Abstract
Purpose:
Although the efficacy of programmed cell death–1 (PD-1) blockade is generally poor for non–small cell lung cancer (NSCLC) with activating mutations of the epidermal growth factor receptor (EGFR) gene, EGFR tyrosine kinase inhibitors (TKIs) may improve the tumor immune microenvironment. We performed a randomized study to assess whether nivolumab improves outcome compared with chemotherapy in such patients previously treated with EGFR-TKIs.
Patients and Methods:
Patients with EGFR-mutated NSCLC who acquired EGFR-TKI resistance not due to a secondary T790M mutation of EGFR were randomized 1:1 to nivolumab (n = 52) or carboplatin–pemetrexed (n = 50). The primary endpoint was progression-free survival (PFS).
Results:
Median PFS and 1-year PFS probability were 1.7 months and 9.6% for nivolumab versus 5.6 months and 14.0% for carboplatin–pemetrexed [log-rank P < 001; hazard ratio (HR) of 1.92, with a 60% confidence interval (CI) of 1.61–2.29]. Overall survival was 20.7 and 19.9 months [HR, 0.88 (95% CI, 0.53–1.47)], and response rate was 9.6% and 36.0% for nivolumab and carboplatin–pemetrexed, respectively. No subgroup including patients with a high tumor mutation burden showed a substantially longer PFS with nivolumab than with carboplatin-pemetrexed. The T-cell–inflamed gene expression profile score (0.11 vs. −0.17, P = 0.036) and expression of genes related to cytotoxic T lymphocytes or their recruitment were higher in tumors that showed a benefit from nivolumab.
Conclusions:
Nivolumab did not confer a longer PFS compared with carboplatin-pemetrexed in the study patients. Gene expression profiling identified some cases with a favorable tumor immune microenvironment that was associated with nivolumab efficacy.
Translational Relevance.
This first randomized phase II study for nivolumab versus standard cytotoxic chemotherapy in patients with metastatic EGFR-mutated non–small cell lung cancer previously treated with EGFR tyrosine kinase inhibitors found no survival benefit of nivolumab therapy. Gene expression profiling identified some patients with a favorable tumor immune microenvironment that was associated with nivolumab efficacy.
Introduction
Non–small cell lung cancer (NSCLC) is the most common cause of death from cancer worldwide (1). Treatment for advanced NSCLC depends on the molecular characteristics of the tumor. Mutations of the epidermal growth factor receptor (EGFR) gene are present in approximately 32% of Asians and approximately 7% of individuals of other ethnic groups with NSCLC, with deletions in exon 19 and an L858R point mutation in exon 21 accounting for approximately 90% of such genetic alterations detected at diagnosis (2).
In patients with advanced or recurrent EGFR mutated NSCLC, EGFR tyrosine kinase inhibitors (TKIs) are considered the standard initial treatment based on their demonstrated definite benefit (3, 4). Eventually, all treated patients develop resistance to EGFR-TKIs, due to several types of resistance pattern including a T790M secondary mutation of EGFR (5). Third-generation EGFR-TKIs such as osimertinib have been found to confer a survival benefit compared with cytotoxic chemotherapy in T790M-positive tumors (6). In addition, osimertinib provided significantly longer PFS and OS compared with standard EGFR-TKIs in the overall population (7, 8).
Cancer immunotherapy including the administration of immune checkpoint inhibitors (ICIs) has markedly changed the treatment paradigm for NSCLC. Programmed cell death–1 (PD-1) and its ligands, PD-L1, a receptor expressed on the surface of activated T and B cells (9), play an important suppressive role in the immune system by preventing the activation of T cells (10–12). Nivolumab is a human immunoglobulin G4 (IgG4) monoclonal antibody to PD-1 that inhibits its binding to PD-L1. Based on the clinical trial results (13–15), ICIs have emerged as a standard of care for advanced NSCLC without oncogenic driver mutations.
Subgroup analysis of such clinical trials has suggested that inhibition of the PD-1–PD-L1 axis is less effective in patients positive for EGFR mutations than in those wild-type for EGFR (16). However, cases of EGFR mutation–positive lung cancer are diverse, with some likely to benefit from ICI treatment. Our previous findings have suggested that subgroups of patients with EGFR-mutated NSCLC—such as those with tumors that are positive for PD-L1, that have a high tumor mutation burden (TMB), or that have acquired resistance to EGFR-TKIs by a mechanism other than development of the T790M mutation achieve a benefit from treatment with ICIs (17). We also recently showed that EGFR-TKI treatment was associated with an increase in both PD-L1 expression on tumor cells and TMB, suggesting that such treatment induces changes in the tumor immune microenvironment of EGFR-mutated NSCLC that might increase the efficacy of subsequent ICI therapy (18). We have therefore now performed a randomized phase II trial to evaluate the efficacy and safety of nivolumab compared with the combination of carboplatin and pemetrexed as a standard cytotoxic chemotherapy for patients with EGFR-mutated NSCLC who develop T790M-independent resistance to EGFT-TKIs.
Patients and Methods
Study design
This prospective, randomized phase II trial (jRCTs051180133, WJOG8515L) was performed at 37 sites of West Japan Oncology Group (WJOG). The full study protocol is available in the Appendix. As previously described (19), patients were randomly assigned on a 1:1 basis to receive nivolumab in an experimental arm or to receive the combination of carboplatin and pemetrexed in a control arm. Random assignment was stratified according to (i) institution, (ii) smoking history (current or former versus never), (iii) history of treatment with a third-generation EGFR-TKI (yes vs. no), (iv) progression-free survival (PFS) for previous EGFR-TKI therapy as an initial treatment (≥270 vs. <270 days), and (v) age (≥75 vs. <75 years old).
Patients
Eligibility criteria included a histologically confirmed diagnosis of locally advanced, metastatic, or recurrent nonsquamous NSCLC positive for an activating mutation of EGFR, including L858R, an exon-19 deletion, G719X, L861Q, or S768I, as well as no previous systemic therapy other than EGFR-TKI treatment for advanced disease. At the time the study was launched, patients who developed resistance after treatment with a first- or second-generation EGFR-TKI but who were negative for T790M were included. The protocol was subsequently amended, however, to include patients with T790M-positive tumors after such treatment who developed resistance after subsequent therapy with a third-generation EGFR-TKI or those who developed resistance after initial treatment with a third-generation EGFR-TKI, according to a shift in the standard of care for EGFR-TKI treatment of EGFR-mutated NSCLC. All patients had measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Additional eligibility criteria included an Eastern Cooperative Oncology Group performance status of 0 or 1, no symptomatic brain metastasis, and adequate organ function. Patients were excluded if they had contraindications for nivolumab. The study protocol was approved by the institutional review board at central certified review board of Wakayama Medical University, and the study was conducted in accordance with the provisions of the Declaration of Helsinki. All patients provided written informed consent before study entry.
