Abstract
Adolescence is characterized by significant changes in several domains, including brain structure and function, puberty, and social and environmental factors. Some of these changes serve to increase the likelihood of psychosis onset during this period, while others may buffer this risk. This review characterizes our current knowledge regarding the unique aspects of adolescence that may serve as risk factors for schizophrenia spectrum disorders. In addition, we provide potential future directions for research into adolescent specific developmental mechanisms that impart vulnerability to psychosis as well as the possibility of interventions that capitalize on adolescents’ unique characteristics. Specifically, we explore the ways in which grey and white matter develop throughout adolescence in typically developing youth as well as those with psychosis spectrum disorders. We also discuss current views on the function that social support and demands, as well as role expectations, play in risk for psychosis. We further highlight the importance of considering biological factors such as puberty and hormonal changes as areas of unique vulnerability for adolescents. Finally, we discuss cannabis use as a factor that may have a unique impact during adolescent neurodevelopment, and subsequently potentially impact psychosis onset. Throughout, we include discussion of resilience factors that may provide unique opportunities for intervention during this dynamic life stage.
Keywords: psychosis, schizophrenia, adolescence, development, social function, cannabis
Introduction
Adolescence is a period of rapid development in brain structure and function (1–3), which supports the transition to the varied demands of adult life. Special opportunities for growth, particularly in areas such as social function, also characterize this time (4, 5). However, adolescence also is associated with a vulnerability to psychopathology, whether it be depression, anxiety, or, the subject of this review, psychosis (6, 7). While the prenatal period represents the first window of vulnerability for increasing risk of psychosis, in which early life insults act as diathesis factors in the later development of psychosis (8), the adolescent stage represents a second risk period rife with stressors more proximal to symptom onset (9).
While peak onset of schizophrenia falls between ages 15–25 (10), the incidence of schizophrenia shows an increase beginning around age 14 (6), with approximately 39% of male and 23% of female patients developing schizophrenia before age 19 (11). Furthermore, epidemiologic studies indicate that prevalence of subthreshold psychotic symptoms during late childhood and adolescence is higher than in adulthood (12). Thus, across a spectrum of symptom severity, adolescence is a significant period for emergence of psychosis (for recent review, see (13)). However, despite epidemiological data showing that adolescent-onset psychosis is not an anomaly, but rather reflects the course of illness in a substantial subset of patients, there is a general perception that psychosis in those below age 18 is markedly different from the expected illness trajectory (14, 15). As a result, while there is growing research on developmental factors in the prodromal phase, there is limited research on developmental factors in individuals diagnosed with psychotic disorders. This is important, as intervention within the first two years of onset of fully psychotic symptoms is associated with better functional outcome and maintenance of symptom remission (16), underscoring the critical need to characterize developmental mechanisms early in the course of illness in order to optimize interventions.
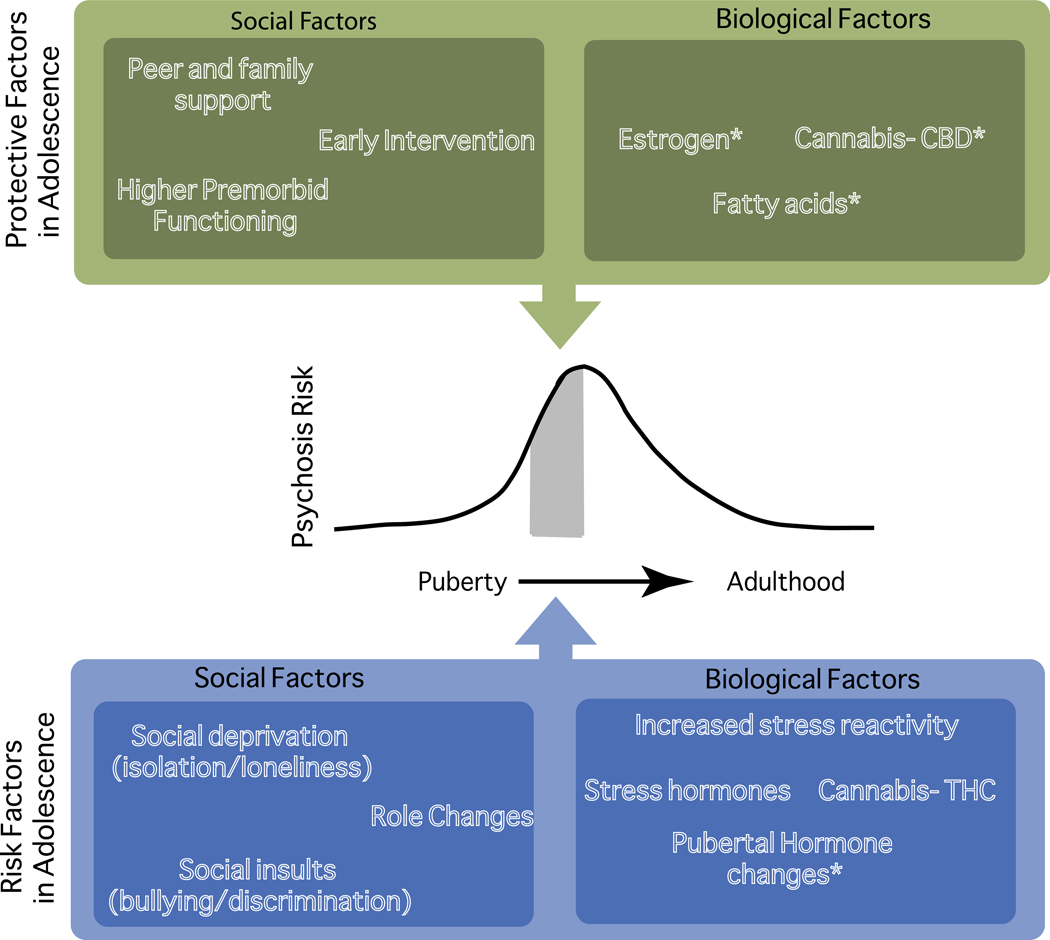
Although schizophrenia is often described as a putatively developmental disorder and relationships have been hypothesized between neurodevelopment and psychosis onset (17), the precise nature of any etiological links is still under investigation. Earlier age of onset, as in adolescent-onset psychosis, has been associated with lower premorbid function(18), more hospitalizations(19), poorer cognitive function(20), and poor prognosis(21). Thus, psychosis during adolescence may confer special risk. However, it is also possible that in addition to vulnerability, the neurodevelopmental changes and unique adolescent environment may confer special opportunities for interventions that could be uniquely effective during this dynamic period. This review examines adolescent development, both as it typically occurs and as it may be altered in adolescents with psychosis and those identified to be at clinical high risk (CHR) for psychotic disorders. We discuss several examples of biological and social risk factors that uniquely or disproportionately affect adolescents, as well as the importance of early intervention in psychosis (Table 1; Figure 1). While we discuss adolescence as a unitary construct, in part reflecting the state of available research, it represents a dynamic period, and further study of stages of development within adolescence is merited. Additionally, labels of “psychosis” and “CHR” reflect a spectrum of psychotic symptomatology that spans from increased risk through diagnosis of psychosis (for recent review, see (13)).
Table 1.
