Abstract
MppA is a periplasmic binding protein in Escherichia coli essential for uptake of the cell wall murein tripeptide l-alanyl-γ-d-glutamyl-meso-diaminopimelate. We have found serendipitously that E. coli K-12 strains carrying a null mutation in mppA exhibit increased resistance to a wide spectrum of antibiotics and to cyclohexane. Normal sensitivity of the mppA mutant to these agents is restored by mppA expressed from a plasmid. As is observed in the multiple antibiotic resistance phenotype in E. coli cells, the mppA null mutant overproduces the transcriptional activator, MarA, resulting in expression of the membrane-bound AcrAB proteins that function as a drug efflux pump. Reduced production of OmpF similar to that observed in the multiple antibiotic resistance phenotype is also seen in the mppA mutant. These and other data reported herein indicate that MppA functions upstream of MarA in a signal transduction pathway to negatively regulate the expression of marA and hence of the MarA-driven multiple antibiotic resistance. Overproduction of cytoplasmic GadA and GadB and of several unidentified cytoplasmic membrane proteins as well as reduction in the amount of the outer membrane protein, OmpP, in the mppA null mutant indicate that MppA regulates a number of genes in addition to those already known to be controlled by MarA.
mppA codes for the precursor of a periplasmic binding protein essential for import of murein tripeptide into Escherichia coli (29). MppA utilizes membrane and cytoplasmic component(s) of the oligopeptide permease to transfer its bound ligand into the cytoplasm of the cell (29). However, very little free murein tripeptide is transported into the cell (28); in fact, nearly all murein tripeptide is transported into the cell via the AmpG permease (18) in the form of N-acetylglucosaminyl-β-1,4-anhydro-N-acetylmuramyl-tripeptide (15), which is then cleaved by AmpD amidase (14, 16) to release the murein tripeptide. This caused us to wonder why E. coli has a minor pathway via MppA to transport free murein tripeptide (29). Could the binding protein, complexed with the tripeptide, serve a signaling function reporting a change in the periplasmic environment where MppA resides rather than simply providing a small additional amount of tripeptide to the cell? A clue to such a possible function arose when we observed that the mppA mutant was significantly more resistant to tetracycline and cefoxitin than the isogenic wild type. Results presented in this report demonstrate that in the absence of MppA, the cell exhibits all of the properties associated with the multiple antibiotic resistance (MAR) phenotype (1). MAR is primarily controlled by the multiple antibiotic resistance (mar) operon (1). The mar locus consists of two divergently positioned transcriptional units that flank the operator marO in E. coli (2) and in Salmonella typhimurium (36). One unit encodes MarC, a putative integral inner membrane protein whose function is unknown. The other unit is an operon comprising marRAB, encoding the MarR repressor, which binds to marO and negatively regulates expression of marRAB (24, 33); MarA, a transcriptional activator (1), which activates expression of other genes, notably, acrAB, encoding the multidrug efflux pump (1, 21) and the mar regulon itself (9, 33); and MarB, coding for a small protein of unknown function.
A reduction in the amount of the outer membrane porin, OmpF, is associated with MAR (3) and is also observed in the mppA mutant. Another outer membrane protein, the OmpP protease, is present in greatly reduced amounts in the mppA mutant. The mppA null mutant also overproduces cytoplasmic glutamate decarboxylases GadA and GadB and several inner membrane proteins that may or may not be associated with MAR. Thus, MppA appears to play a central role in the regulation of a number of proteins including those responsible for MAR.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The E. coli K-12 strains used in this study are listed in Table 1. All bacterial strains were grown at 37°C with shaking at 240 rpm in L medium (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl per liter) supplemented with 50 μg of diaminopimelic acid (Dap) per ml when required. The antibiotics ampicillin (100 μg/ml), kanamycin (25 μg/ml), and chloramphenicol (10 μg/ml) were used for selective media. Plasmid pMLD1285 (ptrc::mppA+) (Ampr) expresses the mppA gene under the control of the isopropyl-β-d-thiogalactoside (IPTG)-inducible trc promoter (ptrc) (29). Unless otherwise stated, 0.5 mM IPTG was present in cultures of cells containing pMLD1285, although sufficient expression of mppA occurs in the absence of IPTG to allow growth of a dapD2 mppA null mutant with murein tripeptide as the source of Dap (29).
TABLE 1.
