Abstract
Infection is the second leading cause of death in patients with chronic kidney disease (CKD). Adequate humoral (antibody) and cellular (T cell-driven) immunity are required to minimize pathogen entry and promote pathogen clearance to enable infection control. Vaccination can generate cellular and humoral immunity against specific pathogens and is used to prevent many life-threatening infectious diseases. However, vaccination efficacy is diminished in patients with CKD. Premature ageing of the immune system and chronic systemic low-grade inflammation are the main causes of immune alteration in these patients. In the case of SARS-CoV-2 infection, COVID-19 can have considerable detrimental effects in patients with CKD, especially in those with kidney failure. COVID-19 prevention through successful vaccination is therefore paramount in this vulnerable population. Although patients receiving dialysis have seroconversion rates comparable to those of patients with normal kidney function, most kidney transplant recipients could not generate humoral immunity after two doses of the COVID-19 vaccine. Importantly, some patients who were not able to produce antibodies still had a detectable vaccine-specific T cell response, which might be sufficient to prevent severe COVID-19. Correlates of protection against SARS-CoV-2 have not been established for patients with kidney failure, but they are urgently needed to enable personalized vaccination regimens.
Subject terms: Allotransplantation, Infection, End-stage renal disease, SARS-CoV-2
Effective vaccination strategies are crucial to mitigate the high risk of infection-associated morbidity and mortality in patients with kidney failure. Here, the authors examine vaccine-induced immunity in these patients, in particular their responses to COVID-19 vaccination, in the context of the immune impairment induced by kidney dysfunction and the use of immunosuppressive medications.
Key points
Patients with chronic kidney disease (CKD) have diminished vaccination responses owing to CKD-associated premature ageing of the immune system and chronic systemic low-grade inflammation.
Both humoral (B cell-driven) and cellular (T cell-driven) immunity are generally required to control infections. Antibodies are particularly efficient in preventing viral infections, whereas T cells have a key role in viral clearance and help to prevent severe disease.
T cell responses against the SARS-CoV-2 spike protein can be detected even in patients who are not able to produce antibody owing to primary or secondary immune deficiency.
The glomerular filtration rate correlates with the severity of COVID-19 — among patients with kidney disease, the worst COVID-19 outcomes are observed in patients with kidney failure.
Correlates of protection against SARS-CoV-2 have not been established for patients with kidney failure but they are urgently needed to enable personalized vaccination regimens. Patients with absent or low responses should be identified for targeted additional booster doses or pre-emptive therapy with monoclonal antibodies.
Vaccine responses in patients with kidney failure might also be increased through the use of higher vaccine doses, adjuvants or intradermal vaccination; temporary reduction of immunosuppressive drugs before vaccination might also be feasible.
Introduction
Chronic kidney disease (CKD) is associated with premature ageing compared with the general population1. This accelerated ageing process leads to alterations in the immune system that predispose individuals to the subsequent development of infections, cancer, autoimmunity and cardiovascular disease2. The high rate of morbidity and mortality associated with CKD, and especially with kidney failure, calls for urgent measures to prevent such life-threatening complications. As a preventive measure, vaccination has saved more lives than any other medical intervention3. However, vaccine-induced immune responses can vary greatly between individuals. For example, the antibody response to the inactivated seasonal influenza vaccine can vary up to 100-fold, and antibody responses against conjugated pneumococcal vaccines can vary up to 40-fold in the general population4. The reasons for such enormous differences are not completely understood. Many studies have investigated risk factors for failed immune responses following vaccination, which include host factors such as sex, age, genetic background, pre-existing immunity, co-morbidities, medication and microbiota; environmental factors such as geographic location, social status, lifestyle and nutrition; and vaccine-specific factors such as vaccine type, dose, schedule, administration route and adjuvants5.
Given their vulnerability to infection, effective vaccination strategies are of paramount importance to protect patients with CKD, especially those with kidney failure6. Similar to their diminished immune response observed in the context of natural infection, in general, patients with CKD mount lower responses to vaccination than healthy individuals7. Among patients with CKD, including those with kidney failure, the number of vaccination non-responders is considerable, which has led to adjustments in their vaccination regimens, especially for vaccines against hepatitis B virus (HBV) or the H1N1 influenza A virus subtype8. The optimal vaccination regime for several other pathogens (such as pneumococcal, herpes zoster, hepatitis A or meningococcal) still requires further evaluation. An effective SARS-CoV-2 vaccination strategy is of particular importance in these patients, owing to the positive association between COVID-19 severity and the grade of CKD9 among these patients; those with kidney failure experience the most severe COVID-19 symptoms10. Patients with kidney failure seem to mount an adequate cellular and humoral response to natural SARS-CoV-2 infection as the magnitude and functionality of SARS-CoV-2-specific immunity, including the T cell and neutralizing antibody responses, were comparable in these patients and individuals with normal kidney function11–14 leading to high expectations for vaccination success in this vulnerable patient cohort. However, the current phase III trials of COVID-19 vaccines have not included patients with kidney failure15,16; therefore, many questions about optimal vaccination doses, boosting regimen and side effects remain unanswered.
In this Review, we provide an overview of vaccine-induced immunity in patients with kidney failure, with a special focus on COVID-19 vaccine-specific immunity. We examine COVID-19 vaccine efficacy, including vaccine immunogenicity and ability to prevent disease, in patients with kidney failure compared with individuals with normal kidney function, and in the context of data available on responses to vaccination for other pathogens. We also discuss the role of the cellular and humoral arms of the adaptive immune response and consider possible mechanisms underlying impaired vaccine-induced immunity in patients with kidney failure, including the effects of immunosuppression.
Adaptive immunity in natural infection
The generation of antibodies following an antigenic challenge is widely acknowledged as a central mechanism underlying vaccine-induced protection. T cells have a crucial role in humoral immunity by providing help to B cells, which is required for their maturation and antibody production. However, less is known about the role of other effector functions of T cells, such as direct cell cytotoxicity, which can also be induced through vaccination. In the context of viral infections, studies on patients with immunodeficiency demonstrated the essential role of T cells in reducing viral load, whereas antibodies were required to prevent viral entry into the host cells17. In SARS-CoV-2 infections, the protective role of T cells is evident from case studies18,19 of patients with agammaglobulinaemia and COVID-19. Despite their general lack of immunoglobulins, patients with X-linked or autosomal-recessive agammaglobulinaemia could recover from COVID-19 without needing mechanical ventilation or intensive care. Similar observations were reported for varicella zoster virus infections — the lack of antibodies failed to protect patients against the infection but their post-infection recovery was similar to that observed in immunocompetent individuals. By contrast, patients with T cell deficiency developed uncontrolled and fatal infections17. These observational data suggest the important role of B cells and antibodies for preventing infection or reducing inoculum size, whereas T cell responses might be sufficient for the clearance of pathogens18,19.
Immune alterations in CKD and kidney failure
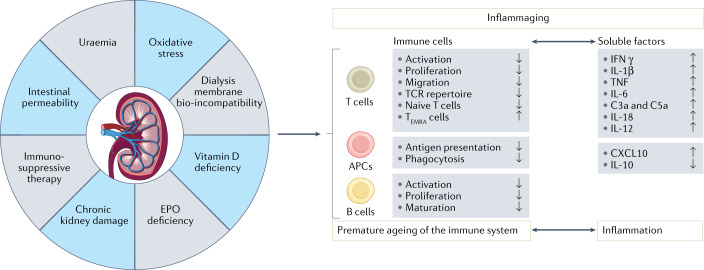
Different factors contribute to the immune alterations observed in patients with CKD, including kidney failure. These factors include accelerated ageing, kidney dysfunction-induced uraemia, oxidative stress, intestinal permeability and metabolic disorders, bio-incompatibility of dialysis membranes, and the use of immunosuppressive therapies in transplant recipients and patients with autoimmune disorders (Fig. 1).
Fig. 1. Inflammaging is a key immune alteration in kidney failure that impairs vaccine responses.
Several factors associated with kidney failure and/or kidney replacement therapy — uraemia, intestinal permeability, oxidative stress, erythropoietin (EPO) and vitamin D deficiency, chronic kidney damage, bio-incompatibility of haemodialysis membranes and immunosuppressive therapy — contribute to immune alterations in patients. These factors interfere with processes involved in the generation of antigen-specific immune response, including antigen uptake and presentation by antigen-presenting cells (APCs), such as dendritic cells and/or macrophages, and the activation and proliferation of antigen-specific T and B cells. Moreover, the systemic changes that occur in patients with kidney failure act on immune cells to induce the production of the pro-inflammatory cytokines IL-1β, TNF, IFNγ, IL-6, IL-12, IL-18, CXC-chemokine ligand 10 (CXCL10), and the complement effector molecules C3a and C5a, while decreasing levels of anti-inflammatory IL-10. These alterations lead to the development of chronic systemic inflammation. In the long term, persistent immune cell activation can also induce exhaustion, which leads to functional impairment and contributes to premature ageing of the immune system. This process, termed inflammaging, is considered a hallmark of immune alteration in chronic kidney disease. Specifically, the effects of uraemia and microinflammation on thymic tissue accelerate the age-associated reduction in the generation of naive T cells and thus contribute to the loss of diversity in the T cell receptor repertoire. In addition, persistent immune activation expands the terminally differentiated effector T (TEMRA) cell population. These highly differentiated T cells contain preformed pro-inflammatory molecules, are often self-reactive and can degranulate in response to unspecific signals, further contributing to inflammation.
Kidney dysfunction-induced immune impairment
In CKD, impaired excretory renal function leads to the accumulation of solutes that would otherwise be eliminated by healthy kidneys. Levels of these nitrogenous substances increase as kidney excretory function decreases, which exacerbates their toxic effects. These uraemic toxins can be categorized into small water-soluble compounds, middle molecules and protein-bound molecules, according to their physico-chemical properties20. Uraemic toxins can affect several essential immune cell functions including activation, migration, cytotoxicity and apoptosis. For example, the small water-soluble compound phenyl acetic acid increases the activation of leukocytes and reduces their apoptosis, thereby promoting an inflammatory state in uraemia21. Another key uraemic toxin, indoxyl sulfate, induces monocyte secretion of tumour necrosis factor (TNF) and production of reactive oxygen species (ROS), which promote inflammation and oxidative stress, respectively22,23. In addition, p-cresyl sulfate enhances phagocytosis in macrophages but decreases their ability to present antigens to T cells, which compromises the adaptive immune response in CKD24.
Oxidative stress — another important immune modulator in CKD — can be caused by disturbances in the balance between oxidant and antioxidant molecules, often owing to the excessive production of ROS and reactive nitrogen species, and the depletion of antioxidants25. The overproduction of ROS and reactive nitrogen species results in the oxidation of lipids, proteins and nucleic acids, which promotes the expression of pro-inflammatory cytokines and the recruitment of immune cells (reviewed in Duni et al.26). In vitro studies also demonstrated that ROS can increase TNF production in macrophages27.
