Abstract
Plantaricin EF and JK are both two-peptide bacteriocins produced by Lactobacillus plantarum C11. The mechanism of plantaricin EF and JK action was studied on L. plantarum 965 cells. Both plantaricins form pores in the membranes of target cells and dissipate the transmembrane electrical potential (Δψ) and pH gradient (ΔpH). The plantaricin EF pores efficiently conduct small monovalent cations, but conductivity for anions is low or absent. Plantaricin JK pores show high conductivity for specific anions but low conductivity for cations. These data indicate that L. plantarum C11 produces bacteriocins with complementary ion selectivity, thereby ensuring efficient killing of target bacteria.
Many lactic acid bacteria produce bacteriocins. Bacteriocins are peptides or proteins that kill bacteria that are related to the producer strain. Bacteriocins are useful to their producer by killing bacteria that compete for the same niche. Plantaricin EF (PlnEF) and plantaricin JK (PlnJK) are both two-peptide bacteriocins that belong to the large group of small, heat-stable nonlantibiotics termed class II bacteriocins (10).
Lactobacillus plantarum C11 secretes plantaricin A (PlnA), a small peptide that consists of 26 amino acids and that exhibits both bactericidal (7) and pheromone activity (4, 7). An all-d-amino-acid PlnA was found to be as bactericidal as an all-l-amino-acid PlnA. In contrast, the pheromone activity was found to be stereospecific and only observed with PlnA composed of l-amino acids (7). Therefore, the antimicrobial activity of PlnA appears not to involve chiral interactions and seems to depend only on the strong amphiphilic α-helical structure of this peptide. PlnA induces the transcription of five operons, i.e., plnABCD, plnEFI, plnJKLR, plnMNOP, and plnGHSTUV (5). The first operon contains the structural gene for PlnA itself. plnB, plnC, and plnD encode proteins that are involved in signal transduction, i.e., a two-component regulatory system that consists of the membrane-associated histidine protein kinase, PlnB, and two response regulators, PlnC and PlnD. PlnA is thought to interact with PlnB to trigger expression of the other structural genes (3, 6). The plnEFI and plnJKLR operons encode two two-peptide bacteriocins, PlnEF and PlnJK. PlnI, PlnL, PlnM, and PlnP are thought to confer immunity to the bacteriocin-producing cells (5). Based on sequence similarity, plnGH is thought to encode subunits of an ABC transporter that secretes and processes the bacteriocin precursors. The functions of PlnO (a 399-amino-acid [aa] hydrophilic peptide), PlnN (a bacteriocin-like peptide), PlnR (a 50-aa hydrophobic, cationic peptide), and PlnSTUV (hydrophobic peptides of 99, 140, 222, and 44 aa) are not known.
PlnE, PlnF, PlnJ, and PlnK are cationic peptides that consist of 33, 34, 25, and 32 amino acids and have molecular weights of 3,703, 3,545, 2,929, and 3,503, respectively (5). These peptides have the propensity to form an amphiphilic α-helical structure in a membrane-mimicking environment (8). The antimicrobial activity of PlnF is enhanced more than 1,000-fold by the equimolar presence of PlnE and vice versa. Likewise, PlnJ and PlnK are efficient antimicrobials when present together. Strikingly, none of the other combinations of these four peptides enhanced the antimicrobial activity (2, 8). The amphiphilic structure of these peptides is believed to play a role in pore formation (8, 9). The complementary peptides (PlnEF or PlnJK) interact, because the simultaneous addition of both of the peptides synergistically promoted the formation of their α-helical structure in the presence of dioleoylphosphatidyl-glycerol liposomes (8). In the present study, we show that PlnEF and PlnJK form pores in the target membranes that differ in ion selectivity. These results might explain why some bacteria are more sensitive to PlnEF, while others are more sensitive to PlnJK (2). The combined and complementary action of these bacteriocins warrants efficient killing of target cells.
MATERIALS AND METHODS
Materials.
