Abstract
Tendon is a bradytrophic and hypovascular tissue, hence, healing remains a major challenge. The molecular key events involved in successful repair have to be unravelled to develop novel strategies that reduce the risk of unfavourable outcomes such as non-healing, adhesion formation, and scarring. This review will consider the diverse pathophysiological features of tendon-derived cells that lead to failed healing, including misrouted differentiation (e.g. de- or transdifferentiation) and premature cell senescence, as well as the loss of functional progenitors. Many of these features can be attributed to disturbed cell-extracellular matrix (ECM) or unbalanced soluble mediators involving not only resident tendon cells, but also the cross-talk with immigrating immune cell populations. Unrestrained post-traumatic inflammation could hinder successful healing. Pro-angiogenic mediators trigger hypervascularization and lead to persistence of an immature repair tissue, which does not provide sufficient mechano-competence. Tendon repair tissue needs to achieve an ECM composition, structure, strength, and stiffness that resembles the undamaged highly hierarchically ordered tendon ECM. Adequate mechano-sensation and -transduction by tendon cells orchestrate ECM synthesis, stabilization by cross-linking, and remodelling as a prerequisite for the adaptation to the increased mechanical challenges during healing. Lastly, this review will discuss, from the cell biological point of view, possible optimization strategies for augmenting Achilles tendon (AT) healing outcomes, including adapted mechanostimulation and novel approaches by restraining neoangiogenesis, modifying stem cell niche parameters, tissue engineering, the modulation of the inflammatory cells, and the application of stimulatory factors.
Cite this article: Bone Joint Res 2022;11(8):561–574.
Keywords: Achilles tendon, Tendon healing, Cell plasticity, Tendon-derived stem cells, tendons, inflammation, stiffness, stem cells, extracellular matrix, strength, neoangiogenesis, tissue engineering
Article focus
Cell biological peculiarities and molecular events which influence the healing response in the Achilles tendon (AT).
Open questions concerning factors which cause unsuccessful AT healing.
Outlook on strategies to augment AT healing.
Key messages
The AT shows a limited healing response.
Unrestrained inflammation, hypervascularization, and changes in stem and progenitor cell niches might contribute to an inappropriate healing response.
Individually adapted mechanostimulation is an important prerequisite for regenerative healing.
Strengths and limitations
This review provides a cell biological point of view on novel aspects of the pathophysiology of AT healing.
This overview on current literature remains narrative, and does not systematically evaluate the experimental works cited.
Tendon – structure and cells
Macroscopical structure
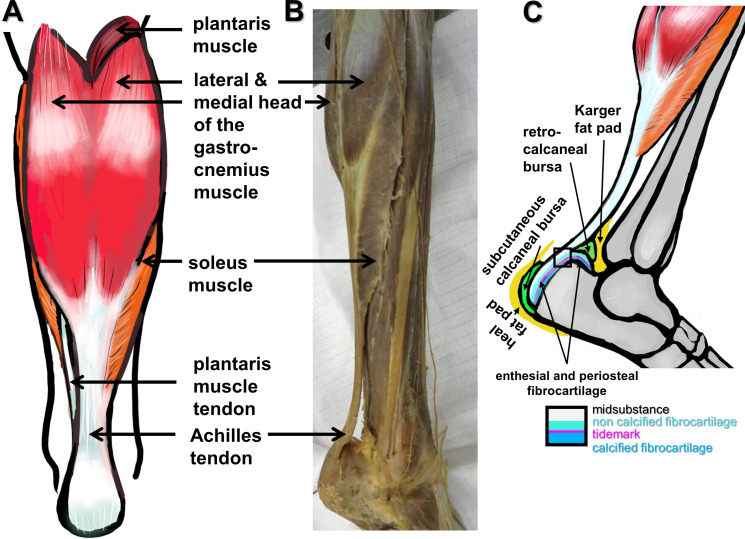
The Achilles tendon (AT) is the strongest tendon in the human body, 1,2 gaining its crucial importance with the upright gait of humans. It connects the triceps surae muscle, consisting of the medial and lateral heads of the gastrocnemius muscle and the soleus muscle forming the dorsal shape of the calf with the calcaneus bone tuberosity (Figures 1a and 1b). 1,2 In 65% of cases the tendon of the plantaris muscle associates laterally with the AT (Figure 1a). 2 Thereby, the AT allows the transmission of forces generated by the triceps surae muscle, high enough to compensate a multitude of loading of the body weight 3 as main actor at the ankle joint mediating plantar flexion. 4 Hence, the tendon is highly stressed in sport disciplines associated with jumping, and sudden accelerations and stops, such as football, basketball, and running. 5 The AT is supported by two bursae (retrocalcaneal and subcutaneous calcaneal) protecting it from wear and tear and attached via a fibrocartilaginous enthesis at the calcaneal tuberosity (Figure 1c). 6 These structures are prone to overuse and the bursae and associated fatty tissues (Karger and heal fat pads, Figure 1c) might contribute to inflammatory responses and healing. Intra-articular and joint-associated fat pads including the Karger fat pad (Figure 1c) have multiple functions: they are paracrine-active, modulating inflammation, but also exert biomechanical functions by distribution of loading and protection of neighbouring structures. 7 In addition, they contain sensory nerve endings (mediating proprioception and sensation of pain), and hence can protect tissues from overload during the healing process. 8 Tendons including the AT 9 generally have a poor blood supply. The AT receives its blood supply mainly from the posterior tibial artery and to a lesser extent from the fibular (peroneal) artery. 10 Their branches run in the paratenon. 11 Three territories of vascular supply can be distinguished, 9 with the mid third being nourished by the fibular artery, and the proximal and distal thirds receiving nutrition from the posterior tibial artery. The mid third (around 2 to 6 cm above the calcaneal insertion) of the AT is more hypovascular than both other regions of the AT, and hence prone to injury. 10,12 In the AT there is a central area mostly devoid of blood vessels, and through twisting of the fibre bundles this area can be exposed to compressive forces. 13,14 Individual subtendon twisting patterns of the AT influence energy storage capacity and stiffness. 15 During gait, transverse rotatory forces are low compared to the tensile stretching forces of the AT but under load, the AT also becomes externally rotated relative to its calcaneal insertion. 16 Despite the blood supply being increased during exercise in this region, 17 the AT is at highest risk of rupturing and failures in healing at its midsection. However, blood supply is further diminished with ageing. 12 Individuals who are characterized by poorer blood supply of the AT midsection have a higher risk of AT rupture. 10
Fig. 1.
Macroscopical anatomy of the Achilles tendon (AT). a) Scheme of a dorsal view. b) and c) Dorsolateral views: b) dissection photograph; and c) scheme of the AT, bursae, fat pads, and enthesis zones (inset). a) and c) The images were created by G. G. Schulze-Tanzil using Krita 4.1.7 (Krita Foundation, The Netherlands).
Microscopic structure
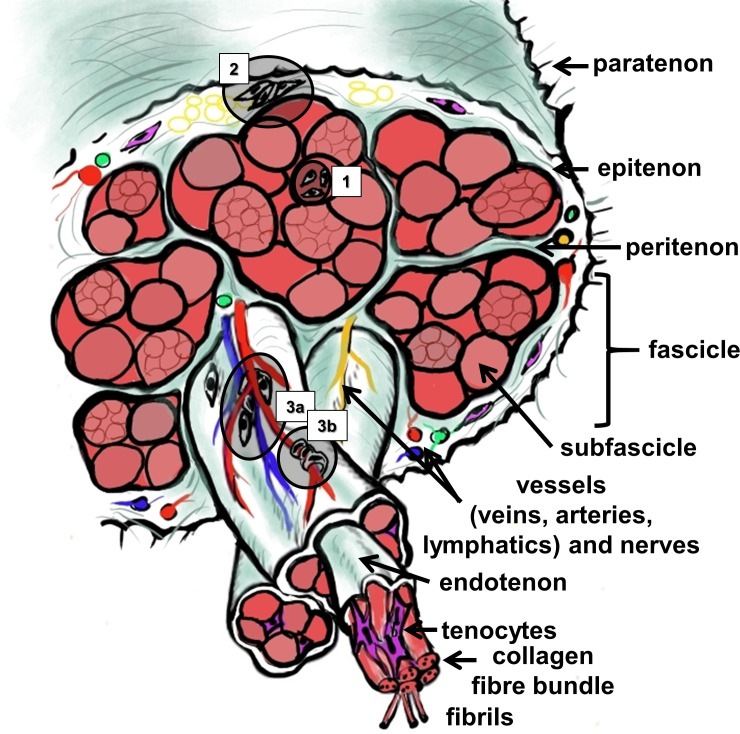
Tendon is a highly hierarchically composed tissue consisting of fascicles and subfascicles, 18 each surrounded by loose connective tissue sheathes (named peri- and endotenon) containing blood and lymphatic vessels and nerves, and the collagen fibre bundles (Figure 2). The entire AT is covered by a connective tissue sheath called epitenon and surrounded by a paratenon, the latter connecting it to the surrounding subcutaneous and adjacent tissue (Figure 2). With regard to force transmission, tendon fascicles with their interfascicular membrane belonging to the peritenon acted as independent units in the AT and lateral force transmission between fascicles was negligible. 19 Nevertheless, the interfascicular ECM in the AT plays an important role in mediating isoelastic properties and, hence, biomechanics of this tendon. 20 Tendon is a hypocellular tissue consisting mainly of the ECM. 21 The ECM contains large amounts of collagen (mainly of type 1, 70% of dry weight), 22 elastic fibres, few proteoglycans (at least 1% of dry weight), 23 and various glycoproteins such as tenomodulin (TNMD) and tenascin C. 24 The composition of the ECM differs between the midportion and enthesis parts (the latter is mostly fibrocartilaginous) of the AT. 25 Types I, III, V, and VI collagens, decorin, biglycan, fibromodulin, and lumican, were found in both areas with partly differing distribution, whereas type II collagen and aggrecan were only found in the enthesis but versican exclusively in the midportion. This diverse distribution of ECM components correlates with differing mechanical cues. 25 The highly aligned type I collagen, as the main collagen in tendon, is mainly responsible for the mechanical characteristics of the tendon. The thinner type III collagen fibrils build a network with collagen type I, and their occurrence is associated with inferior tensile mechanical properties. 26 Further ECM proteins affect the mechanical properties, but also cross-linking of the proteins. 27,28 The interfascicular ECM provides sliding of tendon fascicles probably among other components supported by its content of lubricin and elastin. 29,30
Fig. 2.
Scheme of the microscopic anatomy of the Achilles tendon. The stem cell niches are numbered (1: within the tendon proper; 2: within the epi/paratenon; interfascicular niches comprise 3a: perivascular; and 3b: niches within the wall of small vessels, containing pericytes). The image was created by G. G. Schulze-Tanzil using Krita 4.1.7 (Krita Foundation, The Netherlands).