E. coli strains used
| Strain | Genotype or description | Source or reference |
|---|---|---|
| AT980 | dapD2 relA1 spoT1 thi-1 Hfr(defective) | E. coli Genetic Stock Center (Yale University, New Haven, Conn.), isolate 4545 |
| AG100K | AG100 ΔmarORAB::Kanr | 23 |
| HSL5 | AT980 ΔmarCORAB::Kanr | P1(AG100K) × AT980 |
| HSL6 | AT980 ΔacrAB::Tn903 Kanr | P1(JZM120) × AT980 |
| TP982 | AT980 oppB::mini-Tn10Cm ampG::Kan | 29 |
| HSL2 | AT980 oppB::mini-Tn10Cm | P1(TP982) × AT980 |
| TP985 | AT980 mppA::mini-Tn10Cm | 29 |
| HSL3 | TP985 ΔacrAB::Tn903 Kanr | P1(JZM120) × TP985 |
| HSL4 | TP985 ΔmarCORAB::Kanr | P1(AG100K) × TP985 |
| AG100 | argE3 thi-3 rpsL xyl mtl supE44 Δ(gal-uvrB) | 10 |
| AG102 | AG100 marR1 | 10 |
| JZM120 | argE3 hisG4 leuB6 Δ(gpt-proA)62 thr-1 thi-1 ara-14 galK2 lacY1 mtl-1 xyl-1 kdgK51 tsx-33 recB21 recC22 sbcB15 supE44 rpsL31 rac ΔacrAB::Tn903 Kanr | 21 |
| JF568 | aroA357 ilv-227 metB65 his-53 purE41 cyc-1 xyl-14 lacY29 rpsL77 tsxN63 | 7 |
| JF701 | JF568 ompC264 | 7 |
| JF703 | JF568 ompF254 aroA+ | 7 |
MICs of antibiotics.
The MICs of various antibiotics were determined by plating several hundred cells on L-Dap agar containing a range of concentrations of antibiotics that varied by a factor of 2.
Tolerance to cyclohexane.
The test was done essentially as described by White et al. (39). L-Dap agar plates, dried at 37°C for several hours, were spotted with 5 μl of cultures that contained about 105 late-log- or stationary-phase cells per ml. After the drop had dried thoroughly, the agar was flooded with cyclohexane to a depth of 2 or 3 mm and incubated at 30°C overnight in a sealed container. Tolerance was indicated by a visible lawn of growth. Sensitive strains produced no visible lawn.
Preparation of cytoplasmic and membrane protein fractions.
Cytoplasmic and membrane proteins were separated by the method of Koski et al. (19), with some modifications. Cells were grown in 40 ml of L-Dap medium from a 1% inoculum. When needed, 0.5 mM IPTG was added in early log phase, and growth was continued for approximately 4 h. Cells were harvested at 4°C when the optical density at 600 nm of cultures reached 0.7 to 1. Cells were washed with 10 ml of cold 10 mM HEPES-KOH buffer (pH 7.4) (buffer A). The cell pellet was suspended in 3 ml of the same buffer and disrupted by sonication with cooling. The unbroken cells were removed by centrifugation at 3,000 × g for 5 min, and the supernatant was centrifuged at 4°C for 60 min at 180,000 × g. The supernatant (cytoplasmic and periplasmic proteins) was stored on ice. The pellet (cell envelope fraction) was washed once with cold buffer A, resuspended in 1 ml of buffer A containing 1% (wt/vol) sodium lauryl sarcosinate (sarcosyl), and incubated at room temperature for 30 min. The anionic detergent sarcosyl solubilizes the proteins of the inner membrane while leaving the major outer membrane proteins insoluble (6). The sarcosyl-insoluble outer membrane proteins and peptidoglycan were sedimented by centrifugation at 180,000 × g as before, and the supernatant containing the cytoplasmic membrane proteins was saved. The pellet was washed once with 1 ml of buffer A, reextracted with sarcosyl for 5 min, pelleted by centrifugation as before, and finally resuspended in 200 μl of distilled water.
Protein techniques.
Proteins were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) according to the method of Laemmli (20). For N-terminal amino acid sequencing, the proteins were transferred from the SDS-polyacrylamide gel to Immobilon-P by the Western blotting method of Towbin et al. (38). The blots were washed in 50 mM Tris-HCl (pH 7.5)–0.15 M NaCl–0.05% (vol/vol) Tween 20, rinsed with distilled water, and stained with 0.025% aqueous Coomassie brilliant blue R-250 for 5 min. The blot was destained with water and then rinsed quickly with 40% methanol–10% acetic acid to facilitate visualization and excision of the band of interest. The N-terminal amino acid sequences of the proteins in the excised bands were determined at the Tufts University Core Facility with an Applied Biosystems model 477A pulsed/liquid-phase sequencer coupled to an on-line high-performance liquid chromatography model 120A analyzer. Data were analyzed by the Genetics Computer Group program.
The Western blots were immunostained with anti-MarA polyclonal rabbit antibody (32), and the MarA band was visualized with a Renaissance chemiluminescence detection kit as instructed by the manufacturer (NEN Life Sciences Products, Boston, Mass.).
