ABSTRACT
Acetovanillone is a major aromatic monomer produced in oxidative/base-catalyzed lignin depolymerization. However, the production of chemical products from acetovanillone has not been explored due to the lack of information on the microbial acetovanillone catabolic system. Here, the acvABCDEF genes were identified as specifically induced genes during the growth of Sphingobium sp. strain SYK-6 cells with acetovanillone and these genes were essential for SYK-6 growth on acetovanillone and acetosyringone (a syringyl-type acetophenone derivative). AcvAB and AcvF produced in Escherichia coli phosphorylated acetovanillone/acetosyringone and dephosphorylated the phosphorylated acetovanillone/acetosyringone, respectively. AcvCDE produced in Sphingobium japonicum UT26S carboxylated the reaction products generated from acetovanillone/acetosyringone by AcvAB and AcvF into vanilloyl acetic acid/3-(4-hydroxy-3,5-dimethoxyphenyl)-3-oxopropanoic acid. To demonstrate the feasibility of producing cis,cis-muconic acid from acetovanillone, a metabolic modification on a mutant of Pseudomonas sp. strain NGC7 that accumulates cis,cis-muconic acid from catechol was performed. The resulting strain expressing vceA and vceB required for converting vanilloyl acetic acid to vanillic acid and aroY encoding protocatechuic acid decarboxylase in addition to acvABCDEF successfully converted 1.2 mM acetovanillone to approximately equimolar cis,cis-muconic acid. Our results are expected to help improve the yield and purity of value-added chemical production from lignin through biological funneling.
IMPORTANCE In the alkaline oxidation of lignin, aromatic aldehydes (vanillin, syringaldehyde, and p-hydroxybenzaldehyde), aromatic acids (vanillic acid, syringic acid, and p-hydroxybenzoic acid), and acetophenone-related compounds (acetovanillone, acetosyringone, and 4′-hydroxyacetophenone) are produced as major aromatic monomers. Also, base-catalyzed depolymerization of guaiacyl lignin resulted in vanillin, vanillic acid, guaiacol, and acetovanillone as primary aromatic monomers. To date, microbial catabolic systems of vanillin, vanillic acid, and guaiacol have been well characterized, and the production of value-added chemicals from them has also been explored. However, due to the lack of information on the microbial acetovanillone and acetosyringone catabolic system, chemical production from acetovanillone and acetosyringone has not been achieved. This study elucidated the acetovanillone/acetosyringone catabolic system and demonstrates the potential of using these genes for the production of value-added chemicals from these compounds.
KEYWORDS: Sphingobium sp. strain SYK-6, acetophenone, biotin-dependent carboxylase, cis, cis-muconic acid, lignin
INTRODUCTION
The use of lignocellulosic biomass is expected to build a decarbonized society that breaks away from fossil resources. The development of lignin utilization has become particularly important in the utilization of lignocellulose. Lignin content in lignocellulose ranges from 9 to 32% (1), and gymnosperm (softwood) lignins are composed of guaiacyl (G) units with small amounts of p-hydroxyphenyl (H) units, whereas angiosperm (hardwood) lignins are composed of G units and syringyl (S) units (2, 3). Grass lignins contain G and S units and more H units than gymnosperm lignins (2, 3). Because of lignin’s complex and heterogeneous structure, it is difficult to selectively extract specific compounds even when it is degraded, and it has been ineffectively used so far (1, 4). Recently, “biological funneling,” in which a heterogeneous mixture of low-molecular-weight aromatic compounds obtained from chemocatalytic depolymerization of lignin is converged to specific value-added chemicals, such as cis,cis-muconic acid (ccMA) and 2-pyrone-4,6-dicarboxylic acid using microbial catabolic functions, has attracted attention (5–12).
In the alkaline oxidation of lignin, aromatic aldehydes (vanillin, syringaldehyde, and p-hydroxybenzaldehyde), aromatic acids (vanillic acid, syringic acid, and p-hydroxybenzoic acid), and acetophenone-related compounds (acetovanillone [AV], acetosyringone [AS; a syringyl-type acetophenone derivative], and 4′-hydroxyacetophenone [a p-hydroxyphenyl-type acetophenone derivative]) are produced as major aromatic monomers (13–15). Base-catalyzed Indulin AT (a pine kraft lignin) depolymerization resulted in the formation of vanillin, vanillic acid, guaiacol, and AV as major aromatic monomers, similar to the alkaline oxidation of Lignoboost lignin (a softwood kraft technical lignin) (8, 16–19). A black liquor produced in a softwood kraft pulping process, Lignoforce, contained guaiacol, vanillin, and AV as major aromatic monomers (20). Among these major aromatic monomers, microbial catabolic systems of vanillin, vanillic acid, and guaiacol have been characterized (21–29). In addition, value-added chemical production from vanillin, vanillic acid, and guaiacol has also been explored (12, 18, 30–36). However, due to the lack of information on the microbial AV catabolic system, chemical production from AV has not been reported (16, 18, 19). Therefore, chemical production from all of the major aromatic monomers produced by oxidative/base-catalyzed lignin depolymerization has been unachieved. Recently, Eltis and coworkers isolated Rhodococcus rhodochrous GD01 and GD02, which can utilize AV in addition to vanillin, vanillic acid, and guaiacol in the black liquor produced during the Lignoforce kraft pulping of softwood (20). Based on the observation that apkC was induced when GD02 was cultured in black liquor extracts containing AV, the genes apkA, apkB, and apkC, presumed to encode biotin-dependent carboxylase, were predicted to be involved in AV catabolism. However, the enzymatic system encoded by these genes is unknown. In addition, in Arthrobacter sp. strain TGJ4, 4′-hydroxyacetophenone and AV are converted to 4-hydroxybenzoic acid and vanillic acid, respectively; however, the enzymatic system has been unclarified (37).
Sphingobium sp. strain SYK-6 can utilize various lignin-derived dimers, such as β-aryl ether, biphenyl, phenylcoumaran, and diarylpropane, as well as monomers, including ferulic acid, vanillin, vanillic acid, and AV (11, 16, 20, 38, 39). In SYK-6 cells, stereoisomers of β-aryl ether model compound, guaiacylglycerol-β-guaiacyl ether, are stereospecifically converted to achiral β-hydroxypropiovanillone (HPV) via Cα-oxidation, ether cleavage, and glutathione removal (Fig. 1) (38, 40–44). HPV and β-hydroxypropiosyringone (HPS; a syringyl-type β-aryl ether metabolite) are oxidized to vanilloyl acetic acid (VAA) and 3-(4-hydroxy-3,5-dimethoxyphenyl)-3-oxopropanoic acid (SAA), respectively, and further catabolized via vanillic acid and syringic acid. (Fig. 1) (45). In addition, we identified vceA and vceB, which encode an acetyl coenzyme A (acetyl-CoA)-dependent VAA/SAA-converting enzyme and a vanilloyl-CoA/syringoyl-CoA thioesterase, respectively (5). However, these enzyme genes were found to be not essential for VAA/SAA catabolism in SYK-6. It has been reported that VAA is chemically unstable and can be converted to AV by a nonenzymatic decarboxylation (46, 47). Indeed, when an SYK-6 cell extract was incubated with HPV, a small amount of AV, along with VAA, was generated (Fig. 1) (45).
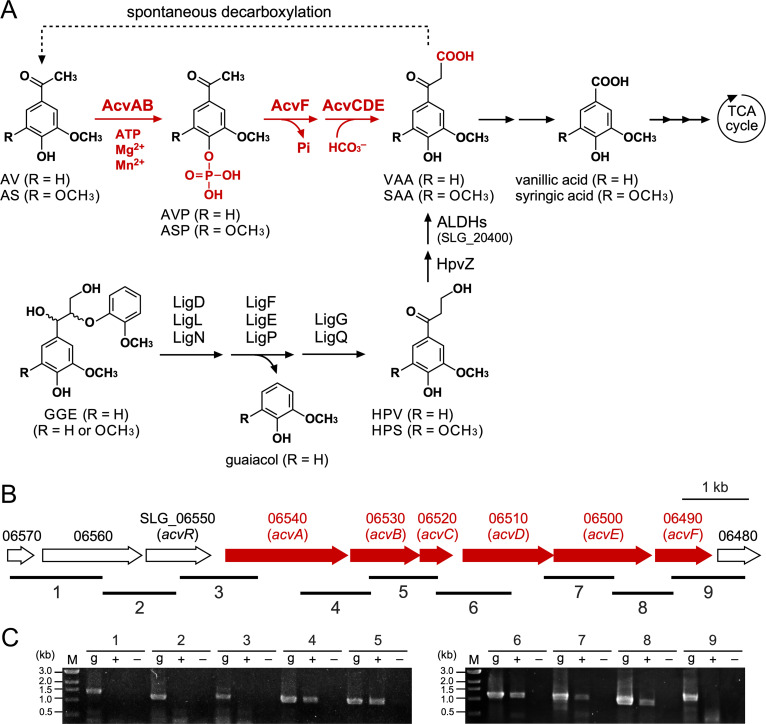
FIG 1.
(A) Catabolic pathway of AV and AS in Sphingobium sp. strain SYK-6. The pathways for both guaiacyl (R = H)- and syringyl (R = OCH3)-type compounds are shown. VAA, an intermediate metabolite of GGE, has been suggested to be spontaneously decarboxylated to AV (45, 46). Enzymes: AcvAB, AVP/ASP synthetase; AcvF, AVP/ASP phosphatase; AcvCDE, biotin-dependent carboxylase; LigD, LigL, and LigN, Cα-dehydrogenases; LigF, LigE, and LigP, β-etherases; LigG and LigQ, glutathione S-transferases; HpvZ, HPV/HPS oxidase; ALDHs, aldehyde dehydrogenases; SLG_20400, vanilloyl acetaldehyde dehydrogenase. AV, acetovanillone; AS, acetosyringone; AVP, 4-acetyl-2-methoxyphenylphosphate; ASP, 4-acetyl-2,6-dimethoxyphenylphosphate; VAA, vanilloyl acetic acid; SAA, 3-(4-hydroxy-3,5-dimethoxyphenyl)-3-oxopropanoic acid; GGE, guaiacylglycerol-β-guaiacyl ether; HPV, β-hydroxypropiovanillone; HPS, β-hydroxypropiosyringone. (B) Gene organization of acvABCDEF. Arrows indicate the genes from SLG_06570 to SLG_06480. (C) RT-PCR analysis of acvABCDEF. Total RNA used for cDNA synthesis was isolated from SYK-6 cells grown in Wx-SEMP containing 5 mM AV. The regions to be amplified are indicated by black bars below the genetic map. Lanes: M, molecular size markers; g, control PCR with the SYK-6 genomic DNA; + and −, RT-PCR with and without reverse transcriptase, respectively.
Here, the catabolic pathway of AV and AS in SYK-6 was determined, an SYK-6 gene cluster consisting of six novel genes involved in AV and AS catabolism was identified, and their functions were clarified (Fig. 1). Furthermore, these genes were expressed in a derivative strain of Pseudomonas sp. strain NGC7 (34), which is expected to be a chassis microorganism for converting lignin-derived aromatic compounds, and this engineered strain successfully produced ccMA from AV.
RESULTS
Determination of AV catabolism pathway in Sphingobium sp. strain SYK-6.
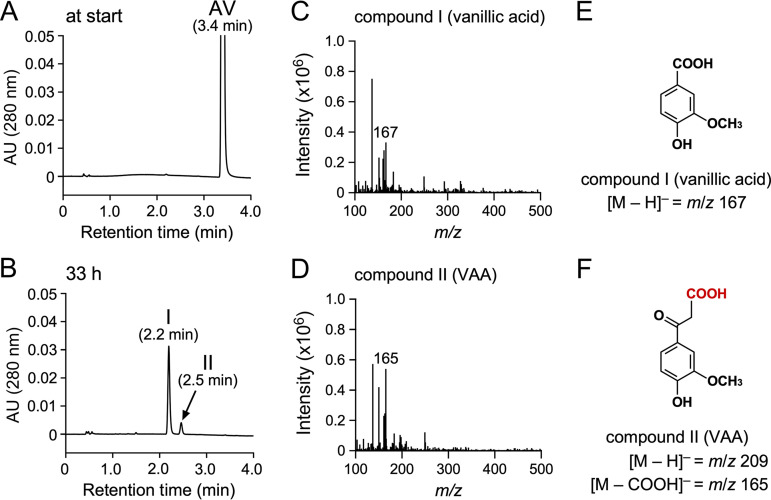
Intermediate metabolites generated during SYK-6 incubation with AV were identified to determine the catabolic pathway of AV in SYK-6. SYK-6 cells grown with AV were incubated with 1 mM AV in Wx minimal medium (48) for 33 h, and high-performance liquid chromatography-mass spectrometry (HPLC-MS) analyzed the supernatant of the reaction mixture. This analysis indicated that AV disappeared and was converted into compounds I and II with retention times of 2.2 and 2.5 min, respectively (Fig. 2A and B). Based on the comparison of the retention time and the mass spectrum of compound I with those of the authentic sample, this compound was identified as vanillic acid (molecular weight [MW], 168) (Fig. 2C and E; see also Fig. S1 in the supplemental material). Furthermore, compound II was found to be VAA (MW, 210) by comparing with the retention time and the mass spectrum of the authentic sample (Fig. 2D; see also Fig. S1). These results suggest that AV was carboxylated, converted to VAA, and catabolized via vanillic acid in SYK-6 (Fig. 1).
FIG 2.
HPLC-MS analysis of AV metabolites. Cells of SYK-6 grown with AV (OD600 = 0.2) were incubated with 1 mM AV in Wx medium. Portions of the reaction mixtures were collected at the start (A) and after 33 h (B) of incubation and then analyzed by HPLC-MS. The ESI-MS spectra of compounds I and II (negative mode) are shown in panels C and D, respectively. (E and F) Chemical structures of compound I (vanillic acid) and compound II (VAA), respectively.