Tenocytes and progenitor cells
Tendon cells (Table I) are responsible for maintaining tendon homeostasis by sensation of mechanical signals, thereby allowing the ECM to adapt to mechanical challenges by a fine modulated remodelling process. The main cell population (90%) in tendon consists of tenocytes and tenoblasts. 3 Tenocytes, representing a highly specialized type of fibroblast, are arranged in rows, embracing collagen fibre bundles with their long communicating cytoplasmic extensions. Tenoblasts are less differentiated cells which are still able to divide, and hence can reconstitute micro-damages of the tissue. Recent data of single cell analysis indicate that tenocytes show some heterogeneity representing several subpopulations. 31 In addition to tenocyte populations, fibrochondrocytes, endothelial cells, pericytes, and stem cells (SCs) can be observed in tendon. 31,32 Fibrochondrocytes are localized at areas of the midsubstance of tendon exposed to compressive forces or the enthesis where the AT inserts into bone. Endothelial cells and pericytes are associated with the small capillaries. Pericytes are contractile cells surrounding the capillaries in an abluminal position, able to contribute to blood flow regulation. Moreover, SC capacities can be attributed to them in the AT (Figure 2). 31 Interfascicular SCs and pericytes are characterized by CD146 expression and recruited in AT injury. 33 SCs have been thoroughly described in tendons including the AT. 34,35 However, they seem not to represent a uniform population since their specific localization, with its unique niche characteristics as well as anatomical distribution in the AT, 36 and differentiation capacity, might substantially differ. There are three main regions where tendon stem/progenitor cells (TSPCs) have been localized, namely in the tendon fascicles (proper-derived TSPCs, Figure 2 (region 1)), in the epitenon (paratenon/epitenon-derived TSPCs, Figure 2 (region 2)), and in the vascularized region of the tendon (perivascular TSPCs, Figure 2 (regions 3a to 3b)) (reviewed by Walia and Huang 37 and Huang et al 38 ). TSPCs localized in tendon fascicles did not only express the same cell surface markers as bone marrow mesenchymal stem cells (BMSCs), but also CD90, SC antigen (SCA)-1, CD44, scleraxis (SCX), tenascin-C, and TNMD as shown for the patellar tendon or the AT. 34,39 Furthermore, TSPCs confined in the paratenon/epitenon region are characterized by the expression of Tubulin polymerization-promotion protein family member 3 (Tppp3) as shown in the patellar tendon and AT, 40,41 laminin, α-smooth muscle actin (αSMA), platelet-derived growth factor receptor α (PDGFRα), 37,41 and osteocalcin. 41,42 Moreover, detailed transcriptome analysis identified SCX, Mohawk (MKX), thrombospondin (Thbs)4, and Wnt10a as genes to distinguish tendon proper progenitors from peritenon progenitors in AT. 43 Recently, using single-cell analysis of non-specified mouse tendons, nestin (Nes) was found to be a novel marker for a perivascular subpopulation of TSPCs with strong tenogenic potential. 44 In addition, perivascular-TSPCs of the supraspinatus tendon expressed CD146, CD133, Endomucin (Emcn), Musashi1 (Msi1), 45 p75 neurotrophin receptor in patellar tendons, 46 and co-expressed αSMA; 37 the latter could also be shown in those of the porcine AT. 41 Moreover, recent publication based on multiomics single cell analysis of human tendons of different origin including AT identified a perivascular cell population that coexpressed high levels of CD90 and CD146, as well as five distinct collagen type I-expressing tenocyte populations in addition to endothelial cells, T-cells, and monocytes. These five collagen (COL)1A1/2-expressing tenocyte cell populations consisted of keratin-7 (KRT) KRT7/SCX-positive cells, pentraxin-related protein (PTX)3-positive cells, TPPP3/proteoglycan 4 (PRG4)-positive chondrogenic cells, apolipoprotein D (APOD)-positive fibro-adipogenic cells, and integrin α7 (ITGA7)-positive smooth muscle cells. 47 A further possible source of progenitor cells that might be involved in the healing of tendon tissue is the bursa, a friction-reducing tissue covering the joint capsule or underlying tendons or ligaments. Cells with a SC character were identified in the subacromial bursa of the glenohumeral joint. 48-50 Bursa tissue, namely the subcutaneous and retrocalcaneal bursae, is also associated with the AT (Figure 1), and it remains to be investigated whether both bursae could contribute to healing. 50
Table I.
Markers shown in cell types of Achilles tendon.
| Resident cell type in AT | Marker expression | References |
|---|---|---|
| Tenoblast/tenocyte | Scleraxis (SCX), Mohawk, TNMD, thrombospondin 4, and Wnt family member, five collagen (COL) type I-expressing tenocyte cell populations: keratin-7/SCX-positive cells, pentraxin-related protein 3-positive cells, TPPP3/proteoglycan 4-positive chondrogenic cells, apolipoprotein D-positive fibro-adipogenic cells, and integrin α7-positive smooth muscle cells. | 34,47 |
| Fibrochondrocyte | COL types II, IX, XI, aggrecan, Sox9 | 25 |
| Endothelial cells, pericytes | CD31, CD34 | |
| SCs/TSPCs | ||
| - interfascicular | CD146 | 33 |
| - perivascular | Nestin, α-smooth muscle actin (αSMA), CD146*, CD90++* | 44,47 |
| - intrafascicular | CD90, stem cell antigen-1, CD44, SCX, tenascin-C, and TNMD | 33,39 , 34 34 |
| - epi-/paratenon | TPPP3, laminin, αSMA, platelet-derived growth factor receptor α, and osteocalcin | 37,41,47 |
High level.
AT, Achilles tendon; COL, collagen; SC, stem cell; SCX, scleraxis; Sox9, SRY box transcription factor 9; TNMD, tenomodulin; TPPP3, tubulin polymerization-promotion protein family member 3; TSPC, tendon stem/progenitor cell; αSMA, α smooth muscle actin.
Joint-associated fat pads such as the Kager fat pad might, as with the infrapatellar fat pad, 51 contain SCs which can directly contribute to healing by exerting trophic functions, engraftment into tissue defects, and lineage differentiation. The expression profile of SCs in the Kager fat pad and their contribution to AT healing have not been characterized, but their contribution to AT tendinopathy has been outlined. 7
Tendon healing
The AT is one of the most frequently ruptured tendons and its rupture represents nearly 20% of all injuries in large tendons, with increasing rates of occurrence. 5,10 It is more common in males and affects men most often at an age between 40 and 50 years. 5 AT rupture often leads to the end of a professional sports career. The AT midsection, characterized by weakest vascularization, is the region of most frequent rupture, 2 to 6 cm proximal of its insertion. 5 Usually, a degeneration of the AT precedes rupture. Eriksen et al 52 found an increased amount of type III collagen at the site of rupture, possibly due to preceding microtraumata and healing associated with lower tensile strength. These unfavourable tissue conditions might limit the subsequent healing process.
Healing phases
The phases of AT healing can be divided into three main phases: inflammation, proliferation, and remodelling (Table II). With the rupture of the tendon, vessels also rupture and a haematoma forms. The haematoma contains immune cells, such as platelets, neutrophils, monocytes, and macrophages. They secrete proinflammatory factors necessary to initiate the repair process (Figure 3). Tenocytes at the rupture undergo apoptosis, while progenitor cells and tenocytes from adjacent tissue invade the rupture zone, proliferate, and differentiate. In the remodelling phase, collagen type III of the initial ECM of the repair callus is replaced by collagen type I. During all three phases, various pro- and anti-inflammatory cytokines, inflammation-resolving factors, and growth factors are expressed, and the timely balanced abundance of the factors is important for the healing progress (for review see Molloy et al 53 and Thomopoulos et al 54 ).
Table II.
Tissue changes in the healing Achilles tendon.
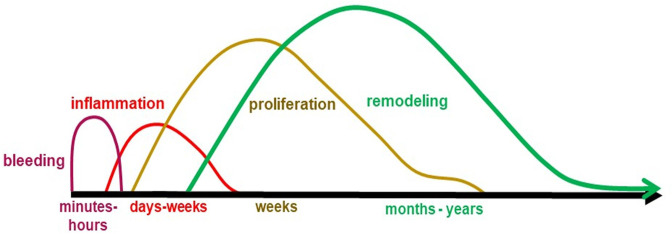
| |||
|---|---|---|---|
| Crucial features in healing AT | Effects | Factors | References |
| Bleeding (hours) | Bleeding and coagulation, cell apoptosis, necrosis | 52-58 | |
| Inflammatory phase (few days) |
Emigration of leucocytes from vessels into tissue (leukodiapedesis), particularly macrophages, phagocytosis: removal of cell and ECM debris, release of diverse proinflammatory mediators | TNFα, IL-6, IL-1β, IL-12, IL-17, and growth factors such as IGF-1, TGFβ, PDGF, bFGF, VEGF |
|
| Proliferation phase (weeks) |
Intrinsic and extrinsic (from the epitenon) cell activation, cell migration, proliferation, myofibroblast formation, angiogenesis, precursor cell commitment and differentiation. Release of anti-inflammatory mediators and growth factors Comprises the so-called granulation phase: formation of a cell-rich vascularized repair tissue Resolution of inflammation |
Anti-inflammatory and inflammation-modulating mediators such as IL-10, PGE2 and growth factors such as IGF-1, TGFβ, VEGF, PDGF, FGF | |
| Remodelling phase (months-years) “callus maturation” |
Myofibroblast contraction, neo-ECM synthesis (collagen type III > I, later: type I > III) and degradation to allow ECM reorganization, cell and ECM alignment, neo-ECM stabilization by cross-linking | Collagen type III > I, later: type I > III, increase in elastin ECM degrading enzymes, e.g. matrix metalloproteinases (MMPs) |
|
AT, Achilles tendon; (b)FGF, basic fibroblast growth factor; ECM, extracellular matrix; IGF, insulin-like growth factor; IL, interleukin; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; PGE2, Prostaglandin E2; TGF, transforming growth factor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.
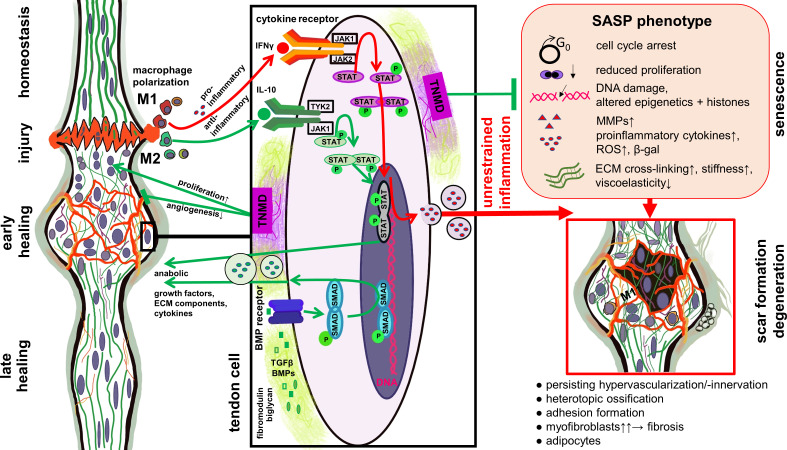
Fig. 3.