Several studies suggest that, compared with healthy individuals, patients with CKD have increased levels of lipopolysaccharide (LPS) endotoxin owing to an uraemia-induced increase in intestinal permeability, which allows endotoxin translocation from the intestinal lumen into the circulation (reviewed in Steiger et al.28). LPS is a broad and potent immune stimulator of various immune cells that induces the release of several pro-inflammatory cytokines. Paradoxically, persistent innate immune activation induced by the prolonged presence of low-grade levels of LPS in patients receiving dialysis can trigger the production of immunoregulatory mediators such as IL-10, which suppress innate and adaptive immunity29. This effect is similar to the ‘endotoxin tolerance’ or ‘immune paralysis’ that is observed in patients with advanced sepsis or chronic infections, and underlies impaired immunity in these patients. Such an immune paralysis effect could impair the development of pathogen-specific immune responses in patients with kidney failure.
In addition to its excretory function, the kidney has several important metabolic functions, including the production of vitamin D and erythropoietin (EPO), both of which can have immunomodulatory effects. Beyond its regulation of calcium and phosphate homeostasis, the vitamin D metabolite calcitriol (that is, biologically active 1,25-dihydroxyvitamin D) regulates the proliferation and differentiation of immune cells. For example, vitamin D promotes tolerogenicity of dendritic cells by downregulating their expression of MHC, adhesion molecules and pro-inflammatory IL-12, while increasing the production of anti-inflammatory IL-10 (refs.30,31). Furthermore, treatment of T cells with vitamin D reduced the secretion of pro-inflammatory T helper 1 (TH1) cell mediators (for example, IL-2, IFNγ and TNF) while increasing the production of TH2 cell cytokines (including IL-3, IL-4, IL-5 and IL-10) in several human and animal studies32,33. Thus, reduced antigen presentation in combination with a shift of T cell differentiation from a TH1 cell to a TH2 cell profile induced by vitamin D treatment can diminish potential tissue damage associated with TH1 cell responses. In addition, activated T cells exposed to vitamin D produced less pro-inflammatory IL-17 and TH1 cytokines and significantly increased the expression of regulatory T cell cell-specific genes and molecules34, suggesting the induction of an anti-inflammatory T cell phenotype. Chronic excess of parathyroid hormone in patients with CKD patients caused by chronic stimulation of the parathyroid gland by hypocalcaemia and hyperphosphataemia can also lead to decreased T cell activation owing to a persistent elevation of intracellular calcium35. Although calcium influx is required for DC migration, calcium overload considerably impairs chemokine-dependent DC migration. The resulting impairment of DC migration into secondary lymphoid organs can limit T cell activation35. In mice and humans, EPO seems to have a direct inhibitory effect on effector and memory T cells, while promoting the differentiation of regulatory T cells. Available data support the idea that EPO synthesis within the kidney has evolved as a mechanism of peripheral immune tolerance to protect this organ from inflammatory responses induced by exposure to the urinary microbiome, high concentration of non-infectious antigens and sodium (reviewed in Cantarelli et al.36). Consequently, EPO and vitamin D deficiency observed in CKD patients might contribute to a diminished anti-inflammatory and increased pro-inflammatory status. Such a state of chronic inflammation might impair the generation of pathogen-specific immunity.
Treatment-induced immune impairment
Patients with CKD and kidney failure might require treatment with immunosuppressive drugs (discussed in more detail later) to mitigate autoimmunity-induced tissue damage or prevent graft rejection. In addition to the immune inhibition induced by the use of such immunosuppressive drugs, treatments such as dialysis can also have immunomodulatory effects. The haemodialysis procedure is known to induce inflammation and several mechanisms are involved in this process, including bio-incompatibility issues with dialysis membranes, which can affect the host immune system as blood comes into direct contact with artificial materials during extracorporeal circulation. Interaction with a haemodialysis membrane can induce the activation of peripheral blood mononuclear cells, with subsequent production of the pro-inflammatory cytokines TNF and IL-1β37. These cytokines can act in an autocrine manner to enhance cell survival but TNF can also induce apoptosis in activated antigen-presenting cells (APCs)38; the degree of apoptosis is related to the degree of membrane bio-incompatibility39, which is still a problem despite improvements in dialysis membrane biocompatibility40. In addition to these effects of contact with dialysis membranes, any bacterial endotoxins that might be present in dialysis fluids, and complement-derived anaphylatoxins such as C3a, can induce cell activation and secretion of pro-inflammatory cytokines37. Of note, the cleavage of C3 into its active fragments, C3a and C3b, can be directly triggered by haemodialysis biomaterials41.
Premature immune ageing
The ability to mount effective immune responses to a variety of novel pathogens depends on the presence of naive T cells, which develop in the thymus, with a diverse TCR repertoire42. However, under physiological conditions, the number of circulating naive T cells decreases gradually after the age of 40 years owing to age-associated thymic involution and the consequent decrease in thymic output43. In patients with CKD, loss of kidney function is associated with a similar loss of TCR diversity to that observed with ageing, even at early stages of disease44. One study reported that the immunological age of T cells was 20–30 years higher than the physiological age in patients with kidney failure45. Furthermore, uraemia not only affects thymic output but also leads to the expansion of terminally differentiated CD4+ and CD8+ T cells2,44. These highly differentiated T cells contain preformed pro-inflammatory molecules, are often self-reactive and can degranulate without a requirement for antigen-specific stimulation, therefore strongly contributing to inflammation; these cells also progressively lose co-stimulatory molecules that are important for their activation after antigen stimulation (reviewed in Betjes46). These highly differentiated T cells, in combination with the accumulation of uraemic toxins, metabolic deviations, gut leakage and oxidative stress associated with CKD, as well as the bio-incompatibility processes during dialysis treatment, promote the overproduction of pro-inflammatory cytokines, leading to chronic low-grade systemic inflammation in patients with uraemia47,48. Of note, chronic inflammation promotes premature immune system ageing and immune cell dysfunction directly, creating a vicious circle47. Furthermore, T cells have an increased susceptibility to activation-induced apoptosis compared with patients with normal kidney function49.
Vaccine-specific immunity in kidney failure
Successful vaccination depends on the ability of a vaccine to induce the generation of antibodies and cytotoxic T cells that can counteract a subsequent infection. The vaccine elicits a primary immune response, which leads to the differentiation of memory B and T cells that enable long-term protective immunity via antigen-specific antibody-mediated and cellular cytotoxicity following antigen re-challenge (Fig. 2). The aforementioned immune alterations in patients with CKD — especially premature ageing of immune systems and low-grade systemic inflammation50,51 (Fig. 1) — interfere with the generation of protective immunity, either in the course of natural infection or following vaccination52, which increases the incidence and severity of infectious diseases in these patients53,54.
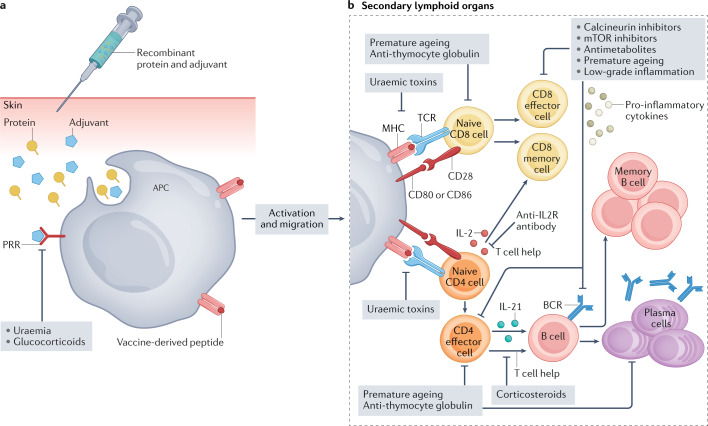
Fig. 2. Generation of vaccine-specific immunity and potential mechanisms of alteration in kidney failure..
a | Impaired vaccine-induced immunity in patients with kidney failure is multifactorial. To identify possible underlying mechanisms of alterations, it is important to review the single steps within the generation of vaccine–specific immune response. Pathogen-derived proteins are taken up by antigen-presenting cells (APCs), processed into peptides and presented on MHC molecules on the cell surface. Adjuvants are commonly added to vaccines to promote APC activation, thereby enhancing antigen uptake and presentation. These compounds act as danger signals by engaging pattern recognition receptors (PRRs). Following activation, APCs migrate to local lymph nodes where they present their MHC–peptide complexes to T cells. Kidney disease-induced factors such as uraemia and immunosuppressive drugs such as corticosteroids can impair APC activation and therefore compromise vaccine-induced immunity. b | Binding of a cognate T cell receptor (TCR) to an MHC–peptide complex, in combination with co-stimulatory signals provided by binding of CD80 and/or CD86 on APCs to CD28 on T cells, induces T cell activation. Activated CD4+ T cells provide help to B cells, to promote their differentiation into effector B cells and the production of antibodies with high affinity for the vaccine antigens, which are key components of humoral immunity. Some activated B cells differentiate into memory B cells to enable a long-term memory immune response, whereas others become plasma cells that actively secrete antibodies. Effector CD4+ T cells and CD8+ T cells activated by migrating APCs proliferate and produce effector cytokines and cytotoxic molecules that contribute to the elimination of infected cells and therefore drive cellular immunity; some of these cells also differentiate into memory T cells. Almost every step during the generation of immune response can be affected by immunosuppressive drugs or factors associated with kidney failure-induced immune alterations, such as premature ageing and low-grade inflammation.
COVID-19 vaccination
Antigen recognition triggers the development of an immune response. The choice of the right antigenic epitope is therefore crucial for vaccine immunogenicity and to induce a sustainable vaccine-specific immune response. During the first year of the COVID-19 pandemic, several vaccine candidates were developed using different platforms, which included nucleic acid (that is, DNA and RNA), viral vector, viral-like particle, peptide, recombinant protein, live-attenuated virus and inactivated virus modalities55. Six of these vaccines have been approved by at least one WHO-recognized authority (reviewed in Cevik et al.56) — the mRNA vaccines BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna), the viral vector vaccines ChAdOx-1 nCoV-19 (University of Oxford/AstraZeneca) and Ad26.COV2.S (Janssen), a recombinant spike protein (S-protein) subunit vaccine NVX-CoV2373 (Novavax) and an inactivated vaccine CoronaVac (Sinovac Biotech). Four of these vaccines (the mRNA-and viral vector-based vaccines) have been widely administered in most high-income countries.
The BNT162b2 and mRNA1273 vaccines are lipid nanoparticle (LNP)-encapsulated mRNA-based vaccines that encode full-length S-proteins, which mediates the fusion between SARS-CoV-2 particles and cell membranes, and is therefore crucial to viral entry into host cells57. The vaccines thus consist primarily of RNA, water, salt, sugar and lipids58. Notably, the LNPs in these vaccines encapsulate the mRNA to facilitate cell entry and prevent RNA degradation, and the mRNA was also modified to enhance protein production while avoiding overstimulation of immune system58. Both of these mRNA vaccines have been deployed through a prime–boost approach. By contrast, the adenovirus vectors in the ChAdOx-1 nCoV-19 and Ad26.COV2.S vaccines carry the full-length DNA sequences for the SARS-CoV-2 S-protein and are replication defective. ChAdOx-1 nCoV-19 has been deployed as a prime–boost vaccine, whereas the Ad26.COV2.S vaccine is administered as a single inoculation of viral particles.58
All of these vaccines are based on the SARS-CoV-2 S-protein but the membrane (M) and nucleocapsid (N) proteins of SARS-CoV-2 are also immunogenic12,59–61. Responses against the three SARS-CoV-2 proteins — S, M and N — varied between individuals infected with the virus, but in one study, the M protein induced an immune response in patients with acute COVID-19 most frequently and elicited the highest frequencies of CD4+ T cells12. Importantly, analysing only S-reactive T cells to assess the presence of antiviral immunity might lead to an underestimation; one study reported that 19% of analysed individuals had CD4+ T cells that were reactive against N protein or M protein but not against S protein12. These data therefore suggest that, in addition to S-protein epitopes, conserved epitopes within the M and N proteins might be promising vaccination targets to ensure neutralizing responses to a broad spectrum of SARV-COV-2 variants.