Study treatment
Patients in the experimental arm received nivolumab (3 mg/kg) on day 1 every 2 weeks until disease progression or the development of unacceptable toxicity. Those in the control group received pemetrexed at 500 mg/m2; and carboplatin at an area under the curve of 6 mg/mL per minute on day 1 every 3 weeks for four cycles followed by optional indefinite pemetrexed maintenance therapy, with the exception that older patients (≥75 years of age) received carboplatin at an area under the curve of 5 mg/mL per minute, as previously described (20, 21).
Evaluation of response and safety
A computed tomography or magnetic resonance imaging scan of the brain, computed tomography scans of the chest and abdomen, a bone scan or positron emission tomography scan, and an electrocardiogram were required before initiation of study treatment. Patients underwent tumor assessment at baseline, every 6 weeks during the first 12 months, and every 12 weeks thereafter. Tumor response was evaluated in accordance with RECIST (version 1.1). PFS was defined as the time from enrollment to the date of confirmation of progressive disease or the date of death from any cause (whichever occurs earlier). Overall survival (OS) was defined as the time from enrollment until death from any cause. Duration of response was defined as the time from the date a confirmed response is detected to the date of confirmation of progressive disease or the date of death from any cause (whichever occurs earlier). Adverse events (AEs) were recorded on the basis of the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Statistical analysis
Analysis of efficacy was performed with the intent-to-treat population. The purpose of the primary analysis was to test the hypothesis that nivolumab is superior to carboplatin-pemetrexed with regard to PFS. The expected 1-year PFS probability for patients with EGFR-mutated NSCLC who receive standard platinum-pemetrexed chemotherapy is 10% on the basis of a Japanese phase II clinical trial of the combination of carboplatin and pemetrexed (22). A sample size of 94 patients, 47 per arm, was determined to provide 80% power (at an overall one-sided 20% significance level) for detection of an increase in 1-year PFS probability from 10% in the control arm to 20% in the experimental arm. Allowing for protocol deviation in 5% of patients, we planned the total number of patients as 50 per arm.
Secondary end points included OS, objective response rate (ORR), duration of response, safety, as well as OS and PFS according to PD-L1 expression. Both OS and PFS were estimated with the Kaplan-Meier method and were compared between arms with the stratified log-rank test and with (i) institution, (ii) smoking history, (iii) history of treatment with a third-generation EGFR-TKI, (iv) PFS for initial EGFR-TKI treatment (≥270 vs. <270 days), and (v) age (≥75 vs. <75 years old) as stratification factors. Hazard ratios (HRs) were derived with stratified Cox proportional hazards (PHs) models. Subgroup analysis of OS and PFS was performed with unstratified Cox regression models. ORR and the disease control rate were compared between arms. For the primary analysis of PFS, a one-sided P value was calculated, with a significance level of 0.20, and both 60% and 95% confidence intervals (CIs) were estimated for HR. For subgroup analysis and other end points, only 95% CIs were estimated and calculated P values were two-sided, with the significance level being undefined. Statistical analysis was performed with SAS (version 9.4), SPSS version 25 (IBM), and GraphPad Prism version 9 (GraphPad) software.
Assessment of tumor immune characteristics
Protocols for immunohistochemistry of PD-L1 and assessment of TMB and immune-related gene expression profile (GEP) are described in Supplementary Patients and Methods.
Results
Patient characteristics
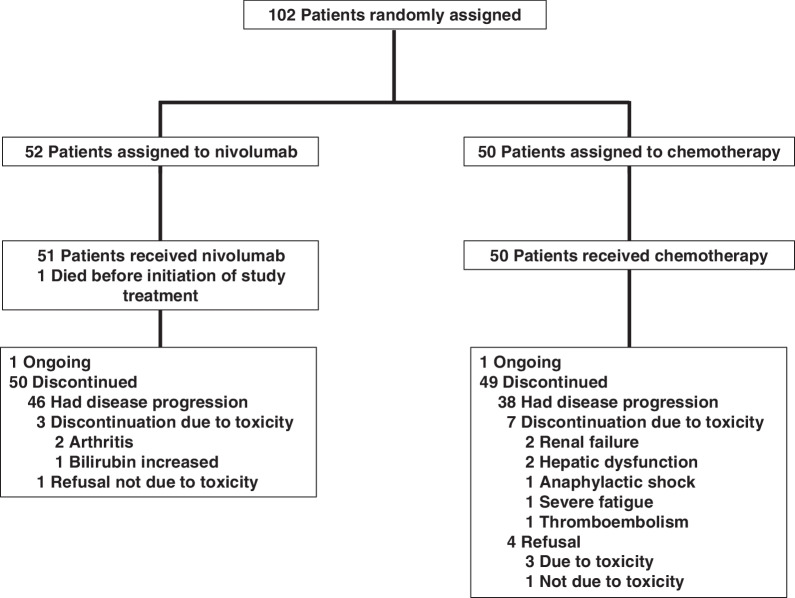
Between April 2016 and June 2019, 102 patients were enrolled and randomized. Patient flow is summarized in Fig. 1, and the trial scheme is summarized in Supplementary Fig. S1. Although age was significantly higher in the nivolumab arm (P = 0.023, Mann–Whitney test), demographic characteristics were otherwise well balanced between the two arms of the study (Table 1). PD-L1 expression on tumor cells was evaluated for 37 patients in the nivolumab arm and 40 patients in the carboplatin-pemetrexed arm.
Figure 1.
CONSORT diagram.
Table 1.