Role of Janus kinase signal transducer and activator of transcription (JAK/STAT) and bone morphogenetic protein (BMP) pathways in tendon healing and degeneration. Simplified scheme of selected signalling pathways involved in tendon healing. The balance of M1 and M2 macrophage polarization plays a central role in resolving the inflammatory phase and affects the outcome of tendon healing. Proinflammatory cytokines released during dominant and prolonged M1 macrophage polarization stimulate molecular factors released in response of the JAK/STAT pathway activation, which can trigger the senescence-associated secretory phenotype (SASP) shift of tendon cells. SASP is associated with degenerative features in healing tendons. Mediators released during M2 macrophage polarization, including anti-inflammatory cytokines such as interleukin (IL)-10, stimulate other parts of the JAK/STAT pathway. Proteoglycans such as biglycan and fibromodulin can bind and stabilize growth factors activating the BMP pathway. Tenomodulin produced by the tendon cells can exert pro-proliferative effect as well as protective roles against cellular senescence and unrestrained angiogenesis. The image was created by G. G. Schulze-Tanzil using Krita 4.1.7 (Krita Foundation, The Netherlands). β-gal, β-galactosidase; ECM, extracellular matrix; IFNγ, interferon γ; MMP, matrix metalloproteinase; ROS, reactive oxygen species; TGFβ, transforming growth factor β; TNMD, tenomodulin.
Healing outcome
AT healing could bear diverse drawbacks with functional deficits. Unsatisfying outcomes observed in AT repair can comprise an increased length of the AT combined with reduced calf muscle volume and persisting deficits in plantar flexion strength after surgically assisted repair of ruptured AT. Loss in strength and muscle volume of the triceps muscle is partly compensated by hypertrophy of the flexor hallucis longus muscle acting as a synergist, but 11% to 13% deficits in triceps surae muscle volumes and 12% to 18% loss in the strength of plantar flexion persist even after long-term observation. 59 Moreover, shear wave elastography (SWE) revealed lower shear wave velocity (SWV) in unilaterally ruptured AT compared to contralateral non-injured and healthy AT, even two years after rupture. 60 SWV is proportional to Young’s modulus and therefore quantifies AT stiffness and material elastic properties. 61 The calculation of tissue stiffness using SWE is based on the principle that shear waves travel faster through stiffer (less elastic material) than through softer tissue. Moreover, stiffness is directly proportional to Young’s modulus; hence, lower SWV is indicative for lower modulus and softer tissue. 62 Interestingly, a pilot study using the same technique, but focusing on acute AT ruptures, showed an elevated bi-sigmoidal SWV over time. 63 A significant increase in tendon elastic properties was observed between the third and the sixth week after AT rupture and a second less substantial one after the ninth week, displaying a time correlation of biomechanical properties with the biological healing stages of tendon tissue. 63 Similarly, Zhang et al 64 showed that repaired tendons gradually became stiffer postoperatively and that tendon functional outcome positively correlated with the elasticity of the repaired AT. While the majority of rehabilitation protocols allow increased weightbearing over time, there is still an enormous variability in rehabilitation protocols for treating AT ruptures (operative or nonoperative, weightbearing, range of motion, physiotherapy, and choice of orthosis), underpinning the necessity for further research of AT response to biomechanical challenges to improve AT healing and avoid degeneration. 65,66
Degeneration of AT: impact on healing
Degenerative tendon diseases are a common cause of chronic disorders and pain, and eventually promote tendon rupture. In this way, degeneration and ageing affect multiple aspects of the anatomy and physiology of ATs. They lower the metabolic activity of the tendon, as shown for the patellar tendon, 67 and tissue regenerative potential. Tendinopathy due to degeneration therefore describes a complex multifaceted pathology of the tendon, clinically characterized by activity-related tendon pain, decline in function, restricted mobility, and disability. In the course of the disease, morphological changes occur within the tendon tissue. Since the tensile strength of the tendon decreases during the degeneration, it can lead to spontaneous tendon rupture, which means ruptures without an appropriate trauma.
Inappropriate and insufficient healing of micro-damages can lead to AT degeneration. Degeneration of tendons post injury could be associated with an increase in vascularization with randomly arranged blood vessels and a disordered collagen fibre arrangement with inhomogeneous distribution of collagen, irregular crimp formation of fibre bundles, and an elevated fraction of collagen type III compared to type I. 52 Many studies have shown that degenerative tendon diseases, e.g. in the rotator cuff, preferentially develop in hypovascular regions. 68 Degeneration reduces the mechanical properties of tendons and predisposes them to re-injury due to disorganization of collagen fibrils and neovascularization in the context of inflammation. Studies on tendinopathy have revealed that vascularization is critical in tendon healing, demonstrating direct impairing effects of neoangiogenesis on biomechanical properties as shown for degenerative patellar tendons. 69 Age-related structural changes such as decrease in collagen content may also impair tendon healing after degeneration and injury. The prominent histological and molecular features of degeneration include tendon thickening, disorganization of collagen fibrils, an increase in the microvasculature and sensory nerve innervation, dysregulated ECM homeostasis, increased immune cells and inflammatory mediators, and enhanced cellular apoptosis. 70,71 The key features of tendon degeneration are summarized in Table III.
Table III.
Key tissue changes in the degenerated Achilles tendon.
| Key features in degenerated AT | Effects | References |
|---|---|---|
| Increase in collagen type III / decrease in type I | Biomechanical stability decreases | 52,52,72 |
| Fibre arrangement: tendon thickening, disorganization of collagen fibrils | Change in biomechanical properties (> stiffness, < elasticity) | 70,71,73 |
| Increased angiogenesis, irregular arrangement | Impaired biomechanical properties, VEGF↑, MMP-3↑ | 69,72 |
| Increased nerve ingrowth | Pain | 74 |
| Misrouted SC differentiation | Ossification, adipogenesis | 75,76,77 |
| Transdifferentiation, e.g. myofibroblast transition | Contraction, scarring | 78,79 |
AT, Achilles tendon; MMP, matrix metalloproteinase; SC, stem cell; VEGF, vascular endothelial growth factor.
Inflammation in AT healing
Inflammation is decisive for the outcome of AT healing and bears the risk of AT degeneration. Like in every tissue of the body, immune cells can also be found in AT. Tendon pain, oedema, and inflammation represent the immediate response to injuries associated with emigration of leucocytes from vessels into tissue (leukodiapedesis) and an early healing response. 80 In a very elaborative study, Ribitsch et al 81 compared fetal and adult tendon healing and saw regeneration in the fetal tendons, while the adult tendons formed scar tissue. In contrast to fetal tissue, adult tissue was characterized by fewer macrophages but increased neutrophils associated with an increased detection of neutrophil-specific proteins. In human ATs, rupture affects the expression of inflammatory cytokines with decreased expression of IL-33, but increased expression of IL-6, IL-10, CD68, and macrophage inhibitory factor-1 (MIF-1) compared to an intact tendon. 82 Dakin et al 83 summarized in a review that not only inflammation, a natural process during healing, but the resolution of inflammation is also important for tendon healing. Inflammation activates a highly regulated process with specialized pro-resolving mediators allowing healing. The group furthermore showed that pro-resolving mediators, such as lipoxin and maresin, modulate the inflammatory status of stromal cells from AT ruptures. 84 IL-1β stimulated cells co-incubated with the pro-resolving mediators showed reduced levels of inflammation initiating factors and increased levels of pro-resolving mediators. To improve healing, exosomes derived from tendon SCs were injected, and notably reduced inflammation (by modulating macrophage polarization and cytokine expression) and apoptosis were seen in healing rat ATs. 85 Characterization of the effect of exosomes revealed a dosage-dependent stimulation of tenocytes’ proliferation and migration. 85 The influence of inflammation on the performance of tendon SCs and tendon healing is summarized by Vinhas et al. 86
Tenocyte cross-talk with immune cells in AT healing
The exchange of exosomes and soluble mediators might also play a role in tenocyte communication with leucocytes. Tenocytes are well known to interact with blood-derived leucocytes via soluble mediators. The presence of peripheral blood mononuclear cells (PBMCs) induced tenocyte proinflammatory gene expression (IL-1β, tumour necrosis factor (TNF)-α, IL-6) in an autologous Achilles tenocyte/PBMC indirect co-culture system; this suggested that the interplay between both cell types was mediated by the exchange of soluble factors. 87 Tenogenesis of equine adipose tissue-derived SCs, e.g. shown by SCX expression and proliferation, was compromised in the presence of leucocytes. 88 Macrophage tenocyte interactions in particular occur in tendon injury and healing processes. An abnormal macrophage number and profile accompanied by hypervascularization, as well as erroneous ECM deposition, was identified in the early phase of tendon repair following surgically induced AT injury in mice deficient of TNMD (a mature gene marker for tendons). 89 Macrophages are required to remove damaged cells and ECM fragments. However, it was observed that M1-polarized macrophages mediate tendon inflammation and degeneration. 90 In contrast, anti-inflammatory M2 macrophages supported AT healing. 91 They could be generated by exposing macrophages to MSC-derived exosomes. 91 An improvement in AT healing could be achieved by reducing M1-polarized macrophages and generating a shift to the M2 phenotype to stimulate regenerative processes. 91 The direct but also indirect interaction (via soluble mediators) of macrophages with co-cultured tenocytes derived in this case from the supraspinatus tendon led to an increase in CD80, but reduced HLA-DR expression on macrophages, indicating their mixed type of polarization, and evoked an increased release of IL-6, IL-8, and monocyte chemoattractant protein-1, predominantly after inflammatory pre-stimulation of tenocytes. 92 In general, proinflammatory cytokines, as well as MMPs and chemokines, are part of the senescence-associated phenotype shift to a senescence-associated secretory phenotype (SASP, Figure 3), which affects paracrine senescence, immune cell invasion, and chronic inflammation. 93
Pathophysiology of tendon tissue and cells affecting AT repair
De- and transdifferentiation in tendon healing
Tenocytes are known to undergo phenotypic shifts under pathophysiological conditions and during the first passages of in vitro culture, as observed in tenocytes isolated from ruptured AT. 94 In cultured chick embryonic tenocytes, a maximum of four passages was recommended to avoid phenotypic shift and senescence. 95 The transition from terminally differentiated cell to a less-differentiated state within the same lineage is known as dedifferentiation. 96 In tendon biology, this effect has been described with tenocytes reverting to tenoblasts during healing. 97 Tan et al 98 showed recently, using a tissue-specific conditional TGFβ type II receptor-knockout model (Tgfbr2; ScxCre), that upon the loss of TGFβ signalling most of the mutant tendon cells not only lost SCX, COL type I α 1 chain (Col1a1), and TNMD gene expression, but also acquired some stem/progenitor features such as SCA-1, CD44, and CD34 expression. However, it was also stated that loss of TGFβ might prevent these cells from gaining the total spectrum of stemness or plasticity. 98
The term ‘transdifferentiation’ refers to the cell regression to a point from which a switch to another lineage can occur. 96 Unsuccessful tendon healing associated with aberrant cell differentiation towards myofibroblasts can result in erroneous ECM deposition and fibrosis 78,79 and trauma-induced heterotopic ossification (HO) via chronic inflammation 99 or endochondral ossification (Figure 3). 100 Interestingly, loss of TNMD in mice could lead to misrouted differentiation towards adipocytes in the early repair phase, 89 and osteocytes via endochondral ossification in the late repair phase. 101 The presence of adipocytes might weaken the compact tendon structure, structural integrity, and biomechanical properties, thereby increasing the risk of rupture. 102 In addition, these cells are known to be paracrine-active, 8 hence it can be hypothesized that paracrine signals from adipose tissue-derived cells might also interfere with AT homeostasis. It has been shown that the inflammatory status of adjacent tissue, such as Kager’s fat pad, is increased in patients suffering from tendinopathy. 7 Maintenance of the tissue-specific differentiated phenotype is guaranteed by epigenetic regulators. 103 Changes of epigenetics including the de-/methylation of DNA and modifications of histones, which organize DNA packing and accessibility for transcription as well as non-coding RNAs, might alter SC lineage differentiation by influencing gene activities. Ageing can lead to the inability of cells to maintain their differentiated phenotype and then ultimately to cell senescence, 103 and might therefore reduce the healing capacity of the cells.
