ABSTRACT
The marine bacterium Alcanivorax borkumensis produces a surface-active glycine-glucolipid during growth with long-chain alkanes. A high-performance liquid chromatography (HPLC) method was developed for absolute quantification. This method is based on the conversion of the glycine-glucolipid to phenacyl esters with subsequent measurement by HPLC with diode array detection (HPLC-DAD). Different molecular species were separated by HPLC and identified as glucosyl-tetra(3-hydroxy-acyl)-glycine with varying numbers of 3-hydroxy-decanoic acid or 3-hydroxy-octanoic acid groups via mass spectrometry. The growth rate of A. borkumensis cells with pyruvate as the sole carbon source was elevated compared to hexadecane as recorded by the increase in cell density as well as oxygen/carbon dioxide transfer rates. The amount of the glycine-glucolipid produced per cell during growth on hexadecane was higher compared with growth on pyruvate. The glycine-glucolipid from pyruvate-grown cells contained considerable amounts of 3-hydroxy-octanoic acid, in contrast to hexadecane-grown cells, which almost exclusively incorporated 3-hydroxy-decanoic acid into the glycine-glucolipid. The predominant proportion of the glycine-glucolipid was found in the cell pellet, while only minute amounts were present in the cell-free supernatant. The glycine-glucolipid isolated from the bacterial cell broth, cell pellet, or cell-free supernatant showed the same structure containing a glycine residue, in contrast to previous reports, which suggested that a glycine-free form of the glucolipid exists which is secreted into the supernatant. In conclusion, the glycine-glucolipid of A. borkumensis is resident to the cell wall and enables the bacterium to bind and solubilize alkanes at the lipid-water interface.
IMPORTANCE Alcanivorax borkumensis is one of the most abundant marine bacteria found in areas of oil spills, where it degrades alkanes. The production of a glycine-glucolipid is considered an essential element for alkane degradation. We developed a quantitative method and determined the structure of the A. borkumensis glycine-glucolipid in different fractions of the cultures after growth in various media. Our results show that the amount of the glycine-glucolipid in the cells by far exceeds the amount measured in the supernatant, confirming the proposed cell wall localization. These results support the scenario that the surface hydrophobicity of A. borkumensis cells increases by producing the glycine-glucolipid, allowing the cells to attach to the alkane-water interface and form a biofilm. We found no evidence for a glycine-free form of the glucolipid.
KEYWORDS: Alcanivorax borkumensis, 3-hydroxy fatty acid, oil spill, biosurfactants, glucose, glycine, HPLC, mass spectrometry, glucolipid
INTRODUCTION
Microorganisms produce a large variety of surface-active compounds. These biosurfactants or bioemulsifiers are amphiphilic molecules produced by microorganisms under certain conditions and are oftentimes secreted by the cells. Biosurfactants encompass small molecules like lipopeptides, acylated amino acids, and glycolipids, as well as polymeric high molecular weight substances (1). A carbohydrate moiety is attached to long-chain aliphatic acids or lipopeptides in glycolipids. For example, rhamnolipids, trehalose lipids, and sophorolipids are mono- or disaccharides acylated with long-chain fatty acids or hydroxy-fatty acids (2). The amphiphilic structure of these molecules provides the means to lower the interfacial tension between immiscible fluids (1). In nature, biosurfactants facilitate the utilization of hydrophobic substrates by microorganisms (3). They can have antibacterial or antifungal properties, and they enable attachment to surfaces and subsequent biofilm formation. Furthermore, they are important virulence factors during bacterial pathogenesis, where their production is regulated by quorum sensing mechanisms (4).
In biotechnological applications, biosurfactants have advantages over synthetic surfactants because of biodegradability and low toxicity (5, 6). These characteristics provide the potential to employ biosurfactants in numerous applications such as additives in detergents and food, cosmetics, pharmacology, petroleum recovery, and agriculture (6–11). As a result, interest in biosurfactants has increased in recent years because these natural products are considered both an alternative and additive to synthetic surfactants.
Aerobic strains of Pseudomonas aeruginosa are known to produce and secrete biosurfactants called rhamnolipids. The P. aeruginosa rhamnolipids are composed of a mono- or dirhamnosyl moiety linked to a unit of two 3-hydroxy-fatty acids (7–9). Because these glycolipids show low absorption of UV light, they are often converted into UV-absorbing derivatives before analysis by high-performance liquid chromatography with diode-array detection (HPLC-DAD) (10). An analytical method for the determination of the rhamnolipids from P. aeruginosa by HPLC-DAD has been previously developed (7). In this method, rhamnolipids are converted into phenacyl esters after reaction with p-bromoacetophenone in the presence of triethylamine. This method was modified by replacing the derivatization reagent with 2-bromoacetophenone (11). Rhamnolipid molecular species with different numbers of rhamnose units or fatty acids were separated and analyzed by HPLC-DAD.
In oil-polluted sea areas, most bacteria become nutrient-starved except the species that can metabolize hydrocarbons, namely, hydrocarbonoclastic or oil-degrading bacteria. Various hydrocarbonoclastic bacteria have been identified in marine environments, including Alcanivorax borkumensis, isolated from North Sea sediment (12). It is found in low numbers in unpolluted waters but accumulates in oil-polluted waters and coastlines, where it may comprise 80 to 90% of the oil-degrading microbial community (13). The substrate spectrum of A. borkumensis encompasses aliphatic hydrocarbons, including linear alkanes, cycloalkanes, and isoprenoids (14). Therefore, A. borkumensis is crucial for petroleum bioremediation. To increase the availability of the hydrocarbons during metabolism, A. borkumensis produces a surface-active glycine-glucolipid which consists of glucose attached to a lipid backbone of four 3-hydroxy-fatty acids, with the carboxyl group of the terminal fatty acid linked to glycine (15). In addition, a different structure of the A. borkumensis glucolipid lacking the glycine residue had previously been proposed. It remained unclear whether both forms exist inside the cells or whether one form might be secreted into the culture supernatant (15–17).
The objective of the present study was to develop an HPLC-DAD-based method for the exact quantification of the glycine-glucolipid from A. borkumensis in combination with mass spectrometric analyses of lipid extracts from cells grown under different conditions. We further analyzed the occurrence of the glycine-glucolipid in its glycine-containing or glycine-free forms within the cells or the culture supernatant.
RESULTS
Analysis of the A. borkumensis glycine-glucolipid by direct infusion mass spectrometry.
The glycine-glucolipid was purified from the A. borkumensis cells after growth on pyruvate by solid-phase extraction (SPE) and thin-layer chromatography (TLC) and analyzed by direct infusion mass spectrometry on a quadrupole time of flight (Q-TOF) instrument (Fig. 1A). The glycine-glucolipid is composed of glucose attached to four 3-hydroxy-fatty acids with a glycine bound to the terminal fatty acid in amide linkage (15). The molecular species of the glycine-glucolipid were predominantly composed of Glc(O-10:0)4Gly (peak of the ammonia adduct at m/z 935.6414) and low amounts of Glc(O-8:0)(O-10:0)3Gly, while Glc(O-8:0)2(O-10:0)2Gly was barely detectable (Fig. 1A, Table 1). The MS/MS spectrum of Glc(O-10:0)4Gly fully agreed with the previously reported structure (Fig. 1A and D) (15). Fragmentation of the glycine-glucolipid molecular species containing one 3-hydroxy-octanoic acid, Glc(O-8:0)(O-10:0)3Gly, revealed that the 3-HO-8:0 fatty acid is randomly distributed to the four possible positions of the congener (Fig. 1C).
