IACC as an integral component of a trimodal treatment strategy in patients with an intact lacrimal artery. Protocol elements implemented as designed appear to have improved overall survival and decreased disease relapse in this cohort. This extended long-term IACC dataset suggests that a critical bar of at least 15 years of follow-up is appropriate for assessing the efficacy of current conventional and future globe-sparing bimodal therapies.
INTRODUCTION
LACRIMAL GLAND ADENOID CYSTIC CARCINOMA (LGACC) is a rare orbital malignancy notorious for its unpredictability and universal devastating lethality. The difficulty in achieving a cure for this disease is principally attributable to the complex regional orbital anatomy, the tumor’s aggressive biological behavior, infiltrative growth pattern, distinct propensity for perineural infiltration with retrograde intracranial extension, hematogenous dissemination, and delay in diagnosis. 1–8 The tumor often infiltrates and spreads through bone. 9 , 10 Intracranial involvement and metastatic disease are the principal causes of death. 1 , 11
Controversy remains regarding the optimal treatment strategy for this orbital tumor, ranging from globe-sparing tumor resection, proton beam therapy, bimodal therapy of exenteration with or without adjacent bone removal followed by external beam radiation therapy to radical multidisciplinary intervention with cranio-orbital resection. 8 , 12 , 13–16 Much of the early management philosophy was influenced by Halsted’s doctrine of radical mastectomy for breast cancer: cutting more equals living more. 8 , 9 , 12 , 15 , 16 However, the dogma that cutting more orbital tissues for local disease control in LGACC equals living more has not proven to be true. 17–19 Despite various surgery and radiation therapy permutations, these patients’ survival outcomes remain dismal. 1 , 3–6 , 8 , 9 , 12 , 20–25 Font and Gamel reported an actuarial survival rate of < 50% at 5 years and a bleak 20% at 10 years, regardless of treatment methods, 4 with virtually no patients surviving beyond 15 years with conventional therapies. 26 Improvements in local disease control are unlikely to impact survival until LGACC is viewed as a systemic disease rather than a local orbital disease. A multi-modal treatment strategy integrating a systemic approach needs to be developed to prevent local recurrence and distant metastatic disease.
To address the principal shortcomings of prior conventional bimodal locoregional therapies, Meldrum and associates 27 introduced a trimodal protocol in 1998. This treatment paradigm has 3 integral components: chemotherapy, orbital exenteration, and radiation therapy. The core element of the strategy is neoadjuvant intra-arterial cytoreductive chemotherapy (IACC) – delivery of a high concentration of chemotherapeutic agent to the tumor through an intact lacrimal artery before exenteration. The rationale of regional infusion of cisplatin is the delivery of a concentration of drug that otherwise cannot be safely delivered through the venous route, achieving local drug concentration exceeding the usual blood level to increase efficacy. This firstpass effect may result in the drug concentration overwhelming the tumor’s nucleotide excision repair system to prevent DNA replication. 28–31 The principal intent of the neoadjuvant phase of chemotherapy is to induce tumor cell death and cytoreduction to enhance tumor margin clearance at the time of exenteration and to minimize dissemination of viable tumor cells before and during surgical manipulation. It also offers the theoretical advantage of eliminating occult tumor cells beyond the surgical margins.
In 2013, Tse and associates reported this trimodal therapy’s long-term outcomes in a non-randomized, retrospective treatment comparison study of 19 consecutive patients from a single institution. The 19 patients were stratified into 2 groups in a post-hoc analysis. Group 1 (intact lacrimal artery and protocol adherence) comprised patients with an intact lacrimal artery and no prior tumor resection or surgical disruption of the bone barrier who completed all aspects of the treatment protocol as designed - in sequential order and within an optimal timeframe. Group 2 (non-intact lacrimal artery or protocol non-adherence) comprised patients without an intact lacrimal artery and disruption of the bone barrier from tumor resection before referral for IACC treatment, except 1 patient who failed to adhere to the integral elements of exenteration and adjuvant chemotherapy. The study’s findings demonstrated a beneficial long-term advantage of an IACC-anchored trimodal protocol in achieving local disease control and disease-free survival even for patients with advanced-stage tumors. 32–34
The main objective of the current report is to provide an additional 8 years of follow-up data on the same cohort of patients and to scrutinize the therapeutic benefits of this treatment strategy. Additionally, it proposes a new standard for future survival follow-up time reporting in assessing the treatment efficacy of current and globe-preservation therapies for LGACC. This study provides the longest follow-up time in the literature to evaluate the therapeutic effectiveness of integrating chemotherapy into conventional bi-modal treatment for a disease in which effective therapy has remained elusive. This case series reports that IACC delivered through an intact lacrimal artery appears to pro-long disease-free survival in this cohort of patients with LGACC.
MATERIALS AND METHODS
The University of Miami Miller School of Medicine Institutional Review Board approved the case series review. The treatment was conducted under the Declaration of Helsinki provisions and was performed in compliance with the Health Insurance Portability and Accountability Act.
• PROTOCOL DESCRIPTION:
The chemotherapy arm of the trimodal protocol consists of 6 cycles of chemotherapy - 2 or 3 neoadjuvant cycles and 4 or 3 adjuvant cycles. 27 A neoadjuvant cycle consists of intra-arterial perfusion of cisplatin 100 mg/m 2 on day 1 and intravenous doxorubicin 25 mg/m 2 on days 1 to 3. To avoid direct brain or retina perfusion through an internal carotid artery access, the internal maxillary artery (IMA), a branch of the external carotid artery is cannulated. A second intra-arterial cycle is administered in 3 weeks. Three to 4 weeks after completing the second cycle, MRI or CT of the orbits is obtained to assess tumor response, which typically shows visible shrinkage of tumor volume, in some cases downstaging the tumor to a surgically resectable mass. However, if the posterior tumor margin shrinkage in the orbital apex is incomplete, rendering tumor resection margin clearance less likely, a third cycle is administered to achieve further cytoreduction. The decision to implement the third cycle is also guided by the patient’s tolerance or side effects to the initial cycles. After 2 (or possibly 3) intra-arterial chemotherapy cycles and following hematologic recovery, orbital exenteration is performed without delay to prevent tumor progression. Surgery is followed by adjuvant radiotherapy 2 to 4 weeks later with concomitant weekly IV cisplatin 20 mg/m 2 for radiosensitization. Following chemoradiation therapy, 3 or 4 cycles of adjuvant IV cisplatin 100 mg/m 2 on day 1 and intravenous doxorubicin 20 mg/m 2 on days 1 to 3 every 3 weeks are administered, so patients would optimally receive a total of 6 cycles of systemic chemotherapy.
• PARTICIPANTS:
The clinical records of 19 consecutive patients with LGACC treated with neoadjuvant IACC were retrieved from the Bascom Palmer Eye Institute (BPEI) and University of Miami Hospital and Clinics. The study period extended from 1988 to 2021. The American Joint Committee on Cancer TNM Classification , 7 th edition 35 for tumor staging was added. Seven patients were treated consecutively by conventional therapies between 1967 and 1984, with information gathered for the 2013 study remaining unchanged. This previously reported cohort of patients constituted the institutional comparator of the treatment group (Supplementary Figures 1–3). Disease-related mortality was defined as death due to LGACC. The 2 previously reported cases with alternate histology were excluded.
All the surviving participants from the 2013 study were contacted in January 2021 in a telephone interview regarding their overall medical status, with particular emphasis on disease-free survival. Referring ophthalmologists or medical oncologists were contacted for medical status confirmation, supplemental information relating to late sequelae of chemotherapy complications, or cause of death. The cause of death was confirmed by medical records and the primary treating physician. Information on locoregional recurrence, distant metastases, disease-free survival time, and medical status were updated.
