Abstract
This study aimed to develop novel SSR markers in tomato. Several BAC clones along chromosome 3 in tomato were selected based on their content. The criteria was the availability of genes, either directly or indirectly related to stress response (drought, salinity, and heat) in tomato. A total of 20 novel in silico SSR markers were developed and 96 important nearby genes were identified. The identified nearby genes represent different tomato genes involved in plant growth and development and biotic and abiotic stress tolerance. The developed SSR markers were assessed using tomato landraces. A total of 29 determinate and semi-determinate local tomato landraces collected from diverse environments were utilized. A total of 33 alleles with mean of 1.65 alleles per locus were scored, showing 100% polymorphic patterns, with a mean of 0.18 polymorphism information content (PIC) values. The mean of observed and expected heterozygosity were 0.19 and 0.24, respectively. The mean value of the Jaccard similarity index was used for clustering the landraces. The developed microsatellite markers showed potential to assess genetic variability among tomato landraces. The genetic distance information reported in this study can be used by breeders in future genetic improvement of tomato for tolerance against diverse stresses.
Keywords: BACs, heterozygosity, polymorphic information content, SSRs, stress, tomato
1. Introduction
Tomato (Solanum lycopersicum L.) is one of the most important and widespread vegetable crops around the world. It is the second most consumed vegetable after potato with a total production reaching 187 million tones [1]. Tomato belongs to the family Solanaceae, which includes several agronomic importance crops, such as potato and pepper. Tomato is a diploid (2n = 2x = 24) plant, and its genome is approximately 950 Mb in size [2]. The breeding system in tomato vary from allogamous to facultative allogamous to autogamous [3].
Both biotic and abiotic stresses are important constraints to tomato productivity. These stresses affect plant growth during all developmental stages when imposed either individually or combined [4,5,6,7,8,9,10]. Climate changes, including drought, salinity, and heat, are the most important factors that reduce agricultural crop yields in arid and semi-arid regions, which threaten the level of food security.
There are more than 7500 tomato landraces and varieties successfully bred and grown for various purposes worldwide [11]. These tomato genetic resources have special importance in breeding programs as sources of desired genes for different purposes, such as disease resistance and agronomic traits [12,13], and they serve as a model organism for genetic and developmental studies [14,15]. Study of phenotypic and genetic diversity in tomato landrace collections is important for germplasm preservation, exploitation, and utilization of these genetic resources. In addition, the characterization of highly diversified materials with molecular markers offers a unique opportunity to define significant marker-trait associations with biological and agronomic interest. To this end, various marker techniques have been successfully applied, either individually or in combination, to study the genetic diversity of various plant species [16]. Unfortunately, the gradual disappearance of many tomato landraces in favor of high-yielding cultivars is likely to erode the genetic base of tomato [17].
Simple sequence repeats (SSRs) is an important source of DNA markers due to their high reproducibility, multiallelic nature, co-dominant inheritance, abundance, and wide genome coverage. Development of SSR markers from map-referenced BAC clones was a very effective means of targeting markers to marker scarce-positions in the genome [18]. In silico mining of SSRs from sequence databases [19] provides an attractive alternative to the molecular approaches. Not only is the in silico approach time and cost effective but it also allows for the discovery of SSRs from expressed sequence tags (ESTs) that represent the coding region of genome. SSR markers have been successfully utilized to analyze genetic diversity in tomato [20,21,22,23]. SSR marker are very crucial in breeding and analysis of plant abiotic stresses [24].
We initiated this study to develop, validate, and map new SSR markers based on in silico analysis, which are tightly linked to putative response genes related to biotic and abiotic stresses using tomato landraces.
2. Materials and Methods
2.1. Plant Materials
Seeds from a total of 29 tomato (Solanum lycopersicum L.) landraces were obtained from the National Agricultural Research Center (NARC), Jordan, where the tomato seeds collected from local farmers throughout the country are kept in the medium-term germplasm seed gene bank. These landraces were collected from different geographical origins [25]. Table 1 shows some of the vegetative characters of the landraces included in the study. Seeds were planted in a growth room for 3 weeks and young leaves were collected and stored under −20 °C for genomic DNA extraction.
Table 1.
Vegetative characters of the 29 Jordanian tomato landraces.
| Accession Number | Growth Type | Plant Size | Foliage Density | Growth Habit |
|---|---|---|---|---|
| 951 | Determinate | Large | Dense | Erect |
| 952 | Determinate | Med.–large | Dense | Prostrate branched |
| 955 | Determinate | Med.–large | Intermediate | Prostrate branched |
| 956 | Determinate | Small | Intermediate | Erect |
| 958 | Determinate | Medium | Intermediate | Erect less branched |
| 959 | Determinate | Med.–large | Intermediate | Half erect branched |
| 960 | Determinate | Small | Sparse | Erect less branched |
| 961 | Determinate | Large | Dense | Prostrate |
| 963 | Determinate | Large | Dense | Erect branched |
| 964 | Semi-determinate | Large | Dense | Erect |
| 969 | Determinate | Medium | Intermediate | Prostrate branched |
| 970 | Determinate | Med.–large | Dense | Erect branched |
| 971A | Semi-determinate | Medium | Intermediate | Erect |
| 971B | Determinate | Small-med. | Sparse | Prostrate branched |
| 972 | Determinate | Large | Dense | Erect branched |
| 973 | Determinate | Medium | Intermediate | Prostrate |
| 975 | Determinate | Medium | Sparse | Erect branched |
| 978 | Semi-determinate | Large | Dense | Prostrate |
| 979 | Determinate | Med.–large | Intermediate | Prostrate |
| 980A | Determinate | Medium | Intermediate | Erect branched |
| 983 | Determinate | Small–med. | Intermediate | Prostrate branched |
| 984 | Determinate | Med.–large | Intermediate | Prostrate branched |
| 985 | Determinate | Med.–large | Sparse | Erect branched |
| 987 | Determinate | Large | Dense | Prostrate branched |
| 988 | Determinate | Medium | Dense | Less erect |
| 989 | Semi-determinate | Large | Dense | Half erect branched |
| 994A | Determinate | Large | Dense | Prostrate |
| 995 | Determinate | Small–med. | Intermediate | Prostrate branched |
| 996 | Determinate | Large | Dense | Erect branched |
2.2. DNA Extraction
Genomic DNA was extracted using Wizard genomic DNA purification kit (Promega, Madison, WI, USA) and was used according to instructions provided by the manufacturer. Then, DNA was stored at −20 °C. Genomic DNA was electrophoresed at 0.8% agarose gel containing ethidium bromide and detected under UV-light. DNA concentration was determined using spectrophotometry.