Tendon tissue ageing, cell senescence, and premature cell death
In AT, the natural ageing process contributes directly to and correlates with the occurrence of ruptures, and alters both, the cellular and ECM compartments. 5 Some of the best-described features associated with the ageing process at a cellular and molecular level are cell cycle arrest, 104 the decrease of cell proliferation capacity, and increased cellular senescence. 76,105 Senescence describes ‘cell ageing’, and it is known to be triggered by epigenetic alterations such as DNA methylation and histone modifications in MSCs, 106,107 as well as by DNA damage accumulation caused via reactive oxygen species 108 and by telomere erosion, leading to the activation of the p14ARF and p16INK4A tumour-suppressor pathways in SCs. 109
A detailed comparison of the cellular and molecular characteristics of human TSPCs revealed increased senescence in the cells derived from aged/degenerative AT, namely increased number of β-galactosidase (β–gal) positive cells and enhanced expression of p16INK. 76 Similarly, a recent study demonstrated that a higher number of senescent cells can be isolated from aged rat ATs. 110 Senescence was associated in aged tenocytes with the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway (Figure 3). Inhibition of this pathway reduced cellular senescence and SASP, as shown by reduced expression of inflammatory and catabolic mediators including IL-6, IL-1β, MMP-3, MMP-9, and CXCL12, thereby restoring the cell function. Moreover, it was demonstrated that the incubation of young tenocytes with proinflammatory IFN-γ promoted senescence. 110 Interestingly, Yan et al 111 demonstrated that aged TSPCs are also less competent at forming 3D tendon organoids in vitro, suggesting that such cells will fail in building tendon tissue during repair. Among other features, aged tendons present compositional, structural, and biomechanical modifications that slow down repair. Studies have shown that ECM gene expression of collagen types I, III, and IV, elastin, and lubricin is decreased due to ageing. 112,113 Conversely, the activation of genes related to ECM remodelling such as MMP-2, MMP-8, and MMP-9 is strongly increased with age. 114,115 Analysis of collagen fibril diameter in human and murine ATs from three- and 18-month-old animals revealed increased presence of small fibrils in the neonatal period versus increased content of larger and higher variability of fibril diameters in adults. 73,112 The diminished and inferior viscoelastic properties in aged tendons are associated with the enhanced cross-linking of collagen, 116 leading to an increased cross-sectional area of the AT as well as AT stiffening. 117 Further intensive research is needed to unravel the role of ageing in AT repair.
Loss of instructive cell niches
Regarding cellular niches in tendon, inflammation might influence the performance of tendon SCs and niche characteristics. 86 Also, changes in the tendon ECM properties can affect cell behaviour. For example, Bi et al 39 demonstrated a crucial role of two ECM components – biglycan and fibromodulin. Depletion of these proteoglycans greatly altered TSPC proliferation and differentiation due to alterations in the bone morphogenetic protein signalling pathway. 39
A novel strategy to achieve cell rejuvenation is supplying cells with instructive niches and providing proper surface topography (e.g. by surface roughness), the appropriate micro-nanoscale organization (e.g. fibril size, distribution and alignment), as well as stiffness (Young’s modulus). For example, when provided with a 3D self-assembling nanofibre hydrogel, aged human TSPCs showed a comparable cell survival and proliferation to young TSPCs as well as restored cell morphology and cytoskeletal architecture. 118 Jiang et al 119 could demonstrate that decellularized ECM from young TSPCs enhanced cell proliferation and differentiation potential of aged TSPCs, and reduced cell senescence. Hence, the identification of novel regulatory biochemical, topographical, and biomechanical factors in the tendon cellular niches during tissue homeostasis, and the ageing and healing processes, would be of great relevance for the tendon field. Expanding such knowledge could contribute to the design of smart strategies to steer progenitor/SCs towards desired behaviour and better healing outcomes.
Key mediators and processes during AT healing
Tenomodulin as molecular factor influencing the repair process
A multitude of molecular factors contribute to AT healing depending on the healing phase (Table I). A molecular factor not listed in Table I but known to be important during AT repair is TNMD. This gene encodes a type II transmembrane glycoprotein with a cleavable C-terminal cysteine-rich domain secreted in the tendon ECM, and has a dual function, namely as a pro-proliferative agent in tendon cells 120 and anti-angiogenic factor inhibiting vascular cell migration. 121 AT of TNMD-deficient mice exhibits a substantially reduced cell density and proliferation in vivo, paralleled with pathological thickening of collagen fibrils, 120 which is a phenotypical modification associated with premature tissue ageing. 122 In order to uncover TNMD role during healing, Lin et al 89 surgically induced a complete AT injury in the midsubstance in TNMD-deficient mice. Detailed analysis at day 8 post injury revealed a profound difference in scar organization, with greatly reduced cell proliferation and increased cell apoptosis, senescence, inflammation, and adipocyte and blood vessel accumulation. 89 Interestingly, when assessed at later timepoints of the healing process, TNMD-knockout tendons exhibited markedly increased heterotopic ossification (HO) paralleled by diminished biomechanical and functional properties of the ATs. 101
Neovascularization/angiogenesis/innervation
Unbridled inflammation during healing is associated with an abundance of diverse mediators such as cytokines and chemokines inducing hypervascularization, which is in contrast to the mature healthy AT known as a hypovascular and hypoinnervated tissue. 9,123 Recent research showed that impairing neoangiogenesis in ruptured tendons promotes healing in the AT. 124 Sprouting of sensory nerve endings during healing AT is also guided by inflammatory mediators such as chemokines. 125 Nerve ingrowth into the tendon fascicles, along with time-dependently emerging sensory, autonomic, and glutamatergic mediators, amplifies and modulates tendon inflammation and healing. 74 Nerve branches follow newly formed blood vessels into the healing area of the tendon, but relocate later during the remodelling and reorganization process to the surrounding AT, particularly the paratenon. 74,125 However, painful degenerated tendons remaining after unsuccessful healing might reflect an uncoupled reinnervation process. 74
ECM remodelling
The composition and organization of the ECM determines the mechanical properties of the tendon, and clear histological alterations can be seen after rupture. On a histological level, Maffulli et al 72 showed alterations in AT fibre structure and arrangement, nuclei morphology, and increased cellularity, vascularity, and glycosaminoglycan content, but decreased collagen stainability. As mentioned earlier, the main ECM protein of healthy tendon is collagen. In intact tendons, collagen has a very low turnover rate and it is hypothesized that formation occurs only in the first 17 years of life. 126 Using double quantum filtered nuclear magnetic resonance (NMR) spectroscopy, the maturation of collagen fibres in regenerating rabbit AT was monitored by Ikoma et al. 127 The authors found an ongoing reorientation of the collagen fibres with an increasing alignment of the collagen over time. The data from the non-invasive NMR spectroscopy were supported by the histology. The main collagen subtype in tendon is collagen type I, with lower amounts of types II, III, V, and VI. 25 During human AT healing the amount of collagen type III is increased, which might lead to thinner collagen fibres and decreased mechanical properties. 52 There was also a substantial increase in elastin (from 2% to 4%) content observed in healing AT, influencing mechanical properties. 58 Although tendons have a low ECM metabolism, remodelling takes place in healthy and injured tendons mainly due to the activity and balance of MMPs and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMPs). Analyzing the expression in ruptured human ATs, collagen types I and III, as well as MMP-1, MMP-2, MMP-13, and TIMP-1 increased with time after rupture while MMP-3 and MMP-10 decreased. 57 Improved ECM maturation and superior biomechanical properties after rupture were seen in a rabbit AT model with TGF-β1 gene transfer in comparison to the controls without gene transfer, according to a study by Hou et al. 128 These authors also concluded that there was an improved cross-linking of the collagen, as synthesis of the cross-linking enzyme lysyl oxidase (LOX) can be upregulated by TGF-β1. Cross-linking between collagen and elastin fibres is catalyzed by LOX enzymes resulting in insoluble ECM complexes with modified mechanical characteristics, and this is an important process in intact as well as in healing tissue. 129
Mechanoresponse
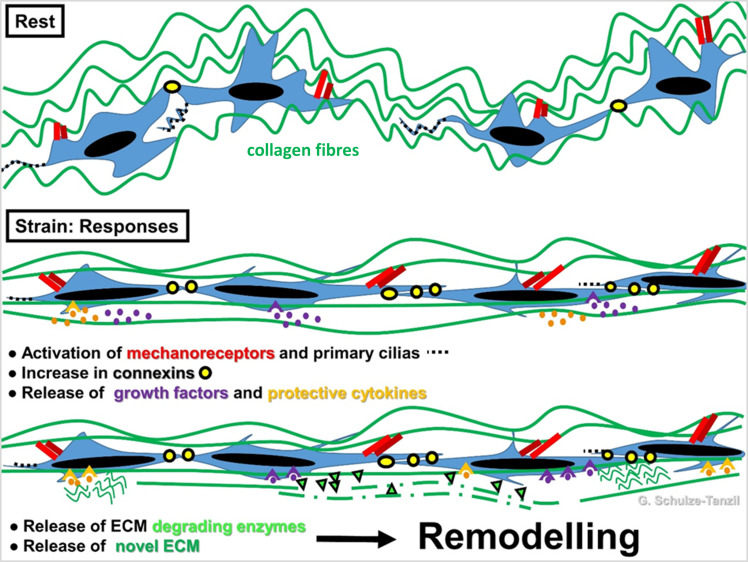
ECM remodelling is strongly influenced by mechanical stimuli (Figure 4), since tenocytes are mechanosensitive cells. 130
Fig. 4.
Scheme of tenocyte mechanoresponse. Stretching at a physiological level leads to activation of mechanoreceptor and primary cilias. Crimping of the collagen fibre bundles disappears (in biomechanical measurement: toe region of a stress-stain curve). Connexin expression is elevated and hence, cell–cell signalling via gap junctions is also elevated. Protective cytokines and anabolic growth factors are released followed by de novo extracellular matrix (ECM) synthesis and ECM-degrading enzyme release, which mediate reorganization of ECM by a remodelling process to adapt the ECM biomechanics according to the stretch direction. The image was created by G. G. Schulze-Tanzil using Krita 4.1.7 (Krita Foundation, The Netherlands).
