Abstract
While the gold-standard for management of localized renal cell carcinoma (RCC) is partial nephrectomy, recent ablative strategies are emerging as alternatives with comparable rates of complications and oncologic outcomes. Thermal ablation, in the form of radiofrequency ablation and cryoablation, is being increasingly accepted by professional societies, and is particularly recommended in patients with a significant comorbidity burden, renal impairment, old age, or in those unwilling to undergo surgery. Maturation of long-term oncologic outcomes has further allowed increased confidence in these management strategies. New and exciting ablation technologies such as microwave ablation, stereotactic body radiotherapy, and irreversible electroporation are emerging. In this article, we review the existing management options for localized RCC, with specific focus on the oncologic outcomes associated with the various ablation modalities.
Keywords: cryoablation, microwave ablation, percutaneous ablation, radiofrequency ablation, renal cell carcinoma, stereotactic ablative body radiotherapy
Introduction
Treatment options for localized renal cell carcinoma
The incidence of renal cell carcinoma (RCC) has increased over the past four decades, with recent estimates predicting 79,000 new cases of kidney cancer in 2022 (1, 2). Stage 1 tumors, which are ≤7 cm and contained to the kidney, comprise 40–50% of new cases (1, 3). This rise is, in part, due to the improved detection of incidental renal tumors, with the advent of and widespread usage of cross-sectional imaging (1, 4).
The management of small renal masses (SRMs), defined as ≤ 4 cm or cT1a tumors, continues to evolve with our improved understanding of tumor biology and application of technology. Treatment options for SRMs include radical or partial nephrectomy (PN), renal mass ablation, and active surveil-lance (5–7). While radical nephrectomy (RN) was once the gold standard for most cases of localized RCC, a shift toward minimally invasive surgery and interest in nephron-sparing procedures led to the popularization of PN as standard of care for localized RCC (8). However, in select patients with localized RCC, focal ablative therapies and active surveillance have gained traction as possible alternatives (8).
Although surgery for localized RCC is the most common treatment and has traditionally been associated with higher cancer-specific survival (CSS), emerging evidence has demonstrated comparable oncologic outcomes with ablative therapy (7, 9–11). Clinicians must engage in shared decision-making with their patients, considering the risks and possible complications paired with patient factors, their treatment goals, and institutional capabilities (5, 6). Review articles play a vital role in keeping patients and clinicians abreast to changing clinical paradigm, especially when considering topics such as the management of SRMs. The advent of numerous ablative modalities and their subsequent outcomes studies necessitates periodic, in-depth examination of the evidence. In this review, we discuss various ablative modalities for localized RCC, their outcomes, and how the current literature supports the integration of ablation into clinical practice. We initially conducted a broad literature review through PubMed (Supplemental Figure 1). Articles were further examined for inclusion in our review with particular attention paid to randomized clinical trials (RCTs). Ongoing trials and or those without published results were discovered through clinicaltrials.gov.
Patient evaluation and selection
All patients with localized RCC should first undergo a detailed history and physical examination. Clinicians should inquire about patient comorbidities, risk factors for RCC (smoking, hypertension, obesity, diabetes), and performance status (3). Serum creatinine and urine dipstick may be utilized to determine chronic kidney disease (CKD) stages (3). Cross-sectional imaging, either computed tomography (CT) or magnetic resonance imaging (MRI), should be obtained, if not already done (3).
Treatment selection for localized RCC can be nuanced and may be influenced by patient, provider, and tumor characteristics (5, 8). Decision aids or risk calculators incorporating patient and tumor characteristics may be used cautiously to personalize counseling and assess morbidity and mortality risk of each treatment option (5, 6). For example, Psutka et al. devised a novel risk calculator which considered patient age, sex, body mass index, CKD stage, ECOG performance status, Charlson comorbidity index, and renal mass diameter to determine the probability of complication or death for RN, PN, ablation, active surveillance, and other causes. Clinicians should engage in shared decision-making with their patients, discussing their desire for invasive treatment and goals for oncologic control, nephron preservation, and minimizing treatment morbidity. Providers should guide patients in carefully balancing the risks and benefits of each treatment option (5, 6).
Ablative therapies may be offered to a variety of patients with SRMs or localized RCC after considering specific patient, tumor, and provider characteristics (5–7). Ideal candidates for ablation include those of older age, with poor performance status, numerous comorbidities, compromised renal function, or a high risk of morbidity from surgical extirpation but are unwilling to accept surveillance (5, 7). Tumors amenable to ablation are small (cT1a masses) and located posteriorly (5, 7). Finally, providers offering ablative therapies should be doing so at a medical center with experience in ablative techniques and access to multidisciplinary care (i.e., interventional radiology, nephrology, medical oncology, etc.) (5). Also, patients who do not desire surgical intervention or want to avoid general anesthesia may be considered for renal mass ablation. For patients selecting ablation, renal mass biopsy should be performed to assist in risk stratification and guide follow-up surveillance (12, 13).
Guideline recommendations
According to the American Urological Association (AUA), thermal ablation, via either radiofrequency or cryoablation, can be considered for treatment of SRMs <3 cm in size (Table 1) (12). If pursuing thermal ablation, a percutaneous procedure is preferred over the laparoscopic approach. Prior to undergoing ablative therapy, clinicians should counsel patients regarding the increased risk of local recurrence or tumor persistence compared to conventional operative intervention. Patients should also undergo a renal mass biopsy to direct management post intervention (12, 13).
Table 1:
Recent professional urological society guidelines on the use of focal therapy in localized renal cell carcinoma.
| Society | Year | Recommendation |
|---|---|---|
| American Society of Clinical Oncology (ASCO) | 2017 | Percutaneous thermal ablation should be considered an option if complete ablation can reliably be achieved.a |
| American Urological Association (AUA) | 2021 | Physicians should consider thermal ablation for cT1a renal masses < 3 cm.b |
| European Association of Urology (EAU) | 2021 | Offer active surveillance or thermal ablation (TA) to frail and/or comorbid patients with small renal masses. Do not routinely offer TA for tumors > 3 cm and cryoablation for tumors > 4 cm.c |
| National Comprehensive Care Network (NCCN) | 2022 | Thermal ablation is an option for patients with cT1 disease, but may be associated with higher rates of recurrence or persistence in tumors > 3 cm.d |
Level of evidence – moderate; bLevel of evidence – conditional recommendation, grade C; cLevel of evidence – weak; dLevel of evidence – 2A.
These guidelines align with those of the European Association of Urology (EAU), which recommend offering thermal ablation as an alternative to surgery for frail and comorbid patients with SRMs. However, the EAU does not explicitly recommend ablative therapies for any other group, and rather states that thermal ablation and cryoablation should not be routinely utilized for tumors less than 3 and 4 cm, respectively. Like the AUA, the EAU also mentions the importance of preablation renal mass biopsy and proper counseling regarding oncologic and procedural risks (14).
To date, both societies acknowledge their recommendations stem from somewhat low-quality evidence with limited follow-up (12–14).
Ablation modalities
Various ablation modalities, including cryoablation, radiofrequency ablation (RFA), microwave ablation (MWA), and stereotactic ablative radiotherapy (SABR), are currently employed in the treatment of localized RCC. Each method may be performed either percutaneously with image guidance or laparoscopically with direct visualization. Here we review the mechanism of action for each ablative strategy as well as outcomes and current societal recommendations.
Cryoablation
Proposed mechanism
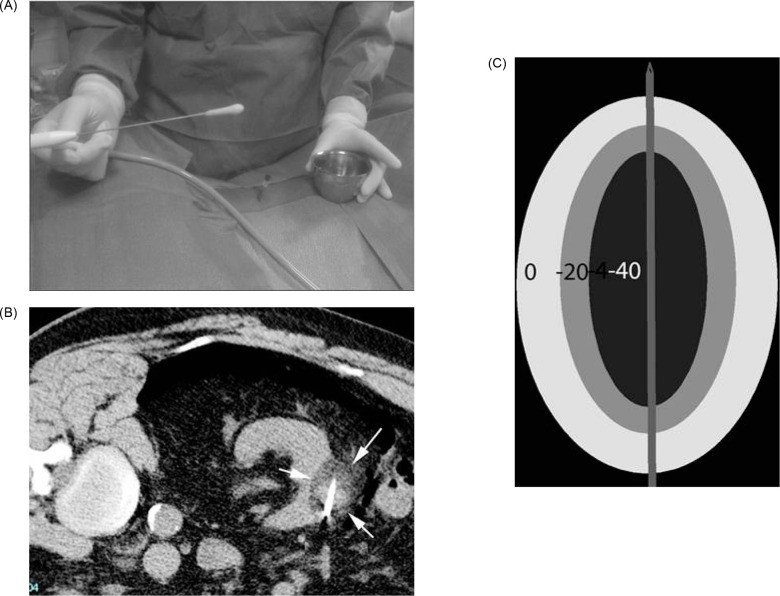
In cryoablation, the tumor is cooled to a temperature sufficient to obtain cell necrosis. Cryogen gas (typically argon) is depressurized, causing a decrease in temperature at the tip of an antenna—a phenomenon termed the Joule–Thomson effect. Through the tip of the antenna, passive thermal diffusion acts on tumor cells. Intracellular and extracellular crystals are produced by slow and fast freezing cycles (generally two), and these cycles cause cell death through cellular dehydration, vascular thrombosis, and membrane rupture (15). While −20°C is the proven temperature at which cell necrosis occurs in cryoablation, −40°C is the typical temperature achieved in real world practice. The target temperature is obtained at least 3.1 mm inside the ice ball, with shorter distances insufficient for achieving cell death (16) (Figures 1A–1C).
