ABSTRACT
Global climate changes cause extreme temperatures and a significant reduction in crop production, leading to food insecurity worldwide. Temperature extremes (including both heat and cold stresses) is one of the most limiting factors in plant growth and development and severely affect plant physiology, biochemical, and molecular processes. Biostimulants like melatonin (MET) have a multifunctional role that acts as a “defense molecule” to safeguard plants against the noxious effects of temperature stress. MET treatment improves plant growth and temperature tolerance by improving several defense mechanisms. Current research also suggests that MET interacts with other molecules, like phytohormones and gaseous molecules, which greatly supports plant adaptation to temperature stress. Genetic engineering via overexpression or CRISPR/Cas system of MET biosynthetic genes uplifts the MET levels in transgenic plants and enhances temperature stress tolerance. This review highlights the critical role of MET in plant production and tolerance against temperature stress. We have documented how MET interacts with other molecules to alleviate temperature stress. MET-mediated molecular breeding would be great potential in helping the adverse effects of temperature stress by creating transgenic plants.
KEYWORDS: Biostimulants, climate change, cold stress, crosstalk, food security, freezing temperature, genetic engineering
Graphical Abstract
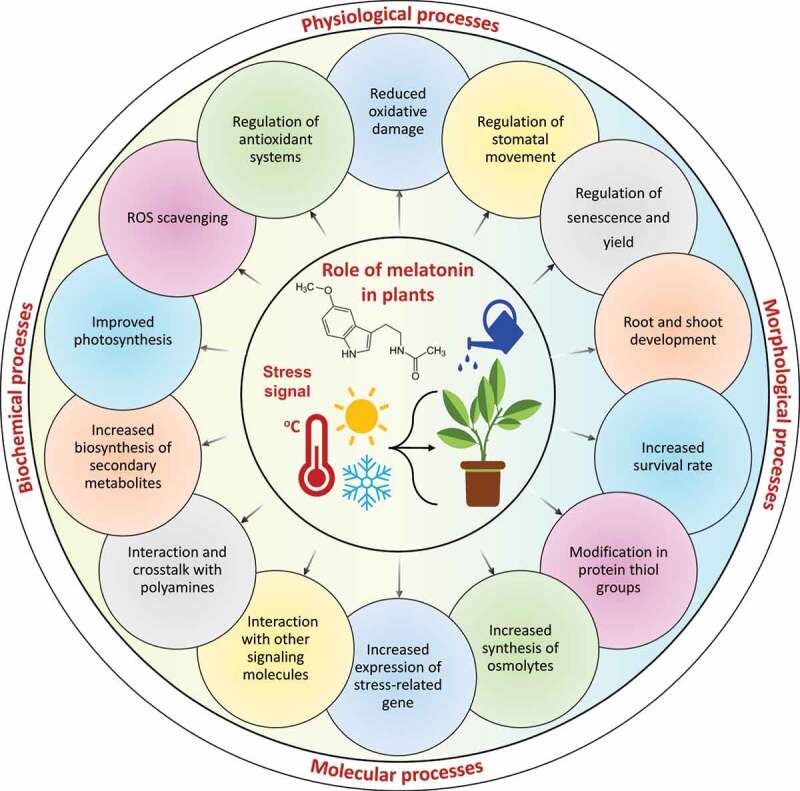
1. Introduction
The influences of climate change are extremely threatening the crop production, crop phenology, plant vulnerability, and livelihood of the 2.8 billion rural peoples.1 While global warming and climate change increase the Earth’s air temperature (Fig. 1), negatively influencing crop growth and productivity.2–6 Current climate change comprises both global warming and its impacts on Earth’s climate patterns. There have been prior phases of climate change, but the present alterations are clearly quicker and not due to natural causes. Due to climate change, deserts are increasing, while heat waves and wildfires are becoming more widespread (Fig. 1).7 Climate warming and the subsequent rise in extreme temperatures notably augment drought incidence, interval, and strength. This expansion of drought attributes causes variations in rainfall regimes, atmospheric water vapor fluxes, and soil humidity, as well as excess and river emissions.2,8,9 In recent times, climate predictions warned that increases in temperature, irregular rainfall and global CO2 emission could have a considerable negative influences on the productivity of economically important field crops.1,7,10 Consequently, a better understanding on the link between crop phenology and temperature is important for exploring superior insight of biodiversity growth, natural activities, and inherent plant systems and their modifications in response to environmental factors.
Figure 1.
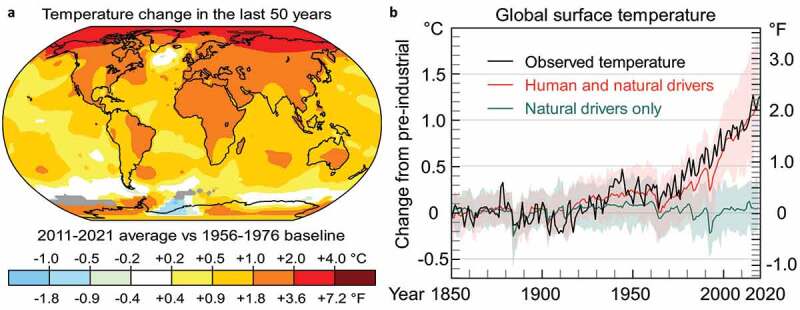
Impact of climate change on modification of environmental temperature. (a) Average surface air temperatures from 2011 to 2020 compared to the 1951–1980 average. Source: NASA (https://data.giss.nasa.gov/gistemp/, Retrieved 11 July 2022). (b) Change in average surface air temperature since the industrial revolution, plus drivers for that change. Human activity has caused enhanced temperatures, with natural forces adding some unpredictability. Source: IPCC (https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Full_Report.pdf; Retrieved 11 July 2022).2
Plants grow in a situation that implements various environmental factors (abiotic and biotic stresses), and discrepancy in any of these factors can hinder several morphological, physiological, and molecular variations at many steps; ultimately, plant growth and production get hampered by these stresses.11–13 Among different stresses, temperature extremes (i.e., cold stress (CS) such as chilling 0–15°C, and freezing <0°C, and heat stress (HS) >25°C) are causing damage to crop production and levying substantial risks to food security globally. Plants are infrequently affected by certain factors, and temperature stress alone or combined with other stresses may trigger oxidative impairment in plants. Consequently, HS and CS impede plant development as a consequence of cellular injury or even cause cell death by reducing membrane fluidity and antioxidant enzyme activities, biosynthesis of diverse proteins and secondary metabolites, and amendments in hormonal signaling after continuing exposure.3–6,14
Plants are required to breed and flourish to prolong their survival under temperature stress. Thus, there are numerous advances for retaining a balance between plant growth, development, and stress tolerance.5,6,11 To cope with temperature stress, plants modify their morphology, physiology, biochemistry, and gene expression, which alters their growing activities to grow and tolerate HS and CS conditions.5,6,11,15 Temperature extremes (HS and CS) can cause overgeneration of reactive oxygen species (ROS) viz singlet oxygen (1O2), hydroxyl (OH) radicals, superoxide (O2−), and hydrogen peroxide (H2O2).16,17 Especially, ROS are exceptionally sensitive to cellular objects and can cause critical oxidative impairment to metabolic events and thus restricting plant growth and development under temperature stress.17–19
Due to the sessile nature of plants, they are unable to escape temperature stress merely by moving to an appropriate stress-free environment. Therefore, plants have evolved a series of mechanisms to cope with undesirable stress conditions by modifying their developmental, physiological, biochemical, and molecular processes to changing environments.5,20 To improve plant growth and productivity under stress conditions, chemical approaches, i.e., exogenous treatment with several substances of both natural and synthetic origin, like phytohormones, osmolytes, gaseous molecules, etc., are getting much attention.21–23 These substances help in mitigating the adverse effect of stress conditions.
Among various substances, melatonin (MET) is considered as a master regulator. MET is an indole compound derived from serotonin (5-hydroxytryptamine); it was first discovered in animal tissues playing an important role in multiple physiological responses.24 MET mediates diverse functions, including organogenesis, flowering, photosynthesis, reproduction, circadian rhythm, fruit ripening and plant acclimatization to changing environments.25 Recent evidence on MET studies highlights the beneficial role of MET in plant stress tolerance and is considered as a regulatory hub of phytohormones.26–28 So, MET appears to be multi-regulatory molecules, as small molecular weight indoleamine (also known as a natural antioxidant) that takes part in various abiotic stresses, including HS and CS.24,29–31 Plants’ introduction to HS/CS triggers a rise in endogenous MET levels, resulting in up-regulation of MET biosynthesis genes and increased MET content. Exogenous MET improves plant tolerance against HS and CS in two aspects, i.e., either by directly scavenging ROS molecules or indirectly by improving antioxidant enzyme activities, photosynthetic efficacy, metabolite contents, and up-regulating the expression levels of MET-related and stress-inducible genes in plants.24,29,31–34 Thus, firstly we discussed MET biosynthesis and metabolism in response to stress conditions. The beneficial role of exogenous MET in understanding how plants respond, adapt and tolerate temperature stress has been widely explained. MET crosstalk with other molecules has also been documented under HS and CS conditions. Finally, the genetic engineering of MET-related genes has also been highlighted, indicating that the modifications in MET biosynthesis and endogenous levels can significantly improve the temperature stress tolerance by rising transgenic plants.
2. Metabolism and Biosynthesis of Melatonin in Plants
MET (N-acetyl-5-methoxytryptamine) was firstly isolated in 1958 in the bovine pineal gland.35 MET is an indolic compound derived from serotonin whose biosynthetic pathway has been widely investigated in animals and plants.32,33 MET has a potent natural antioxidant capacity which justifies its exogenous supply in plants subjected to stressed conditions biostimulating the plant growth under adverse conditions.24,32,33
The metabolic biosynthesis in plants has a higher complexity than in animals. It is also necessary to point out that plants are able to synthesize more amounts of this biomolecule than animals.36 In plants, mitochondria and chloroplasts are the main organelles in which MET is synthesized (Fig. 2).37 The main site of MET biosynthesis is the chloroplasts, but if the chloroplast pathway is disrupted, the mitochondrial pathway will be energized for MET generation to preserve homeostasis.36,37 The levels of MET in plants can vary from picograms to micrograms per gram of tissue, depending on the tissue and the crop analyzed. Therefore, higher concentrations supplied, such as 100 mM are not desirable since it may result in toxic effects.29,36,37
Figure 2.
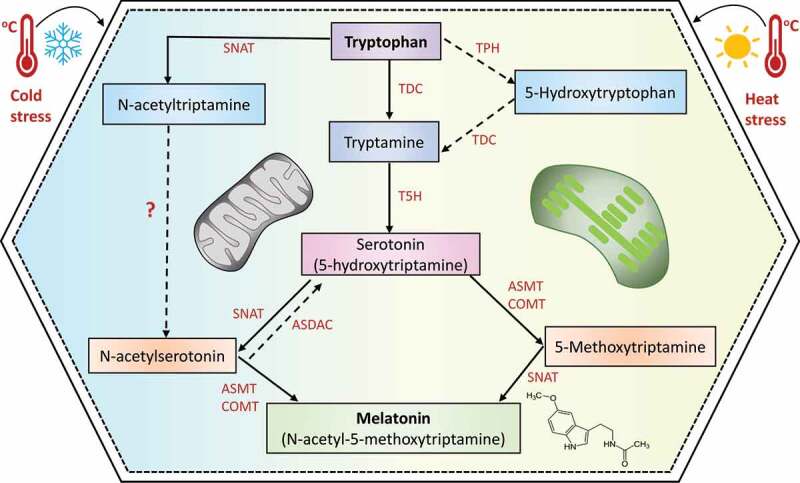
Biosynthetic pathway of melatonin in plants. The enzymes participating in this metabolism are tryptophan decarboxylase (TDC), tryptophan hidroxylase (TPH), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT), N-acetylserotonin methyltransferase (ASMT), caffeic acid O-methyltransferase (COMT), and N-acetylserotonin deacetylase (ASDAC). Black arrows (TDC-T5H-SNAT-ASMT/COMT or TDC-T5H-ASMT/COMT-SNAT) are the common pathways; the dotted arrows (TPH-TDC) represent an uncommon pathway; the dotted arrow (ASDAC) represents a reverse pathway, and the dotted arrows (SNAT-?) represent the uncompleted pathway.