2.3. SSR Marker Development
Mainly, tomato chromosome three was selected because it showed potential responsive biomarkers for both biotic and abiotic stresses [7,9]. Nonetheless, some SSR markers were found on other chromosomes (1, 1 and 2 on chromosomes 4, 10, and 12, respectively). Bacterial artificial chromosome (BAC) clones along chromosome 3 were retrieved from Sol Genomics Network (SGN) (https://solgenomics.net/) (accessed on 1 June 2022) and corresponding Genbank accession numbers were determined (NCBI, https://www.ncbi.nlm.nih.gov/) (accessed on 1 June 2022). Whole BAC sequences were searched for stress related genes. Concurrently, the same BACs were screened for available SSR sequences using the Simple Sequence Repeat Identification Tool (SSRIT) [19]. Adjacent flanking sequences for SSR loci were selected and then used to develop SSR specific PCR primers (Table 2). Detected SSR markers were viewed with Jbrowse available in SGN (2022), and adjacent stress responsive genes were retrieved and tabulated.
Table 2.
The 20 novel microsatellite loci developed in the study along with their related PCR information.
| BAC Accession | Clone Name | SSR Locus | Chr | Primer Sequence (5’–3’) | Repeat Motif | Allele Size (s) (bp) | Tm (°C) |
|---|---|---|---|---|---|---|---|
| AC235792 | C03HBa0029M12 | ju003 | 3 | F-ATGGTGTGTCAGTCCTTTCATC | 8 GA | 254 | 50.4 |
| R-AAAGGTTAAGGGTCCTGCTAGC | 52.9 | ||||||
| AC235795 | C03HBa0036B17 | ju004 | 3 | F-TCGATGTCATTACTCACGTTCC | 5 CA | 238, 242 | 51.7 |
| R-GATACCAAAACGCAGCAAGTTG | 54.1 | ||||||
| AC235804 | C03HBa0224P23 | ju006 | 10 * | F-CATTTCATGAAAGGGGAATTCTAG | 10 TG | 201, 277 | 53.1 |
| R-ACATTTCGTGTTAGCTGGGTTC | 52.6 | ||||||
| AC238438 | C03HBa0031F10 | ju 007 | 3 | F-GAGTTTGATAAAGCAAAAGGC | 6 AG | 163, 182 | 48.2 |
| R-AACAGAACCCGAGTTTGGAC | 50.5 | ||||||
| AC238439 | C03HBa0031P17 | ju008 | 3 | F-CAATTATTAGACAGCCAACCAAG | 5 AAT | 264 | 50.5 |
| R-GGCATTTATTTGGTCAGAAAGC | 52.5 | ||||||
| AC238450 | C03HBa0114P24 | ju010 | 3 | F-TACCCTTTCGTTTACCCAAATTG | 11 AT | 282 | 54.1 |
| R-AATTGACCGATTTTCCCTTCTC | 53.1 | ||||||
| AC238451 | C03HBa0121O11 | ju011 | 3 | F-GTGAAATGATGTTTCCTCTGACAAG | 5 AAC | 246, 253 | 53.7 |
| R-CTTTCGACATCCTTTTGACTCG | 53.2 | ||||||
| AC238457 | C03HBa0166B15 | ju014 | 3 | F-CGGCAATGTAAGAGTTGAGCTC | 6 GA | 243 | 53.4 |
| R-ATCATCCCAAGCGTCAAAATAG | 52.7 | ||||||
| AC238459 | C03HBa0176B05 | ju015 | 3 | F-ACTCTTCATCCGTTGTACAATTC | 6 TTC | 264, 276 | 49.5 |
| R-TTCACTCGGATGATTGTAATCG | 51.9 | ||||||
| AC238462 | C03HBa0203H10 | ju017 | 3 | F-GATTTTATTGGGTGTCTGTTGTC | 5 TGT | 248 | 49.8 |
| R-AGGGAGAAAAGATGAACAGTATC | 48.1 | ||||||
| AC238468b | C12HBa0270F24 | ju022 | 12 ** | F-ATGGATTTACTGTAACAGTGTGAAC | 6 TTC | 293 | 49 |
| R-GTCCAAATTAATAACAGATCCATAG | 48.4 | ||||||
| AC238468c | C12HBa0270F24 | ju023 | 12 ** | F-AATTATTCGTAAGTTTCCGTCTGTC | 25 AT | 308, 320 | 52.2 |
| R-CCTTTATGAATGACCAAAAGCTAC | 51.3 | ||||||
| AC238560 | C03HBa0030A11 | ju026 | 3 | F-AATCAATATCATCGCTTCACTG | 19 TA | 246, 292 | 48.9 |
| R-ATGTTGTGGTATTATTGACTTATGAG | 48.7 | ||||||
| AC238561 | C03HBa0031M05 | ju027 | 3 | F-ATGCTTAAGGTCTCCAAACACC | 5 CAA | 250 | 51.8 |
| R-CTCTCTACTTTTGGGATTACGC | 49.7 | ||||||
| EU124730 | C03HBa0001E24 | ju029 | 3 | F-TGCTGTACATACTGCATAAATGG | 7 TG | 350 | 50.1 |
| R-AACCTGCTGAATTAACTTGTAGTG | 49.5 | ||||||
| EU124737 | C03HBa0054O21 | ju035 | 3 | F-GTTATATAGAAAGACAAGGTAGAAGGTC | 25 AT | 288, 293 | 49.7 |
| R-GGTAGACTTTTTATGTGTTGTTGC | 49.7 | ||||||
| EU124739 | C03HBa0233O20 | ju037 | 3 | F-AAAATTGTTGGTCAACATGGTG | 7 TAT | 241, 246 | 51.6 |
| R-TTATCTCCTTTCCCTTTCATTC | 49 | ||||||
| EU124741 | C04HBa0318C22 | ju039 | 4 | F-GATGGTGTCATAGATCTAGCCTTAG | 6 TTAA | 355, 421 | 50.4 |
| R-TGGGGAATTATGTAGTGTTGAG | 48.7 | ||||||
| EU124742 | C03HBa0323D22 | ju040 | 3 | F-GCGATCCTGTTTGAGAAGAAGG | 5 CA | 340, 345 | 54.6 |
| R-ATGAACAAATGCTTAAGAGGGG | 52 | ||||||
| EU124743 | C03HBa0007J09 | ju041 | 3 | F-TTCCAAAAACACTTACGAAAGTTAG | 26 AT | 292, 316, 330 | 51.4 |
| R-CATGTAAGTCAAAAGAATGGAGG | 50.2 |
* The original clones was assigned to chromosome number 3 (clone number C03HBa0224P23). ** The original clones was assigned to chromosome number 3 (clone number C03HBa0270F24).
