Abstract
Purpose:
The majority of CRC research using Fecal Immunochemical Test (FIT) have studied short term screening results in predominantly urban areas.
The purpose of this study was to evaluate the effectiveness of two outreach strategies embedded in a health literacy intervention on repeat CRC screening in rural community clinics.
Methods:
A two-arm randomized controlled trial was conducted in four rural clinics in Louisiana. During a regularly scheduled clinic visit participants ages 50–75 received a FIT kit and brief educational intervention. Participants were randomized to receive an automated call or a personal call by a prevention counselor after four weeks and eight weeks if FIT kits were not returned. In year 2, all materials were mailed, and follow-up calls were conducted as in year 1. The primary outcome was repeat FIT, which was the return of the FIT kit in both years.
Participants:
Of 568 eligible participants, 55% were female, 67%, African American and 39% had low health literacy.
Findings:
Repeat FIT rates were 36.5% for those receiving the automated call and 33.6% for those receiving a personal call (p=0.45). No annual FITs were returned in 30% of participants, while only one FIT was returned by 35% of participants (31% only year 1 and 4% only year 2).
Conclusion:
Sustaining CRC screening with FIT is challenging in rural clinics. A lower cost automated call was just as effective as the personal call in promoting annual screening. However, more intensive strategies are needed to improve long term with FIT screening among rural participants.
Keywords: Repeat Colorectal Cancer Screening, Fecal Immunochemical Test, Telephone follow-up strategies, Health Literacy, Rural Community Clinics
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the U.S. with 51,020 deaths expected in 2019.1 Early detection and treatment of CRC through screening has remarkable potential to decrease mortality from the disease.1 Yet despite significant gains in CRC screening promotion, little improvement has been seen in reducing CRC screening disparities in rural areas and among populations with lower income, educational attainment, health literacy, or race.2–6 Rural areas face significant disadvantages compared to urban areas including higher rates of poverty, lower educational attainment, a higher proportion of elderly and uninsured adults and limited access to health services and specialist.7–10 Despite almost twenty percent of the population living in rural areas only 3% of recent NCI cancer control grants have had a rural focus.11–12
Rural disparities in CRC incidence, mortality and screening have been well documented.8–12 Screening rates are even lower in isolated rural counties with high poverty rates.9 This has serious consequences, as CRC mortality rates in rural counties are 16% higher than metropolitan counties.9,11–12 Healthy People 2020 has set a target of 70.5%5 and the American Cancer Society (ACS) and CRC Screening Roundtable have called for increasing screening rates to 80% by 2018.13 Such an accomplishment would potentially prevent an estimated 200,000 deaths within 20 years.14 With these initiatives, efforts to narrow the gaps in screening disparities and lower overall mortality have been prioritized.15
Among CRC screening modalities colonoscopy is the most frequently used in the U.S. and is considered the gold standard as it allows for direct visual inspection of the entire colon and same session detection biopsy and removal of polyps.16–17 Although it is usually only needed every 10 years, it is invasive, requires cumbersome test preparation, is expensive, and has limited availability in rural areas.16,18 Fecal Occult Blood Tests (FOBT) are the most commonly used CRC screening test in rural settings4,19–21 and the Fecal Immunochemical Test (FIT) is recommended.17–18 The FIT is non-invasive, can be performed at home, is inexpensive, acceptable and alleviates barriers in rural areas such as transportation to screening facilities, insufficient number of colonoscopy facilities and shortage of trained personal.18–21 However, to be effective the test must be completed annually.4,16–17
The majority of CRC studies using FIT have only looked at short term effects and were conducted in urban areas.22–25 A recent systematic review of 27 interventions to increase FOBTs for CRC in low income and rural populations found the most effective were clinic-based, and included multiple components such as one-on-one education and distribution of FIT in clinic, mailed reminders and FIT kits with preaddressed stamped envelopes.26 All studies were limited to initial screening.26 In the few studies assessing two-year FIT completion, all were urban and the most effective included a mailed test kit with reminder letter and if needed a stepped care approach with texts, automated and personal calls to prompt completion.27 The limited amount of rural cancer control research coupled with growing cancer disparities has made rural cancer control research a priority of the National Cancer Institute.11,28
The current study was designed for community clinics in rural areas with limited resources. All participants received health literacy education and FIT with simplified instructions. Participants were then randomized to receive automated follow-up call or a personal call by a prevention coordinator. First year FIT completion rates previously reported were 69% for those receiving an automated call and 67% for those receiving a personal call.29 The purpose of this report is to compare the two telephone outreach strategies to promote repeat annual screening with FIT and report on the patterns of FIT return over two years
Methods
A two-arm randomized controlled trial to evaluate the effectiveness of two approaches to improve annual CRC screening was implemented February 2015-October 2018 in four community health clinics in rural Louisiana. The clinics that were chosen were all in isolated rural areas and had a high volume of participants over age 50. The population of these rural communities ranged from 666 to 33,000. According to clinic health records, CRC completion rates before study implementation ranged from 1% to 3%.