RESULTS
MAR phenotype of the mppA null mutant.
We found serendipitously that the mppA mutant, TP985, was significantly more resistant to tetracycline and cefoxitin than its isogenic parent. This caused us to examine the sensitivity of the mppA mutant to a wider spectrum of antibiotics. The mppA mutation in TP985 resulted in a fourfold increase in resistance to penicillin G and nalidixic acid as well as to tetracycline and cefoxitin compared to its wild-type parent AT980 (Table 2; compare lines 1 and 5). Introduction of pMLD1285, expressing mppA, restored the mutant strain to wild-type sensitivity (Table 2, line 8). The antibiotic resistance of AG102, which has a point mutation in marR, the repressor gene in the marRAB operon, and consequently overexpresses the transcriptional activator, MarA (2), was three- to sixfold greater than that of its parent, AG100, under our conditions (data not shown).
TABLE 2.
MIC test
| Line | Strain | Relevant genotype | Concn (μg/ml)
|
|||
|---|---|---|---|---|---|---|
| Tetracycline | Nalidixic acid | Cefoxitin | Penicillin G | |||
| 1 | AT980 | Wild type | 2 | 2 | 2 | 20 |
| 2 | HSL5 | ΔmarCORAB | 2 | 2 | 1 | 20 |
| 3 | HSL6 | ΔacrAB | 0.5 | 0.5 | 1 | 10 |
| 4 | HSL2 | oppB::Cm | 1 | 2 | 2 | 20 |
| 5 | TP985 | mppA::Cm | 8 | 8 | 16 | 80 |
| 6 | HSL4 | mppA::Cm ΔmarCORAB | 2 | 2 | 2 | 10 |
| 7 | HSL3 | mppA::Cm ΔacrAB | 0.5 | 1 | 2 | 20 |
| 8 | TP985 | mppA::Cm/pACYC177 mppA+ | 4 | 2 | 2 | NDa |
| 9 | TP985 | mppA::Cm/pACYC177 | 8 | 8 | 16 | ND |
ND, not done.
Interestingly, AG102 (marR1) does not exhibit increased resistance to kanamycin. Likewise, TP985 (mppA::Cm) is not more resistant to kanamycin than its isogenic parent (data not shown).
The marA overexpression mutant, AG102 (marR1), has been shown to grow under a lake of cyclohexane (1, 39). We found that TP985 (mppA::Cm) is similarly able to grow under a lake of cyclohexane and that the plasmid expressing mppA restores its sensitivity to cyclohexane (data not shown). Thus, an mppA mutant mirrors the resistance of a marA overexpression strain, strongly indicating that MppA plays a role in the negative regulation of MAR.
The mar operon is required for the ΔmppA MAR phenotype.
As shown in Table 2 (line 6 versus lines 5 and 1), in the absence of the mar operon, the antibiotic sensitivity of TP985 returns to that of its parent. Thus, the lack of MppA cannot activate the MAR phenotype unless the mar operon is present. This result indicates that MppA acts exclusively on the mar operon to regulate MAR.
Opp permease is not involved in the negative regulation controlled by MppA.
MppA, in its murein tripeptide transport function, utilizes the membrane and cytoplasmic components of the Opp permease, OppBCDF (29). However, strain HSL2, carrying mini-Tn10Cm in oppB, does not express the MAR phenotype (Table 2, line 4). This suggests that OppBCDF is not involved in the regulatory function of MppA.
Increased abundance of MarA and involvement of AcrAB in the MAR phenotype of TP985 (mppA::Cm).
It is known that MAR is associated with an increase in MarA, which then activates the acrAB operon to produce AcrA and AcrB, which constitute the drug efflux pump primarily responsible for MAR (1, 21, 27). SoxS and Rob can also up-regulate acrAB (22) but are not involved here, since, as already shown, in the absence of the mar operon, mppA::Cm has no effect on antibiotic sensitivity. If MppA is involved in the negative regulation of marA, the MarA and AcrAB contents of the mppA mutant should increase relative to its parent. Figure 1 is a Western blot of soluble E. coli proteins probed with anti-MarA antibody. As can be seen, TP985 (mppA::Cm) exhibits a greater than wild-type amount of MarA, and complementing the mutant with pMLD1285 (expressing mppA) down-regulates the amount of MarA in the mutant to the wild-type level. AG102 (marR1), overexpressing marA, produces 50% more MarA than does TP985 (mppA::Cm) (Fig. 1, lanes 2 and 5).
FIG. 1.
Overproduction of MarA by the mppA mutant and by a marR mutant as determined by Western blotting with anti-MarA antibody. Lanes: 1, AT980; 2, TP985 (mppA::Cm); 3, TP985/pMLD1285 (mppA+); 4, AG100; 5, AG102 (marR1).