PCR reactions were performed in a 10 μL volume consisting of 20 ng of DNA, 1.0 unit of DNA Taq Polymerase (Promega), 1 × PCR buffer (Promega), 1.5 mM MgCl2, 0.2 mM of each dNTP (Promega), 0.5 μM of tailed forward primer (Integrated DNA technology, Coralville, IA, USA), 0.03 μM tailed labeled with IRD700 (Integrated DNA technology), and 1.5 μM of reverse primer (Integrated DNA technology). The forward primer was “tailed” by the inclusion of 19 extra nucleotides at the 5′ end, which facilitated the labeling of the products. The reactions were carried out in a thermo cycler Perkin-Elmer 9700 (Applied Biosystems, Waltham, MA, USA), with the following profile: 95 °C for 5 min, 20 cycles at 95 °C for 20 s, annealing temperature (65 °C) for 30 s, decreasing 0.5 °C/cycle, extension temperature 72 °C for 30 s; followed by 20 cycles at 95 °C for 30 s, annealing temperature 55 °C for 30 s, and 72 °C for 30 s with a final extension at 72 °C for 10 min. SSR markers were profiled using a LI-COR Bioscience 4300 DNA Analyzer, 1 μL of the product was loaded onto a 6% polyacrylamide gel after mixing with 0.5 μL stop solution (Li-COR), and electrophoresed at 1500 Volts.
2.4. Data Analysis
The Jaccard similarity matrix was used for cluster analysis, using the unweighted pair group method arithmetic average to study the genetic relationships among the cultivars [26]. These coefficients were used to construct dendrogram, using the unweighted pair group method with arithmetic average (UPGMA); the robustness of internodes was assessed by bootstrap analysis with 1000 replicates and principal coordinate analysis (PCoA) was performed using the PAST program [27]. For each primer pair, Microsatellite-Toolkit for Excel [28] was used for estimating mean number of alleles, observed and expected heterozygosities (Ho, He) [29], polymorphism information content (PIC) according to [30], power of discrimination was calculated with the formula PD = 1 − Σgi2, where gi is the frequency of the cultivar at locus I [31].
3. Results
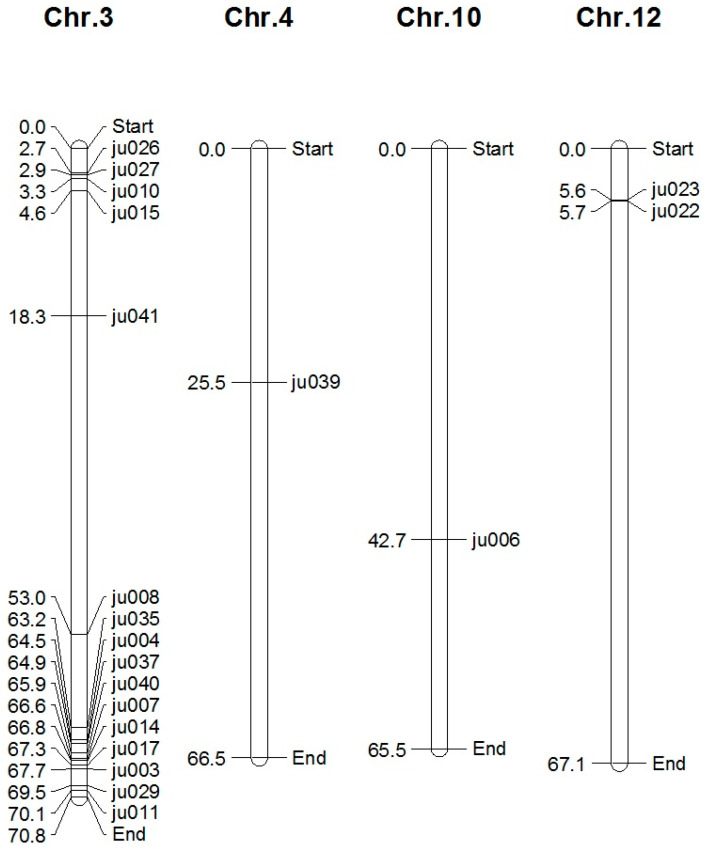
A total of 20 novel microsatellites were developed in silico. The microsatellites are distributed among chromosomes 3, 4, 10, and 12 (Figure 1). In total, 16 loci were located on chromosome 3, while 1, 1, and 2 loci were located in chromosomes 4, 10, and 12, respectively. All original BAC clones were assigned to chromosome 3 in tomato (when this study was carried out). However, new data curation remapped several clones to other chromosomes. For example, 2 loci (ju022 and ju023) were found to be located on chromosome 12 based on the clone C12HBa0270F24, which was originally mapped on chromosome 3 based on the clone number C03HBa0270F24. In addition, the ju006 marker found in the original clones (number C03HBa0224P23) was assigned to chromosome number 3 but found recently to be located on chromosome 10. Nonetheless, 4 loci are clustered at the telomeric end of the short arm of chromosome 3, while 10 loci are clustered at the telomeric end of the long arm of the same chromosome, similarly, 2 other loci were found in the telomeric region of the short arm of chromosome 12.