Figure 1:
(A) Ice ball seen on the end of cryoablation probe. (B) Ice ball seen in renal tumor during cryoablation. (C) Depiction of isotherms of ice ball.
Outcomes
The literature of cryoablation for the management of SRMs is limited. In this section, we highlight the particular studies that have large sample sizes and or sufficient long-term follow-up. Breen et al. performed a retrospective single-institution analysis regarding efficacy of image-guided cryoablation of T1 renal masses. Major complications (Clavien–Dindo grade ≥ III) occurred in 4.9% (23 out of 473) procedures. Of all 433 patients with T1 renal masses OS was reported as 91.7% (95% CI: 87.5%, 94.5%) and 78.8% (95% CI: 71.1%, 84.6%) at 3 and 5 years, respectively. Recurrence-free survival (RFS) and metastasis-free survival (MFS) were evaluated in a subset of 220 patients with sporadic biopsy-proven RCC. At 3 years, local RFS and MFS were reported as 97.2% (95% CI: 92.6%, 99.0%) and 97.7% (95% CI: 93.3%, 99.1%), respectively. At 5 years, RFS and MFS were calculated at 93.9% (95% CI: 85.8%, 97.4%) and 94.4% (95% CI: 86.7%, 97.7%), respectively (17). Similarly, Pickersgill et al. conducted a single-institution retrospective analysis from 2005 to 2015 of percutaneous cryoablation (PCA) for SRMs to study long-term oncologic outcomes and factors predicting disease recurrence. This study included 308 patients with a mean tumor size of 2.7 ± 1.3 cm. Disease progression rates at a mean follow-up of 38 months were 10.1 and 6.2%, for local recurrence and new lymphadenopathy or metastasis, respectively. After excluding patients with a solitary kidney or Von Hippel–Lindau syndrome, local recurrence and new lymphadenopathy or metastasis occurred in 8.6 and 1.9% of cases, respectively. Disease-free survival (DFS) of PCA was estimated to be 92.5% at 1 year, 89.3% at 2 years, and 86.7% at 3 years. The risk of disease progression increased by 32% with every 1 cm increase in tumor size (18). Additional outcomes of recent observational studies investigating PCA are described in Table 2. Taken together, these studies suggest that cryoablation of SRMs have favorable oncologic outcomes in carefully selected patients. Tumor size can best predict those who will have disease recurrence (17, 18).
Table 2:
Recent observational studies investigating the role of percutaneous cryoablation in the treatment of localized renal cell carcinoma.
| Study | Year | Intervention | Experimental design | Patient population | Number of patients |
Outcomes | Media and follow-up | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Henderickx et al. | 2020 | PCA | Retrospective Single institution |
Clinical T1 RCC |
165 | OS DFS RFS |
60 months | 5-yr OS = 74.0% 5-yr DFS = 96.9% 5-yr RFS = 95.4% |
| Bhagavatula et al. | 2020 | PCA | Retrospective Single institution |
Biopsy-proven, T1 RCC |
307 | OS DSS DFS LPFS |
95 months | 10-yr OS = 78% 10-yr DSS = 99% 10-yr DFS = 88% 10-yr LPFS = 95% |
| Morkos et al. | 2020 | PCA | Retrospective Propensity-matched |
Biopsy-proven, Stage 1 RCC |
134 | OS RFS DSS |
88.8 months | 5-yr OS = 87% 5-yr DSS = 94% 10-yr OS = 72% |
| Gobara et al. | 2021 | PCA | Prospective Single institution |
Biopsy-proven, T1a RCC |
33 | CSS OS |
60.1 months | 5-yr CSS = 100%5-yr OS = 96.8% |
CSS, cancer-specific survival; DFS, disease-free survival; DSS, disease-specific survival; LPFS, local progression–free survival; OS, overall survival; PCA, percutaneous cryoablation; RCC, renal cell carcinoma; RFS, recurrence-free survival.
Several groups have also reported on the difference in laparoscopic and PCA. Kim et. al compared PCA in 123 tumors with laparoscopic cryoablation (LCA) in 167 tumors. Both groups shared no difference in decline in glomerular filtration rate (GFR) or complication rates. In terms of oncologic outcomes, 5-year OS and RFS rates were 86.3 and 86.3%, respectively, for PCA, and 79.3 and 85.5%, respectively, for LCA. Multivariate Cox proportional hazards analysis demonstrated that cryoablation approach regardless of mechanism was not predictive of overall mortality or disease recurrence (P = 0.36 and 0.82, respectively), concluding that oncologic outcomes were not fully attributed to cryoablation approach (19). Other authors have corroborated these findings as well (20).
To date, there are currently two registered clinical trials studying cryoablative techniques for RCC. NCT04506671 is a prospective, nonrandomized trial comparing local recurrence in patients with T1b renal tumor receiving cryoablation or PN. The primary outcome is local recurrence for up to 5 years, while secondary outcomes include metastatic progression, quality of life, renal function, rate of adverse events, blood loss, length of stay, and pain scores (21). NCT04040530 is a prospective cohort study comparing patients with T1 biopsy-proven RCC who undergo treatment with PN or cryoablation. Main outcomes include change in quality of life, rehabilitation from treatment, complication and readmission rates, and treatment success after 3 months (22). These studies will hopefully inform how to best include cryoablation in our armamentarium for treating SRMs. Cryoablation in contrast to radiofrequency and MWA does not have a coagulative effect theoretically leading to increased bleeding complications, although this has not been clinically relevant. In addition, unlike microwave and RFA, cryoablation does not destroy collagen, resulting in less long-term injury to the collecting system. As with all ablative therapies long-term, active follow-up is essential.
Guideline recommendations
AUA guidelines acknowledge cryoablation as an option for patients who select ablative therapy instead of surgery as treatment for their renal mass. They recognize that there is no significant difference between cryoablation and RFA in complications, metastatic progression, or CSS (12, 13). The American Society of Clinical Oncology (ASCO) similarly recommend, “percutaneous thermal ablation should be considered an option for patients who possess tumors such that complete ablation will be achieved” (23).
Radiofrequency Ablation
Mechanism
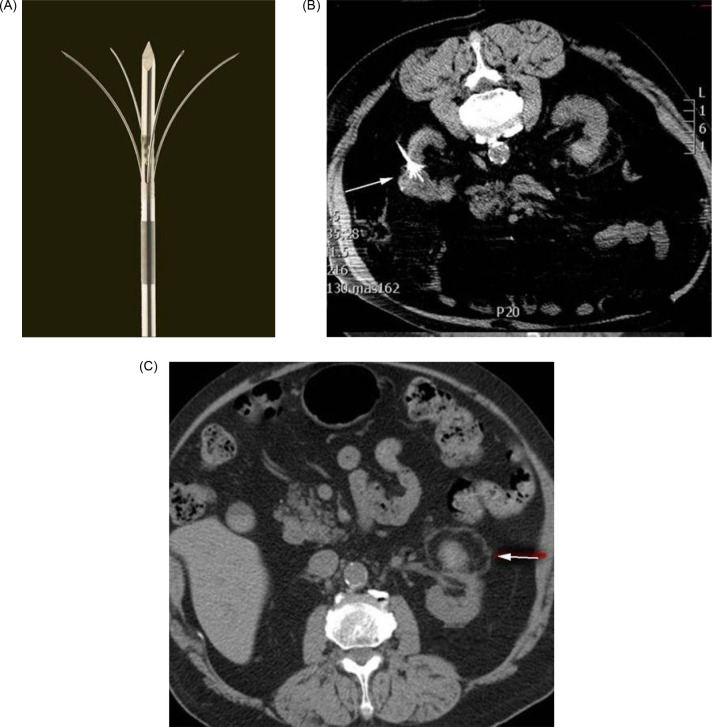
Radiofrequency ablation uses an electrical current in the radio frequency range, between 3 Hz and 300 GHz, to heat tissue resulting in coagulative necrosis. The alternating current is delivered through antennas placed directly into the tumor, with the electrical circuit completed through grounding pads, such as those seen with the Bovie cautery device (24). As current passes through the tissue, heat is generated due to tissue resistance, a concept termed “resistive heating” (Figure 2) (25). Cell death occurs instantly at temperatures above 60°. Notably, effective delivery of RFA relies on the electrical and thermal conductivity in tissue, which can be adversely affected by overheating and desiccation of the tissue directly adjacent to the electrode, resulting in an insulating barrier of charred tissue (26). RFA is often not recommended adjacent to large vasculature, due to inability to overcome the heat sink effect leading to increased risk of incomplete necrosis, possibly impacting oncologic efficacy (27). Over the years, a variety of techniques have been developed to monitor tissue temperature and impedance to limit desiccation and increase the zone of ablation (28). Similarly, multi-tined, expandable, and perfusion electrodes, and bipolar electrode systems are available technologies used to modulate the size and shape of the ablated tissue (26) (Figures 3A–3C).