Participants
The study design and participant outreach materials have been published previously.29 Inclusion criteria included: 1) a participant of the identified clinics, 2) age 50 to 75 (based on national guidelines)16, and 3) English speaking. Exclusion criteria include: 1) previous history of cancer other than non-melanoma skin cancer, 2) up to date with CRC screening according to national guidelines17 (FOBT every year, sigmoidoscopy every 5 years, or colonoscopy every 10 years), 3) a first relative family history that requires a more complete history and possible colonoscopy because of their risk factor (these participants will be referred to their provider for follow-up), 4) too ill to participate. The study was approved by the Institutional Review Board.
Theoretical Framework
The intervention components were designed following health literacy best practices and the theory of health learning capacity to simplify the complexity of independently completing the FIT.30–33 The Health Belief Model and Social Cognitive theories guided the framing of intervention content to address the salience of sustained CRC with FIT screening and the need to take complete the test annually.32–33 The clinic based educational strategy was designed to overcome key participant barriers to CRC screening, such as access to tests, limited knowledge, negative beliefs, poor self-efficacy and lack of motivation. The education materials were developed with FQHC participants and providers to help insure they were useful, understandable, appealing and cultural appropriate. The telephone follow-up was included as an intervention strategy to determine the added benefit of prompting participants and to encourage FIT completion.
Structured Survey
The structured baseline interview reported previously29,34 included demographic and CRC screening items from validated questionnaires used previously by the authors.35–36 Health literacy was assessed using the Rapid Estimate of Adult Literacy in Medicine (REALM).37
Interventions
At enrollment, after completing the structured interview, the clinic based research assistant (RA) gave participants the FIT test with simplified instructions and a brief literacy-informed educational intervention that included a CRC pamphlet written on a 5th grade level and step by step demonstration of the FIT instructions and ‘teach back’ to confirm participants’ understanding. Using a participant 1:1 permuted block randomization scheme stratified by participants were then randomized to either the personal call (PC) arm or the automated call (AC) arm.
Personal Call Arm.
Participants randomized to the PC arm, were given a personal call by a centralized prevention coordinator to encourage completion if they had not returned their FIT to the central lab within 4 weeks. If needed the prevention coordinator would go over the FIT instructions and address any barriers identified. The same procedure was followed at 8 weeks if needed.
Automated Call Arm.
Participants randomized to the AC arm who did not return their FIT received an automated reminder call at four weeks and if needed again at eight weeks after enrollment. The participant-friendly AC was a culturally appropriate recording in a conversational tone rather than computer-synthesized speech. There was an option where the participant could request another FIT kit if needed.
Participants used preaddressed postage paid envelops to mail their FIT kits to a central laboratory for processing. The centrally located RA at the university-based medical center checked the lab website daily for results and recorded both positive and negative results in the tracking system to update the follow-up database. The RA updated the AC and PC follow-up system to ensure follow-up calls for only those who did not return their FIT (Fig 1).
Figure 1.