Figure 1.
Distribution of novel microsatellite loci in different tomato chromosomes. Loci (right to chromosome) and their corresponding distance in Mb (left to chromosome).
However, ju041 was located inside chromosome 3 as well as ju008. Likewise, ju039 and ju006 are also located toward the middle of chromosomes 4 and 10, respectively.
The chromosomal coordinates for the 20 novel developed microsatellite were determined using the Sol Genomics network (Table S1). Ninety six nearby genes for the novel developed microsatellite were identified. The identified nearby genes representing different tomato genes, for example, loci ju006, ju014, ju015, ju017, and ju035 are located near Serine/Threonine protein kinase-related gene and loci ju010 and ju035 are located near MYB transcription factor gene, whereas ju014 is located near AP2-like ethylene-responsive transcription factor (Table S1).
Genetic diversity was examined in Jordanian tomato landraces to validate novel SSR markers. In total, 33 polymorphic alleles with a 100 polymorphism percentage was achieved. The mean number of alleles per locus was 1.65. The highest value for observed heterozygosity was 0.81 recorded for ju007, while the lowest was 0.0 with an average of 0.19. Expected heterozygosity per locus ranged from 0.00 to 0.54, with an average of 0.24. The average value of PIC for the primer sets was 0.18, ranging from (0.0) to (0.38). PD varied from 0.32 for ju003 to 0.96 for ju023 and ju026, with an average of 0.68 (Table 3).
Table 3.
SSR locus name, number of alleles, (Ho), (He) observed and expected heterozygosities, polymorphic information content (PIC), and discrimination power (PD) values for 22 developed polymorphic microsatellite loci in a sample of 29 tomato landraces.
| Locus | No. of Alleles | Ho | He | PIC | PD |
|---|---|---|---|---|---|
| ju003 | 1 | 0 | 0 | 0 | 0.32 |
| ju004 | 2 | 0.24 | 0.41 | 0.32 | 0.72 |
| ju006 | 2 | 0.59 | 0.49 | 0.36 | 0.64 |
| ju007 | 2 | 0.81 | 0.5 | 0.37 | 0.34 |
| ju008 | 1 | 0 | 0 | 0 | 0.61 |
| ju010 | 1 | 0 | 0 | 0 | 0.73 |
| ju011 | 2 | 0.31 | 0.27 | 0.23 | 0.68 |
| ju014 | 1 | 0 | 0 | 0 | 0.52 |
| ju015 | 2 | 0.32 | 0.27 | 0.23 | 0.75 |
| ju017 | 1 | 0 | 0 | 0 | 0.37 |
| ju022 | 1 | 0 | 0 | 0 | 0.48 |
| ju023 | 2 | 0 | 0.42 | 0.32 | 0.96 |
| ju026 | 2 | 0.71 | 0.54 | 0.38 | 0.96 |
| ju027 | 1 | 0 | 0 | 0 | 0.73 |
| ju029 | 1 | 0 | 0 | 0 | 0.52 |
| ju035 | 2 | 0 | 0.42 | 0.32 | 0.94 |
| ju037 | 2 | 0 | 0.5 | 0.37 | 0.92 |
| ju039 | 2 | 0.48 | 0.37 | 0.3 | 0.72 |
| ju040 | 2 | 0.09 | 0.09 | 0.08 | 0.83 |
| ju041 | 3 | 0.26 | 0.51 | 0.38 | 0.91 |
| Total | 33 | - | - | - | |
| Mean | 1.65 | 0.19 | 0.24 | 0.18 | 0.68 |
| Max | 3 | 0.81 | 0.54 | 0.38 | 0.96 |
| Min | 1 | 0 | 0 | 0 | 0.32 |
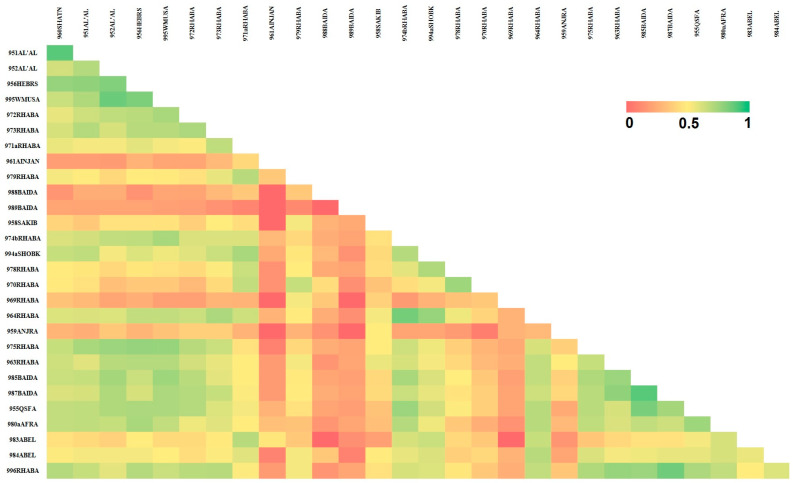
Pair-wise similarity values ranged from 0.00 to 0.89 and the overall accessions similarity showed an average of 0.47. The maximum similarity index (0.89) was recorded between accessions of 951AL’AL and 960SHATANA, while low values of genetic similarity between 961AINJNA and 988BAIDA, 989BAIDA, and 958SAKIB were reported (Figure 2).
Figure 2.
Jaccard’s similarity index (0–1) represented as a heatmap generated from developed SSR marker data for a collection of 29 tomato landraces (depicted as accession number and collection site).