Figure 2:
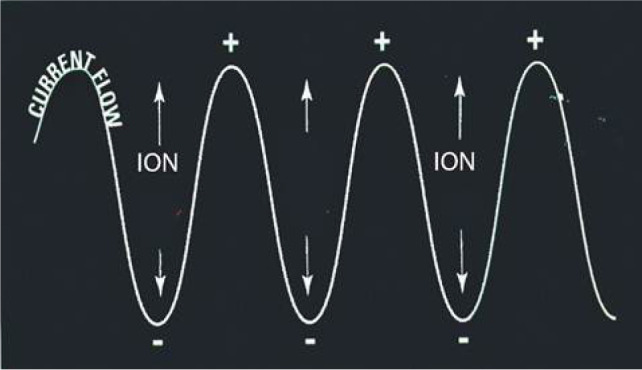
Depiction of radiofrequency causing ionic agitation and subsequent frictional heating.
Figure 3:
(A) Multi-tined radiofrequency ablation antenna. (B) Radiofrequency ablation antenna expanded within tumor. (C) Typical tissue changes after successful radiofrequency ablation including pseudo-capsule, subcapsular fat, and nonenhancing lesion.
Outcomes
Most data supporting the role of RFA is retrospective in nature and suggests that RFA is feasible in most instances (Table 3). A study by Zhou et al. explored the therapeutic and renal function outcomes of 297 patients who underwent image-guided percutaneous ablation of T1a biopsy-proven RCC between 2006 and 2016 (29). Of their cohort of 297 patients, 244 (82%) of patients underwent RFA. Technical success, defined as the ability to successfully perform an ablative procedure, was achieved in 100% of patients, with a 16% rate of adverse events. At 1-month, primary efficacy, defined as the ability to successfully treat a tumor with one ablative session, was achieved in 233 of 244 patients (95%), with secondary efficacy after repeat ablation achieved in 100% of patients. During their follow-up of 2 years, there were no reported instances of local recurrence. With regard to renal function, they found no difference between preablation and postablation GFR (29).
Table 3:
Recent publications investigating oncologic outcomes in patients undergoing RFA for localized renal carcinoma.
| Study | Year | Experimental design | Intervention | Patient population | Number of patients | Outcomes | Mean/Median follow-up | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Clark et al. |
2007 | Phase I clinical trial | MWA | Solid, enhancing renal mass on imaging No size limitations |
Total = 10 | Ablation size Presence of skip areas |
Not reported | Single probe ablation size = 4.1 × 2.7 × 2.2 cm Triple probe ablation size = 5.7 × 4.7 × 3.8 cm No skip areas in any patient |
| Liang et al. |
2008 | Phase I clinical trial | MWA | Biopsy proven cT1a RCC | Total = 12 | Feasibility Safety Efficacy |
Median = 11 months | No complications 100% technical success rate |
| Bartoletti et al. | 2012 | Phase I clinical trial Multi-institution |
MWA | Solid, enhancing renal mass on imaging Candidate for open radical nephrectomy |
Total = 14 | Coagulation parameters Bleeding Ablation size |
Mean = 27.4 months | No significant effects of coagulation No local bleeding after MWA Mean ablation size = 44.14 mm (±22.59 mm) |
| Guan et al. |
2012 | RCT | MWA | Solitary, unilateral, cT1a | Total = 102 MWA = 48 PN = 54 |
RFS | Median MWA = 32 months Median PN = 36 months |
In the pathologically confirmed subgroup =3-yr RFS: 90.4% vs 96.6%; P = 0.465 |
| Yu et al. | 2015 | Retrospective Single institution |
MWA vs RN | Biopsy-proven T1a RCC | Total = 426 MWA = 98 RN = 328 |
OS CSS |
Median MWA = 25.8 months Median RN = 26.1 months |
5-yr OS = 82.6 vs 98.6%; P = 0.0004 5-yr CSS = 97 vs 98%; P = 0.38 |
| Vanden Berg | 2021 | Retrospective Single institution |
MWA | Biopsy-proven RCC (including oncocytic) | Total = 101 Biopsy-proven RCC = 82 |
RFS | Median = 12.4 months | 40 months, RFS in biopsy-proven RCC = 93.3% |
| Yu et al. | 2020 | Retrospective Propensity matched |
MWA vs PN | Biopsy-proven T1a RCC | Total = 1955 MWA = 185 PN = 1770 |
LTP CSS Metastasis |
Median = 40.6 months | LTP = 3.2% vs 0.5%; P = 0.10 CSS = 2.2% vs 3.8%; P = 0.24 Metastases = 4.3% vs 4.3%; P = 0.76 |
| Yu et al. | 2022 | Retrospective Multi-institution |
MWA | Biopsy-proven T1 RCC | Total = 323 cT1a = 275 cT1b = 48 |
LTP DFS CSS OS |
cT1a Median = 66 months cT1b Median =30.4 months |
10-yr OS = 67.5% 10-yr DFS = 71.8% 10-yr CSS = 87.4% 10-yr LTP = 1.9% 5-yr OS = 89.2% 5-yr DFS = 69.1% 5-yr CSS = 91.4% 5-yr LTP = 11.3% |
CSS, cancer-specific survival; DFS, disease-free survival; LTP, local tumor progression; MWA, microwave ablation; OS, overall survival; RCC, renal cell carcinoma; RFS, recurrence-free survival; PN, partial nephrectomy; RN, radical nephrectomy.
Data regarding oncologic outcomes for RFA are promising. Olweny et al. retrospectively compared the oncologic outcomes of RFA to PN, the current gold standard of localized RCC (30). Despite increased age and comorbidity burden, there was no difference in OS in RFA vs PN at 5 years (97.2% vs 100%; P = 0.31) in their cohort of biopsy-proven RCC patients. Neither age, tumor size, histology nor duration of follow-up was a significant predictor of oncologic outcomes. Another long-term study by Psutka et al. concluded that RFA can result in durable local control with low risk of recurrence (10). Johnson et al. also explored long-term outcomes in patients who underwent RFA and found that DFS and CSS were 89 and 96%, respectively, at 6 years for those with tumors less than 3 cm. However, in individuals with tumors greater than 3 centimeters, DFS decreased significantly to 68% (31). In their sub-group analysis of individuals with biopsy- proven RCC with at least 10-years of follow-up, MFS and CSS were both 94%. Similar, recent, retrospective studies have shown comparable long-term onco-logic outcomes in those undergoing RFA vs PN (32–34).
However, despite the encouraging body of literature, RCTs are needed to clarify the role of RFA. Unfortunately, there are no published RCTs’ data comparing RFA to PN for SRMs. However, NCT00019955, a phase II trial assessing the feasibility of RFA in slowing destroying tumor tissue and preventing tumor growth, has completed enrollment. No results have been posted to date (35).
Guideline recommendations
Strong retrospective results in the setting of a lack of available randomized trial data have resulted in relatively broad guideline recommendations for the use of RFA. The AUA notes that thermal ablation, “should be considered as an alternative approach for the management of cT1a renal masses < 3 cm”(12, 13). The EAU states that thermal ablation can be offered, “to frail and/or comorbid patients with SRMs.” They caution that, “low quality studies suggest high disease recurrence rates after RFA of tumors > 3 cm (14) (Figures 4A and 4B).