Biological and Psychosocial Factors That May Contribute to Psychosis Risk in Adolescence
| Example Risk/Resilience Factors | Potential Mechanism(s) in Adolescence | Impact on Psychosis Risk | Areas for Future Study |
|---|---|---|---|
|
| |||
| Biological Factors | |||
|
| |||
| Accelerated Pruning | Alters cortical thickness, particularly in prefrontal regions believed to support development of higher order cognitive function, which in turn may contribute to cognitive deficits Disrupts excitatory–inhibitory balance in brain via impact on glutamatergic neuron functioning |
↑ | Validation of presynaptic protein marker findings in adolescent psychosis samples Identification of moderators of GM decreases Evaluation of interaction of antipsychotic medication use and GM developmental processes |
|
| |||
| Blunted Myelination | Alters maturation of long-range tracts associated with higher order cognitive function | ↑ | Evaluation of efficacy of WM treatments such as fatty acid administration during the adolescent developmental period Characterization of relationship between structural and functional connectivity specifically in adolescent psychosis samples |
|
| |||
| Pubertal Sex Hormones | Testosteronea Affects organizational and activational processes in the brain, which may make the brain more sensitive to environmental input Moderates dopaminergic signaling, associated with changes in thalamo-cortico-striatal networks |
– | Validation of accurate puberty measures and evaluation of hormone effects using multimodal measures Investigation of hormonal correlates in adolescent psychosis samples Evaluation of interaction of HPG and HPA axes |
|
| |||
| Estrogena Buffers loss of excitatory synapses and changes in dendritic spine density |
↓ | ||
|
| |||
| Cannabis Use: THC | Promotes downregulation of CB1 receptors on neurons, which alters maturation of PFC GABAergic neurons Promotes upregulation of CB2 receptors on microglia, which alters synaptic pruning |
↑ | Continued investigation of the longevity and intensity of THC-induced brain alteration Evaluation of moderators of THC-related brain change, including age at first use and frequency of use |
|
| |||
| Cannabis Use: CBD | Acts as antagonist for CB1 and CB2 receptorsa Acts as a D2 antagonist, similar to atypical antipsychotic medicationsa |
– | Evaluation of mixed THC/CBD use Investigation of longevity and intensity of CBD-related symptom relief and brain change |
|
| |||
| Psychosocial Factors | |||
|
| |||
| Prophylactic Intervention in At-Risk Individuals | Reduces potential neurotoxicity due to dopaminergic hypersensitivitya Decreases oxidative stress, which in turn may limit reductions in PFC interneuron activitya Decreases stress responsivity/anxiety response, which in turn may reduce hippocampal damage believed to partially drive dopamine dysfunctiona |
↓ | Validation of animal models of psychosis risk states Continued research on biological changes associated with early intervention in humans Mapping of interventions onto biological mechanisms in human studies |
|
| |||
| Social Stressors (e.g., Defeat, Isolation) | Alters dopamine signaling following defeat Alters oligodendrocyte maturation, subsequently alters myelination in prefrontal regions following isolation |
↑ | Evaluation of interaction of diathesis and adolescent stressors on psychosis emergence Exploration of mechanisms linking social functioning and neural change (e.g., myelination) in humans |
|
| |||
| Role Stressors (e.g., Increased Academic Demands) |
Alters cortisol release, which in turn modulates dopamine response Changes hippocampal morphology due to cortisol response |
↑ | Identification of developmental effects of stress in adolescent psychosis samples |
CBD, cannabidiol; GABA, gamma-aminobutyric acid; GM, gray matter; HPA, hypothalamic-pituitary-adrenal; HPG, hypothalamic-pituitary-gonadal; PFC, prefrontal cortex; THC, tetrahydrocannabinol; WM, white matter.
Areas of limited research support.
Figure 1.
Protective factors, risk factors, and psychosis risk in adolescence. CBD, cannabidiol; THC, tetrahydrocannabinol. *Areas of limited research support.
Brain Development
Grey Matter Development and Psychosis
During adolescence, although brain volume remains relatively stable (22), grey (GM) and white matter (WM) undergo substantial microstructural changes. GM changes during adolescence (e.g. changes in cortical thickness) are considered to be due to reductions in synaptic density via pruning. Synaptic pruning is facilitated by microglia, which are extremely active in synaptic maintenance and refine circuitry in an activity-dependent manner(23). Synaptic density peaks in early life and shows age-related decreases (24), with developmental trajectories varying by brain region. Lateral frontal, parietal, and occipital cortical regions show an initial increase in cortical thickness in childhood with a peak around age 10, followed by a period of decline in adolescence and stabilization in adulthood (2). Prefrontal pruning is believed to be ongoing into the third decade of life (25), with synaptic density changes associated with age-related improvements in cognitive ability (26). Puberty coincides with a rapid decrease in synaptic density, which is believed to underlie age-related decreases in GM (27). Adolescence is a key period for prefrontal pruning in particular, along with changes in glutamatergic and GABA-ergic neurotransmitter systems (28). Notably, these late-maturing regions support higher level cognitive functions which both continue to mature across adolescence and are impacted in psychosis. In adolescence, stressors (e.g. social stress, cannabis use) that affect microglial functioning may have ramifications for appropriate development of neural circuits and excitatory-inhibitory balance in the brain.
Disruptions in normal developmental pruning processes have long been implicated in the pathogenesis of schizophrenia (29). Documented reductions in cortical GM in schizophrenia are hypothesized to be due to either early (e.g. reduced synaptogenesis early in life) or late (e.g. exaggerated pruning in adolescence) developmental insults (30), or a combination of both. GM differences have even been documented in CHR youth, with progressive reduction in GM associated with transition to psychosis, consistent with an accelerated pruning model (31). In adult patients, cortical GM volume reductions relative to controls have been demonstrated(32) along with decreased density of dendritic spines (33). Recent research in adult schizophrenia samples has validated postmortem findings of lower levels of presynaptic protein markers such as SV2A (34), putatively reflecting an overall lack of synaptic terminals due to excessive pruning. However, evidence suggests that GM decreases are not unilateral across the brain, with region-specific differences in increased versus decreased volume (35). Additionally, GM reductions may be characteristic of a subtype of psychotic illness (36). However, much evidence is from MRI and thus necessarily inferential, and evidence in adolescent samples is severely limited.
Both atypical and typical antipsychotics have been associated with brain volume change over time (37). Though antipsychotics have been associated with GM decreases, medication-naïve samples have also shown this pattern (38), and findings on the impact of antipsychotics on synaptic density are mixed. The potential interaction of antipsychotics and development has spurred discussion on appropriateness of antipsychotic use in pediatric populations and whether antipsychotics impact brain development. Recently, there has been a growing interest in synaptic pruning as a potential treatment target (39), which exemplifies how the dynamic adolescent period might be leveraged to develop age-targeted treatment strategies.
In addition to synaptic pruning, differences in gyrification have been documented in adolescent schizophrenia patients (40). One theory posits that tension produced by neural connections plays a key role in cortical folding (41), suggesting that pruning-induced alterations in structural connectivity particularly during adolescence would impact gyrification. Though we focus on pruning here, we acknowledge the complexity of GM changes in psychosis. Future reviews may provide key synthesis of extant literature, inclusive of multiple GM developmental processes.