It is well known that immobilization of a healing tendon is detrimental to the healing process. Disturbed mechanoresponse and mechanotransduction in tendon cells can lead to failure in healing. Runesson et al 36 demonstrated that exercise elevated in vivo cell proliferation in rapidly dividing cells, whereas the stem/progenitor population did not change in number. Moreover, exercise has also been shown to influence the immune response in tendons. 66 Eliasson et al 131 performed several in vivo studies to investigate the effect of load on rat AT healing. In a study from 2009, they investigated the effect of mechanical unloading (by botox injection into the gastrocnemius muscle) on gene expression during healing. Unloading resulted in a reduced transverse tendon area and reduced mechanical properties. On the gene expression level, an increased expression of TNF-α, TGF-β1, LOX, and procollagens types I and III was seen in the unloaded healing tendons at the early timepoints. At the later timepoints, most investigated markers had increased expression in loaded tendons. In a subsequent study, the AT of tail-suspended rats was transected and gene expression after one episode of loading was analyzed. 132 Strong upregulation of 86 genes and downregulation of 64 genes were seen three hours after loading, while only a few genes were regulated after 48 hours. Upregulated genes were associated with inflammation and coagulation, while proteoglycans and collagens were downregulated. Loading also resulted in an increased peak force at day 7. Applying different loading regimens, Hammerman et al 133 were able to show that mild load (around 10 Newtons in animal experiments) increased the mechanical properties without affecting the expression of inflammatory genes, which were activated after strong loading.
Future perspectives
Tendon ruptures are common injuries, and the AT is affected in 20% of cases. The tendon heals by the formation of an inferior scar tissue, and the function is often impaired. Epidemiological investigations have shown that the incidence is increasing in both athletic and occupational populations. The incidence of AT ruptures ranges between six and 37 per 100,000 inhabitants in cohorts from the European Union and Canada. 134-136 The overall incidence has been rising in recent decades, based on an increasing incidence of this condition in the older population due to degeneration, 136 and elastic properties of a healed AT are inferior even a long time after rupture. 60 To optimize and coordinate the AT healing process as early as possible, a mechanostimulation protocol adapted to the individual patient’s healing progress would be highly advantageous. Studies have revealed that in patients with chronic tendinopathy, eccentric training and local administration of a sclerosing agent, polidocanol, improved the sport activity level and pain by reducing the number of neovessels. 137 Eccentric training describes the muscle work when a muscle continuously elongates during load-bearing, for example by slowly releasing a carried load to the floor. Preclinical results with non-invasive electromagnetic stimulation of AT during healing of tendinopathy are promising, 138 and could be beneficial for clinical treatment. These clinical findings are in line with the results showing increased neovascularization and VEGF synthesis in affected tendons, and previous observations where vascularization was directly correlated to biomechanical properties of the tendon. 139 Considering the pivotal role that the successful resolution of inflammation plays during AT healing, an anti-inflammatory treatment might present a future approach to reduce unrestrained neovascularization and overflowing innervation associated with pain. 124 In vivo monitoring, as well as controlling and modulating vascularization during tendon regeneration, might be a promising treatment approach in the future. 69
MSC-derived exosomes could present a strategy to resolving overflowing inflammation in healing AT. 91 Understanding how different cell populations engage and are regulated by the discrete niches they reside in would also be very beneficial in identifying novel strategies to navigate cell behaviour during repair. Modifying niche parameters could further contribute to more satisfactory healing outcomes. Taking into account that in cases of critical AT defects healing might not be successful at all, and that suitable autografts are limited, tissue engineering could provide an approach to bridge these defects, e.g. based on allogenic or xenogenic AT ECMs or synthetic biomaterials. 140,141 Biomaterial-free cell sheets made from SCs could also be of advantage to improving biomechanical properties of healing ATs. 142 Recently, it was found that tenocytes differentiated from induced progenitor SCs could successfully be used to augment AT healing and restore function. 143 All in all, based on the complexity of the AT, as well as due to demographic changes and rising incidences of tendon pathologies, a multifactorial approach combining different imaging, biochemical, cellular, and biomechanical aspects is necessary to improve AT repair and achieve major breakthroughs in tendon injury management.
Author contributions
G. G. Schulze-Tanzil: Conceptualization, Writing – original draft, Visualization, Writing – review & editing.
M. Delgado Cáceres: Writing – original draft, Writing – review & editing.
R. Stange: Writing – review & editing.
B. Wildemann: Conceptualization, Writing – original draft, Writing – review & editing.
D. Docheva: Conceptualization, Writing – original draft, Writing – review & editing.
Funding statement
The authors received no financial or material support for the research, authorship, and/or publication of this article.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Gundula G. Schulze-Tanzil, Email: gundula.schulze@pmu.ac.at.
Manuel Delgado Cáceres, Email: manuel.delgado-caceres@klinik.uni-regensburg.de.
Richard Stange, Email: richard.stange@ukmuenster.de.
Britt Wildemann, Email: Britt.Wildemann@med.uni-jena.de.
Denitsa Docheva, Email: denitsa.docheva@klinik.uni-regensburg.de.
References
- 1. O’Brien M . The anatomy of the achilles tendon . Foot Ankle Clin . 2005. ; 10 ( 2 ): 225 – 238 . 10.1016/j.fcl.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 2. Ballal MS , Walker CR , Molloy AP . The anatomical footprint of the achilles tendon: a cadaveric study . Bone Joint J . 2014. ; 96-B ( 10 ): 1344 – 1348 . 10.1302/0301-620X.96B10.33771 [DOI] [PubMed] [Google Scholar]
- 3. Doral MN , Alam M , Bozkurt M , et al. . Functional anatomy of the Achilles tendon . Knee Surg Sports Traumatol Arthrosc . 2010. ; 18 ( 5 ): 638 – 643 . 10.1007/s00167-010-1083-7 [DOI] [PubMed] [Google Scholar]
- 4. Szaro P , Witkowski G , Smigielski R , Krajewski P , Ciszek B . Fascicles of the adult human achilles tendon - an anatomical study . Ann Anat . 2009. ; 191 ( 6 ): 586 – 593 . 10.1016/j.aanat.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 5. Hess GW . Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention . Foot Ankle Spec . 2010. ; 3 ( 1 ): 29 – 32 . 10.1177/1938640009355191 [DOI] [PubMed] [Google Scholar]
- 6. Rufai A , Ralphs JR , Benjamin M . Structure and histopathology of the insertional region of the human achilles tendon . J Orthop Res . 1995. ; 13 ( 4 ): 585 – 593 . 10.1002/jor.1100130414 [DOI] [PubMed] [Google Scholar]
- 7. Pingel J , Petersen MCH , Fredberg U , et al. . Inflammatory and Metabolic Alterations of Kager’s Fat Pad in Chronic Achilles Tendinopathy . PLoS One . 2015. ; 10 ( 5 ): e0127811 . 10.1371/journal.pone.0127811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Labusca L , Zugun-Eloae F . The unexplored role of intra-articular adipose tissue in the homeostasis and pathology of articular joints . Front Vet Sci . 2018. ; 5 : 35 . 10.3389/fvets.2018.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmed IM , Lagopoulos M , McConnell P , Soames RW , Sefton GK . Blood supply of the achilles tendon . J Orthop Res . 1998. ; 16 ( 5 ): 591 – 596 . 10.1002/jor.1100160511 [DOI] [PubMed] [Google Scholar]
- 10. Chen TM , Rozen WM , Pan W-R , Ashton MW , Richardson MD , Taylor GI . The arterial anatomy of the Achilles tendon: anatomical study and clinical implications . Clin Anat . 2009. ; 22 ( 3 ): 377 – 385 . 10.1002/ca.20758 [DOI] [PubMed] [Google Scholar]
- 11. Zantop T , Tillmann B , Petersen W . Quantitative assessment of blood vessels of the human achilles tendon: an immunohistochemical cadaver study . Arch Orthop Trauma Surg . 2003. ; 123 ( 9 ): 501 – 504 . 10.1007/s00402-003-0491-2 [DOI] [PubMed] [Google Scholar]
- 12. Mazzone MF , McCue T . Common conditions of the achilles tendon . Am Fam Physician . 2002. ; 65 ( 9 ): 1805 – 1810 . [PubMed] [Google Scholar]
- 13. Shim VB , Handsfield GG , Fernandez JW , Lloyd DG , Besier TF . Combining in silico and in vitro experiments to characterize the role of fascicle twist in the achilles tendon . Sci Rep . 2018. ; 8 ( 1 ): 13856 . 10.1038/s41598-018-31587-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prosenz J , Rath C , Hadrovic-Avdic M , Hirtler L . The twist of the achilles tendon - associations of torsions in the lower extremity . Clin Anat . 2018. ; 31 ( 7 ): 1085 – 1091 . 10.1002/ca.23247 [DOI] [PubMed] [Google Scholar]
- 15. Knaus KR , Blemker SS . 3D models reveal the influence of achilles subtendon twist on strain and energy storage . Front Bioeng Biotechnol . 2021. ; 9 : 539135 . 10.3389/fbioe.2021.539135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Obst SJ , Renault JB , Newsham-West R , Barrett RS . Three-dimensional deformation and transverse rotation of the human free Achilles tendon in vivo during isometric plantarflexion contraction . J Appl Physiol (1985) . 2014. ; 116 ( 4 ): 376 – 384 . 10.1152/japplphysiol.01249.2013 [DOI] [PubMed] [Google Scholar]
- 17. Langberg H , Bülow J , Kjaer M . Blood flow in the peritendinous space of the human achilles tendon during exercise . Acta Physiol Scand . 1998. ; 163 ( 2 ): 149 – 153 . 10.1046/j.1365-201X.1998.00361.x [DOI] [PubMed] [Google Scholar]
- 18. Fang F , Lake SP . Experimental evaluation of multiscale tendon mechanics . J Orthop Res . 2017. ; 35 ( 7 ): 1353 – 1365 . 10.1002/jor.23488 [DOI] [PubMed] [Google Scholar]
