ABSTRACT
Targeted protein degradation allows targeting undruggable proteins for therapeutic applications as well as eliminating proteins of interest for research purposes. While several types of degraders that harness the proteasome or the lysosome have been developed, a technology that simultaneously degrades targets and accelerates cellular autophagic flux remains unavailable. In this study, we developed a general chemical tool by which given intracellular proteins are targeted to macroautophagy for lysosomal degradation. This platform technology, termed AUTOTAC (AUTOphagy-TArgeting Chimera), employs bifunctional molecules composed of target-binding ligands (TBLs) linked to autophagy-targeting ligands (ATLs). Upon binding to targets via the TBL, the ATL binds the ZZ domain of the otherwise dormant autophagy receptor SQSTM1/p62 (sequestosome 1), which activates SQSTM1 associated with targets and sequesters them into oligomeric species for autophagic targeting and lysosomal degradation. AUTOTACs were used to degrade various oncoproteins or aggregation-prone proteins in neurodegeneration both in vitro and/or in vivo. We suggest that AUTOTAC provides a platform for selective proteolysis as a research tool and in drug development.
KEYWORDS: Chemical tools, selective autophagy, protein quality control, proteolysis, N-degron pathway, N-terminal arginylation, SQSTM1/p62, targeted protein degradation (TPD), neurodegeneration, proteinopathy
Recent advances in genetic bioengineering, exemplified by CRISPR, TALEN, and siRNA, have enabled selective destruction and functional silencing of DNA and RNA. In contrast to the universally applicable nature of genetic editing, no general tools exist by which proteins are selectively recognized and targeted for destruction. Such a technology, if available, will be applied not only as a research tool but also as degrader-type drugs. Targeted protein degradation (TPD) that uses heterobifunctional chimeric molecules or molecular glues is an emerging therapeutic modality in drug discovery. The majority of degraders, some of which are now under clinical trials, target intracellular and/or extracellular proteins to the proteasome or lysosome (Figure 1).
Figure 1.
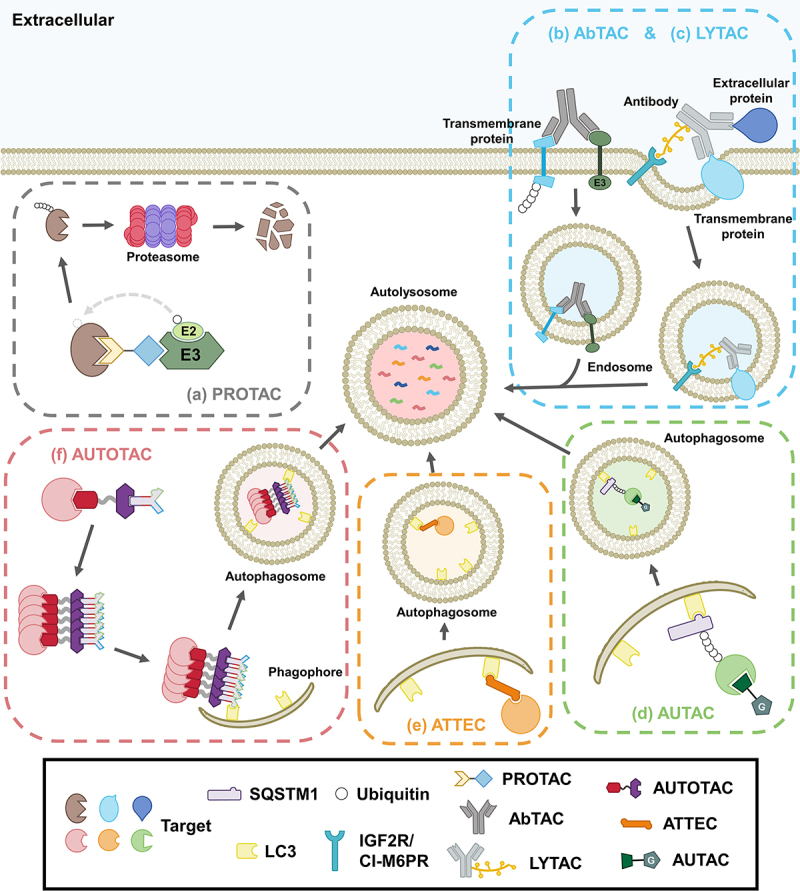
Schematic models of current targeted protein degradation (TPD) platforms that target a protein of interest (POI). Intracellular TPD platforms include (a) the proteasome-based PROTAC, (b) transmembrane E3 ligase-targeting antibody-based AbTAC, (c) lysosomal-targeting receptor-based LYTAC, (d) S-guanylation/autophagy-based AUTAC, (e) LC3/autophagy-based ATTEC, and (f) SQSTM1/autophagy-based AUTOTAC.