White Matter Development and Psychosis
Myelination of white matter tracts begins in-utero(42) and increases through the second and third decades of life, as assayed by postmortem (42) and imaging studies (3). Recent studies using diffusion imaging techniques have found that WM fractional anisotropy (FA; a putative metric of WM integrity) and mean diffusivity (MD) peak in the majority of regions by age 35 (3), or earlier (43, 44), although many of these regions reach near-mature levels in adolescence, with myelination slowing as adulthood approaches (3). Adolescent WM maturation has been linked to pubertal stage and hormone levels (45). Myelination of WM tracts occurs in a caudal-cranial, posterior-anterior arc (46) such that long-range association tracts and frontaltemporal tracts, which are associated with higher order cognition (43), are undergoing development during adolescence. Importantly, these regions also undergo relatively later pruning processes (as discussed above). While there is preclinical support for FA as an index of myelination (47), some imaging metrics capture additional adolescent changes in WM structure, such as changes in axon diameter (48) and neurite density (49), that have received less attention. Experience-driven changes in WM, including altered oligodendrocyte function and myelination following social deprivation (50), new myelination and remodeling of existing myelin with sensory stimulation (51), and FA increases following motor learning (52), are likely to be important during adolescence, as changes in social and role functions as well as hormonal changes and biological maturation (reviewed below) expose adolescents to a variety of formative new experiences. Finally, GM and WM changes are not fully independent; increases in neural activity may foster increases in myelination (53, 54), however, the relevance of these interactions to psychosis is not well understood.
Psychotic spectrum disorders are commonly regarded as disorders of dysconnectivity, with reductions in FA across nearly all major tracts (54, 55). Although some evidence suggests post-conversion adulthood decline in WM FA among schizophrenia patients (56), reduced FA in long-range association tracts (e.g., inferior longitudinal fasciculus, superior longitudinal fasciculus, inferior fronto-occipital fasciculus) is evident in adolescents with psychosis (57–59), CHR individuals (60, 61), and adolescents with psychotic-like experiences (PLEs) (62, 63). Evidence from cross-sectional and longitudinal studies across the spectrum have suggested these deficits are due to blunting of expected age-related increases in WM volume and FA (59, 64) (see (65) for critical review of these findings related to early vulnerability and developmental models of psychosis). Further research is needed to elucidate the nuanced causal relationship between WM development in adolescence and psychotic symptoms, including how altered WM development relates to other vulnerabilities, like cognitive (43) and social (66) deficits. Given these changes, myelination is another potential target for age-related treatments (54). One area of interest has been administration of fatty acids, as omega-3 polyunsaturated fatty acids are critical for myelin formation (67); studies have shown mixed effects (68), potentially because it is important to intervene during times of active myelination, such as adolescence.
Functional Connectivity and Psychosis
Similar to structural connectivity, functional connectivity undergoes significant development and reorganization during adolescence (e.g., (69, 70)), yet less work has focused on functional connectivity development in adolescent psychosis (71). Extant studies have found that adolescents endorsing PLEs show functional connectivity alterations similar to adult psychosis patients (72, 73), and that age-related connectivity changes are altered in CHR and PLE-endorsing adolescents in amygdalar (73, 74) and working memory circuits (75). Additional work, including findings from large developmental datasets and adolescent psychosis samples, is needed.
Adolescence as a Window for Intervention
The considerable changes in brain, body, and environment during adolescence suggest a period of greater plasticity relative to adults later in the course of illness, which may be leveraged for unique opportunities to intervene. Decades of research emphasizing early intervention (16) are consistent with the notion of adolescence as a particularly optimal time for intervention.
For example, reducing duration of untreated psychosis (DUP) via early intervention has been consistently associated with greater treatment response, functional improvement, and stable treatment gains (76, 77). It has been proposed that early intervention may limit neurotoxic effects of dopaminergic hyperactivity; however, evidence to support this as the primary mechanism by which DUP contributes to poor outcomes is mixed (78). Given the average age of onset, the optimal DUP during which intervention is most likely to be effective coincides with the adolescent developmental period. Early intervention may be bolstered by or utilize the developmental plasticity of adolescence. In particular, adolescent-focused intervention potentially provides the opportunity to alter the developmental illness trajectory. Despite a compelling theoretical basis (79), empirical data on developmental interventions for psychosis in humans are limited. However, animal studies have provided evidence for the efficacy of developmentally sensitive intervention, with adolescent-focused interventions in animal models of schizophrenia leading to long-term, stable gains maintained in adulthood (80, 81).
Factors Particularly Relevant during Adolescence
Social and Familial Environment
Adolescence is an intense period of social change as adolescents navigate individuation and increasing autonomy. Familial relationships take a less central – and more contentious – social role (82, 83) in favor of peer and romantic relationships (84) that become increasingly hierarchical and complex particularly through early adolescence (83). Peer relationships similarly become more influential in adolescence, with greater impact on mood (85), decision-making (86), risk-taking (4), and substance use (87). Adolescents also show increased neuroendocrine and cardiovascular reactivity to social stressors relative to children (88) and increased subcortical activation following social rejection compared to adults (89), suggesting special vulnerability to social stress. Chronic social stress begets feelings of loneliness, which peak in adolescence, as incidence of psychotic disorders increases (90).
Individuals who develop psychosis have been shown to have poorer premorbid social functioning in childhood and adolescence(91), and poor social functioning and reduced friend networks are associated with worsening functioning and increased risk of conversion among CHR youth (92, 93). Conversely, PLEs and WM abnormalities among children and adolescents also predict poor social functioning and social competence, respectively (94). Acute social stressors, including peer victimization, during adolescence are associated with increased PLEs in adulthood (95), and CHR youth report higher physical and psychological bullying (96).
Preclinical experiments suggest possible biological mechanisms, including altered dopamine signaling following social defeat (97) and altered oligodendrocyte maturation and myelination in PFC following juvenile social isolation (50). These provide tentative explanations for the associations between WM abnormalities in late-developing regions and social functioning in subsyndromal and prodromal adolescents (66, 94), although this has yet to be thoroughly tested in humans. Additionally, diathesis-stress models suggest that genetic and early life insults increase adolescent vulnerability to stress (8), so tests of interactions between diathesis factors and adolescent stressors on neurodevelopment or psychosis emergence are an important future direction.
Though social changes in adolescence may confer risk, the social context of the adolescent, and specifically the family context, may provide unique opportunity to scaffold treatment that may not be possible once individuals live independently. Existing family interventions for psychosis focus on reducing expressed emotion (EE), the hostile family environment that can emerge when a family member is diagnosed with severe mental illness. Family-focused interventions targeting EE can improve the relationship between caregivers and CHR youth, have prophylactic efficacy, and reduce relapse in fully psychotic youth (98). Beyond EE interventions, adolescent-specific modifications of psychosocial treatments for psychosis are limited. However, interventions for other severe mental illnesses provide compelling evidence that the family environment can be uniquely leveraged during treatment. Interpersonal and Social Rhythm Therapy (IPSRT), an evidence-based psychotherapy for the treatment of bipolar disorder, focuses on the relationship between mood and stressors, regulating sleep-wake and social rhythms, and identifying precipitants to worsening mood(99). In the adolescent modification (IPSRT-A), parents are flexibly involved throughout treatment and reinforce the adolescent’s successful use of treatment strategies (100). Though IPSRT-A is indicated for bipolar disorder, benefits of family support in implementation of therapeutic strategies at home may generalize to psychosis interventions and may help improve treatment adherence.