Figure 4:
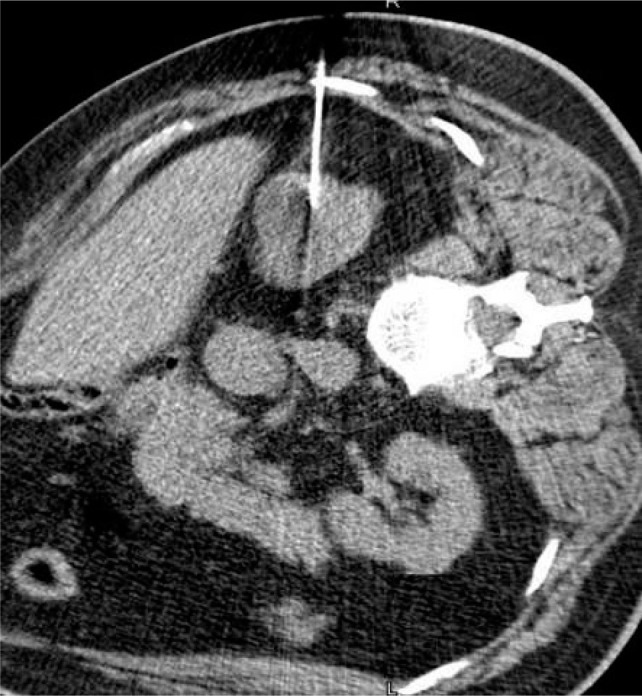
(A) Microwave antenna seen in 3 cm, upper pole renal mass. (B) Follow-up at 3 months shows residual enhancement of the superior aspect of the lesion consistent with residual tumor, demonstrating the importance of follow-up.
Microwave Ablation
Mechanism
Microwave ablation is a form of electromagnetic radiation that typically oscillates between 900–2500 MHz (36). Like RFA, this fluctuation causes the continued realignment of polar molecules within tissue producing an increase in kinetic energy. This phenomenon, termed “dielectric hysteresis,” translates to rising temperature within the tissue, often above 100°C (15, 36). In MWA, these electromagnetic waves are delivered through one or more antennae inserted into the tissue (27). In contrast to RFA, MWA does not rely on tissue conductivity, and can effectively heat a variety of tissues regardless of electrical conductivity (37). This field effect provides for uniform heat generation (Figure 5). Thus, it is effective compared to RFA for lung, bone, and other tissues with high electrical impedance (37). Further, MWA has an increased ability to overcome the heat sink effect (38). This is an advantage for efficacy within highly vascular organs such as the kidneys, where increased blood flow can lead to the dispersion of thermal energy (27).
Figure 5:
Depiction of microwave antenna generating a field of heat.
Outcomes
There is limited data regarding the efficacy of MWA. In an early study by Liang et al. that evaluated the feasibility, safety, and efficacy of MWA for RCC, a total of 12 patients with biopsy-proven RCC from 1.3 cm to 3.8 cm in diameter underwent MWA (Table 4) (39). All tumors were completely ablated with a single session of MWA. No complications were reported, and no recurrence was observed at a median follow-up of 11 months. Similarly, two phase I studies, with fewer than 15 patients who underwent MWA prior to nephrectomy, concluded that MWA can safely and quickly ablate renal tumors (40, 41).
Table 4:
Recent publications evaluating efficacy and safety of microwave ablation for the treatment of localized renal cell carcinoma.
| Study | Year | Experimental design | Intervention | Patient population | Number of patients | Outcomes | Mean/Median follow-up | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Olweny et al. |
2012 | Retrospective Single institution |
RFAvs PN | Biopsy-proven, T1a RCC | RFA cohort = 37 PN cohort = 37 |
OS CSS RFS DFS MFS |
RFA Median = 6.5 years PN Median = 6.1 years |
5-yr OS = 97.2 vs 100%; P = 0.31 5-yr CSS = 97.2 vs 100%; P = 0.31 5-yr DFS = 89.2 vs 89.2%; P = 0.78 5-yr RFS = 91.7 vs 94.7%; P = 0.96 5-yr MFS = 97.2 vs 91.5%; P = 0.35 |
| Psutka et al. |
2013 | Retrospective Single institution |
RFA | cT1 RCC | RFA cohort = 185 | RFS MFS CSS DFS OS |
Median = 6.43 years | 5-yr RFS = 95.2% 5-yr MFS =99.4% 5-yr CSS = 99.4% 5-yr DFS =87.6% 5-yr OS = 73.3% 10-yr RFS T1a = 93.2% |
| Chang et al. |
2015 | Retrospective Single institution |
RFA vs PN | Biopsy-proven, T1b RCC | RFA cohort = 27 PN cohort = 29 |
OS CSS DFS |
RFA Mean = 65.9 months PN Mean = 70.2 months |
5-yr OS = 85.5 vs 96.6%; P = 0.14 5-yr CSS = 92.6 vs 96.6; P = 0.49 5-yr DFS = 81.0 vs 89.7%; P = 0.36 |
| Ji et al. | 2016 | Retrospective Single institution |
RFA vs PN | Biopsy-proven, T1a RCC | RFA cohort = 105 PN cohort = 74 |
OS CSS DFS |
RFA = Median 78 months PN = Median 82 months |
5-yr OS = 93.3 vs 94.6%; P > 0.05 5-yr CSS = 98.0 vs 98.5; P > 0.05 5-yr DFS = 97.1 vs 97.3%; P > 0.05 |
| Andrews et al. | 2019 | Retrospective Single institution |
RFA vs PN vs PCA | Clinical T1 RCC | RFA cohort = 180 PN cohort = 1055 PCA cohort = 187 |
CSS | RFA Median = 7.5 years PN Median = 9.4 years Cryo Median = 6.3 years |
5-yr CSS cT1a = 95.6 vs 99.3 vs 100% 5-yr CSS cT1b = N/A vs 98% vs 91% |
| Park et al. | 2019 | Retrospective Single institution |
RFA vs PN | Biopsy-proven, T1a RCC |
RFA cohort = 62 | OS | RFA Mean = 60 months | 5-yr OS = 98.4 vs 100%; P = 0.360 |
| Johnson et al. | 2019 | Retrospective Single institution |
RFA | Contrast enhancing, nonmetastatic renal mass | Total = 106 Biopsy-proven w/ 10-yr f/u = 39 |
MFS CSS |
Median = 79 months | 10-yr biopsy proven MFS = 94% 10-yr biopsy proven CSS = 94% |
| Zhou et al. | 2019 | Retrospective Single institution | RFA vs PCA vs MWA | Biopsy proven, T1a RCC | RFA cohort = 244 PCA cohort = 26 MWA cohort = 27 |
DFS MFS CSS |
Median = 24 months | 2-yr DFS = 100 vs 100 vs 100% 2-yr MFS = 100 vs 100 vs 100% 2-yr CSS = 100 vs 100 vs 100% |
CSS, cancer-specific survival; DFS, disease-free survival; MFS, Metastasis-free survival; MWA, microwave ablation; OS, overall survival; PCA, percutaneous cryoablation; PN, partial nephrectomy; RCC, renal cell carcinoma; RFA, radiofrequency ablation; RFS, recurrence-free survival.
More recently, Yu et al. retrospectively compared the efficacy of MWA (n = 98) and laparoscopic RN (n = 328) in patients with RCC ≤ 4 cm. At 5 years, CSS was comparable between MWA and laparoscopic RN (97% vs 98%; P = 0.38) (42). An even larger study by Yu et al. comparing 1955 propensity-matched patients with cT1a RCC undergoing MWA or laparoscopic RN found no difference in local progression (3.2% vs 0.5%, respectively; P = 0.10) and CSS rates (2.2% vs 3.8%, respectively; P = 0.24) (43). MWA was associated with decreased decline in GFR (6.2 vs 16.4%; P < 0.001). However, when compared to laparoscopic RN, MWA was associated with worse OS (HR = 2.4; 95%CI 1.0–5.7; P = 0.049) and DFS (82.9% vs 91.4%; P = 0.003). These findings may be attributed to poor overall health and increased comorbidities within the patient population selected to undergo MWA. The most recent study by Yu et al. further reinforces that MWA is a safe, reliable option for patients with cT1 RCC. After a median follow-up of 66 months for patients with T1a patients, CSS, DFS, and OS rates were 87.4, 71.8, and 67.5%, respectively. For T1b patients with a median follow-up of 30.4 months, CSS, DFS, and OS rates were 91.4, 69.1, and 89.2%, respectively. Interestingly, technical success was achieved in 97% of patients despite over 40% of tumors inhabiting dangerous locations (near bowel or the collecting system) (44).