86Rb+ (10 mCi/mg), [14C]choline+ (55 mCi/mmol), [14C]glutamic acid (251 mCi/mmol), and 33Pi (3,000 Ci/mmol) were obtained from Amersham Life Science, Little Chalfont, Buckinghamshire, United Kingdom). The separated components of PlnEF and PlnJK were synthesized and purified as described previously (8). Peptides were suspended in a solution containing 40% (vol/vol) 2-propanol, 0.1% (vol/vol) trifluoracetic acid (TFA), and 59.9% (vol/vol) H2O. This mixture without peptide was used in control experiments and is indicated as “solvent.” The complementary components were added together in a ratio of 1:1 to give either PlnEF or PlnJK. Gramicidin A′ (a mixture of around 80% gramicidin A, 15% gramicidin B and 5% gramicidin C) was obtained from Sigma.
Bacterial strains and culture conditions.
Lactobacillus plantarum 965 was grown at 30°C in MRS (3a) supplemented with 0.1% (vol/vol) Tween 80, but without sodium acetate and triammonium citrate. Lactose was replaced by 1% (wt/vol) glucose. In the case of phosphate efflux experiments, cells were grown in MRS broth either without (33Pi experiments) or with 27 g of K2HPO4 per ml (unlabelled Pi experiments). Cells grown up to the exponential growth phase were harvested by centrifugation (Eppendorf centrifuge, 2 min at 6,000 rpm), washed twice at 4°C, and used directly.
Uptake and efflux measurements.
L. plantarum 965 cells were suspended in the buffers indicated in the figure legends, and uptake of radiolabelled compounds was monitored after energization with 0.5% (wt/vol) glucose. At indicated times, either the solvent, ionophores, or plantaricins (0.1 to 0.3 volume%) were added. In the case of choline efflux experiments, cells (500 μg of protein) were suspended in 30 μl of M17 broth (Difco) without glucose and loaded with 3.6 μCi of [14C]choline by overnight incubation at 4°C. Subsequently, cells were diluted in 4.2 ml of 50 mM sodium phosphate (pH 7.0) in the presence or absence of solvent, valinomycin, and nigericin or plantaricins. Samples were applied to 45-μm-pore-size cellulose nitrate filters and washed twice with ice-cold 50 mM sodium phosphate (pH 7.0) (choline efflux) or 100 mM LiCl. The radioactivity that was retained on the filter was measured by liquid scintillation counting in a Packard Tri-Carb 460 CD counter (Packard Instruments Corp.).
To measure efflux of unlabelled phosphate, cells (1.3 mg of protein/ml) suspended in 50 mM NaOH Mes [2-(N-morpholino)ethanesulfonic acid, pH 7.0] were incubated with solvent; nisin (1.4 μM); valinomycin and nigericin (250 nM each); the individual PlnE (1.4 μM), PlnF (1.4 μM), PlnJ (2.8 μM), and PlnK (2.8 μM) peptides; PlnEF (0.7 μM each peptide); or PlnJK (1.4 μM each peptide). To avoid cell lysis and/or continued efflux during centrifugation, samples were transferred to the top of a 20% (wt/vol) sucrose layer and directly centrifuged (2 min at 8,000 rpm in an Eppendorf centrifuge). The upper layer, containing the released Pi, was dried, subjected to destruction (30 min, 180°C in 70% perchloric acid), and analyzed for inorganic phosphorus (20). All experiments were performed at 30°C unless stated otherwise.
Proton motive force measurements.
The transmembrane electrical potential, Δψ, was monitored by means of DiSC3(5) fluorescence (excitation wavelength, 643 nm; emission wavelength, 666 nm; slit width excitation and emission, 10 nm) (21). The intracellular pH of cells was measured by using 2′,7′-bis-(2-carboxy-ethyl)-5(and-6)-carboxyfluorescein (BCECF) as a pH-sensitive dye (excitation, 502 nm; slit width, 5 nm; emission, 525 nm; slit width, 15 nm) (13). The data were corrected for the PlnJK-induced BCECF efflux by subtraction of the PlnJK-induced fluorescence increases (15.4% ± 0.9% of the fluorescence increase after energization and valinomycin addition).
Miscellaneous.
Protein measurements were performed according to the method of Lowry et al. (11).
RESULTS
PlnEF and PlnJK dissipate Δψ.