Role Changes
In addition to social changes, adolescents are tasked with navigating role changes, especially increasing academic and work demands. Across cultures, the transition to “adulthood” is marked by full assumption of adult roles and responsibilities (101). Despite this, the research base that focuses on how role changes affect typically developing adolescents’ mood and later functioning is much smaller than that on social change, often focusing on early adulthood and large role changes such as graduation or entering the workforce (102). Role responsibilities and transitions, especially in academics, are commonly cited as the most significant stressors for adolescents (103). However, moderating this stress is important, as academic functioning and educational attainment (primarily associated with adolescence) have been found to correlate with higher levels of life satisfaction and health even decades after this life stage ends (104). Research investigating the predictive power of role changes for later psychopathology and symptom severity is more limited than for social functioning. Findings around role changes and psychopathology are mixed, potentially due to inconsistencies in measurement (105). However, academic performance has been a research target for understanding the relationship between role functioning and adolescent psychosis (106). Findings are mixed (106), but suggest that premorbid academic performance differs among psychosis patients, first-degree relatives, and healthy controls (107). More broadly, role functioning in CHR youth appears to be predicted by WM abnormalities (66), and to in turn predict severity of psychosis symptomatology (108). Additionally, stressors, such as role changes, are believed to contribute to the emergence or worsening of psychotic symptoms (109). Stress exposure leads to cortisol release which is related acutely to dopamine release (110) and chronically with potential neurotoxic effects, particularly on hippocampal morphology (reviewed in (111)).
Pubertal Risk Factors
One definition of the onset of adolescence is with puberty (112), which begins with adrenarche, the release of androgens from the adrenal gland (113, 114). Androgen levels reach adult levels in the late teens or early 20’s (115) and are associated with secondary sex characteristic development (114). Gonadarche is the activation of the hypothalamic-pituitary-gonadal (HPG) axis (114), which stimulate ovaries and testes to produce estrogen and testosterone, ultimately resulting in sexual maturity. However, while hormones may seem attractive as a measure of the timing of adolescence, the issue is complex, as such measures relate to, but do not perfectly mirror, physical development (112). Moreover, hormone measures vary widely, both within and between pubertal stages (116). Thus, multimodal assessments are critical for accurate assessment. In addition to impacting the body, hormonal changes during puberty may profoundly affect neurodevelopment (114). Effects can include organizational effects (structural changes) and activational effects (fluctuating activity) (117). During typical adolescent development, dramatic increases in hormones (114), such as testosterone and estradiol (118), contribute to initiation of a period of structural reorganization involving GM volume decreases and increases in WM volume and integrity (45), consistent with cortical pruning and myelination. Moreover, such hormonal changes in have been associated with cognitive functions developing during adolescence such as affective reactivity and reward processing (118, 119). In addition to gonadal hormones, stress hormones such as cortisol also increase in basal levels during puberty (120), as does cortisol release in response to stress (121). The neurological effects of stress and cortisol exposure in adolescence also seem to be longer-lasting and more pervasive compared to adulthood (122).
Support for a role of hormones in psychosis comes from longstanding findings of a difference in incidence and onset in males and females (6, 123), as well as altered hormone levels in adult patients (124, 125). One hypothesis is that estrogen may be neuroprotective (126, 127), potentially through buffering the loss of excitatory synapses (128), and influence on dendritic spine density (129). In addition to potential estrogen effects, there is also growing evidence for reduced testosterone in adults with schizophrenia(123). Given overlap between the post-pubertal period and psychosis, there is also interest in age-specific impacts of hormonal changes in adolescence, both in terms of risk and protective factors. For example, sex hormones may be particularly relevant to brain-behavior relationships, as activational effects may increase sensitivity of neural circuits to environmental input (114). In addition, elevated dopaminergic signaling during adolescence may be moderated by testosterone (130), which also predicts structural connectivity of thalamo-striato-cortical networks (131) that have been implicated in schizophrenia and CHR youth. Recent work has investigated potential hormonally-based interventions, but not in adolescent patients (123). In addition to gonadal hormones, the role of HPA axis function and development in psychosis onset has garnered significant study (e.g., (111, 132, 133)), and there is evidence for coupling of HPA and HPG axes (134). Increased basal cortisol and blunted cortisol reactivity have been found in psychosis populations (reviewed in (133)). While theoretical links have been drawn between hormonal changes, brain maturation, and psychosis onset (135, 136), empirical data are scarce and further research is needed (125). Further investigation of how hormonal changes, broadly construed, intersect or interact with early stages of psychosis will be critical for our understanding of divergent developmental trajectories (137, 138).
Adolescent Cannabis Use
Substance use, particularly alcohol and cannabis, is often initiated in adolescence (139). Though alcohol has been associated with increased psychosis risk, the neurodevelopmental mechanisms are unknown (140). For the purposes of this review, we focus on cannabis given the depth of literature focused on neurodevelopmental impacts of cannabis. Furthermore, cannabis use has become of particular interest as access to cannabis has changed with legalization of medical and recreational cannabis use in several states in the US. Additionally, cannabis use may have distinct neural effects in adolescence.
Cannabis acts on the brain’s endocannabinoid system, which, in part, plays a key role in PFC maturation. Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, is associated with cannabinoid receptor type 1 (CB1) downregulation on neurons and cannabinoid receptor type 2 (CB2) upregulation on microglia (141). CB1 mediates developmental processes, including the GABA-ergic PFC neuron maturation believed to support development of inhibitory control (142). Appropriate development of the GABA system is key to excitatory/inhibitory balance, with disruptions leading to PFC dysfunction (143). GABAergic dysfunction and prefrontal hypofunction are indicated in psychosis pathophysiology, and the impact of cannabis use on pruning and the GABA system may exacerbate risk for or contribute to psychotic onset (143). Given the role of microglia in pruning, adolescent THC exposure in rodents potentially alters the neurodevelopmental trajectory (142), with microglia alterations and related cognitive deficits persisting into adulthood (141). Alternatively, a recent meta-analysis of human studies suggests cannabis use has a small effect on cognitive functioning in adulthood, which is reduced by discontinuing use (144). While existing literature provides a compelling rationale for adverse effects of adolescent cannabis use, intensity and longevity of its effects remains an empirical question.