- 19. Haraldsson BT , Aagaard P , Qvortrup K , et al. . Lateral force transmission between human tendon fascicles . Matrix Biol . 2008. ; 27 ( 2 ): 86 – 95 . 10.1016/j.matbio.2007.09.001 [DOI] [PubMed] [Google Scholar]
- 20. Patel D , Zamboulis DE , Spiesz EM , et al. . Structure-function specialisation of the interfascicular matrix in the human achilles tendon . Acta Biomater . 2021. ; 131 : 381 – 390 . 10.1016/j.actbio.2021.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peffers MJ , Fang Y , Cheung K , Wei TKJ , Clegg PD , Birch HL . Transcriptome analysis of ageing in uninjured human Achilles tendon . Arthritis Res Ther . 2015. ; 17 : 33 . 10.1186/s13075-015-0544-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birch HL , Bailey JV , Bailey AJ , Goodship AE . Age-related changes to the molecular and cellular components of equine flexor tendons . Equine Vet J . 1999. ; 31 ( 5 ): 391 – 396 . 10.1111/j.2042-3306.1999.tb03838.x [DOI] [PubMed] [Google Scholar]
- 23. Benjamin M , Qin S , Ralphs JR . Fibrocartilage associated with human tendons and their pulleys . J Anat . 1995. ; 187 ( Pt 3) : 625 – 633 . [PMC free article] [PubMed] [Google Scholar]
- 24. Jo CH , Lim HJ , Yoon KS . Characterization of tendon-specific markers in various human tissues, tenocytes and mesenchymal stem cells . Tissue Eng Regen Med . 2019. ; 16 ( 2 ): 151 – 159 . 10.1007/s13770-019-00182-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waggett AD , Ralphs JR , Kwan AP , Woodnutt D , Benjamin M . Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon . Matrix Biol . 1998. ; 16 ( 8 ): 457 – 470 . 10.1016/s0945-053x(98)90017-8 [DOI] [PubMed] [Google Scholar]
- 26. Buckley MR , Evans EB , Matuszewski PE , et al. . Distributions of types I, II and III collagen by region in the human supraspinatus tendon . Connect Tissue Res . 2013. ; 54 ( 6 ): 374 – 379 . 10.3109/03008207.2013.847096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reddy GK . Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon . Exp Diabesity Res . 2004. ; 5 ( 2 ): 143 – 153 . 10.1080/15438600490277860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gautieri A , Passini FS , Silván U , et al. . Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue . Matrix Biol . 2017. ; 59 : 95 – 108 . 10.1016/j.matbio.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 29. Thorpe CT , Peffers MJ , Simpson D , Halliwell E , Screen HRC , Clegg PD . Anatomical heterogeneity of tendon: Fascicular and interfascicular tendon compartments have distinct proteomic composition . Sci Rep . 2016. ; 6 : 20455 . 10.1038/srep20455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thorpe CT , Karunaseelan KJ , Ng Chieng Hin J , et al. . Distribution of proteins within different compartments of tendon varies according to tendon type . J Anat . 2016. ; 229 ( 3 ): 450 – 458 . 10.1111/joa.12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Micheli AJ , Swanson JB , Disser NP , et al. . Single-cell transcriptomic analysis identifies extensive heterogeneity in the cellular composition of mouse Achilles tendons . Am J Physiol Cell Physiol . 2020. ; 319 ( 5 ): C885 – C894 . 10.1152/ajpcell.00372.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benjamin M , Ralphs JR . Tendons and ligaments--an overview . Histol Histopathol . 1997. ; 12 ( 4 ): 1135 – 1144 . [PubMed] [Google Scholar]
- 33. Marr N , Meeson R , Kelly EF , et al. . CD146 Delineates an Interfascicular Cell Sub-Population in Tendon That Is Recruited during Injury through Its Ligand Laminin-α4 . Int J Mol Sci . 2021. ; 22 ( 18 ): 9729 . 10.3390/ijms22189729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mienaltowski MJ , Adams SM , Birk DE . Regional differences in stem cell/progenitor cell populations from the mouse achilles tendon . Tissue Eng Part A . 2013. ; 19 ( 1–2 ): 199 – 210 . 10.1089/ten.TEA.2012.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lui PPY . Markers for the identification of tendon-derived stem cells in vitro and tendon stem cells in situ - update and future development . Stem Cell Res Ther . 2015. ; 6 : 106 . 10.1186/s13287-015-0097-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Runesson E , Ackermann P , Brisby H , Karlsson J , Eriksson BI . Detection of slow-cycling and stem/progenitor cells in different regions of rat Achilles tendon: response to treadmill exercise . Knee Surg Sports Traumatol Arthrosc . 2013. ; 21 ( 7 ): 1694 – 1703 . 10.1007/s00167-013-2446-7 [DOI] [PubMed] [Google Scholar]
- 37. Walia B , Huang AH . Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair . J Orthop Res . 2019. ; 37 ( 6 ): 1270 – 1280 . 10.1002/jor.24156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang Z , Yin Z , Xu J , et al. . Tendon stem/progenitor cell subpopulations and their implications in tendon biology . Front Cell Dev Biol . 2021. ; 9 : 631272 . 10.3389/fcell.2021.631272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bi Y , Ehirchiou D , Kilts TM , et al. . Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche . Nat Med . 2007. ; 13 ( 10 ): 1219 – 1227 . 10.1038/nm1630 [DOI] [PubMed] [Google Scholar]
- 40. Harvey T , Flamenco S , Fan CM . A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis . Nat Cell Biol . 2019. ; 21 ( 12 ): 1490 – 1503 . 10.1038/s41556-019-0417-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang J , Li F , Williamson KM , et al. . Characterization of the structure, vascularity, and stem/progenitor cell populations in porcine Achilles tendon (PAT) . Cell Tissue Res . 2021. ; 384 ( 2 ): 367 – 387 . 10.1007/s00441-020-03379-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y , Zhang X , Huang H , et al. . Osteocalcin expressing cells from tendon sheaths in mice contribute to tendon repair by activating Hedgehog signaling . Elife . 2017. ; 6 : e30474 . 10.7554/eLife.30474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mienaltowski MJ , Cánovas A , Fates VA , et al. . Transcriptome profiles of isolated murine Achilles tendon proper- and peritenon-derived progenitor cells . J Orthop Res . 2019. ; 37 ( 6 ): 1409 – 1418 . 10.1002/jor.24076 [DOI] [PubMed] [Google Scholar]
- 44. Yin Z , Hu J-J , Yang L , et al. . Single-cell analysis reveals a nestin+ tendon stem/progenitor cell population with strong tenogenic potentiality . Sci Adv . 2016. ; 2 ( 11 ): e1600874 . 10.1126/sciadv.1600874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tempfer H , Wagner A , Gehwolf R , et al. . Perivascular cells of the supraspinatus tendon express both tendon- and stem cell-related markers . Histochem Cell Biol . 2009. ; 131 ( 6 ): 733 – 741 . 10.1007/s00418-009-0581-5 [DOI] [PubMed] [Google Scholar]
- 46. Xu W , Sun Y , Zhang J , et al. . Perivascular-derived stem cells with neural crest characteristics are involved in tendon repair . Stem Cells Dev . 2015. ; 24 ( 7 ): 857 – 868 . 10.1089/scd.2014.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kendal AR , Layton T , Al-Mossawi H , et al. . Multi-omic single cell analysis resolves novel stromal cell populations in healthy and diseased human tendon . Sci Rep . 2020. ; 10 ( 1 ): 13939 . 10.1038/s41598-020-70786-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Utsunomiya H , Uchida S , Sekiya I , Sakai A , Moridera K , Nakamura T . Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears . Am J Sports Med . 2013. ; 41 ( 3 ): 657 – 668 . 10.1177/0363546512473269 [DOI] [PubMed] [Google Scholar]
- 49. Steinert AF , Kunz M , Prager P , et al. . Characterization of bursa subacromialis-derived mesenchymal stem cells . Stem Cell Res Ther . 2015. ; 6 : 114 . 10.1186/s13287-015-0104-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klatte-Schulz F , Bormann N , Voss I , et al. . Bursa-derived cells show a distinct mechano-response to physiological and pathological loading in vitro . Front Cell Dev Biol . 2021. ; 9 : 657166 . 10.3389/fcell.2021.657166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Han Y , Li H , Zhou R , et al. . Comparison between intra-articular injection of infrapatellar fat pad (IPFP) cell concentrates and IPFP-mesenchymal stem cells (MSCs) for cartilage defect repair of the knee joint in rabbits . Stem Cells Int . 2021. ; 2021 : 9966966 . 10.1155/2021/9966966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eriksen HA , Pajala A , Leppilahti J , Risteli J . Increased content of type III collagen at the rupture site of human achilles tendon . J Orthop Res . 2002. ; 20 ( 6 ): 1352 – 1357 . 10.1016/S0736-0266(02)00064-5 [DOI] [PubMed] [Google Scholar]
- 53. Molloy T , Wang Y , Murrell G . The roles of growth factors in tendon and ligament healing . Sports Med . 2003. ; 33 ( 5 ): 381 – 394 . 10.2165/00007256-200333050-00004 [DOI] [PubMed] [Google Scholar]
- 54. Thomopoulos S , Parks WC , Rifkin DB , Derwin KA . Mechanisms of tendon injury and repair . J Orthop Res . 2015. ; 33 ( 6 ): 832 – 839 . 10.1002/jor.22806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arvind V , Huang AH . Reparative and maladaptive inflammation in tendon healing . Front Bioeng Biotechnol . 2021. ; 9 : 719047 . 10.3389/fbioe.2021.719047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cui Q , Fu S , Li Z . Hepatocyte growth factor inhibits TGF-β1-induced myofibroblast differentiation in tendon fibroblasts: role of AMPK signaling pathway . J Physiol Sci . 2013. ; 63 ( 3 ): 163 – 170 . 10.1007/s12576-013-0251-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Minkwitz S , Schmock A , Kurtoglu A , et al. . Time-dependent alterations of mmps, timps and tendon structure in human achilles tendons after acute rupture . Int J Mol Sci . 2017. ; 18 ( 10 ): E2199 . 10.3390/ijms18102199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Svärd A , Hammerman M , Eliasson P . Elastin levels are higher in healing tendons than in intact tendons and influence tissue compliance . FASEB J . 2020. ; 34 ( 10 ): 13409 – 13418 . 10.1096/fj.202001255R [DOI] [PubMed] [Google Scholar]
- 59. Heikkinen J , Lantto I , Piilonen J , et al. . Tendon length, calf muscle atrophy, and strength deficit after acute achilles tendon rupture: long-term follow-up of patients in a previous study . J Bone Joint Surg Am . 2017. ; 99-A ( 18 ): 1509 – 1515 . 10.2106/JBJS.16.01491 [DOI] [PubMed] [Google Scholar]
- 60. Frankewycz B , Penz A , Weber J , et al. . Achilles tendon elastic properties remain decreased in long term after rupture . Knee Surg Sports Traumatol Arthrosc . 2018. ; 26 ( 7 ): 2080 – 2087 . 10.1007/s00167-017-4791-4 [DOI] [PubMed] [Google Scholar]