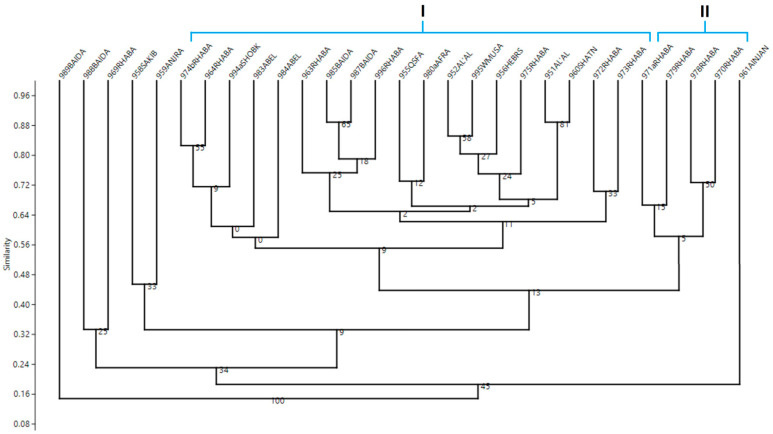
The UPGMA cluster analysis of the accessions based on SSR data exhibited moderate clustering relationships, except for 951AL’AL and 960SHATANA (bootstrapping value 81%), two accessions from Ain AL-Baida (985BAIDA and 987BAIDA) with bootstrapping value of 65%, 952AL’AL and 995WMUSA with bootstrapping value of 58%, and the two accessions 964RHABA and 974bRHABA with 55% of bootstrapping value. At 50% of similarity value, two major groups were formed (Figure 3). Subclusters were revealed for major branches separating accessions from the same geographical distribution. Cluster 1 contained four accessions of Rhaba region and cluster 2 was further subdivided to subgroups compassed 19 accessions. In one subgroup two accessions from Rhaba (972RHABA and 973RHABA) grouped corresponding with their geographical area. Except 995WMUSA, which is cultivated in the southern part of Jordan, five accessions were grouped in a second subgroup, including two accessions from Al’al (951AL’AL and 952AL’AL), 975RHABA, 960SHATNA, and 956HEBRS, all were cultivated in the northern part of Jordan. Two accessions from the northern part of Jordan, which were 963RHABA and 996RHABA, and two from the south, 985BAIDA and 987BAIDA, were grouped in a third subgroup. A fourth subgroup contained three accessions from the southern part (983ABELand 984ABEL and 994aSHOBK) and two from the northern part (964RHABA and 974bRHABA). 955QSFA and 980aAFRA representing two diverse region formed a fifth subgroup. The remaining six accessions failed to form clusters and were individually separated.
Figure 3.
Dendrograms generated using unweighted pair group method with arithmetic average (UPGMA) analysis, showing relationships between 29 tomato landraces (Accession number and collection site), using SSR data based on Jaccard genetic similarity index. Bootstrap support value is given above each branch.
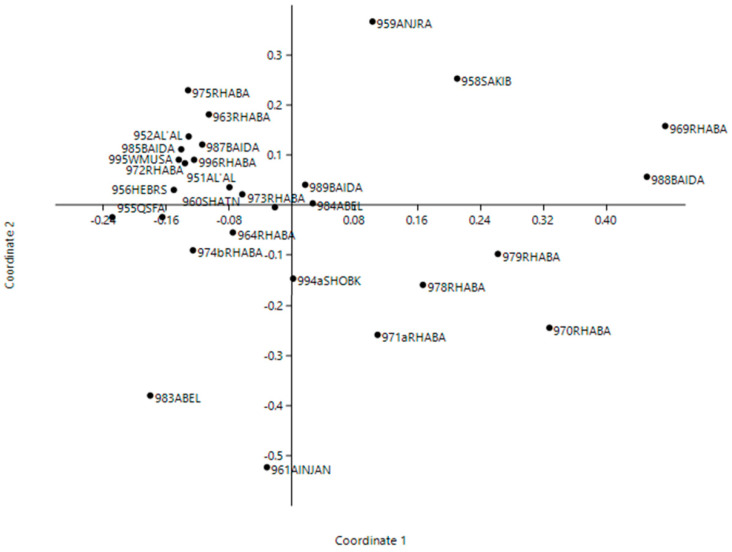
Principal coordinate analysis (PCoA) was performed to validate genetic relationships among 29 accessions (Figure 4). The first two axes explained 37.9% of the variation, where the first coordinate accounted for 21.1% variation, while the second axis explained a 16.8% variation. Following the same trend of the dendrogram, moderate relationships and no specific geographic relationships were obtained. However, PCoA showed that all samples were distributed to the four parts of the coordinates with no specific aggregations.
Figure 4.
Principal coordinate analysis (PCoA) of 29 Jordanian tomato landraces (Accession number and collection site) based on 20 microsatellite loci. The first and second coordinate accounting for 21.1% and 16.8% variation, respectively.
Of all developed SSR markers, di-nucleotide were the most abundant with 12 loci, of these 4 loci are of AT repeat motif, followed by tri-nucleotide with 7 loci and tetra-nucleotide with 1 locus (Table 2).
4. Discussion
A total of 14 out of 20 novel developed microsatellites are clustered in telomeric and subtelomeric regions of chromosome 3. Furthermore, two other SSR loci were found located at sub-telomeric region of chromosome 12. The result is inconsistent with results, emphasizing that plant genes show clustering in telomeric and subtelomeric regions. Ref. [32] reported that the avenacin cluster (12 genes) lies in a subtelomeric region at the end of the long arm of chromosome 1.
Important genes for plant growth and development, disease resistance, and abiotic stress tolerance along with many other crucial processes in plants were found. For example, MYB transcription factor, which is located near loci ju010 and ju035, plays a key role in plant development, secondary metabolism, hormone signal transduction, disease resistance, and abiotic stress tolerance, [33,34,35], while calmodulin-binding heat shock protein, ap2-like ethylene-responsive transcription factor, and WRKY transcription factor, which are located near locus ju027, locus ju014, and the locus ju023, respectively, are involved in plant responses to abiotic stresses [36,37,38]. On the other hand, leucine-rich repeat family protein, which is located near locus ju007, provide recognition of pathogen products of avirulence (AVR) genes [39]. Other loci are located near other important genes (Table 4).
Table 4.