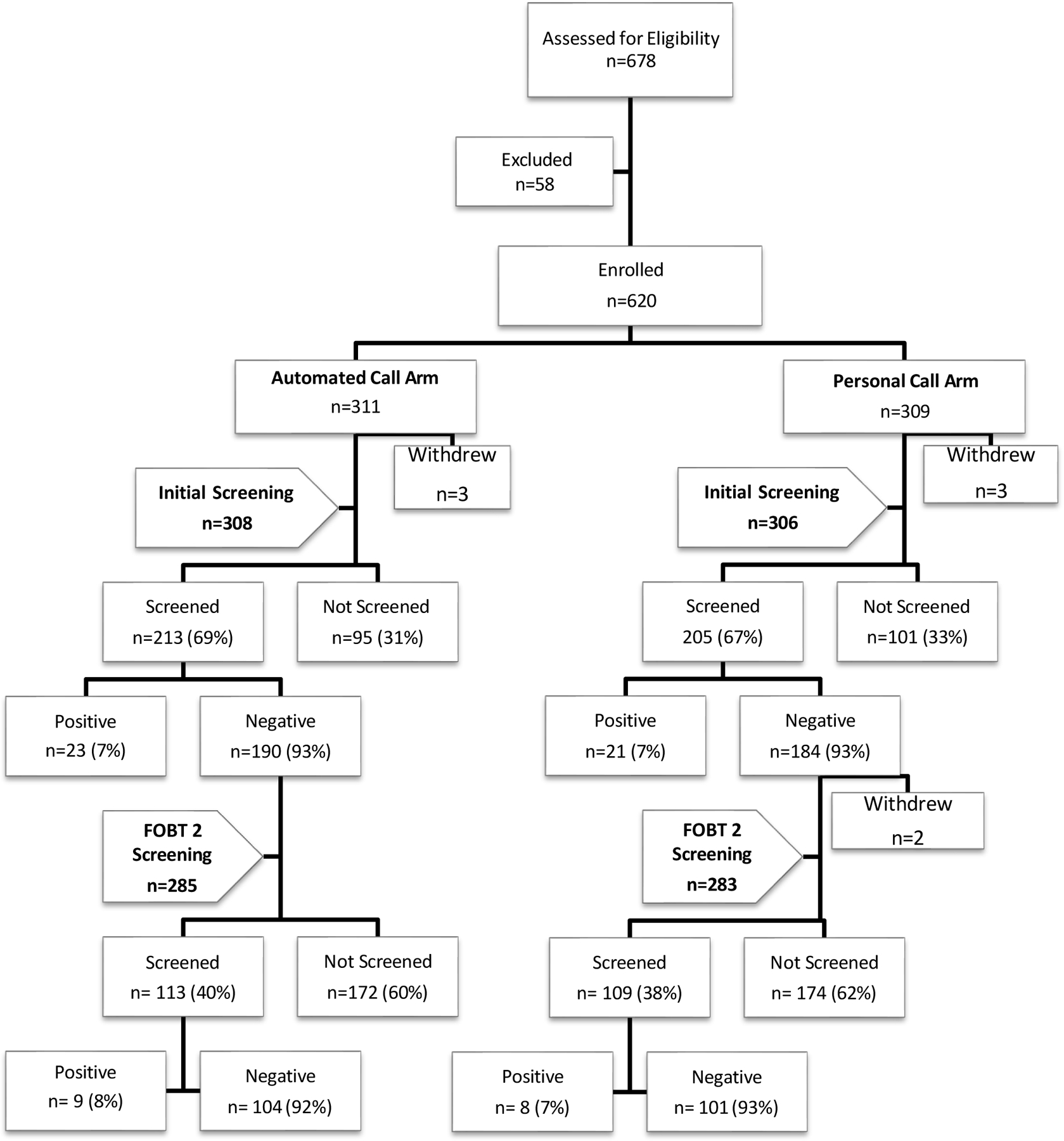
Flowchart of Initial and Repeat Screening (those who completed initial screening)
In year 2 participants were mailed a letter on their clinic letterhead to encourage them to complete their CRC screening which would be mailed the following week. The central RA mailed the FIT with the CRC pamphlet and simplified instructions given at enrollment. Participants who complete year 1 FIT were sent the materials one year after their test was returned. For those who did not complete FIT in year one, materials were sent 12 months after enrollment. The follow-up call protocol was the same as year 1.
Protocol for FIT Results.
For participants with negative results, the central RA sent the results to the designated clinic nurse at each study site daily to record the screening information in the participants’ medical records. Participants were mailed a letter from their clinic by the central RA informing them of negative results.
In the event of a positive result, the central RA called the clinic nurse and spoke directly with her to ensure participants were called by the clinic and followed-up for additional testing using the clinic’s protocol. Participants with positive FITs received an appointment for a follow-up with their provider (who then recommended a colonoscopy). All participants with a positive screen were followed for the length of the study to track CRC follow-up and outcomes but were not sent additional FIT screening.