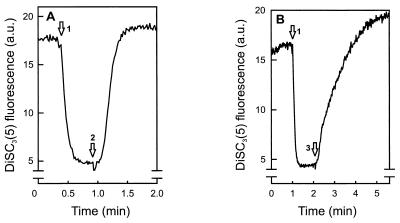
The capacity of PlnEF and PlnJK to dissipate the Δψ in the sensitive strain L. plantarum 965 was determined. Cells were suspended in 50 mM sodium phosphate, and Δψ was generated by the addition of the potassium ionophore valinomycin. PlnEF (Fig. 1A) and PlnJK (Fig. 1B) both efficiently dissipated the Δψ. PlnEF appeared more efficient than PlnJK. The individual components had little (PlnJ, PlnK, and PlnF) or hardly detectable (PlnE) activity (data not shown).
FIG. 1.
PlnEF (A) and PlnJK (B) dissipate the Δψ in L. plantarum 965. Cells (22 μg of protein/ml) were suspended in 50 mM sodium phosphate (pH 7.0). The arrows indicate the addition of valinomycin (250 nM) (arrow 1), PlnEF (60 nM each peptide) (arrow 2), and PlnJK (100 nM each peptide) (arrow 3). a.u., arbitrary units.
In order to study the effect of pH on the PlnEF- and PlnJK-induced dissipation of the Δψ, the time needed to recover 50% of the Δψ-induced DiSC3(5) fluorescence change was measured. Both plantaricins showed a marked pH optimum around pH 6 to 6.5 (data not shown). These data demonstrate that both PlnJK and PlnEF are capable of dissipating the Δψ of target cells.
PlnJK and PlnEF dissipate ΔpH.
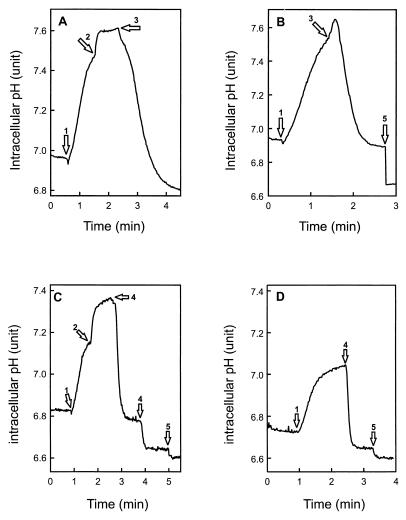
Intracellular BCECF can be used to monitor the intracellular pH and transmembrane pH gradient (ΔpH), provided that a correction is made for the fluorescence changes due to extrusion or loss of BCECF from the cell (13, 14). Since the addition of PlnJK caused a considerable release of the cellular BCECF (see below), this correction was essential for accurate intracellular pH determinations. PlnEF or PlnJK was added to glucose-energized, BCECF-loaded cells that were incubated with (Fig. 2A and C) or without (Fig. 2B and D) valinomycin to dissipate the Δψ. In the presence of valinomycin, PlnEF (Fig. 2A) and PlnJK (Fig. 2C) caused an immediate dissipation of the ΔpH. In the absence of valinomycin (i.e., in the presence of Δψ), PlnJK induced a direct dissipation of the ΔpH (Fig. 2D), while PlnEF elicited a transient increase in ΔpH followed by its dissipation (Fig. 2B). Dissipation of the ΔpH by PlnJK is faster than that by PlnEF. The individual peptides caused only a marginal loss of the ΔpH (data not shown). These data suggest that PlnJK dissipates the ΔpH directly, whereas dissipation of ΔpH by PlnEF might be indirect.
FIG. 2.
PlnEF and PlnJK both dissipate the ΔpH. Cells (22 μg of protein/ml) loaded with BCECF were diluted into 50 mM potassium phosphate (pH 6.5) and energized with 0.5% (wt/vol) glucose (arrow 1). Subsequently, valinomycin (arrow 2) (250 nM), PlnEF (A and B, arrow 3) (60 nM each peptide), PlnJK (C and D, arrow 4) (100 nM each peptide), or nigericin (arrow 5) (250 nM) was added.
PlnJK causes efflux of specific anions.