COVID-19 vaccine-specific immunity in patients receiving dialysis
Several studies have analysed the efficacy and safety of COVID-19 vaccines in patients receiving dialysis. The data are heterogeneous, which might be explained by differences in cohort size, co-morbidity prevalence, control cohorts and vaccination strategy. Although in most studies the humoral response was lower than that observed in control cohorts, the rate of seroconversion and the number of patients with detectable S-protein-reactive T cell immunity was very high (59–97%) (Supplementary Table 1). Of note, patients with kidney failure who are being treated with dialysis and immunosuppressive drugs (for example, with autoimmunity-driven CKD) might have a significantly diminished immune response following vaccination (discussed below). A 2021 mechanistic study assessed the humoral response to the BNT162b2 vaccine in patients with kidney failure and found defective memory B cell differentiation, including the generation of vaccine-specific plasmablasts and mature memory B cells, in patients receiving dialysis and in kidney transplant recipients62. In this study, the frequencies of antigen-specific circulating plasmablasts correlated with the total IgG response. This observation contrasts with data obtained from earlier studies of vaccination against other pathogens, in which IgG titres did not correlate with the frequencies of circulating antibody-secreting cells (ASCs) and/or plasmablasts63,64 owing to ASC migration to bone marrow or inflamed tissue64.
The safety of COVID-19 vaccination does not seem to differ between patients receiving dialysis and the general population15,16. Despite isolated case reports of de novo glomerulonephritis after COVID-19 vaccination65, data from large cohorts of patients with chronic inflammatory diseases did not show an increase in the relapse rate of these chronic inflammatory diseases66.
COVID-19 vaccine-specific immunity in kidney transplant patients
In contrast to the satisfactory vaccination-induced immunity detected in patients receiving dialysis, the efficacy of COVID-19 vaccination in kidney transplant recipients is suboptimal (Supplementary Table 1). A meta-analysis of 27 studies on the immunogenicity of COVID-19 vaccination in patients with kidney failure demonstrated that, after two doses of COVID-19 vaccine, kidney transplant patients had significantly lower seropositivity (26.1%) than patients receiving haemodialysis (84.3%) or peritoneal dialysis (92.4%), although higher seroconversion rates among transplant recipients have been reported in other studies67,68. Vaccine side effects in transplant recipients were comparable with those reported in phase III studies of healthy individuals15,16 and no donor-specific antibodies (DSA) developed after vaccination.
Despite this low humoral response, the prevalence of transplant recipients with detectable S-protein-reactive T cells was comparable with that of healthy controls69. Although the magnitude of the S-protein-specific CD4+ T cell response (assessed by flow cytometry) in kidney transplant recipients was significantly lower than that observed in healthy controls, the frequencies of S-protein-reactive CD4+ T cells were similar between the two groups after COVID-19 convalescence70. Multiple studies have confirmed this discrepancy between cellular and humoral immune responses in transplant recipients (Supplementary Table 1). These data suggest that a substantial proportion of kidney transplant recipients might fail to mount an adequate humoral response but might develop robust S-protein-reactive T cell responses. These antigen-reactive T cells produced cytokines in response to antigen stimulation ex vivo and should therefore, despite the lack of antibodies, mediate antiviral response activity and protect patients from severe COVID-19. Moreover, these data demonstrate that detection of S-protein-binding antibodies is not sufficient to assess the overall vaccination response. Consequently, current serological correlates of protection are not reliable and might underestimate existing antiviral protection in patients with a failed humoral response but with effective cellular immunity. Defining COVID-19 vaccine-specific correlates of protection for patients with kidney failure could facilitate the personalized management of these vulnerable patients during the pandemic. Of note, in the general population, detection of 264 binding antibody units in an antigen-binding assay, or a pseudovirus neutralization assay titre of 26 IU/ml, corresponded to a vaccine efficacy of 80 % against symptomatic COVID-19 (Alpha variant of concern (VOC))71; a threshold for asymptomatic infections could not be determined.
Risk factors for impaired vaccine responses
Identifying risk factors for failed immune responses after COVID-19 vaccination is currently of particular importance. A systematic review and meta-analysis of 32 studies on COVID-19 vaccination in patients receiving dialysis demonstrated that a higher prevalence of type 2 diabetes correlated significantly with lower vaccine immunogenicity (assessed via antibody response)72. Immunogenicity did not correlate significantly with mean age, sex and dialysis vintage in these patients. By contrast, a prospective study of COVID-19 mRNA vaccination in patients receiving dialysis demonstrated that immunosuppressive treatment, longer dialysis vintage, lower serum haemoglobin and albumin concentrations, and lower leukocyte counts were all predictors of a lack of response to vaccination in a univariate analysis73.
In accordance with the known role of patient age for de novo generation of pathogen-specific immune responses, the rate of seroconversion correlated negatively with age in a 2021 study of transplant recipients74. Type 2 diabetes, treatment with anti-thymocyte globulins in the previous year and longer time post-transplantation were also associated with a failed immune response68,75. Of note, risk factors should be evaluated separately in transplant recipients and patients receiving dialysis, owing to the differences in the use of immunosuppressive drugs in these two cohorts.
Immunosuppressive medications
Commonly used immunosuppressive medications inhibit the generation of antiviral and vaccine-specific immunity, for example, by interfering with the maturation of dendritic cells and therefore reducing the activation of specific T cells76 (Fig. 2); many of these drugs, such as glucocorticoids, have a broad immunosuppressive effect. Immunosuppressive antimetabolites such as azathioprine, mycophenolate mofetil, or its active form mycophenolic acid (MPA), have a clear inhibitory effect on seroconversion67,68,74,77. These drugs not only modulate the maturation, differentiation and early proliferation of B cells but also inhibit T cells78–80. However, one study reported that low-level MPA (≤360 mg/day) was associated with a better vaccination response (according to antibody titres) than a higher dose81. Rituximab treatment, which is used in patients with autoimmune kidney disease, prior to ABO-incompatible transplantation with a high level of HLA antibodies and in cases of post-transplant Epstein–Barr virus-associated lymphoma, reduces seroconversion significantly owing to its broad elimination of B cells and the consequent low or zero B cell count. Moreover, calcineurin inhibitors (CNIs) and mammalian target of rapamycin (mTOR) inhibitors modulate the activation and proliferation of T and B cells82,83. The number of serological non-responders to COVID-19 vaccination was higher among patients treated with a high dose of CNIs (tacrolimus >7 ng/ml or ciclosporin >150 ng/ml) than among those receiving lower doses81. By contrast, the role of mTOR inhibitors in SARS-CoV-2 vaccine responses remains unclear. Although mTOR can downregulate the proliferation of T and B cells82,83, some studies did not report a negative effect of mTOR inhibition on vaccination efficacy68,81. Furthermore, studies of influenza vaccination in older individuals demonstrated that mTOR inhibitors enhanced vaccination responsiveness84. The treated patients had a lower frequency of CD4+ and CD8+ T cells that express programmed death 1 (PD-1), which inhibits T cell proliferation, cytokine production and cytolytic function following antigenic stimulation85. This effect has not been investigated in the context of COVID-19 vaccination.
Belatacept is a cytotoxic T lymphocyte antigen 4 (CTLA4) fusion protein with high affinity for CD80 and CD86. This drug inhibits T cell activation by competitively blocking the binding of the co-stimulatory molecule CD28 to CD80 and CD86 (ref.86). Patients treated with belatacept had the worst vaccination response after two doses of COVID-19 vaccine (seroconversion rate 0–5%) than other patients with kidney failure who were treated with alternative immunosuppressive drugs87–90. Belatacept can inhibit S-protein-reactive T helper (TH) cells that express CD28 constitutively69, preventing them from providing B cell help and leading to a failed humoral response. This co-stimulation blockade also disrupts B cell–follicular helper T crosstalk, which reduces germinal centre formation91,92. Belatacept might also decrease the expression of the transcription factor PR domain zinc finger protein 1 (also known as BLIMP1), which regulates plasma cell differentiation and function91. Of note, belatacept conversion was associated with a high incidence of opportunistic infections, including cytomegalovirus, Epstein–Barr virus and pneumocystis, in a multicentre cohort study of kidney transplant recipients93.
Protein translation and efficacy of nucleic acid-based COVID-19 vaccines
The humoral immune response following COVID-19 vaccination is far below that observed with influenza94–96, which is also administered in a prime–boost regimen. For example, seroprotection after influenza vaccination in a transplant population was 63–92%, depending on the study and the influenza strains analysed. Importantly, in contrast to traditional whole organism and subunit vaccines, in nucleic acid vaccines the antigen has to be produced in the host cells before it can be presented by APCs. Immunosuppressive drugs might interfere with the processes involved in the generation and release of the S protein, thereby enhancing their inhibitory effect on this vaccine platform (Fig. 3). MPA reportedly interferes with translational processes in yeast97 and inhibits RNA polymerase III (Pol III) in mammalian cells, which is required for transcription of 5 S ribosomal RNA, transfer RNA98 and other small RNAs99,100. Pol III inhibition can lead to ribosomal disturbances and a rapid decline of transfer RNA, which is crucial to the correct translation of mRNA codons; rapamycin can also suppress Pol III activity101. Furthermore, in animal studies, the CNIs ciclosporin A and tacrolimus inhibited protein synthesis102.
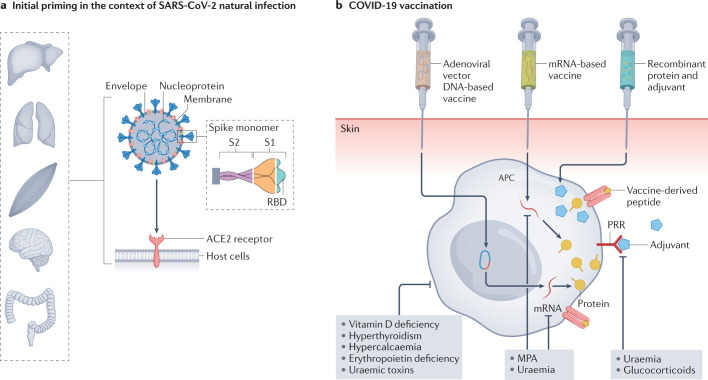
Fig. 3. SARS-CoV-2 natural infection and COVID-19 vaccination.
a | SARS-CoV-2 is transmitted via contaminated air droplets or by contact with contaminated surfaces. The virus encodes four major structural proteins: spike (S), membrane (M), envelope (E) and nucleocapsid (N). The S protein mediates entry into the host cell by binding to angiotensin-converting enzyme 2 (ACE2), which is expressed on different epithelial cells including lung, kidney, gastrointestinal tract, brain and muscle cells. Consequently, multiple organs are susceptible to SARS-CoV-2 infection. The S protein comprises a short intracellular tail and a large ectodomain that includes the receptor-binding domain (RBD) within the S1 subunit and the membrane-fusing S2 subunit. The S, M and N proteins are all known to elicit an immune response. b | Vaccination can be developed using different vaccine platforms, including subunit vaccines or nucleic acid-based (DNA or mRNA) vaccines. In addition to the relevant pathogen-derived antigens, vaccines typically contain adjuvants that stimulate pattern recognition receptors (PRRs). These compounds act on antigen-presenting cells (APCs) to improve antigen uptake, upregulation of MHC molecules, presentation of processed epitopes on MHC molecules and migration to draining lymph nodes, where they present MHC–antigen complexes to T cells. In mRNA-based vaccines, the protein antigen that will be used to activate adaptive immune cells still needs to be produced within host cells (via translation of the vaccine-delivered mRNA) before it can be processed and presented by APCs. Viral vector vaccines require an additional step, as the vaccine-delivered DNA must first be transcribed into mRNA within the host cells, before the aforementioned translation, processing and presentation steps. Under conditions of uraemia and/or immunosuppression, antigenic priming can be impaired through inhibition of APC activation, as well as inhibition of protein synthesis in nucleic acid-based vaccines. MPA, mycophenolic acid.