FIG 1.
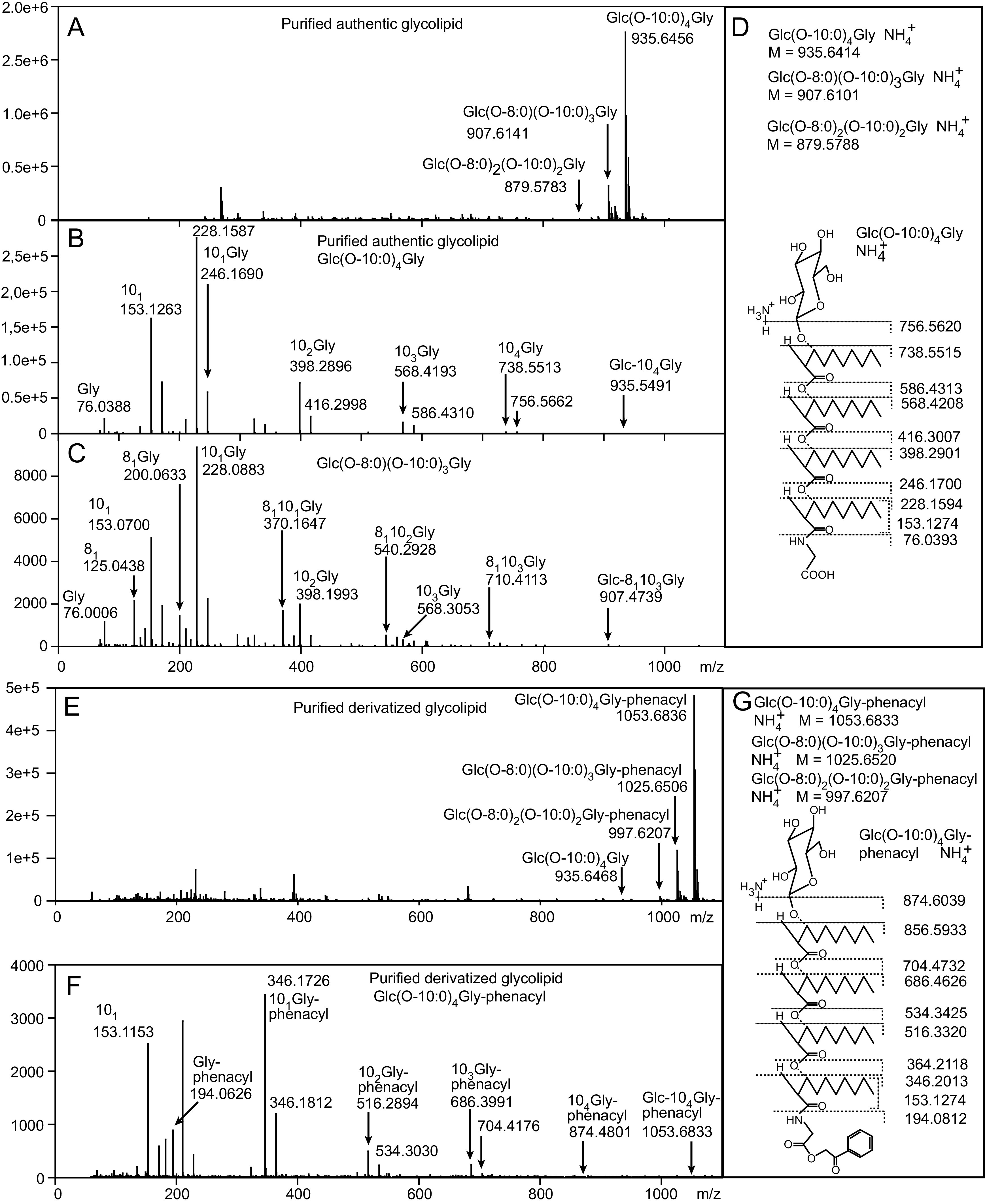
Analysis of authentic and derivatized A. borkumensis glycine-glucolipid by mass spectrometry. The glycine-glucolipid was purified by SPE and TLC and analyzed by direct infusion Q-TOF mass spectrometry. (A) Total ion count spectrum of the purified authentic glycine-glucolipid. (B) MS/MS spectrum after fragmentation of the main glycine-glucolipid species Glc(O-10:0)4Gly. Each neutral loss of an acyl group is represented by two fragment peaks differing by one H2O group (m/z = 18). (C) MS/MS spectrum after fragmentation of the glycine-glucolipid species Glc(O-8:0)(O-10:0)3Gly. The m/z for 3-HO-10:0 and 3-HO-8:0 differ by 28. Note that only the fragments representing the losses of the acyl moieties including H2O are depicted. (D) Calculated masses of ammonium adducts of different molecular species and fragments generated from Glc(O-10:0)4Gly of the authentic glycine-glucolipid. After the loss of the sugar head group with ammonia, the lipid backbone fragments exist as H+ adducts. Fragmentation within the lipid backbone results in radical ions with m/z differences originating from the loss of one H2O molecule. The glycine ion with m/z = 76.0393 represents the H+ adduct. (E) Total ion count spectrum of the purified glycine-glucolipid after conversion into phenacyl esters. (F) MS/MS spectrum after fragmentation of the main species Glc(O-10:0)4Gly-phenacyl. (G) Calculated masses of ammonium adducts of molecular species and fragments generated from Glc(O-10:0)4Gly-phenacyl. Glc, glucose; Gly, glycine; O-8:0 or “8,” 3-hydroxy-octanoic acid; O-10:0 or “10,” 3-hydroxy-decanoic acid.
TABLE 1.
Relative amounts of molecular species of the A. borkumensis glycine-glucolipid after growth on pyruvate- or hexadecane-containing mediuma
| Glycine-glucolipid molecular species | Growth on pyruvate |
Growth on hexadecane |
||
|---|---|---|---|---|
| Relative amount (%) (HPLC) | Relative amount (%) (Q-TOF) | Relative amount (%) (HPLC)b | Relative amount (%) (Q-TOF) | |
| Glc(O-8:0)2(O-10:0)2Gly | 4.0 ± 0.4 | 4.0 ± 0.1 | n.d. | 0.5 ± 0.2 |
| Glc(O-8:0)(O-10:0)3Gly | 25.3 ± 0.3 | 24.5 ± 0.4 | 11.1 ± 1.0 | 3.1 ± 1.5 |
| Glc(O-10:0)4Gly | 70.8 ± 0.6 | 71.4 ± 0.4 | 88.9 ± 1.0 | 96.3 ± 1.7 |
The glycine-glucolipid was extracted from the whole culture broth of bacteria grown on pyruvate or hexadecane. Relative amounts of molecular species were determined by HPLC-DAD or Q-TOF mass spectrometry.
n.d., not detected. Mean ± SD, n = 3.
Quantification of the A. borkumensis glycine-glucolipid by HPLC-DAD.
The A. borkumensis glycine-glucolipid is devoid of strong chromophores (15). Hence, HPLC-DAD can barely detect it using a UV/visible light detector. We therefore chose an HPLC method previously developed to analyze P. aeruginosa rhamnolipids, another class of glycolipids that do not contain strong chromophores for UV/visible light detection (10). The original protocol included a derivatization step of the terminal fatty acid of the rhamnolipid with p-bromoacetophenone, later replaced with 2-bromoacetophenone (7, 11). In the A. borkumensis glycine-glucolipid, the terminal 3-hydroxy fatty acid is bound to glycine in amide linkage and therefore cannot be derivatized. However, the terminal glycine of the glycine-glucolipid possesses a free carboxy group, which might be accessible for conversion into its phenacyl ester (Fig. 2A). As an internal standard, we selected undecanoic acid, a free fatty acid readily converted into its phenacyl ester (18).