• STATISTICAL ANALYSES:
The efficacy of treatments with Kaplan-Meier survival analyses for 3 endpoints was compared: mortality from all causes (overall survival), mortality due to LGACC with patients censored at death due to other reasons (disease-specific survival), and time to recurrence (progression-free survival). There is some controversy in the oncology literature regarding the most appropriate assessment of mortality: all-cause or disease-specific. 36,37 This is a particular issue for small retrospective studies wherein 2 considerations come into play: (1) the uncertainty of obtaining accurate cause of death information from contacting relatives and (2) the reduction in statistical power associated with fewer endpoints in cause-specific mortality. For both reasons, all-cause mortality is preferred but disease-specific mortality is also reported. The survival time was calculated as the number of days from protocol initiation to the endpoint, last follow-up examination, or contact date. Statistical significance was assessed with the log-rank test. The reporting of progression-free survival and overall survival are well accepted standards for assessing cancer therapies in oncologic literature and they are universally utilized to compare data across studies. Instead of demonstrating overall survival advantages, especially for diseases with multiple lines of treatment or long-term survivorship, which may take years to confirm, progression-free survival has been used as a surrogate endpoint for which new treatments for malignant disease have been FDA approved. These end-points are therefore reported here, consistent with clinical trial assessments.
Patients were divided into 2 groups receiving IACC: group 1 and group 2, depending on the integrity of the lacrimal artery. The latter group included 1 patient (case 8) with an intact lacrimal artery who received neoadjuvant therapy but deviated from protocol by refusing exenteration and adjuvant treatment. Survival was measured by specific cumulative proportions illustrated in the Kaplan-Meier curves, the benchmark statistic for interpreting survival. 38 In addition to standard median follow-up (F/U) time-all for all patients, median F/U time-alive only for patients surviving at the end of the time of this report was also reported. Because LGACC mortality manifests only after many years, 26 recently treated patients with short follow-up contribute little to treatment efficacy assessment. The median F/U time-alive is a gauge for identifying studies including patients with short follow-up.
Cumulative proportions were compared with the log-rank test of statistical significance. The risk ratio associated with positive tumor margins on each endpoint was studied with univariate Cox survival regression stratified by group and employing the likelihood ratio test for statistical significance.
RESULTS
The patient demographics, treatment characteristics, clinical data, TNM stage at presentation, length of follow-up, and cause of death are summarized in Table 1 . No patients were lost to follow-up.
TABLE 1.
Patient Characteristics of IA Cytoreductive Chemotherapy for Lacrimal Gland Adenoid Cystic Carcinoma
| Patient No. | Age/Gender/Affected Side | ACC Subtype/Perineural/Bone Infiltration | IACC Cycle/Total Cycles | Prior Tumor Resection | Radiographic Tumor Response/Tissue Margins | TNM Treatment | Survival Time in Months (Years) and Status at Last Follow-Up | Time (Months) of Local Recurrence/Met | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||
| 1 | 29/M/OD | Basa/P/B | 3/4 | NO | Yes/- | T4cN0M0 Ex/RT | 389 (32.4); A,ND | NED | Intracranial extension |
| 3 | 73/F/OS | Basa/P | 2/6 | NO | Yes/- | T4bN0M0 Ex/RT | 222 (18.5); D,ND | NED | Kidney transplant, died SCC |
| 4 | 58/M/OS | Crib/P | 2/6 | NO | Yes/- | T4cN0M0 Ex/RT/GK | 238 (19.8); D,ND | NED | Intracranial, ethmoid sinus ACC, died of aspiration pneumonia |
| 5 | 35/M/OS | Crib/P | 3/6 | NO | Yes/+ | T4aN0M0 Ex/RT | 251 (20.9); A,ND | NED | Non-healing apex; free flap |
| 7 | 30/M/OS | Basa/P | 2/4 | NO | Yes/- | T2aN0M0 Ex/B/RT | 216 (18); A,ND | NED | |
| 10 | 29/F/OD | Basa | 2/6 | NO | Yes/- | T4bN0M0 Ex/RT | 198 (16.5); A,ND | NED | |
| 17 | 53/F/OD | Basa/B | 2/6 | NO | Yes/- | T4cN0M0* Ex/RT | 131 (10.9); A,ND | NED | Bone erosion, dural enhancement, - neck dissection |
| 18 | 44/M/OS | Basa/Crib/P/B | 3/6 | NO | Yes/+ | T4cN0M0 Ex/RT | 98 (8.17); D,D | (74); Lung | Pitting of lateral wall; bone burring, jaw |
| Group 2 | |||||||||
| 2 | 32/M/OD | Crib/P/B | 3/6 | YES | Yes/- | T4bN0M0 Ex/B/RT | 159 (13.25); D,ND | NED | HIV, died of oral SCC |
| 6 | 42/F/OD | Crib/P/B | 3/6 | YES | Yes/- | T4bN0M0 Ex/B/RT | 135 (11.25); D,D | (50); Lung | Bone necrosis, hearing deficit; 7th N palsy |
| 8 | 36/F/OS | Basa/P/B | 2/2 | NO | Yes/+ | T4bN0M0 Ex/RT | 32 (2.7); D,D | (20); LR/Liver | Delayed exent, refused final 4 cycles |
| 9 | 64/F/OD | Crib/B | 2/2 | YES | Yes/+ | T4cN0M0 Ex/B/RT | 120 (10); D,ND | (13); LR/Sinus | ACC in sinus mucosa, died of breast CA |
| 11 | 54/M/OS | Crib/Basa | 2/6 | YES | Yes/+ | T4bN0M0 Ex/B/TM/RT | 88 (7.3); D,D | (20); LR/Lung/Brain | Extensive disease at presentation |
| 12 | 32/F/OD | Crib | 2/5 | YES | Yes/+ | T3aN0M0 Ex/B/RT/GK | 134 (11.25); D,ND | NED | Died of leukemia |
| 13 | 34/M/OD | Crib | 2/6 | YES | Yes/- | T4bN0M0 Ex/B/RT | 190 (15.8); A,D | (168); Lung | Lung nodules |
| 14 | 49/M/OS | Crib/Basa | 2/5 | YES | Yes/- | T2aN0M0 Ex/B/RT | 184 (15.3); A,D | (57); Lung | Lung nodule resection; Lung and liver metastases after disease-free for 10.8 years |
| 15 | 20/M/OD | Crib/Basa/P/B | 3/5 | YES | Yes/- | T3aN0M0 Ex/B/RT | 175 (14.6); A,ND | NED | |
| 16 | 38/F/OS | Basa | 2/6 | YES | Yes/+ | T4aN0M0 Ex/RT | 154 (12.8); A,ND | NED | |
| 19 | 56M/OS | Basa/P | 5/5 | YES | Yes/+ | T4cN0M0 Ex/RT | 33 (2.75); D,D | (22) | Globe-sparing resection of a T2 tumor and radiotherapy. Recurrence as a T4c tumor with intracranial and ethmoid sinus involvement |
The patient number is the chronologic order of treatment and is displayed by the treatment group.
stage changed from T4b upon re-review of scans; A = alive; A,D = alive, with disease; A,ND = alive, no disease; ACC = adenoid cystic carcinoma; B = bone infiltration; Basa = basaloid subtype; Crib = cribriform subtype; D = died; D,D = died, with disease; D,ND = died, no disease; Exent + bone + RT = exenteration with removal of lateral orbital wall fragment plus postoperative radiation therapy; Exent + RT = exenteration plus postoperative radiation therapy; GK = gamma knife therapy; LR = local recurrence; N/A = not applicable; NED = no evidence of disease; P = perineural infiltration; Radiographic Tumor Response/Tissue Margins = radiographic evidence of tumor shrinkage after IA chemotherapy cycles/presence of tumor cells at soft tissue surgical margins; Resect = primary resection of mass; SCC = squamous cell carcinoma; 7th N = facial nerve palsy; TM = temporalis muscle resection; TNM = AJCC TNM Classification 7th edition criteria
• TREATMENT-RELATED ADVERSE EVENTS:
The toxicities of therapy were summarized in the 2013 paper as supplemental data (Table 5, available at http://aaojournal.org ). The toxicities were those expected from exposure to aggressive chemotherapy consisting of repeated cycles of cisplatin and doxorubicin, including cytopenias (anemia and thrombocytopenia at times requiring transfusion), neutropenic fever, elevated creatinine, and tinnitus. There were no catheter placement or infusion complications, including stroke (embolic or ischemic), CNS hemorrhage, or bleeding at the arterial puncture site. There were no episodes of cardiac failure. Toxicities unique to the treatment program included trismus, facial nerve palsy, and hearing deficit identified in a minority of patients – none were permanent. One patient (case 3) required renal transplant 5 years after treatment and died of unrelated cancer at 18.5 years after diagnosis. The etiology of renal impairment was not directly related to the administration of chemotherapy. There were no additional unexpected toxicities since the 2013 report.