Importantly, immunosuppressive therapy does not seem to significantly affect natural immunity in transplant recipients infected with SARS-CoV-2 — the magnitude and functional characteristics of SARS-CoV-2-reactive T cells, as well as levels of S-protein-specific binding and neutralizing antibodies in transplant patients were comparable with those of individuals who were not being treated with immunosuppressive drugs14,70,103,104. The systemic immune response triggered by the natural infection, therefore, seems to be more resistant to the inhibitory effects of immunosuppressive drugs than vaccine-induced responses. One of the main differences between the natural infection and vaccination is the nature of antigen stimulation and the initial interaction between the antigen and the immune system. Given the aforementioned potential effects of immunosuppressive drugs on protein translation, mRNA or vector-based vaccination strategies might be especially inhibited owing to an effect on the amount of antigenic protein produced. Such an effect might also contribute to the observed stronger immunogenicity of mRNA-1273 compared with BNT162b2 given that the mRNA dose is three times higher in the mRNA-1273 vaccine, which might result in higher levels of antigen translation68,105,106. The crucial effect of factors specific to natural infection is also highlighted by the observation that, in patients with established SARS-CoV-2 immune induced by natural infection, the seroconversion rate was 100% after a single dose of mRNA vaccine, with antibody levels that were comparable with those of healthy volunteers107. These data confirm that secondary antibody responses are less affected by immunosuppression than primary responses in naive patients3; one main difference is that activation of secondary immune responses requires lower antigenic stimuli than primary immune activation.
Breakthrough infections in kidney failure
Over the past few months of the pandemic, the incidence of SARS-CoV-2 infections has started to rise again. However, beyond single-centre case series, data on the rate of breakthrough COVID-19 in vaccinated kidney transplant recipients are scarce. Nonetheless, the rate and severity of COVID-19 breakthrough infections in vaccinated kidney transplant recipients and patients receiving dialysis seem to be moderate, perhaps owing to the antiviral protective capacity of T cells in these patients81,108–110. Of note, breakthrough infection is associated with low antibody titres in most reports109–111. For example, in a 2021 study of 55 transplant recipients with breakthrough COVID-19, SARS-CoV-2 antibody data were available for 25 patients and 24 had negative serology111. In another study, among ~1,680 fully vaccinated kidney transplant recipients109 (at least 14 days after the second vaccine dose) who were being treated with a CNI and mycophenolate mofetil, 8 developed COVID-19. Of these infected patients, only 3 required short-term hospitalization (3–5 days) but none required mechanical ventilation or oxygen at discharge. By contrast, 12 of 181 vaccinated kidney transplant recipients treated with belatacept110 developed COVID-19. Eight of these patients required hospitalization, including 3 who required treatment in the intensive care unit; 6 patients died.
In addition to the effect of immunosuppressive drugs, viral mutations and the development of new viral VOCs contribute to the emergence of breakthrough COVID-19. The most commonly used COVID-19 vaccines have focused on the SARS-CoV-2 S-protein and the receptor-binding domain (RBD) contained within the S1 subunit of the S protein is currently the major focus of vaccine development (Fig. 3). This domain has an essential role in binding to host cells and blocking antibodies should therefore prevent viral entry into host cells. However, the RBD domain is especially susceptible to mutations, which increases the risk that novel viral strains might be able to escape the neutralizing capacity of antibodies directed against the original non-mutated RBD. The most recently reported SARS-CoV-2 VOC — named Omicron — contains up to 36 mutations in the S protein, 15 of which have been identified within the RBD112. These mutations are probably responsible for the failure of neutralizing antibodies and the high incidence of breakthrough infections in vaccinated individuals who are currently being observed113. Data from the general population showed that Omicron neutralization was undetectable for almost all individuals vaccinated with a prime–boost regimen, whereas in patients who had received an additional vaccine booster within the last 3 months, neutralization of Omicron was potent and only 4–6-fold lower than that observed with the original (wild type) SARS-CoV-2 variant112. These data suggest that the cross-reactivity of neutralizing antibodies is enhanced by an additional booster immunization, at least in the short term. However, the use of vaccines that target multiple S protein antigenic sites other than the RBD could be important, as previously demonstrated for the Middle East respiratory syndrome coronavirus114,115. Specifically, in addition to epitopes located on the RBD, an epitope located on the N-terminal domain elicited efficient neutralizing activity114. Furthermore, the addition of monoclonal antibodies specific for epitopes in RBD and non-RBD regions of Middle East respiratory syndrome coronavirus delayed the emergence of escape mutations in a cell-based virus in vitro escape assay115.
Breakthrough COVID-19 infections in fully vaccinated patients receiving dialysis have also been increasingly reported owing to suboptimal immunity against SARS-CoV-2 VOCs. In healthy individuals, the peak of neutralizing antibody titres after BNT162b2 vaccination was 4- to 6-fold lower in response to infection with the beta and delta VOC compared with the wild type or alpha VOC116. Similarly, only 63% of patients receiving dialysis had neutralizing antibodies against Beta VOC after two doses of BNT162b2 (ref.117) and breakthrough COVID-19 cases increased among fully vaccinated patients when the Delta VOC became dominant118. Interestingly, the breakthrough COVID-19 rate among fully vaccinated patients receiving dialysis was higher (1.6%) than that reported for fully vaccinated transplant recipients (0.8%)118, perhaps reflecting the higher SARS-CoV-2 exposure risk in patients attending dialysis centres. High exposure represents an important risk factor that should be considered in patient care, especially in the context of reduced vaccine-induced immunity. Another study of patients receiving dialysis reported that, despite 79% seroconversion rates and the detection of neutralizing antibodies against wild type SARS-CoV-2 in 56% of patients following two doses of mRNA vaccine, only 35% and 26% of patients had neutralizing antibodies against Alpha and Beta VOCs, respectively119. Levels of detectable neutralizing antibodies against the Omicron VOC were also significantly lower than those against the Delta or wild type variants in transplant recipients after three doses of mRNA vaccine120.
Importantly, currently available commercial serological tests do not reflect humoral immunity to VOCs adequately in the general population and especially in patients with kidney failure119,121. Consequently, relying on vaccine-induced seroconversion of enzyme-linked immunosorbent assay SARS-CoV-2 IgG or a positive anti-RBD response as a correlate of protection might be misleading, especially for protection against the Omicron variant. Of note, although humoral protection against VOCs in patients receiving dialysis and the general population is relatively low, cellular immunity against all analysed VOCs, including Omicron, seems to be detectable and functional in most study participants119,122,123.
Strategies to improve vaccination responses
Considering the severe clinical course and high mortality of patients with kidney failure and infections, particularly SARS-CoV-2 infection, improving seroconversion in patients with primary vaccination failure is crucial. The low vaccination response in kidney transplant recipients described above, the decline of antibody titres over time124, breakthrough COVID-19 in fully vaccinated patients118,125,126 and the uncertainty of protection against VOCs led to the rollout of additional primary vaccination doses in high-risk groups, including patients receiving dialysis and kidney transplant recipients. In contrast to a booster vaccine dose, which is given after the immunity of the initial prime–boost vaccination starts to wane, additional primary vaccine doses have been provided to patients with compromised immunity to improve their initial vaccination response. Additional primary vaccination doses had a clear benefit on the titres of S-protein-binding antibodies in a series of studies in patients with kidney failure with an absent or low response to two COVID-19 vaccine doses (Table 1). Of note, a significant decline in the SARS-CoV-2-specific humoral and cellular immune response observed 1 month after the third primary vaccine dose prompted the proposal of a fourth primary vaccine dose127. Some studies demonstrate that humoral immunity improves after a fourth primary vaccination dose in patients receiving dialysis and transplant recipients (Table 2). Accordingly, a fourth primary vaccine dose has been recommended for this vulnerable patient group128.
Table 1.
Immune responses in patients with kidney failure after three primary COVID-19 vaccine doses
| KRT | Vaccine | Humoral response after 2nd dose | Post-vaccination time (weeks) | Sample (n) | Humoral responsed (%) | T cell response (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Tx | mRNA-1273 | Absent to low | 4 | 159 | 49.1 | NA | 129 |
| Absent | 95 | 27.4 | NA | ||||
| HD | BNT162b2 | Absent to high | 4 | 38 | 94.7 | NA | 175 |
| PD | 31 | 100.0 | NA | ||||
| Tx | BNT162b2 | Absent to high | 4 | 80 | 61.3 | 70.0e | 130 |
| Absent to low | 50 | 38.0 | NA | ||||
| Tx | BNT162b2 | Absent to low | 2 | 66 | 42.4 | NA | 131 |
| Absent | 35 | 17.1 | NA | ||||
| Tx | BNT162b2 | Absent | 4 | 62c | 6.5 | NA | 110 |
| HD | BNT162b2 | Absent to high | 3 | 66 | 92.4 | NA | 176 |
| Absent to low | 34 | 97.1 | NA | ||||
| Absent | 11 | 54.5 | NA | ||||
| Tx | BNT162b2 | Absent to high | 4 | 396 | 67.9 | NA | 151 |
| Absent | 232 | 45.3 | NA | ||||
| HD | BNT162b2 | Absent to high | 4 | 45 | 88.9 | NA | 177 |
| Absent | 5 | 40.0 | NA | ||||
| HD | BNT162b2 | Absent to high | 2 | 75 | 92.0 | 64.0f | 140 |
| Absent | 12 | 50.0 | 33.3f | ||||
| Txa | mRNA-1273 | Absent to high | 4 | 60 | NA | NA | 132 |
| Tx | BNT162b2 | Absent to high | 4 | 76 | 67.1 | NA | 133 |
| HD | BNT162b2 | Absent | 4 | 12 | 41.7 | NA | 178 |
| Tx | BNT162b2 and mRNA-1273 | Absent to high | 4–11 | 158 | 46.2 | NA | 134 |
| Absent | 10 | 30.0 | NA | ||||
| Tx | BNT162b2 | Absent to high | 4 | 61 | 62.3 | NA | 135 |
| Absent | 34 | 32.4 | NA | ||||
| Tx | BNT162b2 | Absent to high | 4 | 136 | 69.1 | NA | 136 |
| HD | BNT162b2 | Absent to low | 4 | 17 | 70.6 | NA | 179 |
| Absent | 7 | 28.6 | NA | ||||
| Tx | AZD1222 and BNT162b2 | Absent | 1 | 25 | 36.0 | 95.0f | 137 |
| HDb | BNT162b2 and mRNA-1273 | Absent to low | 3–5 | 23 | 87.0 | NA | 121 |
| Tx | BNT162b2 | Absent to high | 4 | 48 | 30.0 | 26.0c | 138 |
| Tx | BNT162b2, mRNA-1273 and Ad26.COV2.S | Absent to low | 2 | 30 | 46.7 | NA | 153 |
| Absent | 24 | 33.3 | NA | ||||
| Txa | BNT162b2 | Absent | 4 | 10 | 60.0 | 90.0g | 139 |
HD, haemodialysis; KRT, kidney replacement therapy; NA, not available; Tx, transplantation. Some studies only included patients without seroconversion after the second vaccination dose, whereas others also included patients with a low humoral response or even all patients regardless of their immune response. aNeutralizing humoral immune response in 60% of patients. bNeutralizing humoral immune response in 86.4% of patients. cAll patients in this cohort were treated with belatacept. dDetermined by enzyme-linked immunosorbent assay-based assessment of antibody binding. eAnti-viral T cell immune response based on IFNγ ELISpot. fAnti-viral T cell immune response based on IFNγ release assay. gAnti-viral CD4+ T cell immune response based on flow cytometry.