To date, there has only been a single published prospective, randomized controlled trial investigating MWA vs PN. The authors randomized 102 patients with a renal mass ≤ 4 cm to open PN (n = 19), laparoscopic PN (n = 35), open MWA (n = 20), or laparoscopic MWA (n = 28). At median follow-up of 32 and 36 months for the MWA and PN, respectively, there was no significant difference in local RFS (91.3% vs 96.0%; P = 0.4650 (45). This limited length of follow-up underscores the need for additional, long-term studies to effectively evaluate oncologic outcomes of MWA for SRMs. To our knowledge there are no active, prospective RCTs investigating the role of MWA. While MWA is best reserved for T1a lesion, recent reports have appeared for MWA inT1b lesions. In one such single-center, retrospective study including 23 patients with T1b RCC, primary technical success was achieved in 20/23 (87%) patients (46), and secondary technical success was achieved in 3/3 (100%) patients. Local tumor progression-free survival (PFS) was 100.0, 90.9, and 90.9% at 1, 2, and 3 years, respectively. Overall survival (OS) was 95.2, 85.7, and 71.4% at 1, 2, and 3 years, respectively.
Guideline recommendations
Current EAU guidelines classify MWA as experimental, but comment that the data seems to support equivalence to RFA and cryoablation in terms of safety and oncologic outcomes over the short term. However, the EAU panel cites inadequate data regarding the clinical efficacy of thermal ablation compared to PN (47). AUA guidelines also classify MWA as investigational, but further clarify that percutaneous ablation techniques can be considered as an alternate approach to manage cT1a masses (12, 13). However, many institutions have adopted MWA as a suitable replacement for RFA.
Stereotactic Ablative Radiotherapy
Mechanism
In contrast to laparoscopic or percutaneous ablation techniques, stereotactive body radiotherapy (SBRT) is completely noninvasive, without need for an anesthetic procedure, and is characterized by delivery of high-dose, hypo-fractionated ionizing radiation to the tumor (48). Similar to other ablative therapies, SBRT is associated with low toxicity and is available in the outpatient setting (49).
Historically thought to be radioresistant, the higher dose, hypo-fractionated nature of SBRT has been effective in treating RCC (50), challenging current treatment paradigms. The precise mechanism of SBRT is unclear, but some hypothesize that the antitumor effects are mediated through acid sphingomyelinase, which under the effects of the single-fraction, high-dose radiation characteristic of SBRT translocates to the cell membrane where it catalyzes lysis of sphingomyelin to ceramide, an intracellular messenger known to coordinate proapoptotic signaling (51). Besides from inducing direct cellular damage (52), SBRT may induce expression of MHC I, adhesion molecules, heat shock proteins, and other inflammatory and immunomodulators, resulting in a local tumor response (53). These immunomodulatory effects of SBRT can extend beyond the local irradiated area to affect meta-static sites (54). In 1953, this phenomenon was termed the “abscopal effect” and has been reported in numerous types of malignancies including RCC (54, 55).
Outcomes
While SBRT has historically had a role in the treatment of lung, liver, and bone tumors, it is becoming increasingly important in the treatment of RCC (51). To evaluate the role of SBRT in primary RCC, Correa et al. performed a meta-analysis including 26 studies and 372 patients, 80% (n = 300) of whom had localized disease (Table 5) (56). The study estimated a local control rate of 97.2% (95% CI 93.9– 99.5%) for the entire cohort, a finding the authors note to be comparable to thermal ablation, when considering tumor size. Furthermore, Yamamoto et al. investigated long-term oncologic outcomes in clinical or recurrent T1 RCC patients who underwent SBRT. In their cohort of 29 patients, 5-year localized control (LC), loco-regional control (LRC), PFS, DFS, and OS were 94, 88, 50, 96, and 68%, respectively (57). In the nonmetastatic, T1b RCC population treated with SBRT, Siva et al. observed a 4-year CSS, OS, and PFS of 91.4, 69.2, and 64.9%, respectively. However, OS and PFS are dominated by death from other causes in this cohort. Local, distance, and any failure at 4 years were 2.9, 11.1, and 12.1%, respectively (58).
Table 5:
Recent retrospective studies on the oncologic outcomes of stereotactic body radiotherapy to treat localized renal cell carcinoma.
| Study | Year | Experimental design | Intervention | Patient population | Number of patients/studies | Outcomes | Follow-up | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Correa et al. | 2019 | Systematic review, meta-analysis | SBRT | All stages, RCC | Studies = 26 Patients = 372 |
LC | Median = 28 months | LC = 97.2% |
| Siva et al. | 2020 | Retrospective Multi-institution |
SBRT | ≥ T1b, nonmetastatic RCC | Patients = 95 | CSS OS PFS |
Median = 2.7 years | 4-yr CSS = 91.4% 4-yr OS = 69.2% 4-yr PFS = 64.9% |
| Yamamoto et al. | 2021 | Retrospective Single institution |
SBRT | Clinical or recurrent T1 RCC | Patients = 29 | LC LR CPF DSS OS |
Median = 57 months | 5-yr LC = 94% 5-yr LRC = 88% 5-yr PFS = 50% 5-yr DSS = 96% 5-yr OS = 68% |
CSS, cancer-specific survival; DSS, disease-specific survival; LC, local control; LRC, loco-regional control; OS, overall survival; PFS, progression-free survival; RCC, renal cell carcinoma; SBRT, stereotactive body radiotherapy.
This encouraging retrospective data has spurred interest in prospective clinical studies (Table 6). One such study, FAS-TRACK (NCT01676428), recruited 37 patients with T1a (n = 13), T1b (n = 23), and T2a (n = 1) RCC (59). Distant progression-free survival was 97% (95% CI: 91–100%) at 1 year and 89% (95% CI: 78–100%) at 2 years. These results led to the development of FASTRACK II (NCT02613819), a phase II, single-arm, interventional trial investigating the role of SBRT in patients with biopsy-confirmed RCC ≤ 8 cm who were medically inoperable, at high risk, or were declined surgery (60). Like FASTRACK II, AQuOS-RCC (NCT03108703) and NCT03811665 are active, non-recruiting phase I studies (61, 62). AQuOS-RCC primarily aims to assess the quality of life in patients undergoing SBRT for primary renal lesions ≥ 2.5 cm or recurrent lesions following local ablative therapy. In addition, there are two phase II trials examining SBRT in the setting of localized RCC: AQuOS-II NCT05023265 and NCT02141919 (63, 64). Notably, there are two actively recruiting trials, NCT01890590 and NCT04115254, studying the use of Cyberknife® and stereotactic magnetic resonance–guided radiation therapy (SMART) in the setting of Stage 1 RCC (65, 66).
Table 6:
Prospective interventional clinical trials evaluating the oncologic outcomes of SBRT and similar radiotherapy for the treatment of localized renal cell carcinoma.
| Study | Status | Experimental design | Intervention | Patient population | Patient enrollment | Outcomes | Follow-up | Conclusions |
|---|---|---|---|---|---|---|---|---|
| FASTRACK NCT01676428 | Complete | Phase I Single-arm Interventional trial |
SBRT | T1a–T2a RCC and Medically inoperable or High risk for surgery or Decline surgery |
Final Enrollment = 37 | AE LPFS DPFS OS |
Median = 24 months | Grades 1 & 2 toxicity = 78% Grade 3-4 toxicity = 3% Grades 4 & 5 toxicity = 0% 2-yr LPFS = 100% 2-yr DPFS = 89% 2-yr OS = 92% |
| FASTRACK II NCT02613819 | Active, not recruiting | Phase II Single-arm Interventional trial |
SBRT | Biopsy-confirmed RCC ≤ 8 cm and Medically inoperable or High risk or Decline surgery |
Current Enrollment = 71 | PFS | Ongoing | Ongoing |
| AQuOS-RCC NCT03108703 | Active, not recruiting | Phase I Single-arm Interventional trial |
SBRT | Lesion ≥ 2.5 cm or Recurrent lesion following local ablative therapy | Enrollment Goal = 30 | QOL LC PFS OS AE |
Ongoing | Ongoing |
| AQuOS-II NCT05023265 | Not yet recruiting | Phase II Single-arm Interventional trial |
SBRT | Primary lesion 3–20 cm and Medically inoperable or Decline surgery | Enrollment Goal = 46 | LC PFS OS QOL AE |
Ongoing | Ongoing |
| NCT01890590 | Recruiting | Phase II Single-arm Interventional trial |
Cyberknife | Stage I RCC | Enrollment Goal = 46 | LPFS QOLAE |
Ongoing | Ongoing |
| NCT03811665 | Active, not recruiting | Phase I Two-arm Interventional trial |
SBRT vs RFA | Biopsy-confirmed T1a RCC | Enrollment Goal = 24 | Treatment failure OSDFSRFS |
Ongoing | Ongoing |
| NCT04115254 | Recruiting | Phase I-II Parallel arms Interventional trial |
SMART | Confirmed malignancy and Tumor ≤ 7 cm | Enrollment Goal = 1000 | Delivery success Tumor visualization Plan creation Tumor control |
Ongoing | Ongoing |
| NCT02141919 | Active, not recruiting | Phase II Single-arm Interventional trial |
SBRT | Biopsy-proven RCC (including oncocytoma) and Tumor ≤ 5 cm |
Current Enrollment = 16 | Tumor growth AE Renal function PFS |
Ongoing | Ongoing |
AE, adverse events; DFS, disease-free survival; DPFS, disease progression–free survival; LC, local control; LPFS, local progression–free survival;OS, overall survival; PFS, progression-free survival; QOL, quality of life; RCC, renal cell carcinoma; RFA, radiofrequency ablation; RFS, recurrence-free survival; SBRT, stereotactic body radiotherapy; SMART, stereotactic magnetic resonance–guided radiation therapy.