• AMERICAN JOINT COMMITTEE ON CANCER CLASSIFICATION AND HISTOPATHOLOGICAL FEATURES:
The 7th American Joint Committee on Cancer classification at initial diagnosis was as follows: T2aN0M0, 2 patients; T3aN0M0, 2 patients; T4aN0M0, 2 patients; T4bN0M0, 7 patients; and T4cN0M0, 6 patients. Histopathological examination of the surgical specimens demonstrated perineural infiltration in 11 patients, bone invasion in 8 patients, and positive resection margins in 8 patients. Tumor histological subtypes of the 19 patients were as follows: cribriform, 7 patients; basaloid, 8 patients; and mixed type with cribriform and basaloid, 4 patients (Table 1 ).
• SURVIVAL OUTCOMES – GROUP 1:
Of the 8 patients in group 1, 7 (88%) had advanced T4a-c tumors (Table 1 ). Three patients in this group have died, 2 from unrelated diseases; 1 (case 18) with T4c disease developed LGACC recurrence at 74 months (6.2 years) and succumbed to the disease 2 years later. Cumulative proportions surviving at 10 and 15 years (Table 2 ) are 87.5% and 87.5%, respectively, including 2 > 20-year survivors, 4 > 15-year survivors, and 1 > 10-year survivor (Table 1 ). The group 1 median F/U time-all was 219 months (18.3 years) (98–389 + months). Of the 5 patients in group 1 who are alive, the median F/U time-alive was 216 + months (18.0 years) (range 131 + to 389 + months). Case 1 is the longest disease-free survivor at 32.4 + years whose T4c disease with intracranial involvement was initially judged to be inoperable.
TABLE 2.
Survival Summary by Treatment and Endpoint. Cumulative Proportion Surviving (Standard Error).
| Treatment Group | Endpoint 1, Survival (All-Cause Mortality) | Endpoint 2, Survival Censored At Death Not Due To LGACC | Endpoint 3, Recurrence-Free Disease-Specific Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| 5 years | 10 years | 15 years | 5 years | 10 years | 15 years | 5 years | 10 years | 15 years | |
| IACC per protocol | 100% (na) | 87.5% (11.7%) | 87.5% (11.7%) | 87.5% (11.7%) | 87.5% (11.7%) | 87.5% (11.7%) | 87.5% (11.7%) | 87.5% (11.7%) | 87.5% (11.7%) |
| IACC protocol deviation | 81.8% (11.6%) | 63.6% (14.5%) | 34.1% (15.0%) | 81.8% (11.6%) | 72.7% (13.4%) | 60.6% (15.7%) | 45.5% (15.0%) | 45.5% (15.0%) | 45.5% (15.0%) |
| IACC combined | 89.5% (7.0%) | 73.3% (10.1%) | 56.1% (11.8%) | 89.5% (7.0%) | 78.9% (9.4%) | 72.4% (10.6%) | 73.7% (10.1%) | 68.4% (10.7%) | 68.4% (10.7%) |
Abbreviations: IACC = intra-arterial cytoreductive chemotherapy; LGACC = lacrimal gland adenoid cystic carcinoma
• SURVIVAL OUTCOMES – GROUP 2:
Eleven patients were in group 2; 10 had excisional biopsy by a lateral orbitotomy approach for presumed pleomorphic adenoma without a prior incisional biopsy. The diagnosis of LGACC was made after tumor resection. Ten patients (90.9%) had ≥ T3 disease (Table 1 ). Intra-arterial chemotherapy was delivered to the surgical bed without an intact lacrimal artery. Seven of 11 patients have died. Four (36.4%) patients (cases 6, 8, 11, and 19) died of metastatic LGACC disease, all with T4b-c disease at presentation. The median disease-free survival time for these 4 deceased patients was 60.5 months (5.0 years) (range 32–135 months). Three (cases 2, 9, and 12) died from an unrelated malignant disease. The median duration of disease-free survival time for these 3 deceased patients was 134 months (11.2 years) (range 120–159 months). Two of the 4 patients (cases 13, 14, 15, and 16) alive in group 2 developed late disease relapse. Case 13 had T4b disease and developed lung metastases after 168 months of disease-free survival. Case 14 presented with T2 disease, had tumor resection with bone take-down for a presumed pleomorphic adenoma, developed a lung nodule at 57 months after IACC, and underwent metastasectomy; he was disease-free until 184 months (15.3 years) when lung and liver nodules were noted. Two cases (15 and 16) are disease-free at 14.6 and 12.8 years, respectively. Cumulative proportions surviving at 10 and 15 years were 63.6% and 34.1%, respectively. The group 2 median F/U time-all was 135 months (11.3 years) (32–190 months). Of the 4 patients in group 2 who are alive, the median F/U time-alive is 179.5 + months (15.0 years) (range 154–190 months). Compared to historical bimodal therapy group 3, group 2 was associated with a significantly lower cause-specific mortality than the institutional comparator group (P = .039) (Supplementary Figure 2).
• ENDPOINT COMPARISON BETWEEN GROUPS 1 AND 2:
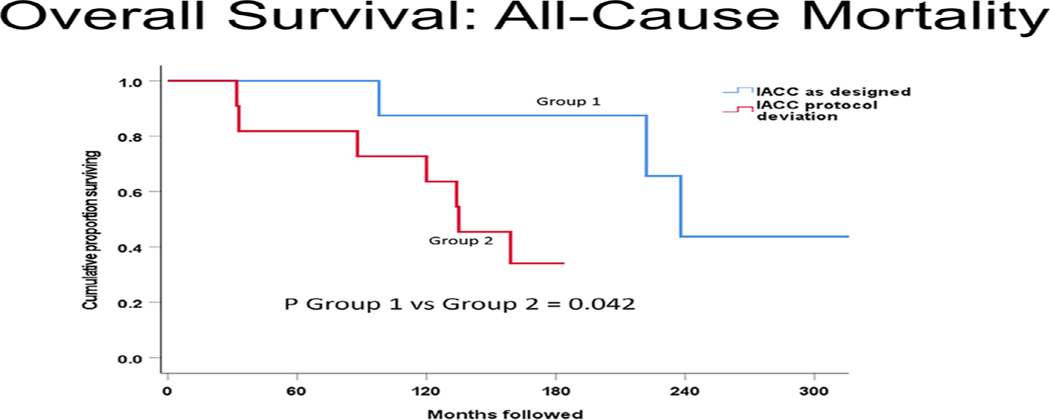
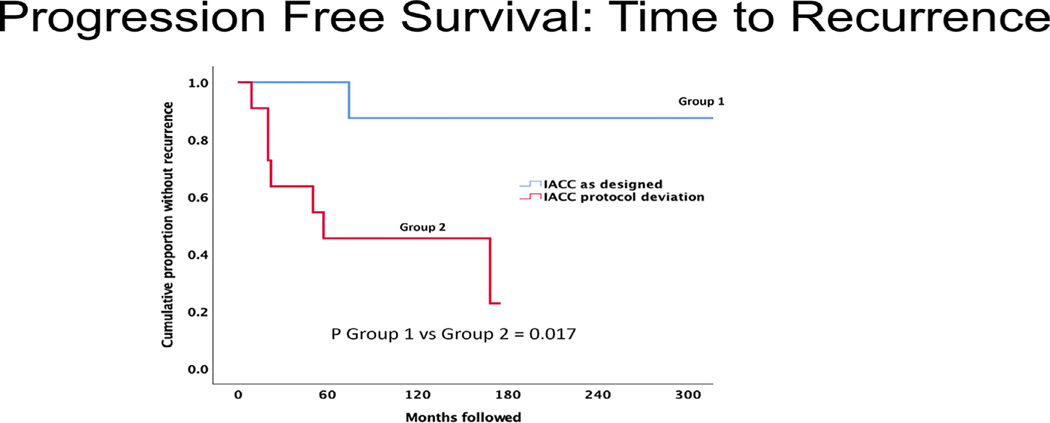
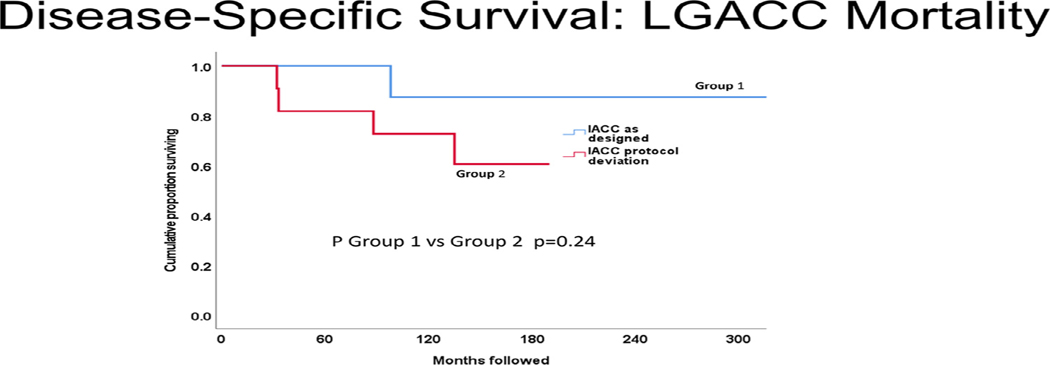
Figures 1 to 3 display cumulative proportions of patients surviving for each endpoint by the group. Group 1 was superior to group 2 with respect to both all-cause mortality (P = .042) and recurrence-free survival (P = .017). Similar in character to the other endpoints, disease-specific mortality was greater in group 2 than group 1, but not significantly so (P = .23).