FIG 2.
Detection of the A. borkumensis glycine-glucolipid by HPLC-DAD after conversion into phenacyl esters. (A) The glycine-glucolipid was converted into its phenacyl ester after incubation with 2-bromoacetophenone in the presence of triethylamine. Undecanoic acid (11:0) was employed as an internal standard. (B) HPLC-DAD chromatogram of a crude lipid extract from A. borkumensis, after derivatization. (C) HPLC-DAD chromatogram of isolated A. borkumensis glycine-glucolipid (purified by SPE and TLC) after derivatization. Glc, glucose; Gly, glycine; O-8:0, 3-hydroxy-octanoic acid; O-10:0, 3-hydroxy-decanoic acid.
Figure 2B and C show the HPLC-DAD chromatograms of a crude lipid extract and the purified glycine-glucolipid from A. borkumensis grown on pyruvate after derivatization. Three peaks eluting at 15.5 min, 17.5 min, and 19.5 min were identified, together with the internal standard, which elutes at 12.1 min. The peak fractions containing the three derivatized lipids were collected from the HPLC column and individually analyzed by direct infusion mass spectrometry. The major peak eluting at 19.5 min showed an m/z of 1,053.6836, in agreement with the addition of the phenacyl group on the parental ion [Glc(O-10:0)4Gly-phenacyl + NH4+] as compared with the nonderivatized species of [Glc(O-10:0)4Gly + NH4+] with an m/z of 935.6414 (Fig. 1E). The fragmentation pattern of [Glc(O-10:0)4Gly-phenacyl + NH4+] indicated that the phenacyl moiety was attached to the carboxylate of the glycine (Fig. 1F and G). The peaks eluting at 15.5 min and 17.5 min corresponded to the molecular species of Glc(O-8:0)2(O-10:0)2Gly-phenacyl and Glc(O-8:0)(O-10:0)3Gly-phenacyl, respectively. The amounts of the three molecular species of Glc(O-8:0)2(O-10:0)2Gly-phenacyl, Glc(O-8:0)(O-10:0)3Gly-phenacyl, and Glc(O-10:0)4Gly-phenacyl were determined by HPLC-DAD as 4.0 ± 0.4%, 25.3 ± 0.3%, and 70.8 ± 0.1%, respectively (Table 1).
In addition to the derivatized peaks of the major molecular species Glc(O-10:0)4Gly-phenacyl, a minor nonderivatized peak corresponding to Glc(O-10:0)4Gly was observed in the total ion count spectrum (Fig. 1E). Taking the peak sizes as a measure of abundance, the degree of derivatization was calculated to be 97.2% ± 0.8% and 98.9% ± 0.4% for the sum of the three glycine-glucolipid species in the crude lipid extract and the purified glycine-glucolipid, respectively (n = 3).
Analysis of the glycine-glucolipid-derived 3-hydroxy fatty acids by GC-MS.
The determination of the 3-hydroxy fatty acids by gas chromatography-mass spectrometry (GC-MS) was assessed as an alternative method for quantifying the glycine-glucolipid. The glycine-glucolipid contains four 3-hydroxy fatty acids, which are bound to the 3-hydroxy group of the adjacent fatty acid by ester linkages, while the first one is linked to the glucose moiety by a glycosidic linkage, and the terminal 3-hydroxy fatty acid is linked to glycine by an amide linkage. For GC-MS measurements, the 3-hydroxy fatty acids were cleaved and transmethylated with methanolic HCl (Fig. 3A). Because its amide-linked fatty acids are more resistant to transmethylation reactions than ester-linked fatty acids, the reaction was optimized to ensure that all four 3-hydroxy fatty acids were released from the glycine-glucolipid (Fig. 3B). Transmethylation reactions were complete after 5 h with 3 N HCl. Subsequently, the 3-hydroxy groups of the fatty acids were converted into trimethylsilyl groups. Quantification by GC-MS was achieved using 3-hydroxy-dodecanoic acid (HO-12:0) as the internal standard. Fig. 3B shows that the purified glycine-glucolipid contains 93.0% HO-10:0 and 7.0% 3-HO-8:0.
FIG 3.
Analysis of 3-hydroxy-fatty acids derived from the A. borkumensis glycine-glucolipid by GC-MS. (A) The four 3-hydroxy-fatty acids in the A. borkumensis glycine-glucolipid were transmethylated, and the free hydroxy groups were converted into trimethylsilyl (TMS) ethers. (B) GC-MS chromatogram (total ion count) of the A. borkumensis glycine-glucolipid, which was purified by SPE and TLC, and the 3-hydroxy fatty acids were converted into methyl esters/TMS ethers, in the presence of the internal standard 3-hydroxy dodecanoic acid (HO-12:0).
A. borkumensis cells show a higher growth rate on pyruvate compared with hexadecane as carbon source.
To study the growth rates of the A. borkumensis cells in different media, the cells were incubated with pyruvate or hexadecane in the Kuhner TOM shaker, a device for online transfer rate measurement of O2 and CO2 (oxygen transfer rate [OTR] and carbon dioxide transfer rate [CTR]) in shake flasks (Fig. 4A and B). A. borkumensis cells with hexadecane as a carbon source needed about 92.6 h to grow to stationary phase, compared with 18.4 h on pyruvate. The determination of transfer rates provides the means to calculate growth rates independent of optical density, to avoid interference by turbidity caused by insoluble nutrients like hexadecane. The growth rate on pyruvate (0.25 h−1) was almost twice as high as on hexadecane (0.14 h−1). More CO2 was produced with pyruvate (148.0 mmol L−1) than with hexadecane (58.6 mmol L−1). Furthermore, more O2 was consumed with hexadecane (178.8 mmol L−1), since it is a much more reduced carbon source than pyruvate (131.4 mmol L−1).
FIG 4.
Growth of A. borkumensis cultures under different conditions. (A) O2 (OTR), CO2 (CTR), and (B) integrals of OTR, CTR of cultures grown in hexadecane or pyruvate medium. The cells were cultivated and monitored in a TOM shaker until the stationary phase was reached. Mean values of two individual cultivations are shown. The error bands indicate the values obtained from the individual cultures. (C) Growth curves (OD600) of A. borkumensis cells grown with pyruvate and phosphate, with hexadecane and phosphate, with pyruvate without phosphate, or with hexadecane without phosphate. The plot shows individual, representative growth curves repeated at least two times with similar results.
The glycine-glucolipid accumulates on A. borkumensis cells grown on hexadecane.
Q-TOF mass spectrometry and HPLC-DAD were employed to quantify the glycine-glucolipid produced by A. borkumensis grown under different conditions. Biosurfactant production can be regarded as a response to alkanes present in the environment and may allow the producing bacteria to access these water-insoluble compounds as carbon sources. Therefore, A. borkumensis was grown with pyruvate or hexadecane as carbon sources (12, 19). In addition, some bacteria adjust their lipid composition during phosphate deprivation by replacing phospholipids with glycolipids or other nonphosphorous lipids to save phosphate for other cellular processes, and it was possible that the glycine-glucolipid also showed some responsiveness to phosphate deprivation (20, 21). Thus, the cells were also grown in the presence or absence of phosphate, both with pyruvate or hexadecane as a carbon source, to assess whether the glycine-glucolipid accumulation was stimulated by the presence of hexadecane or in response to phosphate deprivation or a mixture of both. In line with the results obtained with the TOM shaker, the A. borkumensis cells grew faster with pyruvate than hexadecane, and they reached a higher cell density in the stationary phase (Fig. 4C). Growth under phosphate deprivation was compromised compared with the respective controls (pyruvate/hexadecane).