Search for genes involved in the conversion of AV using microarray analysis.
The AV conversion rate was measured using SYK-6 cells grown in Wx containing 10 mM sucrose, 10 mM glutamic acid, 0.13 mM methionine, and 10 mM proline (Wx-SEMP; the noninducing condition) and Wx-SEMP containing 5 mM AV (the inducing condition) to investigate the inducibility of converting AV in SYK-6. The conversion rate of 1 mM AV by cells grown under the inducing condition was ~27-fold higher than that of cells grown under the noninducing condition (see Fig. S2). Thus, the AV-converting enzyme gene(s) was induced by AV and/or its intermediates.
The AV-converting enzyme gene(s) was searched based on the induction profiles of the entire SYK-6 genes analyzed using data from a previous DNA microarray experiment (49). This analysis indicated that six consecutive genes consisting of SLG_06540, SLG_06530, SLG_06520, SLG_06510, SLG_06500, and SLG_06490 were induced 17- to 76-fold, during SYK-6 growth with AV (Fig. 1B; see also Table S1). Among these genes, SLG_06540 and SLG_06530 showed 44 and 41% amino acid sequence identities, respectively, with proteins 1 and 2, which comprise phenylphosphate synthase involved in biotin and thiamine diphosphate-independent phenol carboxylation in the process of anaerobic phenol metabolism by Thauera aromatica K172 (see Table S2) (50–53). SLG_06520 and SLG_06510 showed 36 and 50% amino acid sequence identities with a biotin carboxyl carrier protein (BCCP; AccB) and biotin carboxylase (BC; AccC) of biotin-dependent acetyl-CoA carboxylase of Bacillus subtilis 168, respectively (see Table S2) (54). SLG_06500 showed 26% amino acid sequence identity with the carboxyltransferase (CT; PycB) of biotin-dependent pyruvic acid carboxylase of Methanothermobacter thermautotrophicus ΔH (see Table S2) (55). These facts suggest that SLG_06520, SLG_06510, and SLG_06500 encode BCCP, BC, and CT, of biotin-dependent carboxylase, respectively (56). The proteomic analysis of the Aromatoleum aromaticum EbN1 grown with 4′-hydroxyacetophenone revealed the upregulation of XccA and XccB, presumably biotin-dependent carboxylase components, and xccA, xccC, and xccB are predicted to be involved in 4′-hydroxyacetophenone carboxylation (57). XccA (CT), XccC (BC), and XccB (BCCP) have 55, 63, and 45% amino acid sequence identities with SLG_06500, SLG_06510, and SLG_06520, respectively (see Table S2). Furthermore, SLG_06500, SLG_06510, and SLG_06520 exhibited 42, 51, and 38% amino acid sequence identities, respectively, with apkA (CT), apkB (BC), and apkC (BCCP), the recently predicted AV catabolic enzyme genes in R. rhodochrous GD02 (see Table S2) (20). SLG_06490 showed 23% amino acid sequence identity with NagD, a ribonucleotide monophosphatase of E. coli K-12, belonging to the haloacid dehydrogenase (HAD) superfamily (see Table S2) (58).
In T. aromatica K172, phenol is phosphorylated to phenylphosphate by phenylphosphate synthase (proteins 1 and 2) before the carboxylation, facilitated by an accessory protein (protein 3) (50–52). Subsequently, the core enzyme composed of α, β, and γ components catalyzing the carboxylation using CO2 as a substrate and the δ subunit catalyzing the dephosphorylation are involved in converting phenylphosphate into 4-hydroxybenzoic acid via phenolate anion (53, 59, 60). Based on the findings presented above, AV carboxylation in SYK-6 was expected to proceed as follows. (i) Putative 4-acetyl-2-methoxyphenylphosphate (AVP) synthetase encoded by SLG_06540 and SLG_06530 phosphorylates AV. (ii) Putative phosphatase encoded by SLG_06490 dephosphorylates the phosphorylated AV. (iii) Putative biotin-dependent carboxylase encoded by SLG_06520, SLG_06510, and SLG_06500 carboxylates the AV dephosphorylated anion intermediate.
Reverse transcription-PCR (RT-PCR) analysis was conducted using total RNA prepared from the SYK-6 cells grown in the presence of AV to examine whether SLG_06540–SLG_06490 form a transcription unit. Specific amplification was observed from SLG_06540 to SLG_06490, indicating that SLG_06540−SLG_06490 form an operon (Fig. 1B and C).
SLG_06540–SLG_06490 are involved in AV and AS catabolism.
To examine whether SLG_06540 and SLG_06490 (SLG_06540−SLG_06490) are involved in AV catabolism in SYK-6, disruption mutants of SLG_06540−SLG_06490 (Δ06540–Δ06490) were created via homologous recombination (see Fig. S3). The growth of Δ06540–Δ06490 cells on AV was evaluated. Since SYK-6 cannot grow at a concentration of several millimolar of AV, 1 mM AV was added to the Wx medium at the beginning of cultivation, and another 1 mM AV was added after 52 h of incubation. The optical density at 660 nm (OD660) of SYK-6 increased after 20 h of cultivation, and further growth was observed when AV was added after 52 h. In contrast, all mutants completely lost their capacity to grow on AV (see Fig. S4A). In addition, each mutant also completely lost the capacity to grow on AS (see Fig. S4B). These results indicate that SLG_06540–SLG_06490 are essential for AV and AS catabolism; thus, we designated these genes acvA to acvF.
SLG_06550 is the transcriptional regulator of acvABCDEF.
SLG_06550, located just upstream of acvA, showed 25% amino acid sequence identity with NphR, an AraC-type transcriptional regulator that positively regulates the 4-nitrophenol monooxygenase gene (nphA1A2) of Rhodococcus sp. strain PN1 (61). To examine whether SLG_06550 is involved in the transcriptional acvABCDEF regulation in SYK-6, an SLG_06550 disruption mutant (Δ06550) was created and its capacity to grow on Wx medium containing AV was examined (see Fig. S5A and B). Δ06550 completely lost its capacity to grow on AV (see Fig. S5C), suggesting that SLG_06550 positively regulates the acvABCDEF operon, and this gene was named acvR.
acvABCDEF confers a host strain the capacity to carboxylate AV and AS.
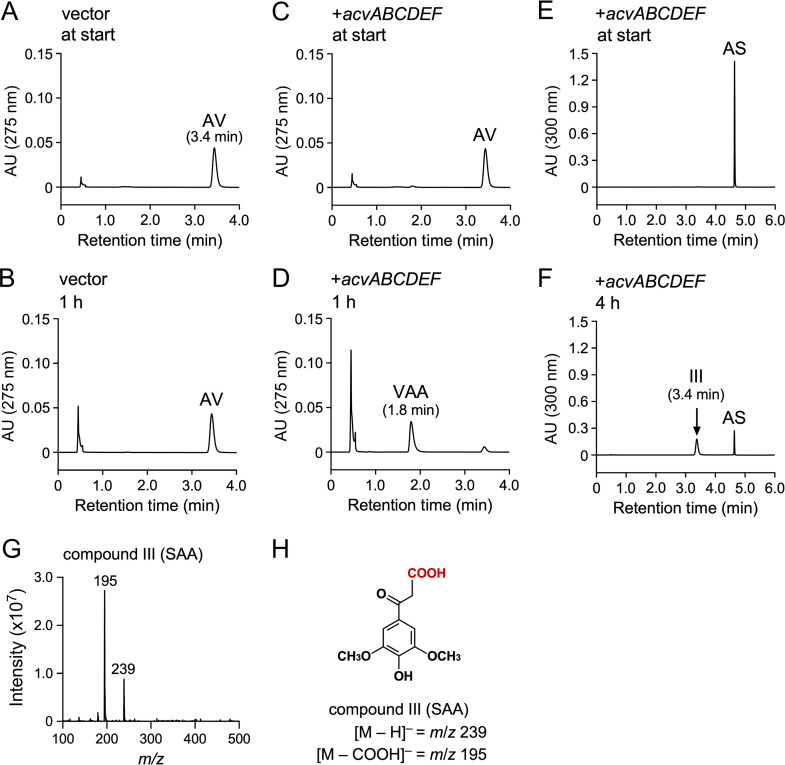
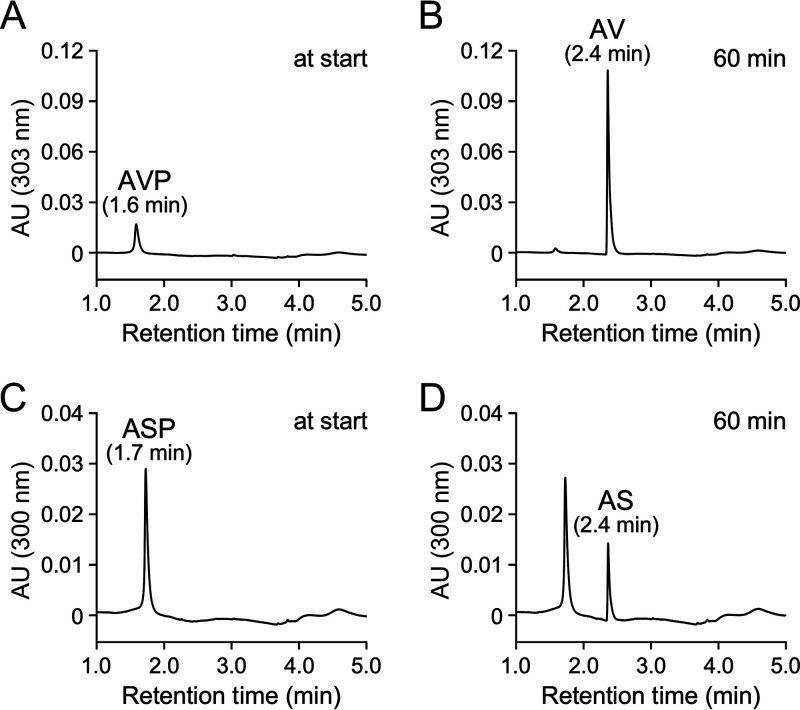
To examine whether acvABCDEF encode the AV and AS carboxylase system, a plasmid (pJBacv) carrying acvABCDEF in pJB861 was introduced into a host strain, Sphingobium japonicum UT26S, which is incapable of AV and AS conversion. The UT26S genome contains genes that show 32% (SJA_C1-33200) and 50% (SJA_C1-33210) amino acid sequence identity with acvC and acvD, encoding putative BCCP and BC, respectively, but no ortholog of acvA and acvB (encoding putative AVP synthetase), acvE (encoding putative CT), and acvF (encoding putative phosphatase). The resting cells of UT26S expressing acvABCDEF were incubated with 200 μM AV for 1 h. HPLC-MS analysis showed that VAA was produced (Fig. 3A to D). When the same resting cells were incubated with 200 μM AS for 4 h, compound III with a retention time of 3.4 min was produced (Fig. 3E and F). Negative electrospray ionization-MS (ESI-MS) analysis of compound III showed a major fragment at m/z 239 (Fig. 3G and H), suggesting that compound III was SAA (MW, 240). These results indicate that acvABCDEF encodes components of the carboxylase system required for AV and AS catabolism.
FIG 3.
Conversions of AV and AS by resting cells of S. japonicum UT26S carrying acvABCDEF. Resting cells of UT26S harboring pJB861 (OD600 = 10.0; A and B) and resting cells of UT26S harboring pJBacv (OD600 = 10.0; C to F) were incubated with AV (200 μM; A to D) or AS (200 μM; E and F). Portions of the reaction mixtures were collected at the start (A, C, and E), after 1 h (B and D), and after 4 h (F) of incubation and analyzed by HPLC-MS. The ESI-MS spectrum of compound III (negative mode) is shown in panel G. (H) Chemical structure of compound III (SAA).
A mixture of AcvA and AcvB catalyzes the phosphorylation of AV and AS.
Proteins 1 and 2 (phenylphosphate synthase) of T. aromatica K172, which show amino acid sequence similarity with acvA and acvB, respectively, convert phenol to phenylphosphate when both are present in the assay (51). Mg-ATP is essential as a phosphoryl donor for this conversion and Mn2+ promotes catalytic activity (51). Each acvA and acvB fused with a His tag at the 5′ terminus was expressed in E. coli to characterize the function of acvA and acvB. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed the production of 71- and 40-kDa proteins in cell extracts of E. coli expressing His-tagged acvA and acvB, respectively (see Fig. S6).
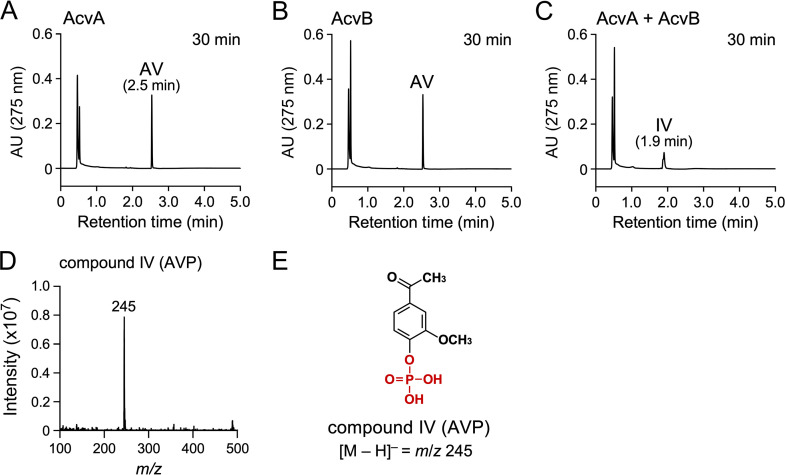
Crude AcvA (500 μg/mL), AcvB (500 μg/mL), and AcvA+AcvB (500 μg/mL each) reacted with 200 μM AV, respectively, in the presence of 2 mM ATP, 2 mM MgCl2, and 200 μM MnCl2. HPLC-MS analysis showed that AV was completely converted into compound IV (1.9 min) after 30 min of incubation when AcvA and AcvB were added together (Fig. 4A to C). Negative ESI-MS analysis of compound IV showed a fragment at m/z 245. Compound IV was identified as AVP (MW, 246) based on the molecular weight deduced from the fragment ion (Fig. 4D and E). These data prove that AcvAB phosphorylated the hydroxy group of AV.