Mainly, size enzymes are involved in the conversion of tryptophan to MET, including tryptophan decarboxylase (TDC), tryptophan hidroxylase (TPH), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT), N-acetylserotonin methyltransferase (ASMT), and caffeic acid O-methyltransferase (COMT) (Fig. 2).29,34 The starting material for MET biosynthesis in plants is tryptophan, generated via the shikimic acid pathway.38 The biosynthesis of MET in plants can be achieved using two different pathways, which are activated under different conditions. For instance, under normal conditions, tryptophan is converted to MET through catalysis by TDC, T5H, SNAT, and ASMT. Nevertheless, under senescence, the catalytic process for the conversion of tryptophan to MET is carried out through TDC, T5H, ASMT, and SNAT.39
There are four-step reactions involved in MET biosynthesis.39,40 The first-step reaction is the decarboxylation of tryptophan into tryptamine through TDC. One rate-limiting step in MET biosynthesis is the participation of TDC.41 The second-step reaction is the hydroxylation of tryptamine via the cytochrome P450 enzyme T5H to generate serotonin.39 It is necessary to point out that besides the conversion of tryptophan to serotonin via TDC and T5H, there is another pathway reported in St. John’s wort (Hypericum perforatum) in which tryptophan is converted to 5-hydroxytryptophan through TPH and then by TDC to generate serotonin.42 Once generated serotonin, there are two pathways to catalyze the conversion to MET. One of these pathways is the conversion of serotonin to N-acetylserotonin via serotonin-N-acetyltransferase, which is then converted to MEL through the catalytic process via N-acetylserotonin methyltransferase (ASMT) or caffeic acid O-methyltransferase (COMT) (pathway known as NM). The second pathway is based on the conversion of serotonin to 5-methoxytryptamine catalyzed by ASMT/COMT, and finally, the synthesis of MET via SNAT (pathway known as MN).43,44
To describe biosynthesis and mode of action of MET, in rice, it was reported that the presence of a reverse MET pathway where N-acetylserotonin is converted to serotonin through N-acetyl- serotonin deacetylase (ASDAC).45 The mode of action of MET in plants is dependent on the subcellular localization of the enzymes involved. For instance, SNAT is present in mitochondria and chloroplasts. The cytoplasm possesses TPH, ASMT/COMT, and TDC, while T5H is present only in the endoplasmic reticulum.46,47 Considering this fact, some authors have reported that N-acetylserotonin is generated in chloroplast and then moved to the cytoplasm for O-methylation to synthesize MET. Conversely, other authors have noted that the methoxylation of serotonin to produce methoxytriptamine occurs in the cytoplasm. Then, this molecule is transported to the chloroplast for acetylation by SNAT to generate MET.36,45
3. Potential of Exogenous Melatonin in Managing Temperature Stress in Plants
The plant needs an ideal temperature for normal growth and development. Changing temperature can inhibit plant growth and severely affect its developmental mechanisms. Especially, CS and HS devastatingly conquer plant growth and development by unsettling several morphological, biochemical, physiological, molecular and cellular processes (Fig. 3). Therefore, plants have evolved several MET-induced physiological, biochemical, and molecular processes to respond, adapt and attain tolerance against temperature stress (CS and HS) (Fig. 4). In the below segments, we have largely studied the defensive role of exogenous MET application in improving the adverse effect of temperature stress in various plant species.
Figure 3.
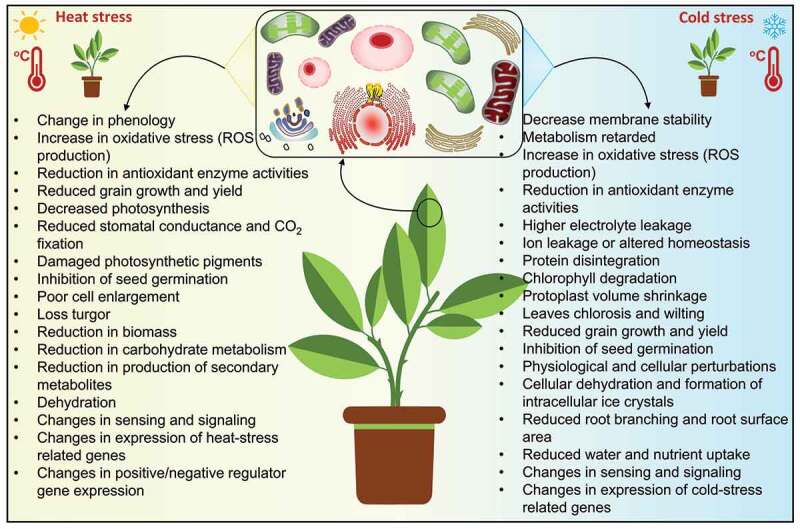
An overview of the impact of temperature stress on plant’s morphological, physiological, biochemical, molecular and cellular processes.
Figure 4.
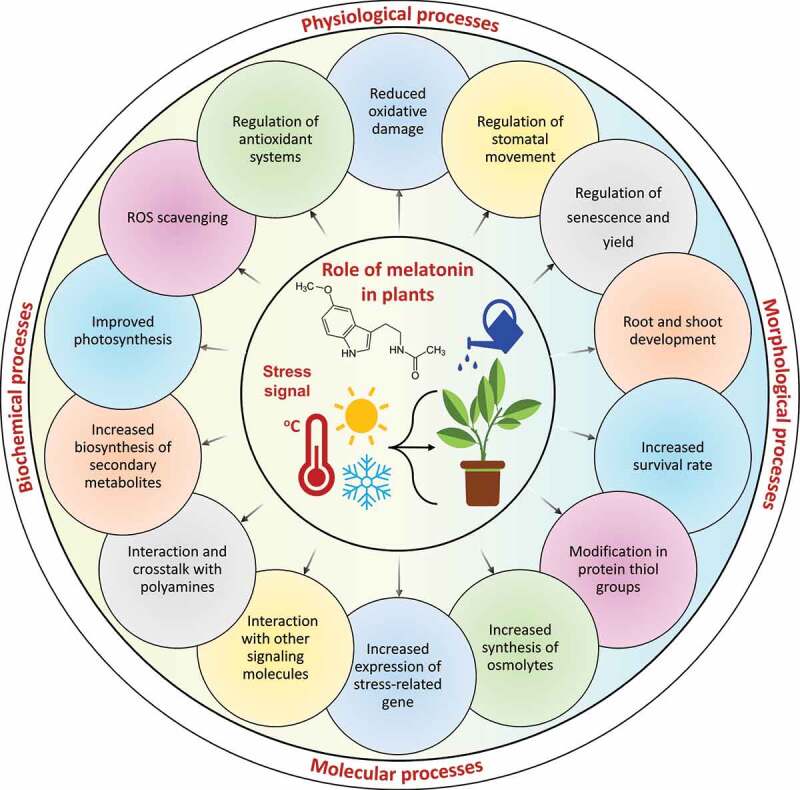
An overview of the beneficial role of melatonin in plants under temperature stress. Melatonin mainly improves several physiological, biochemical, morphological, cellular, and molecular processes and plants’ survival against stressful environments. Within the circle, the small chemical structure represents melatonin.
3.1. Cold Stress
Cold stress (CS; including chilling and freezing temperatures) exerts multiple effects and leads to changes in biochemistry, physiology, and molecular biology in plants (Fig. 3).4,5,48 Cold stress also affects the photosynthetic machinery inducing photo-inhibition at both PSI and PSII.4,5,14,48,49 Melatonin (N-acetyl-5-methoxytryptamine), first reported in plants in 1995, protects plants against various environmental stresses, including CS (Table 1).57 In major crops, fruits and vegetables, cold stress responses are linked with both unique as well as common elements between chilling and freezing temperatures. As the optimum chilling and freezing temperature ranges vary from crop to crop, fruit to fruit and vegetables to vegetables, the following sections have given an overview of the protective roles of exogenous MET in plants under temperature stresses.
Table 1.
Summary of some recent examples of melatonin-mediated temperature stress tolerance in different plants.
| Plant specie | Stress condition | Dose | Protective role | References |
|---|---|---|---|---|
| Cold stress | ||||
| Barley (Hordeum vulgare L.) | 2 ± 0.5°C | 1 mM | Exogenous MET application increased the drought priming-induced cold tolerance (DPICT) by modulating subcellular antioxidant systems and ABA levels | 50 |
| Maize (Zea mays L.) | 5°C, 14 d | 50 and 500 μM | MET pretreatment-induced modifications improve the respiratory/energetic metabolism of the conditioned seeds, and these changes could be crucial for efficient stress amelioration | 51 |
| Cucumber (Cucumis sativus L.) | 10°C, 7 d | 50 and 500 μM | MET pretreatment improved the antioxidant defense, especially SOD and GSSG-R, stimulating glutathione’s de novo synthesis and augmenting other antioxidants’ activities (GSH/GSSG ratio) | 52 |
| Elymus nutans | 4°C | 0, 1, 10, 50, 100, 300 μM | MET application improved ABA production and up-regulated the CS-responsive genes expression in an ABA-independent manner | 53 |
| Rice (Oryza sativa L.) | 12°C | 20 or 100 μM | MET pretreatment relieved the stress-induced inhibitions to photosynthesis, and PSII activities and also increased the antioxidant enzyme activities and non-enzymatic antioxidant levels | 54 |
| Watermelon (Citrullus lanatus L.) | 4°C | 50, 150, 300, 500, or 800 μM | MET induced cold tolerance via long-distance signaling, and such induction was associated with an enhanced antioxidant capacity and optimized defense gene expression | 55 |
| Cucumber (Cucumis sativus L.) | 15/8°C day/night | 200 μM | MET alleviated chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism | 56 |
| Tea (Camellia sinensis L.) | −5°C, 3 h | 100 µM | MET treatment mitigated the CS-induced reductions in photosynthetic capacity by reducing oxidative stress through enhanced antioxidant potential and redox homeostasis | 57 |
| Tea (Camellia sinensis L.) | 4°C | 100 µM | MET pretreatment alleviated the ROS burst, decreased MDA levels, maintained high photosynthetic efficiency, and increased the activities of SOD, POD, APX, and CAT | 58 |
| Rice (Oryza sativa L.) | 15/15°C | 150 μM | MET alleviated low-temperature stress through AB15-mediated signals during seed germination. | 59 |
| Alfalfa (Medicago truncatula L.) | 4°C | 75 μM | MET pretreatment enhanced the antioxidative ability by improving the activities of POD, SOD, CAT and APX, helping the plants counteract CS-mediated damage by strengthening the non-enzymatic antioxidant system | 48 |
| Barley (Hordeum vulgare L.) | 5°C | 1 μM | MET pretreatment improved the activities of SOD and CAT and also helped plants sustain stable redox homeostasis | 60 |
| Pepper (Capsicum annuum L.) | 5/10 ± 0.5°C, 72 h | 5 µM | MET treatment improved water relations, photosynthetic parameters, and antioxidant enzymes’ activities while lowered MDA and H2O2 contents and membrane permeability | 61 |
| Strawberry (Fragaria × ananassa L.) | 0/−4 ◦C (16/8 h), 2 d | 100 μM | MET pretreatment protected plants from the cold damages induced through enhanced antioxidant defense potential and modulated the DREB/CBF – COR pathway. | 62 |
| Pepper (Capsicum annuum L.) | 15°C/5°C | 200 µmol L−1 | MET alleviated low temperature-induced stress by GA3, IAA, and ZT accumulation while decreased ABA level | 63 |
| Eggplant (Solanum melongena L.) | 5°C/10°C (night/day), 3 d | 1, 5 or 25 μM | MET alleviated adverse effects of chilling stress, and increased the APOX, POX, CAT, and photosynthetic activities | 64 |
| Banana (Musa spp.) | 4°C | 50, 100, 150 and 200 μM | MET improved the electron transfer rate, total antioxidant capacity, CAT and SOD activities and proline and soluble sugar contents and significantly reduced the accumulations of MDA, superoxide anion and H2O2 | 65 |
| Heat stress | ||||
| Perennial ryegrass (Lolium perenne L.) | 38/33°C | 20 μM | MET treatment alleviated growth inhibition and leaf senescence, increasing the melatonin and CK content’s endogenous content and decreasing ABA content. It also up-regulated the CK biosynthesis genes expression, while the biosynthesis and signaling genes involved in ABA were down-regulated | 66 |
| Kiwifruit (Actinidia deliciosa) | 45 ◦C | 200 µM | MET alleviated heat-induced oxidative harm through reducing H2O2 content and increasing proline content, raised ascorbic acid levels, and the activity of antioxidant enzymes, including SOD, CAT, and POD | 67 |
| Tall fescue (Festuca arundinacea) | 42°C | 1 mM and 50 mM | MET treatment decreased ROS, electrolyte leakage, and MDA but increased Chl, total protein, and antioxidant enzyme activities | 68 |
| Tomato (Solanum lycopersicum L.) | 28 ± 1°C, 36 h | 100 μM | MET pretreatment reduced the oxidative stress by controlling the over-accumulation of O2•− and H2O2, lowering the lipid peroxidation content and less membrane injury index | 69 |
| Tomato (Solanum lycopersicum L.) | 40°C, 9 h | 10 μM | Endogenous MET alleviated the heat-induced oxidative stress by maintaining an efficient enzymatic antioxidant system and redox homeostasis. | 70 |
| Radish (Raphanus sativus L.) | 35°C/30°C day/night | 0, 11.6, 17.4, 29.0, 34.8 and 67.0 mg L−1 | MET treatment increased the antioxidant enzyme activity, APX, Chl a, and carotenoid contents compared with the control. The auxin and ABA contents were also increased significantly | 71 |
| Wheat (Triticum aestivum L.) | 42 ◦C | 100 µM | MET treatment reduced oxidative stress by preventing the over-accumulation of H2O2, lowering lipid peroxidation, MDA, and increasing proline biosynthesis. It also increased the activities of antioxidant enzymes, such as SOD, CAT, and POD | 72 |
| Pinellia ternata | 35°C day/30°C | 100 μM | MET treatment increased Chl content and relative water content and decreased MDA and electrolyte leakage. Also, activated HSPs, ribosomal proteins, and ROS-scavenging enzymes | 73 |
| Tall fescue (Festuca arundinacea) | 42°C | 20 µM | MET reduced the heat-caused damaging effects on Chl a, Chl b, carotenoid, and protein synthesis machinery. It also enhanced the activities of antioxidant enzymes, protein, and lipid molecules and favored the lower production of H2O2 and MDA | 74 |
| Strawberry (Fragaria × ananassa Duch.) | 35°C and 40°C | 0, 50, and 100 μM | MET treatment decreased heat injury symptoms and induced antioxidant mechanisms, also up-regulated the expression of defense HSF (FaTHsfA2a, FaTHSFB1a) and HSP (HSP90) genes | 75 |
| Rice (Oryza sativa L.) | 38 ◦C | 0, 20, 100, 500 µM | MET treatment improved the heat tolerance of rice seeds by enhancing the activity of the antioxidant enzymes and significantly reducing the MDA content | 76 |
| Tomato (Solanum lycopersicum L.) | 40°C, 7 d | 50 mM | MET alleviated the oxidative damage of PSII by balancing the electron transfer of the donor side, reaction center, and receptor side | 77 |
| Rice (Oryza sativa L.) | 38°C/28°C | 250 mL of 200 μmol L−1 | MET improved the stress resistance by enhancing the scavenging efficiency of ROS and improved the leaf photosynthetic and heat-resistance properties. | 78 |
Abbreviations: Abscisic acid (ABA); ascorbate peroxidase (APX); cold stress (CS); cytokinin (CK); chlorophyll (Chl); catalase (CAT); glutathione reductase (GSSG-R); gibberellic acid (GA3); heat stress (HS); hydrogen peroxide (H2O2); heat shock proteins (HSPs); indole-3-acetic acid (IAA); malondialdehyde (MDA); melatonin (MET); peroxidase (POD); reactive oxygen species (ROS); superoxide dismutase (SOD); zeatin (ZT).