Outcomes
The primary outcome was completion of a repeat FIT, (completion of an initial FIT within 12 months of enrollment plus the completion of a year 2 FIT within 12–18 months of the initial FIT). Screening ratios were defined as the PC to AC ratio of repeat FIT completion rates. Additional patterns of FIT return were also summarized, specifically the rates and screening ratios for completing no FITS or completing only year 1 FIT or only year 2 FIT. The fidelity of the calls was measure by tracking with the automated call system that measured the number of calls to each participant and their responses and tracking by the prevention coordinator in the PC arm.
Statistical Analysis
To examine whether participants in the study arms differed on continuous baseline characteristics of age, analysis of variance was used. Chi-square tests were performed for categorical factors, including health literacy level. Multivariate analyses adjusting for age, race, gender, and health literacy level were done using generalized linear models. A test for interaction between each of literacy level, age, gender, race and study arm was performed to determine whether treatment effect differed by levels of these factors. An unadjusted test for the main effect of each of health literacy level, age, gender, race and study arm was performed to determine whether screening rates or number of follow-up calls differed by levels of these factors. Four indicator variables were defined to assess the pattern for FIT return over two years (yes/no for each year). Each of these variables was analyzed using a generalized linear model.
Results
In all, 678 participants were identified as meeting age (50-75) and screening criteria, of these 58 (8.9%) refused to participate and 6 withdrew before completing a screening (Fig 1). A total of 614 participants were consented and enrolled. Of these, 44 had a positive FIT result and were excluded from the second FIT screening and 2 withdrew after year 1 resulting in a sample size of 568 for the 2nd year screening analysis.
Baseline characteristics of participants eligible for the FIT in year 2 are compared among study arms in Table 1. Approximately half of the participants (55%) were female. The majority (67%) were African American. Over one in three (35%) had not graduated from high school and 39% had limited literacy (i.e. < 9th grade reading level). There were no significant differences across arms for race/ethnicity, marital status and literacy.
Table 1.
Baseline characteristics of study sample eligible for repeat FIT, by Study Arm
| Characteristic | All Participants (n=568) |
|
||
|---|---|---|---|---|
| Automated (n=285) |
Personal (n=283) |
p-value | ||
| Age, Mean (sd), n=562 | 58.4 (6.1) | 58.2 (5.9) | 58.5 (6.2) | 0.51 |
| N (%) | N (%) | N (%) | ||
| Age Categories | ||||
| 50–59 | 347 (62) | 178 (63) | 169 (61) | 0.16 |
| 60–69 | 185 (33) | 95 (34) | 90 (32) | |
| 70–85 | 30 (5) | 10 (4) | 20 (7) | |
| Female | 312 (55) | 154 (54) | 158 (56) | 0.67 |
| Years of Education | ||||
| Less than high school | 196 (35) | 94 (33) | 102 (36) | 0.90 |
| High school grad | 265 (47) | 133 (47) | 132 (47) | |
| Some College | 60 (11) | 32 (11) | 28 (10) | |
| ≥ College Graduate | 36 (6) | 20 (7) | 16 (6) | |
| Refused/Don’t Know | 8 (1) | 4 (1) | 4 (1) | |
| Race | ||||
| African-American | 376 (67) | 193 (68) | 183 (65) | 0.37 |
| Caucasian/Hispanic | 188 (33) | 89 (32) | 99 (35) | |
| Marital Status | ||||
| Single | 194 (35) | 99 (35) | 95 (34) | 0.25 |
| Married | 209 (37) | 96 (34) | 113 (40) | |
| Separated | 32 (6) | 20 (7) | 12 (4) | |
| Divorced | 73 (13) | 42 (15) | 31 (11) | |
| Widowed | 54 (10) | 25 (9) | 29 (10) | |
| Literacy Level | ||||
| Low/Marginal (0–60) | 224 (39) | 110 (39) | 114 (40) | 0.68 |
| Adequate (61–66) | 344 (61) | 175 (61) | 169 (60) | |
In year 2, when FIT and literacy appropriate materials were mailed, 39% of participants completed screening. Screening rates were not significantly different for those receiving an automated call (39.7%) or personal call (38.5%) (Table 2). Screening rates were not significantly different between arms when analyzed by literacy (Table 3). In the 2nd year 75% of participants needed at least one personal call to complete screening and 65% needed two calls. These were not different by type of call. The need for follow-up calls in year 2 was greater than in year 1 where 40% required a follow-up call to complete screening. In year two 18 (9%) of FITs were positive and these were followed up by their clinic.