Characteristics of the study patients (n = 102).
| Nivolumab (n = 52) | Carboplatin–pemetrexed (n = 50) | |||
|---|---|---|---|---|
| Characteristics | No. | % | No. | % |
| Age (y) | ||||
| Median | 70.5 | 67 | ||
| Range | 51–84 | 45–83 | ||
| Sex | ||||
| Male | 24 | 46.2 | 19 | 38.0 |
| Female | 28 | 53.8 | 31 | 62.0 |
| Smoking history | ||||
| Never | 29 | 55.8 | 28 | 56.0 |
| Past or current | 23 | 44.2 | 22 | 44.0 |
| ECOG performance status | ||||
| 0 | 17 | 32.7 | 18 | 36.0 |
| 1 | 35 | 67.3 | 32 | 64.0 |
| Stage | ||||
| IIIB | 2 | 3.8 | 2 | 4.0 |
| IV | 41 | 78.8 | 40 | 80.0 |
| Postoperative recurrence | 9 | 17.3 | 8 | 16.0 |
| Metastatic sites | ||||
| Brain | 18 | 34.6 | 14 | 28.0 |
| Treated with radiotherapy | 7 | 13.5 | 4 | 8.0 |
| Histology | ||||
| Adenocarcinoma | 52 | 100 | 50 | 100 |
| EGFR mutation | ||||
| Exon-21 L858R | 28 | 53.8 | 24 | 48.0 |
| Exon-19 deletion | 24a | 46.2 | 24 | 48.0 |
| Other | 1b | 1.9 | 2c | 4.0 |
| T790M on enrollment in study | ||||
| Positive | 18 | 34.6 | 15 | 30.0 |
| Negative | 34 | 65.4 | 35 | 70.0 |
| EGFR-TKI as first-line therapy | ||||
| Gefitinib | 16 | 30.8 | 18 | 36.0 |
| Erlotinib | 18 | 34.6 | 14 | 28.0 |
| Afatinib | 11 | 21.2 | 11 | 22.0 |
| Osimertinib | 4 | 7.7 | 5 | 10.0 |
| Other | 2d | 3.8 | 2d | 4.0 |
| PFS for first-line EGFR-TKI | ||||
| ≥270 d | 36 | 69.2 | 33 | 66.0 |
| <270 d | 16 | 30.8 | 17 | 34.0 |
| PD-L1 TPS | ||||
| <1% | 19 | 36.5 | 27 | 54.0 |
| 1%–49% | 10 | 19.2 | 10 | 20.0 |
| ≥50% | 8 | 15.4 | 3 | 6.0 |
| Unknown | 15 | 28.8 | 10 | 20.0 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; PFS, progression-free survival; PD-L1, programmed cell death–ligand 1; TPS, tumor proportion score.
aOne case with two mutations: an exon-19 deletion and L858R.
bExon-18 G719X plus exon-20 S768I.
cExon-18 G719X (n = 1) and exon-18 G719S plus exon-21 L861Q (n = 1).
dInvestigational EGFR-TKI.
Efficacy
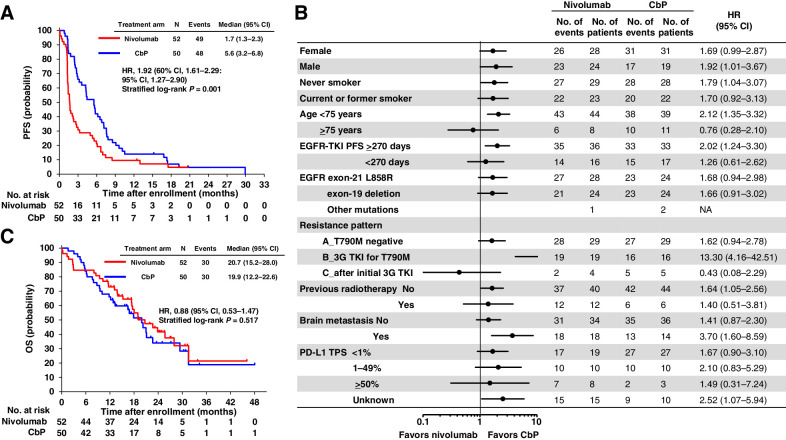
The data cutoff date for the efficacy analysis was June 30, 2020. Median follow-up time was 25.5 months (range, 0.1–46.1 months) for the nivolumab arm and 23.4 months (range, 1.6–48.0 months) for the carboplatin-pemetrexed arm. At the cut-off date, there were 49 (94.2%) patients in the nivolumab arm with an event (progressive disease or death), compared with 48 (96.0%) patients in the carboplatin–pemetrexed arm. Median PFS was 1.7 months (95% CI, 1.3–2.3 months) and 5.6 months (95% CI, 3.2–6.8 months) in the nivolumab and carboplatin–pemetrexed arms, respectively (stratified log-rank test P = 0.001; stratified Cox PH model HR of 1.92, with a 60% CI of 1.61–2.29 and 95% CI of 1.27–2.90), and the study thus did not meet its primary end point (Fig. 2A). The 1-year PFS probability was 9.6% and 14.0% in the nivolumab and carboplatin–pemetrexed arms, respectively. No definite benefit of nivolumab with regard to PFS was apparent in any patient subset, including those based on PD-L1 tumor proportion score (TPS) or TKI resistance pattern (Fig. 2B; Supplementary Figs. S2–S5).
Figure 2.
Efficacy of nivolumab relative to that of carboplatin–pemetrexed. A, Kaplan–Meier plots for PFS of patients treated with nivolumab or carboplatin–pemetrexed (CbP). CI, confidence interval; HR, hazard ratio. B, Forest plots of the hazard ratio for nivolumab versus carboplatin-pemetrexed with regard to PFS in patient subsets. Resistance patterns A, B, and C refer to patients who developed resistance to treatment with a first- or second-generation epidermal growth factor receptor–tyrosine kinase inhibitor (EGFR-TKI) but who were negative for T790M, those with T790M-positive tumors after such treatment and who developed resistance after subsequent therapy with a third-generation EGFR-TKI (3G TKI), and those who developed resistance after initial treatment with a third-generation EGFR-TKI, respectively. PD-L1, programmed cell death–ligand 1; TPS, tumor proportion score; NA, not applicable. C, Kaplan–Meier plots for overall survival (OS) of patients treated with nivolumab or carboplatin-pemetrexed.