FIG 4.
Conversion of AV by crude AcvA and AcvB. AV (200 μM) was incubated with a mixture of cell extracts of E. coli BL21(DE3) harboring pE16acvA and E. coli BL21(DE3) harboring pET-16b (500 μg protein/mL each; A), a mixture of cell extracts of E. coli BL21(DE3) harboring pE16acvB and E. coli BL21(DE3) harboring pET-16b (500 μg protein/mL each; B), and a mixture of cell extracts of E. coli BL21(DE3) harboring pE16acvA and E. coli BL21(DE3) harboring pE16acvB (500 μg protein/mL each; C). Reactions were performed in the presence of 2 mM ATP, 2 mM MgCl2, and 200 μM MnCl2. Portions of the reaction mixtures were collected after 30 min of incubation and analyzed by HPLC. The ESI-MS spectrum of compound IV (negative mode) is shown in panel D. (E) Chemical structure of compound IV (AVP).
AcvA and AcvB were purified to near homogeneity by Ni affinity chromatography from the cell extracts of E. coli expressing His-tagged acvA and acvB, respectively (see Fig. S6). However, because the specific activity of purified AcvA+AcvB was lower than that of crude AcvA+AcvB, the AcvAB enzyme properties were investigated using crude enzymes.
To examine the AcvAB cofactor requirement, crude AcvA+AcvB (50 to 1,000 μg protein/mL each) was incubated with 100 μM AV in the presence and absence of cofactors (2 mM ATP, 2 mM MgCl2, 200 μM MnCl2, 2 mM ATP + 2 mM MgCl2, 2 mM ATP + 200 μM MnCl2, 2 mM MgCl2 + 200 μM MnCl2, and 2 mM ATP + 2 mM MgCl2 + 200 μM MnCl2). The highest activity (ca. 111 nmol min−1 mg−1) was obtained in the presence of ATP + Mg2+ + Mn2+, whereas 42 and 3% activities were observed with ATP + Mg2+ and ATP + Mn2+, respectively. No activity was observed in the presence of the other cofactors. These results indicate that AcvAB used Mg-ATP as a phosphoryl donor for AV phosphorylation similar to the phenylphosphate synthase of T. aromatica K172, and that phosphorylation activity is promoted in the presence of Mn2+.
Crude AcvA+AcvB (10 to 500 μg protein/mL each) was incubated with 100 μM AV, AS, acetophenone, 4′-hydroxyacetophenone, 3′-hydroxyacetophenone, 3′,4′-dihydroxyacetophenone, 3′-hydroxy-4′-methoxyacetophenone, 3′,4′-dimethoxyacetophenone, 3′,4′,5′-trimethoxyacetophenone, 4′-hydroxypropiophenone, 4′-hydroxybuthyrophenone, 4′-hydroxyvalerophenone, guaiacol, vanillic acid, 4-hydroxybenzoic acid, and phenol, respectively, in the presence of 2 mM ATP + 2 mM MgCl2 + 200 μM MnCl2 to examine the substrate range of AcvAB (see Fig. S7). HPLC-MS analysis showed that AcvAB converted AS into 4-acetyl-2,6-dimethoxyphenylphosphate (ASP; see Table S3 and Fig. S8A to C). AcvAB showed the highest specific activity toward 4′-hydroxyacetophenone and exhibited activity toward compounds with the hydroxy group at the 4 position of the aromatic ring except for vanillic acid and 4-hydroxybenzoic acid (see Table S3 and Fig. S8). AcvAB also showed activity toward 3′-hydroxyacetophenone, but it showed no activity toward 3′-hydroxy-4′-methoxyacetophenone (see Table S3 and Fig. S8). In addition, AcvAB showed conversion ability for phenol, the substrate of phenylphosphate synthase of T. aromatica K172, which is involved in the anaerobic carboxylation of phenol, but no reaction product was detected.
AcvF catalyzes the dephosphorylation of AVP and ASP.
Because AcvF showed 23% amino acid sequence identity with NagD of E. coli K-12, belonging to the HAD superfamily, including phosphatases, AcvF is expected to have dephosphorylation activities for AVP and ASP (58). The AcvF function was characterized by expressing acvF fused with a His tag at the 5′ terminus in E. coli. SDS-PAGE analysis showed a 32-kDa protein production in cell extract of E. coli expressing His-tagged acvF, and AcvF was purified to near homogeneity by Ni affinity chromatography (see Fig. S6). To investigate the AVP and ASP dephosphorylation ability of AcvF, AVP and ASP were prepared by incubating 200 μM AV and AS, respectively, with crude AcvA+AcvB in the presence of ATP + MgCl2 + MnCl2. In addition, 4-acetyl-phenylphosphate was also prepared from 4′-hydroxyacetophenone. HPLC analysis confirmed that AV, AS, and 4′-hydroxyacetophenone were completely converted into AVP, ASP, and 4-acetyl-phenylphosphate, respectively, and the filtrate obtained via ultrafiltration (MW cutoff, 10 kDa) was used as the substrates. The prepared AVP was stable without spontaneously dephosphorylated into AV even after 35 h of incubation at 30°C. Purified AcvF (5 μg protein/mL) was incubated with 100 μM AVP, ASP, and 4-acetyl-phenylphosphate for 60 min. HPLC analysis of the reaction mixtures showed that AcvF converted AVP and ASP into AV and AS, respectively (Fig. 5). Similarly, AcvF converted 4-acetyl-phenylphosphate into 4′-hydroxyacetophenone (see Fig. S10A and B). These results indicated that AcvAB phosphorylates AV, AS, and 4′-hydroxyacetophenone, which is then dephosphorylated by AcvF, respectively.
FIG 5.
Conversions of AVP and ASP by AcvF. AVP (100 μM; A and B) and ASP (100 μM; C and D) were incubated with purified AcvF (5 μg protein/mL). Portions of the reaction mixtures were collected at the start (A and C) and 60 min (B and D) of incubation and analyzed by HPLC.
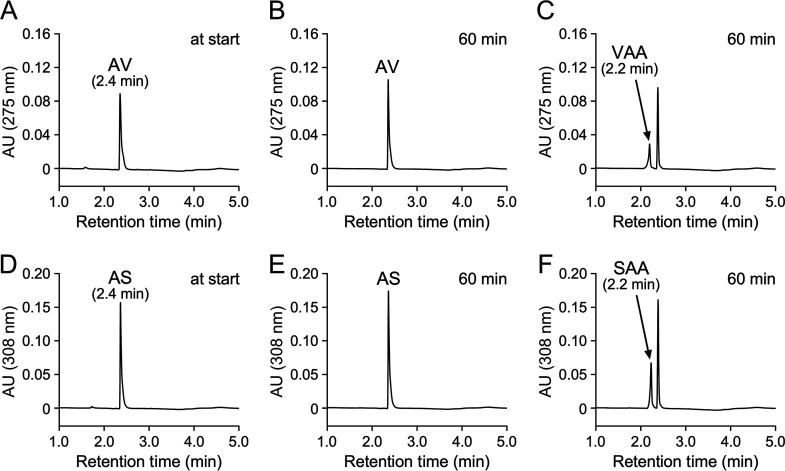
Carboxylation of AV and AS by a mixture of AcvAB, AcvF, and AcvCDE.
It was expected that AcvC-AcvD-AcvE could carboxylate the acetyl groups of AV and AS since acvC, acvD, and acvE were predicted to encode biotin-dependent carboxylase components, BCCP, BC, and CT, respectively (56). acvCDE were expressed in E. coli with a His-tag fused to the 5′ terminus of acvC, characterizing the function of acvC, acvD, and acvE. However, SDS-PAGE analysis showed no clear AcvD and AcvE production, except for AcvC (see Fig. S9). Furthermore, the resulting cell extract of E. coli expressing acvCDE (1 mg protein/mL) was incubated with 100 μM AV in the presence of crude AcvA+AcvB (1 mg protein/mL each), purified AcvF (10 μg protein/mL), 2 mM ATP + 2 mM MgCl2 + 200 μM MnCl2, and 10 mM NaHCO3 for 60 min; however, no reaction product was observed (data not shown). Therefore, acvCDE (without His tag) was expressed under the control of the Q5 promoter of the pQF vector using Sphingobium japonicum UT26S as a host (see Fig. S9). The resulting cell extract of UT26S expressing acvCDE (crude AcvCDE) was incubated with 100 μM AV and AS under the same reaction conditions as described above. The conversion product was undetected when the cell extract of UT26S harboring pQF vector was used (Fig. 6A, B, D, and E), whereas VAA and SAA were detected in the reaction with crude AcvCDE (Fig. 6C and F). In addition, compound V was detected when 4′-hydroxyacetophenone was used as a substrate (see Fig. S10C and D). Negative ESI-MS analysis of compound V showed fragments at m/z 179 and 135 (see Fig. S10E), showing that compound V was 3-(4-hydroxyphenyl)-3-oxopropanoic acid (MW, 180; see Fig. S10F). These results suggest that AcvCDE catalyzes the carboxylation of AV, AS, and 4′-hydroacetophenone.
FIG 6.
A mixture of AcvA-AcvB, AcvF, and AcvC-AcvD-AcvE catalyzed carboxylation of AV and AS. AV (100 μM; A to C) and AS (100 μM; D to F) were incubated with a cell extract of S. japonicum UT26S harboring pQF (1 mg protein/mL; A, B, D, and E) or a cell extract of UT26S harboring pQFacvCDE (1 mg protein/mL; C and F) in the presence of AcvA-AcvB and AcvF. Specifically, the reactions were performed in the presence of a cell extract of E. coli BL21(DE3) harboring pE16acvA (1 mg protein/mL), a cell extract of E. coli BL21(DE3) harboring pE16acvB (1 mg protein/mL), purified AcvF (10 μg protein/mL), 2 mM ATP, 2 mM MgCl2, 200 μM MnCl2, and 10 mM NaHCO3. Portions of the reaction mixtures were collected at the start (A and D) and after 60 min (B, C, E, and F) of incubation and analyzed by HPLC.
To examine whether AcvCDE directly carboxylates AV, crude AcvCDE (1 mg protein/mL) was incubated with 100 μM AV in the presence of 2 mM ATP + 2 mM MgCl2 + 200 μM MnCl2, and 10 mM NaHCO3 for 60 min, but no VAA formation was observed (see Fig. S11A and B). Conversely, when AVP was used as a substrate, VAA was generated under the same reaction condition except for the substrate (see Fig. S11C and D). These results indicate that AcvCDE could not directly carboxylate AV. Although acvF is essential for SYK-6 growth on AV, crude AcvCDE converted AVP into VAA in the absence of AcvF. When AVP was incubated with a cell extract of UT26S harboring pQF, conversion of AVP into AV was shown (see Fig. S11E). Therefore, enzyme(s) present in UT26S appear to complement the AcvF function. Furthermore, a cell extract of UT26S expressing acvCDEF (see Fig. S9) produced more VAA than the cell extract of UT26S expressing acvCDE when 100 μM AV was incubated with these cell extracts in the presence of crude AcvA + AcvB (1 mg protein/mL each), 2 mM ATP + 2 mM MgCl2 + 200 μM MnCl2, and 10 mM NaHCO3 for 2 h (see Fig. S12). These results suggest that the dephosphorylation of AVP catalyzed by AcvF is necessary for AV carboxylation.
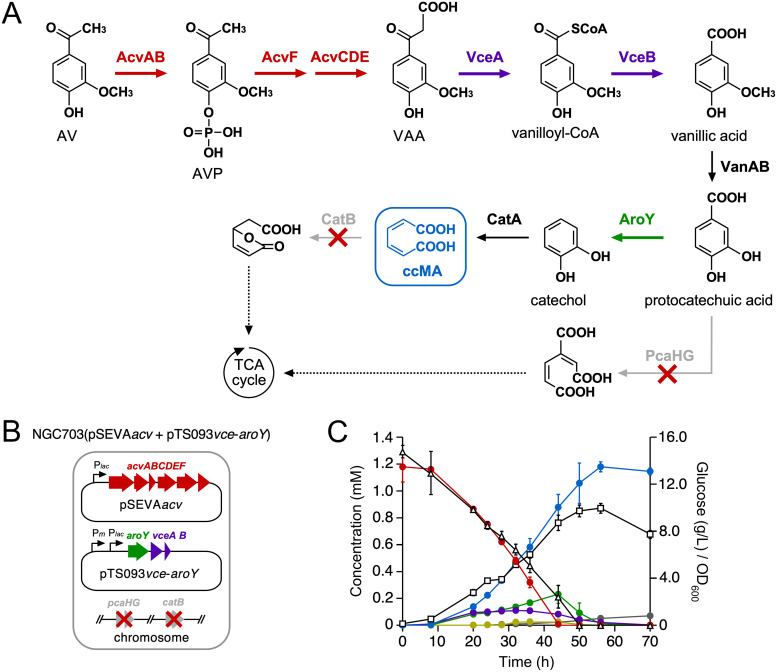
Expression of acvABCDEF with vceA, vceB, and aroY in Pseudomonas sp. NGC703 enables ccMA production from AV.