Table 2.
Return of Year 2 screening only within 12–18 months. p-values control for age (in years), race (African American vs Caucasian and Hispanic), gender, and literacy (2 categories).
| Characteristic |
All Participants (n=568) |
|
|
|---|---|---|---|
| Automated (n=285) |
Personal (n=283) |
||
| Repeat FIT returned (Screened) | 222 (39) | 113 (39.7) | 109 (38.5) |
| Repeat FIT not returned | 346 (61) | 172 (60.3) | 174 (61.5) |
| Unadjusted | |||
| Screening Ratio (P to A) | 0.97 (0.79 – 1.19) | ||
| p-value | 0.78 | ||
| Adjusted | |||
| Screening Ratio (P to A) | 0.97 (0.79 – 1.19) | ||
| p-value | 0.75 | ||
Table 3.
Return of repeat FIT within 12–18 months, by literacy level.
| Limited Literacy All (n=224) |
|
||
|---|---|---|---|
| Automated (n=110) |
Personal (n=114) |
||
| Repeat FIT returned (Screened) | 80 (36) | 41 (37.3) | 39 (34.2) |
| Repeat FIT not returned | 144 (64) | 69 (62.7) | 75 (65.8) |
| Screening Ratio (P to A) | 0.92 (0.65 – 1.30) | ||
| p-value | 0.63 | ||
|
| |||
|
Adequate Literacy All (n=344) |
|
||
|
Automated (n=175) |
Personal (n=169) |
||
|
| |||
| Repeat FIT returned | 142 (41) | 72 (41.1) | 70 (41.4) |
| Repeat FIT not returned | 202 (59) | 103 (58.9) | 99 (58.6) |
| Screening Ratio (P to A) | 0.97 (0.76 – 1.22) | ||
| p-value | 0.77 | ||
When the treatment effect (screening rates by type of call) was investigated by participant health literacy, race, age and gender, there were differences by age but not by literacy, race and gender. In older participants, age 60 and over, the personal call was more effective (44%) than the automated call (37%) but in the younger participants, age 50–59, the automated call (37%) was more effective than the personal call (27%), interaction p=0.01.
When screening rates (regardless of treatment) were investigated by participant health literacy, race, age and gender, there were differences by literacy and age, but not by race and sex. Screening rates were higher in participants with adequate literacy compared to those with limited literacy (39% vs 30%, p=0.03). Screening rates were higher in participants age 60 and over compared to those age 50–59 (41% vs 32%, p=0.036). There were no differences in the number of follow-up calls by literacy, age, sex or race.
Repeat FITs (a FIT completed in year 1 and 2) were returned by 35% of all eligible participants. Screening rates were once again not significantly different for those receiving an automated call (36.5%) or personal call (33.6%) (Table 4). No annual FITs were returned in 30% of participants, while only one FIT was returned by 35% (31% only in year 1 and 4% only in year 2, Table 4). None of these varied by the type of reminder call they received.
Table 4.
Pattern of FIT return over a two-year period.
(FIT1 is FIT in first year, FIT2 is FIT in second year.)
| All Participants (n=568) |
|
|||
|---|---|---|---|---|
| Automated (n=285) |
Personal (n=283) |
p-value | ||
| Both FIT1 and FIT2 NOT returned | 171 (30.1) | 86 (30.2) | 85 (30.0) | 0.84 |
| Screening Ratio 95% Confidence Interval | 1.03 (0.80– 1.32) |
|||
| FIT1 returned, FIT2 not returned | 175 (30.8) | 86 (30.2) | 89 (31.5) | 0.68 |
| Screening Ratio 95% Confidence Interval | 1.05 (0.82– 1.35) |
|||
| FIT1 not returned, FIT2 returned | 23 (4.0) | 9 (3.2) | 14 (5.0) | 0.30 |
| Screening Ratio 95% Confidence Interval | 1.53 (0.68– 3.47) |
|||
| Both FIT1 and FIT2 returned | 199 (35.0) | 104 (36.5) | 95 (33.6) | 0.45 |
| Screening Ratio 95% Confidence Interval | 0.92 (0.73– 1.15) |
|||
Screening ratios and p-values control for age (in years), race (African American vs Caucasian and Hispanic), gender and literacy (limited vs adequate).