Among TPDs, PROteolysis-TArgeting Chimera (PROTAC) employs a chimera that serves as a bridge to increase the spatiotemporal proximity between a protein of interest (POI) and an E3 ligase of the ubiquitin-proteasome system (UPS). Upon forming a ternary complex, the E3 catalyzes polyubiquitination of the target, leading to proteasomal degradation. Antibody-based PROTAC (AbTAC) uses engineered antibodies that target membrane-associated POIs and the transmembrane E3 RNF43. In doing so, RNF43 polyubiquitinates the POI and induces the internalization of the RNF43-AbTAC-POI complex into endocytic vesicles, leading to lysosomal degradation. LYsosome-TArgeting Chimera (LYTAC) utilizes an engineered antibody chemically linked to an oligoglycopeptide motif. While the antibody binds a transmembrane POI, the oligoglycopeptide motif binds IGF2R/CI-M6PR (insulin-like growth factor 2 receptor), leading to internalization and degradation via the endosome-lysosome pathway. In a next-generation version of LYTAC, a warhead that binds an extracellular POI is chemically linked to acetylgalactosamine that binds ASGR/ASPGR (asialoglycoprotein receptor) for internalization. AUtophagy-TArgeting Chimera (AUTAC) employs a nucleotide-based degradation tag to induce the S-guanylation and subsequent lysine 63-linked polyubiquitination of POI, which recruits autophagic receptors for macroautophagic proteolysis. AuTophagy-TEthering Compound (ATTEC) uses a molecular glue that directly interacts with LC3, tethering POI to maturing autophagic membranes for macroautophagic degradation.
In this study, we aimed to develop a general platform that targets any given intracellular protein to SQSTM1-dependent macroautophagy for lysosomal degradation. To this end, we took advantage of our recent findings that autophagic proteolysis is critically regulated by the N-degron pathway that modulates the half-lives of substrates based on the identity of their N-terminal destabilizing residues, called N-degrons. N-degrons include the N-terminal arginine (Nt-Arg) residue of proteins that has been generated through post-translational conjugation of the amino acid L-Arg by ATE1-encoded Arg-tRNA transferases (R-transferases; EC 2.3.2). In response to accumulating cargoes such as misfolded proteins and their aggregates, the Nt-Arg on various proteins is neosynthesized and binds the ZZ domain of the autophagic receptor SQSTM1. As an N-recognin, otherwise inactive SQSTM1 is conformationally activated into an autophagy-compatible form, accelerating self-oligomerization in complex with its cargoes and SQSTM1 interaction with LC3. In parallel, a subpopulation of ligand-bound SQSTM1 migrates to the endoplasmic reticulum and induces omegasome biogenesis in preparation for receiving incoming cargoes.
The AUTOTAC chemical platform, developed in this study [1], employs a chimera composed of target-binding ligand (TBL) linked to autophagy-targeting ligand (ATL), the latter being a chemical mimic of the Nt-Arg (Figure 2). We performed molecular docking analysis of the ATL scaffold to identify several SQSTM1-ZZ binders that exhibit the selective engagement with SQSTM1 as well as the activity to augment autophagosome biogenesis and autophagic flux. As a proof-of-concept approach, we chose PHTPP, vinclozolinM2, and fumagillol as TBLs to degrade respectively ESR2/ERβ (estrogen receptor 2 (beta)), AR (androgen receptor), and METAP2 (methionine aminopeptidase 2). The oncoprotein-targeting AUTOTACs bind and activate SQSTM1 to induce SQSTM1-dependent autophagic degradation of POIs independently of ubiquitin. These degraders exhibit a sustained degradative efficacy, indicative of the recycling of AUTOTACs from the lysosome. These results verified the feasibility of the AUTOTAC platform for targeted proteolysis of intracellular oncoproteins via autophagy.
Figure 2.