Median OS was 20.7 months (95% CI, 15.2–28.0 months) and 19.9 months (95% CI, 12.2–22.6 months) in the nivolumab and carboplatin-pemetrexed arms, respectively (stratified log-rank test P = 0.517; stratified Cox PH model HR of 0.884, with a 95% CI of 0.53–1.47; Fig. 2C). No subgroup showed an obvious difference in OS between nivolumab and carboplatin–pemetrexed (Supplementary Fig. S6). Details of treatment after disease progression during trial therapy are shown in Supplementary Table S1. Among the 52 patients in the nivolumab arm, five patients (9.6%) had a partial response, 15 patients (28.8%) stable disease, and 30 patients (57.7%) had progressive disease as their best overall response. Two patients were not evaluable, whereas one patient (2.0%) had a complete response, 17 patients (34.0%) a partial response, 20 patients (40.0%) stable disease, and 12 patients (24.0%) had progressive disease in the carboplatin-pemetrexed arm (n = 50). The ORR was 9.6% and 36.0% in the nivolumab and carboplatin–pemetrexed arms, respectively, and the median duration of response was similar for nivolumab and carboplatin–pemetrexed [5.3 months (95% CI, 4.6 months to not reached) versus 5.5 months (95% CI, 2.9–8.0 months), respectively; HR of 0.42 (95% CI, 0.12–1.48); Supplementary Figs. S7 and S8].
Safety
AEs related to study treatment occurred in 60.8% (grade 3–5, 9.8%) and 82.0% (grade 3–5, 12.0%) of patients in the nivolumab and carboplatin–pemetrexed arms, respectively (Table 2). The most common AEs of grade 3 or 4 were fatigue and skin toxicity (3.8% each) in the nivolumab arm and neutropenia (32.0%), anemia (28.0%), leukopenia (22.0%), and thrombocytopenia (16.0%) in the carboplatin–pemetrexed arm. Serious AEs were observed in 25.5% and 16.0% of patients in the nivolumab and carboplatin–pemetrexed arms, respectively. Treatment discontinuation due to AEs occurred in three patients in the nivolumab arm (two with arthritis and one with bilirubin increased) and seven patients in the carboplatin-pemetrexed arm (two each with renal failure and hepatic dysfunction as well as one each with anaphylactic shock, severe fatigue, and thromboembolism). There was one case of treatment-related interstitial lung disease (ILD) of grade 1 in the nivolumab arm. Treatment-related death was not apparent in either arm.
Table 2.
Treatment-related adverse events of any grade in ≥10% of patients.
| Nivolumab (n = 52) | Carboplatin–pemetrexed (n = 50) | |||||||
|---|---|---|---|---|---|---|---|---|
| All grades | Grade ≥3 | All grades | Grade ≥3 | |||||
| Adverse event | No. | % | No. | % | No. | % | No. | % |
| Nonhematologic | ||||||||
| Fatigue | 15 | 28.8 | 2 | 3.8 | 23 | 46.0 | 0 | 0 |
| Skin toxicity | 8 | 15.4 | 2 | 3.8 | 8 | 16.0 | 1 | 2.0 |
| Anorexia | 7 | 13.5 | 1 | 1.9 | 25 | 50.0 | 1 | 2.0 |
| ALT increased | 6 | 11.5 | 1 | 1.9 | 27 | 54.0 | 1 | 2.0 |
| Nausea | 6 | 11.5 | 0 | 0 | 24 | 48.0 | 3 | 6.0 |
| AST increased | 4 | 7.7 | 0 | 0 | 23 | 46.0 | 2 | 4.0 |
| ALP increased | 4 | 7.7 | 0 | 0 | 11 | 22.0 | 1 | 2.0 |
| Hematologic | ||||||||
| WBC decreased | 2 | 3.8 | 1 | 1.9 | 39 | 78.0 | 11 | 22.0 |
| Neutrophil decreased | 2 | 3.8 | 1 | 1.9 | 43 | 86.0 | 16 | 32.0 |
| Thrombocytopenia | 2 | 3.8 | 0 | 0 | 43 | 86.0 | 8 | 16.0 |
| Anemia | 4 | 7.7 | 0 | 0 | 40 | 80.0 | 14 | 28.0 |
Note: Adverse events are defined according to MedDRA preferred terms.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; WBC, white blood cell.
TMB
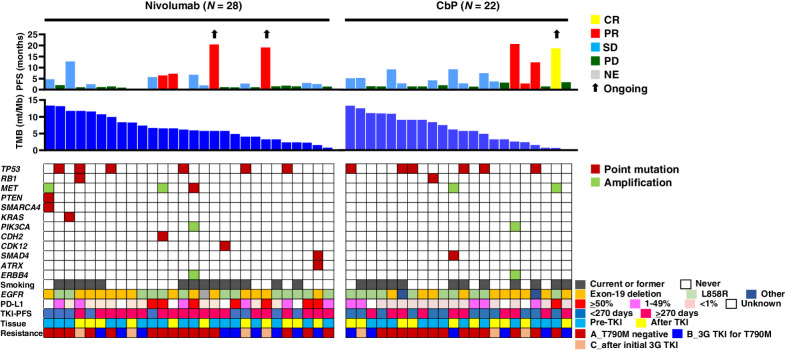
To investigate the relation of TMB or specific oncogenic mutations to nivolumab efficacy, we performed targeted sequencing for 50 patients (28 in the nivolumab arm and 22 in the carboplatin–pemetrexed arm). The overall results are shown in Fig. 3. The most common genetic alterations were in TP53 [12 patients (24.0%)], MET [five patients (10.0%)], and RB1, PIK3CA, ERBB4, and SMAD4 [two patients each (4.0%)], and the median TMB was 6.2 mutations/Mb. Consistent with previous findings (23), TMB tended to be higher in patients without (n = 36) than in those with (n = 14) the T790M mutation (7.0 vs. 5.6 mutations/Mb). There was no significant difference in PFS between nivolumab and carboplatin–pemetrexed for patients with a high or low TMB (with the median as cutoff; Supplementary Fig. S9).
Figure 3.
Individual treatment outcome and response for 50 patients with specimens available for analysis of tumor mutation burden (TMB) according to tumor characteristics. Progression-free survival (PFS) and best objective response (CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable) for nivolumab or carboplatin-pemetrexed (CbP) are shown in the top panel. Black arrows indicate ongoing response at data cutoff date. TMB (mutations/Mb) is shown in the middle panel. The distribution of selected gene alterations, smoking status, epidermal growth factor receptor gene (EGFR) mutation type, programmed cell death–ligand 1 (PD-L1) tumor proportion score, PFS for initial EGFR tyrosine kinase inhibitor (TKI) treatment, timing for collection of tumor tissue for analysis (before or after initial EGFR-TKI treatment), and pattern of EGFR-TKI resistance (see legend of Fig. 2 for explanation of A, B, and C) are shown in the bottom panel. 3G, third-generation.