Cells were harvested and crude lipid extracts were prepared for glycine-glucolipid measurements by HPLC-DAD after conversion into phenacyl esters. The total glycine-glucolipid (sum of Glc(O-8:0)2(O-10:0)2Gly, Glc(O-8:0)(O-10:0)3Gly, and Glc(O-10:0)4Gly-phenacyl) amounted to ~0.8 nmol per 1010 cells (equivalent to 4.2 nmol per mg protein) when cells were grown with pyruvate in the presence or absence of phosphate (Fig. 5B). The glycine-glucolipid content was higher in cells grown with phosphate and hexadecane (~1.3 nmol per 1010 cells), but it was only ~0.4 nmol per 1010 cells when phosphate was omitted from the hexadecane medium. Therefore, phosphate deprivation did not increase the glycine-glucolipid content, indicating that it is not involved in the phosphate deprivation response of A. borkumensis. In contrast, growth on hexadecane resulted in increased glycine-glucolipid accumulation per 1010 cells by > 50%, indicating that its synthesis was stimulated under conditions of alkane metabolization. It should be noted that the yield per volume of the glycine-glucolipid in pyruvate-grown cells (8 nmol per mL culture) was higher compared with hexadecane-grown cells (3 nmol per mL culture), since the cells grow to a higher density in pyruvate medium (Fig. 4C). Furthermore, the molecular species composition of the glycine-glucolipid from hexadecane-grown cells was shifted in comparison with pyruvate-grown cells, because the relative contents of Glc(O-8:0)(O-10:0)3Gly and Glc(O-10:0)4Gly amounted to 11.1% ± 1.0% and 88.9% ± 1.0%, respectively, while Glc(O-8:0)2(O-10:0)2Gly was not detectable, in hexadecane-grown cells (Table 1).
FIG 5.
Quantification of the glycine-glucolipid after growth in different media and in cell pellets and supernatants of A. borkumensis cultures. (A) The lipid extracts were prepared from A. borkumensis cells grown on pyruvate or hexadecane in the presence (+P) or absence (-P) of phosphate and analyzed by direct infusion Q-TOF mass spectrometry. The peak sizes were used for relative quantification. (B) The lipid extracts were derivatized with 2-bromoacetophenone and the glycine-glucolipid quantified by HPLC-DAD. (C) Lipids were extracted from the total culture broth of A. borkumensis cells grown in pyruvate or hexadecane. After centrifugation, lipids were extracted from the cell pellets or the supernatants (Super). The lipid extracts were analyzed by direct infusion Q-TOF mass spectrometry, and the peak sizes were used for relative quantification. (D) The lipid extracts were derivatized with 2-bromoacetophenone and the glycine-glucolipid quantified by HPLC-DAD. All glycine-glucolipid-containing peaks (Glc(O-10:0)4Gly, Glc(O-8:0)(O-10:0)3Gly, and Glc(O-8:0)2(O-10:0)2Gly) were summarized. The amounts of glycine-glucolipids are expressed in total ion counts per 1010 cells (Q-TOF) or in nmol per 1010 cells and in nmol per mg protein (HPLC) Data show means and SD (n = 3). Student T-test, *, P < 0.05; **, P < 0.01; n.s., not significant.
Next, the glycine-glucolipid amount was measured by Q-TOF mass spectrometry. Because of the lack of an internal standard, relative amounts of the glycine-glucolipid were determined based on the peak sizes. The total amounts of the glycine-glucolipid of the cells grown under different conditions agreed with the amounts determined by HPLC-DAD. (Fig. 5A).
The glycine-glucolipid accumulates exclusively in a form bound to A. borkumensis cells.
Commonly, glycolipid biosurfactants are recovered from the extracellular medium of producing bacterial cultures (22). Previous studies suggested that a certain amount of the glycine-glucolipid of A. borkumensis might also be secreted into the medium after cleavage of the glycine moiety, where it might associate with hydrocarbons and aid in the solubilization, uptake, and metabolization of alkanes (15, 17, 23, 24). To determine the relative distribution of the glycine-glucolipid between the cells and the secreted fraction, the cells were harvested by centrifugation, and the glycine-glucolipid was measured in the whole culture broth, the cell pellet, and the cell-free supernatant. The experiment was conducted in the presence of pyruvate or hexadecane as carbon sources, and the glycine-glucolipid was measured by HPLC-DAD and Q-TOF mass spectrometry. As shown before (Fig. 5A and B), the amount of the glycine-glucolipid per cell increased when the cells were grown in the presence of hexadecane (Fig. 5C and D). The predominant fraction of the glycine-glucolipid was detected in the cell pellet, while only minute amounts were found in the supernatant, both when the cells were grown on pyruvate or hexadecane. Therefore, most of the glycine-glucolipid accumulates in a form bound to the bacteria, and only very low amounts are found in the supernatant.
The structures of the glycine-glucolipid in the whole culture broth, the cell pellet, and the supernatant were studied by Q-TOF mass spectrometry. In all fractions, the main peaks in the mass spectrum showed the glycine-containing form of the glycine-glucolipid carrying four fatty acids—Glc(O-10:0)4Gly, Glc(O-8:0)(O-10:0)3Gly, and Glc(O-8:0)2(O-10:0)2Gly as NH4+ adducts with m/z 935.6414, 907.6101, and 879.5788, respectively. No evidence was found for the occurrence of the glycine-glucolipid form lacking the glycine residue (Glc[O-10:0]4 as NH4+ adducts with m/z 878.6199) (16).
DISCUSSION
Different bacteria, including Pseudomonas and Alcanivorax species, produce surface-active glycolipids to solubilize hydrophobic compounds during metabolism. In this study, we present an HPLC-DAD-based method for the absolute quantification of the glycine-glucolipid from A. borkumensis. Quantification is based on an internal standard and normalization using response factors obtained from measuring the 3-hydroxy-fatty acids by GC-MS. The method can easily be adapted to other bacterial species for the quantitative analysis of related glycolipids composed of sugar head groups bound to 3-hydroxy fatty acids. Using chloroform/methanol, glycolipids are extracted from the membranes or the cell walls of bacteria, and even secreted lipids can be measured. The derivatization represents an additional step prior to quantification, and measurement by UV detection provides only moderate sensitivity. On the other hand, lipid analysis by liquid chromatography MS provides much higher sensitivity, as shown for the analysis of rhamnolipids, albeit at increased expenses (25).
It should be noted that the measurement of 3-hydroxy fatty acids by GC-MS after transmethylation cannot be used for glycine-glucolipid quantification in crude cell extracts. Many bacteria accumulate polyhydroxyalkanoate (PHA) during excess carbon supply and imbalanced nutrient conditions (i.e., with high C, but low N or P supply) (26). A. borkumensis produces mostly polyhydroxybutyrate (poly-HO-4:0, PHB) when grown on pyruvate, but accumulates high amounts of PHA containing HO-8:0, HO-10:0, and HO-12:0 during growth on octadecane (27). In the present study, strongly increased amounts of 3-hydroxy fatty acids were found in cells grown on hexadecane (with or without phosphate) or pyruvate (without phosphate), presumably derived from increased PHA accumulation.