Under CS, treatment of D. odorifera seedlings with exogenous MET and Ca2+ (0.6 mM + 5 mM CaCl2) improved the plant growth and relieved injuries.79 Exogenous MET and Ca2+ enhanced photosynthetic and antioxidant activities and decreased lipid peroxidation, electrolyte leakage, and ROS generation under CS (3°C). In addition, exogenous MET and Ca2+ intensely raised the phytohormones levels like gibberellin (GA3) and auxin (IAA); however, abscisic acid (ABA) was decreased. The GA3 and IAA contents were improved respectively by 22% and 17% in seedlings when treated with combined MET-Ca2+ than MET alone.79 In a study, hulless barley seeds were grown under different temperatures (25°C, 15°C, and 5°C) and treated with MET (1 µM) solution for 12 hours before the seeds germinated.80 Exogenous MET (1 µM) treatment alleviated the growth inhibition caused by CS and also restored the circadian rhythmic oscillation of circadian clock genes HvCCA1 and HvTOC1, whose circadian rhythmic phenotypes were lost by CS. The findings confirmed that MET treatment also lessened the soluble sugars and malondialdehyde (MDA) contents.80 Pepper seedlings and flowering were pre-treated with 5 µM MET and then were exposed to CS at 5°C/10°C (night/day) for 3 days.61 The results revealed that MET treatment improved CS tolerance by improving photosynthetic parameters, water relations, and antioxidant enzyme activities while lowering H2O2 and MDA contents and membrane permeability.61 In another study, the exogenous role of MET was investigated on soybean seedlings when exposed to CS.81 The results revealed that CS enhanced oxidative damage by ROS accumulation, affecting the growth and development of soybean. However, 5 µmol L−1 MET treatment alleviated the oxidative damage by increasing the transcript abundance of antioxidant-related genes.81
A recent study investigated the effect of 100 μM MET on bell pepper while storing at 4°C for 20 days and afterward shelf at 20°C for 3 days.82 The findings revealed that MET treatment lessened the cell structure damage and lightened the increase in chilling injury incidence, MDA content, and membrane permeability under CS conditions. Moreover, MET activated the antioxidant defense system to fight oxidative damage by up-regulating the expression levels of CaSOD, CaPOD, CaCAT, and CaAPX genes.82 During cold storage of pomegranate fruit at 4°C for 120 days, different doses of MET (0, 1, 10, 100, and 1000 μM) confer CS tolerance.83 The results confirmed that treatment with MET attenuates the H2O2 accumulation by improving GR, APX, CAT, and SOD activity, maintaining membrane integrity by hindering LOX and PLD activity. Therefore, exogenous MET is a safe strategy for providing CS tolerance in pomegranate fruit.83
Cut anthurium flowers could develop chilling injury during low-temperature storage, manifested as spathe browning.84 MET treatment (1, 10, 100, and 1000 μM) ameliorated chilling injury in cut anthurium flowers by 11, 29, 51, and 31%, respectively, than untreated flowers. Furthermore, MET triggers the proline synthesis and enhances ROS avoiding and scavenging activity, increasing GR, CAT, APX, and SOD activities.84 In another study, the authors studied the impact of exogenous MET treatment on chilling injury in tomatoes during cold storage.85 They found that 100 μM of MET application alleviated chilling injury and provided enough intracellular ATP by higher H+-ATPase, Ca-ATPase, cytochrome C oxidase (CCO), and succinate dehydrogenase (SDH) enzyme activity. It also protected membrane integrity due to a higher unsaturated/saturated fatty acids ratio as a consequence of higher FAD3 and FAD7 genes expression, which coincided with lower PLD and LOX genes expression and enzyme activity.85 Cold stress decreased the biomass, photosynthetic pigments, and mineral nutrients of Medicago truncatula plants. The authors studied that exogenous MET and Rhizobium inoculation (RI) attenuated CS-induced injuries and reduced oxidative damage.48 MET pretreatment also enhanced the antioxidative ability by improving the activities of APX (42%), CAT (140%), SOD (50%), and POD (8%), also increased osmolyte accumulation, nutrient uptake, and nitrate reductase activity.48
Recent studies also showed that MET plays a vital role in maintaining the fruit’s health during cold storage. For instance, during cold storage at 0°C for 45 days, sweet cherry fruits were treated with different MET levels (1, 10, 100, and 1000 µM). MET treatment retarded the fruit senescence and improved the antioxidant potential.86 The results showed that 1000 µM of MET treatment exhibited the lowest flesh browning and decayed incidence after 45 days of storage at 0°C. Moreover, exogenous MET enhanced the activities of APX, SOD, GR, and CAT while lowered the activities of lipoxygenase and phospholipase D.86 Another study demonstrated that the plum fruit treatment with 1.0 mmol L−1 MET mitigated the fruit chilling injury by reducing flesh-reddening and ‘ethylene burst.’ The MET application also repressed fruit softening and maintained energy status. In contrast, MET reduced the accumulation of cold-induced secondary metabolites.87 Likewise, MET application in kiwifruit alleviated chilling injury during cold storage.88 MET treatment strongly repressed the activity of lignin metabolism enzymes (PAL, 4CL, and C4H) and the expression of structural genes, whereas improved the activity of antioxidant enzymes (SOD, CAT, APX, and GR) and the accumulation of antioxidant substances (AsA and GSH). The outcomes propose that MET actively participates in cold tolerance and lignin accumulation through enzyme activity regulation in postharvest kiwifruit.88
Another study evaluated the influence of MET application on the chilling injury and quality of guavas during storage at 4 ± 1°C.89 MET treatment showed lower weight loss, cell membrane permeability, and chilling injury index also delayed the decreases of fruit firmness, sucrose, total soluble sugar, vitamin C, titratable acidity, and total soluble solids. These findings suggest that MET treatment could improve chilling tolerance and retain the quality of cold-stored guavas.89 Likewise, MET treatment confers chilling tolerance in mango fruit.90 The MET application increased polyamine accumulation and GABA shunt pathway activity.90 MET application also increased the accumulation of endogenous polyamines such as ADC and ODC and lowered DAO and PAO activities in the peel and pulp.90 In a recent study, the authors investigated the effects of exogenous MET on the ripening and decay incidence of plum fruit.91 The results presented that MET could slow the ripening process as directed by the firmness, respiration rate, and ethylene production and reduced the weight loss and decay incidence of plum fruit in room storage.91 In addition, MET application triggered the phenylpropanoid pathway by enhancing the activities of phenylalanine ammonia-lyase (PAL), 4-coumarate-coenzyme A ligase (4CL), cinnamate-4-hydroxylase (4CH) and peroxidase (POD), and accompanied by higher contents of total phenols and lignin, which might be contributed to improving the temperature tolerance in plum fruit during storage.91 In a nutshell, all these examples suggest that exogenous MET significantly improves CS tolerance by regulating several physiological, biochemical, and molecular mechanisms in different fruits and crop plant species.
3.2. Heat Stress
Heat stress (HS) is one of the major limiting factors for plant growth and causes a severe decline in global crop production. Heat stress accelerates crop senescence in extreme environments, decreasing crop productivity and even may cause death.4–6,92,93 HS also disturbs the respiration and photosynthesis-related metabolic processes leading to disruption of the carbon economy of the plants under HS condition (Fig. 3).93–95 MET acts as a plant growth regulator and plays an essential role in maintaining many physio-biochemical and molecular processes to cope with HS conditions (Table 1).