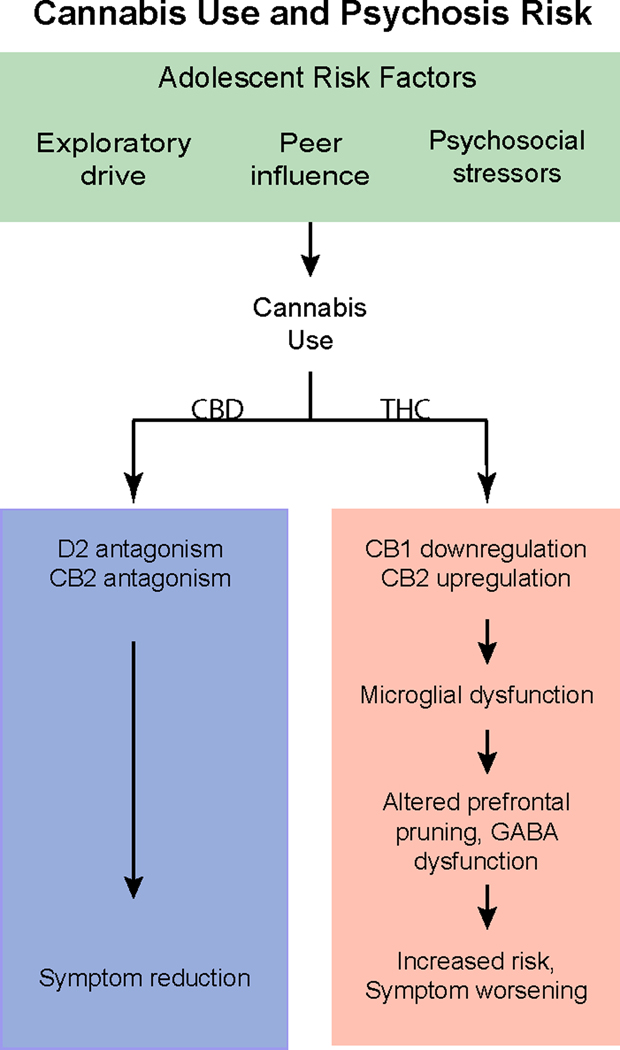
Despite growing evidence for psychogenic properties of THC, the relationship between adolescent cannabis use and psychosis risk is not straightforward, namely due to the differential effects of THC compared to other cannabinoids, such as cannabidiol (CBD) (Figure 2). CBD acts as an antagonist for CB1 and CB2 receptors and may have opposite neural effects as THC, and thus may not have the same psychosis-inducing or risk-heightening effects (145, 146). CBD can potentially alleviate psychotic symptoms and may act as a D2 antagonist, in a way that is analogous to atypical antipsychotics (147). Peripubertal CBD exposure prevents onset of psychosis-like behaviors in a rodent model of psychosis risk (148).
Figure 2.
Cannabis use and psychosis risk in adolescence. CB1, CB1, receptor; CB2, CB2 receptor; CBD, cannabidiol; GABA, gamma-aminobutync acid; THC, tetrahydrocannabinol.
Evidence suggests that cannabis can impact specific brain developmental processes in adolescence, indicating a sensitive period for adverse effects of cannabis use on psychosis risk particularly in light of differential effects in adults versus adolescents. Adolescent cannabis use may also interact with genetic risk for psychosis (149). However, the relationship between adolescent cannabis use and psychosis risk is complex, and the impact of potentially opposing effects of THC and CBD, along with environmental, genetic, and behavioral moderators requires further study.
Conclusions
A greater understanding of the opportunity and challenges that accompany adolescence has driven a push to carve out adolescent medicine, and adolescent mental health, as unique focus areas (150). Work focusing on adolescence has potential to not only reveal truths about the etiology of complex illnesses that arise during this period, but also to identify aspects of the adolescent experience that can be leveraged to improve outcomes. We have endeavored here to focus on neural changes, social and role changes, onset of adrenarche and gonadarche, and increased use of cannabis that construct a unique window of vulnerability in which the adolescent is highly susceptible to the onset of psychosis. We also have endeavored to highlight further research questions, including evaluation of the effects of many other risk factors that exist for adolescents (e.g., trauma), continued research into diathesis factors and interaction with stressors that contribute to heightened vulnerability, and investigation into factors that may moderate the effects of these stressors on the development and severity of symptoms (e.g. early intervention, medication). Moreover, adolescence is not a unitary period, and it is likely that all of these risk and protective factors fluctuate and interact across this age range. Gaining a finer understanding of the relative timing of brain development, hormonal changes, risk factors, and symptoms, is going to be critical for identifying age appropriate or age targeted intervention points. In addition, research that not only identifies developmentally specific treatment targets, but that takes advantage of the unique strengths of the adolescent social and family environment to maximize existing treatments, may be able to make strides towards improving some of the struggles faced by these youth.
Acknowledgements
This work was supported by MH116433 (KHK) and MH101506 (KHK).
Footnotes
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilmore JH, Knickmeyer RC, Gao W (2018): Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 19:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. (2008): Neurodevelopmental trajectories of the human cerebral cortex. Journal of neuroscience. 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008): Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- 4.Chein J, Albert D, O’Brien L, Uckert K, Steinberg L (2011): Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev Sci. 14:F1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blakemore SJ, Mills KL (2014): Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 65:187–207. [DOI] [PubMed] [Google Scholar]
- 6.Dalsgaard S, Thorsteinsson E, Trabjerg BB, Schullehner J, Plana-Ripoll O, Brikell I, et al. (2019): Incidence Rates and Cumulative Incidences of the Full Spectrum of Diagnosed Mental Disorders in Childhood and Adolescence. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 62:593–602. [DOI] [PubMed] [Google Scholar]
- 8.Walker E, Kestler L, Bollini A, Hochman KM (2004): Schizophrenia: etiology and course. Annu Rev Psychol. 55:401–430. [DOI] [PubMed] [Google Scholar]
- 9.Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, et al. (2003): Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 29:653–669. [DOI] [PubMed] [Google Scholar]
- 10.Sham PC, MacLean CJ, Kendler KS (1994): A typological model of schizophrenia based on age at onset, sex and familial morbidity. Acta Psychiatr Scand. 89:135–141. [DOI] [PubMed] [Google Scholar]
- 11.Loranger AW (1984): Sex difference in age at onset of schizophrenia. Arch Gen Psychiatry. 41:157–161. [DOI] [PubMed] [Google Scholar]
- 12.Linscott RJ, van Os J (2013): An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 13.Mennigen E, Bearden CE (2019): Psychosis Risk and Development: What Do We Know From Population-Based Studies? Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollis C (2000): Adult outcomes of child-and adolescent-onset schizophrenia: diagnostic stability and predictive validity. American Journal of Psychiatry. 157:1652–1659. [DOI] [PubMed] [Google Scholar]
- 15.Ballageer T, Malla A, Manchanda R, Takhar J, Haricharan R (2005): Is adolescent-onset first-episode psychosis different from adult onset? Journal of the American Academy of Child & Adolescent Psychiatry. 44:782–789. [DOI] [PubMed] [Google Scholar]
- 16.Kane JM, Robinson DG, Schooler NR, Mueser KT, Penn DL, Rosenheck RA, et al. (2016): Comprehensive Versus Usual Community Care for First-Episode Psychosis: 2-Year Outcomes From the NIMH RAISE Early Treatment Program. Am J Psychiatry. 173:362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, et al. (2008): Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 20:1297–1327. [DOI] [PubMed] [Google Scholar]
- 18.Stentebjerg-Olesen M, Pagsberg AK, Fink-Jensen A, Correll CU, Jeppesen P (2016): Clinical Characteristics and Predictors of Outcome of Schizophrenia-Spectrum Psychosis in Children and Adolescents: A Systematic Review. J Child Adolesc Psychopharmacol. 26:410–427. [DOI] [PubMed] [Google Scholar]
- 19.Immonen J, Jaaskelainen E, Korpela H, Miettunen J (2017): Age at onset and the outcomes of schizophrenia: A systematic review and meta-analysis. Early Interv Psychiatry. 11:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajji T, Ismail Z, Mulsant B (2009): Age at onset and cognition in schizophrenia: meta-analysis. The British Journal of Psychiatry. 195:286–293. [DOI] [PubMed] [Google Scholar]
- 21.Lay B, Blanz B, Hartmann M, Schmidt MH (2000): The psychosocial outcome of adolescent-onset schizophrenia: a 12-year followup. Schizophr Bull. 26:801–816. [DOI] [PubMed] [Google Scholar]
- 22.Dekaban AS (1978): Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 4:345–356. [DOI] [PubMed] [Google Scholar]
- 23.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. (2012): Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huttenlocher PR (1979): Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Res. 163:195–205. [DOI] [PubMed] [Google Scholar]
- 25.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, et al. (2011): Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharitonova M, Martin RE, Gabrieli JD, Sheridan MA (2013): Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Developmental cognitive neuroscience. 6:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drzewiecki CM, Willing J, Juraska JM (2016): Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: a role for pubertal onset. Synapse. 70:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caballero A, Granberg R, Tseng KY (2016): Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci Biobehav Rev. 70:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinberg I (1982): Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of psychiatric research. 17:319–334. [DOI] [PubMed] [Google Scholar]