Table 2.
Immune responses in patients with kidney failure after four primary COVID-19 vaccine doses
| KRT | Vaccine | Humoral response after 2nd dose | Post-vaccination time (weeks) | Sample (n) | Binding humoral response (%) | Ref. |
|---|---|---|---|---|---|---|
| Tx | BNT162b2, mRNA-1273 and Ad26.COV2.S | Absent to high | 2–6 | 18 | 83.3 | 141 |
| Absent | 6 | 50.0 | ||||
| Txa | mRNA-1273 | Low | 4 | 58 | 100.0 | 142 |
| Tx | BNT162b2 and mRNA-1273 | Absent to low | 4 | 92 | 50.0 | 143 |
| HD | BNT162b2 | Absent to high | 6–8 | 19 | 100.0 | 144 |
| Tx | BNT162b2 | Absent to low | 4 | 37 | 51.4 | 145 |
| Absent | 31 | 41.9 | ||||
| Tx | BNT162b2 and others | Absent | 5 | 49 | 42.9 | 146 |
| Tx | BNT162b2, mRNA-1273, ChAdOx1-S and Ad26.COV2.S | Absent to high | 2 | 250 | 55.6 | 147 |
| Tx | Ad26.COV2.S | Absent to low | 4 | 20 | 45.0 | 148 |
| Absent | 13 | 15.4 | ||||
| Tx | BNT162b2 | Absent | 4 | 29 | 76.0 | 149 |
HD, haemodialysis; KRT, kidney replacement therapy; NA, not available; Tx, transplantation. Note that some studies only included patients without seroconversion after the second or third vaccine dose, whereas others also included patients with a low humoral response or even all patients regardless of their immune response. a63.8% of patients had a neutralizing response against the Delta SARS-CoV-2 variant.
However, in contrast to patients receiving dialysis, the immunogenicity of a third or fourth primary vaccine dose is limited in transplant recipients. Nonetheless, studies of transplant recipients with an absent or low response to prime–boost vaccination110,129–149 showed that the seroconversion rate and antibody titres increased after additional primary vaccine doses (Tables 1,2). The observed seroconversion rate after the third and fourth primary vaccine doses ranged from 30 to 60% and from 15 to 76%, respectively, in initial non-responders. Neutralization assays were performed in a few studies and confirmed that the detected antibodies had a neutralizing capacity in most patients132,139. In addition to improvements in humoral immunity, several studies demonstrated enhanced cellular immunity; the range of detectable vaccine-specific T cell responses after the third primary vaccine dose was 25–95%. The broad variability in these responses might be at least partly explained by methodological differences, for example, the use of flow cytometry, ELISpot or IFNγ-release assay to detect T cell responses, or differences in assay sensitivity and limits of detection. Of note, in two studies that used flow cytometry, S-protein-reactive T cells were detected in 90% and 95%, respectively137,139. Importantly, detectable levels of anti-RBD IgG antibodies and S-protein-reactive CD4+ T cells were predictive of a high response to the third primary vaccine dose131. By contrast, <10% of patients with failed seroconversion and negative IFNγ-release assay responded to a third dose131.
With regard to safety, no adverse events other than those reported for phase III studies in the general population, such as fatigue, headache, local pain, injection site reactions and myalgia were observed110,132,135,139,150. Moreover, the incidence of acute rejection did not increase in transplant recipients after a third primary vaccine dose132,133,135,151. Of note, we only identified two single case reports of acute rejection after a second and a third primary mRNA vaccine dose in a kidney transplant and a heart transplant recipient, respectively152,153. Importantly, higher immunogenicity, for example, with the mRNA-1273 vaccine compared with BNT162b2, did not increase the incidence of acute rejection. These data are in line with those of previous studies demonstrating that the higher antigen concentration in HBV or influenza vaccines was not associated with an increased incidence of acute rejections154–157.
In addition to the administration of multiple primary doses in cases of failed seroconversion after the standard vaccination regimen, transient discontinuation of immunosuppressive drug therapies that have a strong inhibitory effect on vaccine-induced immunity could also be considered. For patients on a standard triple immunosuppressive regimen and at a low risk of allograft rejection, temporary discontinuation of antimetabolites should be tolerated without rejection episodes158. In patients treated with belatacept, a temporary switch to a CNI or an mTOR inhibitor might be necessary. Of note, the potential inhibitory effect of immunosuppression on the translation of vaccine-delivered mRNA, might be overcome by using alternative vaccine platforms that are independent of the translation of mRNA antigens, but this hypothesis has not been tested.
Several prospective vaccination studies and retrospective analyses compared the efficacy and immunogenicity of different mRNA vaccines in patients receiving dialysis and kidney transplant recipients (Table 3). The mechanisms underlying the differences in the immunogenicity of COVID-19 vaccines have not been identified but might include differences in antigen dose. Higher antigen doses provide a stronger immune stimulus and subsequently induce a stronger immune response159–161. Accordingly, individuals with compromised immunity such as patients with CKD, transplant recipients or older individuals, receive higher antigen doses than those given to the general population when they are vaccinated against HBV154,155 and influenza162,163. Specifically, patients with kidney failure receive an HBV vaccination dose that is four times higher than the standard dose164. Moreover, immunogenicity (according to the antibody response) was significantly better in transplant recipients that received a high dose of influenza vaccination compared with the standard dose165. Of note, despite its higher mRNA concentration, which does not seem to have safety implications, the efficacy of the mRNA-1273 vaccine data was similar to that of the BNT162b2 vaccine in the general population15,16. However, in almost all reported studies in patients receiving dialysis and kidney transplant recipients, vaccination with mRNA-1273 had the best outcomes defined by the incidence of breakthrough COVID-19 and antibody titres (Table 3). Importantly, increasing vaccination doses could reduce vaccine stocks and compromise vaccine availability55.
Table 3.
Efficacy of prime–boost COVID-19 vaccination in patients with kidney failure
| KRT | Post-vaccination time (weeks) | Sample (n) | Efficacy parameter | BNT162b2 | mRNA-1273 | Ad26.COV2.S | ChAdOx-1 nCoV-19 | Ref. |
|---|---|---|---|---|---|---|---|---|
| HD | 2 | 519 | Frequency of detectable humoral response | 90.4% | 97.2% | 16.7% | NA | 180 |
| HD | 2–3 | 143 | Frequency of detectable neutralizing response | 34.1% | 77.3% | NA | NA | 181 |
| HD | 4 | 94 | Frequency of detectable humoral response | 81.8% | NA | 70.6% | NA | 182 |
| Txa | 4 | 649 | Frequency of detectable humoral response | 48.8% | 59.6% | NA | NA | 67 |
| HD | 3 | 205 | Frequency of detectable humoral response | 92.0% | 98.0% | NA | NA | 73 |
| HD | >7 | 5,318 | Positive PCR incidence rate (per 1,000 patient-weeks) | 1.4 | NA | 1.5 | NA | 183 |
| Tx | 2–3 | 142 | Frequency of detectable humoral response | 47.6% | 62.2% | NA | NA | 184 |
| Magnitude titre (AU/ml) | <111 | 255 | NA | NA | ||||
| HD | 2–4 | 1,583 | Frequency of detectable humoral response | 95.7% | 97.4% | 41.6% | NA | 185 |
| HD | 2 | 1,528 | Frequency of detectable humoral response | 87.0% | 96.1% | 25.5% | NA | 186 |
| PD | NA | |||||||
| Tx | 2–6 | 25 | Frequency of detectable humoral response | 25.0% | 25.0% | NA | NA | 187 |
| HD | 3 | 116 | Frequency of detectable humoral response | 94.9% | 98.7% | NA | NA | 188 |
| Magnitude titre (BAU/ml) | 676 | 1507 | NA | NA | ||||
| HD | 2–4 | 148 | Frequency of detectable humoral response | 85.8% | 93.3% | NA | NA | 189 |
| HD | 12–22 | 10,576 | Positive PCR incidence rate (per 10,000 patient-days) | 1.47 | 0.91 | 2.42 | NA | 118 |
| PD | NA | |||||||
| HD | 2–4 | 64 | Frequency of detectable humoral response | 93.0%b | 33.0% | NA | 190 | |
| Tx | 4 | 768 | Frequency of detectable humoral response | 65.6% | NA | NA | 43.6% | 191 |
| Frequency of detectable cellular response | 17.5% | NA | NA | 5.1% | ||||
| Magnitude titre (BAU/ml) | 58 | NA | NA | 7.1 | ||||
| HD | 4–5 | 1,136 | Frequency of detectable humoral response | 87.5% | 97.0% | NA | NA | 68 |
| Tx | 333 | 49.0% | 26 | NA | NA | |||
| HD | 4–5 | 543 | Frequency of detectable humoral response | 76.5% | 87.8% | NA | NA | 106 |
| Magnitude titre (AU/ml) | 258 | 1,230 | NA | NA | ||||
| Magnitude cellular response | 0.497 | 0.809 | NA | NA | ||||
| HD | 3–4 | 138 | Frequency of detectable humoral response | 76.9% | 100.0% | NA | NA | 105 |
| PD | NA | NA | ||||||
| HD | Magnitude titre (AU/ml) | 16.6 | 16.2 | NA | NA | |||
| PD | NA | NA | ||||||
| Tx | 133 | Frequency of detectable humoral response | 56.0% | 76.2% | NA | NA | ||
| Magnitude titre (AU/ml) | 12.4 | 5.6 | NA | NA | ||||
AU, antibody unit; BAU, binding antibody unit; HD, haemodialysis; KRT, kidney replacement therapy; NA, not available; Tx, transplantation. Only patients with a complete vaccination course and no known history of SARS-CoV-2 infection were considered for this summary. aThis cohort included patients with non-renal transplantation. bResponse reported for mRNA vaccines as a group without specific data for each vaccine.
