Abstract
Aims
The aim of this study was to determine the risk of tibial eminence avulsion intraoperatively for bi-unicondylar knee arthroplasty (Bi-UKA), with consideration of the effect of implant positioning, overstuffing, and sex, compared to the risk for isolated medial unicondylar knee arthroplasty (UKA-M) and bicruciate-retaining total knee arthroplasty (BCR-TKA).
Methods
Two experimentally validated finite element models of tibia were implanted with UKA-M, Bi-UKA, and BCR-TKA. Intraoperative loads were applied through the condyles, anterior cruciate ligament (ACL), medial collateral ligament (MCL), and lateral collateral ligament (LCL), and the risk of fracture (ROF) was evaluated in the spine as the ratio of the 95th percentile maximum principal elastic strains over the tensile yield strain of proximal tibial bone.
Results
Peak tensile strains occurred on the anterior portion of the medial sagittal cut in all simulations. Lateral translation of the medial implant in Bi-UKA had the largest increase in ROF of any of the implant positions (43%). Overstuffing the joint by 2 mm had a much larger effect, resulting in a six-fold increase in ROF. Bi-UKA had ~10% increased ROF compared to UKA-M for both the male and female models, although the smaller, less dense female model had a 1.4 times greater ROF compared to the male model. Removal of anterior bone akin to BCR-TKA doubled ROF compared to Bi-UKA.
Conclusion
Tibial eminence avulsion fracture has a similar risk associated with Bi-UKA to UKA-M. The risk is higher for smaller and less dense tibiae. To minimize risk, it is most important to avoid overstuffing the joint, followed by correctly positioning the medial implant, taking care not to narrow the bone island anteriorly.
Cite this article: Bone Joint Res 2022;11(8):575–584.
Keywords: Bi-unicondylar, Combined partial knee arthroplasty, Avulsion fracture, Finite element, unicondylar knee arthroplasty, avulsion fractures, tibial eminence, UKA, strained, anterior cruciate ligament (ACL), tibial bone, total knee arthroplasty (TKA), lateral collateral ligament (LCL), medial collateral ligament (MCL)
Article focus
Finite element analysis assessing the risk of tibial eminence avulsion fracture during bi-unicondylar knee arthroplasty (Bi-UKA).
Effect of overstuffing, variations to surgical cuts, and bony anatomy were investigated.
Comparisons to avulsion risk in isolated medial unicondylar knee arthroplasty (UKA-M) and bicruciate-retaining total knee arthroplasty contextualize results.
Key messages
There is a similar risk of tibial eminence avulsion fracture in Bi-UKA as in UKA-M. Risk is increased in smaller and less dense tibiae.
Overstuffing the joint and cuts narrowing the bone island anteriorly should be avoided to reduce avulsion fracture risk.
Strengths and limitations
The strengths of the study are the use of multiple specimen-specific experimentally validated tibia models, and a wide range of scenarios were investigated.
The limitations of this study are that fracture propagation was not modelled, and the effect of relative femoral translation on anterior cruciate ligament (ACL) strain was not included.
Introduction
Knee osteoarthritis (OA) is a debilitating ailment that affects 16% of the global population. 1-3 One-third of people with knee OA have damage limited to just two compartments of the knee, 4 and as such there has been a renewed interest in combined partial knee arthroplasty (CPKA) to treat knee OA in a limited manner. 5-14 In particular, bi-unicondylar arthroplasty (Bi-UKA) is of interest for patients with a healthy patellofemoral compartment and functional cruciate ligaments, and either ipsilateral medial and lateral tibiofemoral OA, 4,5,11,12 or a well-functioning unicondylar arthroplasty (UKA) with subsequent degeneration in the other tibiofemoral compartment. 13
Recent evidence suggests that Bi-UKA patients have superior outcomes and biomechanics compared to those with a similar disease pattern but treated with total knee arthroplasty (TKA). 11-13,15 However, a potential barrier to the wider use of this procedure is a perceived risk of compromising the anterior cruciate ligament (ACL) if the seemingly narrow bone island between the two tibial components fractures under ACL tension. Indeed, some authors have suggested that intraoperative tibial eminence avulsion fracture may occur in 4% to 9% of Bi-UKAs. 16,17 Bicruciate-retaining total knee arthroplasty (BCR-TKA) leaves behind a similar island to which the cruciates attach, although with bone also removed anteriorly. Intraoperative tibial island fractures with BCR-TKA also occurred at a rate of 9% in development. 18 There is little information available to delineate which factors increase or decrease the risk of ACL avulsion.
Finite element (FE) analysis has proved a valuable tool for assessing fracture risk, 19,20 bone mechanics, 21-24 joint kinematics, 25,26 and ligament mechanics 27 following unicompartmental arthroplasty. Therefore, the aim of this study was to investigate the risk of tibial eminence avulsion following Bi-UKA using FE analysis to determine the effects of implant positioning, overstuffing, and bony anatomy. To contextualize the findings, data were compared to tibiae implanted with isolated medial unicompartmental knee arthroplasty (UKA-M) and a bridged implant akin to BCR-TKA.
Methods
Bone model preparation
Two (one male, one female) experimentally validated specimen-specific FE models of right-sided proximal tibiae cut at mid-shaft were analyzed (Table I). 28 Bone volume meshes were created in 3-matic (Materialise NV, Belgium) with ten-noded tetrahedral elements of 2.4 mm global edge-length. The meshes were further refined locally at the resected bone surfaces to 0.95 mm edge-length, with a growth rate of 10% from these surfaces, to achieve mesh convergence within 5%. The bones were anatomically aligned with lateral x-axis, anterior y-axis, proximal z-axis, and origin at the midpoint between the condylar centres. 29 Distal cortical bone was modelled with type 127 ten-noded tetrahedral elements in MARC (MSC Software Corporation, USA). Proximally, the thin tibial cortex was modelled with type 22 one-side collapsed quadratic quadrilateral 0.2 mm thick shell elements to reduce partial volume effects arising from the bone material allocation method.
Table I.