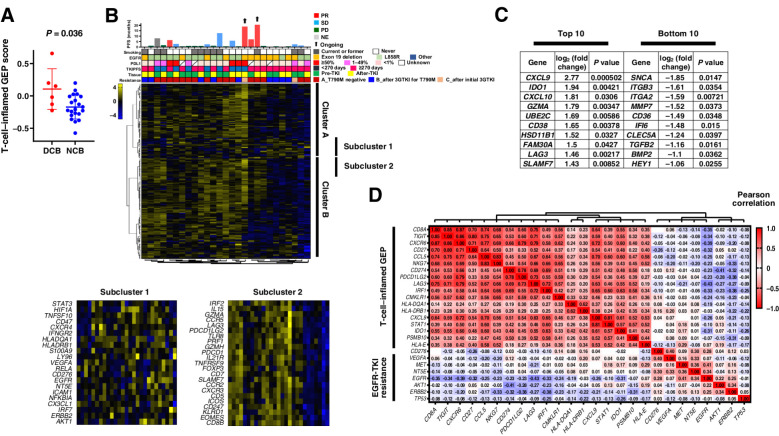
Immune-related GEP
To investigate the role of the immunologic phenotype of the tumor microenvironment in the response to nivolumab in EGFR-mutated NSCLC, we performed immune-related gene expression analysis for tumor samples from 27 and 28 patients in the nivolumab and carboplatin-pemetrexed arms, respectively. Among the 27 patients treated with nivolumab, those (n = 6) with a durable clinical benefit (DCB), defined as a PFS of >6 months, showed a higher T-cell–inflamed GEP score than did those (n = 21) with no clinical benefit (NCB), with mean (95% CI) values of 0.11 (0.06–0.15) and −0.17 (−0.16 to −0.18), respectively (Mann–Whitney test, P = 0.036; Fig. 4A), implicating immune phenotype as a determinant of nivolumab efficacy in the study population. To identify immune cell types associated with nivolumab response, we performed an unsupervised clustering analysis for 183 selected genes. Hierarchical clustering for the 27 nivolumab-treated and 28 carboplatin–pemetrexed–treated patients is shown in Fig. 4B and Supplementary Fig. S10, respectively. Whereas no clear association was apparent between clusters of cases and response to therapy in the carboplatin-pemetrexed arm, nivolumab-treated patients with a longer PFS tended to be present in neighboring clusters. On the basis of this hierarchical clustering for the 27 nivolumab-treated patients, we selected two gene subclusters that were expressed at a higher level in the NCB group (subcluster 1) or in the DCB group (subcluster 2; Fig. 4B). Genes for receptor tyrosine kinases (such as ERBB2 and EGFR) and their downstream signaling molecules (such as STAT3 and AKT1) as well as genes for angiogenesis-related factors (such as HIF1A and VEGFA) were included in subcluster 1. In contrast, subcluster 2 contained CD8}, genes for cytolytic molecules expressed in cytotoxic T cells and natural killer cells (such as GZMA and PRF1), as well those for costimulatory molecules including PDCD1 (encoding PD-1), PDCD1LG2 (encoding PD-L2), and LAG3. In addition, genes corresponding to a lymphoid compartment pathway tended to be expressed at a higher level in the DCB group than in the NCB group (Supplementary Fig. S11).
Figure 4.
Immune-related gene expression analysis of tumor specimens. A, Dot plots for the T-cell–inflamed gene expression profile (GEP) score according to nivolumab efficacy for 27 patients. The mean and standard error of the mean values are also shown, and the P value was determined with the Mann-Whitney test. DCB, durable clinical benefit, defined as a partial response or stable disease lasting >6 months; NCB, no clinical benefit. B, Heat map of immune-related gene expression for 27 patients treated with nivolumab (middle panel). Hierarchical clustering of the 27 tumors was performed according to the expression of 183 selected immune-related genes. A dendrogram was generated by clustering, resulting in the identification of several clusters, with two main clusters being designated A and B. The details of two representative subclusters (subclusters 1 and 2) of these two clusters are shown expanded in the bottom panels because of their potential importance for a biological explanation of nivolumab efficacy based on their constituent genes. The color scale represents the Z score for the expression of each individual gene, with the highest expression shown in yellow, medium in black, and lowest in blue. Progression-free survival (PFS) and best objective response for nivolumab as well as other patient characteristics are presented in the top panel as in Fig. 3. C, Lists of the top 10 and bottom 10 genes whose expression was associated with PFS for nivolumab as revealed by comparison of single-gene expression between DCB and NCB groups (Supplementary Fig. S12). D, Correlation between expression of the 18 genes constituting the T-cell–inflamed GEP and that of genes related to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) resistance in 55 patients of the current trial. The color scale indicates Pearson correlation coefficient.
We also performed single-gene analysis with the 27 specimens from nivolumab-treated patients (Fig. 4C; Supplementary Fig. S12). The expression of genes for chemokines related to recruitment of CD8+ T cells (CXCL9 and CXCL10) (24) as well as that of GZMA was upregulated in the DCB group, whereas that of genes related to epithelial-mesenchymal transition, especially of cancer-associated fibroblasts (such as ITGB3, ITGA2, MMP7, and CD36) (25), was upregulated in the NCB group.
Finally, to explore the mechanism underlying the general lack of response to nivolumab in EGFR mutation–positive NSCLC, we examined the relation between the expression of genes that constitute the T-cell–inflamed GEP and that of those related to the EGFR signaling pathway and EGFR-TKI resistance with the use of all 55 specimens from the study patients (Fig. 4D). Whereas genes related to cytotoxic T lymphocytes, chemokines, and cell-mediated cytotoxicity were strongly correlated with each other, those for receptor tyrosine kinases and downstream signaling proteins were negatively correlated with the immune modulatory genes.
Discussion
In the current randomized study, we have evaluated the efficacy of nivolumab relative to that of platinum-doublet chemotherapy in patients with EGFR-mutated NSCLC who developed EGFR-TKI resistance mediated by various mechanisms. Our results show that PFS for nivolumab was inferior to that for the standard cytotoxic chemotherapy in these patients.