- 61. Ruan Z , Zhao B , Qi H , et al. . Elasticity of healthy Achilles tendon decreases with the increase of age as determined by acoustic radiation force impulse imaging . Int J Clin Exp Med . 2015. ; 8 ( 1 ): 1043 – 1050 . [PMC free article] [PubMed] [Google Scholar]
- 62. Davis LC , Baumer TG , Bey MJ , Holsbeeck MV . Clinical utilization of shear wave elastography in the musculoskeletal system . Ultrasonography . 2019. ; 38 ( 1 ): 2 – 12 . 10.14366/usg.18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Frankewycz B , Henssler L , Weber J , et al. . Changes of material elastic properties during healing of ruptured achilles tendons measured with shear wave elastography: a pilot study . Int J Mol Sci . 2020. ; 21 ( 10 ): E3427 . 10.3390/ijms21103427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang L , Wan W , Wang Y , et al. . Evaluation of elastic stiffness in healing achilles tendon after surgical repair of a tendon rupture using in vivo ultrasound shear wave elastography . Med Sci Monit . 2016. ; 22 : 1186 – 1191 . 10.12659/MSM.895674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Frankewycz B , Krutsch W , Weber J , Ernstberger A , Nerlich M , Pfeifer CG . Rehabilitation of Achilles tendon ruptures: is early functional rehabilitation daily routine? Arch Orthop Trauma Surg . 2017. ; 137 ( 3 ): 333 – 340 . 10.1007/s00402-017-2627-9 [DOI] [PubMed] [Google Scholar]
- 66. Yang Y , Wu Y , Zhou K , et al. . Interplay of forces and the immune response for functional tendon regeneration . Front Cell Dev Biol . 2021. ; 9 : 657621 . 10.3389/fcell.2021.657621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Almekinders LC , Deol G . The effects of aging, antiinflammatory drugs, and ultrasound on the in vitro response of tendon tissue . Am J Sports Med . 1999. ; 27 ( 4 ): 417 – 421 . 10.1177/03635465990270040301 [DOI] [PubMed] [Google Scholar]
- 68. Funakoshi T , Iwasaki N , Kamishima T , et al. . In vivo visualization of vascular patterns of rotator cuff tears using contrast-enhanced ultrasound . Am J Sports Med . 2010. ; 38 ( 12 ): 2464 – 2471 . 10.1177/0363546510375536 [DOI] [PubMed] [Google Scholar]
- 69. Sahin H , Tholema N , Petersen W , Raschke MJ , Stange R . Impaired biomechanical properties correlate with neoangiogenesis as well as VEGF and MMP-3 expression during rat patellar tendon healing . J Orthop Res . 2012. ; 30 ( 12 ): 1952 – 1957 . 10.1002/jor.22147 [DOI] [PubMed] [Google Scholar]
- 70. Millar NL , Silbernagel KG , Thorborg K , et al. . Author Correction: Tendinopathy . Nat Rev Dis Primers . 2021. ; 7 ( 1 ): 10 . 10.1038/s41572-021-00251-8 [DOI] [PubMed] [Google Scholar]
- 71. Millar NL , Silbernagel KG , Thorborg K , et al. . Tendinopathy . Nat Rev Dis Primers . 2021. ; 7 ( 1 ): 1 . 10.1038/s41572-020-00234-1 [DOI] [PubMed] [Google Scholar]
- 72. Maffulli N , Barrass V , Ewen SW . Light microscopic histology of achilles tendon ruptures. A comparison with unruptured tendons . Am J Sports Med . 2000. ; 28 ( 6 ): 857 – 863 . 10.1177/03635465000280061401 [DOI] [PubMed] [Google Scholar]
- 73. Strocchi R , De Pasquale V , Guizzardi S , et al. . Human Achilles tendon: morphological and morphometric variations as a function of age . Foot Ankle . 1991. ; 12 ( 2 ): 100 – 104 . 10.1177/107110079101200207 [DOI] [PubMed] [Google Scholar]
- 74. Ackermann PW , Franklin SL , Dean BJF , Carr AJ , Salo PT , Hart DA . Neuronal pathways in tendon healing and tendinopathy--update . Front Biosci (Landmark Ed) . 2014. ; 19 ( 8 ): 1251 – 1278 . 10.2741/4280 [DOI] [PubMed] [Google Scholar]
- 75. Lui PPY , Wong CM . Biology of tendon stem cells and tendon in aging . Front Genet . 2019. ; 10 : 1338 . 10.3389/fgene.2019.01338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kohler J , Popov C , Klotz B , et al. . Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration . Aging Cell . 2013. ; 12 ( 6 ): 988 – 999 . 10.1111/acel.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu L , Li Z , Chen S , et al. . BRD4 promotes heterotopic ossification through upregulation of LncRNA MANCR . Bone Joint Res . 2021. ; 10 ( 10 ): 668 – 676 . 10.1302/2046-3758.1010.BJR-2020-0454.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dyment NA , Hagiwara Y , Matthews BG , Li Y , Kalajzic I , Rowe DW . Lineage tracing of resident tendon progenitor cells during growth and natural healing . PLoS One . 2014. ; 9 ( 4 ): e96113 . 10.1371/journal.pone.0096113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Howell K , Chien C , Bell R , et al. . Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing . Sci Rep . 2017. ; 7 : 45238 . 10.1038/srep45238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bianchi E , Ruggeri M , Rossi S , et al. . Innovative strategies in tendon tissue engineering . Pharmaceutics . 2021. ; 13 ( 1 ): 89 . 10.3390/pharmaceutics13010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ribitsch I , Bileck A , Aldoshin AD , et al. . Molecular mechanisms of fetal tendon regeneration versus adult fibrous repair . Int J Mol Sci . 2021. ; 22 ( 11 ): 5619 . 10.3390/ijms22115619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Klatte-Schulz F , Minkwitz S , Schmock A , et al. . Different achilles tendon pathologies show distinct histological and molecular characteristics . Int J Mol Sci . 2018. ; 19 ( 2 ): E404 . 10.3390/ijms19020404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dakin SG , Dudhia J , Smith RKW . Resolving an inflammatory concept: the importance of inflammation and resolution in tendinopathy . Vet Immunol Immunopathol . 2014. ; 158 ( 3–4 ): 121 – 127 . 10.1016/j.vetimm.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dakin SG , Colas RA , Newton J , et al. . 15-Epi-LXA4 and MaR1 counter inflammation in stromal cells from patients with achilles tendinopathy and rupture . FASEB J . 2019. ; 33 ( 7 ): 8043 – 8054 . 10.1096/fj.201900196R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang M , Liu H , Cui Q , et al. . Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon . Stem Cell Res Ther . 2020. ; 11 ( 1 ): 402 . 10.1186/s13287-020-01918-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vinhas A , Rodrigues MT , Gomes ME . Exploring stem cells and inflammation in tendon repair and regeneration . Adv Exp Med Biol . 2018. ; 1089 : 37 – 46 . 10.1007/5584_2018_258 [DOI] [PubMed] [Google Scholar]
- 87. Al-Sadi O , Schulze-Tanzil G , Kohl B , et al. . Tenocytes, pro-inflammatory cytokines and leukocytes: a relationship? Muscles Ligaments Tendons J . 2011. ; 1 ( 3 ): 68 – 76 . [PMC free article] [PubMed] [Google Scholar]
- 88. Brandt L , Schubert S , Scheibe P , et al. . Tenogenic Properties of Mesenchymal Progenitor Cells Are Compromised in an Inflammatory Environment . Int J Mol Sci . 2018. ; 19 ( 9 ): E2549 . 10.3390/ijms19092549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lin D , Alberton P , Caceres MD , Volkmer E , Schieker M , Docheva D . Tenomodulin is essential for prevention of adipocyte accumulation and fibrovascular scar formation during early tendon healing . Cell Death Dis . 2017. ; 8 ( 10 ): e3116 . 10.1038/cddis.2017.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sunwoo JY , Eliasberg CD , Carballo CB , Rodeo SA . The role of the macrophage in tendinopathy and tendon healing . J Orthop Res . 2020. ; 38 ( 8 ): 1666 – 1675 . 10.1002/jor.24667 [DOI] [PubMed] [Google Scholar]
- 91. Chamberlain CS , Clements AEB , Kink JA , et al. . Extracellular vesicle-educated macrophages promote early achilles tendon healing . Stem Cells . 2019. ; 37 ( 5 ): 652 – 662 . 10.1002/stem.2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stolk M , Klatte-Schulz F , Schmock A , Minkwitz S , Wildemann B , Seifert M . New insights into tenocyte-immune cell interplay in an in vitro model of inflammation . Sci Rep . 2017. ; 7 ( 1 ): 9801 . 10.1038/s41598-017-09875-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Birch J , Gil J . Senescence and the SASP: many therapeutic avenues . Genes Dev . 2020. ; 34 ( 23–24 ): 1565 – 1576 . 10.1101/gad.343129.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yao L , Bestwick CS , Bestwick LA , Maffulli N , Aspden RM . Phenotypic drift in human tenocyte culture . Tissue Eng . 2006. ; 12 ( 7 ): 1843 – 1849 . 10.1089/ten.2006.12.1843 [DOI] [PubMed] [Google Scholar]
- 95. Nguyen PK , Deng F , Assi S , et al. . Phenotype stability, expansion potential, and senescence of embryonic tendon cells in vitro . J Orthop Res . 2021. ; Epub ahead of print . 10.1002/jor.25180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jopling C , Boue S , Izpisua Belmonte JC . Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration . Nat Rev Mol Cell Biol . 2011. ; 12 ( 2 ): 79 – 89 . 10.1038/nrm3043 [DOI] [PubMed] [Google Scholar]
- 97. Davidson CJ , Ganion LR , Gehlsen GM , Verhoestra B , Roepke JE , Sevier TL . Rat tendon morphologic and functional changes resulting from soft tissue mobilization . Med Sci Sports Exerc . 1997. ; 29 ( 3 ): 313 – 319 . 10.1097/00005768-199703000-00005 [DOI] [PubMed] [Google Scholar]
- 98. Tan G-K , Pryce BA , Stabio A , et al. . Tgfβ signaling is critical for maintenance of the tendon cell fate . Elife . 2020. ; 9 : e52695 . 10.7554/eLife.52695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sorkin M , Huber AK , Hwang C , et al. . Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing . Nat Commun . 2020. ; 11 ( 1 ): 722 . 10.1038/s41467-019-14172-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Feng H , Xing W , Han Y , et al. . Tendon-derived cathepsin K-expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification . J Clin Invest . 2020. ; 130 ( 12 ): 6354 – 6365 . 10.1172/JCI132518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Delgado Caceres M , Angerpointner K , Galler M , et al. . Tenomodulin knockout mice exhibit worse late healing outcomes with augmented trauma-induced heterotopic ossification of Achilles tendon . Cell Death Dis . 2021. ; 12 ( 11 ): 1049 . 10.1038/s41419-021-04298-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang Y , He G , Wang F , et al. . Aspirin inhibits adipogenesis of tendon stem cells and lipids accumulation in rat injury tendon through regulating PTEN/PI3K/AKT signalling . J Cell Mol Med . 2019. ; 23 ( 11 ): 7535 – 7544 . 10.1111/jcmm.14622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bitman-Lotan E , Orian A . Nuclear organization and regulation of the differentiated state . Cell Mol Life Sci . 2021. ; 78 ( 7 ): 3141 – 3158 . 10.1007/s00018-020-03731-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tsai W-C , Chang H-N , Yu T-Y , et al. . Decreased proliferation of aging tenocytes is associated with down-regulation of cellular senescence-inhibited gene and up-regulation of p27 . J Orthop Res . 2011. ; 29 ( 10 ): 1598 – 1603 . 10.1002/jor.21418 [DOI] [PubMed] [Google Scholar]