Bone model characteristics.
| Model | Age, yrs | Height, cm | Weight, kg | Mean bone density, gcm-3 | Bone volume, cm3 | Medial implant size | Lateral implant size |
|---|---|---|---|---|---|---|---|
| Male | 65 | 183 | 64 | 0.344 | 199 | C | E |
| Female | 81 | 152 | 91 | 0.296 | 154 | B | D |
The tibiae were CT-scanned (Definition AS+, Siemens, Germany) with slice thickness of 0.6 mm, and cross-section voxels 0.5 × 0.5 mm, and a calibration phantom. Cancellous bone material properties were applied heterogeneously based on empirical measures relating Hounsfield units (HU) to density and elastic moduli from quantitative CT of each specimen using Mimics (Materialise NV). 20 All other materials were homogeneous, isotropic, and linear elastic (Table II). The stiffness of the thin cortex covering the proximal tibia (which is thinner than the resolution of the scan) was captured by applying shell elements proximally, which were assigned homogeneous material properties of 18 GPa (large, dense, male tibia) or 14 GPa (smaller, less denser, female tibia).
Table II.
Material properties and mesh size.
| Material | Density, (ρ gcm-3) | Elastic Modulus, E (MPa) | Poisson’s ratio | No. elements |
|---|---|---|---|---|
| Cancellous | ρ = 0.04 + 0.00092 HU | 0.3 | 685,667 | |
| Cortical | N/A | 18,000 | 0.33 | 46,841 |
| Cortex | 18,000 | 0.33 | 4,595 | |
| CoCr implant | 210,000 | 0.33 | 39,589 | |
| PE bearing | 600 | 0.3 | 32,258 | |
| PMMA cement | 1800 | 0.33 | 48,496 |
CoCr, cobalt-chromium; HU, Hounsfield unit; N/A, not applicable; PE, polyethylene; PMMA, polymethyl methacrylate.
Implants
The Oxford medial and fixed lateral UKA implants were used (Zimmer Biomet, USA). For both UKA-M and Bi-UKA, implants were sized (Table I) and positioned using surgical planning software (Embody Orthopaedic, UK), following the senior surgical author’s clinical practise (JPC). All interfaces were glued, with sensitivity studies confirming that this contact condition was acceptable for the static load cases applied.
Boundary conditions and loading
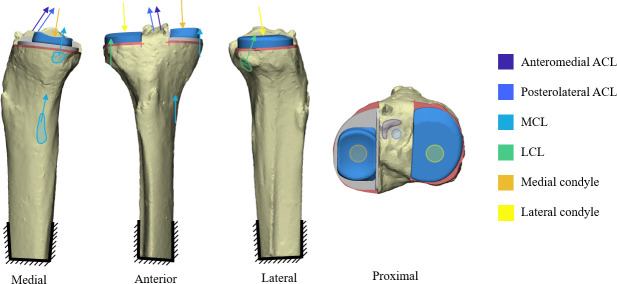
Tibiae were fixed distally and intraoperative loading at full extension was simulated, as the ACL is most strained in extension. 30 Sensitivity studies determined that force contributions from the ACL, medial collateral ligament (MCL), and lateral collateral ligament (LCL) should be simulated, with the corresponding joint reaction force. No single source could provide all data needed; loads were estimated from clinical data, intraoperative/in vitro measurements, and calculations (Table III). 30-35 Two loadcases (Figure 1) were investigated: 1) baseline loading for a balanced knee that replicated native knee ligament strain; and 2) an overstuffed knee. The latter was estimated by calculating the additional strain and load that would occur if 2 mm oversized meniscal bearings were used (Table III). As both load cases were intraoperative, no muscle forces were simulated.
Table III.
Boundary conditions and sources.
| Boundary conditions | Balanced knee | Overstuffed by 2 mm | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Magnitude, N | Direction | Magnitude, N | Direction | ||||||
| i | j | k | i | j | k | ||||
| AM bundle ACL | 38.0 | 0.16 | -0.55 | 0.82 | 176.4 | 0.15 | -0.53 | 0.84 | 30,32,35 |
| PL bundle ACL | 31.0 | 0.17 | -0.56 | 0.81 | 216.4 | 0.16 | -0.53 | 0.83 | 30,32,35 |
| MCL | 120.8 | 0 | -0.19 | 0.98 | 304.0 | 0 | -0.18 | 0.98 | 31,33-35 |
| LCL | 68.6 | 0 | 0-33 | 0.95 | 175.5 | 0 | 0.31 | 0.95 | 31,33-35 |
| Medial condyle | 145.9 | -0.047 | 0.16 | -0.99 | 493.2 | -0.076 | 0.25 | -0.96 | 31,35 |
| Lateral condyle | 97.3 | -0.047 | 0.16 | -0.99 | 328.8 | -0.076 | 0.25 | -0.96 | 31,35 |
ACL, anterior cruciate ligament; AM, anteromedial; LCL, lateral collateral ligament; MCL, medial collateral ligament; PL, posterolateral.
Fig. 1.
Bi-unicondylar knee arthroplasty baseline with representation of loading. ACL, anterior cruciate ligament; LCL, lateral collateral ligament; MCL, medial collateral ligament.
The effects of positioning
The typical variation associated with use of manual surgical instruments (Table IV) 36,37 was simulated. Either the medial implant was moved by two standard deviations (SD), the lateral implant by two SD, or both the medial and lateral implant moved by one SD simultaneously. Deviations that would make the bone island narrower and taller (and hence may increase risk of avulsion) were simulated. Bone overcuts were simulated, based on typical variation for manual instruments: 2 mm transversely, and 2.4 mm sagittally. 38 The overcuts were 1 mm wide, 19 corresponding to the width of sawblades used in surgery, and ran parallel to each implant.
Table IV.