At the time the current study was designed, the efficacy of ICIs for EGFR mutation–positive NSCLC was uncertain. Meta-analysis for subgroups of prior phase 3 trials had revealed a lower efficacy of PD-1/PD-L1 inhibitor treatment for EGFR-mutated NSCLC compared with EGFR–wild-type NSCLC, but the OS inferiority for such treatment compared with cytotoxic chemotherapy was still not clear, with little detailed efficacy information, including results for long-term efficacy outcome, having been reported (16). Our current data, obtained with a randomized study design, provide more reliable evidence to support the notion that, in general, ICI monotherapy does not confer a clinical benefit for patients with EGFR mutation–positive NSCLC. A small phase II trial that investigated the efficacy of the PD-1–targeted antibody pembrolizumab in EGFR-TKI–naïve patients with EGFR-mutated NSCLC did not detect a response or achieve sustained duration of treatment for >1 year, although it was restricted to patients with a high PD-L1 TPS (≥50%; ref. 26). In the current study, which was performed with EGFR-TKI–treated patients, five of 52 patients (9.6%) treated with nivolumab achieved a PFS of >1 year. Randomized controlled trials of cytotoxic chemotherapy combined with an antibody to PD-1 for EGFR mutation–positive NSCLC are currently ongoing (NCT02864251 and NCT03515837). Given that combination treatment with an antibody to PD-L1 and cytotoxic chemotherapy confers prolonged survival and has become the standard of care for patients with extensive-stage small cell lung cancer (27), for which PD-1/PD-L1 inhibitors alone show a 1-year PFS probability of only approximately 10% (28), the potential for combination treatment with cytotoxic chemotherapy and a PD-1 inhibitor in patients with EGFR mutation–positive NSCLC remains.
Although the number of cases compared was small, disease control by nivolumab tended to be more durable for patients who developed T790M-negative resistance to first- or second-generation EGFR-TKIs or for those who progressed during initial treatment with a third-generation EGFR-TKI than for those who became resistant to third-generation EGFR-TKI treatment after the initial development of T790M-positive resistance, all of whom showed tumor progression within 3 months (Supplementary Fig. S4). We previously found that patients with T790M mutation–positive NSCLC have a poor tumor immune microenvironment and show a poor response to antibodies to PD-1 (17, 18), and our current results suggest that this is also the case for T790M-positive tumors after subsequent treatment with a third-generation TKI. These observations may be explained in part by the lower TMB of T790M-positive NSCLC compared with T790M-negative tumors (18, 23). However, our current results did not detect an association between TMB and nivolumab efficacy, suggesting that other factors might also play a role.
The toxicity of nivolumab in the current study was consistent with previous findings, with the drug being well tolerated. The frequency of ILD was previously found to be 38% in EGFR-mutated NSCLC patients treated concurrently with the PD-L1–targeted antibody durvalumab and osimertinib (29). In addition, EGFR-TKI therapy immediately after PD-1/PD-L1 inhibitor treatment has been associated with a high incidence of ILD (30). Lung toxicity of nivolumab administered after EGFR-TKI treatment was thus a potential concern in the current study. However, only one case of immune-related pneumonitis (grade 1) was apparent in the nivolumab arm, indicating that the safety of PD-1 inhibition after EGFR-TKI treatment is acceptable.
Studies of various types of cancer including NSCLC have implicated TMB and immune-related gene expression as potential biomarkers for prediction of ICI efficacy (31). Furthermore, characterization of gene expression patterns may identify factors that act to suppress or promote antitumor immunity in EGFR-mutated NSCLC. We have now found that the T-cell–inflamed GEP score was significantly higher in patients who benefited from nivolumab therapy, whereas, unexpectedly, no clear association between TMB and nivolumab efficacy was apparent. Of note, analysis of the expression of individual genes and pathway analysis for the lymphoid compartment suggested that cytotoxic T cells and the chemokines that recruit them contribute to an active tumor immune microenvironment in EGFR-mutant NSCLC that may confer nivolumab efficacy in some cases. With regard to the underlying causes of the poor tumor immune microenvironment in most such patients, activation of the EGFR signaling pathway in EGFR mutation–positive NSCLC has been found to result in tumor immune evasion through various mechanisms (32, 33). EGFR signaling thus induces downregulation of the transcription factor IRF1 concurrently with that of its target gene for CXCL10 through the PI3K–AKT signaling pathway (32). We also found that the expression of EGFR and AKT1 showed a weak negative correlation with that of IRF1 in tumor specimens of the study patients. Of interest, genes for angiogenesis-related proteins such as VEGFA and that (NT5E) for CD73, which produces the immunosuppressive mediator adenosine (34, 35), were found in close proximity to EGFR in subcluster 1 of the hierarchical clustering analysis (Fig. 4B). The combination of agents that target these molecules with an ICI might thus be a promising treatment approach. However, individualized treatment strategies will be required given the diverse factors that determine the immunosuppressive tumor microenvironment in EGFR-mutated NSCLC.
Our trial has several limitations. First, it included patients who became resistant to EGFR-TKIs at various stages of their treatment and as a result of different mechanisms. Although randomization yielded a clear negative result overall for the efficacy of nivolumab in these patients, the small number of subjects may limit the conclusions that can be drawn from the subgroup and biomarker analyses. Further validation analysis for immune-related gene expression in an independent dataset for EGFR-mutated NSCLC is needed. Second, PD-L1 expression on tumor cells, which has been established as an important predictive factor for the efficacy of PD-1–targeted antibodies in NSCLC, was not evaluated in all patients and could not be used for stratification. And third, tissue specimens subjected to genetic and pathological analyses were obtained at various times including before and after EGFR-TKI treatment, which might limit interpretation of the biomarker analysis results. Nevertheless, our prospective study has important implications for the future development of immunotherapy in oncogenic driver mutation–positive NSCLC, given that it has shown that, even among such patients, some individuals possess a favorable tumor immune microenvironment for a durable response to ICIs.
In conclusion, nivolumab was clearly inferior to standard platinum-combination chemotherapy with regard to PFS in patients with EGFR-mutated NSCLC with acquired EGFR-TKI resistance. Nevertheless, a small proportion of such patients experienced a long-term response to nivolumab, and biomarker analysis identified potential target molecules for future immunotherapy in this patient population.