FIGURE 1.
All-cause mortality comparison of intra-arterial cytoreductive chemotherapy (IACC) as designed versus protocol deviation. group 1 (IACC as designed) had significantly better overall survival (P = .042) than group 2 (IACC protocol deviation).
FIGURE 3.
Time to recurrence comparison of intra-arterial cytoreductive chemotherapy (IACC) as designed versus protocol deviation. Group 1 (IACC as designed) had significantly better progression-free survival (P = .017) than group 2 (IACC protocol deviation); that is: group 2 had a significantly shorter time to recurrence.
• ALL-CAUSE MORTALITY OF PATIENTS WITH STAGE T3 AND T4 TUMORS:
In addition to the analysis of all patients described above, including T2 patients, an ancillary analysis was performed for the higher risk T3 and T4 tumors. Group 1 comprised 7 patients (88%) with T4 tumors and 1 patient (12%) with a T2 tumor. Group 2 comprised 8 patients (73%) with T4 tumors, 2 patients (18%) with T3 tumors, and 1 patient (9%) with a T2 tumor. Among the subset of patients with ≥ T3 tumors, group 1 had overall survival superior to group 2 (P = .025, log-rank test). After 10 years of follow-up, cumulatively, 85.7% of group 1 patients survived versus 50.0% in group 2. After 15 years of follow-up, the cumulative proportion of patients surviving in group 1 remained 85.7%, while group 2 dropped to 18.8% (Table 2 ).
• EXTRAORBITAL OR INTRACRANIAL EXTENSION (T4C) AND CYTOREDUCTION:
Four patients in group 1 presented with tumor margins extending beyond the orbital confines through the superior orbital fissure to involve the intracranial compartment (cases 1, 4, and 17) or through the inferior orbital fissure to infiltrate the infratemporal fossa (case 18). Cases 1 and 4 were judged to be inoperable for a cure because of intracranial extension. After 2 IACC cycles, a substantial reduction in the intracranial mass was noted in both cases, converting an unresectable disease to a resectable condition. Of the 2 remaining patients, case 17 at presentation had dural enhancement consistent with tumor involvement but displayed no enhancement after completing IACC. Exenteration specimen margins and dura biopsy were negative. Case 18 had a positive margin at the inferior orbital fissure and succumbed to the disease at 98 months.
Thus, 3 patients with apparent unresectable disease at presentation achieved complete resection after cytoreductive chemotherapy. In group 2, 1 (case 9) of 2 patients with T4c disease (extraorbital) survived > 10 years without recurrence but died of an unrelated cause.
• RISK OF RECURRENCE WITH POSITIVE MARGINS:
Two of 8 patients in group 1 and 6 of 11 patients in group 2 had positive tissue margins (Table 1 ) at resection. The finding of positive tumor margin(s) after definitive resection increased the risk of disease-specific mortality by a factor of 8.0 (P = .043, stratified Cox proportional hazards regression) compared to that of negative margins. The relative risk of tumor recurrence (risk ratio) with positive tumor margins was 4.0, but this did not reach statistical significance (P = .083). This apparent paradox may be associated with the small sample size – a type 2 error due to a lack of statistical power. In this limited case series, a P -value only marginally > .05 associated with a risk ratio of 4.0 suggested that positive margins are likely associated with recurrence. Perineural infiltration, bone invasion, and basaloid histology were not associated with increased risk of any of the endpoints (all P > .05, stratified Cox survival regression) in the way that positive margins were.
DISCUSSION
This report updates the original paper from 2013 on the 19 patients with LGACC managed by a trimodal treatment protocol (neoadjuvant intra-arterial chemotherapy, exenteration, concurrent chemoradiation, and adjuvant intravenous chemotherapy) in a single center by the same surgeon and medical oncologist. Eight additional years is a substantial increase in follow-up time for patients with this disease, primarily covering the critical period when many patients suffer mortality, in which 37% live for 10 years, and essentially none live beyond 15 years. 26 In a study cohort maintaining a 100% follow-up with no dropouts, the median follow-up time for the entire group was 13 years, and for surviving patients, 15 years. All survivors were followed for > 10 years, ranging from 10.9 to 32.4 years – the most extended longitudinal study for any LGACC study cohorts. These findings affirm that integrating neoadjuvant intra-arterial delivery of a high concentration of chemotherapy through an intact lacrimal artery in a coordinated treatment strategy is pivotal in improving disease-free survival and reducing disease relapse for this lethal malignancy. Adherence to all elements of the IACC protocol as designed for patients whose tumor had an intact lacrimal artery (group 1) is vital in achieving significantly better outcomes. This is supported by the demonstration that all-cause mortality (P = .042) and recurrence (P = .017) measures in group 2 patients are inferior to group 1 patients with extended follow-up, accompanied by a note-worthy, though not statistically significant, reduced disease-specific mortality in group 1. Table 2 and Supplementary Figures 1 to 3 present outcomes for the current groups 1 and 2 and 3 historical control comparators that were used in the 2013 report (BPEI historical control) 17 , 25 , along with data from 2 cohorts receiving globe-sparing treatment to be addressed below. 39,40 Group 1 significantly outperformed all 3 historical control comparators for all endpoints for which data were available in the previously published reports. Thus, the presence of an intact lacrimal artery appears to be an integral characteristic of optimal treatment outcomes.
As illustrated in Supplementary Figures 1 to 3, survival endpoint metrics for group 2 are significantly better than BPEI historical bimodal treatment (all P < .05, log-rank test), demonstrating that the integration of chemotherapy into “standard” therapies appears to improve outcomes, even in patients with disrupted lacrimal artery anatomy. In particular, there is a steep initial drop off in the historical bimodal comparator series during the first few years not seen with the IACC groups, suggesting that while the absence of an intact lacrimal artery (group 2) may not prolong survival as in group 1 (Figures 1 and 2 ), inclusion of chemotherapy in the treatment algorithm may delay recurrence and therefore increase survival times, shifting the survival curves to the right. These findings would seem to validate the anatomical and chemotherapeutic rationales underpinning the design of the chemotherapy component of the protocol. The stratification of patients into 2 groups reinforces the benefits of IACC for patients with intact lacrimal arteries who receive the entire course of treatment.