Surgical errors simulated, measured from the baseline, planned positions.
| Error type | Medial (2 SD) | Lateral (2 SD) | Compound (1 SD each) | |
|---|---|---|---|---|
| Medial | Lateral | |||
| Translations, mm | ||||
| Lateral | +2.9 | -5.5 | +2.2 | -3.5 |
| Proximal | -2.6 | -2.6* | -0.6 | -0.6* |
| Rotations, ° | ||||
| Flexion | -11.4 | -7.5 | -8.1 | -4.5 |
| Varus | +1.1 | +2.3 | +2.05 | +0.3 |
| Internal | -7.4 | +7.4 | -1.7 | +0.4 |
Duplicated error from UKA-M reference, as no error acting in the detrimental direction measured for UKA-L was available.
SD, standard deviation; UKA-L, isolated lateral unicondylar knee arthroplasty; UKA-M, isolated medial unicondylar knee arthroplasty.
Comparison to UKA-M and the effects of anterior bone cuts
The risk of fracture (ROF) following Bi-UKA was compared to other ACL-preserving arthroplasty procedures: isolated UKA-M, and a bridged implant akin to a BCR-TKA. For the UKA-M analyses, lateral condylar loading was distributed to simulate a healthy meniscus. Implant positioning was again varied by two SD of typical clinical variation (Table IV). 36,37 The BCR-TKA analyses focused on the effects of the anterior bone removal as this is a key difference between Bi-UKA and BCR-TKA. To isolate the effects of anterior bone cuts from other variations in tray footprint specific to the implant manufacturer, a pseudo BCR-TKA implant was created by bridging the UKA-M and isolated lateral unicondylar knee arthroplasty (UKA-L) in their surgeon-planned positions. Two bridge sizes were analyzed, requiring removal of either 5 or 10 mm of anterior bone. A 7 mm radius was applied to the bridge to eliminate stress-concentrating sharp corners.
Data analysis
Strains were observed qualitatively throughout the model and peak strains quantified. The 95th and 99th percentile maximum principal elastic strains (MPES) were quantified in the region of interest. For the Bi-UKA implant positioning analyses, this region included the bone island and adjacent cuts: it extended 10 mm distal to the transverse bone cuts, 5 mm medially/laterally of the medial/lateral sagittal cuts, respectively, and included all bone elements in the anteroposterior (AP) direction. These analyses found that in all cases the strain was largest around the medial implant bone cuts. Thus, when comparing Bi-UKA and UKA-M the region of interest was narrowed to capture only the medial half of the spine providing two benefits: 1) a more focused region of interest; and 2) a similar number of nodes for the Bi-UKA and UKA-M models in the region of interest; an important factor when comparing 95th and 99th percentile strains.
Bone failure is related to a yield strain criterion; 39 in proximal tibial bone, tensile yield strain has been measured to be + 6500µε. 40 Hence, ROF 19,41 was calculated as follows:
ROFs are reported in the main text, with MPES-95, MPES-99, and peak strains tabulated in the Supplementary Material.
Sensitivity analyses
The sensitivity of the model to key modelling decisions was evaluated: firstly the sensitivity to material assignment, and secondly the effects of ACL load distribution.
Results
In all cases simulated, the highest tensile strains occurred on the anterior portion of the face of the medial sagittal cut, just proximal to the junction between the medial sagittal and transverse cuts, and distal to the ACL attachment (Figure 2). Strain concentrations were also observed on the anterior portion of the face of the lateral sagittal cut, but these were 44% to 84% lower than that on the medial cut.
Fig. 2.
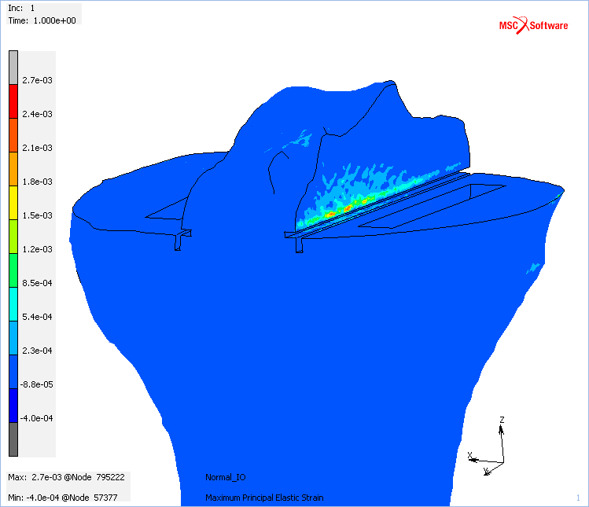
Maximum principal elastic strain in the male baseline bi-unicondylar knee arthroplasty model for the balanced loadcase. Blue indicates low/compressive strains and red indicates the highest predicted tensile strains.
Effect of surgical positioning
For all variations in surgical positioning, except varus/valgus which had negligible effect, a 2 SD variation in positioning the medial implant had a greater effect on ROF than a 2 SD variation in positioning the lateral implant in Bi-UKA (Figure 3). Varying the position of the lateral implant alone had little effect on ROF for all cases except medial translation, which resulted in a 21% increase in ROF. In general, variation that narrowed the bone island medially and anteriorly had the largest effect: lateral translation of the UKA-M in isolation, or combined with medial translation of the UKA-L, had the greatest effect, increasing ROF by 43%. The next largest effect was external rotation of the UKA-M, increasing ROF by 22%. Distal translation of the UKA-M increased ROF by 12%. All other variations resulted in changes in ROF < 10%.
Fig. 3.
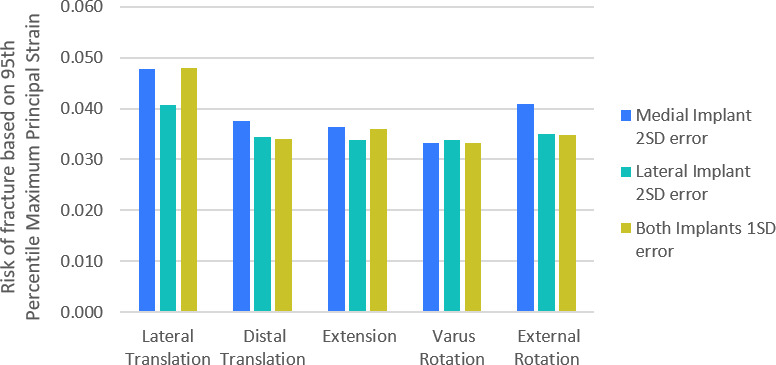
Bar graph showing the percentage difference in risk of fracture as the positions of the isolated medial unicondylar knee arthroplasty (UKA-M) and isolated lateral unicondylar knee arthroplasty (UKA-L) components were varied from the planned bi-unicondylar knee arthroplasty based on the typical accuracy achieved with manual instruments. A 2 standard deviation (SD) variation of the UKA-M implant only (blue), 2 SD variation in the UKA-L only (green), and 1 SD variation of both implants (yellow) are shown. In all cases the variation acts to narrow the bone island and/or make it taller.