Potential SSR markers and nearby genes.
| Locus | Nearby Gene | Functions | Reference |
|---|---|---|---|
| ju014, ju015, ju035 | Serine/threonine-protein kinase | Central processor unit (cpu): accepting input information from receptors that sense environmental conditions, phytohormones, and other external factors, and converting it into appropriate outputs, such as changes in metabolism, gene expression, and cell growth and division | [40] |
| ju017 | Serine carboxypeptidase | Stress response, growth, development, and pathogen defense | [41] |
| ju023 | Pirin | Role in seed germination and transcription of a light- and ABA-regulated gene under specific conditions in Arabidopsis thaliana | [42] |
| ju010. ju029 | F-box family protein | Plant hormonal signal transduction, floral development, secondary metabolism, senescence, circadian rhythms, and responses to both biotic and abiotic stresses | [43] |
| ju014, ju040 | Polygalacturonase | Major role in cell wall degradation and fruit softening | [44] |
The high percentage of polymorphism (100%) obtained in the study was similar with other studies conducted in tomato landraces. Ref. [45] obtained 100% of polymorphism for 4 SSR primers out of 5 for 39 Jordanian tomato landraces. Ref. [46] reported 100% polymorphism using 9 tomato landraces form Jordan along with 1 commercial cultivar using SSR markers and [25] obtained 60% of polymorphism for the same landraces used in this study using ISSR markers. A high percentage of polymorphism for tomato landraces using different molecular markers from different countries was obtained from Saudi Arabia [47,48], Turkey, and Iran [49].
The observed distribution of repeat motif in SSR markers developed: 60% for di-nucleotide, 35% for tri-nucleotide, and 5% for tetra-nucleotide was in accordance with other studies about the nature of repeat motif in SSR markers in plant genomes. Ref. [50] reported that SSRs existed primarily as dinucleotide repeats and trinucleotide repeats, accounting for 97.59% of all SSRs. Dinucleotide repeats (74.56%) were the most abundant repeat unit, followed by tr- (23.08%) and tetra-(2.04%) in the Camellia japonica genome. Furthermore, out of the 15,498 SSR markers analyzed in the Platostoma palustre genome [51], 71.96%, 26.26%, and 1.52% was SSR with di-nucleotide, tri-nucleotide, and tetra-nucleotide repeat motif, respectively.
A strong correlation between genetic similarity values and geographical distribution were recorded between Jordanian tomato landraces included in the study. Our results were in agreement with other results for genetic diversity studies using tomato landraces from different parts of the world. For example, Ref. [20] proved that 14 florescent SSR markers were able to separate 15 local tomato landraces from Campania region (Southern Italy) according to their geographical distribution, and the UPGMA dendrogram supported by principal coordinate analysis (PCoA) revealed clusters of Saudi tomato landraces according to their geographical origin using SDS-PAGE and sequence-related amplified polymorphism (SRAP) markers [47].
Although, the dendrogram and the principle coordinate analysis shows a moderate relationship between landraces grouping and geographical region, some evidences of correlations between landraces and geographical region was observed. In the two main subclusters, which comprised 23 landraces out of 29 formed at 50% of genetic similarity, many landraces from the same geographical region are clustered together similar to the landraces in subcluster 1, which are from RHABA region and 5 landraces in the second subgroup of subcluster II. The obtained results could be supported by other results conducted by [20,25,47], using different tomato landraces and different molecular markers. In cases where landraces grouped according to geographical region, it could be explained by a reduced admixture of the gene pool between local farmers.
5. Conclusions
In this study, we found that most of the developed SSR markers are located in the telomeric region of both short and long arms of chromosome 3 and 12. Many important genes are identified near the developed SSR markers, a major group of these nearby genes are important responsive factors for abiotic tolerance and biotic resistance, whereas other genes are important for plant growth and development. The newly developed SSR markers were validated using a collection of Jordanian tomato landraces comprised of 29 landraces from different geographical areas of the country. A high percentage of polymorphism were found for all alleles. Some landraces showed most of the developed SSR markers, while others showed some of these SSR markers. In this regard, potential landraces were further selected for salinity stress analysis, using DGE of salinity responsive genes, to be used in our breeding program.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biotech11030034/s1, Table S1: Nearby genes for developed 20 SSR markers along with their description.
Author Contributions
Conceptualization, M.B., H.M., N.H. and M.T.S.; methodology, M.B., L.A.-Q., H.H. and A.A.; software, M.B. and M.T.S.; writing—original draft preparation, M.B., L.A.-Q., H.H., H.M., N.H. and M.T.S.; writing—review and editing, M.B., L.A.-Q., H.H., H.M., N.H., A.A. and M.T.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by scientific and innovative research fund/Ministry of Higher Education and Scientific Research.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAOSTAT 2020. [(accessed on 1 June 2022)]. Available online: http://faostat.fao.org.