Pretreatment of tomato seedlings with 100 μM MET and then were exposed to HS (42°C) for 24 h.69 After heat shock, the molecular analysis showed that MET treatment effectively lessened the oxidative stress by regulating the over-accumulation of superoxide (O2•−) and hydrogen peroxide (H2O2), reducing the lipid peroxidation content and less membrane injury index. So, MET alleviated HS injuries by improving antioxidant defense mechanisms, such as the ascorbate–glutathione cycle, and reprogramming the NO biosynthesis and PAs metabolic pathways.69 Cherry radish was cultured at 35°C/30°C day/night high temperature and applied different MET concentrations, i.e., 0, 11.6, 17.4, 29.0, 34.8, and 67.0 mg L−1.71 The results showed that MET treatment significantly increased cherry radish biomass by 13%, and the soluble solid and soluble protein were improved by 9% and 18%, respectively. MET treatment also enhanced the activities of antioxidant enzymes, ascorbate, Chl a, carotenoid contents, ABA, and auxin contents. Thus, MET application positively affected cherry radish growth under HS.71
In a recent study, it was found that MET promotes photosynthesis and biomass accumulation in tea plants and improves tea quality under HS.96 MET treatment reduced the polyphenol to free amino acid ratio by finely refining the concentrations of polyphenols and amino acids. The qRT-PCR analysis discovered that MET amplified the transcript levels of catechins biosynthesis genes, including CsCHS, CsCH1, CsF3H, CsDFR, CsANS, CsLAR, and CsANR, under HS.96 Pretreatment of wheat seedlings with 100 µM MET followed by exposure to HS resulted in efficiently reduced oxidative stress by avoiding the over-accumulation of H2O2, dropping the MDA content, and growing proline biosynthesis.72 Moreover, the activities of antioxidant enzymes, like SOD, CAT, and POD, were increased substantially. Furthermore, the expression of ROS-related genes TaSOD, TaPOD, and TaCAT, and anti-stress responsive genes, such as TaMYB80, TaWRKY26, and TaWRKY39, were also induced in MET-treated seedlings.72
In Mentha × piperita L. and Mentha arvensis L. plants, a group of researchers assessed the chemical profile changes of essential oil and antioxidant enzymes activity in response to HS.97 The findings revealed that MET treatment plays an essential role in regulating physiological processes and alleviating HS. External MET applications also triggered a significant rise in enzymatic antioxidants, including SOD, POX, CAT, and APX peroxidase, and non-enzymatic antioxidants like ascorbic acid and vitamin E, causing reduced ROS levels and lipid peroxidation under HS.97 In another study, it was reported that irrigation treatment with 20 µM of MET significantly alleviated HS-induced pollen inactivation in tomatoes.98 MET treatment alleviated the ROS synthesis by up-regulating the transcription and activities of antioxidant enzymes under HS conditions. The results suggest that MET protects pollen activity and reduces ROS accumulation by inducing antioxidant enzyme activities under HS.98 In a recent study, regulatory networks of MET in chrysanthemum seedlings were explored in response to HS.99 The findings revealed that MET treatment decreased the H2O2 content and MDA accumulation, promoting the proline and soluble protein, GSH, and AsA accumulation, and antioxidant enzymes activities, including SOD and POD CAT, and APX under HS. Moreover, MET improved the dry weight, fresh weight, photosynthesis rate, Chl content, and gas exchange parameters.99
In a recent study, the authors found that 100 μM of MET application enhanced tomatoes’ CO2 assimilation and photosynthetic pigment content under HS. MET protects the PSI and PSII reaction center and lessens photo-inhibition.100 Likewise, HS (42°C) enhanced the ROS and lowered the photosynthesis efficiency in soybean.101 While pretreatment with 100 µmol of MET improved the photosynthetic pigment (Chl a and Chl b), plant growth and reduced the oxidative stress via scavenging superoxide and H2O2 and reducing the electrolyte leakage and MDA contents.101 A recent study with meta-analysis revealed that MET treatment improves the maize root length, plant height, leaf area, shoot weight, fresh and dry root weight, CAT, POD, SOD and APX activities, protein, and soluble sugar under stress conditions.102 In contrast, MET treatment lowered the levels of H2O2, O2−, MDA, and electrolyte leakage. In conclusion, MET relieves oxidative damage by refining stress tolerance, regulating the antioxidant defense system, and improving leaf Chl content compared to control.102 The effect of MET priming on the photosynthetic electron transport of PSII against heat stress was evaluated in tall fescue. The results revealed that MET weakened the electron transfer efficiency of PSII per light reaction center at the receptor and donor sides. In contrast, it improved the number of reaction centers per unit cross-sectional area. In brief, MET regulates the photoelectric conversion of PSII of tall fescue under heat stress and improves its survival rate after heat shock.103 In conclusion, exogenous MET treatment improves plant growth and development by inducing the antioxidant defense mechanisms, interacting with other chemicals, and reprogramming the biochemical metabolism.
4. Melatonin-mediated Genetic Engineering in Improving Temperature Stress Tolerance in Plants
4.1. A Brief Overview of Genetic Engineering in Plants
Biotechnology has given marvelous and fantastic scope for crop development, crop protection, crop quality management, and enhancing other agricultural attributes.104 In certain plants, genetic engineering has allowed for the integration of specifically desired features. Isolating a gene of interest, ligating that gene with a suitable vector to generate a recombinant-DNA molecule, and then transferring that gene into the plant genome to develop a novel function are all examples of genetic engineering.104,105 Transgenic technology has been dubbed “agriculture’s fastest expanding technology.” It refers to a collection of approaches for transferring desirable gene(s) across taxonomic boundaries into a specific plant via non-conventional means from any source (plants, animals, microbes, or even artificially generated genes).106,107 The cornerstone and essence of sustainable agriculture are combating numerous sorts of abiotic stressors like temperature. The primary benefits of transgenic technology are that the genes regulating many agronomically significant features may be derived from any organism – plants, bacteria, etc., and used to alter plants. Novel features from any background can thus be easily integrated into the target plant.106,107
The clustered regularly interspaced short palindromic repeats (CRISPR)/associated protein 9 (Cas9) system is a valuable genome editing tool having specificity, high efficiency, and processing a wide range of applications.108 CRISPR/Cas9 is more rapid, less expensive, precise, and highly effective in editing genomes even at the multiplex level than other genome editing technologies like transcriptional activator-like effector nucleases (TALENs) and zinc finger nucleases (ZFNs). CRISPR/Cas9 has shown the most significant promise for addressing emerging challenges in agriculture.109 Targeted knock-out/in, substitution, insertion, and deletion mutations created by CRISPR/Cas9 system have explored the regulatory functions of genes and their impact on other biochemical processes and helped to improve crops under abiotic stresses by their scavenging capacity.108 CRISPR/Cas9 can be easily programmed to include double-strand breaks at any desired target site. CRISPR/Cas9-based genome editing helps to create novel cultivars with desirable traits such as yield improvement, enhancement in yield quality and higher resistance to biotic and abiotic stresses.110 Moreover, through this genetic engineering methodology, genetic breeders can eliminate unfavorable traits or add favorable traits in a straightforward process needing only one single generation.108 Recently, CRISPR/Cas9-based genome editing have been widely utilized to improve temperature tolerance in different plant species.111–115
4.2. Melatonin Enhances Temperature Tolerance in Transgenic Plants
Genetic engineering of MET-encoding genes is a fascinating area that can also help improve the temperature stress tolerance in plants. However, only a few studies have looked at the effect of MET in heat-stressed crops at the genetic/molecular level. MET increased the production of heat shock proteins in tomato cells in response to HS, which controlled cell structure and protects cellular protein structure and stability under HS.116 Tryptophan decarboxylase (TDC), the first gene involved in MET production, has been overexpressed in rice. MET buildup in TDC3 transgenic lines is seed-specific; transgenic seeds had 31-fold greater MET concentrations than wild-type seeds.117
High temperatures and dark conditions increased MET levels of the final two enzymes in the MET production process.118 This suggests that MET has a function in temperature regulation. Microarray examination of global gene expression patterns in transgenic rice seedlings overexpressing the sheep SNAT gene resulted in an endogenous rise in MET levels relative to the wild type.119 Because of the increased levels of endogenous MET, the MET-rich transgenic rice plants showed higher oxidative stress resistance against cold. According to the microarray analysis, the endogenous MET increase in rice resulted in a moderate level of gene expression changes, with 464 genes differentially expressed.120 According to a previous report, genome-wide transcriptome analysis showed that nearly 4000 transcripts were differentially expressed as a result of MET exposure in transgenic bermudagrass (Cynodon dactylon). MET had many favorable impacts on transgenic bermudagrass under CS (4°C).121 Moreover, it was reported sheep (Ovis aries) SNAT (OaSNAT) gene is expressed in tobacco leaves. OaSNAT was found to be transiently expressed and exhibited a bright red fluorescence, indicating that the animal’s OaSNAT protein was expressed in the plant under HS (30°C).119
A previous study discovered that the development of ubiquitinated protein in the insoluble protein aggregates of MET treated and ASMT overexpressed tomato plants was greatly reduced compared to WT plants after 9 hours of HS (40°C) treatment.116 These results imply that either exogenous MET or endogenous MET manipulation via ASMT overexpression decreases HS-induced protein aggregation and ubiquitination. Heat stress significantly increased transcript levels of HSP17.4, HSP20, HSP20-1, HSP21, HSP70, and HSP90 in WT plants within 3 hours. More importantly, the expression of these HSPs was increased in MET-treated and ASMT plants following HS, implying that MET activated HSPs to aid in the refolding of denatured proteins.116 Under HS (28°C), MET concentrations in transgenic rice seeds were 31 times greater than those in WT seeds. Both in homologous and ectopic TDC overexpression lines, the level of MET intermediates was likewise elevated and improved the HS tolerance in transgenic rice lines.122 In plants treated with MET, the relative normalized expression of COR15a was seven times greater, and a four-fold induction was seen in MET-treated plants after 0.5 hours of CS treatment.123 These results suggest that the up-regulation of CS-related genes in transgenic plants by MET may stimulate the biosynthesis of cold-shielding compounds and improve plants growth treated with exogenous MET under CS.
Interestingly, MET treatment also plays key role in gene expression and cell suspension under temperature stress. For instance, it was reported that pIPKb002-TDC3 binary vector was used to create the TDC3 transgenic rice line, which was able to synthesize MET when compared to three separate transgenic rice plants that expressed each TDC isogene separately for MET production.117 While the WT and other TDC genotypes showed considerable expression of TDC under HS (28°C), there was specific TDC overexpression for each TDC isogene.117 In another study, preincubation with MET prior to CS treatment (2–3°C) protected carrot suspension cells from dying, as detected by trypan blue staining.124 When cells were preincubated with 86 nm MET before being exposed to cold, there was only a small drop in cell viability, after a 7-day CS, the proportion of TUNEL-positive cells reduced from 83.6 to 12.8% as MET concentrations increased.124
Furthermore, a previous study found that when tomatoes were subjected to a drought treatment at 20°C, WT plants lost water faster than transgenic ‘Micro‐Tom’ tomato overexpressing ovine AANAT and ovine HIOMT genes.125 The average mass ratio of transgenic lines declined from 100% to around 65% after 4 hours of HS treatment. This is substantially higher than the WT, which experienced a reduction from 100% to 50%. In fact, the three transgenic lines had a higher mass ratio than the WT at all times during HS (20°C) treatment, and transgenic plants overexpressing ovine HIOMT were more drought tolerant.125 According to scientists, at – 20°C, MET triggered the synthesis and breakdown of cellulose, pectin, and xylan, suggesting that MET may alleviate the effects of temperature stress based on the trials above.30 Previously, it was opined that the leaves of WT and snat mutant lines were inoculated with Pst-avrRpt2 to see how decreased MET production caused by the knockout of SNAT gene function affected pathogen resistance in Arabidopsis thaliana under HS (28°C).126 The knockout mutant lines were more susceptible to pathogen assault than WT plants, suggesting the direct involvement of MET as a signal molecule in pathogen resistance, mainly under HS conditions.126
Through the dual regulation of leaf senescence and vascular development, a recent study discovered the involvement of a caffeic acid O-methyltransferase (OsCOMT) gene in modulating rice grain yield.127 The results revealed that OsCOMT is involved in the production of MET.127 According to transgenic assays, OsCOMT dramatically slowed down the senescence of leaves at the grain-filling stage by preventing the breakdown of Chl and chloroplast, which boosted the effectiveness of photosynthesis. Briefly, MET-mediated leaf senescence and vascular development offer a potential method for genetically enhancing rice grain output.127 In watermelon, 16 putative O-methyltransferase (ClOMT) genes were identified. Among them, ClOMT03 (Cla97C07G144540) was considered a potential COMT gene (renamed ClCOMT1) based on its high identity (60.00–74.93%) to known COMT genes involved in MET production, expressed in nearly all tissues, and upregulation during abiotic stressors.128 Watermelon calli with ClCOMT1 overexpression had considerably higher MET concentrations than those with ClCOMT1 deletion using the CRISPR/Cas-9 technology. These results suggest that ClCOMT1 plays an essential role in MET biosynthesis in watermelon. Cold, drought and salt stress also increased MET concentrations while up-regulated ClCOMT1 expression in watermelon. ClCOMT1 is a positive regulator of plant tolerance to abiotic stimuli, as shown by the improved tolerance of transgenic Arabidopsis to such challenges when ClCOMT1 was overexpressed.128 So far, MET-encoding genes have not been widely edited using CRISPR/Cas system; therefore, more efforts are required to apply the genome editing application to fully unravel the MET role against temperature stress. The use of CRISPR/Cas9 for modifying plant genomes is fast growing. To offset the harmful effects of climate change and secure the future food security of growing populations in tropical nations, the CRISPR/Cas9 system is evolving into a user-friendly tool for the generation of non-transgenic genome-edited crop plants.