AcrB is a cytoplasmic membrane protein with 12 transmembrane segments that, together with AcrA (a periplasmic lipoprotein) and TolC, is the efflux pump largely responsible for MAR (21, 27). JZM120 (ΔacrAB) is supersensitive to hydrophobic antibiotics and detergents but only slightly more sensitive to many other antibiotics (21). When the ΔacrAB mutation was introduced into TP985 (mppA::Cm), the resultant strain HSL3 became as sensitive or slightly more sensitive to antibiotics than the parent strain (Table 2; compare lines 5 and 7 and lines 1 and 3). ΔacrAB also rendered AT980 and TP985 (mppA::Cm) at least 300-fold more sensitive to SDS, consistent with the report by Ma et al. (21) that deletion of acrAB increased the sensitivity of two other E. coli K-12 strains to SDS more than 150-fold. Taken together, these results support the notion that MppA affects MAR primarily by regulating acrAB expression through its control of marA.
Increased expression of acrAB in the mppA null mutant.
Recently, Rhee et al. reported the crystal structure of MarA bound to the marO sequence and proposed a consensus sequence for MarA binding based on 10 mar regulon promoters (30). We recognized a near-perfect MarA binding site 27 nucleotides upstream of promoter 1 of the acrAB operon. The presence of a MarA binding site upstream of the acrAB promoter strongly suggests that overexpression of MarA in TP985 (mppA::Cm) should lead to increased levels of the AcrAB proteins that constitute the efflux pump and hence to the MAR phenotype.
A cytoplasmic enzyme, glutamate decarboxylase (Gad), is overproduced in TP985 (mppA::Cm).
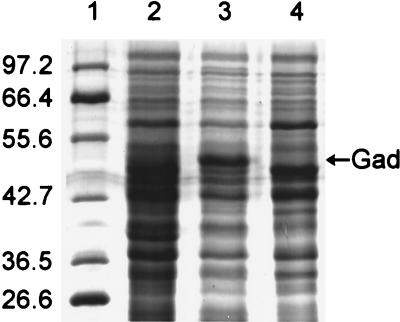
The cytoplasmic proteins from TP985, its isogenic parent strain AT980, and TP985/pMLD1285 (ptrc::mppA+) were separated by SDS-PAGE. A remarkably intense band (representing up to 17% of the total soluble protein) was present in TP985 (Fig. 2, lane 3). In contrast, this band was only faintly detectable in the soluble fraction from AT980 and TP985/pMLD1285 (ptrc::mppA+) (Fig. 2, lanes 2 and 4). To identify the protein in this band, the N-terminal amino acid sequence of this protein band was determined. The sequence revealed that the band consisted of two proteins, GadA and GadB. These proteins represent isotypes of glutamate decarboxylase differing only in five amino acid residues, three of which are located within the first six N-terminal residues (34). The N-terminal sequence analysis MD(K or Q)K(L or Q)(L or V) indicated that the sample contained a mixture of these two nearly identical proteins. From the quantitation of the amino acids recovered in the N-terminal amino acid analysis, it was concluded that the band contained about 25% more GadA than GadB. To confirm that overexpression of gadA and gadB indeed occurs in the mppA mutant, Gad enzymatic activity, assayed by the method of Rice et al. (31), was at least eightfold higher in TP985 (mppA::Cm) than in AT980 and TP985/pMLD1285 (data not shown). We also observed overproduction of Gad in AG102.
FIG. 2.
SDS–10% acrylamide gel of E. coli soluble proteins showing overproduction of Gad by the mppA mutant TP985. Lanes: 1, molecular weight standards (sizes indicated in kilodaltons); 2, AT980; 3, TP985 (mppA::Cm); 4, TP985/pMLD1285 (mppA+).
To ascertain if gadA and gadB overexpression in TP985 could be a consequence of marA overexpression, we initially examined the promoter regions upstream of gadA and gadBC for putative MarA binding sites. Both genes were found to have potential, though imperfect, MarA binding sites about 60 nucleotides upstream of the coding sequence. This observation is consistent with activation of the glutamate decarboxylase genes by MarA. However, when the Gad content of HSL4, the mppA Δmar double mutant, was compared with that of the mppA mutant, both were found to equally overproduce Gad. From this result, it seems clear that MppA regulation of Gad does not depend upon the mar operon. Whether overexpression of Gad is related to the MAR phenotype is not clear at present.
Outer membrane protein changes associated with the mppA mutation.