Effect of overstuffing
Overstuffing the joint with bearings two sizes larger resulted in ~600% increases in ROF regardless of implant positioning. The worst case was the combination of overstuffing with lateral translation of the UKA-M (Figure 4).
Fig. 4.
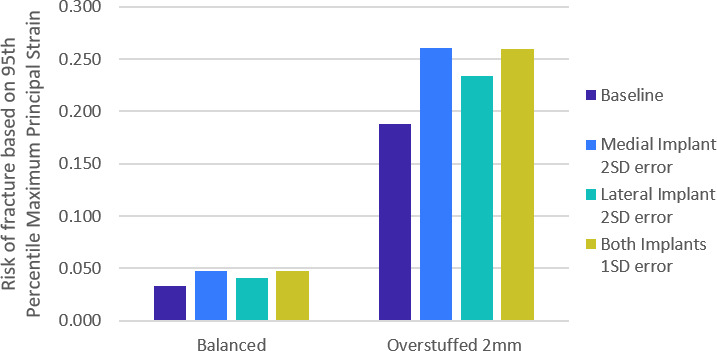
Bar graph showing the risk of fracture in bi-unicondylar knee arthroplasty (Bi-UKA) when the knee is balanced, and overstuffed by 2 mm. Dark blue bars show risk of fracture with Bi-UKA in the planned positions, while the variations displayed are of mediolateral translation of each implant. This is representative of all the directions of surgical variations.
Sensitivity to anatomy
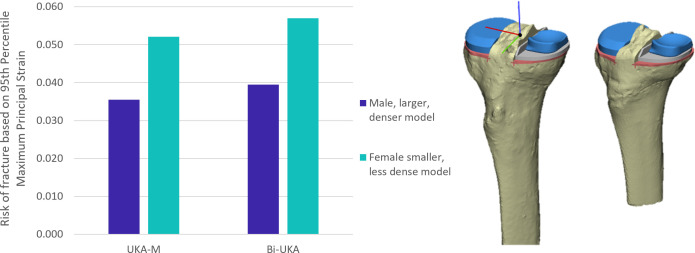
Following Bi-UKA, the ROF predicted for the smaller, less dense female model was 43% higher than the male model (Figure 5).
Fig. 5.
Bar graph showing the risk of fracture in isolated medial unicondylar knee arthroplasty (UKA-M) and bi-unicondylar knee arthroplasty (Bi-UKA) when the knee is balanced in the male (dark blue) and female (turquoise) tibiae.
Comparison to UKA-M and the effect of anterior bone cuts for BCR-TKA
For both the male and female tibiae, ROF in Bi-UKA was predicted to be 9% to 13% higher than in UKA-M for the planned positions and all variations simulated (Figure 5 and Figure 6). Anterior bone cuts for BCR-TKA increased ROF (Figure 7): 55% for a 5 mm cut, and 105% for a 10 mm cut.
Fig. 6.
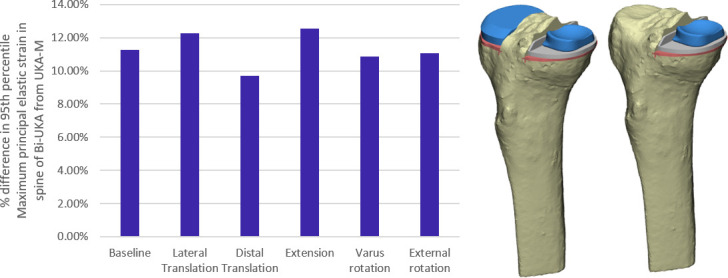
Bar graph showing the percentage difference in maximum principal elastic strain (MPES)-95 measured in the medial portion of the spine of bi-unicondylar knee arthroplasty (Bi-UKA) from isolated medial unicondylar knee arthroplasty (UKA-M), when the implants are positioned as planned, and with typical surgical variation applied to the medial implant.
Fig. 7.
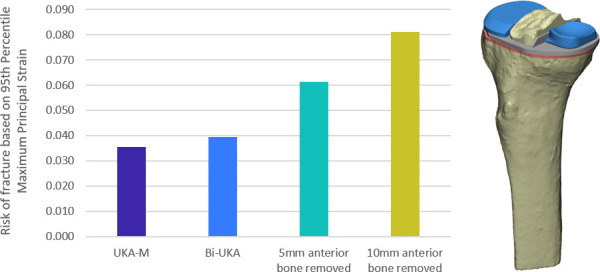
Bar graph showing the risk of fracture in isolated medial unicondylar knee arthroplasty (UKA-M) and bi-unicondylar knee arthroplasty (Bi-UKA) when the knee is balanced, and when 5 mm and 10 mm of bone is removed anteriorly.
Sensitivity analyses
The conclusions drawn from the model were found to be insensitive to the material property assignment, and choice of ACL load distribution (Supplementary Material).
Discussion
The most important finding from this study was that there was only a small increase in the risk of tibial eminence avulsion fracture in Bi-UKA compared to UKA-M for both the larger, denser male tibia (Figure 6) and the female tibia (Figure 5). The predicted strains were an order of magnitude below the 6,500 µε fracture limit, and thus this FE analysis supports the use of Bi-UKA. For all experiments, the highest strains in the tibia were seen on the anterior portion of the face of the medial sagittal cut, distal to the ACL attachment (Figure 2). The initiation of failure at this point is intuitive, correlating with the anatomy of the ACL, which pulls posteriorly and laterally on the tibial eminence.