5. Melatonin Interaction and Crosstalk with Other Molecules under Temperature Stress
Melatonin (MET) acts as a multifunctional molecule that is widely involved in plant growth, development and stress responses. Numerous studies reveal that MET interacts with other signaling molecules, including ROS, nitric oxide (NO) and hydrogen sulfide (H2S).22,129–131 The interactions among MET, ROS, NO and H2S demonstrate the responses to abiotic stress through a series of signaling networks, which confer the plant adaption and tolerance. Moreover, MET is able to interact jointly with H2O2 and NO, resulting in post-translational changes and regulation of hormonal activities.129–132 There is also established crosstalk between MET and phytohormones such as auxin, cytokinin, gibberellins, ethylene and ABA.133 In this section, we demonstrated the significance of MET interaction, regulation of redox homeostasis via antioxidant system, and protection of plants from oxidative stress-induced cellular injuries in response to temperature stress.
Under stressed conditions like HS and CS, the interplay between hormones and MET has been widely reported, which is interrelated and mitigates the damages caused by these adverse conditions.26,31,36,45 Plants respond to the temperature stress by using multiple signal pathways, in which MET plays a key role and works synergistically and/or antagonistically with phytohormones as well as other molecules for regulating plant growth, development and defense response (Fig. 5). For instance, an experiment was carried out in which rice plants were subjected to different temperature regimes.59 To ameliorate the damages caused by HS, the exogenous supply of MET played a significant impact on plant adaption and stress tolerance.59 Exogenous MET treatment altered the NO level, while NO influenced the endogenous MET content.59 The interplay of MET, NO and ROS induces diverse physiological behaviors in plants through interaction mechanisms.129,130 MET can interact synergistically or antagonistically with phytohormones and regulates their endogenous levels, and these interactions lead to enhancing plant defense under stressful condition. Induction of endogenous phytohormones or MET level due to application or presence of exogenous MET may regulate differentially in different plant species. In the following sections, we updated the studies on how the exogenous supply of MET regulates the level of phytohormones and plant adaption.
Figure 5.
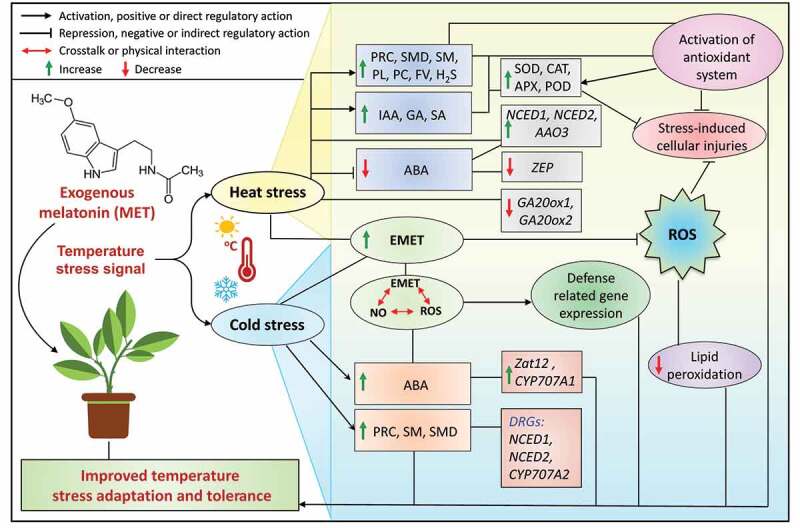
Interaction between melatonin and other biomolecules in plants under temperature stress conditions. Exogenous MET-mediated induction of exogenous MET and other biomolecules occurs in response to heat stress, which inhibits abscisic acid induction. The cold stress shows the opposite effect on abscisic acid regulation compared to heat stress in plants. Several candidate genes respond differently to cold and heat stresses, particularly defense-related genes, which help to plant stress tolerance. Endogenous MET inhibits superoxide molecules, and there is some interaction between endogenous MET, nitric oxide, and reactive oxygen species. Melatonin activates the antioxidant system during stress by increasing antioxidants; the combined mechanisms help to reduce lipid peroxidation, inhibit oxidative stress-induced cellular injury, and improve plant temperature stress tolerance. On the left side, the small chemical structure represents MET. Abbreviations: MET, melatonin; EMET; endogenous melatonin; ABA, abscisic acid; GA, gibberellic acid; IAA, indole-3-acetic acid; SA, salicylic acid; SOD, superoxide dismutase, CAT, catalase; APX, Ascorbate peroxidase; POD, peroxidase; NO, nitric oxide; ROS, reactive oxygen species, PRC, putrescine; SMD, spermidine; SM, spermine; PL, proline, PC, phenolic compound; FV, flavonoid; H2S, hydrogen sulfide; DRGs, differentially regulated genes. LpZEP, LpNCED1, CsZat12, CsCYP707A1, CsNCED1, CsNCED2, and CsCYP707A2 genes regulated in response to MET under temperature stress.
Tomato is one of the most important horticultural crops worldwide, and both HS and CS reduce the optimal physiological performance of tomatoes.134 In this sense, it was noted that the foliar spraying of tomato plants with 80 mL of 100 μM MET each day for one week before exposure to HS (42°C) for 24 h.69 After HS, MET-treated plants showed an increase in the endogenous levels of several polyamines such as putrescine, spermidine, and spermine. Moreover, the findings revealed an increase in NO content.69 Working on the same crop, the foliar sprays with 100 μM MET in tomato plants subjected to HS (38/28°C and photoperiod of 16/8 h) for 5 days resulted in a decline in ABA concentration in tomato leaves.135 The mRNA levels of several genes such as NCED1, NCED2, and AAO3 involved in ABA biosynthesis were assessed, showing a significant increase under HS and MET application.135 On the contrary, GA content in leaves was elevated under HS with MET application. The determination of relative expression of GA biosynthesis genes (GA20ox1 and GA20ox2) were downregulated under HS, while the responses were reversed after the addition of MET supplementation with HS conditions in tomato plants.135
According to recent reports, there are other studies focused on the amelioration of exogenous feeding with MET in temperature-stressed plants.101 The irrigation with a volume of 30 mL of 100 µM MET twice daily for 6 days in soybean plants subjected to HS (42°C) for 3 and 7 days resulted in an increase in proline, phenolics compounds, and flavonoids concentration as well as in polyamines such as spermine, spermidine, and putrescine.101 Moreover, the application of MET reduced ABA content whereas increased the level of salicylic acid.101 On the other hand, a study reported that the supply of 100 µM MET under HS in wheat seedlings resulted in an overaccumulation of proline concentration.72
The ameliorative effect of exogenous application of MET under HS has also been reported in perennial ryegrass (Lolium perenne L.).66 This positive effect can be related to the decrease in ABA synthesis, mainly ascribed to the down-regulation of two ABA biosynthesis genes (LpZEP and LpNCED1). Though, a study found an increased level of auxin and ABA in cherry radish subjected to HS and an exogenous supply of MET.71 This study is a bit exceptional in terms of ABA induction in response to MET-mediated HS. Moreover, they reported a positive effect of MET in temperature-stressed plants.71 Regarding other biomolecules, the exposure of wheat plants to HS (40°C) for six hours for 15 days was ameliorated by the exogenous application of MET, which led to an increase in hydrogen sulfide contents.136 Regarding the interaction with ABA, it was also reported that the exogenous application of MET under CS in Elymus nutans resulted in an increase in ABA synthesis.53
Under CS, the exogenous MET (200 µM) in cucumber seedlings led to an increase in the polyamines contents (such as putrescine, spermine, and spermidine) and also modulated the expression of the key ABA biosynthesis genes (CsNCED1 and CsNCED2) as well as genes involved in ABA catabolism (CsCYP707A1 and CsCYP707A2).56 A recent study reported that CS significantly reduced root length, plant height, leaf surface area, and Chl contents, and increased the ROS levels that led to induced oxidative damage, lipid peroxidation and electrolyte leakage. However, these parameters were significantly reverted after supplementation of MET enhanced stress tolerance.137 Recently, it has been reported that there is a correlation between MET with MeJA, which modulate H2O2 levels and increase CS tolerance in watermelon.138 In short, all these examples suggest that MET interacts and crosstalk with hormones, polyamines, and other gaseous molecules to improve temperature stress tolerance by regulating several physio-biochemical and molecular mechanisms in plants. In the near future, more in-depth studies should be carried out to fully reveal the mode of action that can help plants to cope with temperature stress conditions.
6. Conclusion and Outlooks
Temperature stress (both heat and cold stress) causes multiple problems at the molecular and physiological levels in plants, resulting in massive productivity losses worldwide. MET is a nontoxic indolic molecule that plays important roles in animals (circadian rhythm, antioxidant activity, and immunity) and plants (photosynthesis enhancement, biomass generation, and plant osmoregulation under abiotic and biotic stressed conditions). The current review has focused on the metabolism and biosynthesis of MET in plants, the beneficial effects of the exogenous supply of MET in crops exposed to low and high temperatures, the recent engineering techniques in melatonin biosynthesis in crops as well as the interaction of this molecule with other hormones under changing temperature conditions. Exogenous MET application improves plant growth under temperature stress by maintaining membrane integrity, improving photosynthetic pigment synthesis, and increasing water and nutrient uptake. MET treatment alleviates the cold-induced osmotic imbalance by enhancing the accumulation of hormones, osmolytes, and secondary metabolites. MET mitigates the detrimental effects of heat stress on the photosynthetic machinery of plants. The production of MDA and H2O2 was lowered in MET-treated plant seedlings under heat stress, signifying the positive modulation of the antioxidant enzyme defense system. MET effectively scavenges a variety of ROS that protects cells and tissues of organisms from the deteriorated effects of oxidative stress.
MET works synergistically and/or antagonistically to phytohormones as well as other molecules for regulating plant growth, development, and defense response. MET crosstalk and interacts with hormones, polyamines, and gaseous molecules to mitigate the damages caused by temperature stress. The current research outcomes specify that MET, H2S, Ca2+, H2O2, NO, and other signaling molecules and the MAP kinase cascade pathway are essential in MET-induced plant tolerance to stress. In addition, MET-mediated genetic engineering could provide a promising approach to unraveling the molecular basis of temperature stress tolerance. Genetic modifications can also improve the MET synthesis in transgenic plants and improve tolerance to temperature stress. Future research is required to know the genetic mechanisms and metabolic pathways involved during recovery under stress conditions on exposure to MET. The information about this topic is vast; nevertheless, there is a complexity of the metabolic pathways and their synthesis in two organelles (chloroplasts and mitochondria) that should be considered. A strong effort is required to elucidate these bottlenecks and provide clarification on several fundamental points. Future research should focus on a deeper understanding of the core MET metabolism by generating overexpressing and knockout mutants via CRISPR/Cas system in different crops of the genes involved in the metabolic pathways.
Acknowledgments
The author wants to thank several colleagues for the scientific discussion, which helped improve the manuscript’s content.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Author’s contribution
AR conceived the idea. AR, SC, PGC, VHO, MAR, and FS contributed to the literature search and writing the manuscript. AR, PGC, MAR and WJ reviewed and edited the manuscript. All authors have read and approved the final version of the manuscript.
Disclosure statement
The authors declare that they have no competing interests.