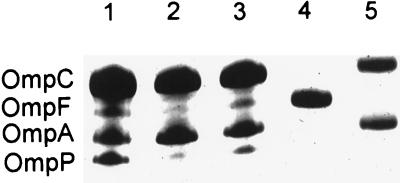
The outer membrane proteins from TP985 (mppA::Cm), its isogenic parent AT980, and TP985/pMLD1285 (ptrc::mppA+) were compared by SDS-PAGE. Outer membranes prepared from strains lacking OmpC or OmpF were used as standards (Fig. 3, lanes 4 and 5). As can be seen in Fig. 3, the OmpF content in TP985 was decreased significantly, as is known to occur in marR mutants such as AG102 (marR1) (4). MicF is known to inhibit the formation of OmpF by hybridizing to its mRNA (26). MicF is up-regulated by MarA and is therefore responsible for the reduced OmpF content of this mutant (4). When plasmid IV (26), also known as pmicB21, bearing the micF promoter fused to lacZ was introduced into AT980 and TP985, the mppA mutant was found to produce about 50% more β-galactosidase than AT980, indicating that more micF message was produced. This may account for the reduced amount of OmpF in TP985 (data not shown).
FIG. 3.
Reduced amounts of OmpF and OmpP in the mppA mutant as revealed by SDS-PAGE on a 12.5% acrylamide gel. Lanes: 1, AT980; 2, TP985 (mppA::Cm); 3, TP985/pMLD1285 (mppA+); 4, JF701 (ompC); 5, JF703 (ompF).
Another outer membrane protein, that migrates slightly faster than OmpA, was also markedly reduced in quantity in TP985 relative to its isogenic parent. Both proteins were restored to normal levels by expression of mppA from pMLD1285 (ptrc::mppA+) (Fig. 3, lane 3). The N-terminal amino acid sequence (SDFFGP) of the protein that migrated slightly faster than OmpA identified it as OmpP (17).
Inner membrane protein changes associated with the mppA mutation.
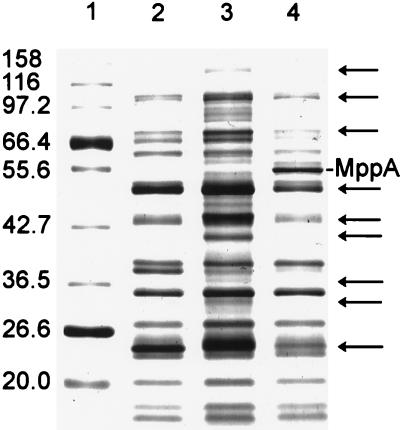
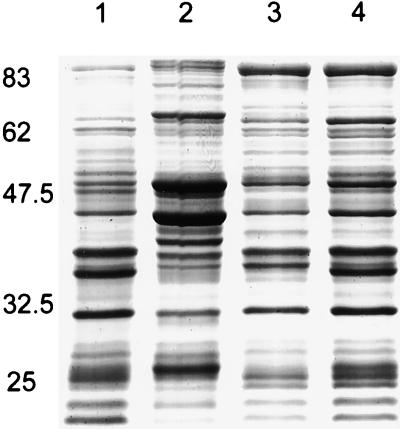
The inner membrane protein profile of the mppA mutant, TP985, compared by SDS-PAGE with AT980 and TP985/pMLD1285(ptrc::mppA+) is shown in Fig. 4. Compared with AT980 and TP985/pMLD1285, TP985 (mppA::Cm) expresses at least nine proteins at increased levels. Of these nine, AG102 (marR1) clearly overexpresses the three major proteins (molecular masses of approximately 50, 43, and 24 kDa), but overexpression of the other proteins was not apparent (data not shown). The up-regulation may not be readily seen in the AG100 background because of strain differences. However, MppA may be involved in negative regulation of genes other than those controlled by the mar regulon. To test this more directly, the inner membrane protein profile of the mppA Δmar double mutant was compared with that of the mppA mutant. As shown by SDS-PAGE in Fig. 5, all of the proteins overproduced by the mppA mutant are no longer overproduced when the mar operon is absent. Thus, we conclude that negative regulation of the mar operon by MppA results in greatly reduced expression of nine or more inner membrane proteins. Incidentally, careful inspection of Fig. 5 also reveals that the mar deletion by itself causes overexpression of several proteins relative to its isogenic parent.
FIG. 4.
SDS–7.5% acrylamide gel of inner membrane proteins showing overproduction of inner membrane proteins by the mppA mutant. Lanes: 1, molecular weight standards (sizes indicated in kilodaltons); 2, AT980; 3, TP985 (mppA::Cm); 4, TP985/pMLD1285 (mppA+). Arrows point to inner membrane proteins that are overproduced by TP985.
FIG. 5.
SDS–10% acrylamide gel of inner membrane proteins showing overproduction of many proteins by the mppA mutant and lack of overproduction when the mar operon is absent. Lanes: 1, AT980; 2, TP985 (mppA::Cm); 3, HSL4 (mppA::Cm ΔmarCORAB); 4, HSL5 (ΔmarCORAB). Sizes are indicated in kilodaltons.