Additional adjuvants and intradermal application of vaccines might also improve vaccine immunogenicity165. For example, the addition of the MF59 adjuvant improved the rate of seroconversion in response to an influenza vaccine in patients receiving haemodialysis166. Of note, LNPs can provide danger signals and stimulate innate immunity through pattern recognition receptors. mRNA-1273 LNPs seem to induce a higher pro-inflammatory response than those used in the BNT162b2 vaccine167. mRNA modifications also affect protein production, therefore modulating immunogenicity58. For example, in both mRNA-1273 and BNT162b2 vaccines, N1-methyl-pseudourine substitutes all uridines throughout the S-protein mRNA sequence. To the best of our knowledge, no differences in mRNA modification between the two vaccines have been reported168.
Intradermal vaccine delivery also led to a higher rate of seroconversion than intramuscular delivery in several studies of HBV and influenza vaccination in patients receiving haemodialysis and transplant recipients169,170. However, data on the efficacy of all of these approaches in the context of COVID-19 vaccination are rare and further studies are lacking.
In patients at a high risk of severe disease with COVID-19 immunization failure, especially kidney transplant recipients, monoclonal antibody therapy might be effective in preventing severe COVID-19, especially if the treatment is initiated early in the course of infection. In the future, administration of long-lasting monoclonal antibody preparations might also enable passive immunization to be provided in patients with refractory immunization failure. Of note, although several antibody preparations with a high neutralization capacity against Alpha, Beta and Gamma SARS-CoV-2 VOC were not protective against Omicron, the monoclonal antibody sotrovimab provided in vitro neutralization against Delta and some subvariants of Omicron171–173.
Conclusions
The generation of vaccine-specific immune responses is a complex process and many of its steps can be disrupted in patients with kidney failure. Further studies are required to elucidate the mechanisms underlying the immune alterations in these patients and to identify potential strategies to improve vaccination outcomes in this population. Thus far, no phase III studies have evaluated the efficacy and safety of COVID-19 vaccination in patients receiving kidney replacement therapy. However, several real-life multicentre vaccination trials in this population suggest that vaccination safety is similar to that of the general population. Importantly, correlates of vaccine-specific immune protection have not been defined. However, the data available so far indicate that full protection requires both arms of adaptive immunity — the cellular and the humoral responses. Further studies are needed to assess the degree of vaccine-induced COVID-19 protection in patients with CKD, especially in those with kidney failure. Although the immunogenicity of COVID-19 vaccination seems to be mostly satisfactory for patients receiving dialysis, the response rate in kidney transplant recipients is low and requires additional measures to ensure optimal protection in this vulnerable group. This low level of seroconversion in transplant recipients was unexpected because vaccination against other pathogens is typically more immunogenic. Given that the main difference between COVID-19 vaccines and other common vaccines is the vaccine platform, immunosuppressant-induced inhibition of transcription and/or translation in vector or mRNA-based vaccines might have a role. Therefore, the potential benefits of subunit or recombinant SARS-CoV-2 protein-based vaccines should be investigated, especially for immunosuppressed patients. Other strategies for improving vaccine responses that should be investigated in patients with kidney failure include the use of adjuvants, intradermal vaccine administration, higher vaccine doses, and additional primary or booster doses.
The currently high rate of breakthrough COVID-19 in fully vaccinated patients can be explained by the waning immune response post-vaccination (current data demonstrate that antibody levels wane over time, including in the general population) in combination with differences in the antigenic structure between the SARS-CoV-2 wild type strain, on which current vaccines are based, and SARS-CoV-2 VOCs. Additional primary vaccine doses or the administration of boosters improved the response to VOCs significantly144. Notably, in the general population, only individuals who had received a booster within the last 3 months had potent neutralization of the Omicron VOC112. In addition, for patients with certain MHC genotypes, immune responses might be mainly directed against SARS-CoV-2 proteins other than the S protein, such as the M or N protein. In such patients, S-protein-based immunization might not elicit an immune response capable of preventing a breakthrough infection174.
Supplementary information
Author contributions
N.B. researched data for the article, made substantial contributions to discussions of the content and wrote the article. All the authors reviewed or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Nephrology thanks D. Bertrand, D. Ducloux, L. Harper and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41581-022-00617-5.
References
- 1.Kooman JP, Kotanko P, Schols AMWJ, Shiels PG, Stenvinkel P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014;10:732–742. doi: 10.1038/nrneph.2014.185. [DOI] [PubMed] [Google Scholar]
- 2.Crépin T, et al. Uraemia-induced immune senescence and clinical outcomes in chronic kidney disease patients. Nephrol. Dial. Transpl. 2020;35:624–632. doi: 10.1093/ndt/gfy276. [DOI] [PubMed] [Google Scholar]
- 3.Kotton CN. Immunization after kidney transplantation — what is necessary and what is safe? Nat. Rev. Nephrol. 2014;10:555–562. doi: 10.1038/nrneph.2014.122. [DOI] [PubMed] [Google Scholar]
- 4.Lynn DJ, Benson SC, Lynn MA, Pulendran B. Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nat. Rev. Immunol. 2021 doi: 10.1038/s41577-021-00554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019;32:e00084-18. doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma BM, et al. Vaccination in patients with chronic kidney disease — review of current recommendations and recent advances. Nephrology. 2021;26:5–11. doi: 10.1111/nep.13741. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, et al. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krueger KM, Ison MG, Ghossein C. Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am. J. Kidney Dis. 2020;75:417–425. doi: 10.1053/j.ajkd.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Uribarri A, et al. Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) Registry. J. Nephrol. 2020;19:1–9. doi: 10.1007/s40620-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kant S, et al. The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation. BMC Nephrol. 2020;21:1–13. doi: 10.1186/s12882-020-02112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anft M, et al. COVID-19-induced ARDS is associated with decreased frequency of activated memory/effector T cells expressing CD11a++ Mol. Ther. 2020;28:2691–2702. doi: 10.1016/j.ymthe.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thieme CJ, et al. Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep. Med. 2020;1:100092. doi: 10.1016/j.xcrm.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartzell S, et al. Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am. J. Transplant. 2020;20:3149–3161. doi: 10.1111/ajt.16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candon S, et al. T cell and antibody responses to SARS-CoV-2: experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am. J. Transplant. 2021;21:854–863. doi: 10.1111/ajt.16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinti I, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J. Allergy Clin. Immunol. 2020;146:211. doi: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soresina A, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020;31:565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanholder R, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 21.Cohen G, Raupachova J, Hörl WH. The uraemic toxin phenylacetic acid contributes to inflammation by priming polymorphonuclear leucocytes. Nephrol. Dial. Transpl. 2013;28:421–429. doi: 10.1093/ndt/gfs454. [DOI] [PubMed] [Google Scholar]
- 22.Yu M, Kim YJ, Kang DH. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin. J. Am. Soc. Nephrol. 2011;6:30–39. doi: 10.2215/CJN.05340610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HY, et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD) Sci. Rep. 2017;7:3057. doi: 10.1038/s41598-017-03130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azevedo MLV, et al. p-Cresyl sulfate affects the oxidative burst, phagocytosis process, and antigen presentation of monocyte-derived macrophages. Toxicol. Lett. 2016;263:1–5. doi: 10.1016/j.toxlet.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Daenen K, et al. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019;34:975–991. doi: 10.1007/s00467-018-4005-4. [DOI] [PubMed] [Google Scholar]
- 26.Duni A, Liakopoulos V, Roumeliotis S, Peschos D, Dounousi E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: untangling Ariadne’s thread. Int. J. Mol. Sci. 2019;20:3711. doi: 10.3390/ijms20153711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanlioglu S, et al. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-α secretion through IKK regulation of NF-κB. J. Biol. Chem. 2001;276:30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- 28.Steiger S, Rossaint J, Zarbock A, Anders HJ. Secondary immunodeficiency related to kidney disease (SIDKD) — definition, unmet need, and mechanisms. J. Am. Soc. Nephrol. 2022;33:259–278. doi: 10.1681/ASN.2021091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–1016. doi: 10.1038/ki.2012.440. [DOI] [PubMed] [Google Scholar]
- 30.Lang C-L, Wang M-H, Chiang C-K, Lu K-C. Vitamin D and the immune system from the nephrologist’s viewpoint. ISRN Endocrinol. 2014;2014:105456. doi: 10.1155/2014/105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 2009;70:345–352. doi: 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J. Nutr. 1995;125:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 33.Boonstra A, et al. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 34.Jeffery LE, et al. 1,25-Dihydroxyvitamin D 3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massry SG, Smogorzewski M. Dysfunction of polymorphonuclear leukocytes in uremia: role of parathyroid hormone. Kidney Int. 2001;59:195–196. doi: 10.1046/j.1523-1755.2001.07845.x. [DOI] [PubMed] [Google Scholar]
- 36.Cantarelli C, Angeletti A, Cravedi P. Erythropoietin, a multifaceted protein with innate and adaptive immune modulatory activity. Am. J. Transplant. 2019;19:2407–2414. doi: 10.1111/ajt.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libetta C, Sepe V, Canton AD. Bio-incompatibility and Th2 polarization during regular dialysis treatment. Int. Rev. Immunol. 2010;29:608–625. doi: 10.3109/08830185.2010.522282. [DOI] [PubMed] [Google Scholar]
- 38.Zanoni I, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 39.Carracedo J, et al. Cell apoptosis and hemodialysis-induced inflammation. Kidney Int. Suppl. 2002;61:89–93. doi: 10.1046/j.1523-1755.61.s80.17.x. [DOI] [PubMed] [Google Scholar]
- 40.Fukushi T, Yamamoto T, Yoshida M, Fujikura E, Miyazaki M. Enhanced neutrophil apoptosis accompanying myeloperoxidase release during hemodialysis. Sci. Rep. 2020;10:21747. doi: 10.1038/s41598-020-78742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kourtzelis I, et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. 2010;116:631–639. doi: 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang F, et al. Decreased peripheral naïve T cell number and its role in predicting cardiovascular and infection events in hemodialysis patients. Front. Immunol. 2021;12:1–9. doi: 10.3389/fimmu.2021.644627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naylor K, et al. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 44.Litjens NHR, Van Druningen CJ, Betjes MGH. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin. Immunol. 2006;118:83–91. doi: 10.1016/j.clim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Betjes MGH, Langerak AW, Van Der Spek A, De Wit EA, Litjens NHR. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011;80:208–217. doi: 10.1038/ki.2011.110. [DOI] [PubMed] [Google Scholar]
- 46.Betjes MGH. Uremia-associated ageing of the thymus and adaptive immune responses. Toxins. 2020;12:224. doi: 10.3390/toxins12040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebert T, et al. Inflammation and premature ageing in chronic kidney disease. Toxins. 2020;12:227. doi: 10.3390/toxins12040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen G. Immune Dysfunction in Uremia 2020. Toxins. 2020;12:439. doi: 10.3390/toxins12070439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier, P., Dayer, E. & Blanc, E. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease.13, 204–212 (2002). [DOI] [PubMed]
- 50.Stavropoulou E, et al. Microbiome, immunosenescence, and chronic kidney disease. Front. Med. 2021;8:1–8. doi: 10.3389/fmed.2021.661203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39:84–92. doi: 10.1159/000368940. [DOI] [PubMed] [Google Scholar]
- 52.Mathew R, Mason D, Kennedy JS. Vaccination issues in patients with chronic kidney disease. Expert. Rev. Vaccines. 2014;13:285–298. doi: 10.1586/14760584.2014.874950. [DOI] [PubMed] [Google Scholar]