Discussion
Few studies have focused on long term CRC screening in rural areas. This study found sustaining screening with FIT is challenging. Of rural participants who completed a FIT in year 1, approximately half (53%) completed a 2nd annual in year 2. In year 1 our clinic-based health literacy-directed intervention yielded high rates of initial screening (68%).29 However in year 2, when mailing the kit with a reminder letter about a third (35%) of all eligible participants completed screening in both years.
The reminder call was more important in prompting screening in year 2 than in year 1; participants being less likely to return the FIT without a call to prompt them. As in year 1 the low-cost automated call was equally as effective as a personal call in encouraging screening completion. Not surprisingly, older participants were more responsive to the personal call and younger participants the automated call.
The most effective multi-model two-year strategy previously conducted was reported by Baker and colleagues27 in a FQHC system in Chicago with predominately female Latino participants. This FQHC system had previously developed a quality improvement plan to promote CRC screening which served as the enhanced usual care arm. The intervention arm added reminders by mail, automated calls and texts, and a 3-month personal call. Of participants randomized to the enhanced usual care, 37% completed an annual 2nd year FIT while participants randomized to the intervention arm had an 82% completion rate for the 2nd year FIT.27 Combining their two groups, a 60% repeat screening rate was seen after an initial screen was done, while in our study, an overall 53% repeat screening rate was seen given the initial screen was done. It is important to note that at the time our study began, rural health clinics in LA did not have an electronic health record (EHR) system and there were no requirements for rural clinic CRC screening quality measurements to be reported.
An important finding from our study is that in both year 1 and year 2 there was no difference in completion rates among those who received a personal call and those who received a lower cost automated call. In year 2, fewer participants return FIT without a call to prompt completion. The type of call is informative in looking at subgroups. The personal call was more effective with participants over 60 and the automated call was more effective with younger patents. In our study older participants and those with adequate literacy were more likely to be screened. Our findings also indicate that the 2nd year intervention was most effective with those who had completed the test the previous year.
Recent longitudinal studies in safety-net health systems in metropolitan areas compared three-year CRC screening outcomes of FIT and colonoscopy.38–40 In both studies initial screening was highest with FIT, however three-year CRC screening outcomes were higher with colonoscopy compared to FIT – 38% to 28% among participants in Dallas and 38% to 14% in San Francisco.38–40 Despite these findings to offer participants an option of CRC screening tests, offering the option of colonoscopy at the time of our study, was not feasible due to low-income participants lack insurance coverage and distance to a colonoscopy center.
Limitations
Our study has limitations related to the generalizability of our results. We included English speaking rural participants receiving care in community clinics in only one state in the southern United States. A strength of this rural clinic study is there was limited loss of follow-up due to participants moving or seeking care elsewhere. In some rural areas, the FQHC is the only available health care services in the area.
Conclusion
The low-cost reminder automated call was equally as effective as a personal call in prompting annual screening. More follow-up calls (both personal and automated) were needed in year 2. Older participants were more responsive to the personal call and younger participants the automated call. In year 2, with mailed FIT kits, simplified materials, and follow-up phone calls, a small percentage of participants who had not completed their FIT in year one completed it in year two. Resource poor clinics could take advantage of their more recent use of EHR’s to identify eligible participants and use the system to generate letters and automated calls or texts to remind participants to complete screening annually. Future studies to address rural and socioeconomic barriers to CRC screening may need to educate newly insured Medicaid patients about screening options and take advantage of increasing availability of colonoscopy services in rural areas. More intensive strategies to engage participants that fail to complete initial screening are also needed.
Acknowledgements
We appreciate the opportunity to partner with the Teche Action Clinics and with the Varnado Family Practice. The study would not have been possible without our research assistants, Connie Thompson-Fly, Charlene Williams, Anreka Key, Linda Gauthier, and Angela LeBlanc. We also appreciate our prevention coordinators Kathryn Davis Penna, MPH and Ja Sae Gatlin, MS.