Overstuffing increased ROF the most: a 2 mm distraction of the joint led to strains six times higher than when native joint laxity was restored (Figure 4). Trial meniscal bearings should be chosen conservatively, working upwards in thickness to minimize likelihood of overstuffing. Slow extension of the knee when trialling bearings, and close attention to resistance to extension, may also help to avoid these higher joint loads. Moving the UKA-M away from the planned position led to higher ROF than the same degree of variation of the UKA-L. Furthermore, variations that narrowed the island anteromedially resulted in the largest increase (i.e. UKA-M external rotation and lateral translation) due to the posterolateral directed ACL tension. Positional variation that made the bone island taller also increased fracture risk, but to a lesser extent (i.e. extension and distal translation), a finding that is likely a consequence of bone stiffness decreasing distally from the joint line. These results highlight the need to position the medial implant correctly. The removal of anterior bone also acted to increase ROF in comparison to Bi-UKA, with 10 mm of bone removed more than doubling the tensile strains in the spine. This demonstrated that BCR-TKA and Bi-UKA are fundamentally different, with Bi-UKA a lower-risk procedure for maintaining ACL function, as the anterior bone is not compromised. The peak tensile strains in the smaller, less dense female tibia were approximately 1.5 times higher than in the male specimen. The increase in fracture risk was similar for both the larger, denser male (11.3%) and smaller, less dense female (9.3%) models with Bi-UKA compared to UKA-M. While all ROFs measured in this study were less than 1, only two bones were analyzed; this finding indicates that a combination of a smaller tibia and less dense bone may lead to an appreciable risk for some patients, particularly if the joint was overstuffed intraoperatively. Hence, additional care may be needed in planning Bi-UKA for osteoporotic, small females, as well as intraoperatively.
To the authors’ knowledge, this is the first study to examine tibial eminence avulsion fracture risk following arthroplasty, so there are no studies that provide a direct comparison. The modelling methods employed are similar to those used in recent FE studies focused on tibial strains following UKA-M. Pegg et al 19 investigated similar surgical cut parameters in their probabilistic FE study looking at condylar fracture risk post-UKA. They considered gait-loading rather than intraoperative loads, and so saw larger condylar loads as well as muscle-loading leading to a generally compressive strain field, rather than the tensile field induced in the spine intraoperatively. Despite this, they also found that the corner where the transverse and sagittal cuts met was at high risk of fracture. Danese et al 42 looked at the effect of malalignment on proximal tibial strains for UKA-M, using a similar number of models and similar modelling parameters to this study, and found that varus/valgus alignment had a greater effect on compressive overstraining under UKA-M compared to internal/external rotation. Thus, it appears that the varus/valgus positioning is important to avoid postoperative fractures during functional activity, and rotational positioning is important to avoid avulsion fractures intraoperatively. A recent study determined that surgical cut errors in UKA-M were effected by limb posture. 43 They found similar errors to those used in this study, however they found significantly higher errors in the internal rotation of the medial component when the traditional supine leg position was used, rather than the manufacturer-recommended hanging leg position. The extreme of the internal rotation error associated with the supine position was greater than the error assessed in this study. The magnitude of the errors used in this study was representative of traditional surgical techniques, however both compound translational and compound rotational errors have been shown to be reduced with robot-assisted surgery. 36 As such, the results of this study may overestimate the risk of ACL avulsion compared to that which might be expected in robot-assisted Bi-UKA, for which there has been a growing interest. 14,44
The models used for this research were originally developed and validated for UKA-M under compressive loading, for medial cortical strains distal to the implant and bone-implant micromotions. It was assumed that the utility of this model could be extended to also include UKA-L, with an additional tensile ACL load applied, and examining regions of interest closer to the implants. Other researchers have made similar assumptions, using cortical strain measurements as a basis for validation of bone FE models investigating fracture. 19,45-48 While direct data to validate peri-implant bone strains were not available, the internal stress distribution predicted by the model was found to be similar to recently published experimental measurements, 49 verifying that the predicted peri-implant cancellous bone stresses were representative of reality (Supplementary Figure b). This research did not study fracture propagation, 50 with MPES-95 used as a surrogate measure to determine whether fracture would occur. None of the simulations resulted in MPES-95 that exceeded the tensile yield strain of proximal tibial bone, although the absolute peak tensile strains in overstuffed Bi-UKA did exceed this threshold (Supplementary Material), and hence such a fracture could propagate with the progressive deactivation of overstrained elements and may explain the clinical observation that ACL avulsion can occur intraoperatively. Gait load cases were not simulated, as there have been no reports of avulsion postoperatively. Further, postoperative condylar fracture during gait was not studied, as this has been analyzed with the FE method by others for UKA-M. One limitation was that the femur was not modelled; a steeper posterior slope (flexed implant) can lead to anterior tibial translation and consequently increased ACL strain. 51 Omitting the femur and a continuum model of the ACL allowed for a computationally cheaper model, and so more simulations could be run; the effect of relative femoral translations may be a topic of future work. The magnitude of the typical variations in implant positions simulated were taken from two Sawbones studies, one looking at UKA-M and the other UKA-L. There have been no studies quantifying the variations in Bi-UKA and so it was assumed that the errors from each of the unicompartmental procedures would be the same in bicompartmental surgery. The authors of these studies suggested that surgeons would take greater care in positioning the implants in a real surgery, and so expected the errors to be smaller than those measured. 38 However, the limited exposure intraoperatively may also serve to increase errors compared to the synthetic bone, capsule, and four-ligament model. 37 Finally, ligament loading necessitated the combination of multiple studies, and hence is a representative model of intraoperative loading, rather than an exact model for the two tibiae simulated.
In conclusion, the risk of tibial eminence avulsion fracture associated with Bi-UKA is similar to UKA-M, suggesting that any perceived risk of avulsion should not weigh heavily in decision-making regarding knee arthroplasty procedures. The risk was higher for a smaller and less dense female tibia. To minimize risk, it is most important to avoid overstuffing of the joint, followed by correctly positioning the medial implant, taking care not to narrow the bone island anteriorly.