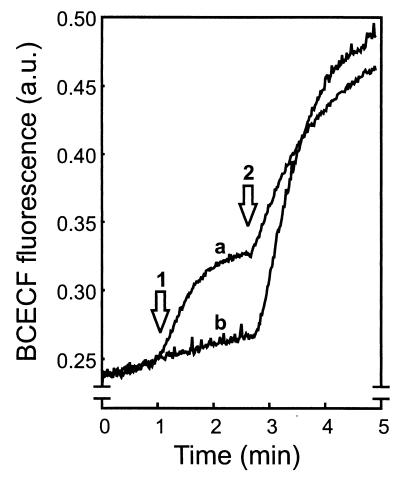
Addition of PlnJK to BCECF-loaded, nonenergized cells resulted in an increase in BCECF fluorescence (Fig. 3, arrow 1). The latter is due to decrease in the fluorescence self-quenching caused by efflux of some of the BCECF. Complete efflux of BCECF could be effected by the lantibiotic nisin (Fig. 3, arrow 2). The BCECF released by the cells was recovered in the supernatant after centrifugation of the PlnJK- or nisin-treated cells. On the other hand, neither solvent nor the individual PlnJ and PlnK peptides, PlnEF, the ionophore gramicidin A′, or the protonophore CCCP caused BCECF efflux. The PlnJK-induced BCECF efflux was more pronounced at pH 6.5 than at pH 7.0 and was almost completely absent at pH 8.0. These data show that in contrast to PlnEF, PlnJK is able to allow BCECF efflux from the cells.
FIG. 3.
PlnJK causes the efflux of the fluorescent dye BCECF. Cells (22 μg of protein/ml) loaded with BCECF were diluted into 50 mM potassium phosphate (pH 7.0). Arrow 1 indicates the addition of PlnJK (100 nM each peptide) (trace a) or solvent alone (trace b). At arrow 2, nisin (0.4 μM) was added to release all of the BCECF. a.u., arbitrary units.
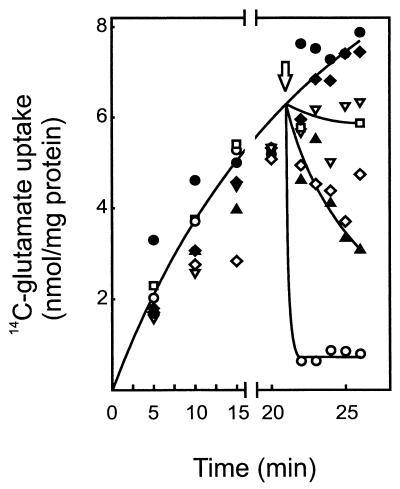
To investigate whether other anions can pass through the PlnJK pores, uptake and efflux of glutamate were measured. Although the exact mechanism of glutamate uptake has not been studied in L. plantarum, it is likely mediated by a system that resembles the ATP-driven, unidirectional system of Lactococcus lactis (19). When cells, after energization, were first allowed to accumulate [14C]glutamate, addition of PlnJK caused a rapid and complete release of the glutamate from the cells (Fig. 4). In contrast, PlnEF and PlnJ caused a slow release of the glutamate, while the other individual components caused no release at all. Glutamate uptake was arrested by the addition of the ionophores valinomycin and nigericin, but the accumulated glutamate was retained by the cell, analogous to previous studies with L. lactis (19). This indicates that indeed in L. plantarum, glutamate uptake takes place via a unidirectional ATP-driven mechanism. These data demonstrate that PlnJK efficiently conducts efflux of glutamate, whereas PlnEF (and PlnJ) is poorly active.
FIG. 4.
PlnJK causes efficient glutamate efflux. Cells (22 μg of protein/ml) suspended in 50 mM potassium phosphate (pH 7.0) were supplemented with 3.2 μM glutamate and energized with 0.5% (wt/vol) glucose. As indicated by the arrow, either valinomycin and nigericin (▿) (250 nM each) or the following plantaricins were added: PlnE (●) (120 nM), PlnF (⧫) (120 nM), PlnEF (▴) (60 nM each peptide), PlnJ (◊) (200 nM), PlnK (□) (200 nM), or PlnJK (○) (100 nM each peptide).