Our previous study demonstrated ccMA production from vanillic acid using Pseudomonas sp. NGC703 (a mutant of Pseudomonas sp. NGC7 deficient in the protocatechuic acid 3,4-dioxygenase gene [pcaHG] and ccMA cycloisomerase gene [catB]) cells harboring pTS084, comprising the protocatechuic acid decarboxylase (aroY), flavin prenyltransferase (kpdB), vanillic acid O-demethylase (vanAB), and catechol 1,2-dioxygenase (catA) genes (Fig. 7A) (34). This study examined whether ccMA production from AV is possible using NGC7 as a platform.
FIG 7.
Production of ccMA from AV through the engineered metabolic pathway constructed in Pseudomonas sp. NGC7. (A) Engineered route for ccMA production from AV. Enzymes: AcvAB, AVP/ASP synthetase; AcvF, AVP/ASP phosphatase; AcvCDE, biotin-dependent carboxylase; VceA, VAA/SAA-converting enzyme; VceB, vanilloyl-CoA/syringoyl-CoA thioesterase; VanAB, vanillate O-demethylase (the vanillic acid-converting enzyme gene has not yet been identified); AroY, protocatechuic acid decarboxylase; PcaHG, protocatechuic acid 3,4-dioxygenase; CatA, catechol 1,2-dioxygenase; CatB, ccMA cycloisomerase. Abbreviations: AV, acetovanillone; AVP, 4-acetyl-2-methoxyphenylphosphate; VAA, vanilloyl acetic acid; ccMA, cis,cis-muconic acid. (B) Schematic representations of the NGC703 recombinant strain, which contains pSEVAacv and pTS093vce-aroY. (C) Conversion of 1.2 mM AV by NGC703(pSEVAacv + pTS093vce-aroY) cells during growth in MMx-3 medium containing 15 g/L glucose. The concentrations of AV (red), VAA (purple), vanillic acid (green), protocatechuic acid (mustard), ccMA (blue), and cis,trans-muconic acid (gray) were periodically measured by HPLC. The concentration of glucose (triangles) was measured by a glucose electrode. Cell growth (squares) was monitored by measuring the OD600. All experiments were performed in triplicate, and values represent the averages ± standard deviations.
We constructed pSEVAacv (pRO1600/ColE1 ori), which carries acvABCDEF under the control of the lac promoter and introduced pSEVAacv into NGC7. When resting cells of NGC7(pSEVAacv) (OD600 = 10.0) were incubated with 200 μM AV, AV was converted into VAA (see Fig. S13A and B). In our previous study, we found VceA, which converts VAA and SAA into vanilloyl-CoA and syringoyl-CoA, respectively, and VceB, which converts vanilloyl-CoA and syringoyl-CoA into vanillic acid and syringic acid, respectively, from SYK-6, and that VAA can be converted into vanillic acid by these enzymes (Fig. 7A) (5). Thus, we constructed pTS093vce (RK2 ori), which carries vceA and vceB under the control of the lac promoter and introduced pTS093vce into NGC7(pSEVAacv). When resting cells of NGC7(pSEVAacv + pTS093vce) (OD600 = 10.0) were incubated with 200 μM AV, VAA and vanillic acid were formed and gradually reduced to almost nothing (see Fig. S13C). To produce ccMA from AV, we constructed pTS093vce-aroY (RK2 ori), which carries aroY with vceA and vceB under the control of the lac promoter, and then introduced pTS093vce-aroY into NGC703 with pSEVAacv (Fig. 7B). When the resulting strain was grown on an MMx-3 medium [34.2 g/L Na2HPO4·12H2O, 6.0 g/L KH2PO4, 1.0 g/L NaCl, 2.5 g/L (NH4)2SO4, 490 mg/L MgSO4·7H2O, 14.7 mg/L CaCl2·2H2O, and 5 mg/L FeSO4·7H2O] containing 15 g/L glucose as a carbon source, 1.2 mM AV could be converted into ccMA with 96% yield (mol ccMA/mol AV) (Fig. 7C). These results demonstrate that combining acvABCDEF with vceA and vceB is useful for the value-added chemical production from AV, a major aromatic monomer produced in oxidative/base-catalyzed lignin depolymerization.
DISCUSSION
AV and AS are major lignin-derived aromatic monomers produced in alkaline oxidative depolymerization (13–16) and base-catalyzed depolymerization of lignin (17–19, 62–68). They are also contained in the black liquor produced during the kraft pulping process (20). This study identified acvABCDEF that encodes components of biotin-dependent AV and AS carboxylase and showed that these genes help the production of value-added chemicals from AV.
As phenylphosphate synthases, proteins 1 and 2 of T. aromatica K172 (51), PpsABGM of Geobacter metallireducens ATCC 53774 (69), and PpsABFP of Ferroglobus placidus DSM 10642 (70) have been reported. These enzymes are responsible for producing a phosphorylated intermediate essential for phenol carboxylation. Although not an example of carboxylation, 4-methylbenzyl phosphate synthase (CreHI) from Corynebacterium glutamicum produces a phosphorylated intermediate essential for oxidizing the 4-cresol methyl group (71). Based on the amino acid sequence similarity, AcvA corresponds to protein 1, PpsAGM, PpsAFP, and CreH (40 to 44% amino acid sequence identity), while AcvB corresponds to protein 2, PpsBGM, PpsBFP, and CreI (39 to 42% amino acid sequence identity) (see Table S2). Among these phenylphosphate synthases, the reaction mechanism has been proposed in proteins 1 and 2 of T. aromatica K172 (52). The phenol phosphorylation by proteins 1 and 2 is similar to the ping-pong mechanism proposed for phosphoenolpyruvate synthase and is thought to proceed by the following two-step reactions. (i) Protein 2 transfers the phosphoryl group from Mg-ATP to His569 of protein 1, producing His569-β-phosphate-γ-phosphate. (ii) The terminal γ-phosphate is proposed to be irreversibly hydrolyzed from protein 1, and then the phosphate group of His569-β-phosphate is transferred to phenol to form phenylphosphate. AcvB, protein 2, PpsBGM, PpsBFP, and CreI have an ATP binding domain (PPDK_N; PF01326) (see Fig. S14). In addition, AcvA, PpsAGM, PpsAFP, and CreH contained a His residue corresponding to protein 1 His569 (see Fig. S15). These facts suggest that AcvAB, PpsABGM, PpsABFP, and CreHI phosphorylate the hydroxy group of each substrate by a mechanism common to proteins 1 and 2 of T. aromatica K172.
AcvF dephosphorylated AVP and ASP produced from AV and AS, respectively. In T. aromatica K172, phenylphosphate produced by proteins 1 and 2 is dephosphorylated by the δ subunit of phenylphosphate carboxylase or nonenzymatic reaction generating phenolate anion. This intermediate is used as a substrate for carboxylation by the core enzyme [(αβγ)3] of phenylphosphate carboxylase (53, 59). Although AcvF and the δ subunit of phenylphosphate carboxylase have no significant similarity with each other (7% identity), both AcvF and the δ subunit are classified in the HAD-like superfamily. Relatively low overall sequence similarity has been reported for this superfamily of enzymes (72). Therefore, AcvF probably dephosphorylates AVP/ASP, similar to the phenylphosphate carboxylase δ subunit, producing an anionic intermediate for AV/AS.
Crude AcvCDE converted AVP into VAA (see Fig. S10C and D). This result suggests that phosphatase(s) present in UT26S complemented the AcvF function. Since acvF is essential for SYK-6 growth on AV/AS and coexpression of acvF and acvCDE in S. japonicum UT26S enhanced AV carboxylation, the dephosphorylation of AVP/ASP catalyzed by AcvF appears crucial for AV/AS carboxylation. To verify this, AcvCDE purification and functional analysis using the purified enzyme are necessary for the future.
AcvC, AcvD, and AcvE were predicted to be BCCP, BC, and CT, respectively, based on the amino acid sequence similarity. In addition to these components, biotin-dependent carboxylases require biotin-protein ligase (BPL), which specifically adds biotin to a lysine residue of BCCP (73). A search for putative BPL genes in SYK-6 revealed the presence of SLG_23040, which showed 23% amino acid sequence identity with BirA of B. subtilis 168 (74). AVP was converted to VAA via acvCDE expression in S. japonicum UT26S, suggesting that AcvC (BCCP) was biotinylated by BPL present in UT26S. SJA_C1-13370, which showed 44% amino acid sequence identity with SLG_23040, may have functioned as BPL. xccA, xccC, and xccB, encoding putative biotin-dependent carboxylase components of A. aromaticum EbN1, are thought to be involved in 4′-hydroxyacetophenone carboxylation, which is structurally similar to AV (57). In addition, apkA, apkB, and apkC, encoding putative biotin-dependent carboxylase components of R. rhodochrous GD02, were recently predicted to be involved in AV catabolism (20). Orthologs of acvA, acvB, and acvF are also present in EbN1 and GD02, and the gene order of acvABCDEF in GD02 is conserved. The Xcc and Apk systems are likely functionally similar to the Acv system in SYK-6. Phenol carboxylase [(αβγ)3 and (δ)3] of T. aromatica K172 (50–53, 59, 60, 75) and acetophenone carboxylase [Apc(αα′βγ)2 core complex and Apcε] of A. aromaticum EbN1 (76, 77) are not biotin-dependent enzymes and show no similarity to AcvC, AcvD, and AcvE. Altogether, AV/AS carboxylation in SYK-6 seems to proceed as follows (see Fig. S16). (i) AV/AS is converted into AVP/ASP by transferring the phosphate group from Mg-ATP to the hydroxy group of AV/AS by AcvAB. (ii) AcvF dephosphorylates the resulting AVP/ASP to produce an anionic intermediate. (iii) The anionic intermediate is converted to VAA/SAA by transferring the carboxyl group from the carboxylated biotin by AcvCDE.
In SYK-6, AV/AS is converted to VAA/SAA, an intermediate metabolite in the β-aryl ether catabolism (Fig. 1). Therefore, we investigated whether β-aryl ether catabolism genes and AV/AS catabolism genes coexist in the genomes of Altererythrobacter sp. strain Root672, Altererythrobacter atlanticus 26DY36, Erythrobacter sp. strain SG61-1L, Sphingobium sp. strain 66-54, and Sphingobium sp. strain B12D2A, which have orthologs of the β-aryl ether catabolic genes (see Table S4). Orthologs of acvABCDEF showing more than 45% amino acid sequence identity were found in all strains. Particularly, the gene order of acvABCDEF in A. atlanticus 26DY36, Sphingobium sp. 66-54, and Sphingobium sp. B12D2A was conserved. Therefore, these strains may have evolved an AV/AS catabolic pathway by connecting the AV/AS carboxylase and the downstream pathway enzymes of the β-aryl ether catabolism.