Author contributions
J. C. Stoddart: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualisation, Writing – original draft, Writing – review & editing.
A. Garner: Conceptualization, Methodology, Writing – review & editing.
M. Tuncer: Methodology, Validation, Writing - review & editing.
J. P. Cobb: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
R. J. van Arkel: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: funding from the Peter Stormonth Darling Charitable Trust, and donation of CAD models of standard implants from Zimmer Biomet in kind.
ICMJE COI statement
J. Cobb reports institutional royalties or licences, and stock or stock options (paid to Imperial College London) from Embody Orthopaedic, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Zimmer Biomet, not related to this study.
Acknowledgements
The authors are grateful to Zimmer Biomet, in particular Michael Malon and Christopher Friend, for supporting our modelling research.
Open access funding
The authors report that they received open access funding for their manuscript from the Imperial Open Access Fund.
Follow A. Garner @dramygarner
Follow J. P. Cobb @orthorobodoc and @MSkLab1
Follow R. Jan. van Arkel @ICBiomechanics
Supplementary material
Numerical results for each experiment are presented for different percentiles of the maximum principal elastic strain. Sensitivity studies referred to in the main text are explained further.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Jennifer C. Stoddart, Email: jcs113@ic.ac.uk.
Amy Garner, Email: a.garner@imperial.ac.uk.
Mahmut Tuncer, Email: mac@tuncer.co.uk.
Justin P. Cobb, Email: j.cobb@imperial.ac.uk.
Richard J. van Arkel, Email: r.vanarkel@imperial.ac.uk.
References
- 1. Felson DT . Epidemiology of hip and knee osteoarthritis . Epidemiol Rev . 1988. ; 10 ( 1 ): 1 – 28 . 10.1093/oxfordjournals.epirev.a036019 [DOI] [PubMed] [Google Scholar]
- 2. Murphy L , Schwartz TA , Helmick CG , et al. Lifetime risk of symptomatic knee osteoarthritis . Arthritis Rheum . 2008. ; 59 ( 9 ): 1207 – 1213 . 10.1002/art.24021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cui A , Li H , Wang D , Zhong J , Chen Y , Lu H . Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies . EClinicalMedicine . 2020. ; 29–30 : 100587 . 10.1016/j.eclinm.2020.100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stoddart JC , Dandridge O , Garner A , Cobb J , van Arkel RJ . The compartmental distribution of knee osteoarthritis - a systematic review and meta-analysis . Osteoarthr Cartil . 2021. ; 29 ( 4 ): 445 – 455 . 10.1016/j.joca.2020.10.011 [DOI] [PubMed] [Google Scholar]
- 5. Garner A , van Arkel RJ , Cobb J . Classification of combined partial knee arthroplasty . Bone Joint J . 2019. ; 101-B ( 8 ): 922 – 928 . 10.1302/0301-620X.101B8.BJJ-2019-0125.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wada K , Price A , Gromov K , Lustig S , Troelsen A . Clinical outcome of bi-unicompartmental knee arthroplasty for both medial and lateral femorotibial arthritis: a systematic review-is there proof of concept? Arch Orthop Trauma Surg . 2020. ; 140 ( 10 ): 1503 – 1513 . 10.1007/s00402-020-03492-6 [DOI] [PubMed] [Google Scholar]
- 7. Amit P , Singh N , Soni A , Bowman NK , Maden M . Systematic Review of Modular Bicompartmental Knee Arthroplasty for Medio-Patellofemoral Osteoarthritis . J Arthroplasty . 2020. ; 35 ( 3 ): 893 – 899 . 10.1016/j.arth.2019.09.042 [DOI] [PubMed] [Google Scholar]
- 8. Biazzo A , Silvestrini F , Manzotti A , Confalonieri N . Bicompartmental (uni plus patellofemoral) versus total knee arthroplasty: a match-paired study . Musculoskelet Surg . 2019. ; 103 ( 1 ): 63 – 68 . 10.1007/s12306-018-0540-1 [DOI] [PubMed] [Google Scholar]
- 9. Elbardesy H , Awad AK , McLeod A , et al. Does bicompartmental knee arthroplasty hold an advantage over total knee arthroplasty? Systematic review and meta-analysis . SICOT J . 2021. ; 7 : 38 . 10.1051/sicotj/2021036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao D , Akram I , Daniilidis K , Labey L , Innocenti B , Tibesku C . Bicompartmental, medial and patellofemoral knee replacement might be able to maintain unloaded knee kinematics . Arch Orthop Trauma Surg . 2022. ; 142 ( 3 ): 501 – 509 . 10.1007/s00402-021-03816-0 [DOI] [PubMed] [Google Scholar]
- 11. Garner AJ , Dandridge OW , Amis AA , Cobb JP , van Arkel RJ . Bi-unicondylar arthroplasty: a biomechanics and clinical outcomes study . Bone Joint Res . 2021. ; 10 ( 11 ): 723 – 733 . 10.1302/2046-3758.1011.BJR-2021-0151.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garner AJ , Dandridge OW , Amis AA , Cobb JP , van Arkel RJ . Partial and Combined Partial Knee Arthroplasty: Greater Anterior-Posterior Stability Than Posterior Cruciate-Retaining Total Knee Arthroplasty . J Arthroplasty . 2021. ; 36 ( 11 ): 3765 – 3772 . 10.1016/j.arth.2021.06.025 [DOI] [PubMed] [Google Scholar]