- 30.Weinberger DR (1987): Implications of normal brain development for the pathogenesis of schizophrenia. Archives of general psychiatry. 44:660–669. [DOI] [PubMed] [Google Scholar]
- 31.Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, et al. (2015): Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biological psychiatry. 77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gur RE, Turetsky BI, Bilker WB, Gur RC (1999): Reduced gray matter volume in schizophrenia. Archives of general psychiatry. 56:905–911. [DOI] [PubMed] [Google Scholar]
- 33.Glantz LA, Lewis DA (2000): Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 57:65–73. [DOI] [PubMed] [Google Scholar]
- 34.Onwordi EC, Halff EF, Whitehurst T, Mansur A, Cotel MC, Wells L, et al. (2020): Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. (2008): Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biological psychiatry. 64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chand GB, Dwyer DB, Erus G, Sotiras A, Varol E, Srinivasan D, et al. (2020): Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain. 143:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011): Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Archives of general psychiatry. 68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah C, Zhang W, Xiao Y, Yao L, Zhao Y, Gao X, et al. (2017): Common pattern of gray-matter abnormalities in drug-naive and medicated first-episode schizophrenia: a multimodal meta-analysis. Psychol Med. 47:401–413. [DOI] [PubMed] [Google Scholar]
- 39.Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. (2019): Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nature neuroscience. 22:374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White T, Andreasen NC, Nopoulos P, Magnotta V (2003): Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biol Psychiatry. 54:418–426. [DOI] [PubMed] [Google Scholar]
- 41.Van Essen DC (1997): A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 385:313–318. [DOI] [PubMed] [Google Scholar]
- 42.Yakovlev P (1967): The myelogenetic cycles of regional maturation of the brain. Regional development of the brain in early life.3–70. [Google Scholar]
- 43.Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, et al. (2014): Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol Psychiatry. 75:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karlsgodt KH, John M, Ikuta T, Rigoard P, Peters BD, Derosse P, et al. (2015): The accumbofrontal tract: Diffusion tensor imaging characterization and developmental change from childhood to adulthood. Hum Brain Mapp. 36:4954–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peper JS, Hulshoff Pol HE, Crone EA, van Honk J (2011): Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 191:28–37. [DOI] [PubMed] [Google Scholar]
- 46.Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, et al. (2010): Longitudinal changes in grey and white matter during adolescence. Neuroimage. 49:94–103. [DOI] [PubMed] [Google Scholar]
- 47.Chang EH, Argyelan M, Aggarwal M, Chandon TS, Karlsgodt KH, Mori S, et al. (2017): The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuroimage. 147:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paus T (2010): Growth of white matter in the adolescent brain: myelin or axon? Brain and cognition. 72:26–35. [DOI] [PubMed] [Google Scholar]
- 49.Chang YS, Owen JP, Pojman NJ, Thieu T, Bukshpun P, Wakahiro ML, et al. (2015): White Matter Changes of Neurite Density and Fiber Orientation Dispersion during Human Brain Maturation. PLoS One. 10:e0123656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makinodan M, Rosen KM, Ito S, Corfas G (2012): A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 337:1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE (2018): Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat Neurosci. 21:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scholz J, Klein MC, Behrens TE, Johansen-Berg H (2009): Training induces changes in white-matter architecture. Nat Neurosci. 12:1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumann N, Pham-Dinh D (2001): Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 81:871–927. [DOI] [PubMed] [Google Scholar]
- 54.Karlsgodt KH (2016): Diffusion Imaging of White Matter In Schizophrenia: Progress and Future Directions. Biol Psychiatry Cogn Neurosci Neuroimaging. 1:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. (2018): Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 23:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters BD, Blaas J, de Haan L (2010): Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? Journal of psychiatric research. 44:993–1004. [DOI] [PubMed] [Google Scholar]
- 57.Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, Chen S, Kumra S (2007): Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Archives of general psychiatry. 64. [DOI] [PubMed] [Google Scholar]
- 58.Epstein KA, Cullen KR, Mueller BA, Robinson P, Lee S, Kumra S (2014): White matter abnormalities and cognitive impairment in early-onset schizophrenia-spectrum disorders. J Am Acad Child Adolesc Psychiatry. 53:362–372 e361–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Epstein KA, Kumra S (2015): White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: A naturalistic diffusion tensor imaging study. Psychiatry Res. 232:34–41. [DOI] [PubMed] [Google Scholar]
- 60.Krakauer K, Nordentoft M, Glenthoj BY, Raghava JM, Nordholm D, Randers L, et al. (2018): White matter maturation during 12 months in individuals at ultra-high-risk for psychosis. Acta Psychiatr Scand. 137:65–78. [DOI] [PubMed] [Google Scholar]
- 61.Vijayakumar N, Bartholomeusz C, Whitford T, Hermens DF, Nelson B, Rice S, et al. (2016): White matter integrity in individuals at ultra-high risk for psychosis: a systematic review and discussion of the role of polyunsaturated fatty acids. BMC Psychiatry. 16:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobson S, Kelleher I, Harley M, Murtagh A, Clarke M, Blanchard M, et al. (2010): Structural and functional brain correlates of subclinical psychotic symptoms in 11–13 year old schoolchildren. Neuroimage. 49:1875–1885. [DOI] [PubMed] [Google Scholar]
- 63.O’Hanlon E, Leemans A, Kelleher I, Clarke MC, Roddy S, Coughlan H, et al. (2015): White Matter Differences Among Adolescents Reporting Psychotic Experiences: A Population-Based Diffusion Magnetic Resonance Imaging Study. JAMA Psychiatry. 72:668–677. [DOI] [PubMed] [Google Scholar]
- 64.Hegarty CE, Jolles DD, Mennigen E, Jalbrzikowski M, Bearden CE, Karlsgodt KH (2019): Disruptions in White Matter Maturation and Mediation of Cognitive Development in Youths on the Psychosis Spectrum. Biol Psychiatry Cogn Neurosci Neuroimaging. 4:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peters BD, Karlsgodt KH (2015): White matter development in the early stages of psychosis. Schizophr Res. 161:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD (2009): White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 66:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peters BD, Voineskos AN, Szeszko PR, Lett TA, DeRosse P, Guha S, et al. (2014): Brain white matter development is associated with a human-specific haplotype increasing the synthesis of long chain fatty acids. J Neurosci. 34:6367–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fusar-Poli P, Berger G (2012): Eicosapentaenoic acid interventions in schizophrenia: meta-analysis of randomized, placebo-controlled studies. J Clin Psychopharmacol. 32:179–185. [DOI] [PubMed] [Google Scholar]