Finally, we successfully achieved the microbial AV conversion (1.2 mM) into ccMA with 96% yield (mol ccMA/mol AV) using Pseudomonas sp. NGC703(pSEVAacv + pTS093vce-aroY), which is a pcaHG catB NGC7 mutant carrying acvABCDEF, vceA, vceB, and aroY (Fig. 7) (5, 34). In producing value-added chemicals from lignin through biological funneling, it is necessary to convert all degradation products obtained by chemical depolymerization of lignin or to degrade compounds that cannot be converted into products and not leave them in the culture medium to facilitate product purification. The results of this study will provide invaluable insights for improving the yield and purity of products in the biological conversion of aromatics obtained by alkaline oxidative and base-catalyzed depolymerization of lignin.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The strains and plasmids used in this study are listed in Table 1. Sphingobium sp. strain SYK-6 and its mutants were grown in lysogeny broth (LB), Wx-SEMP, and Wx-SEMP containing 5 mM AV at 30°C. Sphingobium japonicum UT26S, Pseudomonas sp. NGC7, and their mutants were grown in LB at 30°C. When necessary, 12.5 mg/L nalidixic acid, 100 mg/L streptomycin, 25 to 50 mg/L kanamycin, or 12.5 to 15.0 mg/L tetracycline was added to the cultures. Escherichia coli strains were grown in LB at 37°C. For cultures of cells carrying antibiotic resistance markers, the media for E. coli transformants were supplemented with 100 mg/L ampicillin, 25 mg/L kanamycin, or 12.5 mg/L tetracycline.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| Sphingobium sp. | ||
| SYK-6 | Wild type; NBRC 103272/JCM 17495, Nalr Smr | 84 |
| Δ06490 (SME062) | SYK-6 derivative; ΔSLG_06490 (acvF), Nalr Smr | This study |
| Δ06500 (SME063) | SYK-6 derivative; ΔSLG_06500 (acvE), Nalr Smr | This study |
| Δ06510 (SME064) | SYK-6 derivative; ΔSLG_06510 (acvD), Nalr Smr | This study |
| Δ06520 (SME065) | SYK-6 derivative; ΔSLG_06520 (acvC), Nalr Smr | This study |
| Δ06530 (SME066) | SYK-6 derivative; ΔSLG_06530 (acvB), Nalr Smr | This study |
| Δ06540 (SME067) | SYK-6 derivative; ΔSLG_06540 (acvA), Nalr Smr | This study |
| Δ06550 (SME068) | SYK-6 derivative; ΔSLG_06550 (acvR), Nalr Smr | This study |
| S. japonicum | ||
| UT26S | Type strain, NBRC 101211/JCM17232, γ-hexachlorocyclohexane degradation | 85 |
| Pseudomonas sp. | ||
| NGC7 | Wild type | 34 |
| NGC703 | NGC7 derivative; ΔpcaHG catB | 34 |
| E. coli | ||
| BL21(DE3) | F– ompT hsdSB(rB– mB–) gal dcm (DE3); T7 RNA polymerase gene under the control of the lacUV5 promoter | 86 |
| HB101 | recA13 supE44 hsd20 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 87 |
| NEB 10-beta | Δ(ara-leu)7697 araD139 fhuA ΔlacX74 galK16 galE15 e14- ϕ80dlacZΔM15 recA1 relA1 endA1 nupG rpsL (Smr) rph spoT1 Δ(mrr-hsdRMS-mcrBC) | New England Biolabs |
| Plasmids | ||
| pRK2013 | Tra+ Mob+ ColE1 replicon; Kmr | 88 |
| pAK405 | Plasmid for allelic exchange and markerless gene deletions in sphingomonads; Kmr | 82 |
| pJB861 | RK2 broad-host-range expression vector; Pm xylS; Kmr | 89 |
| pET-21a(+) | Expression vector; T7 promoter; Apr | Novagen |
| pET-16b | Expression vector; T7 promoter; Apr | Novagen |
| pQF | Expression vector; Q5 promoter, codon-optimized cymR; Tcr | 90 |
| pUC118 | Cloning vector; lactose promoter (Plac); Apr | Takara Bio |
| pSEVA241 | pRO1600/ColE1 replicon; Kmr | 83 |
| pTS093 | pJB866 with a 0.2-kb PCR-amplified fragment containing Plac from pUC118 | 12 |
| pTS032 | pUC118 with a 1.6-kb KpnI fragment carrying aroY | 35 |
| pJHV01 | pJB866 with a 4.6-kb BamHI PCR-amplified fragment carrying hpvZ, SLG_20400, vceA, and vceB | 5 |
| pAK06490 | pAK405 with a 1.7-kb deletion cassette carrying up- and downstream regions of acvF | This study |
| pAK06500 | pAK405 with a 1.6-kb deletion cassette carrying up- and downstream regions of acvE | This study |
| pAK06510 | pAK405 with a 1.6-kb deletion cassette carrying up- and downstream regions of acvD | This study |
| pAK06520 | pAK405 with a 1.4-kb deletion cassette carrying up- and downstream regions of acvC | This study |
| pAK06530 | pAK405 with a 1.6-kb deletion cassette carrying up- and downstream regions of acvB | This study |
| pAK06540 | pAK405 with a 1.7-kb deletion cassette carrying up- and downstream regions of acvA | This study |
| pAK06550 | pAK405 with a 1.8-kb deletion cassette carrying up- and downstream regions of acvR | This study |
| pE21acv | pET-21a(+) with a 7.4-kb PCR-amplified fragment carrying acvABCDEF | This study |
| pJBacv | pJB861 with a 7.4-kb NotI fragment carrying acvABCDEF from pE21acv | This study |
| pE16acvA | pET-16b with a 1.8-kb PCR-amplified fragment carrying acvA | This study |
| pE16acvB | pET-16b with a 1.0-kb PCR-amplified fragment carrying acvB | This study |
| pE16acvF | pET-16b with a 0.8-kb PCR-amplified fragment carrying acvF | This study |
| pE16acvCDE | pET-16b with a 3.4-kb PCR-amplified fragment carrying acvCDE | This study |
| pQFacvCDE | pQF with a 3.3-kb PCR-amplified fragment carrying acvCDE | This study |
| pQFacvCDEF | pQF with a 4.4-kb PCR-amplified fragment carrying acvCDEF | This study |
| pSEVA241_Plac | pSEVA241 with a 0.2-kb PCR-amplified fragment containing Plac from pUC118 | This study |
| pSEVAacv | pSEVA241_Plac with a 7.4-kb NotI fragment carrying acvABCDEF from pJBacv | This study |
| pQFvce | pQF with a 1.4-kb PCR-amplified fragment carrying vceA and vceB from pJHV01 | This study |
| pTS093vce | pTS093 with a 1.4-kb PCR-amplified fragment carrying vceA and vceB from pQFvce | This study |
| pTS093vce-aroY | pTS093vce with a 1.6-kb KpnI fragment carrying aroY from pTS032 | This study |
The terms Nalr, Smr, Kmr, Tcr, and Apr indicate resistance to nalidixic acid, streptomycin, kanamycin, tetracycline, and ampicillin, respectively.
Preparation of substrates.
VAA and SAA were prepared as described previously (5). For the preparation of AVP, ASP, and 4-acetyl-phenylphosphate, AV, AS, or 4′-hydroxyacetophenone (final concentration, 200 μM) was incubated in 1 mL 50 mM Tris-HCl buffer (pH 7.5; buffer A) containing the cell extracts of E. coli BL21(DE3) cells harboring pE16acvA and E. coli BL21(DE3) cells harboring pE16acvB (1 mg protein/mL each), 2 mM ATP, 2 mM MgCl2, and 200 μM MnCl2. After incubation for 2 h at 30°C, complete consumption of AV, AS, or 4′-hydroxyacetophenone was confirmed by HPLC, and then the reaction mixtures were filtered using an Amicon Ultra spin filter unit (10-kDa cutoff; Millipore). cis,trans-Muconic acid was prepared by incubating ccMA (1.0 g/L) for 2 h at 60°C in a solution whose pH was adjusted to 4.0 with acetic acid. After the incubation, the pH was adjusted to 7 with sodium hydroxide, and the sample was stored at −80°C until use. Other aromatic compounds were purchased from Tokyo Chemical Ind., Co., Ltd.; Sigma-Aldrich Co., LLC.; and Fujifilm Wako Pure Chemical Corporation.
Identification of the metabolites.
SYK-6 cells grown in LB were inoculated into Wx-SEMP to an OD600 of 0.2 and grown at 30°C. AV (5 mM) was added when the OD600 of the culture reached 0.5, and the culture was then further incubated for 12 h. Cells were collected by centrifugation (5,000 × g for 5 min at 4°C), washed twice with Wx minimal medium, and resuspended in the same medium. The resultant cell suspensions were inoculated into Wx medium containing 1 mM AV to an OD600 of 0.2, followed by incubation for 33 h at 30°C. The reaction mixtures were centrifuged, and the supernatants were collected. The resulting filtered samples were analyzed by HPLC-MS.
HPLC-MS analysis.
HPLC-MS analysis was performed with the Acquity UPLC system coupled with an Acquity TQ detector as described previously (78). The in vivo reaction products of AV and AS were analyzed using a UPLC equipped with an Acquity UPLC BEH C18 column (2.1 by 100 mm; Waters). The flow rate of the mobile phase was 0.5 mL/min. The in vitro reaction products of AV, AS, acetophenone, 4′-hydroxyacetophenone, 3′-hydroxyacetophenone, 3′,4′-dihydroxyacetophenone, 3′-hydroxy-4′-methoxyacetophenone, 3′,4′-dimethoxyacetophenone, 3′,4′,5′-trimethoxyacetophenone, 4′-hydroxypropiophenone, 4′-hydroxybuthyrophenone, 4′-hydroxyvalerophenone, guaiacol, vanillic acid, 4-hydroxybenzoic acid, AVP, and ASP were analyzed using a UPLC equipped with a TSKgel ODS-140HTP column (2.1 by 100 mm; Tosoh). The flow rate of the mobile phase was 0.5 mL/min. The mobile phase was a mixture of solution A (acetonitrile containing 0.1% formic acid) and solution B (water containing 0.1% formic acid) under the following conditions: (i) for detection of in vivo reaction products of AV: 0 to 3.0 min, linear gradient from 5 to 15% A; 3.0 to 4.0 min, decreasing gradient from 15% to 5% A; (ii) for detection of in vivo reaction products of AS: 0 to 3.5 min, 10% A; 3.5 to 4.0 min, linear gradient from 10 to 30% A; 4.0 to 5.0 min, 30% A; 5.0 to 5.1 min, decreasing gradient from 30% to 10% A; 5.1 to 6.0 min, 10% A; and (iii) finally, detection of the in vitro reaction products: 0 to 0.5 min, 2% A; 0.5 to 1.5 min, linear gradient from 2 to 15% A; 1.5 to 3.0 min, linear gradient from 15 to 100% A; 3.0 to 4.0 min, decreasing gradient from 100% to 2% A; 4.0 to 5.0 min, 2% A. AV, AS, acetophenone, 4′-hydroxyacetophenone, 3′-hydroxyacetophenone, 3′,4′-dihydroxyacetophenone, 3′-hydroxy-4′-methoxyacetophenone, 3′,4′-dimethoxyacetophenone, 3′,4′,5′-trimethoxyacetophenone, 4′-hydroxypropiophenone, 4′-hydroxybuthyrophenone, 4′-hydroxyvalerophenone, guaiacol, vanillic acid, 4-hydroxybenzoic acid, phenol, AVP, ASP, 4-acetyl-phenylphosphate, VAA, SAA, and 3-(4-hydroxyphenyl)-3-oxopropanoic acid were detected at 275, 300, 255, 275, 260, 275, 274, 274, 280, 271, 272, 271, 276, 260, 255, 271, 303, 300, 260, 280, 308, and 260 nm, respectively. In the ESI-MS analysis, MS spectra were obtained using the negative-ion and positive-ion modes with the settings reported in our previous study (78).
Resting cell assay.
SYK-6 cells grown in LB were inoculated into Wx-SEMP to an OD600 of 0.2 and grown at 30°C until the OD600 of the culture reached 0.5. After the addition of 5 mM AV, the cells were incubated for a further 12 h as the inducing condition. For the noninducing condition, the culture was incubated for a further 12 h without AV. The cells were collected by centrifugation (5,000 × g for 5 min at 4°C) and then washed twice with buffer A. The cells were resuspended in the same buffer and used as resting cells. Preparation of resting cells of S. japonicum UT26S are described below.
Resting cells of SYK-6 (OD600 of 5.0) were incubated with 1.0 mM AV at 30°C with shaking. Resting cells of UT26S harboring pJB861and UT26S harboring pJBacv (OD600 of 10.0) were incubated with 200 μM AV or AS at 30°C with shaking. Portions of the reaction mixtures were collected, and the amounts of compounds were measured by HPLC.
Analysis of nucleotide and amino acid sequences.
Nucleotide sequences were determined by Eurofins Genomics. Sequence analysis was performed by using the MacVector program (MacVector, Inc.). Sequence similarity searches, pairwise alignments, and multiple alignments were conducted using the BLASTP program (79), the EMBOSS Needle program through the EMBL-EBI server (80), and the Clustal Omega program (81), respectively.
RT-PCR analysis.
SYK-6 cells grown in LB were inoculated into Wx-SEMP to an OD600 of 0.2 and grown at 30°C. AV (5 mM) was added when the OD600 of the culture reached 0.5, and the culture was then further incubated for 6 h. Total RNA was isolated from the cells by using an Illumina RNAspin Mini RNA isolation kit (GE Healthcare). The samples were treated with DNase I to remove any contaminating genomic DNA. Total RNA (4 μg) was reverse transcribed using SuperScript IV reverse transcriptase (Invitrogen) with random hexamer primers. The cDNA was purified using a NucleoSpin Gel and PCR Clean-up kit (TaKaRa Bio, Inc.). PCR was performed with the cDNA, specific primers (Table 2), and Q5 High-Fidelity DNA polymerase (New England Biolabs). The DNA obtained was electrophoresed on a 0.8% agarose gel.
TABLE 2.