- 53.Ishigami J, Matsushita K. Clinical epidemiology of infectious disease among patients with chronic kidney disease. Clin. Exp. Nephrol. 2019;23:437–447. doi: 10.1007/s10157-018-1641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalrymple LS, Go AS. Epidemiology of acute infections among patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2008;3:1487–1493. doi: 10.2215/CJN.01290308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai CC, et al. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expert. Rev. Vaccines. 2021;20:1013–1025. doi: 10.1080/14760584.2021.1949293. [DOI] [PubMed] [Google Scholar]
- 56.Cevik M, Grubaugh ND, Iwasaki A, Openshaw P. COVID-19 vaccines: keeping pace with SARS-CoV-2 variants. Cell. 2021;184:5077–5081. doi: 10.1016/j.cell.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nance KD, Meier JL. Modifications in an emergency: the role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent. Sci. 2021;7:748–756. doi: 10.1021/acscentsci.1c00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grifoni A, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu S, et al. The immunodominant and neutralization linear epitopes for SARS-CoV-2. Cell Rep. 2021;34:108666. doi: 10.1016/j.celrep.2020.108666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swadling L, et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. 2021 doi: 10.1038/s41586-021-04186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rincon-Arevalo H, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021;6:1–13. doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 63.Leyendeckers H, et al. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur. J. Immunol. 1999;29:1406–1417. doi: 10.1002/(SICI)1521-4141(199904)29:04<1406::AID-IMMU1406>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 64.Odendahl M, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 65.Tan HZ, et al. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021;100:467–471. doi: 10.1016/j.kint.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furer V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann. Rheum. Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 67.Boyarsky BJ, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stumpf J, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021 doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sattler A, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Invest. 2021;131:e150175. doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thieme CJ, et al. The magnitude and functionality of SARS-CoV-2 reactive cellular and humoral immunity in transplant population is similar to the general population despite immunosuppression. Transplantation. 2021;105:2156–2164. doi: 10.1097/TP.0000000000003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen JJ, et al. Immunogenicity rates after SARS-CoV-2 vaccination in people with end-stage kidney disease: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4:e2131749. doi: 10.1001/jamanetworkopen.2021.31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Broseta J, et al. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am. J. Kidney Dis. 2021;78:571–581. doi: 10.1053/j.ajkd.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Midtvedt K, et al. Low immunization rate in kidney transplant recipients also after dose 2 of the BNT162b2 vaccine: continue to keep your guard up! Transplantation. 2021;105:E80–E81. doi: 10.1097/TP.0000000000003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cucchiari D, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat. Rev. Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 77.Taylor AL, Watson CJE, Bradley JA. Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit. Rev. Oncol. Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 78.Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14:2–8. doi: 10.1191/0961203305LU2109OA. [DOI] [PubMed] [Google Scholar]
- 79.Haneda M, et al. Comparative analysis of drug action on B-cell proliferation and differentiation for mycophenolic acid, everolimus, and prednisolone. Transplantation. 2014;97:405–412. doi: 10.1097/01.tp.0000441826.70687.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karnell JL, et al. Mycophenolic acid differentially impacts B cell function depending on the stage of differentiation. J. Immunol. 2011;187:3603–3612. doi: 10.4049/jimmunol.1003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rozen-Zvi B, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin. Microbiol. Infect. J. 2021;27:1173.e1–1173.e4. doi: 10.1016/j.cmi.2021.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Broen JCA, van Laar JM. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat. Rev. Rheumatol. 2020;16:167–178. doi: 10.1038/s41584-020-0374-8. [DOI] [PubMed] [Google Scholar]
- 83.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 85.Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C. Partial restoration of T cell function in aged mice by in vitro blockade of the PD-1/PD-L1 pathway. Aging Cell. 2010;9:785–798. doi: 10.1111/j.1474-9726.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gardner D, Jeffery LE, Sansom DM. Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. Am. J. Transpl. 2014;14:1985–1991. doi: 10.1111/ajt.12834. [DOI] [PubMed] [Google Scholar]
- 87.Chavarot N, et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105:E94–E95. doi: 10.1097/TP.0000000000003784. [DOI] [PubMed] [Google Scholar]
- 88.Bertrand D, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J. Am. Soc. Nephrol. 2021;32:2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ou MT, et al. Immunogenicity and reactogenicity after SARS-CoV-2 mRNA vaccination in kidney transplant recipients taking belatacept. Transplantation. 2021;105:2119–2123. doi: 10.1097/TP.0000000000003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terrec F, et al. Belatacept use after kidney transplantation and its effects on risk of infection and COVID-19 vaccine response. J. Clin. Med. 2021;10:5159. doi: 10.3390/jcm10215159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leibler C, et al. Control of humoral response in renal transplantation by belatacept depends on a direct effect on B cells and impaired T follicular helper-B cell crosstalk. J. Am. Soc. Nephrol. 2018;29:1049–1062. doi: 10.1681/ASN.2017060679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am. J. Transpl. 2013;13:2280–2292. doi: 10.1111/ajt.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bertrand D, et al. Opportunistic infections after conversion to belatacept in kidney transplantation. Nephrol. Dial. Transpl. 2020;35:336–345. doi: 10.1093/ndt/gfz255. [DOI] [PubMed] [Google Scholar]
- 94.Scharpé J, et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am. J. Transpl. 2008;8:332–337. doi: 10.1111/j.1600-6143.2007.02066.x. [DOI] [PubMed] [Google Scholar]
- 95.Rambal V, et al. Differential influenza H1N1-specific humoral and cellular response kinetics in kidney transplant patients. Med. Microbiol. Immunol. 2014;203:35–45. doi: 10.1007/s00430-013-0312-3. [DOI] [PubMed] [Google Scholar]
- 96.Birdwell KA, et al. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am. J. Kidney Dis. 2009;54:112–121. doi: 10.1053/j.ajkd.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morillo-Huesca M, Vanti M, Chávez S. A simple in vivo assay for measuring the efficiency of gene length-dependent processes in yeast mRNA biogenesis. FEBS J. 2006;273:756–769. doi: 10.1111/j.1742-4658.2005.05108.x. [DOI] [PubMed] [Google Scholar]
- 98.Jurkiewicz A, et al. Inhibition of tRNA gene transcription by the immunosuppressant mycophenolic acid. Mol. Cell. Biol. 2019;40:e00294-19. doi: 10.1128/MCB.00294-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dieci G, Fiorino G, Castelnuovo M, Teichmann M, Pagano A. The expanding RNA polymerase III transcriptome. Trends Genet. 2007;23:614–622. doi: 10.1016/j.tig.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 100.Graczyk D, Cieśla M, Boguta M. Regulation of tRNA synthesis by the general transcription factors of RNA polymerase III, TFIIIB and TFIIIC, and by the MAF1 protein. Biochim. Biophys. Acta Gene Regul. Mech. 2018;1861:320–329. doi: 10.1016/j.bbagrm.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 101.Orioli A, Praz V, Lhôte P, Hernandez N. Human MAF1 targets and represses active RNA polymerase III genes by preventing recruitment rather than inducing long-term transcriptional arrest. Genome Res. 2016;26:624–634. doi: 10.1101/gr.201400.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sans MD, Williams JA. Calcineurin is required for translational control of protein synthesis in rat pancreatic acini. Am. J. Physiol. Cell Physiol. 2004;287:C310–C319. doi: 10.1152/ajpcell.00534.2003. [DOI] [PubMed] [Google Scholar]
- 103.Benotmane I, et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am. J. Transplant. 2020;20:3162–3172. doi: 10.1111/ajt.16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Favà A, et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am. J. Transplant. 2021;21:2749–2761. doi: 10.1111/ajt.16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wijtvliet VPWM, et al. mRNA-1273 vaccine (Moderna): a better option than BNT162b2 (Pfizer) in kidney transplant recipients and dialysis patients? Nephrol. Dial. Transpl. 2022;37:799–803. doi: 10.1093/ndt/gfab352. [DOI] [PubMed] [Google Scholar]
- 106.Van Praet J, et al. Predictors and dynamics of the humoral and cellular immune response to SARS-CoV-2 mRNA vaccines in hemodialysis patients: a multicenter observational study. J. Am. Soc. Nephrol. 2021;32:3208–3220. doi: 10.1681/ASN.2021070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benotmane I, et al. Strong antibody response after a first dose of a SARS-CoV-2 mRNA-based vaccine in kidney transplant recipients with a previous history of COVID-19. Am. J. Transplant. 2021;21:3808–3810. doi: 10.1111/ajt.16764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grupper A, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021;21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mehta RB, Silveira FP. COVID-19 after two doses of mRNA vaccines in kidney transplant recipients. Am. J. Transplant. 2021 doi: 10.1111/ajt.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chavarot N, et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am. J. Transplant. 2021 doi: 10.1111/ajt.16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caillard S, et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021;100:457–481. doi: 10.1016/j.kint.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garcia-Beltran WF, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:1–10. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen J, Wang R, Gilby NB, Wei G-W. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J. Chem. Inf. Model. 2022 doi: 10.1021/acs.jcim.1c01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Y, et al. A novel neutralizing monoclonal antibody targeting the N-terminal domain of the MERS-CoV spike protein. Emerg. Microbes Infect. 2017;6:1–7. doi: 10.1038/emi.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang L, et al. Importance of neutralizing monoclonal antibodies targeting multiple antigenic sites on the middle east respiratory syndrome coronavirus spike glycoprotein to avoid neutralization escape. J. Virol. 2018;92:1–21. doi: 10.1128/JVI.02002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wall EC, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Speer C, et al. Neutralizing antibody response against variants of concern after vaccination of dialysis patients with BNT162b2. Kidney Int. 2021;100:700–702. doi: 10.1016/j.kint.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manley HJ, et al. SARS-CoV-2 vaccine effectiveness and breakthrough infections in maintenance dialysis patients. Preprint at. medRxiv. 2021 doi: 10.1101/2021.12.20.21268124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thieme CJ, et al. Impaired humoral but substantial cellular immune response to variants of concern b1.1.7 and b.1.351 in hemodialysis patients. J. Am. Soc. Nephrol. 2021;32:2725–2727. doi: 10.1681/ASN.2021050672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kumar D, et al. Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine. Am. J. Transplant. 2022 doi: 10.1111/ajt.17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stervbo U, et al. Improved cellular and humoral immunity upon a second BNT162b2 and mRNA-1273 boost in prime-boost vaccination no/low responders with end-stage renal disease. Kidney Int. 2021 doi: 10.1016/j.kint.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tarke A, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–859.e11. doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao Y, et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 2022;28:472–476. doi: 10.1038/s41591-022-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davidovic T, et al. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: time for a boost. Kidney Int. 2021 doi: 10.1016/j.kint.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aslam S, Adler E, Mekeel K, Little SJ. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl. Infect. Dis. 2021;23:19–21. doi: 10.1111/tid.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qin CX, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105:e265–e266. doi: 10.1097/TP.0000000000003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bertrand D, et al. Waning antibody response and cellular immunity 6 months after third dose SARS-Cov-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Am. J. Transplant. 2022 doi: 10.1111/ajt.16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.ECDC and EMA. Updated joint statement from ECDC and EMA on additional booster doses of COVID-19 vaccines. EMA/635144, (2022).