- 105. Hu C , Zhang Y , Tang K , Luo Y , Liu Y , Chen W . Downregulation of CITED2 contributes to TGFβ-mediated senescence of tendon-derived stem cells . Cell Tissue Res . 2017. ; 368 ( 1 ): 93 – 104 . 10.1007/s00441-016-2552-1 [DOI] [PubMed] [Google Scholar]
- 106. Shibata KR , Aoyama T , Shima Y , et al. . Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion . Stem Cells . 2007. ; 25 ( 9 ): 2371 – 2382 . 10.1634/stemcells.2007-0225 [DOI] [PubMed] [Google Scholar]
- 107. Wagner W , Ho AD , Zenke M . Different facets of aging in human mesenchymal stem cells . Tissue Eng Part B Rev . 2010. ; 16 ( 4 ): 445 – 453 . 10.1089/ten.TEB.2009.0825 [DOI] [PubMed] [Google Scholar]
- 108. Chen J-H , Stoeber K , Kingsbury S , Ozanne SE , Williams GH , Hales CN . Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts . J Biol Chem . 2004. ; 279 ( 47 ): 49439 – 49446 . 10.1074/jbc.M409153200 [DOI] [PubMed] [Google Scholar]
- 109. Sharpless NE , DePinho RA . How stem cells age and why this makes us grow old . Nat Rev Mol Cell Biol . 2007. ; 8 ( 9 ): 703 – 713 . 10.1038/nrm2241 [DOI] [PubMed] [Google Scholar]
- 110. Chen M , Xiao L , Dai G , et al. . Inhibition of JAK-STAT signaling pathway alleviates age-related phenotypes in tendon stem/progenitor cells . Front Cell Dev Biol . 2021. ; 9 : 650250 . 10.3389/fcell.2021.650250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yan Z , Yin H , Brochhausen C , Pfeifer CG , Alt V , Docheva D . Aged Tendon Stem/Progenitor Cells Are Less Competent to Form 3D Tendon Organoids Due to Cell Autonomous and Matrix Production Deficits . Front Bioeng Biotechnol . 2020. ; 8 : 406 . 10.3389/fbioe.2020.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gehwolf R , Wagner A , Lehner C , et al. . Pleiotropic roles of the matricellular protein Sparc in tendon maturation and ageing . Sci Rep . 2016. ; 6 : 32635 . 10.1038/srep32635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kostrominova TY , Brooks SV . Age-related changes in structure and extracellular matrix protein expression levels in rat tendons . Age (Dordr) . 2013. ; 35 ( 6 ): 2203 – 2214 . 10.1007/s11357-013-9514-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yu T-Y , Pang J-H , Wu K-H , Chen M-L , Chen C-H , Tsai W-C . Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes . BMC Musculoskelet Disord . 2013. ; 14 : 2 . 10.1186/1471-2474-14-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wood LK , Brooks SV . Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice . J Orthop Res . 2016. ; 34 ( 2 ): 346 – 353 . 10.1002/jor.22824 [DOI] [PubMed] [Google Scholar]
- 116. Arnesen SM , Lawson MA . Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts . Mech Ageing Dev . 2006. ; 127 ( 9 ): 726 – 732 . 10.1016/j.mad.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 117. Stenroth L , Peltonen J , Cronin NJ , Sipilä S , Finni T . Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo . J Appl Physiol (1985) . 2012. ; 113 ( 10 ): 1537 – 1544 . 10.1152/japplphysiol.00782.2012 [DOI] [PubMed] [Google Scholar]
- 118. Yin H , Strunz F , Yan Z , et al. . Three-dimensional self-assembling nanofiber matrix rejuvenates aged/degenerative human tendon stem/progenitor cells . Biomaterials . 2020. ; 236 : 119802 . 10.1016/j.biomaterials.2020.119802 [DOI] [PubMed] [Google Scholar]
- 119. Jiang D , Xu B , Gao P . Effects of young extracellular matrix on the biological characteristics of aged tendon stem cells . Adv Clin Exp Med . 2018. ; 27 ( 12 ): 1625 – 1630 . 10.17219/acem/75503 [DOI] [PubMed] [Google Scholar]
- 120. Docheva D , Hunziker EB , Fässler R , Brandau O . Tenomodulin is necessary for tenocyte proliferation and tendon maturation . Mol Cell Biol . 2005. ; 25 ( 2 ): 699 – 705 . 10.1128/MCB.25.2.699-705.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Oshima Y , Sato K , Tashiro F , et al. . Anti-angiogenic action of the C-terminal domain of tenomodulin that shares homology with chondromodulin-I . J Cell Sci . 2004. ; 117 ( Pt 13 ): 2731 – 2744 . 10.1242/jcs.01112 [DOI] [PubMed] [Google Scholar]
- 122. Ribitsch I , Gueltekin S , Keith MF , et al. . Age-related changes of tendon fibril micro-morphology and gene expression . J Anat . 2020. ; 236 ( 4 ): 688 – 700 . 10.1111/joa.13125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rajpar I , Tomlinson RE . Function of peripheral nerves in the development and healing of tendon and bone . Semin Cell Dev Biol . 2022. ; 123 : 48 – 56 . 10.1016/j.semcdb.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tempfer H , Kaser-Eichberger A , Lehner C , et al. . Bevacizumab improves achilles tendon repair in a rat model . Cell Physiol Biochem . 2018. ; 46 ( 3 ): 1148 – 1158 . 10.1159/000489057 [DOI] [PubMed] [Google Scholar]
- 125. Stålman A , Bring D , Ackermann PW . Chemokine expression of CCL2, CCL3, CCL5 and CXCL10 during early inflammatory tendon healing precedes nerve regeneration: an immunohistochemical study in the rat . Knee Surg Sports Traumatol Arthrosc . 2015. ; 23 ( 9 ): 2682 – 2689 . 10.1007/s00167-014-3010-9 [DOI] [PubMed] [Google Scholar]
- 126. Heinemeier KM , Schjerling P , Heinemeier J , Magnusson SP , Kjaer M . Lack of tissue renewal in human adult achilles tendon is revealed by nuclear bomb (14)C . FASEB J . 2013. ; 27 ( 5 ): 2074 – 2079 . 10.1096/fj.12-225599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ikoma K , Kusaka Y , Takamiya H , Eliav U , Navon G , Seo Y . Evaluation of collagen fiber maturation and ordering in regenerating tendons employing H-1 double quantum filtered NMR spectroscopy . J Orthop Res . 2003. ; 21 ( 1 ): 149 – 156 . 10.1016/S0736-0266(02)00092-X [DOI] [PubMed] [Google Scholar]
- 128. Hou Y , Mao Z , Wei X , et al. . The roles of TGF-beta1 gene transfer on collagen formation during achilles tendon healing . Biochem Biophys Res Commun . 2009. ; 383 ( 2 ): 235 – 239 . 10.1016/j.bbrc.2009.03.159 [DOI] [PubMed] [Google Scholar]
- 129. Cai L , Xiong X , Kong X , Xie J . The role of the lysyl oxidases in tissue repair and remodeling: a concise review . Tissue Eng Regen Med . 2017. ; 14 ( 1 ): 15 – 30 . 10.1007/s13770-016-0007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Snedeker JG , Foolen J . Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy . Acta Biomater . 2017. ; 63 : 18 – 36 . 10.1016/j.actbio.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 131. Eliasson P , Andersson T , Aspenberg P . Rat achilles tendon healing: mechanical loading and gene expression . J Appl Physiol (1985) . 2009. ; 107 ( 2 ): 399 – 407 . 10.1152/japplphysiol.91563.2008 [DOI] [PubMed] [Google Scholar]
- 132. Eliasson P , Andersson T , Aspenberg P . Influence of a single loading episode on gene expression in healing rat achilles tendons . J Appl Physiol (1985) . 2012. ; 112 ( 2 ): 279 – 288 . 10.1152/japplphysiol.00858.2011 [DOI] [PubMed] [Google Scholar]
- 133. Hammerman M , Dietrich-Zagonel F , Blomgran P , Eliasson P , Aspenberg P . Different mechanisms activated by mild versus strong loading in rat Achilles tendon healing . PLoS One . 2018. ; 13 ( 7 ): e0201211 . 10.1371/journal.pone.0201211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ganestam A , Kallemose T , Troelsen A , Barfod KW . Increasing incidence of acute achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013. A nationwide registry study of 33,160 patients . Knee Surg Sports Traumatol Arthrosc . 2016. ; 24 ( 12 ): 3730 – 3737 . 10.1007/s00167-015-3544-5 [DOI] [PubMed] [Google Scholar]
- 135. Suchak AA , Bostick G , Reid D , Blitz S , Jomha N . The incidence of achilles tendon ruptures in Edmonton, Canada . Foot Ankle Int . 2005. ; 26 ( 11 ): 932 – 936 . 10.1177/107110070502601106 [DOI] [PubMed] [Google Scholar]
- 136. Lantto I , Heikkinen J , Flinkkilä T , Ohtonen P , Leppilahti J . Epidemiology of achilles tendon ruptures: increasing incidence over a 33-year period . Scand J Med Sci Sports . 2015. ; 25 ( 1 ): e133 - 8 . 10.1111/sms.12253 [DOI] [PubMed] [Google Scholar]
- 137. Alfredson H , Ohberg L . Neovascularisation in chronic painful patellar tendinosis--promising results after sclerosing neovessels outside the tendon challenge the need for surgery . Knee Surg Sports Traumatol Arthrosc . 2005. ; 13 ( 2 ): 74 – 80 . 10.1007/s00167-004-0549-x [DOI] [PubMed] [Google Scholar]
- 138. Perucca Orfei C , Lovati AB , Lugano G , et al. . Pulsed electromagnetic fields improve the healing process of achilles tendinopathy: a pilot study in a rat model . Bone Joint Res . 2020. ; 9 ( 9 ): 613 – 622 . 10.1302/2046-3758.99.BJR-2020-0113.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Stange R , Sahin H , Wieskötter B , et al. . In vivo monitoring of angiogenesis during tendon repair: a novel MRI-based technique in a rat patellar tendon model . Knee Surg Sports Traumatol Arthrosc . 2015. ; 23 ( 8 ): 2433 – 2439 . 10.1007/s00167-014-2897-5 [DOI] [PubMed] [Google Scholar]
- 140. Lohan A , Kohl B , Meier C , Schulze-Tanzil G . Tenogenesis of decellularized porcine achilles tendon matrix reseeded with human tenocytes in the nude mice xenograft model . Int J Mol Sci . 2018. ; 19 ( 7 ): E2059 . 10.3390/ijms19072059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Stoll C , John T , Conrad C , et al. . Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation . Biomaterials . 2011. ; 32 ( 21 ): 4806 – 4815 . 10.1016/j.biomaterials.2011.03.026 [DOI] [PubMed] [Google Scholar]
- 142. Maruyama M , Wei L , Thio T , Storaci HW , Ueda Y , Yao J . The effect of mesenchymal stem cell sheets on early healing of the achilles tendon in rats . Tissue Eng Part A . 2020. ; 26 ( 3–4 ): 206 – 213 . 10.1089/ten.TEA.2019.0163 [DOI] [PubMed] [Google Scholar]
- 143. Nakajima T , Nakahata A , Yamada N , et al. . Grafting of iPS cell-derived tenocytes promotes motor function recovery after achilles tendon rupture . Nat Commun . 2021. ; 12 ( 1 ): 5012 . 10.1038/s41467-021-25328-6 [DOI] [PMC free article] [PubMed] [Google Scholar]