Three molecular species of the glycine-glucolipid were separated and quantified by HPLC-DAD, and identified by Q-TOF mass spectrometry. Besides the Glc(O-10:0)4Gly lipid with a relative amount of 70.8% when the cells were grown on pyruvate, we identified Glc(O-8:0)2(O-10:0)2Gly, and Glc(O-8:0)(O-10:0)3Gly with 4.0% and 25.3%, respectively. Several molecular species of the glycine-glucolipid with different proportions of 3-HO-10:0, 3-HO-8:0, or 3-HO-6:0 fatty acids were previously described, but no quantitative data were available (15). The preferred incorporation of 3-HO-10:0, while 3-HO-8:0 is a minor substrate and 3-HO-12:0 is not used at all, might be explained by the specificity of the enzymes involved in glycine-glucolipid production. Notably, the spectrum of congeners identified for the A. borkumensis glycine-glucolipid is much narrower than the structurally related rhamnolipids produced by P. aeruginosa or different Burkholderia species (28). It likewise points to biosynthetic enzymes or other quality control mechanisms that ensure specific 3-hydroxy-fatty acid incorporation.
We consistently observed higher growth rates when A. borkumensis cells were grown on pyruvate compared with hexadecane, both in the TOM shaker experiment and in open flasks (Fig. 4 A, B, C). It is crucial to maintain a neutral pH in the medium because the pH drops when cultivating the cells with hexadecane, and it increases during growth with pyruvate. Therefore, an alkaline, i.e., growth-inhibiting pH, is reached much faster with pyruvate than with hexadecane. Therefore, we used high buffer concentrations (50 mM HEPES) to avoid pH shifts during growth. Previously, Naether et al. (29) observed a higher growth rate on hexadecane compared with pyruvate. This might in part have been caused by interference with turbidity originating from hexadecane in the medium. Furthermore, the medium was buffered with only 5 mM TAPSO (pH 7.6), suggesting a shift to alkaline conditions during growth on pyruvate. On the other hand, our results, which show a higher growth rate on pyruvate than on hexadecane, are consistent with those of Barbato et al. (30) who found a higher cell density (measured by flow cytometry) at the end of the growth experiment on pyruvate compared with dodecane. Furthermore, Manilla-Perez et al. (31) also found that cells grew better with pyruvate or acetate compared with hexadecane or octadecane. The finding that the growth rate in pyruvate medium was higher than in hexadecane-containing medium suggests that pyruvate metabolism is the preferred pathway for A. borkumensis, because a water-soluble substrate is biologically much more easily available than a water-insoluble one.
Furthermore, the amount of the glycine-glucolipid per cell increased when pyruvate was replaced with hexadecane as a carbon source. Interestingly, the composition of molecular species of the glycine-glucolipid changes during growth on hexadecane because large amounts of Glc(O-10:0)4Gly accumulated, while the quantities of Glc(O-8:0)(O-10:0)3Gly were much lower, and Glc(O-8:0)2(O-10:0)2Gly was not detected. During growth on pyruvate, medium-chain length 3-hydroxy fatty acids are presumably derived from intermediates of fatty acid de novo synthesis, in agreement with the finding that 3-hydroxy-acyl groups in the glycine-glucolipid and the intermediates of fatty acid de novo synthesis are in D-configuration (16). During growth on hexadecane, β-oxidation of acyl-CoAs derived from alkane catabolism results in the generation of medium-chain length L-3-hydroxy-fatty acids. In this scenario, an epimerization step to the D-isomer is required before 3-hydroxy-fatty acids can be incorporated into the glycine-glucolipid (27). Interestingly, growth on octadecane also results in an increase in PHA synthesis accompanied by a shift of the predominant chain length from C4 (on pyruvate) to C8, C10, and C12 (on octadecane). This indicates that acyl chains derived from octadecane via β-degradation and isomerization from the L to the D-3-hydroxy fatty acid were used for PHA synthesis (27). In addition to glycine-glucolipid and PHA, wax esters accumulated in A. borkumensis cells grown on hexadecane but not on pyruvate, while triacylglycerol was present in cells grown under both conditions (32). The triacylglycerol and wax ester fractions in hexadecane-grown cells were enriched with palmitic acid (16:0), suggesting that this acyl group was derived from hexadecane. Furthermore, fatty acids derived from alkane degradation were directly incorporated into membrane phospholipids (29).
In some bacteria and plants, the amounts of glycolipids, primarily glucose or galactose moieties linked to diacylglycerol, increase during growth in phosphate-deprived medium, because the glycolipids replace phospholipids in the membranes (20, 21). In contrast, the glycine-glucolipid production in A. borkumensis was not stimulated by phosphate deprivation. The correlation between the amount of the glycine-glucolipid and the carbon source, but a missing effect of phosphate limitation, agrees with the role of the glycine-glucolipid as a bioemulsifier required for association of the cells with the oil-water interphase. The glycine-glucolipid bound to the bacterial cell wall could reduce the oil/water interfacial tension when the cells are exposed to hydrocarbons (24).
Two different forms of the glycolipid were previously described in A. borkumensis. A glucose-containing lipid bound to a congener of four esterified 3-hydroxy decanoic acids lacking a glycine moiety was suggested to be present in the culture broth (16, 33). Later, the glycine-glucolipid isolated from the A. borkumensis cell pellet contained a glucose bound to a congener of four 3-hydroxy-decanoic acids carrying a glycine residue in amide linkage (15). The differences in the structures of glycolipids in the cell pellet and culture broth led to the speculation that the glycine-glucolipid in the cell wall of A. borkumensis might be a precursor for the secreted, glycine-free form needed for alkane solubilization (15, 17, 24). In this study, we only identified the glycine-containing form of the glycine-glucolipid predominantly in the cell pellet; abundance was very low in the cell-free supernatant (Fig. 5). We detected further lipids in the supernatant by Q-TOF mass spectrometry, particularly phosphatidylglycerol (PG), which is an abundant phospholipid in A. borkumensis. The distribution of PG was very similar to that of the glycine-glucolipid, i.e., most PG was found in the cell pellet and only minute amounts in the supernatant. PG in the supernatant presumably derived from damaged cell membranes rather than from active secretion. These findings support the scenario that the A. borkumensis cells are directly attached to the lipid-water interphase (24, 34), and that the glycine-glucolipid is not secreted for the solubilization of alkanes. In line with this finding, even concentrated supernatants of A. borkumensis cells lack any emulsifying activity (33). These data are also in line with previous reports, which showed that A. borkumensis cells grown on hexadecane reveal stronger adhesion to hexadecane and more increased surface tension than cells grown on pyruvate (30). The water contact angle of pyruvate-grown cells (~75°) was considerably lower than that of hexadecane-grown cells (~110°) (29). The hexadecane-grown cells tend to aggregate; they become partially hydrophobic, reduce interfacial tension, and form biofilms at the oil/water interface in contrast to pyruvate grown cells (24, 34). Therefore, the cell-associated glycine-glucolipid may help the bacteria become partially hydrophobic to attach to the oil/water interface, where they reduce interfacial tension and form the early stages of a biofilm around the hexadecane droplets.
Glycolipids have a tremendous market potential as they do not only provide the basis for CO2-neutral production from various feedstocks, but also come with novel structures and hence potentially valuable new properties (35, 36). With sophorolipids and rhamnolipids as prime examples, we will see further developments of glycolipid use. For research and development, but also for the evaluation of ecotoxicity and manufacturing, methods for quantification of the glycolipid are key tools. Here, we present a workbench of analytical methods that allow identification of the congeners and quantification of the glycine-glucolipid of A. borkumensis, which will be instrumental to foster the development of this interesting biomolecule.