DISCUSSION
This report demonstrates that the mppA null phenotype is very similar to the MAR phenotype that results from marA overexpression (1). Compared to the wild-type strain, cells lacking MppA are more resistant to most antibiotics and overproduce MarA and presumably AcrB, the cytoplasmic membrane component of the efflux pump. As in MAR, kanamycin sensitivity is unchanged, the mutant is able to grow in the presence of cyclohexane, and the OmpF porin level is reduced. Reintroduction of the mppA gene on a plasmid restores the wild-type sensitivity in all respects. Thus, MppA plays a critical role in the negative regulation of the MAR phenotype.
Comparison of the proteins produced in the presence or absence of MppA has revealed a number of notable changes in the composition of the inner and outer membrane proteins as well as changes in the soluble protein fraction of the cell. In addition to the cytoplasmic protein, MarA, we observed a marked rise in GadA and GadB levels, such that about 17% of the soluble protein of the cell was represented by these glutamate decarboxylases. This overexpression does not require the mar operon. The E. coli glutamate decarboxylases are pyridoxal phosphate-dependent enzymes that catalyze the alpha-decarboxylation of glutamate to yield γ-aminobutyrate and CO2. The E. coli chromosome contains two genes, gadA and gadB, encoding this enzymatic activity, and they map at distinct loci (5). Glutamate decarboxylase activity has been used for rapid identification of E. coli since it is absent in enteric organisms other than shigellae (31).
The physiological function of Gad is not known, although a possible role in maintaining pH homeostasis was proposed long ago (8). GadC, which is produced by expression of the gadBC operon, is a presumed efflux pump for γ-aminobutyrate and has been shown to be required for development of resistance to acid in E. coli (11). Decarboxylation of glutamate consumes a proton, thus raising the cytoplasmic pH. An alternative possible function for Gad was recently suggested by the demonstration that GadB, together with GadC, generates proton motive force that can be converted to ATP (12). The AcrAB drug efflux pump utilizes membrane potential as a source of energy (21). It is tempting to suggest that MAR in E. coli is driven, in part, by the membrane potential generated by GadBC and utilized by AcrAB.
The reduction in expression of the outer membrane protein, OmpF, in TP985 (mppA::Cm) appears similar to that observed in the MAR phenotype (4). OmpF production involves a posttranscriptional regulatory mechanism mediated by micF. Part of the 174-base micF RNA is complementary to the 5′ end of ompF mRNA and inhibits the translation of ompF mRNA by hybridizing with it (26). From studies with a micF-lacZ fusion or with strains deleted for the micF locus, it was suggested that mutations in both the tolC and the marR loci increased micF expression, causing a posttranslational decrease in the level of OmpF protein (4, 25). The reduction in OmpF content in the mppA mutant is moderate but apparent (Fig. 3). We observed a small increase in expression from the micF-lacZ fusion plasmid in the mppA mutant, which suggests that micF may also be overproduced in the mppA mutant.
Another outer membrane protein, OmpP, is also significantly reduced in amount in TP985 (mppA::Cm) (Fig. 3). OmpP is a protease, 87% identical to the well-known outer membrane protease OmpT (17). Curiously, OmpP is not present in all strains of E. coli K-12 (17). The significance of the OmpP level for the MAR phenotype is not apparent.
It is very likely that the MAR, the resistance to cyclohexane, and the diminished amount of OmpF seen in the mppA null mutant are related to MarA since these changes also occur in the marA overexpression mutant AG102 (marR1). It was surprising to discover that at least nine inner membrane proteins are present in greatly increased amounts in the mppA mutant and that this requires the presence of the mar operon.
How MppA is involved in the regulation of marA remains to be determined. Obviously, a periplasmic protein, MppA, cannot directly contact the cytoplasmic protein, MarR, which interacts with marO to regulate mar expression. One possibility is that a membrane-bound sensor kinase-phosphatase, either directly or via a phosphorelay (35), maintains MarR in the operator-bound, repressor state. Figure 6 compares MarR with several well-studied response regulators and illustrates that MarR contains a sequence element containing the two critical aspartic acid residues and a lysine residue that constitute the signature for two-component response regulators known to undergo reversible aspartyl phosphorylation-dephosphorylation (35). The occurrence of a putative response regulator-like motif in MarR raises the possibility that a sensor kinase is involved in regulation of marA by MppA. While we have not eliminated SoxS or Rob as additional transcriptional activators (22, 39) that may contribute to the intrinsic resistance of E. coli, the fact that the mppA mutant does not express MAR in the absence of MarA clearly indicates that the MAR phenotype of TP985 depends on MarA. Since overproduction of MarA seems sufficient to account for the mutant’s MAR phenotype, this leads us to discuss a model in terms of the mar operon.