- 2.Arumuganathan K., Earle E.D. Nuclear DNA content of some important plant species. Plant Mol. Biol. Rep. 1991;9:208–2018. doi: 10.1007/BF02672069. [DOI] [Google Scholar]
- 3.Rick C.M. Biosystematic studies in Lycopersicon and closely related species of Solanum. In: Hawkes J.G., Lester R.N., Skelding A.D., editors. The Biology and Taxonomy of the Solanaceae. Academic Press; New York, NY, USA: 1979. pp. 667–677. [Google Scholar]
- 4.Alsadon A.A., Sadder M.T., Wahb-Allah M.A. Responsive gene screening and exploration of genotypes responses to salinity tolerance in tomato. Aust. J. Crop Sci. 2013;7:1383–1395. [Google Scholar]
- 5.Sadder M.T., Alsadon A.A., Wahb-Allah M.A. Transcriptomic analysis of tomato lines reveals putative stress-specific biomarkers. Turk. J. Agric. For. 2014;38:700–715. doi: 10.3906/tar-1312-17. [DOI] [Google Scholar]
- 6.Alsadon A.A., Ibrahim A.A., Wahb-Allah M.A., Ali A.A.M., Sadder M.T. Tomato under salinity stress: Correlation between growth and yield components and responsive genes. Acta Hortic. 2015;1081:111–119. doi: 10.17660/ActaHortic.2015.1081.11. [DOI] [Google Scholar]
- 7.Kissoudis C., Chowdhury R., van Heusden S., van de Wiel C., Finkers R., Visser R.G.F., Bai Y., van der Linden G. Combined biotic and abiotic stress resistance in tomato. Euphytica. 2015;202:317–332. doi: 10.1007/s10681-015-1363-x. [DOI] [Google Scholar]
- 8.Kissoudis C., Sunarti S., van de Wiel C., Visser R.G.F., van der Linden G., Bai Y. Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J. Exp. Bot. 2016;67:5119–5132. doi: 10.1093/jxb/erw285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul A., Rao S., Mathur S. The α-crystallin domain containing genes: Identification, phylogeny and expression profiling in abiotic stress, phytohormone response and development in tomato (Solanum lycopersicum) Front. Plant Sci. 2016;7:426. doi: 10.3389/fpls.2016.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichardt S., Piepho H.P., Stintzi A., Schaller A. Peptide signaling for drought-induced tomato flower drop. Science. 2020;367:1482–1485. doi: 10.1126/science.aaz5641. [DOI] [PubMed] [Google Scholar]
- 11.Korir N.K., Diao W., Tao R., Li X., Kayesh E., Li A., Zhen W., Wang S. Genetic diversity and relationships among different tomato varieties revealed by EST-SSR markers. Genet. Mol. Res. 2014;13:43–53. doi: 10.4238/2014.January.8.3. [DOI] [PubMed] [Google Scholar]
- 12.Rick C.M. Genetic relationship between self-incompatibility and floral traits in tomato species. Biol. Zent. Bl. 1982;101:185–198. [Google Scholar]
- 13.Stevens M.A., Rick C.M. Genetics and breeding. In: Atherton J.G., Rudich J., editors. The Tomato Crop. Chapman and Hall; New York, NY, USA: 1986. pp. 35–109. [Google Scholar]
- 14.Tanksley S.D., McCouch S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science. 1997;277:1063–1066. doi: 10.1126/science.277.5329.1063. [DOI] [PubMed] [Google Scholar]
- 15.Hay A., Craft J., Tsiantis M. Plant hormones and homeoboxes: Bridging the gap? BioEssays. 2004;26:395–404. doi: 10.1002/bies.20016. [DOI] [PubMed] [Google Scholar]
- 16.Tam S.M., Mhiri C., Vogelaar A., Kerkveld M., Pearce S.R., Grandbastien M.A. Comparative analyses of genetic diversities within tomato and pepper collections detected by retrotransposon-based SSAP, AFLP and SSR. Theor. Appl. Genet. 2005;110:819–831. doi: 10.1007/s00122-004-1837-z. [DOI] [PubMed] [Google Scholar]
- 17.Qaryouti M.M., Hamdan H.H., Edwan M.A., Hurani O.M., Al-Dabas M.A. Evaluation and Characterization of Jordanian Tomato Landraces. Dirasat Agric. Sci. 2007;34:44–56. [Google Scholar]
- 18.Song Q.J., Marek L.F., Shoemaker R.C., Lark K.G., Concibido V.C., Delannay X., Specht J.E., Cregan P.B. A new integrated genetic linkage map of the soybean. Theor. Appl. Genet. 2004;109:122–128. doi: 10.1007/s00122-004-1602-3. [DOI] [PubMed] [Google Scholar]
- 19.Temnykh S., DeClerck G., Lukashova A., Lipovich L., Cartinhour S., McCouch S. Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): Frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 2001;11:1441–1452. doi: 10.1101/gr.184001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caramante M., Rouphael Y., Corrado G. The Genetic Diversity and Structure of Tomato Landraces from the Campania Region (Southern Italy) Uncovers a Distinct Population Identity. Agronomy. 2021;11:564. doi: 10.3390/agronomy11030564. [DOI] [Google Scholar]
- 21.Al-Shammari A.M., Hamdi G.J. Genetic diversity analysis and DNA fingerprinting of tomato breeding lines using SSR markers. J. Agric. Sci. 2021;32:1–7. doi: 10.15159/jas.21.13. [DOI] [Google Scholar]
- 22.Pidigam S., Thuraga V., Munnam S.B., Amarapalli G., Kuraba G., Pandravada S.R., Nimmarajula S., Sudini H.K. Genetic diversity, population structure and validation of SSR markers linked to Sw-5 and I-2 genes in tomato germplasm. Physiol. Mol. Biol. Plants. 2021;27:695–1710. doi: 10.1007/s12298-021-01037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou R., Wu Z., Cao X., Jiang F.L. Genetic diversity of cultivated and wild tomatoes revealed by morphological traits and SSR markers. Genet. Mol. Res. 2015;14:13868–13879. doi: 10.4238/2015.October.29.7. [DOI] [PubMed] [Google Scholar]
- 24.Younis A., Ramzan F., Ramzan Y., Zulfiqar F., Ahsan M., Lim K.B. Molecular markers improve abiotic stress tolerance in crops: A review. Plants. 2020;9:1374. doi: 10.3390/plants9101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brake M.H., Al-Gharaibeh M.A., Hamasha H.R., Al-Sakarneh N.S., Alshomali I.A., Migdadi H.M., Qaryouti M.M., Haddad N.J. Assessment of genetic variability among Jordanian tomato landrace using inter-simple sequence repeats markers. Jordan J. Biol. Sci. 2021;14:91–95. [Google Scholar]
- 26.Jaccard P. Nouvelles recherches sur la distribution florale. Bull. Société Vaud. Sci. Nat. 1908;44:223–270. [Google Scholar]
- 27.Hammer O., Harper D.A.T., Ryan P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:1–9. [Google Scholar]