MATERIALS AND METHODS
Microorganism and cultivation conditions.
Alcanivorax borkumensis SK2 was grown in Marine Broth 2216 (BD Difco, Fisher Scientific) with 1% (wt/vol) of pyruvate as the carbon source. To study the glycine-glucolipid production under different conditions, the cells were grown in modified ONR7a medium (22.79 g/L NaCl, 0.72 g/L KCl, 3.98 g/L Na2SO4, 0.083 g/L NaBr, 0.031 g/L NaHCO3, 0.027 g/L H3BO3, 0.0026 g/L NaF, 11.18 g/L MgCl2 × 6 H2O, 1.46 g/L CaCl2 × 2 H2O, 0.024 g/L SrCl2 × 6 H2O, 0.01 g/L FeSO4 × 7 H2O, 0.005 g/L MnSO4 × H2O, 0.0064 g/L ZnCl2, 0.0004 g/L CoCl2 × 6 H2O, 0.0002 g/L Na2MoO4 × 2 H2O, 0.01274 g/L Na2EDTA × 2 H2O, 0.46 g/L NaH2PO4, 2 g/L NH4Cl, 50 mM HEPES) with 1% (wt/vol) sodium pyruvate or 1% (vol/vol) hexadecane as carbon source. The pH was 7.0 for pyruvate and 7.5 for hexadecane medium. For phosphate deprivation, NaH2PO4 was omitted from the media. Cultures were incubated at 28°C under shaking at 180 rpm. Due to the agitation during growth, the medium components were homogenously dispersed without any accumulation of nutrient components on the surface, the interior, or the bottom of the culture broth. Cells were harvested for lipid extraction after 3 days, i.e., in the late exponential growth phase. A culture aliquot was used to determine the number of cells by plating serial dilutions and colony counting (OD600 = 1 is equivalent to 1.76 × 1010 cells per mL). Similarly, the amount of protein was measured in a culture aliquot (OD600 = 1 is equivalent to 330 mg protein per L).
For the determination of the oxygen transfer rate (OTR) and carbon dioxide transfer rate (CTR), A. borkumensis cells were inoculated to an OD600 of 0.1 in 25 mL (5% filling volume) of the modified ONR7a medium (for medium composition and pH see above) and 10 g/L pyruvate or 4.83 g/L hexadecane. Cultures were incubated in a TOM shaker (Kuhner, Birsfelden, Switzerland) with 50 mm throw, at 300 rpm and 30°C. The OTR and CTR were measured online in mmol L−1 h−1 in duplicates and averaged, and the values were integrated to obtain integral OTR and CTR. The growth rates we determined from the exponential trend line at the steepest point of the OTR curve.
Lipid extraction and glycine-glucolipid purification.
Samples for OD600 measurement or lipid extraction were taken with a pipette from the interior of the culture broth. Samples were vortexed and transferred into cuvettes, and the OD600 was measured against a blank containing the corresponding medium without cells to quantify the biomass of the cells.
For lipid extraction, the cells were harvested by centrifugation for 30 min at 7,000 × g, resuspended with a minimal volume of deionized H2O, and boiled at 100°C for 15 min. After cooling, two volumes of CHCl3/CH3OH (1:2) were added, and the mixture was shaken at 4°C for 1 h. After centrifugation for 20 min at 2,000 × g, the organic phase was collected. The extraction was repeated two more times with two volumes of (ii) CHCl3/CH3OH (2:1) and (iii) CHCl3. After centrifugation, the organic phases were combined with the first extract and the solvent was evaporated with nitrogen gas. The crude lipid extract was dissolved in 3 mL of CHCl3/CH3OH (2:1) and washed with 0.75 mL of 0.9% NaCl. The organic phase was again concentrated under N2 gas, and the lipids dissolved in CHCl3.
The crude lipid extract dissolved in chloroform was purified by solid-phase extraction (SPE) on silica columns (Strata Silica SI-1, Phenomenex) equilibrated with chloroform. After washing the column with chloroform, the glycine-glucolipid was eluted with acetone/isopropanol (9:1 [vol/vol]). Next, the glycolipid fraction was purified by separation on a silica thin-layer chromatography (TLC) plate (Machery Nagel Kieselgel 60 with concentration zone) developed with CHCl3/CH3OH/CH3COOH/water (90:15:10:3.5, vol/vol/vol/vol). Analytical plates were stained with 2.4% (wt/vol) α-naphthol in 10% sulfuric acid/80% ethanol to locate the glycolipids. For preparative separation, the TLC plates were stained with ammonium-8-anilino-1-naphthalinsulfonate (ANS; 0.2% in methanol) and observed under UV light. The silica material containing the glycine-glucolipid was isolated from the TLC plate, and lipids were extracted with CHCl3/CH3OH (2:1 [vol/vol]).
To unravel whether the glycine-glucolipid can be secreted, A. borkumensis cells were grown for 96 h in 100 mL ONR7a medium with 1% (wt/vol) pyruvate or with 1% (vol/vol) hexadecane. Half of the culture was extracted twice with ethyl acetate (1:1). The other half was centrifuged at 14,000 × g for 15 min. The cell pellet was extracted twice with 50 mL of ethyl acetate. The supernatant was purified by filtration (Filtropur V50; pore size, 0,45 μm; Sarstedt) and extracted twice with ethyl acetate (50 mL). The lipids in the ethyl acetate phases were dried under nitrogen gas and dissolved in 200 μL CHCl3. The glycine-glucolipid was purified by SPE (see above) and measured by HPLC-DAD or direct infusion mass spectrometry.
Glycolipid analysis by direct infusion mass spectrometry.
Total lipid extracts from A. borkumensis or samples collected from HPLC-DAD were dried under nitrogen gas and dissolved in chloroform/methanol/300 mM ammonium acetate (300:665:35 [vol/vol/vol]). The authentic glycine-glucolipid or its phenacyl esters were measured by direct infusion mass spectrometry (Agilent 6530 Accurate-Mass Q-TOF MS with chipcube nanospray source). Lipids were ionized in the positive mode yielding predominantly NH4+ adducts. The instrumental parameters were as follows: drying gas N2, 8 L/min; fragmentor voltage, 200 V; gas temperature, 300°C; HPLC-Chip Vcap, 1700 V; scan rate, 1 spectrum/s; fragmentation energy Glc(O-10:0)4Gly, 37.1 V; Glc(O-10:0)4Gly-phenacyl, 41.5 V. Data were processed using the Agilent MassHunter Qualitative Analysis software and Microsoft Office Excel. Glycolipid abundances were calculated using the peaks of the parental NH4+ adducts.
Fatty acid analysis by GC-MS.
The four 3-hydroxy fatty acids of the glycine-glucolipid can be quantified after methylation and trimethylsilylation by GC-MS in the presence of an internal standard. For the analysis of the crude lipid extract or the glycine-glycolipid purified by SPE and TLC, 50 or 5 μg, respectively, of internal standard (3-hydroxy-dodecanoic acid, 3-OH-12:0) were added, and the solvent was removed with N2 gas. The glycine-glucolipid was dissolved in 1 mL of 3 N methanolic HCl. Methylation was carried out at 80°C for 5 h to convert all 3-hydroxy-fatty acids into methyl esters. The samples were cooled down and 1 mL of hexane and 1 mL of 0.9% NaCl were added (37). After mixing and short centrifugation, the hexane phase was collected and dried with N2 gas. Free hydroxyl groups were silylated with 50 μL of N-methyl-N-(trimethylsilyl)-trifluoroacetamide (MSTFA) and 150 μL of pyridine by incubation at 80°C for 30 min. The solvent was evaporated under N2 gas and the derivatized 3-hydroxy-fatty acids were dissolved in hexane. Samples were injected into an Agilent GC-MS (7890A GC system with 5975C inert XL MSD) equipped with an Agilent HP-5MS column. The following temperature gradient was used: starting at 50°C, increased to 160°C by 25°C/min, to 170°C by 2°C/min, then to 250°C by 10°C/min, and finally decreased to 50°C by 40°C/min. The 3-hydroxy-fatty acids of the glycine-glucolipid (mostly HO-10:0 and HO-8:0) were quantified relative to the internal standard HO-12:0 considering the different response factors (RF), which were determined from the ratio of the peak area (A) to the amount (n, in nmol) determined by weighing.