- 129.Benotmane I, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. J. Am. Med. Assoc. 2021;326:1063–1065. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bertrand D, et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021 doi: 10.1016/j.kint.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Charmetant X, et al. Predictive factors of a viral neutralizing humoral response after a third dose of COVID-19 mRNA vaccine. Am. J. Transplant. 2022 doi: 10.1111/ajt.16990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hall VG, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N. Engl. J. Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kamar N, et al. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N. Engl. J. Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marlet J, et al. Antibody responses after a third dose of COVID-19 vaccine in kidney transplant recipients and patients treated for chronic lymphocytic leukemia. Vaccines. 2021;9:4–9. doi: 10.3390/vaccines9101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Massa F, et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine. 2021;73:103679. doi: 10.1016/j.ebiom.2021.103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Masset C, et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021;100:1132–1135. doi: 10.1016/j.kint.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schrezenmeier E, et al. B and T cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J. Am. Soc. Nephrol. 2021 doi: 10.1681/asn.2021070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stumpf J, et al. Cellular and humoral immune responses after three doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation. 2021;105:267–269. doi: 10.1097/TP.0000000000003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Westhoff TH, et al. A third vaccine dose substantially improves humoral and cellular SARS-CoV-2 immunity in renal transplant recipients with primary humoral nonresponse. Kidney Int. 2021;100:1135–1136. doi: 10.1016/j.kint.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Espi M, et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2022;101:390–402. doi: 10.1016/j.kint.2021.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alejo JL, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105:E280–E281. doi: 10.1097/TP.0000000000003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Benotmane I, et al. A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the delta variant in kidney transplant recipients. Kidney Int. 2022;50:5–8. doi: 10.1016/j.kint.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Caillard S, Thaunat O, Benotmane I, Masset C, Blancho G. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann. Intern. Med. 2022;175:455–456. doi: 10.7326/L21-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cinkilic O, et al. Inferior humoral and sustained cellular immunity against wild-type and omicron variant of concern in hemodialysis patients immunized with 3 SARS-CoV-2 vaccine doses compared with 4 doses. Kidney Int. 2022 doi: 10.1016/j.kint.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kamar N, et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA-based vaccine in recipients of a solid organ transplant. JAMA Netw. Open. 2021;4:e2136030. doi: 10.1001/jamanetworkopen.2021.36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Masset C, et al. A fourth SARS-CoV-2 mRNA vaccine in strictly seronegative kidney transplant recipients. Kidney Int. 2022;101:825–826. doi: 10.1016/j.kint.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Osmanodja B, et al. Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. J. Clin. Med. 2022;11:2565. doi: 10.3390/jcm11092565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roch T, et al. A vector-based vaccine dose after 3 doses of mRNA-based COVID-19 vaccination does not substantially improve humoral SARS-CoV-2 immunity in renal transplant recipients. Kidney Int. Rep. 2022;7:932–934. doi: 10.1016/j.ekir.2022.01.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schrezenmeier E, et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight. 2022;7:e157836. doi: 10.1172/jci.insight.157836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karaba AH, et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am. J. Transplant. 2022;22:1253–1260. doi: 10.1111/ajt.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Del Bello A, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am. J. Transplant. 2021 doi: 10.1111/ajt.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Del Bello A, et al. Acute rejection after anti-SARS-CoV-2 mRNA vaccination in a patient who underwent a kidney transplant. Kidney Int. 2021;100:238–239. doi: 10.1016/j.kint.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Werbel WA, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann. Intern. Med. 2021;174:1330–1332. doi: 10.7326/L21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duchini A, Goss JA, Karpen S, Pockros PJ. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin. Microbiol. Rev. 2003;16:357–364. doi: 10.1128/CMR.16.3.357-364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Danziger-Isakov L, Kumar D. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases community of practice. Clin. Transplant. 2019;33:1–10. doi: 10.1111/ctr.13563. [DOI] [PubMed] [Google Scholar]
- 156.Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin. Infect. Dis. 2018;66:1802–1811. doi: 10.1093/cid/cix1081. [DOI] [PubMed] [Google Scholar]
- 157.Cordero E, et al. Two doses of inactivated influenza vaccine improve immune response in solid organ transplant recipients: results of TRANSGRIPE 1-2, a randomized controlled clinical trial. Clin. Infect. Dis. 2017;64:829–838. doi: 10.1093/cid/ciw855. [DOI] [PubMed] [Google Scholar]
- 158.Pascual J, et al. Three-year observational follow-up of a multicenter, randomized trial on tacrolimus-based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplant. Transplantation. 2006;82:55–61. doi: 10.1097/01.tp.0000225806.80890.5e. [DOI] [PubMed] [Google Scholar]
- 159.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat. Rev. Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 160.Sullivan KM, et al. Immunomodulatory and antimicrobial efficacy of intravenous immunoglobulin in bone marrow transplantation. N. Engl. J. Med. 1990;323:1120–1123. doi: 10.1056/NEJM199009133231103. [DOI] [PubMed] [Google Scholar]
- 161.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miskulin DC, et al. High-dose seasonal influenza vaccine in patients undergoing dialysis. Clin. J. Am. Soc. Nephrol. 2018;13:1693–1702. doi: 10.2215/CJN.03390318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Haddadin Z, et al. Alternative strategies of posttransplant influenza vaccination in adult solid organ transplant recipients. Am. J. Transplant. 2021;21:938–949. doi: 10.1111/ajt.16295. [DOI] [PubMed] [Google Scholar]
- 164.Kosmadakis G, Albaret J, Correia EDC, Somda F, Aguilera D. Vaccination practices in dialysis patients: a narrative review. Semin. Dial. 2018;31:507–518. doi: 10.1111/sdi.12709. [DOI] [PubMed] [Google Scholar]
- 165.Babu TM, Kotton CN. Immunizations in chronic kidney disease and kidney transplantation. Curr. Treat. Options Infect. Dis. 2021;13:47–65. doi: 10.1007/s40506-021-00248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Noh JY, et al. Immunogenicity of trivalent influenza vaccines in patients with chronic kidney disease undergoing hemodialysis: MF59-adjuvanted versus non-adjuvanted vaccines. Hum. Vaccines Immunother. 2016;12:2902–2908. doi: 10.1080/21645515.2016.1191717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tahtinen S, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 2022;23:532–542. doi: 10.1038/s41590-022-01160-y. [DOI] [PubMed] [Google Scholar]
- 168.Morais P, Adachi H, Yu YT. The critical contribution of pseudouridine to mRNA COVID-19 vaccines. Front. Cell Dev. Biol. 2021;9:1–9. doi: 10.3389/fcell.2021.789427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barraclough KA, et al. Intradermal versus intramuscular hepatitis B vaccination in hemodialysis patients: a prospective open-label randomized controlled trial in nonresponders to primary vaccination. Am. J. Kidney Dis. 2009;54:95–103. doi: 10.1053/j.ajkd.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 170.Yousaf F, Gandham S, Galler M, Spinowitz B, Charytan C. Systematic review of the efficacy and safety of intradermal versus intramuscular hepatitis B vaccination in end-stage renal disease population unresponsive to primary vaccination series. Ren. Fail. 2015;37:1080–1088. [PubMed] [Google Scholar]
- 171.Planas D, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 172.Cameroni E, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wilhelm A, et al. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. EBioMedicine. 2022;82:104158. doi: 10.1016/j.ebiom.2022.104158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paniskaki K, et al. Immune response in moderate to critical breakthrough COVID-19 infection after mRNA vaccination. Front. Immunol. 2022;13:1–11. doi: 10.3389/fimmu.2022.816220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bensouna I, et al. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am. J. Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dekervel M, et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 2021 doi: 10.1093/ckj/sfab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100:702–704. doi: 10.1016/j.kint.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Longlune N, et al. High immunogenicity of a messenger RNA-based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol. Dial. Transpl. 2021;36:1704–1709. doi: 10.1093/ndt/gfab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Robert T, et al. Humoral response after SARS-COV2 vaccination in patient undergoing maintenance hemodialysis: loss of immunity, third dose and non-responders. Nephrol. Dial. Transpl. 2021 doi: 10.1093/ndt/gfab299. [DOI] [PubMed] [Google Scholar]
- 180.Anand S, et al. Antibody response to COVID-19 vaccination in patients receiving dialysis. J. Am. Soc. Nephrol. 2021;32:2435–2438. doi: 10.1681/ASN.2021050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bassi J, et al. Poor neutralization and rapid decay of antibodies to SARS-CoV-2 variants in vaccinated dialysis patients. PLoS One. 2022;17:e0263328. doi: 10.1371/journal.pone.0263328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Billany RE, et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: a call to arms. Kidney Int. 2021;99:1492–1494. doi: 10.1016/j.kint.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brunelli ASM, et al. Comparative effectiveness of BNT162b2 versus Ad26.COV2.S for the prevention of COVID-19 among dialysis patients. Preprint at. medRxiv. 2021 doi: 10.1101/2021.10.21.21265339. [DOI] [Google Scholar]
- 184.Dębska-Ślizień A, et al. Predictors of humoral response to mRNA COVID19 vaccines in kidney transplant recipients: a longitudinal study — the COViNEPH project. Vaccines. 2021;9:1165. doi: 10.3390/vaccines9101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Garcia P, et al. COVID19 vaccine type and humoral immune response in patients receiving dialysis. J. Am. Soc. Nephrol. 2021 doi: 10.1681/asn.2021070936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hsu CM, et al. Seroresponse to SARS-CoV-2 vaccines among maintenance dialysis patients. Am. J. Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Husain SA, et al. Postvaccine anti-SARS-CoV-2 spike protein antibody development in kidney transplant recipients. Kidney Int. Rep. 2021;6:1699–1700. doi: 10.1016/j.ekir.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kaiser RA, Haller MC, Apfalter P, Kerschner H, Cejka D. Comparison of BNT162b2 (Pfizer–BioNtech) and mRNA-1273 (Moderna) SARS-CoV-2 mRNA vaccine immunogenicity in dialysis patients. Kidney Int. 2021;100:697–698. doi: 10.1016/j.kint.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lacson E, et al. Immunogenicity of SARS-CoV-2 vaccine in dialysis. J. Am. Soc. Nephrol. 2021;32:2735–2742. doi: 10.1681/ASN.2021040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mulhern JG, et al. Humoral response to mRNA versus an adenovirus vector-based SARS-CoV-2 vaccine in dialysis patients. Clin. J. Am. Soc. Nephrol. 2021 doi: 10.2215/cjn.06450521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Prendecki M, et al. Comparison of humoral and cellular responses in kidney transplant recipients receiving BNT162b2 and ChAdOx1 SARS-CoV-2 vaccines. Preprint at. medRxiv. 2021 doi: 10.1101/2021.07.09.21260192. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.