References
- 1.Bouabdelli S, Zeroual A, Meddi M, and Assani A.. Impact of temperature on agricultural drought occurrence under the effects of climate change. Theor Appl Climatol. 2022;148(1–2):191–209. doi: 10.1007/s00704-022-03935-7. [DOI] [Google Scholar]
- 2.Basavaraj P, and Rane J. Avenues to realize potential of phenomics to accelerate crop breeding for heat tolerance. Plant Physiol Rep. 2020;25(4):594–610. doi: 10.1007/s40502-020-00552-2. [DOI] [Google Scholar]
- 3.Zahra N, Shaukat K, Hafeez MB, Raza A, Hussain S, Chaudhary MT, Akram MZ, Kakavand SN, Saddiq MS, and Wahid A. 2021. Physiological and Molecular Responses to High, Chilling, and Freezing Temperature in Plant Growth and Production: Consequences and Mitigation Possibilities . Harsh Environment and Plant Resilience: Molecular and Functional Aspects. Husen, A. (Springer, Cham; ):235–290. doi: 10.1007/978-3-030-65912-7_10. [DOI] [Google Scholar]
- 4.Raza A, Tabassum J, Kudapa H, and Varshney RK. Can omics deliver temperature resilient ready-to-grow crops? Crit Rev Biotechnol. 2021;41(8):1209–32. doi: 10.1080/07388551.2021.1898332. [DOI] [PubMed] [Google Scholar]
- 5.Giordano M, Petropoulos SA, Rouphael Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture. 2021;11(5):463. doi: 10.3390/agriculture11050463. [DOI] [Google Scholar]
- 6.Farooq MS, Uzaiir M, Raza A, Habib M, Xu Y, Yousuf M, Yang SH, and Ramzan Khan M. uncovering the research gaps to alleviate the negative impacts of climate change on food security: a review. Front Plant Sci. 2022;13:927535 doi: 10.3389/fpls.2022.927535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masson-Delmotte V, Zhai P, Pirani A, Connors S, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis M. Climate change 2021: the physical science basis. contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge (United Kingdom and New York, NY, USA): Cambridge University Press; 2021. [Google Scholar]
- 8.Bouras E, Jarlan L, Khabba S, Er-Raki S, Dezetter A, Sghir F, Tramblay Y. Assessing the impact of global climate changes on irrigated wheat yields and water requirements in a semi-arid environment of Morocco. Sci Rep. 2019;9(1):1–14. doi: 10.1038/s41598-019-55251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai A, Zhao T. Uncertainties in historical changes and future projections of drought. Part I: estimates of historical drought changes. Climatic Change. 2017;144(3):519–33. doi: 10.1007/s10584-016-1705-2. [DOI] [Google Scholar]
- 10.Bond-Lamberty B, Thomson A. Temperature-associated increases in the global soil respiration record. Nature. 2010;464(7288):579–82. doi: 10.1038/nature08930. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Li X-M, Lin H-X, and Chong K. Crop improvement through temperature resilience. Annual Rev Plant Biol. 2019;70:753–80. doi: 10.1146/annurev-arplant-050718-100016. [DOI] [PubMed] [Google Scholar]
- 12.Malhi GS, Kaur M, Kaushik P. Impact of climate change on agriculture and its mitigation strategies: a review. Sustainability. 2021;13(3):1318. doi: 10.3390/su13031318. [DOI] [Google Scholar]
- 13.Shahzad A, Ullah S, Dar AA, Sardar MF, Mehmood T, Tufail MA, Shakoor A, Haris M. Nexus on climate change: agriculture and possible solution to cope future climate change stresses. Environ Sci Pollut Res. 2021;28:14211–32. [DOI] [PubMed] [Google Scholar]
- 14.Yan L, Zeng L, Raza A, Lv Y, Ding X, Cheng Y, and Zou X. Inositol improves cold tolerance through inhibiting CBL1 and increasing Ca2+ Influx in rapeseed (Brassica napus L.). Front Plant Sci. 2022;13:775692. doi: 10.3389/fpls.2022.775692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raza A, Su W, Jia Z, Luo D, Zhang Y, Gao A, Hussain MA, Mehmood SS, Cheng Y, and Lv Y. Mechanistic insights into trehalose-mediated cold stress tolerance in rapeseed (Brassica napus L.) seedlings. Front Plant Sci. 2022;13:857980. doi: 10.3389/fpls.2022.857980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bita C, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasanuzzaman M, Bhuyan M, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants. 2020;9(8):681. doi: 10.3390/antiox9080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittler R. ROS are good. Trends Plant Sci. 2017;22(1):11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Mittler R, Zandalinas SI, Fichman Y, and Van Breusegem F. Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol. : 2022 doi: 10.1038/s41580-022-00499-2. [DOI] [PubMed] [Google Scholar]
- 20.Harfouche AL, Jacobson DA, Kainer D, Romero JC, Harfouche AH, Mugnozza GS, Moshelion M, Tuskan GA, Keurentjes JJ, Altman A. Accelerating climate resilient plant breeding by applying next-generation artificial intelligence. Trends Biotechnol. 2019;37(11):1217–35. doi: 10.1016/j.tibtech.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Sabagh AE, Mbarki S, Hossain A, Iqbal M, Islam M, Raza A, Llanes A, Reginato M, Rahman M, and Mahboob W, et al. Potential role of plant growth regulators in administering crucial processes against abiotic stresses. Front Agron. 2021;3:648694 doi: 10.3389/fagro.2021.648694. [DOI] [Google Scholar]
- 22.Raza A, Tabassum J, Mubarik M, Anwar S, Zahra N, Sharif Y, Hafeez M, Zhang C, Corpas F, and Chen H. Hydrogen sulfide: an emerging component against abiotic stress in plants. Plant Biol. 2022;24(4):540–58. doi: 10.1111/plb.13368. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee S, Corpas FJ. Crosstalk among hydrogen sulfide (H2S), nitric oxide (NO) and carbon monoxide (CO) in root-system development and its rhizosphere interactions: a gaseous interactome. Plant Physiol Biochem. 2020;155:800–14. doi: 10.1016/j.plaphy.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Sun C, Liu L, Wang L, Li B, Jin C, Lin X. Melatonin: a master regulator of plant development and stress responses. J Integr Plant Biol. 2021;63(1):126–45. doi: 10.1111/jipb.12993. [DOI] [PubMed] [Google Scholar]
- 25.Akula R, and Mukherjee S. New insights on neurotransmitters signaling mechanisms in plants. Plant Signal Behav. 2020;15(6):1737450. doi: 10.1080/15592324.2020.1737450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnao MB, and Hernández-Ruiz J. Melatonin: a new plant hormone and/or a plant master regulator? . Trends Plant Sci. 2019;24:38–48. [DOI] [PubMed] [Google Scholar]
- 27.Arnao M, Hernández‐Ruiz J, and Hu Y. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021;23(S1):7–19. doi: 10.1111/plb.13202. [DOI] [PubMed] [Google Scholar]
- 28.Zhao D, Wang H, Chen S, Yu D, Reiter RJ. Phytomelatonin: an emerging regulator of plant biotic stress resistance. Trends Plant Sci. 2021;26(1):70–82. doi: 10.1016/j.tplants.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S, Guo Y-D. Roles of melatonin in abiotic stress resistance in plants. J Exp Bot. 2015;66(3):647–56. doi: 10.1093/jxb/eru336. [DOI] [PubMed] [Google Scholar]
- 30.Wei W, Li Q-T, Chu Y-N, Reiter RJ, Yu X-M, Zhu D-H, Zhang W-K, Ma B, Lin Q, Zhang J-S. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot. 2015;66(3):695–707. doi: 10.1093/jxb/eru392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharif R, Xie C, Zhang H, Arnao MB, Ali M, Ali Q, Muhammad I, Shalmani A, Nawaz MA, Chen P. Melatonin and its effects on plant systems. Molecules. 2018;23(9):2352. doi: 10.3390/molecules23092352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan J, Xie Y, Zhang Z, Chen L. Melatonin: a multifunctional factor in plants. Int J Mol Sci. 2018;19(5):1528. doi: 10.3390/ijms19051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu X, Ren J, Huang X, Zheng X, Tian Y, Shi L, Dong P, Li Z. Melatonin: biosynthesis, content, and function in horticultural plants and potential application. Sci Hortic (Amsterdam). 2021;288:110392. doi: 10.1016/j.scienta.2021.110392. [DOI] [Google Scholar]
- 34.Mannino G, Pernici C, Serio G, Gentile C, Bertea CM. Melatonin and phytomelatonin: chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals—an overview. Int J Mol Sci. 2021;22(18):9996. doi: 10.3390/ijms22189996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerner AB, Case JD, Takahashi Y, Lee TH, and Mori W. ISOLATION OF MELATONIN, THE PINEAL GLAND FACTOR THAT LIGHTENS MELANOCYTES1. J American Chem Soc. 1958;80(10):2587–2587. doi: 10.1021/ja01543a060. [DOI] [Google Scholar]
- 36.Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, and Reiter RJ. Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol. 2019;10:249. doi: 10.3389/fendo.2019.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan D-X, Reiter RJ, Dietz K-J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J Exp Bot. 2020;71(16):4677–89. doi: 10.1093/jxb/eraa235. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Llorca M, Muñoz P, Müller M, and Munné-Bosch S . Biosynthesis, metabolism and function of auxin, salicylic acid and melatonin in climacteric and non-climacteric fruits. Front Plant Sci. 10:136 doi: 10.3389/fpls.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Back K, Tan DX, Reiter RJ. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res. 2016;61(4):426–37. doi: 10.1111/jpi.12364. [DOI] [PubMed] [Google Scholar]
- 40.Hardeland R. Melatonin in plants–diversity of levels and multiplicity of functions. Front Plant Sci. 2016;7:198. doi: 10.3389/fpls.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HY, Byeon Y, Lee K, Lee HJ, Back K. Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. J Pineal Res. 2014;57(4):418–26. doi: 10.1111/jpi.12181. [DOI] [PubMed] [Google Scholar]
- 42.Murch S, KrishnaRaj S, and Saxena P. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000;19(7):698–704. doi: 10.1007/s002990000206. [DOI] [PubMed] [Google Scholar]
- 43.Ye T, Yin X, Yu L, Zheng SJ, Cai WJ, Wu Y, Feng YQ. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography‐mass spectrometry. J Pineal Res. 2019;66(1):e12531. doi: 10.1111/jpi.12531. [DOI] [PubMed] [Google Scholar]
- 44.Mir AR, Faizan M, Bajguz A, Sami F, Siddiqui H, and Hayat S. Occurrence and biosynthesis of melatonin and its exogenous effect on plants. Acta Soc Bot Pol. 89 2 :8922. doi: 10.5586/asbp.8922. [DOI] [Google Scholar]
- 45.Lee K, Lee HY, Back K. Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants. J Pineal Res. 2018;64(2):e12460. doi: 10.1111/jpi.12460. [DOI] [PubMed] [Google Scholar]
- 46.Tan DX, Hardeland R, Back K, Manchester LC, Alatorre‐Jimenez MA, Reiter RJ. On the significance of an alternate pathway of melatonin synthesis via 5‐methoxytryptamine: comparisons across species. J Pineal Res. 2016;61(1):27–40. doi: 10.1111/jpi.12336. [DOI] [PubMed] [Google Scholar]
- 47.Wei Y, Zeng H, Hu W, Chen L, He C, Shi H. Comparative transcriptional profiling of melatonin synthesis and catabolic genes indicates the possible role of melatonin in developmental and stress responses in rice. Front Plant Sci. 2016;7:676. doi: 10.3389/fpls.2016.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irshad A, Rehman RNU, Kareem HA, Yang P, and Hu T. Addressing the challenge of cold stress resilience with the synergistic effect of rhizobium inoculation and exogenous melatonin application in Medicago truncatula. Ecotoxicology and Environmental Safety. 2021;226:112816. doi: 10.1016/j.ecoenv.2021.112816. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Raza A, Huang H, Su W, Luo D, Zeng L, Ding X, Cheng Y, Liu Z, and Li Q. Analysis of Lhcb gene family in rapeseed (Brassica napus L.) identifies a novel member “BnLhcb3. 4” modulating cold tolerance. Environ Exp Bot. 2022;198:104848. doi: 10.1016/j.envexpbot.2022.104848. [DOI] [Google Scholar]