Funding
Funded by American Cancer Society grant “Health Literacy Interventions to Overcome Disparities in CRC Screening” RSG-13–021-01-CPPB. This work was also supported in part by 2 U54 GM104940–02 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
No conflicts are noted by the authors related to the work described.
REFERENCES
- 1.American Cancer Society. Colorectal cancer facts & figures 2017–2019. Available: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf [Accessed May 7, 2019].
- 2.Centers for Disease Control and Prevention (CDC). Behavioral risk factor surveillance system survey data Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2012, 2014, 2016. [Google Scholar]
- 3.Centers for Disease Control and Prevention. National center for health statistics 2011. Available: https://www.healthypeople.gov/2020/data-search/Search-the-Data#objid=4054. [Accessed August 8, 2018].
- 4.Hall IJ, Tangka FK, Sabatino SA, et al. Patterns and trends in cancer screening in the United States. Prev Chronic Dis 2018; 15:170465. doi: 10.5888/pcd15.170465 [Accessed February 1, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy people 2020 Available: https://www.healthypeople.gov/. [Accessed May 12, 2018].
- 6.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA: A Cancer Journal for Clinicians 2017; 67:7–30. Available: 10.3322/caac.21395/full [Accessed February 1, 2019]. [DOI] [PubMed] [Google Scholar]
- 7.Henley SJ, Jemal A Rural cancer control: bridging the chasm in geographic health inequity. Cancer Epidemiol Biomarkers Prev 2018; 27(11):27–143. doi: 10.1158/1055-9965.epi-18-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blake KD, Moss JL, Gaysynsky A, et al. Making the case for investment in rural cancer control: an analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev 2017; 26(7):992–997. doi: 10.1158/1055-9965.epi-17-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahnd W, James A, Jenkins W, et al. Rural-urban difference in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev 2018; 27(11):12–65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollin J, Bellarny G, Ferdinand A, et al. Rural healthy people 2020 new decade, same challenges. Journal of Rural Health 2015; 31(3):326–333. doi: 10.1111/jrh.12116 [DOI] [PubMed] [Google Scholar]
- 11.Kennedy A,Vanderpool R, Croyle R, et al. An overview of the National Cancer Institute’s initiative to accelerate rural cancer control research. Cancer Epidemiol Biomarkers Prev 2018; 27(11). [DOI] [PubMed] [Google Scholar]
- 12.Callaghan TH, Ferdinand AO, Towne SD Jr., et al. Cancer mortality in rural America 1999–2016. South-West Rural Health Research Center 2018. Available: https://www.ruralhealthresearch.org/centers/southwest [Accessed February 1, 2019].
- 13.Karlitz JJ, Oliphant A-LB, Greenwald DA, et al. The American College of Gastroenterology and the 80% by 2018 colorectal cancer initiative: a multifaceted approach to maximize screening rates. The American Journal of Gastroenterology 2017; 112(9):1360–1362. doi: 10.1038/ajg.2017.217 [DOI] [PubMed] [Google Scholar]
- 14.Meester RG, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer 2015; 121(13):2281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montminy EM, Karlitz JJ, Landreneau SW. Progress of colorectal cancer screening in United States: past achievements and future challenges. Preventive Medicine 2019; 120:78–84 [DOI] [PubMed] [Google Scholar]
- 16.U.S. Preventive Services Task Force. Final recommendation statement: colorectal cancer: screening 2016. Available: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2. [Accessed February 1, 2019].