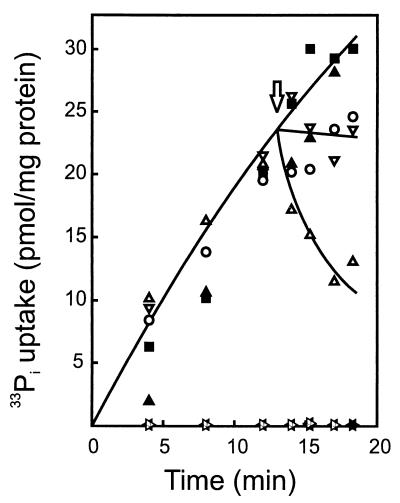
Since PlnJK caused the efflux of the anions BCECF and glutamate, we also studied whether it can cause the release of cellular phosphate. Uptake of phosphate by L. lactis occurs via an ATP-driven, unidirectional system (18), and a similar mechanism is anticipated in L. plantarum. Cells that were energized with glucose rapidly accumulated the externally added 33Pi, whereas no uptake was observed when the cells were first pretreated with PlnEF or PlnJK (see Fig. 5). On the other hand, once the 33Pi had been accumulated by the cells, addition of PlnEF, PlnJK, or the combination of valinomycin and nigericin did not induce release of the phosphate. In contrast, release was observed after the addition of nisin (Fig. 5). A colorimetric analysis of the release of cellular phosphate using a centrifugation assay to separate the cells from the suspension medium also demonstrated considerable release of phosphate only with nisin and not with PlnEF or PlnJK (data not shown). These data suggest that neither PlnEF nor PlnJK is able to conduct phosphate.
FIG. 5.
PlnEF and PlnJK do not induce phosphate efflux. Cells (22 μg of protein/ml) were energized with 0.5% (wt/vol) glucose and incubated with 0.11 nM 33Pi. At the arrow, either solvent (■), nisin (▵) (3.3 μM), valinomycin and nigericin (▿) (250 nM each), PlnEF (▴) (60 nM each peptide), or PlnJK (○) (100 nM each peptide) was added. Alternatively, cells were pretreated from 30 s with PlnEF (★) (60 nM each peptide) or PlnJK (⋆) (100 nM each peptide) prior to the addition of glucose and 33Pi.
PlnEF causes efflux of cations.
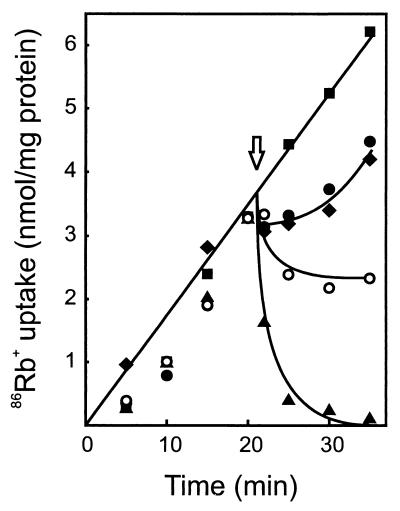
Next, the activity of the plantaricins to elicit cation release was investigated. For this purpose, cells were loaded with [14C]choline by overnight incubation. [14C]choline-loaded cells were subsequently challenged with PlnEF or PlnJK. PlnEF appeared to be more efficient in inducing choline release than PlnJK (data not shown). However, both were more effective than single peptides and the combination of valinomycin and nigericin (data not shown). In a control experiment, no plantaricin-induced Pi efflux was observed, precluding plantaricin-induced lysis. In contrast to [14C]choline, 86Rb+ ions readily accumulated in glucose-energized cells (Fig. 6). PlnEF elicited the rapid release of accumulated 86Rb+, while PlnJK blocked further uptake and caused only a marginal loss of accumulated 86Rb+. Only a slight inhibition of uptake was observed with the individual PlnE and PlnF peptides (Fig. 6), while the individual PlnJ and PlnK peptides had no effect at all (data not shown). The PlnEF-induced 86Rb+ efflux occurred within a broad pH range of 4.5 to 9.5, but at temperatures below 10°C, PlnEF was without effect. The presence of Gd3+ (0.2 mM) or Ca2+ (5 mM) only slightly affected the PlnEF-induced 86Rb+ ion efflux (data not shown). These data suggest that PlnEF is more effective than PlnJK for release of cations from the cells.
FIG. 6.
PlnEF and PlnJK inhibit 86Rb+ uptake. Cells (112 μg of protein/ml) suspended in 50 mM sodium phosphate (pH 7.0) were energized with 0.5% (wt/vol) glucose and incubated with 1.9 μM 86Rb+. At the arrow, the cells were supplemented with solvent (■), PlnE (●) (60 nM), PlnF (⧫) (60 nM), PlnEF (▴) (60 nM each peptide), or PlnJK (○) (120 nM each peptide).