Supplementary Material
Acknowledgments
This study was conducted by WJOG under a funding contract with Ono Pharmaceutical Co. Ltd. (Osaka, Japan) and Bristol Myers Squibb Co. Ltd. (Tokyo, Japan). We thank the patients, their families, WJOG data center staff (especially Koji Takeda, Shinichiro Nakamura, and Seiko Tanaka), and all of the investigators who participated in the study.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
H. Hayashi reports grants and personal fees from Ono Pharmaceutical Co. Ltd. and Bristol Myers Squibb Co. Ltd. during the conduct of the study; grants from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., and Ono Pharmaceutical Co. Ltd.; and personal fees from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Eli Lilly Japan K.K., Kyorin Pharmaceutical Co. Ltd., Merck Biopharma Co. Ltd., MSD K.K., Novartis Pharmaceuticals K.K., Ono Pharmaceutical Co. Ltd., Shanghai Haihe Biopharm, Taiho Pharmaceutical Co. Ltd., Pfizer, and Takeda Pharmaceutical Co. Ltd. outside the submitted work. S. Sugawara reports personal fees from Bristol Myers Squibb, Ono Pharmaceutical, Eli Lilly and Company, AstraZeneca, Chugai Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim, MSD, Pfizer, Taiho Pharmaceutical, Novartis, Kyowa Kirin, and Yakult Honsha outside the submitted work. Y. Fukuda reports grants from Ono Pharmaceutical Co., Ltd., and Bristol Myers Squibb during the conduct of the study; personal fees from AstraZeneca, Chugai Pharmaceutical Co. Ltd., MSD, Taiho Pharmaceutical, Nippon Boehringer Ingelheim, Daiichi Sankyo Company, Limited, Merck Biopharma Co., Ltd., and Ono Pharmaceutical outside the submitted work. D. Fujimoto reports personal fees from Ono Pharmaceutical Co. Ltd., Bristol Myers Squibb Co. Ltd., and Eli Lilly Japan K.K. during the conduct of the study and personal fees from AstraZeneca K.K., Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., MSD K.K., Boehringer Ingelheim Japan Inc., and Novartis Pharma K.K outside the submitted work. S. Miura reports personal fees from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical, Pfizer, Eli Lilly, Boehringer-Ingelheim Japan, Ono Pharmaceutical, AstraZeneca, Novartis, MSD, Bristol Myers Squibb, and Daiichi Sankyo outside the submitted work. Y. Ozawa reports personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Nippon Kayaku, MSD K.K., Ono Pharmaceutical Co. Ltd., Novartis Pharma K.K., and Takeda Pharmaceutical Co. Ltd. outside the submitted work. J. Tanizaki reports personal fees from AstraZeneca K.K., Boehringer-Ingelheim Japan Inc., Bristol Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., MSD K.K., and Taiho Pharmaceutical Co. Ltd. outside the submitted work. K. Azuma reports personal fees from AstraZeneca K.K., Bristol Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., MSD K.K., and Ono Pharmaceutical Co. Ltd. outside the submitted work. S. Omori reports grants and personal fees from Daiichi Sankyo Co., Ltd.; personal fees from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Amgen K.K., AstraZeneca K.K., and Novartis Pharma K.K. outside the submitted work. M. Tachihara reports personal fees from Eli Lilly Japan K.K. and Ono Pharmaceutical Co. Ltd. during the conduct of the study, and personal fees from Eli Lilly Japan K.K., Bristol Myers Squibb, Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., MSD K.K., Boehringer Ingelheim Japan Inc., Taiho Pharmaceutical Co. Ltd., and Pfizer Japan Inc. and grants and personal fees from AstraZeneca K.K. outside the submitted work. K. Nishino reports personal fees from Eli Lilly Japan, Ono Pharmaceutical Co., Ltd., and Bristol Myers Squibb K.K. during the conduct of the study; personal fees from AstraZeneca, Chugai Pharmaceutical, Nippon Boehringer Ingelheim, Roche Diagnostics, Novartis Japan, Pfizer, Merck, and Takeda Pharmaceutical Company Limited outside the submitted work. A. Bessho reports personal fees from Bristol Myers Squibb, Ono Pharmaceutical, and Eli Lilly during the conduct of the study; and personal fees from AstraZeneca, Boehringer Ingelheim, Pfizer, and Chugai Pharmaceutical Co., Ltd. outside the submitted work. K. Haratani reports personal fees from AS ONE CORPORATION, Bristol Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., and Ono Pharmaceutical Co. Ltd. and grants and personal fees from AstraZeneca K.K., and MSD K.K., outside the submitted work. K. Sakai reports personal fees from Roche Diagnostics, Bio-Rad, AstraZeneca, Chugai Pharmaceutical Co., Ltd., and Hitachi outside the submitted work. K. Nishio reports grants from Nippon Boehringer Ingelheim, West Japan Oncology Group, Ignyta, Inc., Korea Otsuka Pharmaceutical, Eli Lilly, Thoracic Oncology Research Group, North East Japan Study Group, Clinical Research Support Center Kyushu, Nichirei Biosciences Inc., and Osakaminami hospital and personal fees from SymBio Pharmaceuticals, Solasia Pharma, Eli Lilly Japan, Otsuka Pharmaceutical, Chugai, Eisai, Pfizer, Boehringer Ingelheim Japan, Novartis Pharma, MSD, Ono Pharmaceutical, Bristol Myers Squibb, Life Technologies Japan, Yakult Honsha, Roche Diagnostics, AstraZeneca, Sanofi, Guardant Health, Amgen, and Merck Biopharma outside the submitted work. N. Yamamoto reports grants from Ono Pharmaceutical Co. Ltd. and Bristol Myers Squibb Co. Ltd., personal fees from MSD K.K., AstraZeneca K.K., Ono Pharmaceutical Co. Ltd., Thermo Fisher Scientific, Daiichi Sankyo Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Boehringer Ingelheim Japan Inc., Novartis Pharmaceuticals K.K., Pfizer Inc., Bristol Myers Squibb, Nippon Kayaku, GlaxoSmithKline K.K., Sanofi K.K., Hisamitsu Pharmaceutical Co., Inc., and Merck Biopharma outside the submitted work. K. Nakagawa reports grants and personal fees from AstraZeneca K.K., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K.K., Bristol Myers Squibb Company, Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., and Merck Biopharma Co. Ltd. and grants, personal fees, and other support from Astellas Pharma Inc., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., and Eli Lilly Japan K.K. during the conduct of the study. K.K. Nakagawa also reports personal fees from MEDICUS SHUPPAN, Publishers Co. Ltd., Care Net Inc., Medical Review Co. Ltd., Roche Diagnostics K.K., Medical Mobile Communications Co. Ltd., 3H Clinical Trial Inc., Nichi-Iko Pharmaceutical Co. Ltd., Yodosha Co. Ltd., Nikkei