- 17.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA: A Cancer Journal for Clinicians 2018. doi: 10.3322/caac.21457. [DOI] [PubMed]
- 18.Pham R, Cross S, Fernandez B, et al. “Finding the right FIT”: Rural patient preferences for fecal immunochemical test (FIT) characteristics. The Journal of the American Board of Family Medicine 2017; 30(5):632–644. doi: 10.3122/jabfm.2017.05.170151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debourcy AC, Lichtenberger S, Felton S, et al. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. Journal of General Internal Medicine 2007; 23(2):169–174. doi: 10.1007/s11606-007-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolan JG, Boohaker E, Allison J, et al. Patients’ preferences and priorities regarding colorectal cancer screening. Medical Decision Making 2012; 33(1):59–70. doi: 10.1177/0272989x12453502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Champion C, Alvarez GG, Affleck E, et al. A systems perspective on rural and remote colorectal cancer screening access. Journal of Cancer Policy 2017; 14:27–32. doi: 10.1016/j.jcpo.2017.09.003. [DOI] [Google Scholar]
- 22.Lasser KE, Murillo J, Medlin E, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: results of a pilot study. BMC Family Practice 2009; 10(1). doi: 10.1186/1471-2296-10-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortuna RJ, Idris A, Winters P, et al. Get screened: a randomized trial of the incremental benefits of reminders, recall, and outreach on cancer screening. Journal of General Internal Medicine 2014; 29(1):90–97. doi: 10.1007/s11606-013-2586-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DH, Feldstein AC, Perrin N, et al. Automated telephone calls to enhance colorectal cancer screening: an economic analysis from a randomized trial. The American Journal of Managed Care 2012; 18(11):691–699. [PMC free article] [PubMed] [Google Scholar]
- 25.Hendren S, Winters P, Humiston S, et al. Randomized, controlled trial of a multimodal intervention to improve cancer screening rates in a safety-net primary care practice. Journal of General Internal Medicine 2014; 29(1):41–49. doi: 10.1007/s11606-013-2506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis MM, Freeman M, Shannon J, et al. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States – How, what and when? BMC Cancer 2018; 18:40. doi: 10.1186/s12885-017-3813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers a randomized clinical trial. JAMA Intern Med 2014; 174(8):1235–1241. doi: 10.1001/jamainternmed.2014.2352 [DOI] [PubMed] [Google Scholar]
- 28.Ghazarian A, Martin N, Lam T Opportunities and challenges in rural cancer research: an epidemiologic perspective. Cancer Epidemiol Biomarkers Prev 2018; 27(11) −143. doi: 10.1158/1055-9965.EPI-18-0962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold CL, Rademaker A, Morris J, et al. Follow-up approaches to a health literacy intervention to increase colorectal cancer screening in rural community clinics: a randomized control trial. Cancer 2019. [DOI] [PMC free article] [PubMed]
- 30.Wolf MS, Wilson EAH, Rapp DN, et al. Literacy and learning in healthcare. Pediatrics 2009; 124(0 3):S275–S281. doi: 10.1542/peds.2009-1162C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merriënboer JJGV, Sweller J Cognitive load theory and complex learning: recent developments and future directions. Educ Psychol Rev 2005; 17(2):147–177. doi: 10.1007/s10648-005-3951-0. [DOI] [Google Scholar]
- 32.Rosenstock IM, Strecher VJ, Becker MH. Social Learning Theory and the health belief model. Health Education Quarterly 1988; 15(2):175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 33.Janz NK, Champion VL, Strecher VJ. The health belief model. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education San Francisco, CA: John Wiley and Sons 2002.
- 34.Davis TC, Morris J, Rademaker A, et al. Barriers and facilitators to colorectal cancer screening among rural women in community clinics by heath literacy. JWomens Health Issues Care 2017; 6(6). doi: 10.4172/2325-9795.1000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis T, Arnold C, Rademaker A, et al. Improving colon cancer screening in community clinics. Cancer 2013; 119(21):3879–3886. doi: 10.1002/cncr.28272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnold CL, Rademaker A, Liu D, et al. Changes in colorectal cancer screening knowledge, behavior, beliefs, self-efficacy, and barriers among community health clinic patients after a health literacy intervention. Journal of Community Medicine & Health Education 2017; 7(1):497. doi: 10.4172/2161-0711.1000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: ashortened screening instrument. Fam Med 2016; 25(6):391–395. [PubMed] [Google Scholar]
- 38.Liang PS, Wheat CL, Abhat A, et al. Adherence to competing strategies for colorectal cancer screening over 3 years. The American Journal of Gastroenterology 2016; 111(1):105–114. doi: 10.1038/ajg.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: a randomized controlled trial in a safety net health system. Cancer 2016; 122(3):456–463. doi: 10.1002/cncr.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singal AG, Gupta S, Skinner CS, et al. Effect of colonoscopy outreach vs fecal immunochemical test outreach on colorectal cancer screening completion. JAMA 2017; 318(9):806. doi: 10.1001/jama.2017.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]