Response factors (area/nmol) for HO-8:0, HO-10:0, HO-12:0 and HO-14:0 were 1,726,916; 4,499,168; 6,296,387; and 6,600,466 respectively.
The relative response factors (RRF) were calculated from the RF of the 3-hydroxy fatty acid relative to the internal standard.
The RF and RRF values were used to calculate the nmol amounts of 3-hydroxy fatty acids in the glycine-glucolipid. The total amount of glycine-glucolipid equals the sum of HO-8:0 and HO-10:0 divided by four.
Lipid analysis by HPLC-DAD.
The glycine-glucolipid was converted into a phenacyl ester for quantification according to a previous protocol with modifications (11). Undecanoic acid (11:0; 5 μg) was added to the lipid extract as an internal standard and the solvent evaporated under N2 gas. The lipid was dissolved in 100 μL of acetonitrile. Different concentrations and ratios of 2-bromoacetophenone and triethylamine were tested for derivatization (10:5, 15:7.5, 20:5, 20:10, 20:15, and 20:20; in mg/mL), and the yield of the derivatized glycine-glucolipid analyzed by HPLC. Best yields of derivatization were obtained by incubation with 15 mg/mL of 2-bromoacetophenone and 7.5 mg/mL of triethylamine, at 80°C for 1 h. The samples were centrifuged, and the supernatants with the glycine-glucolipid phenacyl esters were transferred to HPLC vials. After injection of 25 μL of the sample, lipids were separated by HPLC (Agilent 1100 series with diode array detector, DAD) on a reversed-phase column (Knauer C18, Eurospher II 100-3 C18A, 100 × 3 mm). The HPLC gradient was composed of solvent A (0.01 N H3P04) and solvent B (acetonitrile) with 50% B for 5 min; from 50% B to 100% B in 20 min; and from 100% B to 50% B in 10 min, at a flow rate of 1.0 mL/min. The absorbance of the derivatized glycine-glucolipid was measured at a wavelength of 244 nm.
The 3-hydroxy-fatty acids of an aliquot of the purified glycine-glucolipid were quantified via GC-MS, and the amount n(glycolipid GCMS) was calculated according to the equations shown above. After measuring the same amount of the glycine-glucolipid by HPLC-DAD, the response factor in HPLC-DAD was calculated:
The response factors (area/nmol) for 11:0 and the glycine-glucolipid were 115 and 170, respectively.
The amount of a molecular species n(Glc(O-10:0)4Gly) of an unknown glycine-glucolipid preparation can be measured by HPLC-DAD and calculated according to the peak area related to the internal standard undecanoic acid (11:0, 5 μg), considering the relative response factor of the glycine-glucolipid:
The total amount of glycine-glucolipid measured by HPLC-DAD equals the sum of the amounts of the individual molecular species:
ACKNOWLEDGMENTS
This work was funded by the Bundesministerium für Bildung und Forschung (BMBF, BioProMare, GlycoX project; grant no. 031B0866A, 031B0866B, and 031B0866C).
We thank Yein Cho (University of Bonn) for help with the optimization of the HPLC method.
Footnotes
[This article was published on 8 August 2022 with ORCID IDs not given for Georg Hölzl, Tobias Karmainski, Sonja Kubicki, Lars M. Blank, and Karl-Erich Jaeger. These ORCID IDs were added in the current version, posted on 23 August 2022.]
Contributor Information
Peter Dörmann, Email: doermann@uni-bonn.de.
Laura Villanueva, Royal Netherlands Institute for Sea Research.
REFERENCES
- 1.Desai JD, Banat IM. 1997. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64. 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang S, Wullbrandt D. 1999. Rhamnose lipids–biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol 51:22–32. 10.1007/s002530051358. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi L, Irorere VU, Marchant R, Banat IM. 2018. Marine derived biosurfactants: a vast potential future resource. Biotechnol Lett 40:1441–1457. 10.1007/s10529-018-2602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ron EZ, Rosenberg E. 2001. Natural roles of biosurfactants. Environ Microbiol 3:229–236. 10.1046/j.1462-2920.2001.00190.x. [DOI] [PubMed] [Google Scholar]
- 5.Banat IM, Makkar RS, Cameotra SS. 2000. Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508. 10.1007/s002530051648. [DOI] [PubMed] [Google Scholar]
- 6.Marchant R, Banat IM. 2017. Protocols for measuring biosurfactant production in microbial cultures, p 119–128. In McGenity TJ, Timmis KN, Nogales B (ed), Hydrocarbon and lipid microbiology protocols, vol 34. Springer, Berlin, Heidelberg. [Google Scholar]
- 7.Schenk T, Schuphan I, Schmidt B. 1995. High-performance liquid chromatographic determination of the rhamnolipids produced by Pseudomonas aeruginosa. J Chromatogr A 693:7–13. 10.1016/0021-9673(94)01127-z. [DOI] [PubMed] [Google Scholar]
- 8.Syldatk C, Lang S, Wagner F, Wray V, Witte L. 1985. Chemical and physical characterization of four interfacial-active rhamnolipids from Pseudomonas spec. DSM 2874 grown on n-alkanes. Z Naturforsch C Biosci 40:51–60. 10.1515/znc-1985-1-212. [DOI] [PubMed] [Google Scholar]
- 9.Parra J, Pastor J, Comelles F, Manresa M, Bosch MP. 1990. Studies of biosurfactants obtained from olive oil. Tens Surf Det 27:302–306. 10.1515/tsd-1990-270509. [DOI] [Google Scholar]
- 10.Behrens B, Baune M, Jungkeit J, Tiso T, Blank LM, Hayen H. 2016. High performance liquid chromatography-charged aerosol detection applying an inverse gradient for quantification of rhamnolipid biosurfactants. J Chromatogr A 1455:125–132. 10.1016/j.chroma.2016.05.079. [DOI] [PubMed] [Google Scholar]
- 11.Mata-Sandoval JC, Karns J, Torrents A. 1999. High-performance liquid chromatography method for the characterization of rhamnolipid mixtures produced by Pseudomonas aeruginosa UG2 on corn oil. J Chromatogr A 864:211–220. 10.1016/s0021-9673(99)00979-6. [DOI] [PubMed] [Google Scholar]
- 12.Yakimov MM, Golyshin PN, Lang S, Moore ER, Abraham WR, Lünsdorf H, Timmis KN. 1998. Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int J Syst Bacteriol 48 Pt 2:339–348. 10.1099/00207713-48-2-339. [DOI] [PubMed] [Google Scholar]
- 13.Harayama S, Kishira H, Kasai Y, Shutsubo K. 1999. Petroleum biodegradation in marine environments. J Mol Microbiol Biotechnol 1:63–70. [PubMed] [Google Scholar]
- 14.Harayama S, Kasai Y, Hara A. 2004. Microbial communities in oil-contaminated seawater. Curr Opin Biotechnol 15:205–214. 10.1016/j.copbio.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Abraham W-R, Meyer H, Yakimov M. 1998. Novel glycine containing glucolipids from the alkane using bacterium Alcanivorax borkumensis. Biochim Biophys Acta 1393:57–62. 10.1016/s0005-2760(98)00058-7. [DOI] [PubMed] [Google Scholar]