- 28.Park S.D.E. Ph.D. Thesis. Animal Genomics Laboratory, University College Dublin; Dublin, Ireland: 2001. The Excel Microsatellite Toolkit (Version 3.1) [Google Scholar]
- 29.Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1987;89:583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Botstein D., White R.L., Skolnick M., Davis R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- 31.Kloosterman A.D., Budowle B., Daselaar P. PCR amplification and detection of the human DIS80 VNTR locus. Amplification conditions, population genetics and application in forensic analysis. Int. J. Legal Med. 1993;105:257–264. doi: 10.1007/BF01370382. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Leveau A., Zhao Q., Feng Q., Lu H., Miao J., Osbourn A. Subtelomeric assembly of a multi-gene pathway for antimicrobial defense compounds in cereals. Nat. Commun. 2021;12:1–13. doi: 10.1038/s41467-021-22920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Y., Li K., Li Y., Zhao X., Wang L. MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology. 2020;9:61. doi: 10.3390/biology9030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T., Zhao Y., Wang Y., Liu Z., Gao C. Comprehensive Analysis of MYB Gene Family and Their Expressions Under Abiotic Stresses and Hormone Treatments. Tamarix Hispida. Front. Plant Sci. 2018;9:1303. doi: 10.3389/fpls.2018.01303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He C., Teixeira da Silva J.A., Wang H., Si C., Zhang M., Zhang X., Li M., Tan J., Duan J. Mining MYB transcription factors from the genomes of orchids (Phalaenopsis and Dendrobium) and characterization of an orchid R2R3-MYB gene involved in water-soluble polysaccharide biosynthesis. Sci. Rep. 2019;9:13818. doi: 10.1038/s41598-019-49812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng H., Xu L., Singh A., Wang H., Du L., Poovaiah B.W. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 2015;6:600. doi: 10.3389/fpls.2015.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Z., Nolan T.M., Jiang H., Yin Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019;10:228. doi: 10.3389/fpls.2019.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Li C., Wang H., Guo Z. WRKY transcription factors: Evolution, binding, and action. Phytopathol. Res. 2019;1:13. doi: 10.1186/s42483-019-0022-x. [DOI] [Google Scholar]
- 39.Ng A., Xavier R.J. Leucine-rich repeat (LRR) proteins: Integrators of pattern recognition and signaling in immunity. Autophagy. 2011;7:1082–1084. doi: 10.4161/auto.7.9.16464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardie D.G. Plant Protein Serine/Threonine Kinases: Classification and Functions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:97–131. doi: 10.1146/annurev.arplant.50.1.97. [DOI] [PubMed] [Google Scholar]
- 41.Xu X., Zhang L., Zhao W., Fu L., Han Y., Wang K., Yan L., Li Y., Zhang X.H., Min D.H. Genome-wide analysis of the serine carboxypeptidase-like protein family in Triticum aestivum reveals TaSCPL184-6D is involved in abiotic stress response. BMC Genom. 2021;22:350. doi: 10.1186/s12864-021-07647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orozco-Nunnelly D.A., Muhammad D., Mezzich R., Lee B.S., Jayathilaka L., Kaufman L.S., Warpeha K.M. Pirin1 (PRN1) Is a Multifunctional Protein that Regulates Quercetin, and Impacts Specific Light and UV Responses in the Seed-to-Seedling Transition of Arabidopsis thaliana. PLoS ONE. 2014;9:e93371. doi: 10.1371/journal.pone.0093371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X., Gonzalez-Carranza Z.H., Zhang S., Miao Y., Liu C.J., Roberts J.A. F-Box proteins in plants. Annu. Plant Rev. 2019;2:307–328. doi: 10.1002/9781119312994.apr0701. [DOI] [Google Scholar]
- 44.Sheehy R.E., Kramer M., Hiatt W.R. Reduction of polygalacturonase activity in tomato fruit by antisense RNA. Proc. Natl. Acad. Sci. USA. 1988;85:8805–8809. doi: 10.1073/pnas.85.23.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makhadmeh I.M., Thabet S.G., Ali M., Alabbadi B., Albalasmeh A., Alqudah A.M. Exploring genetic variation among Jordanian Solanum Lycopersicon L. landraces and their performance under salt stress using SSR markers. J. Genet. Eng. Biotechnol. 2022;20:45. doi: 10.1186/s43141-022-00327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Qadumii L.W., Sadder M.T., Migdadi H. Assessment of in silico BAC-based simple sequence repeat (SSR) marker development for tomato (Solanum lycopersicum L.) Afr. J. Biotechnol. 2012;11:13938–13946. doi: 10.5897/AJB11.3472. [DOI] [Google Scholar]
- 47.Al Shaye N., Migdadi H., Charbaji A., Alsayegh S., Daoud S., AL-Anazi W., Alghamdi S. Genetic variation among Saudi tomato (Solanum lycopersicum L.) landraces studied using SDS-PAGE and SRAP markers. Saudi J. Biol. Sci. 2018;25:1007–1015. doi: 10.1016/j.sjbs.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alzahib R.H., Migdadi H.M., Ghamdi A.A.A., Alwahibi M.S., Afzal M., Elharty E.H., Alghamdi S.S. Exploring Genetic Variability among and within Hail Tomato Landraces Based on Sequence-Related Amplified Polymorphism Markers. Diversity. 2021;13:135. doi: 10.3390/d13030135. [DOI] [Google Scholar]
- 49.Henareh M., Dursun A., Abdollahi-Mandoulakani B., Haliloğlu K. Assessment of genetic diversity in tomato landraces using ISSR markers. Genetika. 2016;48:25–35. doi: 10.2298/GENSR1601025H. [DOI] [Google Scholar]
- 50.Li Q., Su X., Ma H., Du K., Yang M., Chen B., Fu S., Fu T., Xiang C., Zhao Q., et al. Development of genic SSR marker resources from RNA-seq data in Camellia japonica and their application in the genus Camellia. Sci. Rep. 2021;11:9919. doi: 10.1038/s41598-021-89350-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Z., Zhang N., Huang Z., Zeng Q., Huang Y., Qi Y. Genome survey sequencing and characterization of simple sequence repeat (SSR) markers in Platostoma palustre (Blume) A.J.Paton (Chinese mesona) Sci. Rep. 2022;12:355. doi: 10.1038/s41598-021-04264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.