Business Publications Inc., Thermo FisherScientific K.K., Yomiuri Telecasting Corporation, Nippon Kayaku Co. Ltd., Hisamitsu Pharmaceutical Co. Inc., NANZANDO Co. Ltd., and Amgen Inc.; personal fees and other support from KYORIN Pharmaceutical Co. Ltd.; grants, personal fees, and other support from Takeda Pharmaceutical Co. Ltd.; grants and personal fees from Taiho Pharmaceutical Co. Ltd., AbbVie Inc., Kyowa Kirin Co. Ltd., and Bayer Yakuhin, Ltd.; and grants from SymBio Pharmaceuticals Limited, ICON JAPAN K.K., Parexel International Corp., Kissei Pharmaceutical Co. Ltd., EPS Corporation, Syneos Health, Pfizer R&D Japan G.K., A2 Healthcare Corp., IQVIA Services Japan K.K., Eisai Co. Ltd., CMIC Shift Zero K.K., EPS International Co. Ltd., Otsuka Pharmaceutical Co. Ltd., PRA Health Sciences, Covance Japan Inc., Medical Research Support, Sanofi K.K., PPD-SNBL K.K, Japan Clinical Research Operations, Sysmex Corporation, Mochida Pharmaceutical Co. Ltd., and GlaxoSmithKline K.K. outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
H. Hayashi: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, investigation, methodology, writing–original draft, writing–review and editing. S. Sugawara: Resources, writing–review and editing. Y. Fukuda: Resources, investigation, writing–review and editing. D. Fujimoto: Resources, writing–review and editing. S. Miura: Resources, writing–review and editing. K. Ota: Resources, writing–review and editing. Y. Ozawa: Resources, writing–review and editing. S. Hara: Resources, writing–review and editing. J. Tanizaki: Resources, writing–review and editing. K. Azuma: Resources, writing–review and editing. S. Omori: Resources, writing–review and editing. M. Tachihara: Resources, writing–review and editing. K. Nishino: Resources, writing–review and editing. A. Bessho: Resources, writing–review and editing. Y. Chiba: Resources, investigation, methodology, writing–review and editing. K. Haratani: Data curation, formal analysis, methodology, writing–original draft. K. Sakai: Data curation, formal analysis, methodology, writing–review and editing. K. Nishio: Resources, supervision, investigation, writing–review and editing. N. Yamamoto: Resources, supervision, funding acquisition, writing–review and editing. K. Nakagawa: Resources, supervision, writing–review and editing.
References
- 1. World Health Organization. Cancer - Key Facts. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer; 2021.
- 2. Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci 2007;98:1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- 4. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- 5. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- 8. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 9. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261–8. [DOI] [PubMed] [Google Scholar]
- 12. Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 2013;14:1212–8. [DOI] [PubMed] [Google Scholar]
- 13. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- 16. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol 2016;12:403–7. [DOI] [PubMed] [Google Scholar]
- 17. Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol 2017;28:1532–9. [DOI] [PubMed] [Google Scholar]
- 18. Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res 2020;26:2037–46. [DOI] [PubMed] [Google Scholar]
- 19. Hayashi H, Chiba Y, Sakai K, Fujita T, Yoshioka H, Sakai D, et al. A randomized phase II study comparing nivolumab with carboplatin-pemetrexed for patients with EGFR mutation-positive nonsquamous non-small-cell lung cancer who acquire resistance to tyrosine kinase inhibitors not due to a secondary T790M mutation: rationale and protocol design for the WJOG8515L study. Clin Lung Cancer 2017;18:719–23. [DOI] [PubMed] [Google Scholar]
- 20. Tamiya A, Tamiya M, Shiroyama T, Kanazu M, Hirooka A, Tsuji T, et al. Dose escalation study of carboplatin-pemetrexed followed by maintenance pemetrexed for elderly patients with advanced nonsquamous nonsmall-cell lung cancer. Ann Oncol 2013;24:980–5. [DOI] [PubMed] [Google Scholar]
- 21. Okamoto I, Nokihara H, Nomura S, Niho S, Sugawara S, Horinouchi H, et al. Comparison of carboplatin plus pemetrexed followed by maintenance pemetrexed with docetaxel monotherapy in elderly patients with advanced nonsquamous non-small cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol 2020;6:e196828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okamoto I, Aoe K, Kato T, Hosomi Y, Yokoyama A, Imamura F, et al. Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naive patients with advanced nonsquamous non-small-cell lung cancer. Invest New Drugs 2013;31:1275–82. [DOI] [PubMed] [Google Scholar]
- 23. Offin M, Rizvi H, Tenet M, Ni A, Sanchez-Vega F, Li BT, et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res 2019;25:1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015;527:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng M, Cai X, Long L, Xie L, Ma H, Zhou Y, et al. CD36 promotes the epithelial-mesenchymal transition and metastasis in cervical cancer by interacting with TGF-beta. J Transl Med 2019;17:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol 2018;13:1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- 28. Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol 2019;14:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol 2020;31:507–16. [DOI] [PubMed] [Google Scholar]
- 30. Kotake M, Murakami H, Kenmotsu H, Naito T, Takahashi T. High incidence of interstitial lung disease following practical use of osimertinib in patients who had undergone immediate prior nivolumab therapy. Ann Oncol 2017;28:669–70. [DOI] [PubMed] [Google Scholar]
- 31. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362:eaar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol 2020;5:eaav3937. [DOI] [PubMed] [Google Scholar]
- 33. Watanabe S, Hayashi H, Haratani K, Shimizu S, Tanizaki J, Sakai K, et al. Mutational activation of the epidermal growth factor receptor down-regulates major histocompatibility complex class I expression via the extracellular signal-regulated kinase in non-small cell lung cancer. Cancer Sci 2019;110:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol 2017;18:1332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumagai S, Koyama S, Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer 2021;21:181–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.