FIGURE 2.
Cause-specific mortality comparison of intra-arterial cytoreductive chemotherapy (IACC) as designed versus protocol deviation. In this analysis, deaths due to causes other than lacrimal gland adenoid cystic carcinoma (LGACC) were censored instead of counted as endpoints. Similar in trend to all-cause mortality, group 1 (IACC as designed) had higher disease-specific survival but was not statistically significantly improved over group 2 (IACC protocol deviation, P = .24). It is essential to note that the lack of a statistically significant difference cannot be equated to a lack of difference in this setting of small sample analyses.
To illustrate the importance of assessing outcomes in the context of the patient group under treatment, the current series was compared with 2 contemporary globe-sparing surgery reports with sufficient raw data to abstract for K-M curve construction: PCSWo 39 and PCSH. 40 PCSWo reports a series of patients treated with proton beam therapy after conservative surgery. 42 The Kaplan-Meier survival curves derived from the 2 reports appear similar to the IACC per-protocol (group 1). However, it is essential to recognize a significant difference in patient selection. In the current population, 17 of 19 patients (89.5%) had ≥ T3 (15 of 19 T4a-c). Of the 10 patients treated by external beam radiation therapy in the Han report (PCSH), 40 there were no patients with T4 and 2 with T3. Likewise, in the Wolkow series (PCSWo), 39 there were no T4 patients, 1 T3 patient, and 17 (94%) < T3. These cohorts represent the opposite spectrum of disease severity. Given these differences in baseline stage with an overwhelming majority of the current patients having locally advanced T4 disease and very few in the other PCS series (no T4), the apparent, comparable outcomes could well be attributed to patient selection.
Another notable difference is identified in follow-up times. In the Han report, 40 the median was 89.5 months. The median time to recurrence was 88.5 months in the current series, with a median follow-up of > 13 years. Since half the patients in the Han series were followed for 89 months (7.4 years), the study would appear to lack the maturity to identify all the potential recurrences in a patient population that tends to relapse late; consider case 13 in the group 2 series who developed disease relapse at 168 months (14 years) following therapy.
In an editorial accompanying the 2013 publication, Bradley and Bradley 26 questioned whether adding neoadjuvant chemotherapy to conventional bimodal therapies in patients with LGACC improves survival. The authors discounted random variability and the potential confounding factor of age as unlikely explanations for the study’s favorable findings. However, they raised the question of tumor size and nodal status in the IACC patients, as tumor staging at presentation has emerged as a determinant of long-term survival in LGACC. 41,42 The current report supplies these data: in group 1, 7 patients (88%) had advanced T4a-c tumors, while in group 2, 10 patients (91%) had ≥ T3 disease, 8 of which (73%) were T4a-c tumors. The disease severity in this population is more advanced than in previously reported studies. In reviewing treatment efficacy of various permutations of conventional therapies, globe-sparing or non-globe-sparing, the current study is the only series with a cohort of 6 patients with an advanced T4a-c tumor achieving disease-free survival of > 15 years - a result unmatched in the literature. Patient safety may be examined in studies with shorter follow-ups, but treatment efficacy can only be studied with long-term follow-ups. Given the outcomes of the established high-risk population reported here with advanced-stage disease and a 15-year cumulative survival proportion of 87.5% in group 1, with a median F/U time-all of 219 months (18.3 years), it is believed that a trimodal strategy improves survival of patients with LGACC.
• TUMOR STAGE – GROUPS 1 AND 2 (TABLE 1):
The intra-arterial chemotherapy delivery was singularly responsible for tumor cytoreduction in contracting the peripheral intracranial tumor margin to within the orbit, such that exenteration achieved tumor margin clearance in 6 of 8 patients in group 1. These data highlight that response to neoadjuvant chemotherapy can produce down-staging of the tumor.
It is believed that optimal management of LGACC requires adherence to all protocol specifications, including rapid cycling of therapy, treatment intensity, and timely exenteration to avoid interval tumor regrowth. 34 More significantly, none had prior bone or tumor mass manipulations, and all had an intact lacrimal artery.
In group 2, at presentation, 3 of 11 patients (27.3%) had stage ≤ T3 disease, while 8 of 11 patients (72.7%) had T4 disease. In addition to extensive disease (T4b-c) at presentation, all 4 patients in group 2 who died of disease (cases 6,8, 11, and 19) had 2 or more risk factors – basaloid histology with perineural or bone infiltration, lateral wall disruption, incomplete primary tumor resection, or positive tumor margins at exenteration. Cases 6 and 19 illustrated the importance of minimizing tumor manipulation and achieving tumor margin clearance at initial tumor resection surgery. Case 6 had lateral wall take-down and incomplete primary tumor resection. Despite undergoing the IACC protocol and achieving tumor margin clearance after 3 IA cycles, the patient developed metastatic lung disease at 50 months. It is unclear whether the metastatic disease had occurred at the time of tumorigenesis or was the result of surgical manipulation in disseminating tumor cells; she survived for > 11 years with the disease. Case 19 represents the possible negative consequence of conservative surgery and the failure of adjuvant radiation for a T2 tumor with basaloid and perineural histologic characteristics. At relapse, the patient presented with a T4c tumor with a large mass in the ipsilateral ethmoid sinus. The recurrent orbital disease was most likely due to incomplete resection, and adjuvant radiation was ineffective in addressing the residual disease in the globe-spared orbit. It was hypothesized that the residual tumor cells most likely gained entrance to the sinus mucosa by retrograde tracking along the lacrimal nerve toward the trunk of the ophthalmic nerve (V 1 ) in the trigeminal ganglion to enter the intracranial compartment. The tumor cells then crossed to the nasociliary nerve, a branch of V 1 in the cavernous sinus, and tracked in an antegrade manner toward the anterior and posterior ethmoidal branches to infiltrate the sinus mucosa, similar to the reported mechanism of tumor cells dissemination in Case 4. 43
Case 8 32 in group 2, along with all 8 patients in group 1 with an intact lacrimal artery, had dramatic radiographic evidence of tumor shrinkage after 2 cycles of IACC through ECA (Table 1 ) – an unequivocal confirmation that the intactness of the lacrimal artery is a sine qua non for the delivery of high-concentration chemotherapy to the tumor. The uniform tumor cytoreduction validates the anastomotic vessel connections of ECA to the lacrimal artery for effective chemotherapy delivery. IACC has less effect in delivering the high concentration of cisplatin to the orbital tumor bed in the absence of an artery after debulking surgery (group 2 patients). Cases 9 and 12 died of diseases unrelated to LGACC; IACC likely prolonged their survival to 120 months (10 years) and 134 months (11.3 years), respectively.
Overall, 1 of 19 patients (5%) with < T3 tumors and 8 of 19 patients (42%) with ≥ T3 tumors developed recurrence. Five of 19 patients (26.3%) with > T3 tumors died of disease, and no-one with < T3 tumors died since the inception of the IACC study period with a maximum follow-up of 32 years.
• POSITIVE TUMOR MARGINS:
Eight of 19 patients (42%) in this study had microscopic positive tumor margins on examining exenteration specimens - 2 in group 1 and 6 in group 2 (Table 1 ). Statistical analysis showed that positive tumor margins increased the risk of all-cause and disease-specific mortality by a factor of 4 and 8, respectively (P < .05, stratified Cox regression analysis). Four patients with positive margins (Table 1 , all with T4 tumors) died of LGACC disease relapse despite receiving chemotherapy with or without an intact lacrimal artery, which raises concerns about patients treated with globe-preserving surgeries with residual disease followed by adjuvant radiotherapy. 43 Case 19 initially had globe-sparing resection of a T2 tumor followed by radiotherapy and recurred as a T4c tumor.