SBRT treatment is exceptionally well tolerated. In a prospective dose-escalation trial, the only acute toxicities were Grade I fatigue (45%) and Grade I nausea (11%). Among the 11 patients, there were two late complications—one Grade 2 with decline in renal function and one Grade 3 with episode of pyelonephritis (67). Similar low toxicity rates were reported by Siva et al. (58).
Guideline recommendations
Current EAU guidelines comment that while stereotactic radiotherapy results are encouraging, additional randomized control data are required to clarify the role in the management of localized RCC (47). AUA guidelines similarly categorize SBRT as investigational, remarking it should be considered for patients who are both medically inoperable and not candidates for traditional thermal ablative modalities (12, 13).
Failure Rates
Although recent studies of focal therapy for the treatment of localized RCC have been promising, a proportion of patients require reintervention due to recurrence or an incomplete eradication of the primary tumor. In a study investigating the long-term oncologic outcomes in patients with T1 RCC, Psutka et al. noted that 13% (n = 24) of patients required retreatment for residual disease (10). Further, 6.5% (n = 12) of patients in their study experienced local recurrence. Small but notable local tumor progression has been observed in those treated with MWA, with Yu et al. showing 5-year local tumor progression rates of 1.9 and 11.3% for patients with cT1a and cT1b RCC, respectively (44). In the context of cryoablation, Bhagavatula et al. reported evidence of recur-rent RCC on imaging after initial treatment in 7.9% (n = 23) of patients. Notably, local progression occurred in 4.5% (n = 13) with 1.0% (n = 3) of patients unfortunately developing metastatic disease (68). In their meta-analysis investigating SBRT, Correa et al. identified a local control rate of 92.2%, but included studies with local controls rates from 70–100%. They clarify that local failure tended to occur in those treated with low-dose SBRT, a finding that has been seen in other malignancies treated with SBRT (56).
It is vital to place these local failure rates in the context of PN. Data comparing ablation with PN are largely retrospective and somewhat heterogeneous. Some studies support worse oncologic results with ablation. For example, Fraisse et al. used radiological evidence and the RENAL nephrometry score to pair similar patients treated by PCA and robotic-assisted partial nephrectomy (RAPN). In their study of 647 patients, the absolute recurrence rates were 2.8% in the RAPN cohort versus 8.4% in the PCA cohort (P = 0.03) (69). However, other studies report more comparable onco-logic outcomes between ablative and surgical therapies. Bianchi et al. associated PN with higher DFS in comparison with thermal ablation (92.8% vs 80.4%, respectively; P = 0.02) but no difference in OS between either treatment modality (70). Andrews et al. reported on 1422 patients withT1a RCC and demonstrated a 5-year CSS to be 99, 96, and 100% for PN, RFA, and cryoablation, respectively (9).
Moving forward, prospective, randomized studies will be critical in accurately assessing local failure rates and discerning oncologic outcomes between ablation and PN. Efforts to refine focal therapy techniques to minimize positive margins and incomplete treatment are also crucial (71).
Future Perspectives
Undoubtedly, it is an exciting time for those involved in the treatment of RCC with the rapid expansion of treatment modalities. In addition to thermal ablation, SABR, and MWA monotherapy, there is a recent, growing interest in combination therapies. In a small series of seven patients with an average tumor size of 6.4 cm, Blitzer et al. used a combination of MWA and SBRT to achieve local control rates of 100% at a median follow-up of 15 months (72).
Novel technologies continue to be utilized for the treatment of RCC. While proton beam therapy has an established role in other malignancies, clinicians are beginning to explore its application in the treatment of RCC. In a multi-institutional study of 22 patients with a median follow-up of 37 months, Fukumitsu et al. reported 3-year OS and 3-year CSS rates of 950 and 100%, respectively, for primary RCC patient undergoing proton therapy. No major complications or effects on serum blood urea, nitrogen, or creatinine were reported (73). Another emerging treatment modality for SRMs is irreversible electroporation (IRE). This technology delivers a pulsed electrical field resulting in irreversible permeabilization of tumor cell membranes (74). Early studies by Wah et al. and Dai et al. have shown promising results with local RFS rates of 91% at 3 years and 81% at 5 years, respectively (75, 76). Additional larger studies of combination therapies and novel therapeutics alongside RFA, PCA, SABR, and MWA are necessary to discern the best ablative therapy options for SRM treatment.
Conclusion
The growing incidence of SRMs and an aging population has increased the demand for novel, nonsurgical therapies. Professional societies are increasingly embracing ablative therapies for treatment of SRMs, particularly in poor surgical candidates or those who do not desire surveillance. RCTs and maturation of long-term oncologic outcomes of up-and-coming technologies such as MWA, IRE, SABR, and others are needed to further expand the armamentarium of urologic oncologists, interventional radiologists, and radiation oncologists.
Footnotes
How to cite: Leopold Z, et al. Modern Management of Localized Renal Cell Carcinoma—Is Ablation Part of the Equation? J Kidney Cancer VHL. 2022; 9(3): 5–23.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
Funding
This work is supported by a grant from the National Cancer Institute (P30CA072720).
Supplementary
Figure S1:
PubMed Search Query.
References
- 1.Turner RM 2nd, Morgan TM, Jacobs BL. Epidemiology of the small renal mass and the treatment disconnect phenomenon. Urol Clin North Am. 2017;44(2):147–54. 10.1016/j.ucl.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3.Sanchez A, Feldman AS, Hakimi AA. Current management of small renal masses, including patient selection, renal tumor biopsy, active surveillance, and thermala. J Clin Oncol. 2018;36(36):3591–600. 10.1200/JCO.2018.79.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai JC, Morgan TN, Moody D, McLaughlin J, Cadeddu JA. Radiofrequency ablation of small renal masses. J Endourol. 2021;35(S2):S38–S45. 10.1089/end.2020.1041 [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekar T, Boorjian SA, Capitanio U, Gershman B, Mir MC, Kutikov A. Collaborative review: Factors influencing treatment decisions for patients with a localized solid renal mass. Eur Urol. 2021;80(5):575–88. 10.1016/j.eururo.2021.01.021 [DOI] [PubMed] [Google Scholar]
- 6.Psutka SP, Gulati R, Jewett MAS, Fadaak K, Finelli A, Legere L, et al. A clinical decision aid to support personalized treatment selection for patients with clinical T1 renal masses: Results from a multi-institutional competing-risks analysis. Eur Urol. 2021. 81(6):576–585. 10.1016/j.eururo.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salagierski M, Wojciechowska A, Zajac K, Klatte T, Thompson RH, Cadeddu JA, et al. The role of ablation and minimally invasive techniques in the management of small renal masses. Eur Urol Oncol. 2018;1(5):395–402. 10.1016/j.euo.2018.08.029 [DOI] [PubMed] [Google Scholar]
- 8.Chan VW, Abul A, Osman FH, Ng HH, Wang K, Yuan Y, et al. Ablative therapies versus partial nephrectomy for small renal masses—A systematic review and meta-analysis. Int J Surg. 2022;97:106194. 10.1016/j.ijsu.2021.106194 [DOI] [PubMed] [Google Scholar]
- 9.Andrews JR, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Oncologic outcomes following partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2019;76(2):244–51. 10.1016/j.eururo.2019.04.026 [DOI] [PubMed] [Google Scholar]