- 16.Passeri A, Schmidt M, Haffner T, Wray V, Lang S, Wagner F. 1992. Marine biosurfactants. IV. Production, characterization and biosynthesis of an anionic glucose lipid from the marine bacterial strain MM1. Appl Microbiol Biotechnol 37:281–286. 10.1007/BF00210978. [DOI] [Google Scholar]
- 17.Martins dos Santos V, Sabirova J, Timmis KN, Yakimov MM, Golyshin PN. 2010. Alcanivorax borkumensis, p 1265–1288. In Timmis KN (ed), Handbook of hydrocarbon and lipid microbiology, vol 1393. Springer Berlin Heidelberg, Berlin, Heidelberg. [Google Scholar]
- 18.Durst HD, Milano M, Kikta EJ, Connelly SA, Grushka E. 1975. Phenacyl esters of fatty acids via crown ether catalysts for enhanced ultraviolet detection in liquid chromatography. Anal Chem 47:1797–1801. 10.1021/ac60361a025. [DOI] [PubMed] [Google Scholar]
- 19.Schneiker S, Martins dos Santos VAP, Bartels D, Bekel T, Brecht M, Buhrmester J, Chernikova TN, Denaro R, Ferrer M, Gertler C, Goesmann A, Golyshina OV, Kaminski F, Khachane AN, Lang S, Linke B, McHardy AC, Meyer F, Nechitaylo T, Pühler A, Regenhardt D, Rupp O, Sabirova JS, Selbitschka W, Yakimov MM, Timmis KN, Vorhölter F-J, Weidner S, Kaiser O, Golyshin PN. 2006. Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nat Biotechnol 24:997–1004. 10.1038/nbt1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minnikin DE, Abdolrahimzadeh H, Baddiley J. 1974. Replacement of acidic phospholipids by acidic glycolipids in Pseudomonas diminuta. Nature 249:268–269. 10.1038/249268a0. [DOI] [PubMed] [Google Scholar]
- 21.Hölzl G, Dörmann P. 2007. Structure and function of glycoglycerolipids in plants and bacteria. Prog Lipid Res 46:225–243. 10.1016/j.plipres.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Kubicki S, Bator I, Jankowski S, Schipper K, Tiso T, Feldbrügge M, Blank LM, Thies S, Jaeger K-E. 2020. A straightforward assay for screening and quantification of biosurfactants in microbial culture supernatants. Front Bioeng Biotechnol 8:958. 10.3389/fbioe.2020.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubicki S, Bollinger A, Katzke N, Jaeger K-E, Loeschcke A, Thies S. 2019. Marine biosurfactants: biosynthesis, structural diversity and biotechnological applications. Mar Drugs 17:408. 10.3390/md17070408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godfrin MP, Sihlabela M, Bose A, Tripathi A. 2018. Behavior of marine bacteria in clean environment and oil spill conditions. Langmuir 34:9047–9053. 10.1021/acs.langmuir.8b01319. [DOI] [PubMed] [Google Scholar]
- 25.Behrens B, Engelen J, Tiso T, Blank LM, Hayen H. 2016. Characterization of rhamnolipids by liquid chromatography/mass spectrometry after solid-phase extraction. Anal Bioanal Chem 408:2505–2514. 10.1007/s00216-016-9353-y. [DOI] [PubMed] [Google Scholar]
- 26.Babel W, Ackermann J-U, Breuer U. 2001. Physiology, Regulation, and Limits of the Synthesis of Poly(3HB), p 125–157. In Babel W, Steinbüchel A (ed), Biopolyesters. Springer Berlin Heidelberg, Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- 27.Sabirova JS, Ferrer M, Lünsdorf H, Wray V, Kalscheuer R, Steinbüchel A, Timmis KN, Golyshin PN. 2006. Mutation in a “tesB-like” hydroxyacyl-coenzyme A-specific thioesterase gene causes hyperproduction of extracellular polyhydroxyalkanoates by Alcanivorax borkumensis SK2. J Bacteriol 188:8452–8459. 10.1128/JB.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel-Mawgoud AM, Lépine F, Déziel E. 2010. Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336. 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naether DJ, Slawtschew S, Stasik S, Engel M, Olzog M, Wick LY, Timmis KN, Heipieper HJ. 2013. Adaptation of the hydrocarbonoclastic bacterium Alcanivorax borkumensis SK2 to alkanes and toxic organic compounds: a physiological and transcriptomic approach. Appl Environ Microbiol 79:4282–4293. 10.1128/AEM.00694-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbato M, Scoma A, Mapelli F, de Smet R, Banat IM, Daffonchio D, Boon N, Borin S. 2016. Hydrocarbonoclastic Alcanivorax isolates exhibit different physiological and expression responses to n-dodecane. Front Microbiol 7:2056. 10.3389/fmicb.2016.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manilla-Pérez E, Reers C, Baumgart M, Hetzler S, Reichelt R, Malkus U, Kalscheuer R, Wältermann M, Steinbüchel A. 2010. Analysis of lipid export in hydrocarbonoclastic bacteria of the genus Alcanivorax: identification of lipid export-negative mutants of Alcanivorax borkumensis SK2 and Alcanivorax jadensis T9. J Bacteriol 192:643–656. 10.1128/JB.00700-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalscheuer R, Stöveken T, Malkus U, Reichelt R, Golyshin PN, Sabirova JS, Ferrer M, Timmis KN, Steinbüchel A. 2007. Analysis of storage lipid accumulation in Alcanivorax borkumensis: Evidence for alternative triacylglycerol biosynthesis routes in bacteria. J Bacteriol 189:918–928. 10.1128/JB.01292-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulz D, Passeri A, Schmidt M, Lang S, Wagner F, Wray V, Gunkel W. 1991. Marine biosurfactants, I. Screening for biosurfactants among crude oil degrading marine microorganisms from the North Sea. Z Naturforsch C J Biosci 46:197–203. 10.1515/znc-1991-3-407. [DOI] [PubMed] [Google Scholar]
- 34.Abbasi A, Bothun GD, Bose A. 2018. Attachment of Alcanivorax borkumensis to hexadecane-In-artificial sea water emulsion droplets. Langmuir 34:5352–5357. 10.1021/acs.langmuir.8b00082. [DOI] [PubMed] [Google Scholar]
- 35.Bator I, Karmainski T, Tiso T, Blank LM. 2020. Killing two birds with one stone - Strain engineering facilitates the development of a unique rhamnolipid production process. Front Bioeng Biotechnol 8:899. 10.3389/fbioe.2020.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiso T, Ihling N, Kubicki S, Biselli A, Schonhoff A, Bator I, Thies S, Karmainski T, Kruth S, Willenbrink A-L, Loeschcke A, Zapp P, Jupke A, Jaeger K-E, Büchs J, Blank LM. 2020. Integration of genetic and process engineering for optimized rhamnolipid production using Pseudomonas putida. Front Bioeng Biotechnol 8:976. 10.3389/fbioe.2020.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Browse J, McCourt PJ, Somerville CR. 1986. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152:141–145. 10.1016/0003-2697(86)90132-6. [DOI] [PubMed] [Google Scholar]