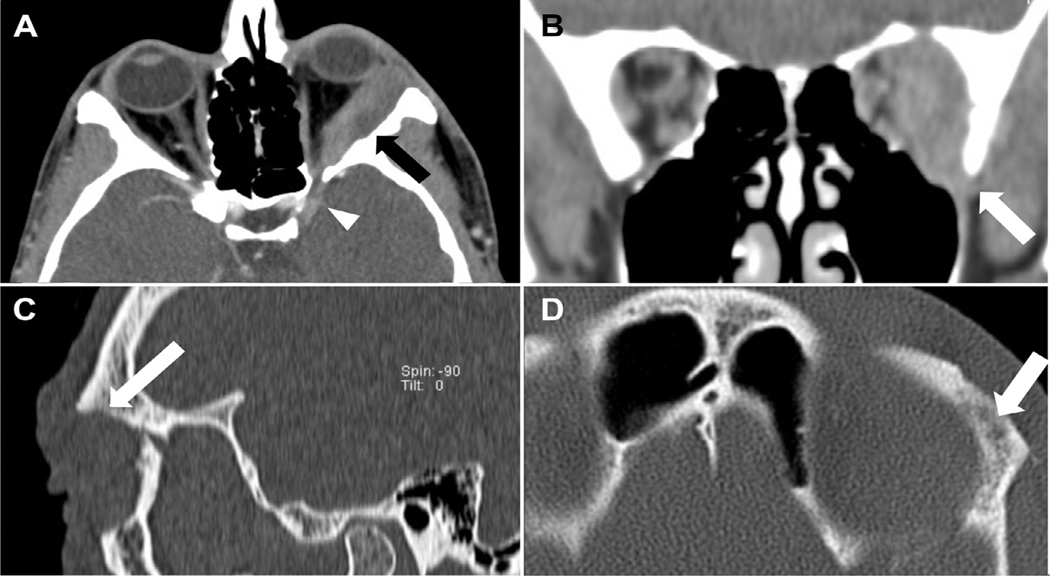
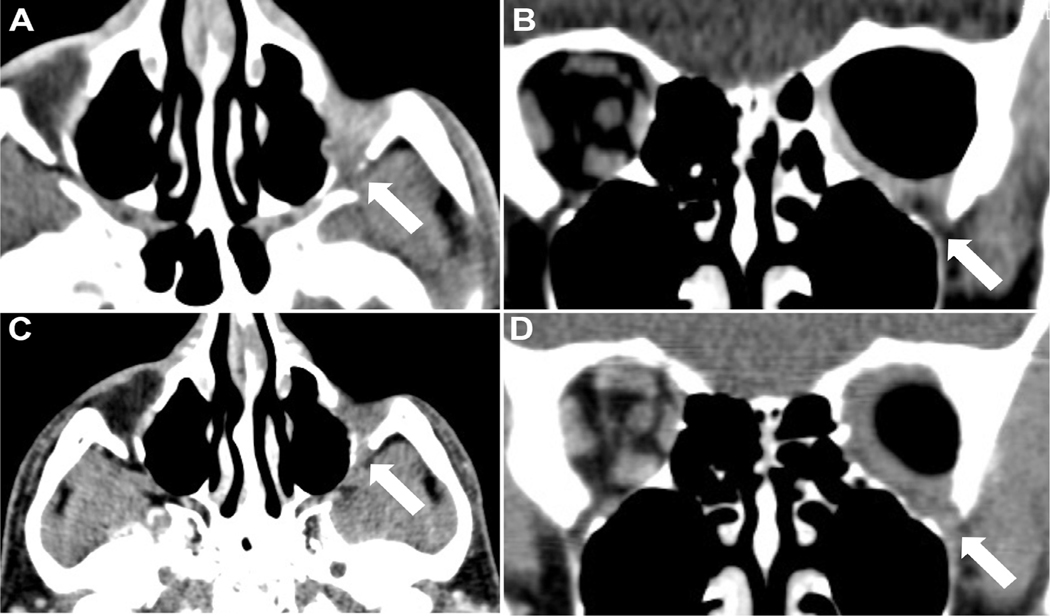
Case 18 illustrates the impact of initially unrecognized intramedullary bone involvement and positive bone tumor margin in a patient with T4c disease and infiltration of the inferior orbital fissure who developed tumor extension to the jaw and metastatic disease to the lungs 74 months (6.2 years) following completion of all elements of the treatment protocol; he is the only patient in group 1 who died of LGACC. Although tumor cytoreduction with 3 cycles of intra-arterial cisplatin was demonstrated on orbital CT scan, multiple lucencies and a permeative pattern involving the intramedullary cavity, subtle signs of deeper tumor infiltration were not initially recognized (Figures 4 and 5 ). 44
FIGURE 4.
Pre-intra-arterial cytoreductive chemotherapy (IACC) treatment tumor appearance of case 18, group 1. A) Axial CT view showing a 3.8×4.6×2.0 cm T4c LGACC tumor of the left orbit. The mass envelops the lateral rectus muscle with extension into the superior orbital fissure. Bony erosions of the lateral orbital wall (arrow) and intracranial linear enhancement (arrowhead) are present. B) Coronal view showing the mass infiltrating the central orbit with extension into the inferior orbital fissure (arrow). Note pitting of the greater wing of the sphenoid bone. C) The sagittal orbital view shows thinning and erosion of the orbital roof (arrow). D) Axial view displaying the permeative pattern of the intramedullary cavity with multiple lucencies (arrow).
FIGURE 5.
Post-intra-arterial cytoreductive chemotherapy (IACC) cytoreductive response and disease relapse of a T4c LGACC. A) There is shrinkage of the tumor mass and retraction of the peripheral tumor margin at the orbital apex and from within the inferior orbital fissure (arrow). B) Contracture of the tumor margin within the inferior orbital fissure after 3 intra-arterial chemotherapy cycles (arrow). C) The patient developed local recurrence in the inferior orbital fissure (arrow) 74 months following completion of all treatment protocol elements. D) Tumor extension to adjacent structures from recurrent lesion within the inferior orbital fissure (arrow).
Microscopic pitting of the greater wing of the sphenoid noted at the time of exenteration was not surgically resected, except for burring the involved cortex due to its proximity to the superior orbital fissure. Tumor cells may have been embedded deep in the Haversian canal system of the medullary bone, rendering it challenging to attain bone tumor margin clearance. This case highlighted the limited effectiveness of penetration depth by radiation or chemotherapy perfusion through bone to address sequestered cancer cells. 9 , 10 Tumor cells harbored within the bone or in the orbital apex soft tissues may have served as a nidus to spawn late locoregional recurrence and hematogenous metastases. Intramedullary bone lucencies accompanied by cortical erosion adjacent to the lacrimal gland mass should heighten concern for disseminating tumor cells at diagnosis. The affected bone is a potential source for treatment failure, and en bloc bone resection should be considered. However, recent data have suggested that cranio-orbital resection with bone removal does not improve patient survival, 19 thus complicating the utility of en bloc bone resection.
In the current intraoperative management approach, if the area of bone involvement is broad and resection is unfeasible or safe, burring the pitted cortical bone is an option. A maneuver mentioned by Rose and associates, 19 though not proven, applies absolute alcohol to bone with microscopic pitting. Instead of bone resection, 2 freeze-thaw cycles of cryotherapy with liquid nitrogen spray are now used after burring to address potential sequestered tumor cells deep within the intramedullary bone. Bone cryosurgery has been well-recognized as a safe and effective limb or joint-sparing surgical treatment for various bone tumors to induce tissue necrosis with ablative intent in obviating the need for resection surgery. 45,46 Only long-term follow-up will determine whether these 2 intraoperative safeguard measures will offer the theoretical advantage in “sterilizing” the concealed tumor cells to minimize late dissemination.
• LATE RELAPSE:
Cases 18 and 13 developed lung metastases at 74 months and 168 months, respectively, after completing all protocol elements as designed. Case 14 developed a lung lesion at 57 months and liver metastases at 184 months. These 3 cases illustrate the clinical reality that despite optimal local disease control, late distant metastases could arise years later as a critical systemic hallmark of this disease. While hematogenous dissemination of tumor cells or seeding into the cranial diploe during debulking surgery may have occurred, 19 cancer cell dormancy is a newer biological insight into disease relapse to consider in LGACC. 47 The incompletely understood cellular dormancy phenomenon appears to mediate through various mechanisms, leading to the down-regulation of Ras/MAPK and PI (3)K/AKT signaling cascades governing cancer cell survival, progression, and treatment resistance. Therapeutic implications of cancer cell dormancy should give rise to the consideration of targeted supplemental adjuvant maintenance therapy guided by the resected tumor’s molecular and genomic profiles. 48
• SURVIVAL DATA REPORTING:
Due to the small number of patients having this uncommon tumor with slow growth, extended follow-up is required to assess treatment efficacy. With conventional treatments 17 , 25 substantial mortality may occur within 5 years of therapy, allowing outcome comparisons with statistical survival methods within this time-frame. However, with IACC and newer eye-preservation proton or photon beam modalities, 43 substantial mortality does not appear until 10 or even 20 years after treatment. This information suggests follow-up until death or survival ≥15 years should be considered a requirement for assessing outcomes of these newer modalities.