Primers used in this study
| Method and gene, plasmid, or strain | Primer | Sequence (5′ to 3′) |
|---|---|---|
| For RT-PCR | ||
| Amplified region 1 | 570-560_F | ATAGCCATGAAAGGGATGCG |
| 570-560_R | AGGAGAACGAGGATTTCCGC | |
| Amplified region 2 | 560-550_F | CGGCAGATATTTCTCCAGCC |
| 560-550_R | CCTTGATGCTACAGATCGGC | |
| Amplified region 3 | 550-540_F | GTGTGCGTCGTCTCGATG |
| 550-540_R | TGCTGGAGACGATGGTCG | |
| Amplified region 4 | 540-530_F | AGGATTGCGGTTTCCACCAT |
| 540-530_R | TACTGCGAGCACTGGTACAC | |
| Amplified region 5 | 530-520_F | GCGTTGATTTCCACCAGTCC |
| 530-520_R | ATGGTGGAAACCGCAATCCT | |
| Amplified region 6 | 520-510_F | CGTTTCCTCCACCAGCTTCT |
| 520-510_R | GGACTGGTGGAAATCAACGC | |
| Amplified region 7 | 510-500_F | CGTGAAACCCGCATCGA |
| 510-500_R | CATGTCGAGGTGCAGGTACT | |
| Amplified region 8 | 500-490_F | CTGTTCGGCGTGAAGAGATG |
| 500-490_R | GCCTATCTCCAGCACCAGTT | |
| Amplified region 9 | 490-480_F | CAGCACGAAATCGAACGGTC |
| 490-480_R | CATCTCTTCACGCCGAACAG | |
| For plasmid construction | ||
| pAK06550 | 06550top_F | GCAAGCTTCCTGTGCGCCTTCATCG |
| 06550bottom_R | GATATTTCTCCAGCCAGCCGAATTCGCTGGAACTCCAGCTATGGTCG | |
| pAK06550 and pAK06540 | 06550bottom-06540top_F | GCGAATTCGGCTGGCTGGAGAAATATC |
| 06550bottom-06540top_R | CGCTTTCCAGCTCATAATGGGATCCCGTGGAACTGGCAGTAATAGGG | |
| pAK06540 and pAK06530 | 06540bottom-06530top_F | CGGGATCCCATTATGAGCTGGAAAGCG |
| 06540bottom-06530top_R | CCCTCGATGGTGATGACCTCTAGACGCGTGTCGAGATCGTTCGG | |
| pAK06530 and pAK06520 | 06530bottom-06520top_F | CGTCTAGACGGTCATCACCATCGAGGG |
| 06530bottom-06520top_R | CACTTCGCCATGCTCCACGAATTCGCGCGTTGATTTCCACCAGTC | |
| pAK06520 and pAK06510 | 06520bottom-06510top_F | GCGAATTCGTGGAGCATGGCGAAGTG |
| 06520bottom-06510top_R | ATTGATGCGGCATTCGATGGGTACCGCATCGTCCAGATCCTCGACC | |
| pAK06510 and pAK06500 | 06510bottom-06500top_F | GCGGTACCCATCGAATGCCGCATCAAT |
| 06510bottom-06500top_R | GGCAACTGGTGCTGGAGATTCTAGACGTGGGGCTCAGCGTGAAGAC | |
| pAK06500 and pAK06490 | 06500bottom-06490top_F | CGTCTAGAATCTCCAGCACCAGTTGCC |
| 06500bottom-06490top_R | GTCTCGACCTTGGGATCAGGATCCCGCTTGTAGCCGCCCAGATTG | |
| pAK06490 | 06490bottom_F | CGGGATCCTGATCCCAAGGTCGAGAC |
| 06490bottom_R | GCGGTACCTGATGCGGGCGGAAATCTC | |
| pE21acv | acvA-acvF_F | AAGGAGATATACAGCGGCCGCGCGCGGAAAAGAGCATATGA |
| acvA-acvF_R | GCTCGAATTCGGATCTTATGCCAGCGGGAATGC | |
| pE16acvA | pE16acvA_F | TCGAAGGTCGTCATATGAGCGAACCGACCAAG |
| pE16acvA_R | GTTAGCAGCCGGATCCTCATGCGTCCTCCAGAAC | |
| pE16acvB | pE16acvB_F | TCGAAGGTCGTCATATGACGGCCGCCGTCTCC |
| pE16acvB_R | GTTAGCAGCCGGATCCTTATCTGCGTCCCCCGAA | |
| pE16acvF | pE16acvF_F | TCGAAGGTCGTCATATGACCGCCCCCTTCACC |
| pE16acvF_R | GTTAGCAGCCGGATCCTCAGCCCAGCAGGCCGAG | |
| pE16acvCDE | pE16acvCDE_F | TCGAAGGTCGTCATATGAGCCTCACGGCAAAG |
| pE16acvCDE_R | GTTAGCAGCCGGATCCTCAGCCATGGCTTTCCAG | |
| pQFacvCDE | pQFacvCDE_F | CTAGTAGAGGAAGCTATGAGCCTCACGGCAAAGGA |
| pQFacvCDE_R | TCACTTCACCGGATCTCAGCCATGGCTTTCCAGTT | |
| pQFacvCDEF | pQFacvCDE_F | CTAGTAGAGGAAGCTATGAGCCTCACGGCAAAGGA |
| pQFacvCDEF_R | TCACTTCACCGGATCTCAGCCCAGCAGGCCGAGCG | |
| pSEVA241_Plac | pSEVA241_Plac_F | TCACACAGGAGGCCGAGCGCCCAATACGCAAACC |
| pSEVA241_Plac_R | GCGCGGCCGCGGCCTCGTAATCATGGTCATAGCTG | |
| pQFvce | pQFvce_F | TGGTCTGTTTGTAACTAGTAGAGGAGCGATTGTAAAAAGTTAAGTAACACACTAAGGAGGTATTTTTATGGCCAAGACCTTCATCAC |
| pQFvce_R | TTTTTTTTTGCGGGTCACTTCACCGTCAGGCAGCGGAGCCGAACA | |
| pTS093vce | pTS093vce_F | TATCCTGCAGGAATTGCGATTGTAAAAAGTTAAGTAA |
| pTS093vce_R | CACCGTACGTCTCGATCAGGCAGCGGAGCCGAA | |
| For colony PCR | ||
| ΔacvR | dis06550_conf_F | ACGGCGATGACGATCAGCTC |
| dis06550_conf_R | CGTTGATGATGCGGTGATCG |
Construction of mutants.
To construct the mutants, the upstream and downstream regions (0.7 to 1.0 kb each) of the genes were amplified by PCR from SYK-6 total DNA using the primer pairs listed in Table 2. The amplified fragments were ligated into pAK405 (82). Each of the resulting plasmids was introduced into SYK-6 cells by triparental mating, and the resulting mutants were selected as described previously (82). Disruption of each gene was examined by Southern hybridization analysis using the digoxigenin system (Roche) or colony PCR using the primer pairs listed in Table 2.
Growth measurement.
SYK-6 and its mutant cells grown in LB were inoculated into Wx-SEMP to an OD600 of 0.2 and grown at 30°C. AV (5 mM) was added when the OD600 of the culture reached 0.5, and the culture was then further incubated for 12 h. Cells were collected by centrifugation (5,000 × g for 5 min at 4°C), washed twice with Wx minimal medium, and resuspended in the same medium. The resultant cell suspensions were inoculated into Wx medium containing 1 mM AV or AS to an OD660 of 0.2. Cells were incubated at 30°C with shaking (60 rpm), and cell growth was monitored every hour by measuring the OD660 with a TVS062CA biophotorecorder (Advantec Co., Ltd.). After 52 h (AV) and 48 h (AS) cultivation, 1 mM AV or AS was added to the culture medium.
Expressions of acvABCDEF in heterologous hosts and enzyme purification.
For expression of acvABCDEF in S. japonicum UT26S, a 7.4-kb fragment carrying acvABCDEF with the NotI site at 5′ terminus and 3′ terminus was amplified by PCR. The amplified fragment was cloned into the BamHI site of pET-21a(+) using In-Fusion HD cloning kit (TaKaRa Bio), and the NotI fragment of the resulting plasmid was then inserted in pJB861 to generate pJBacv. For the expression of acvA, acvB, acvF, and acvCDE in E. coli, DNA fragments carrying each gene were amplified by PCR from the SYK-6 total DNA using the primer pairs shown in Table 2. The amplified fragments were cloned into NdeI and BamHI sites of pET-16b using an In-Fusion HD cloning kit. For expression of acvCDE and acvCDEF in UT26S, DNA fragments carrying acvCDE and acvCDEF were amplified by PCR from the SYK-6 total DNA (Table 2). The amplified fragments were cloned into BamHI site of pQF using an NEBuilder HiFi DNA assembly cloning kit (New England Biolabs) to generate pQFacvCDE and pQFacvCDEF. Nucleotide sequences of the resultant plasmids were then confirmed.
pJBacv, pQFacvCDE, and pQFacvCDEF were introduced into UT26S cells by electroporation. Cells of UT26S harboring pJBacv were inoculated into LB supplemented with 1 mM m-toluic acid as an inducer and grown at 30°C for 24 h. Cells of UT26S harboring pQFacvCDE or pQFacvCDEF were inoculated into LB supplemented with 0.1 mM 4-isopropylbenzoic acid as an inducer and grown at 30°C for 24 h. Cells of E. coli BL21(DE3) harboring pE16acvA, pE16acvB, pE16acvF, or pE16acvCDE were grown in LB at 30°C. Each gene expression was induced for 4 h at 30°C by adding 1 mM isopropyl-β-d-thiogalactopyranoside when the OD600 of the culture reached 0.5. The cells of UT26S and E. coli transformants were then collected by centrifugation (5,000 × g for 5 min at 4°C), washed twice with buffer A, resuspended in the same buffer, and used as resting cells. The cells were then disrupted using an ultrasonic disintegrator. After centrifugation (19,000 × g for 15 min at 4°C), the supernatants were obtained as cell extracts (crude enzymes). AcvA, AcvB, and AcvF were purified from cell extracts of E. coli(pE16acvA), E. coli(pE16acvB), and E. coli(pE16acvF), respectively, using a His SpinTrap column (GE Healthcare). The resultant elution fractions were subjected to desalting and concentration using an Amicon Ultra centrifugal filter unit (30-kDa cutoff; Merck Millipore), and the enzyme preparations were stored at −80°C. SDS-PAGE and protein concentration determination using the Bradford method were performed as described previously (43).
Enzyme activity of AcvAB.
Crude AcvA, crude AcvB, crude AcvA+AcvB (500 μg protein/mL each) or purified AcvA+AcvB (100 μg protein/mL each) were incubated with 100 or 200 μM AV in the presence of 2 mM ATP, 2 mM MgCl2, and 200 μM MnCl2 in buffer A for 10 to 30 min at 30°C. The reactions were stopped by adding acetonitrile to a final concentration of 50%. Protein precipitates were removed by centrifugation (19,000 × g for 15 min). The resulting supernatants were diluted with water to a final concentration of acetonitrile of 25%, filtered, and analyzed using HPLC-MS. The specific activity was expressed in moles of AV converted per minute per milligram of protein.
Enzyme properties of AcvAB.
The enzyme reaction was conducted by incubating crude AcvA+AcvB (20 to 1,000 μg protein/mL each) with 200 μM AV, 2 mM ATP, 2 mM MgCl2, and 200 μM MnCl2 in buffer A for 10 min at 30°C. After incubation, the amounts of AV were measured using HPLC. To examine cofactor requirement of AcvAB, crude AcvA+AcvB (50 to 1000 μg protein/mL each) was incubated with 100 μM AV in the presence or absence of cofactors (2 mM ATP, 2 mM MgCl2, 200 μM MnCl2, 2 mM ATP + 2 mM MgCl2, 2 mM ATP + 200 μM MnCl2, 2 mM MgCl2 + 200 μM MnCl2, and 2 mM ATP + 2 mM MgCl2 + 200 μM MnCl2) for 10 min at 30°C. To determine the substrate range, 100 μM AV, AS, acetophenone, 4′-hydroxyacetophenone, 3′-hydroxyacetophenone, 3′,4′-dihydroxyacetophenone, 3′-hydroxy-4′-methoxyacetophenone, 3′,4′-dimethoxyacetophenone, 3′,4′,5′-trimethoxyacetophenone, 4′-hydroxypropiophenone, 4′-hydroxybuthyrophenone, 4′-hydroxyvalerophenone, guaiacol, vanillic acid, 4-hydroxybenzoic acid, and phenol were used for the reaction, and the conversion of substrates and generation of reaction products were analyzed using HPLC-MS.
Enzyme activity of AcvF.
Purified AcvF (5 μg protein/mL) was incubated with 100 μM AVP, ASP, or 4-acetyl-phenylphosphate in buffer A for 60 min at 30°C. The supernatants were then analyzed using HPLC.
Enzyme activity of AcvCDE.
Cell extract of E. coli BL21(DE3) harboring pE16acvCDE, cell extract of S. japonicum UT26S harboring pQF, cell extract of UT26S harboring pQFacvCDE, or cell extract of UT26S harboring pQFacvCDEF (1 mg protein/mL) was incubated with 100 μM AV, AS, or 4′-hydroxyacetophenone, 2 mM ATP, 2 mM MgCl2, 200 μM MnCl2, and 10 mM NaHCO3 in the presence or absence of crude AcvA+AcvB (1 mg protein/mL each) and purified AcvF (10 μg protein/mL) in buffer A for 60 to 120 min at 30°C. When using AVP as a substrate, cell extract of UT26S harboring pQF or cell extract of UT26S harboring pQFacvCDE (1 mg protein/mL) was incubated with 100 μM AVP, 2 mM ATP, 2 mM MgCl2, 200 μM MnCl2, and 10 mM NaHCO3 in buffer A for 60 min at 30°C. The supernatants were then analyzed using HPLC.
ccMA production from AV.
A 0.2-kb fragment carrying the lac promoter (Plac) from pUC118 was amplified and cloned into the SfiI site of pSEVA241 (83) with In-Fusion HD cloning kit to generate pSEVA241_Plac. The 7.2-kb NotI fragment carrying acvABCDEF from pJBacv was ligated into the corresponding site of pSEVA241_Plac to generate pSEVAacv. A 1.5-kb fragment carrying vceA and vceB was amplified by PCR using pJHV01 (5) and the primer pairs listed in Table 2. The resulting fragment was cloned into the BamHI site of pQF by NEBuilder HiFi DNA assembly cloning kit to generate pQFvce. The 1.5-kb fragment carrying vceA and vceB was amplified by PCR using pQFvce and the primer pairs listed in Table 2. The resulting fragment was cloned into the EcoRI-XhoI site of pTS093 (12) with an In-Fusion HD cloning kit to generate pTS093vce. The 1.6-kb KpnI fragment carrying aroY from pTS032 (35) was ligated into the corresponding sites of pTS093vce to generate pTS093vce-aroY. pSEVA241_Plac, pSEVAacv, or pSEVAacv + pTS093vce was introduced into NGC7 cells by electroporation. pSEVAacv and pTS093vce-aroY were introduced into NGC703 (ΔpcaHG catB) cells by electroporation.
Conversion of AV by NGC7 transformants.
Cells of NGC7 harboring pSEVA241_Plac, NGC7 harboring pSEVAacv, and NGC7 harboring pSEVAacv + pTS093vce were grown in LB containing kanamycin or kanamycin + tetracycline for 16 h. The cells were collected by centrifugation at 9,000 × g for 3 min, washed twice with MMx-3 buffer [34.2 g/L Na2HPO4·12H2O, 6.0 g/L KH2PO4, 1.0 g/L NaCl, and 2.5 g/L (NH4)2SO4], resuspended in the same buffer, and used as resting cells. Resting cells of NGC7 harboring pSEVA241_Plac, NGC7 harboring pSEVAacv, and NGC7 harboring pSEVAacv + pTS093vce (OD600 of 10.0) were incubated with 200 μM AV or AS at 30°C with shaking. Portions of the reaction mixtures were periodically collected, and the reactions were stopped by centrifugation. The resultant supernatants were diluted, filtered, and analyzed using an HPLC instrument 1200 series (Agilent Technologies, Inc.) equipped with a ZORBAX Eclipse Plus C18 column (4.6 by 150 mm; Agilent Technologies, Inc.). The flow rate of the mobile phase was 1.0 mL/min, and the detection wavelength was 280 nm. The mobile phase was a mixture of solution A (50% methanol containing 1% acetic acid) and solution B (5% methanol containing 1% acetic acid) under the following conditions: 0 to 8.0 min, linear gradient from 0 to 20% A; 8.0 to 13.0 min, linear gradient from 20 to 100% A; 13.0 to 18.0 min, 100% A; 18.0 to 23.0 min, decreasing gradient from 100 to 0% A; and 23.0 to 25.5 min, 100% B.