- 69.Uddin LQ, Supekar KS, Ryali S, Menon V (2011): Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 31:18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ernst M, Torrisi S, Balderston N, Grillon C, Hale EA (2015): fMRI functional connectivity applied to adolescent neurodevelopment. Annu Rev Clin Psychol. 11:361–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satterthwaite TD, Baker JT (2015): How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr Opin Neurobiol. 30:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amico F, O’Hanlon E, Kraft D, Oertel-Knochel V, Clarke M, Kelleher I, et al. (2017): Functional Connectivity Anomalies in Adolescents with Psychotic Symptoms. PLoS One. 12:e0169364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jalbrzikowski M, Murty VP, Tervo-Clemmens B, Foran W, Luna B (2019): Age-Associated Deviations of Amygdala Functional Connectivity in Youths With Psychosis Spectrum Disorders: Relevance to Psychotic Symptoms. Am J Psychiatry. 176:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gee DG, Karlsgodt KH, van Erp TG, Bearden CE, Lieberman MD, Belger A, et al. (2012): Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophr Res. 134:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karlsgodt KH, van Erp TG, Bearden CE, Cannon TD (2014): Altered relationships between age and functional brain activation in adolescents at clinical high risk for psychosis. Psychiatry Res. 221:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perkins DO, Gu H, Boteva K, Lieberman JA (2005): Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. American journal of psychiatry. 162:1785–1804. [DOI] [PubMed] [Google Scholar]
- 77.Kane JM, Robinson DG, Schooler NR, Mueser KT, Penn DL, Rosenheck RA, et al. (2015): Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. American Journal of Psychiatry. 173:362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anderson KK, Voineskos A, Mulsant BH, George TP, McKenzie KJ (2014): The role of untreated psychosis in neurodegeneration: a review of hypothesized mechanisms of neurotoxicity in first-episode psychosis. The Canadian Journal of Psychiatry. 59:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marín O (2016): Developmental timing and critical windows for the treatment of psychiatric disorders. Nature medicine. 22:1229. [DOI] [PubMed] [Google Scholar]
- 80.Du Y, Grace AA (2013): Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 38:1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cabungcal J-H, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, et al. (2014): Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 83:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laursen B, Williams VA (1997): Perceptions of interdependence and closeness in family and peer relationships among adolescents with and without romantic partners. New Directions for Child and Adolescent Development. 1997:3–20. [DOI] [PubMed] [Google Scholar]
- 83.Steinberg L, Morris AS (2001): Adolescent development. Annual review of psychology. 52:83–110. [DOI] [PubMed] [Google Scholar]
- 84.Furman W (2002): The emerging field of adolescent romantic relationships. Current directions in psychological science. 11:177–180. [Google Scholar]
- 85.Sebastian C, Viding E, Williams KD, Blakemore SJ (2010): Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 72:134–145. [DOI] [PubMed] [Google Scholar]
- 86.Larson RW, Richards MH, Moneta G, Holmbeck G, Duckett E (1996): Changes in adolescents’ daily interactions with their families from ages 10 to 18: Disengagement and transformation. Developmental psychology. 32:744. [Google Scholar]
- 87.Caouette JD, Ewing SWF (2017): Four mechanistic models of peer influence on adolescent cannabis use. Current addiction reports. 4:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, et al. (2009): Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol. 21:47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vijayakumar N, Cheng TW, Pfeifer JH (2017): Neural correlates of social exclusion across ages: A coordinate-based meta-analysis of functional MRI studies. NeuroImage. 153:359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shovestul B, Han J, Germine L, Dodell-Feder D (2019): Risk factors for loneliness: The high relative importance of age versus other factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, et al. (1997): Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. American Journal of Psychiatry. 154:1544–1550. [DOI] [PubMed] [Google Scholar]
- 92.Cornblatt BA, Carrion RE, Addington J, Seidman L, Walker EF, Cannon TD, et al. (2012): Risk factors for psychosis: impaired social and role functioning. Schizophr Bull. 38:1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robustelli BL, Newberry RE, Whisman MA, Mittal VA (2017): Social relationships in young adults at ultra high risk for psychosis. Psychiatry Res. 247:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DeRosse P, Ikuta T, Karlsgodt KH, Peters BD, Gopin CB, Szeszko PR, et al. (2017): White Matter Abnormalities Associated With Subsyndromal Psychotic-Like Symptoms Predict Later Social Competence in Children and Adolescents. Schizophr Bull. 43:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y, Mendonca M, Johnson S, O’Reilly H, Bartmann P, Marlow N, et al. (2019): Testing the neurodevelopmental, trauma and developmental risk factor models of psychosis using a naturalistic experiment. Psychol Med.1–10. [DOI] [PubMed] [Google Scholar]
- 96.Addington J, Stowkowy J, Cadenhead KS, Cornblatt BA, McGlashan TH, Perkins DO, et al. (2013): Early traumatic experiences in those at clinical high risk for psychosis. Early Interv Psychiatry. 7:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Selten JP, van der Ven E, Rutten BP, Cantor-Graae E (2013): The social defeat hypothesis of schizophrenia: an update. Schizophr Bull. 39:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Claxton M, Onwumere J, Fornells-Ambrojo M (2017): Do family interventions improve outcomes in early psychosis? A systematic review and meta-analysis. Frontiers in psychology. 8:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frank E, Swartz HA, Kupfer DJ (2000): Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biological psychiatry. 48:593–604. [DOI] [PubMed] [Google Scholar]
- 100.Hlastala SA, Kotler JS, McClellan JM, McCauley EA (2010): Interpersonal and social rhythm therapy for adolescents with bipolar disorder: treatment development and results from an open trial. Depression and anxiety. 27:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schlegel A, Barry Iii H (1991): Adolescence: An anthropological inquiry. Free Press; (New York: ). [Google Scholar]
- 102.Cohen P, Kasen S, Chen H, Hartmark C, Gordon K (2003): Variations in patterns of developmental transitions in the emerging adulthood period. Dev Psychol. 39:657–669. [DOI] [PubMed] [Google Scholar]
- 103.American Psychological Association (2013): Stress in America Survey. [Google Scholar]
- 104.Murrell SA, Salsman NL, Meeks S (2003): Educational attainment, positive psychological mediators, and resources for health and vitality in older adults. J Aging Health. 15:591–615. [DOI] [PubMed] [Google Scholar]
- 105.Armanen A, Lahti M, Therman S, Suvisaari J, Lindgren M (2018): Psychological, social and role functioning as predictors of psychosis in an adolescent psychiatric sample. Early Interv Psychiatry. 12:1064–1071. [DOI] [PubMed] [Google Scholar]
- 106.Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttunen T, Rabe-Hesketh S, et al. (1999): School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry. 56:457–463. [DOI] [PubMed] [Google Scholar]
- 107.Bucci P, Galderisi S, Mucci A, Rossi A, Rocca P, Bertolino A, et al. (2018): Premorbid academic and social functioning in patients with schizophrenia and its associations with negative symptoms and cognition. Acta Psychiatr Scand. 138:253–266. [DOI] [PubMed] [Google Scholar]
- 108.Addington J, Penn D, Woods SW, Addington D, Perkins DO (2008): Social functioning in individuals at clinical high risk for psychosis. Schizophrenia research. 99:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, et al. (2003): The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 29:671–692. [DOI] [PubMed] [Google Scholar]