FIG. 6.
Comparison of the MarR amino acid sequence with the signature sequence of representative two-component response regulators. The two aspartates, one of which becomes phosphorylated, and the lysine that are highly conserved are shown in large bold type. The amino acids shown in smaller bold letters form part of a conserved hydrophobic core (35). MarR contains five charged amino acids in the hydrophobic core, as opposed to only two in CheB and none in the others. The numbers indicate the number of amino acids separating the listed amino acids.
According to this hypothetical model, in the absence of MppA or of murein tripeptide bound to MppA, MarR would be phosphorylated and would be inactive as the repressor of the mar operon. The nonphosphorylated form of MarR is predicted to be the active repressor only because purified MalE-MarR fusion protein has been shown to bind tightly to marO, and the instability of the aspartyl-phosphate bond makes it unlikely that the fusion protein retained any phosphate (33). However, it must be stressed that the opposite scenario, i.e., the phosphorylated MarR is the repressor and the absence of MppA causes dephosphorylation of MarR to render it inactive, is also possible.
Presumably the absence of MppA in the null mutant mimics the unliganded state in the wild type. Hence, in this model for sensing stress, murein tripeptide liganded to MppA is the active negative regulator and senses the normal state. Dissociation of the ligand would activate the signal transduction pathway, inactivate MarR, and trigger MAR. The question then becomes, what stress could dissociate the presumed ligand, murein tripeptide, from MppA? One possible scenario that would reduce the concentration of tripeptide in the periplasm is a defective outer membrane; another possibility would be conditions that shut down AmiA and AmiB, the enzymes that release tripeptide from murein or murein degradation products (37).
In conclusion, we have demonstrated that MppA, a periplasmic protein that binds murein tripeptide, is involved in the negative regulation of marA and hence of the MAR phenomenon associated with the mar operon as well as expression of many inner membrane proteins. We have proposed, and are now testing, the hypothesis that MppA exerts its control via a signal transduction pathway.
ACKNOWLEDGMENTS
We thank Michael Alekshun, Nicholas Delihas, Ulf Henning, Stuart Levy, Laura McMurry, and Debabrata RayChaudhuri for generously supplying strains, plasmids, antisera, and proteins and for helpful discussions. We especially thank Debabrata RayChaudhuri for critical advice on the manuscript.
This work was supported in part by Public Health Service grant GM51610 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Alekshun M N, Levy S B. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents and Chemother. 1997;41:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen S P, Hachler H, Levy S B. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J Bacteriol. 1993;175:1484–1492. doi: 10.1128/jb.175.5.1484-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S P, Levy S B, Foulds J, Rosner J L. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J Bacteriol. 1993;175:7856–7862. doi: 10.1128/jb.175.24.7856-7862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen S P, McMurry L M, Levy S B. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J Bacteriol. 1988;170:5416–5422. doi: 10.1128/jb.170.12.5416-5422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Biase D, Tramonti A, John R A, Rossa F. Isolation, overexpression, and biochemical characterization of the two isoforms of glutamic acid decarboxylase from Escherichia coli. Protein Expr Purif. 1996;8:430–438. doi: 10.1006/prep.1996.0121. [DOI] [PubMed] [Google Scholar]
- 6.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foulds J, Chai T-J. New major outer membrane protein found in an Escherichia coli tolF mutant resistant to bacteriophage Tulb. J Bacteriol. 1978;133:1478–1483. doi: 10.1128/jb.133.3.1478-1483.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gale E F. The bacterial amino acid decarboxylases. Adv Enzymol. 1946;6:1–32. [Google Scholar]
- 9.Gambino L, Gracheck S J, Miller P F. Overexpression of the MarA positive regulator is sufficient to confer multiple antibiotic resistance in Escherichia coli. J Bacteriol. 1993;175:2888–2894. doi: 10.1128/jb.175.10.2888-2894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George A M, Levy S B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J Bacteriol. 1983;155:531–540. doi: 10.1128/jb.155.2.531-540.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hersh B M, Farooq F T, Barstad D N, Blankenhorn D L, Slonczewski J L. A glutamate-dependent acid resistance gene in Escherichia coli. J Bacteriol. 1996;178:3978–3981. doi: 10.1128/jb.178.13.3978-3981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higuchi T, Hayashi H, Abe K. Exchange of glutamate and γ-aminobutyrate in a Lactobacillus strain. J Bacteriol. 1997;179:3362–3364. doi: 10.1128/jb.179.10.3362-3364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiles I D, Higgins C F. Peptide uptake by Salmonella typhimurium: the periplasmic binding protein. Eur J Biochem. 1986;158:561–567. doi: 10.1111/j.1432-1033.1986.tb09791.x. [DOI] [PubMed] [Google Scholar]