- 13. Garner AJ , Dandridge OW , van Arkel RJ , Cobb JP . The compartmental approach to revision of partial knee arthroplasty results in nearer-normal gait and improved patient reported outcomes compared to total knee arthroplasty . Knee Surg Sports Traumatol Arthrosc . 2021. . 10.1007/s00167-021-06691-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banger MS , Johnston WD , Razii N , et al. Robotic arm-assisted bi-unicompartmental knee arthroplasty maintains natural knee joint anatomy compared with total knee arthroplasty: a prospective randomized controlled trial . Bone Joint J . 2020. ; 102-B ( 11 ): 1511 – 1518 . 10.1302/0301-620X.102B11.BJJ-2020-1166.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garner A , Dandridge O , Amis AA , Cobb JP , van Arkel RJ . The extensor efficiency of unicompartmental, bicompartmental, and total knee arthroplasty . Bone Joint Res . 2021. ; 10 ( 1 ): 1 – 9 . 10.1302/2046-3758.101.BJR-2020-0248.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parratte S , Pauly V , Aubaniac J-M , Argenson J-N . Survival of bicompartmental knee arthroplasty at 5 to 23 years . Clin Orthop Relat Res . 2010. ; 468 ( 1 ): 64 – 72 . 10.1007/s11999-009-1018-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Confalonieri N , Manzotti A , Cerveri P , De Momi E . Bi-unicompartmental versus total knee arthroplasty: A matched paired study with early clinical results . Arch Orthop Trauma Surg . 2009. ; 129 ( 9 ): 1157 – 1163 . 10.1007/s00402-008-0713-8 [DOI] [PubMed] [Google Scholar]
- 18. Lombardi AV , McClanahan AJ , Berend KR . The bicruciate-retaining TKA: Two is better than one . Semin Arthroplasty . 2015. ; 26 ( 2 ): 51 – 58 . 10.1053/j.sart.2015.08.004 [DOI] [Google Scholar]
- 19. Pegg EC , Walter J , D’Lima DD , Fregly BJ , Gill HS , Murray DW . Minimising tibial fracture after unicompartmental knee replacement: A probabilistic finite element study . Clin Biomech (Bristol, Avon) . 2020. ; 73 : 46 – 54 . 10.1016/j.clinbiomech.2019.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tuncer M , Hansen UN , Amis AA . Prediction of structural failure of tibial bone models under physiological loads: effect of CT density-modulus relationships . Med Eng Phys . 2014. ; 36 ( 8 ): 991 – 997 . 10.1016/j.medengphy.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 21. Scott CEH , Eaton MJ , Nutton RW , Wade FA , Evans SL , Pankaj P . Metal-backed versus all-polyethylene unicompartmental knee arthroplasty: Proximal tibial strain in an experimentally validated finite element model . Bone Joint Res . 2017. ; 6 ( 1 ): 22 – 30 . 10.1302/2046-3758.61.BJR-2016-0142.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simpson DJ , Price AJ , Gulati A , Murray DW , Gill HS . Elevated proximal tibial strains following unicompartmental knee replacement--A possible cause of pain . Med Eng Phys . 2009. ; 31 ( 7 ): 752 – 757 . 10.1016/j.medengphy.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 23. Kwon O-R , Kang K-T , Son J , Suh D-S , Baek C , Koh Y-G . Importance of joint line preservation in unicompartmental knee arthroplasty: Finite element analysis . J Orthop Res . 2017. ; 35 ( 2 ): 347 – 352 . 10.1002/jor.23279 [DOI] [PubMed] [Google Scholar]
- 24. Dai X , Fang J , Jiang L , Xiong Y , Zhang M , Zhu S . How does the inclination of the tibial component matter? A three-dimensional finite element analysis of medial mobile-bearing unicompartmental arthroplasty . Knee . 2018. ; 25 ( 3 ): 434 – 444 . 10.1016/j.knee.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 25. Koh Y-G , Lee J-A , Chung H-S , Kim H-J , Kang K-T . Restoration of normal knee kinematics with respect to tibial insert design in mobile bearing lateral unicompartmental arthroplasty using computational simulation . Bone Joint Res . 2020. ; 9 ( 7 ): 421 – 428 . 10.1302/2046-3758.97.BJR-2019-0384.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee J-A , Koh Y-G , Kim PS , Kang KW , Kwak YH , Kang K-T . Biomechanical effect of tibial slope on the stability of medial unicompartmental knee arthroplasty in posterior cruciate ligament-deficient knees . Bone Joint Res . 2020. ; 9 ( 9 ): 593 – 600 . 10.1302/2046-3758.99.BJR-2020-0128.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Innocenti B , Bilgen ÖF , Labey L , van Lenthe GH , Sloten JV , Catani F . Load sharing and ligament strains in balanced, overstuffed and understuffed UKA. A validated finite element analysis . J Arthroplasty . 2014. ; 29 ( 7 ): 1491 – 1498 . 10.1016/j.arth.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 28. Tuncer M , Cobb JP , Hansen UN , Amis AA . Validation of multiple subject-specific finite element models of unicompartmental knee replacement . Med Eng Phys . 2013. ; 35 ( 10 ): 1457 – 1464 . 10.1016/j.medengphy.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 29. Cobb JP , Dixon H , Dandachli W , Iranpour F . The anatomical tibial axis: reliable rotational orientation in knee replacement . J Bone Joint Surg Br . 2008. ; 90-B ( 8 ): 1032 – 1038 . 10.1302/0301-620X.90B8.19905 [DOI] [PubMed] [Google Scholar]
- 30. Markolf KL , Park S , Jackson SR , McAllister DR . Contributions of the posterolateral bundle of the anterior cruciate ligament to anterior-posterior knee laxity and ligament forces . Arthroscopy . 2008. ; 24 ( 7 ): 805 – 809 . 10.1016/j.arthro.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 31. Cho K-J , Seon J-K , Jang W-Y , Park C-G , Song E-K . Objective quantification of ligament balancing using VERASENSE in measured resection and modified gap balance total knee arthroplasty . BMC Musculoskelet Disord . 2018. ; 19 ( 1 ): 266 . 10.1186/s12891-018-2190-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jordan SS , DeFrate LE , Nha KW , Papannagari R , Gill TJ , Li G . The in vivo kinematics of the anteromedial and posterolateral bundles of the anterior cruciate ligament during weightbearing knee flexion . Am J Sports Med . 2007. ; 35 ( 4 ): 547 – 554 . 10.1177/0363546506295941 [DOI] [PubMed] [Google Scholar]