DISCUSSION
This study indicates that two bacteriocins, PlnEF and PlnJK, both produced by L. plantarum C11, form pores in the cytoplasmic membranes of the target cells. Both plantaricins dissipate the ΔpH and Δψ. PlnEF dissipates the Δψ more rapidly than PlnJK. In contrast, PlnJK dissipates the ΔpH more rapidly than PlnEF. In view of the observed anion selectivity, the immediate dissipation of the ΔpH by PlnJK might be due to influx of hydroxyl ions. Unlike PlnJK, PlnEF first causes an increase in ΔpH before the entire ΔpH collapses. This increase in ΔpH is probably due to an enhanced proton extrusion after the dissipation of the Δψ, in analogy to the effect of valinomycin on the ΔpH. A temporary increase in ΔpH followed by dissipation has also been observed for the ionophore gramicidin A′ (16). Gramicidin A conducts small monovalent cations, including protons (17). We found that PlnEF has high conductivity for monovalent cations: protons and rubidium and choline ions. PlnJK on the other hand efficiently conducts anions, such as glutamate and BCECF. Strikingly, the C terminus of PlnJ (---RAIRR) and the N terminus of PlnK (RRSRK---) are both strongly cationic. It is tempting to speculate that these sequences are involved in the anion selectivity of the PlnJK pore. However, both PlnEF and PlnJK were ineffective in causing phosphate efflux.
PlnEF dissipates Δψ more efficiently than PlnJK. This difference is presumably caused by the higher cation conductance of PlnEF compared to that of PlnJK (Fig. 6). The latter hypothesis is consistent with absence of phosphate conductance by both plantaricins and with the much higher cation concentration (50 mM) than hydroxyl ion concentration (0.1 μM) at pH 7. Both growth inhibition of L. plantarum 965 (2) and ΔpH dissipation (Fig. 2) occur more efficiently by PlnJK than by PlnEF. This suggests that ΔpH dissipation more effectively causes growth inhibition than Δψ dissipation. ΔpH dissipation leads to a drop in intracellular pH and consequent inhibition of metabolism and thus of substrate-level phosphorylation which provides the cell with metabolic energy in the form of ATP.
All two-peptide class II bacteriocins dissipate Δψ (1, 12, 15, 22). On the basis of the ion selectivity, two subgroups of two-peptide bacteriocins can now be identified: (i) monovalent cation-conducting systems such as lactococcin G (15, 16) and PlnEF (lactococcin G does, however, not conduct protons) and (ii) bacteriocins with a preference for anions (i.e., PlnJK and possibly acidocin J1132) (22). In contrast to the bacteriocins described above, lactacin F seems to lead to efflux of both potassium ions and phosphate (1).
In conclusion, L. plantarum C11 produces three antimicrobial peptide systems, i.e., the bacteriocin-like pheromone PlnA, the cation-conducting PlnEF, and PlnJK, which exhibits a high conductivity for specific anions and a low conductivity for cations. Future structural analyses may reveal the molecular bases for the cation and anion selectivity of PlnEF and PlnJK. The combined, complementary activity of PlnEF and PlnJK ensures efficient bactericidal activity.
ACKNOWLEDGMENTS
We thank Dimitris Mantzilas for help in preparing, purifying, and quantitating the bacteriocins.