The inclusion of median time followed for surviving patients is further recommend to describe treatment efficacy and identify the maturity of the patient cohort. Because of improved efficacy with new therapies manifesting longer times until recurrence or death, a critical bar of 15 years of follow-up is suggested for assessing the effectiveness of current conventional and future globe-sparing treatments. Notwithstanding, concerning safety data on new therapies such as early deaths or complications would be reported with short follow-up, as appropriate. Following conventions of recent papers, 32,39,40 survival and recurrence data should be summarized with follow-up-specific cumulative proportions illustrated in Kaplan-Meier curves.
Most studies on survival outcomes of various treatments on LGACC have short-term follow-ups and a lack of completeness of raw data. None of the reported case series provided additional follow-up information on their initially reported cohort of patients. This report is the only long-term study assessing a single unified protocol’s therapeutic efficacy on the same cohort of patients reported earlier. It would be helpful for authors of previous publications to do likewise. For this rare disease, where single centers have difficulty accumulating sufficient cases from which to assess the impact of risk factors and treatments, the publication of raw data sets is encouraged so that others can make use of them to test future hypotheses. The current study’s more comprehensive patient survival data seems to validate the trimodal protocol treatment design. This study’s favorable long-term therapeutic efficacy findings may form the foundation for integrating neoadjuvant intra-arterial chemotherapy as the core element to reduce the tumor mass and to improve resection margin clearance in a future trimodal globe-preservation treatment strategy.
• LIMITATIONS:
The authors acknowledge that the limitations of this study are the small sample size, retrospective review, and lack of randomization. The absence of balanced comparison groups precluded inferences of causality and limited statistical adjustment for potential confounding. In this cohort, most patients were T4, with 4 ≤ T3 tumors. Some patients had previous surgeries by referring surgeons, which was the reason for the disruption of the lacrimal artery and stratification into group 2 - a post hoc analysis. These variables need to be considered in assessing survival and recurrence, but the small sample size makes multivariable statistical approaches both underpowered and susceptible to over-fitting. However, this was a consecutive series of patients with LGACC, and an alternate treatment strategy for lesions with a lesser tumor burden at presentation was not selected.
In this rare disease with late-onset mortality endpoints, the role of a randomized trial in assessing treatment efficacy is limited, unless a consortium of investigators is available and committed to more than a decade of recruitment and follow-up. Post hoc analyses of case series are characteristically performed to mine data for possible insights; alternatively, if substantial progress is made in identifying a surrogate outcome highly associated with the mortality end-point, that parameter could be ascertained after a few years of follow-up, which could lead to the design of a manageable short-term trial.
To conclude, this report describes the outcome of a high-risk and advanced tumor stage population of patients with LGACC treated by a trimodal therapy with neoadjuvant intra-arterial chemotherapy as the core element of the strategy. The strengths of this study are the study duration, follow-up rate, in-depth data analysis incorporating AJCC TNM tumor staging, and comparison of survival outcomes. The report documents lengthy follow-up data to a cumulative duration of 15 years associated with excellent survival outcomes, which are attributed to the integration of neoadjuvant intra-arterial and adjuvant intravenous chemotherapy as the third arm of the trimodal strategy. An intact lacrimal artery appears central to IACC delivery for an optimal survival outcome. An incisional biopsy, not resection, should be performed in patients presenting with a lacrimal gland mass to plan appropriate therapy. Bone take-down and tumor debulking resulting in disruption of the lacrimal artery portend an unfavorable outcome. Tumor cytoreduction with cytotoxic chemotherapy improves resectability and attainment of negative resection margins. Survival for those patients who relapse appears better than comparable patients reported in literature without chemotherapy integrated into the treatment regimen. Future directions include molecular studies to identify biomarkers for possible actionable targets. The authors believe that these results strongly support the hypothesis that chemotherapy improves outcomes and form the basis of hypothesis building to establish future improved treatment.
Supplementary Material
Funding/Support:
This work was supported in part by NIH Center Core Gr ant P30EY014801; Research to Prevent Blindness Unrestricted Grant, Inc. New York, New York GR004596; and the Dr. Nasser Ibrahim Al-Rashid Orbital Vision Research Fund. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Financial Disclosures : No conflicting relationship exists for any author. All authors attest that they meet the current ICMJE criteria for authorship.
Credit author statement : D.T.T.: Conceptualization, methodology, formal analysis, investigation, data curation, validation, writing original draft, writing-review and editing, visualization, project administration, supervision, resources, and funding acquisition. P.W.B.: Conceptualization, methodology, writing - review and editing, and formal analysis. B.C.T.: Methodology, formal analysis, investigation, data curation, writing-review and editing, and project administration. W.J.F.: Methodology, formal analysis, investigation, data curation, and writing review and editing.
REFERENCES
- 1.Lee DA, Campbell RJ, Waller RR, Ilstrup DM. A clinicopathologic study of primary adenoid cystic carcinoma of the lacrimal gland. Ophthalmology. 1985;92(1):128–134 . [DOI] [PubMed] [Google Scholar]
- 2.Font RL, Smith SL, Bryan RG. Malignant epithelial tumors of the lacrimal gland: a clinicopathologic study of 21 cases. Arch Ophthalmol. 1998;116:613–616 . [DOI] [PubMed] [Google Scholar]
- 3.Font RL, Gamel JW. Epithelial tumors of the lacrimal gland: an analysis of 265 cases. In: Jakobiec FA, ed. Ocular and Adnexal Tumors. Birmingham, Alabama: Aesculapius Publishing Co.; 1978:787–805 . [Google Scholar]
- 4.Font RL, Gamel JW. Adenoid cystic carcinoma of the lacrimal gland A clinicopathologic study of 79 cases. In: Nicholson DH, ed. Ocular Pathology. Update . New York: Masson Publishing; 1980:277–283 . [Google Scholar]
- 5.Gamel JW, Font RL. Adenoid cystic carcinoma of the lacrimal gland: the clinical significance of a basaloid histologic pattern. Hum Pathol. 1982;13(3):219–225 . [DOI] [PubMed] [Google Scholar]
- 6.Wright JE, Stewart WB, Krohel GB. Clinical presentation and management of lacrimal gland tumours. Br J Ophthalmol. 1979;63(9):600606 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byers RM, Berkeley RG, Luna M, Jesse RH. Combined therapeutic approach to malignant lacrimal gland tumors. Am J Ophthalmol. 1975;79(1):53–55 . [DOI] [PubMed] [Google Scholar]