- 33. Sanz-Pena I , Zapata GE , Verstraete MA , Meere PA , Walker PS . Relationship Between Ligament Forces and Contact Forces in Balancing at Total Knee Surgery . J Arthroplasty . 2019. ; 34 ( 6 ): 1261 – 1266 . 10.1016/j.arth.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 34. Herzog W , Read LJ . Lines of action and moment arms of the major force-carrying structures crossing the human knee joint . J Anat . 1993. ; 182 ( Pt 2) ( Pt 2 ): 213 – 230 . [PMC free article] [PubMed] [Google Scholar]
- 35. Schmitz A , Piovesan D . Development of an open-source, discrete element knee model . IEEE Trans Biomed Eng . 2016. ; 63 ( 10 ): 2056 – 2067 . 10.1109/TBME.2016.2585926 [DOI] [PubMed] [Google Scholar]
- 36. Jaffry Z , Masjedi M , Clarke S , et al. Unicompartmental knee arthroplasties: robot vs. patient specific instrumentation . Knee . 2014. ; 21 ( 2 ): 428 – 434 . 10.1016/j.knee.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 37. Ng CTJ , Newman S , Harris S , Clarke S , Cobb J . Patient-specific instrumentation improves alignment of lateral unicompartmental knee replacements by novice surgeons . Int Orthop . 2017. ; 41 ( 7 ): 1379 – 1385 . 10.1007/s00264-017-3468-4 [DOI] [PubMed] [Google Scholar]
- 38. Clarius M , Aldinger PR , Bruckner T , Seeger JB . Saw cuts in unicompartmental knee arthroplasty: an analysis of Sawbone preparations . Knee . 2009. ; 16 ( 5 ): 314 – 316 . 10.1016/j.knee.2008.12.018 [DOI] [PubMed] [Google Scholar]
- 39. Pankaj P , Donaldson FE . Algorithms for a strain-based plasticity criterion for bone . Int J Numer Method Biomed Eng . 2013. ; 29 ( 1 ): 40 – 61 . 10.1002/cnm.2491 [DOI] [PubMed] [Google Scholar]
- 40. Morgan EF , Keaveny TM . Dependence of yield strain of human trabecular bone on anatomic site . J Biomech . 2001. ; 34 ( 5 ): 569 – 577 . 10.1016/s0021-9290(01)00011-2 [DOI] [PubMed] [Google Scholar]
- 41. Galloway F , Kahnt M , Ramm H , et al. A large scale finite element study of A cementless osseointegrated tibial tray . J Biomech . 2013. ; 46 ( 11 ): 1900 – 1906 . 10.1016/j.jbiomech.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 42. Danese I , Pankaj P , Scott CEH . The effect of malalignment on proximal tibial strain in fixed-bearing unicompartmental knee arthroplasty . Bone Joint Res . 2019. ; 8 ( 2 ): 55 – 64 . 10.1302/2046-3758.82.BJR-2018-0186.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tapasvi S , Shekhar A , Patil S , Pandit H . Limb position influences component orientation in Oxford mobile bearing unicompartmental knee arthroplasty: an experimental cadaveric study . Bone Joint Res . 2020. ; 9 ( 6 ): 272 – 278 . 10.1302/2046-3758.96.BJR-2019-0258.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blyth MJG , Banger MS , Doonan J , Jones BG , MacLean AD , Rowe PJ . Early outcomes after robotic arm-assisted bi-unicompartmental knee arthroplasty compared with total knee arthroplasty: a prospective, randomized controlled trial . Bone Joint J . 2021. ; 103-B ( 10 ): 1561 – 1570 . 10.1302/0301-620X.103B10.BJJ-2020-1919.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sternheim A , Giladi O , Gortzak Y , et al. Pathological fracture risk assessment in patients with femoral metastases using CT-based finite element methods. A retrospective clinical study . Bone . 2018. ; 110 : 215 – 220 . 10.1016/j.bone.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 46. Leung ASO , Gordon LM , Skrinskas T , Szwedowski T , Whyne CM . Effects of bone density alterations on strain patterns in the pelvis: application of a finite element model . Proc Inst Mech Eng H . 2009. ; 223 ( 8 ): 965 – 979 . 10.1243/09544119JEIM618 [DOI] [PubMed] [Google Scholar]
- 47. Salo Z , Kreder H , Whyne CM . The Impact of an Open-Book Pelvic Ring Injury on Bone Strain: Validation of a Finite Element Model and Analysis Within the Gait Cycle . J Biomech Eng . 2021. ; 143 ( 7 ): 1 – 7 . 10.1115/1.4050459 [DOI] [PubMed] [Google Scholar]
- 48. Inoue S , Akagi M , Asada S , Mori S , Zaima H , Hashida M . The Valgus Inclination of the Tibial Component Increases the Risk of Medial Tibial Condylar Fractures in Unicompartmental Knee Arthroplasty . J Arthroplasty . 2016. ; 31 ( 9 ): 2025 – 2030 . 10.1016/j.arth.2016.02.043 [DOI] [PubMed] [Google Scholar]
- 49. Munford MJ , Stoddart JC , Liddle AD , Cobb JP , Jeffers JRT . Total and partial knee arthroplasty implants that maintain native load transfer in the tibia . Bone Joint Res . 2022. ; 11 ( 2 ): 91 – 101 . 10.1302/2046-3758.112.BJR-2021-0304.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ridzwan MIZ , Sukjamsri C , Pal B , et al. Femoral fracture type can be predicted from femoral structure: A finite element study validated by digital volume correlation experiments . J Orthop Res . 2018. ; 36 ( 3 ): 993 – 1001 . 10.1002/jor.23669 [DOI] [PubMed] [Google Scholar]
- 51. Meier M , Janssen D , Koeck FX , Thienpont E , Beckmann J , Best R . Variations in medial and lateral slope and medial proximal tibial angle . Knee Surg Sports Traumatol Arthrosc . 2021. ; 29 ( 3 ): 939 – 946 . 10.1007/s00167-020-06052-y [DOI] [PubMed] [Google Scholar]