- 50.Li X, Tan DX, Jiang D, Liu F. Melatonin enhances cold tolerance in drought‐primed wild‐type and abscisic acid‐deficient mutant barley. J Pineal Res. 2016;61(3):328–39. doi: 10.1111/jpi.12350. [DOI] [PubMed] [Google Scholar]
- 51.Kołodziejczyk I, Dzitko K, Szewczyk R, and Posmyk MM. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J Plant Physiol. 2016;193:47–56. doi: 10.1016/j.jplph.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Marta B, Szafrańska K, and Posmyk MM. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front Plant Sci. 2016;7:575. doi: 10.3389/fpls.2016.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu J, Wu Y, Miao Y, Xu Y, Zhao E, Wang J, Sun H, Liu Q, Xue Y, Xu Y. Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han Q-H, Huang B, Ding C-B, Zhang Z-W, Chen Y-E, Hu C, Zhou L-J, Huang Y, Liao J-Q, Yuan S. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front Plant Sci. 2017;8:785. doi: 10.3389/fpls.2017.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Chang J, Zheng J, Dong Y, Liu Q, Yang X, Wei C, Zhang Y, Ma J, and Zhang X. Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. via long distance transport. Sci Rep. 2017;7(1):1–15. 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao H, Zhang K, Zhou X, Xi L, Wang Y, Xu H, Pan T, Zou Z, Otani H, Pazour GJ. Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci Rep. 2017;7(1):1–12. 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Wei J-P, Scott ER, Liu J-W, Guo S, Li Y, Zhang L, and Han W-Y. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules. 2018;23(1):165. doi: 10.3390/molecules23010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marques DN, Carvalho MEA, Piotto FA, Batagin-Piotto KD, Nogueira ML, Gaziola SA, and Azevedo RA. Cadmium Tolerance in Plants, Elsevier (Academic Press, Elsevier; ). 2019;423–461 doi: 10.1016/B978-0-12-815794-7.00016-3. [DOI] [Google Scholar]
- 59.Li R, Jiang M, Song Y, and Zhang H. Melatonin alleviateslow-temperature stress via ABI5-mediated signals during seed germination in rice (Oryza sativa L.). Front Plant Sci 12; 2021:727596. doi: 10.3389/fpls.2021.727596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang T-L, Xi Q-Q, Wei X-Y, Xu L, Wang Q-Q, Fu J-Y, Ling C, Zuo Y, Zhao Y, and He H-Y. Rhythmical redox homeostasis can be restored by exogenous melatonin in hulless barley (Hordeum vulgare L. var. nudum) under cold stress. Environ Exp Bot. 194:104756. doi: 10.1016/j.envexpbot.2021.104756. [DOI] [Google Scholar]
- 61.Korkmaz A, Değer Ö, Szafrańska K, Köklü Ş, Karaca A, Yakupoğlu G, Kocacinar F. Melatonin effects in enhancing chilling stress tolerance of pepper. Sci Hortic (Amsterdam). 2021;289:110434. doi: 10.1016/j.scienta.2021.110434. [DOI] [Google Scholar]
- 62.Hayat F, Sun Z, Ni Z, Iqbal S, Xu W, Gao Z, Qiao Y, Tufail MA, Jahan MS, and Khan U. Exogenous melatonin improves cold tolerance of strawberry (Fragaria × ananassa Duch.) through modulation of DREB/CBF-COR pathway and antioxidant defense system. Horticulturae. 2022;8(3):194. doi: 10.3390/horticulturae8030194. [DOI] [Google Scholar]
- 63.Li J, Ding D, Li N, Xie J, Yu J, Lyv J, Bakpa EP, Zhang J, Wang C, and Zhang J. Melatonin enhances the low-temperature combined low-light tolerance of pepper (Capsicum annuum L.) seedlings by regulating photosynthesis, carotenoid, and hormone metabolism. Environ Exp Bot. 99:104868 doi: 10.1016/j.envexpbot.2022.104868. [DOI] [Google Scholar]
- 64.Yakuboğlu G, Ardıç ŞK, Cuci Y, Korkmaz A. Mitigation of chilling stress effects on eggplant seedlings by exogenous application of melatonin. Turkish J. Food Sci. Technol. 2022;10(4):562–68. doi: 10.24925/turjaf.v10i4.562-568.4601. [DOI] [Google Scholar]
- 65.Liu J, Wu H, Wang B, Zhang Y, Wang J, Cheng C, Huang Y. Exogenous melatonin enhances cold resistance by improving antioxidant defense and cold-responsive genes’ expression in banana. Horticulturae. 2022;8(3):260. doi: 10.3390/horticulturae8030260. [DOI] [Google Scholar]
- 66.Zhang J, Shi Y, Zhang X, Du H, Xu B, Huang B. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ Exp Bot. 2017;138:36–45. doi: 10.1016/j.envexpbot.2017.02.012. [DOI] [Google Scholar]
- 67.Liang D, Gao F, Ni Z, Lin L, Deng Q, Tang Y, Wang X, Luo X, Xia H. Melatonin improves heat tolerance in kiwifruit seedlings through promoting antioxidant enzymatic activity and glutathione S-transferase transcription. Molecules. 2018;23(3):584. doi: 10.3390/molecules23030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alam MN, Zhang L, Yang L, Islam MR, Liu Y, Luo H, Yang P, Wang Q, Chan Z. Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24-epibrassinolide. BMC Genomics. 2018;19(1):1–14. doi: 10.1186/s12864-018-4588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jahan MS, Shu S, Wang Y, Chen Z, He M, Tao M, Sun J, and Guo S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019;19(1):1–16. doi: 10.1186/s12870-019-1992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahammed GJ, Xu W, Liu A, and Chen S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ Exp Bot. 2019;161:303–11. doi: 10.1016/j.envexpbot.2018.06.006. [DOI] [Google Scholar]
- 71.Jia C, Yu X, Zhang M, Liu Z, Zou P, Ma J, and Xu Y. Application of melatonin-enhanced tolerance to high-temperature stress in cherry radish (Raphanus sativus L. var. radculus pers). J Plant Growth Regul. 2020;39(2):631–40. doi: 10.1007/s00344-019-10006-1. [DOI] [Google Scholar]
- 72.Buttar ZA, Wu SN, Arnao MB, Wang C, Ullah I, Wang C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants. 2020;9(7):809. doi: 10.3390/plants9070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma G, Zhang M, Xu J, Zhou W, Cao L. Transcriptomic analysis of short-term heat stress response in Pinellia ternata provided novel insights into the improved thermotolerance by spermidine and melatonin. Ecotoxicol Environ Saf. 2020;202:110877. doi: 10.1016/j.ecoenv.2020.110877. [DOI] [PubMed] [Google Scholar]
- 74.Alam MN, Yang L, Yi X, Wang Q, and Robin AHK. Role of melatonin in inducing the physiological and biochemical processes associated with heat stress tolerance in tall fescue (Festuca arundinaceous). Journal of Plant Growth Regulation. : doi: 10.1007/s00344-021-10472-6. [DOI] [Google Scholar]
- 75.Manafi H, Baninasab B, Gholami M, Talebi M, and Khanizadeh S. Exogenous melatonin alleviates heat‐induced oxidative damage in strawberry (Fragaria× ananassa Duch. cv Ventana) Plant. J Plant Growth Regul. 41:52–64 doi: 10.1007/s00344-020-10279-x. [DOI] [Google Scholar]
- 76.Yu Y, Deng L, Zhou L, Chen G, Wang Y. Exogenous melatonin activates antioxidant systems to increase the ability of rice seeds to germinate under high temperature conditions. Plants. 2022;11(7):886. doi: 10.3390/plants11070886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun C, Meng S, Qi M, Li T, Yin Z. Melatonin alleviates oxidative damage of photosynthesis under heat stress in tomato. SSRN. 2022;1–21. doi: 10.2139/ssrn.4030610. [DOI] [Google Scholar]
- 78.Fan X, Zhao J, Sun X, Zhu Y, Li Q, Zhang L, Zhao D, Huang L, Zhang C, Liu Q. Exogenous melatonin improves the quality performance of rice under high temperature during grain filling. Agronomy. 2022;12(4):949. doi: 10.3390/agronomy12040949. [DOI] [Google Scholar]
- 79.Pu Y-J, Cisse EHM, Zhang L-J, Miao L-F, Nawaz M, and Yang F. Coupling exogenous melatonin with Ca2+ alleviated chilling stress in Dalbergia odorifera T. Chen. Trees. 2021;35(5):1541–54. doi: 10.1007/s00468-021-02134-7. [DOI] [Google Scholar]
- 80.Chang T, Zhao Y, He H, Xi Q, Fu J, Zhao Y. Exogenous melatonin improves growth in hulless barley seedlings under cold stress by influencing the expression rhythms of circadian clock genes. PeerJ. 2021;9:e10740. doi: 10.7717/peerj.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bawa G, Feng L, Shi J, Chen G, Cheng Y, Luo J, Wu W, Ngoke B, Cheng P, and Tang Z. Evidence that melatonin promotes soybean seedlings growth from low-temperature stress by mediating plant mineral elements and genes involved in the antioxidant pathway. Funct Plant Biol. 2020;47(9):815–24. doi: 10.1071/FP19358. [DOI] [PubMed] [Google Scholar]
- 82.Kong X-M, Ge W-Y, Wei B-D, Zhou Q, Zhou X, Zhao Y-B, Ji S-J. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol Technol. 2020;170:111315. doi: 10.1016/j.postharvbio.2020.111315. [DOI] [Google Scholar]
- 83.Jannatizadeh A. Exogenous melatonin applying confers chilling tolerance in pomegranate fruit during cold storage. Sci Hortic (Amsterdam). 2019;246:544–49. doi: 10.1016/j.scienta.2018.11.027. [DOI] [Google Scholar]
- 84.Aghdam MS, Jannatizadeh A, Nojadeh MS, Ebrahimzadeh A. Exogenous melatonin ameliorates chilling injury in cut anthurium flowers during low temperature storage. Postharvest Biol Technol. 2019;148:184–91. doi: 10.1016/j.postharvbio.2018.11.008. [DOI] [Google Scholar]
- 85.Jannatizadeh A, Aghdam MS, Luo Z, Razavi F. Impact of exogenous melatonin application on chilling injury in tomato fruits during cold storage. Food Bioproc Tech. 2019;12(5):741–50. doi: 10.1007/s11947-019-2247-1. [DOI] [Google Scholar]
- 86.Sharafi Y, Jannatizadeh A, Fard JR, Aghdam MS. Melatonin treatment delays senescence and improves antioxidant potential of sweet cherry fruits during cold storage. Sci Hortic (Amsterdam). 2021;288:110304. doi: 10.1016/j.scienta.2021.110304. [DOI] [Google Scholar]
- 87.Xu R, Wang L, Li K, Cao J, Zhao Z. Integrative transcriptomic and metabolomic alterations unravel the effect of melatonin on mitigating postharvest chilling injury upon plum (cv. Friar) fruit. Postharvest Biol Technol. 2022;186:111819. doi: 10.1016/j.postharvbio.2021.111819. [DOI] [Google Scholar]
- 88.Jiao J, Jin M, Liu H, Suo J, Yin X, Zhu Q, Rao J. Application of melatonin in kiwifruit (Actinidia chinensis) alleviated chilling injury during cold storage. Sci Hortic (Amsterdam). 2022;296:110876. doi: 10.1016/j.scienta.2022.110876. [DOI] [Google Scholar]
- 89.Chen H, Lin H, Jiang X, Lin M, Fan Z. Amelioration of chilling injury and enhancement of quality maintenance in cold-stored guava fruit by melatonin treatment. Food Chemistry: X. 2022;14:100297. doi: 10.1016/j.fochx.2022.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhardwaj R, Pareek S, Saravanan C, Yahia EM. Contribution of pre-storage melatonin application to chilling tolerance of some mango fruit cultivars and relationship with polyamines metabolism and γ-aminobutyric acid shunt pathway. Environ Exp Bot. 2022;194:104691. doi: 10.1016/j.envexpbot.2021.104691. [DOI] [Google Scholar]
- 91.Yan R, Xu Q, Dong J, Kebbeh M, Shen S, Huan C, and Zheng X. Effects of exogenous melatonin on ripening and decay incidence in plums (Prunus salicina L. cv. Taoxingli) during storage at room temperature. Sci Hortic (Amsterdam). 2022;292:110655. doi: 10.1016/j.scienta.2021.110655. [DOI] [Google Scholar]
- 92.Raza A. Metabolomics: a systems biology approach for enhancing heat stress tolerance in plants. Plant Cell Rep. 2022;41(3):741–63. doi: 10.1007/s00299-020-02635-8. [DOI] [PubMed] [Google Scholar]
- 93.Haider S, Raza A, Iqbal J, Shaukat M, and Mahmood T. Analyzing the regulatory role of heat shock transcription factors in plant heat stress tolerance: a brief appraisal. Mol Biol Rep. 2022;49(6):5771–85. doi: 10.1007/s11033-022-07190-x. [DOI] [PubMed] [Google Scholar]
- 94.Sharma N, Thakur M, Suryakumar P, Mukherjee P, Raza A, Prakash CS, and Anand A. ‘Breathing Out’ under heat stress—respiratory control of crop yield under high temperature. Agronomy. 2022;12(4):806. doi: 10.3390/agronomy12040806. [DOI] [Google Scholar]