- 10.Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63(3):486–92. 10.1016/j.eururo.2012.08.062 [DOI] [PubMed] [Google Scholar]
- 11.Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67(2):252–9. 10.1016/j.eururo.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 12.Campbell SC, Clark PE, Chang SS, Karam JA, Souter L, Uzzo RG. Renal mass and localized renal cancer: Evaluation, management, and follow-up: AUA Guideline: Part I. J Urol. 2021;206(2):199–208. 10.1097/JU.0000000000001911 [DOI] [PubMed] [Google Scholar]
- 13.Campbell SC, Uzzo RG, Karam JA, Chang SS, Clark PE, Souter L. Renal mass and localized renal cancer: Evaluation, management, and follow-up: AUA Guideline: Part II. J Urol. 2021;206(2):209–18. 10.1097/JU.0000000000001912 [DOI] [PubMed] [Google Scholar]
- 14.Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernandez-Pello S, et al. European Association of Urology Guidelines on renal cell carcinoma: The 2019 update. Eur Urol. 2019;75(5):799–810. 10.1016/j.eururo.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 15.Filippiadis D, Mauri G, Marra P, Charalampopoulos G, Gennaro N, De Cobelli F. Percutaneous ablation techniques for renal cell carcinoma: Current status and future trends. Int J Hyperthermia. 2019;36(2):21–30. 10.1080/02656736.2019.1647352 [DOI] [PubMed] [Google Scholar]
- 16.Campbell SC, Krishnamurthi V, Chow G, Hale J, Myles J, Novick AC. Renal cryosurgery: Experimental evaluation of treatment parameters. Urology. 1998;52(1):29–33; discussion 33–4. 10.1016/s0090-4295(98)00169-1 [DOI] [PubMed] [Google Scholar]
- 17.Breen DJ, King AJ, Patel N, Lockyer R, Hayes M. Image-guided cryoablation for sporadic renal cell carcinoma: Three-and 5-year outcomes in 220 patients with biopsy-proven renal cell carcinoma. Radiology. 2018;289(2):554–61. 10.1148/radiol.2018180249 [DOI] [PubMed] [Google Scholar]
- 18.Pickersgill NA, Vetter JM, Kim EH, Cope SJ, Du K, Venkatesh R, et al. Ten-year experience with percutaneous cryoablation of renal tumors: Tumor size predicts disease progression. J Endourol. 2020;34(12):1211–7. 10.1089/end.2019.0882 [DOI] [PubMed] [Google Scholar]
- 19.Kim EH, Tanagho YS, Saad NE, Bhayani SB, Figenshau RS. Comparison of laparoscopic and percutaneous cryoablation for treatment of renal masses. Urology. 2014;83(5):1081–7. 10.1016/j.urology.2013.10.081 [DOI] [PubMed] [Google Scholar]
- 20.Liu HY, Shen SH, Hsu LN, Chiang PH. Comparisons of per-cutaneous versus retroperitoneoscopic cryoablation for renal masses. Int Urol Nephrol. 2018;50(8):1407–15. 10.1007/s11255-018-1925-7 [DOI] [PubMed] [Google Scholar]
- 21.Percutaneous cryoablation versus partial nephrectomy for T1b renal tumor. Cited 04/18/22. Available from: https://clinicaltrials.gov/ct2/show/NCT04506671?term=cryoablation+kidney&draw=2&rank=3
- 22.Patient-reported outcome after nephron-sparing treatment of small renal tumours. Cited 04/18/22. Available from: https://clinicaltrials.gov/ct2/show/NCT04040530?term=cryoablation+renal&draw=2&rank=10
- 23.Finelli A, Ismaila N, Bro B, Durack J, Eggener S, Evans A, et al. Management of small renal masses: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(6):668–80. 10.1200/JCO.2016.69.9645 [DOI] [PubMed] [Google Scholar]
- 24.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7(8):1054–63. 10.4254/wjh.v7.i8.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr Probl Diagn Radiol. 2009;38(3):135–43. 10.1067/j.cpradiol.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong K, Georgiades C. Radiofrequency ablation: Mechanism of action and devices. J Vascular Interventional Radiol. 2010;21(8, Supplement):S179–S86. 10.1016/j.jvir.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 27.Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, et al. Radiofrequency ablation and microwave ablation in liver tumors: An update. Oncologist. 2019;24(10):e990–e1005. 10.1634/theoncologist.2018-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khiatani V, Dixon RG. Renal ablation update. Semin Intervent Radiol. 2014;31(2):157–66. 10.1055/s-0034-1373790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Herwald SE, McCarthy C, Uppot RN, Arellano RS. Radiofrequency ablation, cryoablation, and microwave ablation for T1a renal cell carcinoma: A comparative evaluation of therapeutic and renal function outcomes. J Vasc Interv Radiol. 2019;30(7):1035–42. 10.1016/j.jvir.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 30.Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: Comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61(6):1156–61. 10.1016/j.eururo.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 31.Johnson BA, Sorokin I, Cadeddu JA. Ten-year outcomes of renal tumor radio frequency ablation. J Urol. 2019;201(2):251–8. 10.1016/j.juro.2018.08.045 [DOI] [PubMed] [Google Scholar]
- 32.Chang X, Zhang F, Liu T, Ji C, Zhao X, Yang R, et al. Radio frequency ablation versus partial nephrectomy for clinical T1b renal cell carcinoma: Long-term clinical and oncologic outcomes. J Urol. 2015;193(2):430–5. 10.1016/j.juro.2014.07.112 [DOI] [PubMed] [Google Scholar]
- 33.Ji C, Zhao X, Zhang S, Liu G, Li X, Zhang G, et al. Laparoscopic radiofrequency ablation versus partial nephrectomy for cT1a renal tumors: Long-term outcome of 179 patients. Urol Int. 2016;96(3):345–53. 10.1159/000443672 [DOI] [PubMed] [Google Scholar]
- 34.Park JM, Yang SW, Shin JH, Na YG, Song KH, Lim JS. Oncological and functional outcomes of laparoscopic radiofrequency ablation and partial nephrectomy for T1a renal masses: A retrospective single-center 60 month follow-up cohort study. Urol J. 2019;16(1):44–9. 10.22037/uj.v0i0.4155 [DOI] [PubMed] [Google Scholar]
- 35.ClinicalTrials.gov . A feasibility study for a multicentre randomised controlled trial to compare surgery with needle ablation techniques in people with small renal masses (4 cm) (CONSERVE): National Library of Medicine. 2015. Cited 04/18/22. Available from: https://clinicaltrials.gov/ct2/show/NCT01608165?type=Intr&cond=Renal+Cell+Carcinoma&intr=Radiofrequency+ablation&draw=4&rank=9
- 36.Chu KF, Dupuy DE. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208. 10.1038/nrc3672 [DOI] [PubMed] [Google Scholar]
- 37.Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S192–203. 10.1016/j.jvir.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laeseke PF, Lee FT Jr., Sampson LA, van der Weide DW, Brace CL. Microwave ablation versus radiofrequency ablation in the kidney: High-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol. 2009;20(9):1224–9. 10.1016/j.jvir.2009.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang P, Wang Y, Zhang D, Yu X, Gao Y, Ni X. Ultrasound guided percutaneous microwave ablation for small renal cancer: Initial experience. J Urol. 2008;180(3):844–8; discussion 8. 10.1016/j.juro.2008.05.012 [DOI] [PubMed] [Google Scholar]
- 40.Clark PE, Woodruff RD, Zagoria RJ, Hall MC. Microwave ablation of renal parenchymal tumors before nephrectomy: Phase I study. AJR Am J Roentgenol. 2007;188(5):1212–4. 10.2214/AJR.05.2190 [DOI] [PubMed] [Google Scholar]
- 41.Bartoletti R, Meliani E, Simonato A, Gontero P, Berta G, Dalla Palma P, et al. Microwave-induced thermoablation with Amica-probe is a safe and reproducible method to treat solid renal masses: Results from a phase I study. Oncol Rep. 2012;28(4):1243–8. 10.3892/or.2012.1950 [DOI] [PubMed] [Google Scholar]
- 42.Yu J, Zhang G, Liang P, Yu XL, Cheng ZG, Han ZY, et al. Midterm results of percutaneous microwave ablation under ultrasound guidance versus retroperitoneal laparoscopic radial nephrectomy for small renal cell carcinoma. Abdom Imaging. 2015;40(8):3248–56. 10.1007/s00261-015-0500-2 [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Zhang X, Liu H, Zhang R, Yu X, Cheng Z, et al. Percutaneous microwave ablation versus laparoscopic partial nephrectomy for cT1a renal cell carcinoma: A propensity-matched cohort study of 1955 patients. Radiology. 2020;294(3):698–706. 10.1148/radiol.2020190919 [DOI] [PubMed] [Google Scholar]