- 8.Wright JE. Factors affecting the survival of patients with lacrimal gland tumours. Can J Ophthalmol. 1982;17(1): 3–9 . [PubMed] [Google Scholar]
- 9.Henderson JW. Past, present, and future surgical management of malignant epithelial neoplasms of the lacrimal gland. Br J Ophthalmol. 1986;70:727–731 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naugle T Jr, Tepper DJ, Haik BG. Adenoid cystic carcinoma of the lacrimal gland: a case report. Ophthalmic Plast Reconstr Surg. 1994;10:45–48 . [DOI] [PubMed] [Google Scholar]
- 11.Ni C, Cheng SC, Dryja TP, Cheng TY. Lacrimal gland tumors: a clinicopathological analysis of 160 cases. Int Ophthalmol Clin. 1982;22:99–120 . [DOI] [PubMed] [Google Scholar]
- 12.Byers RM, Berkeley RG, Luna M, Jesse RH. Combined therapeutic approach to malignant lacrimal gland tumors. Am J Ophthalmol. 1975;79:53–55 . [DOI] [PubMed] [Google Scholar]
- 13.Henderson JW, Neault RW. En bloc removal of intrinsic neoplasms of the lacrimal gland. Am J Ophthalmol. 1976;82:905–909 . [DOI] [PubMed] [Google Scholar]
- 14.Rootman J, Lapointe JS. Tumors of the lacrimal gland. In: Rootman J, ed. Diseases of the Orbit; A Multidisciplinary Approach. Philadelphia: JB Lippincott; 1988:384–395 . [Google Scholar]
- 15.Marsh JL, Wise DM, Smith M, Schwartz H. Lacrimal gland adenoid cystic carcinoma: intracranial and extracranial enbloc resection. Plast Reconstr Surg. 1981;68:577–585 . [DOI] [PubMed] [Google Scholar]
- 16.Janecka I, Housepian E, Trokel S, et al. Surgical management of malignant tumors of the lacrimal gland. Am J Surg. 1984;148:539–541 . [DOI] [PubMed] [Google Scholar]
- 17.Esmaeli B, Ahmadi MA, Youssef A, et al. Outcomes in patients with adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg. 2004;20:22–26 . [DOI] [PubMed] [Google Scholar]
- 18.Esmaeli B, Golio D, Kies M, DeMonte F. Surgical management of locally advanced adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg. 2006;22(5):366–370 . [DOI] [PubMed] [Google Scholar]
- 19.Rose GE, Gore SK, Plowman NP. Cranio-orbital resection does not appear to improve survival of patients with lacrimal gland carcinoma. Ophthal Plast Reconstr Surg. 2019;35:77–84 . [DOI] [PubMed] [Google Scholar]
- 20.Henderson JW. Adenoid cystic carcinoma of the lacrimal gland, is there a cure? Trans Am Ophthalmol Soc. 1987;85:312–314 discussion 314–319 . [PMC free article] [PubMed] [Google Scholar]
- 21.Jakobiec FA. Discussion of Henderson JW. Adenoid cystic carcinoma of the lacrimal gland: is there a cure? Trans Am Ophthalmol Soc. 1987;85:314–317 . [PMC free article] [PubMed] [Google Scholar]
- 22.Polito E, Leccisotti A. Epithelial malignancies of the lacrimal gland: survival rates after extensive and conservative therapy. Ann Ophthalmol. 1993;25:422–426 . [PubMed] [Google Scholar]
- 23.Ashton N. Epithelial tumours of the lacrimal gland. Mod Probl Ophthalmol. 1975;14:306–323 . [PubMed] [Google Scholar]
- 24.Zimmerman LE, Sanders TE, Ackerman LV. Epithelial tumors of the lacrimal gland: prognostic and therapeutic significance of histologic types. Int Ophthalmol Clin. 1962;2:337–367 . [Google Scholar]
- 25.Wright JE, Rose GE, Garner A. Primary malignant neoplasms of the lacrimal gland. Br J Ophthalmol. 1992;76:401–407 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley EA, Bradley DJ. Adenoid Cystic Carcinoma of the Lacrimal Gland: Rare . . . Lethal . . . Cured? Ophthalmology. 2022;120:1311–1312 . [DOI] [PubMed] [Google Scholar]
- 27.Meldrum ML, Tse DT, Benedetto P. Neoadjuvant intracarotid chemotherapy for treatment of advanced adenocystic carcinoma of the lacrimal gland. Arch Ophthalmol. 1998;116:315–321 . [DOI] [PubMed] [Google Scholar]
- 28.Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883 . [DOI] [PubMed] [Google Scholar]
- 29.Chaney SG, Sancar A. DNA repair: enzymatic mechanisms and relevance to drug response. J Natl Cancer Inst. 1996;88:1346–1360 . [DOI] [PubMed] [Google Scholar]
- 30.Furuta T, Ueda T, Aune G, Sarasin A, Kraemer KH, Pommier Y. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res. 2002;62:4899–4902 . [PubMed] [Google Scholar]
- 31.Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–316 . [DOI] [PubMed] [Google Scholar]
- 32.Tse DT, Kossler AL, Feuer WF, Benedetto PW. Long-term outcomes of neoadjuvant intra-arterial cytoreductive chemotherapy for lacrimal gland adenoid cystic carcinoma. Ophthalmology. 2013;120:1313–1323 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tse DT, Benedetto P, Dubovy S, Schiffman JC, Feuer WJ. Clinical analysis of the effect of intraarterial cytoreductive chemotherapy in the treatment of lacrimal gland adenoid cystic carcinoma. Am J Ophthalmol. 2006;141(1):44–53 . [DOI] [PubMed] [Google Scholar]
- 34.Tse DT, Kossler AL, Feuer WJ, Benedetto PW. Author reply: To PMID 23582989. Ophthalmology. 2014;121(1):e8–e10 . [DOI] [PubMed] [Google Scholar]
- 35.Edge SB, Byrd DR, Compton CC, et al. . AJCC Cancer Staging Manual. New York: Springer; 2009:569–571 . [Google Scholar]
- 36.Penston J. Should we use total mortality rather than cancer-specific mortality to judge cancer screening programmes? Yes. BMJ. 2011;343:1008–d6395. doi: 10.1136/BMJ.d6395 . [DOI] [PubMed] [Google Scholar]
- 37.Steele RJC, Brewster DH. Should we use total mortality rather than cancer-specific mortality to judge cancer screening programmes? BMJ. 2011;343:2091–d6397. doi: 10.1136/BMJ.d6397 . [DOI] [PubMed] [Google Scholar]
- 38.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolkow N, Jakobiec FA, Lee H, Sutula FC. Long-term outcomes of globe-preserving surgery with proton beam radiation for adenoid cystic carcinoma of the lacrimal gland. Am J Ophthalmol. 2018;195:43–62 . [DOI] [PubMed] [Google Scholar]
- 40.Han J, Kim YD, Woo K, Sobti D. Long-term outcomes of eye-sparing surgery for adenoid cystic carcinoma of lacrimal gland, ophthalmic plastic and reconstructive surgery. Ophthalmic Plast Reconstructr Surg. 2018;34(1):74–78 . [DOI] [PubMed] [Google Scholar]
- 41.Li N, Xu L, Zhao H, et al. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer. 2012;118:3945–3953 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad SM, Esmaeli B, Williams M, et al. American Joint Committee on Cancer classification predicts outcome of patients with lacrimal-gland adenoid cystic carcinoma. Ophthalmology. 2009;116:1210–1215 . [DOI] [PubMed] [Google Scholar]
- 43.Tse DT, Benedetto P, Morcos JJ, Johnson TE, Weed D, Dubovy S. An atypical presentation of adenoid cystic carcinoma of the lacrimal gland. Am J Ophthalmol. 2006;141:187–189 . [DOI] [PubMed] [Google Scholar]
- 44.Hanrahan CJ, Shah LM. MRI of spinal bone marrow: Part 2, T1-weighted imaging-based differential diagnosis. AJR. 2011;197:1309–1321 . [DOI] [PubMed] [Google Scholar]
- 45.Meller I, Weinbroum A, Bickels S, et al. Fifteen years of bone cryosurgery: A single-center experience of 440 procedures and long-term follow-up. Eur J Surg Oncol. 2008;34: 921–927 . [DOI] [PubMed] [Google Scholar]
- 46.Veth R, Schreuder B, van Beem H, et al. Cryosurgery in aggressive, benign, and low-grade malignant bone tumours. Lancet Oncol. 2005;6:25–34 . [DOI] [PubMed] [Google Scholar]
- 47.Yeh AC, Ramaswamy S. Mechanisms of cancer cell dormancy – another hallmark of cancer? Cancer Res. 2015;75(23):5014–5022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doddapaneni R, Tao W, Naranjo A, Nikpoor N, Tse DT, Pelaez D. Fibroblast growth factor receptor 1 (FGFR1) as a therapeutic target in adenoid cystic carcinoma of the lacrimal gland. Oncotarget. 2019;10(4):480–493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.