- 95.Hu S, Ding Y, Zhu C. Sensitivity and responses of chloroplasts to heat stress in plants. Front Plant Sci. 2020;11:375. doi: 10.3389/fpls.2020.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li X, Li M-H, Deng -W-W, Ahammed GJ, Wei J-P, Yan P, Zhang L-P, Fu J-Y, and Han W-Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J Plant Physiol. 2020;253:153273. doi: 10.1016/j.jplph.2020.153273. [DOI] [PubMed] [Google Scholar]
- 97.Haydari M, Maresca V, Rigano D, Taleei A, Shahnejat-Bushehri AA, Hadian J, Sorbo S, Guida M, Manna C, and Piscopo M. Salicylic acid and melatonin alleviate the effects of heat stress on essential oil composition and antioxidant enzyme activity in mentha× piperita and Mentha arvensis L. Antioxidants. 2019;8(11):547. doi: 10.3390/antiox8110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qi Z-Y, Wang K-X, Yan M-Y, Kanwar MK, Li D-Y, Wijaya L, Alyemeni MN, Ahmad P, and Zhou J. Melatonin alleviates high temperature-induced pollen abortion in Solanum lycopersicum. Molecules. 2018;23(2):386. doi: 10.3390/molecules23020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xing X, Ding Y, Jin J, Song A, Chen S, Chen F, Fang W, and Jiang J. Physiological and transcripts analyses reveal the mechanism by which melatonin alleviates heat stress in chrysanthemum seedlings. Front Plant Sci. 2012;12 673236 doi: 10.3389/fpls.2021.673236 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jahan MS, Guo S, Sun J, Shu S, Wang Y, Abou El-Yazied A, Alabdallah NM, Hikal M, Mohamed MH, Ibrahim MF. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol Biochem. 2021;167:309–20. doi: 10.1016/j.plaphy.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 101.Imran M, Aaqil Khan M, Shahzad R, Bilal S, Khan M, Yun B-W, Khan AL, Lee I-J. Melatonin ameliorates thermotolerance in soybean seedling through balancing redox homeostasis and modulating antioxidant defense, phytohormones and polyamines biosynthesis. Molecules. 2021;26(17):5116. doi: 10.3390/molecules26175116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muhammad I, Yang L, Ahmad S, Mosaad IS, Al-Ghamdi AA, Abbasi AM, Zhou X-B. Melatonin application alleviates stress-induced photosynthetic inhibition and oxidative damage by regulating antioxidant defense system of maize: a meta-analysis. Antioxidants. 2022;11(3):512. doi: 10.3390/antiox11030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang G, Xing M, Hu T, Ji M, Li X, Amombo E, Shao A, Xu X, and Fu J. Photosystem II photochemical adjustment of tall fescue against heat stress after melatonin priming. J Plant Physiol. 275:153758. doi: 10.1016/j.jplph.2022.153758. [DOI] [PubMed] [Google Scholar]
- 104.Sonnewald U, Fernie AR. Next-generation strategies for understanding and influencing source–sink relations in crop plants. Current Opinion in Plant Biology. 2018;43:63–70. doi: 10.1016/j.pbi.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 105.Demirer GS, Silva TN, Jackson CT, Thomas JB, W Ehrhardt D, Rhee SY, Mortimer JC, Landry MP. Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nat Nanotechnol. 2021;16(3):243–50. doi: 10.1038/s41565-021-00854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rajeev Kumar S, Anunanthini P, Ramalingam S. Epigenetic silencing in transgenic plants. Front Plant Sci. 2015;6:693. doi: 10.3389/fpls.2015.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhatnagar-Mathur P, Vadez V, Sharma KK. Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep. 2008;27(3):411–24. doi: 10.1007/s00299-007-0474-9. [DOI] [PubMed] [Google Scholar]
- 108.Chen K, Wang Y, Zhang R, Zhang H, Gao C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 2019;70(1):667–97. doi: 10.1146/annurev-arplant-050718-100049. [DOI] [PubMed] [Google Scholar]
- 109.Haque E, Taniguchi H, Hassan MM, Bhowmik P, Karim MR, Śmiech M, Zhao K, Rahman M, Islam T. Application of CRISPR/Cas9 genome editing technology for the improvement of crops cultivated in tropical climates: recent progress, prospects, and challenges. Front Plant Sci. 2018;9:617. doi: 10.3389/fpls.2018.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu H, Li C, Gao C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nature Reviews Molecular Cell Biology. 2020;21(11):661–77. doi: 10.1038/s41580-020-00288-9. [DOI] [PubMed] [Google Scholar]
- 111.Huang TK, Armstrong B, Schindele P, Puchta H. Efficient gene targeting in nicotiana tabacum using CRISPR/SaCas9 and temperature tolerant LbCas12a. Plant Biotechnol J. 2021;19(7):1314–24. doi: 10.1111/pbi.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shen C, Que Z, Xia Y, Tang N, Li D, He R, Cao M. Knock out of the annexin gene OsAnn3 via CRISPR/Cas9-mediated genome editing decreased cold tolerance in rice. J Plant Biol. 2017;60(6):539–47. doi: 10.1007/s12374-016-0400-1. [DOI] [Google Scholar]
- 113.Schindele P, Puchta H. Engineering CRISPR/Lb Cas12a for highly efficient, temperature-tolerant plant gene editing. Plant Biotechnol J. 2020;18(5):1118. doi: 10.1111/pbi.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeng Y, Wen J, Zhao W, Wang Q, Huang W. Rational improvement of rice yield and cold tolerance by editing the three genes OsPIN5b, GS3, and OsMYB30 with the CRISPR–Cas9 system. Front Plant Sci. 2020;10:1663. doi: 10.3389/fpls.2019.01663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li X, Xu S, Fuhrmann-Aoyagi MB, Yuan S, Iwama T, Kobayashi M, and Miura K. CRISPR/Cas9 technique for temperature, drought, and salinity stress responses. Curr Issues Mol Biol. 2022;44(6):2664–82. doi: 10.3390/cimb44060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu W, Cai SY, Zhang Y, Wang Y, Ahammed GJ, Xia XJ, Shi K, Zhou YH, Yu JQ, Reiter RJ. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J Pineal Res. 2016;61(4):457–69. doi: 10.1111/jpi.12359. [DOI] [PubMed] [Google Scholar]
- 117.Byeon Y, Park S, Lee HY, Kim YS, Back K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J Pineal Res. 2014;56(3):275–82. doi: 10.1111/jpi.12120. [DOI] [PubMed] [Google Scholar]
- 118.Byeon Y, Back K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J Pineal Res. 2014;56(4):408–14. doi: 10.1111/jpi.12129. [DOI] [PubMed] [Google Scholar]
- 119.Byeon Y, Lee HY, Lee K, Back K. A rice chloroplast transit peptide sequence does not alter the cytoplasmic localization of sheep serotonin N-acetyltransferase expressed in transgenic rice plants. J Pineal Res. 2014;57(2):147–54. doi: 10.1111/jpi.12151. [DOI] [PubMed] [Google Scholar]
- 120.Byeon Y, Park S, Kim YS, Back K. Microarray analysis of genes differentially expressed in melatonin-rich transgenic rice expressing a sheep serotonin N -acetyltransferase. J Pineal Res. 2013;55(4):357–63. doi: 10.1111/jpi.12077. [DOI] [PubMed] [Google Scholar]
- 121.Shi H, Jiang C, Ye T, Tan D-X, Reiter RJ, Zhang H, Liu R, Chan Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J Exp Bot. 2015;66(3):681–94. doi: 10.1093/jxb/eru373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kanjanaphachoat P, Wei B-Y, Lo S-F, Wang I-W, Wang C-S, Yu S-M, Yen M-L, Chiu S-H, Lai -C-C, and Chen L-J. Serotonin accumulation in transgenic rice by over-expressing tryptophan decarboxlyase results in a dark brown phenotype and stunted growth. Plant Mol Biol. 2012;78(6):525–43. doi: 10.1007/s11103-012-9882-5. [DOI] [PubMed] [Google Scholar]
- 123.Bajwa VS, Shukla MR, Sherif SM, Murch SJ, and Saxena PK. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J Pineal Res. 2014;56(3):238–45. doi: 10.1111/jpi.12115. [DOI] [PubMed] [Google Scholar]
- 124.Lei XY, Zhu RY, Zhang GY, Dai YR. Attenuation of cold‐induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. J Pineal Res. 2004;36(2):126–31. doi: 10.1046/j.1600-079X.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 125.Wang L, Zhao Y, Reiter RJ, He C, Liu G, Lei Q, Zuo B, Zheng XD, Li Q, Kong J. Changes in melatonin levels in transgenic ‘Micro-Tom’ tomato overexpressing ovine AANAT and ovine HIOMT genes. J Pineal Res. 2014;56(2):134–42. doi: 10.1111/jpi.12105. [DOI] [PubMed] [Google Scholar]
- 126.Lee HY, Byeon Y, Tan DX, Reiter RJ, Back K. Arabidopsis serotonin N-acetyltransferase knockout mutant plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. J Pineal Res. 2015;58(3):291–99. doi: 10.1111/jpi.12214. [DOI] [PubMed] [Google Scholar]
- 127.Huangfu L, Chen R, Lu Y, Zhang E, Miao J, Zuo Z, Zhao Y, Zhu M, Zhang Z, Li P. OsCOMT, encoding a caffeic acid O-methyltransferase in melatonin biosynthesis, increases rice grain yield through dual regulation of leaf senescence and vascular development. Plant Biotechnol J. 2022;20(6):1122–39. doi: 10.1111/pbi.13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang J, Guo Y, Yan J, Zhang Z, Yuan L, Wei C, Zhang Y, Ma J, Yang J, and Zhang X. The role of watermelon caffeic acid O-methyltransferase (ClCOMT1) in melatonin biosynthesis and abiotic stress tolerance. Hortic Res. 8:210. doi: 10.1038/s41438-021-00645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He H, He LF. Crosstalk between melatonin and nitric oxide in plant development and stress responses. Physiol Plant. 2020;170(2):218–26. doi: 10.1111/ppl.13143. [DOI] [PubMed] [Google Scholar]
- 130.Martínez-Lorente SE, Pardo-Hernández M, Martí-Guillén JM, López-Delacalle M, Rivero RM. Interaction between melatonin and no: action mechanisms, main targets, and putative roles of the emerging molecule nomela. Int J Mol Sci. 2022;23(12):6646. doi: 10.3390/ijms23126646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gu Q, Xiao Q, Chen Z, Han Y. Crosstalk between melatonin and reactive oxygen species in plant abiotic stress responses: an update. Int J Mol Sci. 2022;23(10):5666. doi: 10.3390/ijms23105666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nawaz MA, Huang Y, Bie Z, Ahmed W, Reiter RJ, Niu M, Hameed S. Melatonin: current status and future perspectives in plant science. Front Plant Sci. 2016;6:1230. doi: 10.3389/fpls.2015.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mukherjee S. Novel perspectives on the molecular crosstalk mechanisms of serotonin and melatonin in plants. Plant Physiol Biochem. 2018;132:33–45. doi: 10.1016/j.plaphy.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 134.Alsamir M, Mahmood T, Trethowan R, and Ahmad N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J Biol Sc. 2021;28(3):1654–63. doi: 10.1016/j.sjbs.2020.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jahan MS, Shu S, Wang Y, Hasan M, El-Yazied AA, Alabdallah NM, Hajjar D, Altaf MA, Sun J, Guo S. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA-and GA-mediated pathways. Front Plant Sci. 2021;12:381. doi: 10.3389/fpls.2021.650955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iqbal N, Fatma M, Gautam H, Umar S, Sofo A, D’ippolito I, Khan NA. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants. 2021;10(9):1778. doi: 10.3390/plants10091778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Turk H, Genisel M. Melatonin-related mitochondrial respiration responses are associated with growth promotion and cold tolerance in plants. Cryobiology. 2020;92:76–85. doi: 10.1016/j.cryobiol.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 138.Li H, Guo Y, Lan Z, Xu K, Chang J, Ahammed GJ, Ma J, Wei C, and Zhang X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic Res. 8:57. doi: 10.1038/s41438-021-00496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