- 14.Höltje J-V, Kopp U, Ursinus A, Wiedemann B. The negative regulator of β-lactamase induction AmpD is a N-acetyl-anhydromuramyl-l-alanine amidase. FEMS Microbiol Lett. 1994;122:159–164. doi: 10.1111/j.1574-6968.1994.tb07159.x. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs C, Huang L-J, Bartowsky E, Normark S, Park J T. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 1994;13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs C, Joris B, Jamin M, Klarsov K, van Beemen J, Mengin-Lecreulx D, van Heijenoort J, Park J T, Normark S, Frere J-M. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-l-alanine amidase. Mol Microbiol. 1995;15:553–559. doi: 10.1111/j.1365-2958.1995.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann A, Stierhof Y-D, Henning U. New outer membrane-associated protease of Escherichia coli K-12. J Bacteriol. 1994;176:359–367. doi: 10.1128/jb.176.2.359-367.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korfmann G, Sanders C C. AmpG is essential for high-level expression of AmpC β-lactamase in Enterobacter cloacae. Antimicrob Agents Chemother. 1989;33:1946–1951. doi: 10.1128/aac.33.11.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koski P, Rhen M, Kantele J, Vaara M. Isolation, cloning, and primary structure of a cationic 16kDa outer membrane protein of Salmonella typhimurium. J Biol Chem. 1989;264:18973–18980. [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 22.Ma D, Alberti M, Lynch C, Nikaido H, Hearst J E. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol Microbiol. 1996;19:101–112. doi: 10.1046/j.1365-2958.1996.357881.x. [DOI] [PubMed] [Google Scholar]
- 23.Maneewannakul K, Levy S B. Identification of mar mutants among quinolone-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1996;40:1695–1698. doi: 10.1128/aac.40.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin R G, Rosner J L. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc Natl Acad Sci USA. 1995;92:5456–5460. doi: 10.1073/pnas.92.12.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirsa R, Reeves P R. Role of micF in tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J Bacteriol. 1987;169:4722–4730. doi: 10.1128/jb.169.10.4722-4730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuno T, Chou M, Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA) Proc Natl Acad Sci USA. 1984;81:1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J T. Turnover and recycling of the murein sacculus in oligopeptide permease-negative strains of Escherichia coli: indirect evidence for an alternative permease system and for a monolayered sacculus. J Bacteriol. 1993;175:7–11. doi: 10.1128/jb.175.1.7-11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J T, RayChaudhuri D, Li H, Normark S, Mengin-LeCreulx D. MppA, a periplasmic binding protein essential for import of the bacterial cell wall peptide l-alanyl-γ-d-glutamyl-meso-diaminopimelate. J Bacteriol. 1998;180:1215–1223. doi: 10.1128/jb.180.5.1215-1223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhee S, Martin R G, Rosner J L, Davies D R. A novel DNA-binding motif in MarA: the first structure for an AraC family transcriptional activator. Proc Natl Acad Sci USA. 1998;95:10413–10418. doi: 10.1073/pnas.95.18.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice E W, Johnson C H, Dunnigan M E, Reasoner D J. Rapid glutamate decarboxylase assay for detection of Escherichia coli. Appl Environ Microbiol. 1993;59:4347–4349. doi: 10.1128/aem.59.12.4347-4349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Seoane A S, Levy S B. Characterization of MarR, the repressor of the multiple antibiotic resistance (mar) operon of Escherichia coli. J Bacteriol. 1995;177:3414–3419. doi: 10.1128/jb.177.12.3414-3419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith D K, Kassam T, Singh B, Elliott J F. Escherichia coli has two homologous glutamate decarboxylase genes that map to distinct loci. J Bacteriol. 1992;174:5820–5826. doi: 10.1128/jb.174.18.5820-5826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stock J B, Stock A M, Mottonen J M. Signal transduction in bacteria. Nature. 1990;344:395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- 36.Sulavik M C, Dazer M, Miller P F. The Salmonella typhimurium mar locus: molecular and genetic analyses and assessment of its requirement for virulence. J Bacteriol. 1997;179:1857–1866. doi: 10.1128/jb.179.6.1857-1866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomioka S, Nikaido T, Miyakawa T, Matsuhashi M. Mutation of the N-acetylmuramyl-l-alanine amidase gene of Escherichia coli K-12. J Bacteriol. 1983;156:463–465. doi: 10.1128/jb.156.1.463-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White D G, Goldman J D, Demple B, Levy S B. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J Bacteriol. 1997;179:6122–6126. doi: 10.1128/jb.179.19.6122-6126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]