REFERENCES
- 1.Abee T, Klaenhammer T R, Letellier L. Kinetic studies of the action of lactacin F, a bacteriocin produced by Lactobacillus johnsonii that forms poration complexes in the cytoplasmic membrane. Appl Environ Microbiol. 1994;60:1006–1013. doi: 10.1128/aem.60.3.1006-1013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderssen E L, Diep D B, Nes I F, Eijsink V G H, Nissen-Meyer J. Antagonistic activity of Lactobacillus plantarum C11: two new two-peptide bacteriocins, plantaricin EF and JK, and the induction factor plantaricin A. Appl Environ Microbiol. 1998;64:2269–2272. doi: 10.1128/aem.64.6.2269-2272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brurberg M B, Nes I F, Eijsink V G H. Pheromone-induced production of antimicrobial peptides in Lactobacillus. Mol Microbiol. 1997;26:347–360. doi: 10.1046/j.1365-2958.1997.5821951.x. [DOI] [PubMed] [Google Scholar]
- 3a.De Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of Lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 4.Diep D B, Håvarstein L S, Nes I F. A bacteriocin-like peptide induces bacteriocin synthesis in Lactobacillus plantarum C11. Mol Microbiol. 1995;18:631–639. doi: 10.1111/j.1365-2958.1995.mmi_18040631.x. [DOI] [PubMed] [Google Scholar]
- 5.Diep D B, Håvarstein L S, Nes I F. Characterization of the locus responsible for the bacteriocin production in Lactobacillus plantarum C11. J Bacteriol. 1996;178:4472–4483. doi: 10.1128/jb.178.15.4472-4483.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diep D B, Håvarstein L S, Nissen-Meyer J, Nes I F. The gene encoding plantaricin A, a bacteriocin from Lactobacillus plantarum C11, is located on the same transcriptional unit as an agr-like regulatory system. Appl Environ Microbiol. 1994;60:160–166. doi: 10.1128/aem.60.1.160-166.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauge H H, Mantzilas D, Moll G N, Konings W N, Driessen A J M, Eijsink V G H, Nissen-Meyer J. Plantaricin A is an amphiphilic α-helical bacteriocin-like pheromone which exerts antimicrobial and pheromone activities through different mechanisms. Biochemistry. 1998;37:16026–16032. doi: 10.1021/bi981532j. [DOI] [PubMed] [Google Scholar]
- 8.Hauge H H, Mantzilas D, Eijsink V G H, Nissen-Meyer J. Membrane-mimicking entities induce structuring of the two-peptide bacteriocins plantaricin E/F and plantaricin J/K. J Bacteriol. 1999;181:740–747. doi: 10.1128/jb.181.3.740-747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauge H H, Nissen-Meyer J, Nes I F, Eijsink V G H. Amphiphilic α-helices are important structural motifs in the α and β peptides that constitute the bacteriocin lactococcin G. Eur J Biochem. 1998;251:565–572. doi: 10.1046/j.1432-1327.1998.2510565.x. [DOI] [PubMed] [Google Scholar]
- 10.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 11.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Marciset O, Jeronimus-Stratingh M C, Mollet B, Poolman B. Thermophilin 13, a nontypical antilisterial poration complex bacteriocin, that functions without a receptor. J Biol Chem. 1997;272:14277–14284. doi: 10.1074/jbc.272.22.14277. [DOI] [PubMed] [Google Scholar]
- 13.Molenaar D, Abee T, Konings W N. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta. 1991;1115:75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- 14.Molenaar D, Bolhuis H, Abee T, Poolman B, Konings W N. The efflux of a fluorescent probe is catalyzed by an ATP-driven extrusion system in Lactococcus lactis. J Bacteriol. 1992;174:3118–3124. doi: 10.1128/jb.174.10.3118-3124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moll G, Ubbink-Kok T, Hauge H H, Nissen-Meyer J, Nes I F, Konings W N, Driessen A J M. Lactococcin G is a potassium ion-conducting, two-component bacteriocin. J Bacteriol. 1996;178:600–605. doi: 10.1128/jb.178.3.600-605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moll G, Hildeng-Hauge H, Nissen-Meyer J, Nes I F, Konings W N, Driessen A J M. Mechanistic properties of the two-component bacteriocin lactococcin G. J Bacteriol. 1998;180:96–99. doi: 10.1128/jb.180.1.96-99.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers V B, Haydon D A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972;274:313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- 18.Poolman B, Nijssen R M J, Konings W N. Dependence of Streptococcus lactis phosphate transport on internal phosphate concentration and internal pH. J Bacteriol. 1987;169:5373–5378. doi: 10.1128/jb.169.12.5373-5378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poolman B, Smid E J, Konings W N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987;169:2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouser G, Fleischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 21.Sims P J, Waggoner A S, Wang C-H, Hoffman J F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974;13:3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- 22.Tahara T, Oshimura M, Umezawa C, Kanatani K. Isolation, partial characterization, and mode of action of acidocin J1132, a two-component bacteriocin produced by Lactobacillus acidophilus JCM 1132. Appl Environ Microbiol. 1996;62:892–897. doi: 10.1128/aem.62.3.892-897.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]