Cells of NGC703(pSEVAacv + pTS093vce-aroY) were grown in LB containing kanamycin and tetracycline for 16 h. The cells were collected by centrifugation at 9,000 × g for 3 min, washed twice with MMx-3 medium, and resuspended in 5 mL of the same medium. The cells were then inoculated into 10 mL of MMx-3 medium containing 15 g/L glucose, 1.2 mM AV, kanamycin, and tetracycline to an OD600 of 0.1 and incubated with shaking for 70 h at 30°C. Cell growth was measured by OD600. Portions of the cultures were periodically collected, and the reactions were stopped by centrifugation. The resultant supernatants were diluted, filtered, and analyzed using a HPLC instrument 1200 series. ccMA yields were calculated as follows: (the produced ccMA [mol]/the consumed AV [mol]) × 100%. The concentrations of glucose in the culture were measured with a BF-5i biosensor (Oji Scientific Instruments, Ltd.).
ACKNOWLEDGMENTS
This study was supported by JST-Mirai program grant JPMJMI19E2 (Japan); a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Rural Biomass Research Project BM-D1310); and JSPS KAKENHI grant JP26850046.
Footnotes
Supplemental material is available online only.
Contributor Information
Eiji Masai, Email: emasai@vos.nagaokaut.ac.jp.
Maia Kivisaar, University of Tartu.
REFERENCES
- 1.Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller M, Langan P, Naskar AK, Saddler JN, Tschaplinski TJ, Tuskan GA, Wyman CE. 2014. Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843. 10.1126/science.1246843. [DOI] [PubMed] [Google Scholar]
- 2.Boerjan W, Ralph J, Baucher M. 2003. Lignin biosynthesis. Annu Rev Plant Biol 54:519–546. 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 3.Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. 2010. Lignin biosynthesis and structure. Plant Physiol 153:895–905. 10.1104/pp.110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM. 2010. The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599. 10.1021/cr900354u. [DOI] [PubMed] [Google Scholar]
- 5.Higuchi Y, Kato R, Tsubota K, Kamimura N, Westwood NJ, Masai E. 2019. Discovery of novel enzyme genes involved in the conversion of an arylglycerol-β-aryl ether metabolite and their use in generating a metabolic pathway for lignin valorization. Metab Eng 55:258–267. 10.1016/j.ymben.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Becker J, Wittmann C. 2019. A field of dreams: lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol Adv 37:107360. 10.1016/j.biotechadv.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Beckham GT, Johnson CW, Karp EM, Salvachúa D, Vardon DR. 2016. Opportunities and challenges in biological lignin valorization. Curr Opin Biotechnol 42:40–53. 10.1016/j.copbio.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Borchert AJ, Henson WR, Beckham GT. 2022. Challenges and opportunities in biological funneling of heterogeneous and toxic substrates beyond lignin. Curr Opin Biotechnol 73:1–13. 10.1016/j.copbio.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CW, Salvachúa D, Rorrer NA, Black BA, Vardon DR, St John PC, Cleveland NS, Dominick G, Elmore JR, Grundl N, Khanna P, Martinez CR, Michener WE, Peterson DJ, Ramirez KJ, Singh P, VanderWall TA, Wilson AN, Yi X, Biddy MJ, Bomble YJ, Guss AM, Beckham GT. 2019. Innovative chemicals and materials from bacterial aromatic catabolic pathways. Joule 3:1523–1537. 10.1016/j.joule.2019.05.011. [DOI] [Google Scholar]
- 10.Linger JG, Vardon DR, Guarnieri MT, Karp EM, Hunsinger GB, Franden MA, Johnson CW, Chupka G, Strathmann TJ, Pienkos PT, Beckham GT. 2014. Lignin valorization through integrated biological funneling and chemical catalysis. Proc Natl Acad Sci USA 111:12013–12018. 10.1073/pnas.1410657111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masai E, Katayama Y, Fukuda M. 2007. Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Biochem 71:1–15. 10.1271/bbb.60437. [DOI] [PubMed] [Google Scholar]
- 12.Sonoki T, Takahashi K, Sugita H, Hatamura M, Azuma Y, Sato T, Suzuki S, Kamimura N, Masai E. 2018. Glucose-free cis,cis-muconic acid production via new metabolic designs corresponding to the heterogeneity of lignin. ACS Sustainable Chem Eng 6:1256–1264. 10.1021/acssuschemeng.7b03597. [DOI] [Google Scholar]
- 13.Villar JC, Caperos A, García-Ochoa F. 2001. Oxidation of hardwood kraft-lignin to phenolic derivatives with oxygen as oxidant. Wood Sci Technol 35:245–255. 10.1007/s002260100089. [DOI] [Google Scholar]
- 14.Zhu Y, Liao Y, Lv W, Liu J, Song X, Chen L, Wang C, Sels BF, Ma L. 2020. Complementing vanillin and cellulose production by oxidation of lignocellulose with stirring control. ACS Sustainable Chem Eng 8:2361–2374. 10.1021/acssuschemeng.9b04837. [DOI] [Google Scholar]
- 15.Schutyser W, Renders T, Van den Bosch S, Koelewijn SF, Beckham GT, Sels BF. 2018. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerization, and upgrading. Chem Soc Rev 47:852–908. 10.1039/c7cs00566k. [DOI] [PubMed] [Google Scholar]
- 16.Abdelaziz OY, Ravi K, Mittermeier F, Meier S, Riisager A, Lidén G, Hulteberg CP. 2019. Oxidative depolymerization of kraft lignin for microbial conversion. ACS Sustainable Chem Eng 7:11640–11652. 10.1021/acssuschemeng.9b01605. [DOI] [Google Scholar]
- 17.Abdelaziz OY, Li K, Tunå P, Hulteberg CP. 2018. Continuous catalytic depolymerization and conversion of industrial kraft lignin into low-molecular-weight aromatics. Biomass Conv Bioref 8:455–470. 10.1007/s13399-017-0294-2. [DOI] [Google Scholar]
- 18.Almqvist H, Veras H, Li K, Garcia Hidalgo J, Hulteberg C, Gorwa-Grauslund M, Skorupa Parachin N, Carlquist M. 2021. Muconic acid production using engineered Pseudomonas putida KT2440 and a guaiacol-rich fraction derived from kraft lignin. ACS Sustainable Chem Eng 9:8097–8106. 10.1021/acssuschemeng.1c00933. [DOI] [Google Scholar]
- 19.Ravi K, Abdelaziz OY, Nöbel M, García-Hidalgo J, Gorwa-Grauslund MF, Hulteberg CP, Lidén G. 2019. Bacterial conversion of depolymerized Kraft lignin. Biotechnol Biofuels 12:56. 10.1186/s13068-019-1397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navas LE, Dexter G, Liu J, Levy-Booth D, Cho M, Jang SK, Mansfield SD, Renneckar S, Mohn WW, Eltis LD. 2021. Bacterial transformation of aromatic monomers in softwood black liquor. Front Microbiol 12:735000. 10.3389/fmicb.2021.735000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunel F, Davison J. 1988. Cloning and sequencing of Pseudomonas genes encoding vanillate demethylase. J Bacteriol 170:4924–4930. 10.1128/jb.170.10.4924-4930.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priefert H, Rabenhorst J, Steinbüchel A. 1997. Molecular characterization of genes of Pseudomonas sp. strain HR199 involved in bioconversion of vanillin to protocatechuate. J Bacteriol 179:2595–2607. 10.1128/jb.179.8.2595-2607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe T, Masai E, Miyauchi K, Katayama Y, Fukuda M. 2005. A tetrahydrofolate-dependent O-demethylase, LigM, is crucial for catabolism of vanillate and syringate in Sphingomonas paucimobilis SYK-6. J Bacteriol 187:2030–2037. 10.1128/JB.187.6.2030-2037.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masai E, Yamamoto Y, Inoue T, Takamura K, Hara H, Kasai D, Katayama Y, Fukuda M. 2007. Characterization of ligV essential for catabolism of vanillin by Sphingomonas paucimobilis SYK-6. Biosci Biotechnol Biochem 71:2487–2492. 10.1271/bbb.70267. [DOI] [PubMed] [Google Scholar]
- 25.Chen HP, Chow M, Liu CC, Lau A, Liu J, Eltis LD. 2012. Vanillin catabolism in Rhodococcus jostii RHA1. Appl Environ Microbiol 78:586–588. 10.1128/AEM.06876-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleige C, Hansen G, Kroll J, Steinbüchel A. 2013. Investigation of the Amycolatopsis sp. strain ATCC 39116 vanillin dehydrogenase and its impact on the biotechnical production of vanillin. Appl Environ Microbiol 79:81–90. 10.1128/AEM.02358-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding W, Si M, Zhang W, Zhang Y, Chen C, Zhang L, Lu Z, Chen S, Shen X. 2015. Functional characterization of a vanillin dehydrogenase in Corynebacterium glutamicum. Sci Rep 5:8044. 10.1038/srep08044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallinson SJB, Machovina MM, Silveira RL, Garcia-Borràs M, Gallup N, Johnson CW, Allen MD, Skaf MS, Crowley MF, Neidle EL, Houk KN, Beckham GT, DuBois JL, McGeehan JE. 2018. A promiscuous cytochrome P450 aromatic O-demethylase for lignin bioconversion. Nat Commun 9:2487. 10.1038/s41467-018-04878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Hidalgo J, Ravi K, Kuré L-L, Lidén G, Gorwa-Grauslund M. 2019. Identification of the two-component guaiacol demethylase system from Rhodococcus rhodochrous and expression in Pseudomonas putida EM42 for guaiacol assimilation. AMB Express 9:34. 10.1186/s13568-019-0759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barton N, Horbal L, Starck S, Kohlstedt M, Luzhetskyy A, Wittmann C. 2018. Enabling the valorization of guaiacol-based lignin: integrated chemical and biochemical production of cis,cis-muconic acid using metabolically engineered Amycolatopsis sp ATCC 39116. Metab Eng 45:200–210. 10.1016/j.ymben.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Otsuka Y, Araki T, Kamimura N, Masai E, Nakamura M, Katayama Y. 2021. Lignin valorization through efficient microbial production of β-ketoadipate from industrial black liquor. Bioresour Technol 337:125489. 10.1016/j.biortech.2021.125489. [DOI] [PubMed] [Google Scholar]
- 32.Tumen-Velasquez M, Johnson CW, Ahmed A, Dominick G, Fulk EM, Khanna P, Lee SA, Schmidt AL, Linger JG, Eiteman MA, Beckham GT, Neidle EL. 2018. Accelerating pathway evolution by increasing the gene dosage of chromosomal segments. Proc Natl Acad Sci USA 115:7105–7110. 10.1073/pnas.1803745115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian Y, Otsuka Y, Sonoki T, Mukhopadhyay B, Nakamura M, Masai E, Katayama Y, Okamura-Abe Y, Jellison J, Goodell B. 2016. Engineered microbial production of 2-pyrone-4,6-dicarboxylic acid from lignin residues for use as an industrial platform chemical. Bioresources 11:6097–6109. 10.15376/biores.11.3.6097-6109. [DOI] [Google Scholar]
- 34.Shinoda E, Takahashi K, Abe N, Kamimura N, Sonoki T, Masai E. 2019. Isolation of a novel platform bacterium for lignin valorization and its application in glucose-free cis,cis-muconate production. J Ind Microbiol Biotechnol 46:1071–1080. 10.1007/s10295-019-02190-6. [DOI] [PubMed] [Google Scholar]
- 35.Sonoki T, Morooka M, Sakamoto K, Otsuka Y, Nakamura M, Jellison J, Goodell B. 2014. Enhancement of protocatechuate decarboxylase activity for the effective production of muconate from lignin-related aromatic compounds. J Biotechnol 192:71–77. 10.1016/j.jbiotec.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Perez JM, Kontur WS, Alherech M, Coplien J, Karlen SD, Stahl SS, Donohue TJ, Noguera DR. 2019. Funneling aromatic products of chemically depolymerized lignin into 2-pyrone-4–6-dicarboxylic acid with Novosphingobium aromaticivorans. Green Chem 21:1340–1350. 10.1039/C8GC03504K. [DOI] [Google Scholar]
- 37.Tanihata Y, Watanabe M, Mitsukura K, Maruyama K. 2012. Oxidative degradation of 4-hydroxyacetophenone in Arthrobacter sp. TGJ4. Biosci Biotechnol Biochem 76:838–840. 10.1271/bbb.110876. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi Y, Takahashi K, Kamimura N, Masai E. 2018. Bacterial enzymes for the cleavage of lignin β-aryl ether bonds: properties and applications, p 226–251. In Beckham GT (ed), Lignin valorization: emerging approaches. Royal Society of Chemistry, London, United Kingdom. [Google Scholar]
- 39.Kamimura N, Takahashi K, Mori K, Araki T, Fujita M, Higuchi Y, Masai E. 2017. Bacterial catabolism of lignin-derived aromatics: new findings in a recent decade: update on bacterial lignin catabolism. Environ Microbiol Rep 9:679–705. 10.1111/1758-2229.12597. [DOI] [PubMed] [Google Scholar]
- 40.Gall DL, Kim H, Lu F, Donohue TJ, Noguera DR, Ralph J. 2014. Stereochemical features of glutathione-dependent enzymes in the Sphingobium sp. strain SYK-6 β-aryl etherase pathway. J Biol Chem 289:8656–8667. 10.1074/jbc.M113.536250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanamura K, Abe T, Kamimura N, Kasai D, Hishiyama S, Otsuka Y, Nakamura M, Kajita S, Katayama Y, Fukuda M, Masai E. 2011. Characterization of the third glutathione S-transferase gene involved in enantioselective cleavage of the β-aryl ether by Sphingobium sp. strain SYK-6. Biosci Biotechnol Biochem 75:2404–2407. 10.1271/bbb.110525. [DOI] [PubMed] [Google Scholar]