- 44.Yu J, Wang H, Cheng ZG, Liu FY, Li QY, He GZ, et al. A multicenter 10-year oncologic outcome of ultrasound-guided percutaneous microwave ablation of clinical T1 renal cell carcinoma: Will it stand the test of time? Eur Radiol. 2022;32(1):89–100. 10.1007/s00330-021-07900-2 [DOI] [PubMed] [Google Scholar]
- 45.Guan W, Bai J, Liu J, Wang S, Zhuang Q, Ye Z, et al. Microwave ablation versus partial nephrectomy for small renal tumors: Intermediate-term results. J Surg Oncol. 2012;106(3):316–21. 10.1002/jso.23071 [DOI] [PubMed] [Google Scholar]
- 46.Guo J, Arellano RS. Percutaneous microwave ablation of stage T1b renal cell carcinoma: Short-term assessment of technical feasibility, short-term oncologic outcomes, and safety. J Endourol. 2020;34(10):1021–7. 10.1089/end.2020.0382 [DOI] [PubMed] [Google Scholar]
- 47.Ljungberg BAL, Bedke J, Bex A, Capitanio A, Giles RH, Hora M, Klatte T, et al. Cited 04/18/22. Available from: https://uroweb.org/guideline/renal-cell-carcinoma
- 48.Kim MS, Kim W, Park IH, Kim HJ, Lee E, Jung JH, et al. Radiobiological mechanisms of stereotactic body radiation therapy and stereotactic radiation surgery. Radiat Oncol J. 2015;33(4):265–75. 10.3857/roj.2015.33.4.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francolini G, Detti B, Ingrosso G, Desideri I, Becherini C, Carta G, et al. Stereotactic body radiation therapy (SBRT) on renal cell carcinoma, an overview of technical aspects, biological rationale and current literature. Crit Rev Oncol Hematol. 2018;131:24–9. 10.1016/j.critrevonc.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 50.Rühle A, Andratschke N, Siva S, Guckenberger M. Is there a role for stereotactic radiotherapy in the treatment of renal cell carcinoma? Clin Transl Radiat Oncol. 2019;18:104–12. 10.1016/j.ctro.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Meerleer G, Khoo V, Escudier B, Joniau S, Bossi A, Ost P, et al. Radiotherapy for renal-cell carcinoma. The Lancet Oncology. 2014; 15(4):e170–e7. 10.1016/S1470-2045(13)70569-2 [DOI] [PubMed] [Google Scholar]
- 52.Song CW, Kim MS, Cho LC, Dusenbery K, Sperduto PW. Radiobiological basis of SBRT and SRS. Int J Clin Oncol. 2014;19(4):570–8. 10.1007/s10147-014-0717-z [DOI] [PubMed] [Google Scholar]
- 53.Finkelstein SE, Timmerman R, McBride WH, Schaue D, Hoffe SE, Mantz CA, et al. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin and Devel Immun. 2011;2011:439752. 10.1155/2011/439752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):104. 10.1186/s13045-018-0647-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26(305):234–41. 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 56.Correa RJM, Louie AV, Zaorsky NG, Lehrer EJ, Ellis R, Ponsky L, et al. The emerging role of stereotactic ablative radio-therapy for primary renal cell carcinoma: A systematic review and meta-analysis. Eur Urol Focus. 2019;5(6):958–69. 10.1016/j.euf.2019.06.00 [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto T, Kawasaki Y, Umezawa R, Kadoya N, Matsushita H, Takeda K, et al. Stereotactic body radiotherapy for kidney cancer: A 10-year experience from a single institute. J Radiat Res. 2021;62(3):533–9. 10.1093/jrr/rrab031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siva S, Correa RJM, Warner A, Staehler M, Ellis RJ, Ponsky L, et al. Stereotactic ablative radiotherapy for ≥T1b primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK). Int J Radiat Oncol Biol Phys. 2020;108(4):941–9. 10.1016/j.ijrobp.2020.06.014 [DOI] [PubMed] [Google Scholar]
- 59.Siva S, Pham D, Kron T, Bressel M, Lam J, Tan TH, et al. Stereotactic ablative body radiotherapy for inoperable primary kidney cancer: A prospective clinical trial. BJU Int. 2017;120(5):623–30. 10.1111/bju.13811 [DOI] [PubMed] [Google Scholar]
- 60.Siva S, Chesson B, Bressel M, Pryor D, Higgs B, Reynolds HM, et al. TROG 15.03 phase II clinical trial of Focal Ablative STereotactic Radiosurgery for Cancers of the Kidney–FASTRACK II.BMC Cancer. 2018;18(1):1030. 10.1186/s12885-018-4916-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ClinicalTrials.gov . Assessment of QoL and Outcomes with SBRT for RCC (AQuOS-RCC): U.S. National Library of Medicine. 2020. Cited 04/18/22. Available from: https://clinical-trials.gov/ct2/show/NCT03108703
- 62.ClinicalTrials.gov . Stereotactic body radiation therapy versus radiofrequency ablation for small renal masses (SBRT vs RFA): U.S. National Library of Medicine. 2021. Cited 04/18/22. Available from: https://clinicaltrials.gov/ct2/show/NCT03811665
- 63.ClinicalTrials.gov . Assessment of quality of life and outcomes in patients with primary renal cell carcinoma treated with SBRT (AQuOS-II): U.S. National Library of Medicine. 2021. Cited 04/18/22. Available from: https://clinicaltrials.gov/ct2/show/NCT05023265
- 64.ClinicalTrials.gov . Stereotactic ablative body radiation therapy for patients with primary renal cancer: U.S. National Library of Medicine. 2021. Cited 04/18/22. Available from: https://clinical-trials.gov/ct2/show/NCT02141919
- 65.ClinicalTrials.gov . A phase II study of cyberknife radiosurgery for renal cell carcinoma: U.S. National Library of Medicine. 2021. Cited 04/18/22. Available from: https://clinicaltrials.gov/ct2/show/NCT01890590
- 66.ClinicalTrials.gov . Stereotactic magnetic resonance guided radiation therapy: U.S. National Library of Medicine. 2021. Cited 04/18/22. Available from: https://clinicaltrials.gov/ct2/show/NCT04115254
- 67.Grubb WR, Ponsky L, Lo SS, Kharouta M, Traughber B, Sandstrom K, et al. Final results of a dose escalation protocol of stereotactic body radiotherapy for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol. 2021;155:138–43. 10.1016/j.radonc.2020.10.031 [DOI] [PubMed] [Google Scholar]
- 68.Bhagavatula SK, Tuncali K, Shyn PB, Levesque VM, Chang SL, Silverman SG. Percutaneous CT-and MRI-guided cryoablation of cT1 renal cell carcinoma: Intermediate-to long-term outcomes in 307 patients. Radiology. 2020;296(3):687–95. 10.1148/radiol.2020200149 [DOI] [PubMed] [Google Scholar]
- 69.Fraisse G, Colleter L, Peyronnet B, Khene ZE, Mandoorah Q, Soorojebally Y, et al. Peri-operative and local control outcomes of robot-assisted partial nephrectomy vs percutaneous cryoablation for renal masses comparison after matching on radiological stage and renal score. BJU Int. 2019;123(4):632–8. 10.1111/bju.14530 [DOI] [PubMed] [Google Scholar]
- 70.Bianchi L, Chessa F, Piazza P, Ercolino A, Mottaran A, Recenti D, et al. Percutaneous ablation or minimally invasive partial nephrectomy for cT1a renal masses? A propensity score-matched analysis. Int J Urol. 2021;29(3):222–8. 10.1111/iju.14758 [DOI] [PubMed] [Google Scholar]
- 71.Singer EA, Bratslavsky G. Management of locally recurrent kidney cancer. Curr Urol Rep. 2010;11(1):15–21. 10.1007/s11934-009-0085-9 [DOI] [PubMed] [Google Scholar]
- 72.Blitzer GC, Wojcieszynski A, Abel EJ, Best S, Lee FT Jr., Hinshaw JL, et al. Combining stereotactic body radiotherapy and microwave ablation appears safe and feasible for renal cell carcinoma in an early series. Clin Genitourin Cancer. 2021;19(5):e313–e8. 10.1016/j.clgc.2021.04.010 [DOI] [PubMed] [Google Scholar]
- 73.Fukumitsu N, Ishikawa H, Arimura T, Wada H, Okimoto T, Sato Y, et al. Proton therapy for primary renal cell carcinoma: The first nationwide retrospective study in Japan. In Vivo. 2020;34(5):2883–9. 10.21873/invivo.12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(2):223–31. 10.1007/s10439-005-8981-8 [DOI] [PubMed] [Google Scholar]
- 75.Wah TM, Lenton J, Smith J, Bassett P, Jagdev S, Ralph C, et al. Irreversible electroporation (IRE) in renal cell carcinoma (RCC): A mid-term clinical experience. Eur Radiol. 2021;31(10):7491–9. 10.1007/s00330-021-07846-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dai JC, Morgan TN, Steinberg RL, Johnson BA, Garbens A, Cadeddu JA. Irreversible electroporation for the treatment of small renal masses: 5-year outcomes. J Endourol. 2021;35(11):1586–92. 10.1089/end.2021.0115 [DOI] [PubMed] [Google Scholar]