- 110.Pruessner JC, Champagne F, Meaney MJ, Dagher A (2004): Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 24:2825–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Walker E, Mittal V, Tessner K (2008): Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 4:189–216. [DOI] [PubMed] [Google Scholar]
- 112.Shirtcliff EA, Dahl RE, Pollak SD (2009): Pubertal development: correspondence between hormonal and physical development. Child Dev. 80:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palmert MR, Boepple PA (2001): Variation in the timing of puberty: clinical spectrum and genetic investigation. J Clin Endocrinol Metab. 86:2364–2368. [DOI] [PubMed] [Google Scholar]
- 114.Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH (2018): Puberty and the human brain: Insights into adolescent development. Neurosci Biobehav Rev. 92:417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Havelock JC, Auchus RJ, Rainey WE (2004): The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 22:337–347. [DOI] [PubMed] [Google Scholar]
- 116.Dorn LD (2006): Measuring puberty. J Adolesc Health. 39:625–626. [DOI] [PubMed] [Google Scholar]
- 117.Arnold AP (2009): The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 55:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peper JS, Dahl RE (2013): Surging Hormones: Brain-Behavior Interactions During Puberty. Curr Dir Psychol Sci. 22:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vijayakumar N, Pfeifer JH, Flournoy JC, Hernandez LM, Dapretto M (2019): Affective reactivity during adolescence: Associations with age, puberty, and testosterone. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elmlinger MW, Kuhnel W, Ranke MB (2002): Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med. 40:1151–1160. [DOI] [PubMed] [Google Scholar]
- 121.Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C (2009): Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 21:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Romeo RD (2010): Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 52:244–253. [DOI] [PubMed] [Google Scholar]
- 123.Owens SJ, Murphy CE, Purves-Tyson TD, Weickert TW, Shannon Weickert C (2018): Considering the role of adolescent sex steroids in schizophrenia. J Neuroendocrinol. 30:453–460. [DOI] [PubMed] [Google Scholar]
- 124.Misiak B, Frydecka D, Loska O, Moustafa AA, Samochowiec J, Kasznia J, et al. (2018): Testosterone, DHEA and DHEA-S in patients with schizophrenia: A systematic review and meta-analysis. Psychoneuroendocrinology. 89:92–102. [DOI] [PubMed] [Google Scholar]
- 125.Trotman HD, Holtzman CW, Ryan AT, Shapiro DI, MacDonald AN, Goulding SM, et al. (2013): The development of psychotic disorders in adolescence: a potential role for hormones. Horm Behav. 64:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wei SM, Berman KF (2019): Ovarian hormones, genes, and the brain: the case of estradiol and the brain-derived neurotrophic factor (BDNF) gene. Neuropsychopharmacology. 44:223–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gogos A, Sbisa AM, Sun J, Gibbons A, Udawela M, Dean B (2015): A Role for Estrogen in Schizophrenia: Clinical and Preclinical Findings. Int J Endocrinol. 2015:615356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Erli F, Palmos AB, Raval P, Mukherjee J, Sellers KJ, Gatford NJF, et al. (2020): Estradiol reverses excitatory synapse loss in a cellular model of neuropsychiatric disorders. Transl Psychiatry. 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gould E, Woolley CS, Frankfurt M, McEwen BS (1990): Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 10:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sato SM, Schulz KM, Sisk CL, Wood RI (2008): Adolescents and androgens, receptors and rewards. Horm Behav. 53:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Asato MR, Terwilliger R, Woo J, Luna B (2010): White matter development in adolescence: a DTI study. Cereb Cortex. 20:2122–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holtzman CW, Trotman HD, Goulding SM, Ryan AT, Macdonald AN, Shapiro DI, et al. (2013): Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 249:172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shah JL, Malla AK (2015): Much ado about much: stress, dynamic biomarkers and HPA axis dysregulation along the trajectory to psychosis. Schizophr Res. 162:253–260. [DOI] [PubMed] [Google Scholar]
- 134.Shirtcliff EA, Dismukes AR, Marceau K, Ruttle PL, Simmons JG, Han G (2015): A dualaxis approach to understanding neuroendocrine development. Dev Psychobiol. 57:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Galdos PM, van Os JJ, Murray RM (1993): Puberty and the onset of psychosis. Schizophr Res. 10:7–14. [DOI] [PubMed] [Google Scholar]
- 136.Saugstad LF (1989): Age at puberty and mental illness. Towards a neurodevelopmental aetiology of Kraepelin’s endogenous psychoses. Br J Psychiatry. 155:536–544. [DOI] [PubMed] [Google Scholar]
- 137.Schimmelmann BG, Conus P, Cotton S, McGorry PD, Lambert M (2007): Pre-treatment, baseline, and outcome differences between early-onset and adult-onset psychosis in an epidemiological cohort of 636 first-episode patients. Schizophr Res. 95:1–8. [DOI] [PubMed] [Google Scholar]
- 138.Amminger GP, Henry LP, Harrigan SM, Harris MG, Alvarez-Jimenez M, Herrman H, et al. (2011): Outcome in early-onset schizophrenia revisited: findings from the Early Psychosis Prevention and Intervention Centre long-term follow-up study. Schizophr Res. 131:112–119. [DOI] [PubMed] [Google Scholar]
- 139.Patrick ME, Schulenberg JE, O’malley PM, Johnston LD, Bachman JG (2011): Adolescents’ reported reasons for alcohol and marijuana use as predictors of substance use and problems in adulthood. Journal of studies on alcohol and drugs. 72:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barkus E, Murray RM (2010): Substance use in adolescence and psychosis: clarifying the relationship. Annu Rev Clin Psychol. 6:365–389. [DOI] [PubMed] [Google Scholar]
- 141.Zamberletti E, Gabaglio M, Prini P, Rubino T, Parolaro D (2015): Cortical neuroinflammation contributes to long-term cognitive dysfunctions following adolescent delta-9-tetrahydrocannabinol treatment in female rats. European Neuropsychopharmacology. 25:2404–2415. [DOI] [PubMed] [Google Scholar]
- 142.Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, et al. (2015): Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiology of disease. 73:60–69. [DOI] [PubMed] [Google Scholar]
- 143.Renard J, Rushlow WJ, Laviolette SR (2018): Effects of adolescent THC exposure on the prefrontal GABAergic system: implications for schizophrenia-related psychopathology. Frontiers in psychiatry. 9:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scott JC, Slomiak ST, Jones JD, Rosen AF, Moore TM, Gur RC (2018): Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA psychiatry. 75:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. (2010): Opposite effects of Δ−9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 35:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leweke F, Piomelli D, Pahlisch F, Muhl D, Gerth C, Hoyer C, et al. (2012): Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Translational psychiatry. 2:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seeman P (2016): Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Translational psychiatry. 6:e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stark T, Ruda-Kucerova J, Iannotti FA, D’Addario C, Di Marco R, Pekarik V, et al. (2019): Peripubertal cannabidiol treatment rescues behavioral and neurochemical abnormalities in the MAM model of schizophrenia. Neuropharmacology. 146:212–221. [DOI] [PubMed] [Google Scholar]
- 149.Estrada G, Fatjó‐Vilas M, Munoz M, Pulido G, Minano M, Toledo E, et al. (2011): Cannabis use and age at onset of psychosis: further evidence of interaction with COMT Val158Met polymorphism. Acta Psychiatrica Scandinavica. 123:485–492. [DOI] [PubMed] [Google Scholar]
- 150.Lee L, Upadhya KK, Matson PA, Adger H, Trent ME (2016): The status of adolescent medicine: building a global adolescent workforce. Int J Adolesc Med Health. 28:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]