- 42.Masai E, Ichimura A, Sato Y, Miyauchi K, Katayama Y, Fukuda M. 2003. Roles of the enantioselective glutathione S-transferases in cleavage of β-aryl ether. J Bacteriol 185:1768–1775. 10.1128/JB.185.6.1768-1775.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higuchi Y, Sato D, Kamimura N, Masai E. 2020. Roles of two glutathione S-transferases in the final step of the β-aryl ether cleavage pathway in Sphingobium sp. strain SYK-6. Sci Rep 10:20614. 10.1038/s41598-020-77462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato Y, Moriuchi H, Hishiyama S, Otsuka Y, Oshima K, Kasai D, Nakamura M, Ohara S, Katayama Y, Fukuda M, Masai E. 2009. Identification of three alcohol dehydrogenase genes involved in the stereospecific catabolism of arylglycerol-β-aryl ether by Sphingobium sp. strain SYK-6. Appl Environ Microbiol 75:5195–5201. 10.1128/AEM.00880-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higuchi Y, Aoki S, Takenami H, Kamimura N, Takahashi K, Hishiyama S, Lancefield CS, Ojo OS, Katayama Y, Westwood NJ, Masai E. 2018. Bacterial catabolism of β-hydroxypropiovanillone and β-hydroxypropiosyringone produced in the reductive cleavage of arylglycerol-β-aryl ether in lignin. Appl Environ Microbiol 84:e02670-17. 10.1128/AEM.02670-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niwa M, Saburi Y. 2002. Vanilloyl acetic acid as an unstable intermediate from β-hydroxypropiovanillone to acetovanillone. Holzforschung 56:360–362. 10.1515/HF.2002.057. [DOI] [Google Scholar]
- 47.Otani H, Lee YE, Casabon I, Eltis LD. 2014. Characterization of p-hydroxycinnamate catabolism in a soil actinobacterium. J Bacteriol 196:4293–4303. 10.1128/JB.02247-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Araki T, Tanatani K, Kamimura N, Otsuka Y, Yamaguchi M, Nakamura M, Masai E. 2020. The syringate O-demethylase gene of Sphingobium sp. strain SYK-6 is regulated by DesX, while other vanillate and syringate catabolism genes are regulated by DesR. Appl Environ Microbiol 86:e01712-20. 10.1128/AEM.01712-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujita M, Mori K, Hara H, Hishiyama S, Kamimura N, Masai E. 2019. A TonB-dependent receptor constitutes the outer membrane transport system for a lignin-derived aromatic compound. Commun Biol 2:432. 10.1038/s42003-019-0676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lack A, Fuchs G. 1994. Evidence that phenol phosphorylation to phenylphosphate is the first step in anaerobic phenol metabolism in a denitrifying Pseudomonas sp. Arch Microbiol 161:132–139. 10.1007/s002030050032. [DOI] [PubMed] [Google Scholar]
- 51.Schmeling S, Narmandakh A, Schmitt O, Gad’on N, Schühle K, Fuchs G. 2004. Phenylphosphate synthase: a new phosphotransferase catalyzing the first step in anaerobic phenol metabolism in Thauera aromatica. J Bacteriol 186:8044–8057. 10.1128/JB.186.23.8044-8057.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narmandakh A, Gad’on N, Drepper F, Knapp B, Haehnel W, Fuchs G. 2006. Phosphorylation of phenol by phenylphosphate synthase: role of histidine phosphate in catalysis. J Bacteriol 188:7815–7822. 10.1128/JB.00785-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schühle K, Fuchs G. 2004. Phenylphosphate carboxylase: a new C-C lyase involved in anaerobic phenol metabolism in Thauera aromatica. J Bacteriol 186:4556–4567. 10.1128/JB.186.14.4556-4567.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marini P, Li SJ, Gardiol D, Cronan JE, Jr, de Mendoza D. 1995. The genes encoding the biotin carboxyl carrier protein and biotin carboxylase subunits of Bacillus subtilis acetyl coenzyme A carboxylase, the first enzyme of fatty acid synthesis. J Bacteriol 177:7003–7006. 10.1128/jb.177.23.7003-7006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukhopadhyay B, Stoddard SF, Wolfe RS. 1998. Purification, regulation, and molecular and biochemical characterization of pyruvate carboxylase from Methanobacterium thermoautotrophicum strain ΔH. J Biol Chem 273:5155–5166. 10.1074/jbc.273.9.5155. [DOI] [PubMed] [Google Scholar]
- 56.Tong L. 2013. Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci 70:863–891. 10.1007/s00018-012-1096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wöhlbrand L, Wilkes H, Halder T, Rabus R. 2008. Anaerobic degradation of p-ethylphenol by “Aromatoleum aromaticum” strain EbN1: pathway, regulation, and involved proteins. J Bacteriol 190:5699–5709. 10.1128/JB.00409-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremblay LW, Dunaway-Mariano D, Allen KN. 2006. Structure and activity analyses of Escherichia coli K-12 NagD provide insight into the evolution of biochemical function in the haloalkanoic acid dehalogenase superfamily. Biochemistry 45:1183–1193. 10.1021/bi051842j. [DOI] [PubMed] [Google Scholar]
- 59.Schmeling S, Fuchs G. 2009. Anaerobic metabolism of phenol in proteobacteria and further studies of phenylphosphate carboxylase. Arch Microbiol 191:869–878. 10.1007/s00203-009-0519-2. [DOI] [PubMed] [Google Scholar]
- 60.Lack A, Fuchs G. 1992. Carboxylation of phenylphosphate by phenol carboxylase, an enzyme system of anaerobic phenol metabolism. J Bacteriol 174:3629–3636. 10.1128/jb.174.11.3629-3636.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takeo M, Murakami M, Niihara S, Yamamoto K, Nishimura M, Kato D, Negoro S. 2008. Mechanism of 4-nitrophenol oxidation in Rhodococcus sp. strain PN1: characterization of the two-component 4-nitrophenol hydroxylase and regulation of its expression. J Bacteriol 190:7367–7374. 10.1128/JB.00742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biswas B, Kumar A, Krishna BB, Bhaskar T. 2021. Effects of solid base catalysts on depolymerization of alkali lignin for the production of phenolic monomer compounds. Renew Energy 175:270–280. 10.1016/j.renene.2021.04.039. [DOI] [Google Scholar]
- 63.Katahira R, Mittal A, McKinney K, Chen X, Tucker MP, Johnson DK, Beckham GT. 2016. Base-catalyzed depolymerization of biorefinery lignins. ACS Sustainable Chem Eng 4:1474–1486. 10.1021/acssuschemeng.5b01451. [DOI] [Google Scholar]
- 64.Prothmann J, Sun MZ, Spégel P, Sandahl M, Turner C. 2017. Ultra-high-performance supercritical fluid chromatography with quadrupole-time-of-flight mass spectrometry (UHPSFC/QTOF-MS) for analysis of lignin-derived monomeric compounds in processed lignin samples. Anal Bioanal Chem 409:7049–7061. 10.1007/s00216-017-0663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kruger JS, Cleveland NS, Zhang ST, Katahira R, Black BA, Chupka GM, Lammens T, Hamilton PG, Biddy MJ, Beckham GT. 2016. Lignin depolymerization with nitrate-intercalated hydrotalcite catalysts. ACS Catal 6:1316–1328. 10.1021/acscatal.5b02062. [DOI] [Google Scholar]
- 66.Roberts VM, Stein V, Reiner T, Lemonidou A, Li X, Lercher JA. 2011. Towards quantitative catalytic lignin depolymerization. Chemistry 17:5939–5948. 10.1002/chem.201002438. [DOI] [PubMed] [Google Scholar]
- 67.Beauchet R, Monteil-Rivera F, Lavoie JM. 2012. Conversion of lignin to aromatic-based chemicals (L-chems) and biofuels (L-fuels). Bioresour Technol 121:328–334. 10.1016/j.biortech.2012.06.061. [DOI] [PubMed] [Google Scholar]
- 68.Lavoie JM, Baré W, Bilodeau M. 2011. Depolymerization of steam-treated lignin for the production of green chemicals. Bioresour Technol 102:4917–4920. 10.1016/j.biortech.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 69.Zhang T, Tremblay PL, Chaurasia AK, Smith JA, Bain TS, Lovley DR. 2013. Anaerobic benzene oxidation via phenol in Geobacter metallireducens. Appl Environ Microbiol 79:7800–7806. 10.1128/AEM.03134-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holmes DE, Risso C, Smith JA, Lovley DR. 2012. Genome-scale analysis of anaerobic benzoate and phenol metabolism in the hyperthermophilic archaeon Ferroglobus placidus. ISME J 6:146–157. 10.1038/ismej.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du L, Ma L, Qi F, Zheng X, Jiang C, Li A, Wan X, Liu SJ, Li S. 2016. Characterization of a unique pathway for 4-cresol catabolism initiated by phosphorylation in Corynebacterium glutamicum. J Biol Chem 291:6583–6594. 10.1074/jbc.M115.695320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seifried A, Schultz J, Gohla A. 2013. Human HAD phosphatases: structure, mechanism, and roles in health and disease. FEBS J 280:549–571. 10.1111/j.1742-4658.2012.08633.x. [DOI] [PubMed] [Google Scholar]
- 73.Chapman-Smith A, Cronan JE. 1999. In vivo enzymatic protein biotinylation. Biomol Eng 16:119–125. 10.1016/S1050-3862(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 74.Bower S, Perkins J, Yocum RR, Serror P, Sorokin A, Rahaim P, Howitt CL, Prasad N, Ehrlich SD, Pero J. 1995. Cloning and characterization of the Bacillus subtilis birA gene encoding a repressor of the biotin operon. J Bacteriol 177:2572–2575. 10.1128/jb.177.9.2572-2575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breinig S, Schiltz E, Fuchs G. 2000. Genes involved in anaerobic metabolism of phenol in the bacterium Thauera aromatica. J Bacteriol 182:5849–5863. 10.1128/JB.182.20.5849-5863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jobst B, Schühle K, Linne U, Heider J. 2010. ATP-dependent carboxylation of acetophenone by a novel type of carboxylase. J Bacteriol 192:1387–1394. 10.1128/JB.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weidenweber S, Schühle K, Demmer U, Warkentin E, Ermler U, Heider J. 2017. Structure of the acetophenone carboxylase core complex: prototype of a new class of ATP-dependent carboxylases/hydrolases. Sci Rep 7:39674. 10.1038/srep39674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fukuhara Y, Inakazu K, Kodama N, Kamimura N, Kasai D, Katayama Y, Fukuda M, Masai E. 2010. Characterization of the isophthalate degradation genes of Comamonas sp. strain E6. Appl Environ Microbiol 76:519–527. 10.1128/AEM.01270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. 2008. NCBI BLAST: a better web interface. Nucleic Acids Res 36:W5–W9. 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li W, Cowley A, Uludag M, Gur T, McWilliam H, Squizzato S, Park YM, Buso N, Lopez R. 2015. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res 43:W580–W584. 10.1093/nar/gkv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaczmarczyk A, Vorholt JA, Francez-Charlot A. 2012. Markerless gene deletion system for Sphingomonads. Appl Environ Microbiol 78:3774–3777. 10.1128/AEM.07347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silva-Rocha R, Martínez-García E, Calles B, Chavarría M, Arce-Rodríguez A, de Las Heras A, Páez-Espino AD, Durante-Rodríguez G, Kim J, Nikel PI, Platero R, de Lorenzo V. 2013. The Standard European Vector Architecture (SEVA): a coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Res 41:D666–D675. 10.1093/nar/gks1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katayama Y, Nishikawa S, Nakamura M, Yano K, Yamasaki M, Morohoshi N, Haraguchi T. 1987. Cloning and expression of Pseudomonas paucimobilis SYK-6 genes involved in the degradation of vanillate and protocatechuate in P. putida. Mokuzai Gakkaishi 33:77–79. [Google Scholar]
- 85.Nagata Y, Kato H, Ohtsubo Y, Tsuda M. 2019. Lessons from the genomes of lindane-degrading sphingomonads. Environ Microbiol Rep 11:630–644. [DOI] [PubMed] [Google Scholar]
- 86.Studier FW, Moffatt BA. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130. [DOI] [PubMed] [Google Scholar]
- 87.Bolivar F, Backman K. 1979. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol 68:245–267. [DOI] [PubMed] [Google Scholar]
- 88.Figurski DH, Helinski DR. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A 76:1648–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blatny JM, Brautaset T, Winther-Larsen HC, Karunakaran P, Valla S. 1997. Improved broad-host-range RK2 vectors useful for high and low regulated gene expression levels in gram-negative bacteria. Plasmid 38:35–51. [DOI] [PubMed] [Google Scholar]
- 90.Kaczmarczyk A, Vorholt JA, Francez-Charlot A. 2013. Cumate-inducible gene expression system for Sphingomonads and other Alphaproteobacteria. Appl Environ Microbiol 79:6795–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S4 and Fig. S1 to S16. Download aem.00724-22-s0001.pdf, PDF file, 10.6 MB (10.6MB, pdf)