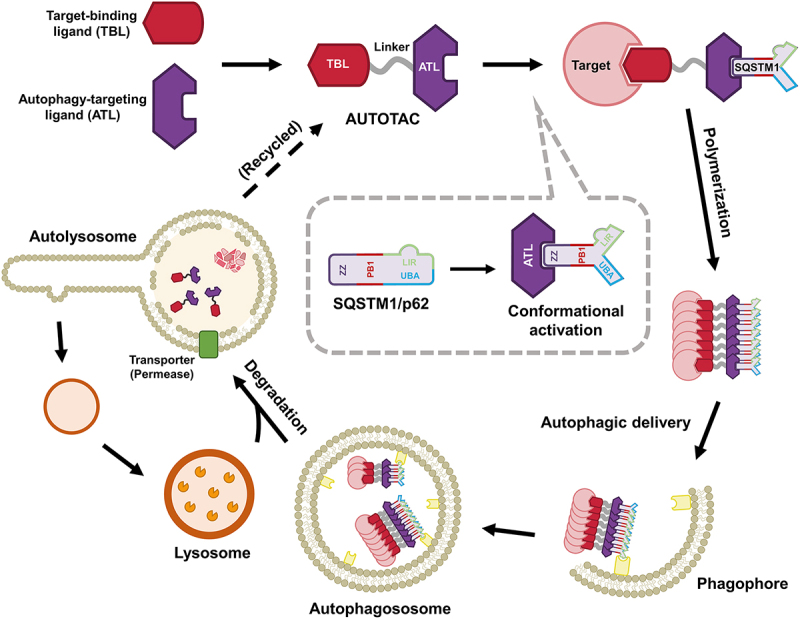
Graphical illustration of the AUTOTAC targeted protein degradation platform and its mechanism of action. The AUTOTAC platform employ bifunctional molecules composed of target-binding ligands (TBLs) linked to SQSTM1-binding autophagy-targeting ligands (ATLs). AUTOTACs simultaneously interact with the protein of interest and the ZZ domain of SQSTM1 via its TBL and ATL, respectively. When the ATL moiety interacts with the SQSTM1-ZZ domain in an Nt-Arg-mimicking manner, inactive SQSTM1 is conformationally and thus biologically activated for self-oligomerization, forming target-SQSTM1 oligomeric complexes near the site of autophagosome formation. Finally, AUTOTACs facilitate ubiquitin- and proteasome-independent degradation of the target-SQSTM1 complexes via macroautophagy and are then recycled from the lysosome for subsequent rounds of degradation, resulting in sustained efficacy.
The vast majority of proteins are misfolded or damaged at least once during their limited lifespans, necessitating timely degradation of misfolded protein aggregates. However, the proteasome has an inner diameter as narrow as 13 Å, which precludes the entrance of protein complexes, oligomers and high-molecular weight aggregates. We therefore asked whether AUTOTACs can degrade UPS-resistant, high-molecular weight oligomers and aggregates of pathological proteins. Two types of target-binding warheads were chosen: the chemical chaperone 4-phenylbutiryl acid (4-PBA) and the oligomeric modulator anle138b. These proteinopathy-targeting AUTOTACs exhibit excellent degradative potency against a wide myriad of misfolded, pathological proteins and/or their aggregates, including MAPT/tau, HTT (huntingtin) and DES (desmin). The degradation of MAPT/tau aggregates is achieved at DC50 values of low nanomoles and t1/2 time of approximately 30 min. Significant degradative potency is maintained even with more than double the length of the PEG linker, suggesting that AUTOTAC-bound SQSTM1 may induce the degradation of the POI without necessarily forming a positively cooperative ternary complex with it. By using a brain-specific tauopathy mouse model, a PBA-based AUTOTAC was demonstrated to selectively eliminate preexisting MAPT/tau aggregates and intracellular neurofibrillary tangles as well as their soluble toxic oligomers from brains. As a follow-up study, an orally administrable anle138b-based AUTOTAC, with proven in vivo efficacy for various pathogenic aggregates, entered preclinical studies to develop drugs for Alzheimer disease, Parkinson disease, amyotrophic lateral sclerosis/ALS, and progressive supranuclear palsy/PSP.
Future studies on AUTOTAC will focus on its pharmacological and mechanistic details in comparison with other TPD platforms such as PROTAC. Currently, most TPD targets are oncoproteins, as exemplified by PROTACs against AR and ESR in P2 and P1 clinical trials, respectively. For certain monomeric and soluble oncoproteins that are hyperactivated and tumorigenic, PROTAC appear to be a suitable means of TPD given the immediate and rapid degradative modality inherent to the UPS. However, UPS-resistant protein complexes and aggregates cause neurodegeneration and many other proteinopathies as well as certain cancers. Lysosome-based TPD technologies such as AUTOTAC provide a powerful means to selectively degrade these UPS-resistant intracellular proteins and other non-proteinaceous materials. One unique mode-of-action in AUTOTAC is SQSTM1 self-oligomerization, which drives sequestration of POI prior to its degradation and, thereby, “cleanses” the cell from pathological cargoes by instantaneously segregating them from the cellular environment. In addition, AUTOTAC is capable of enhancing autophagic flux by boosting up SQSTM1-dependent biogenesis of autophagic membranes, making it an ideal choice to develop TPD-type drugs. After 30 years since the discovery of autophagy genes by several groups in yeast, autophagy is emerging as a major platform to develop drugs that can cure many currently incurable diseases.
Acknowledgments
The authors thank the Y.T.K. and Y.K.K. laboratories’ members and AUTOTAC Bio Inc.’s employees for their comments during this study. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1A5A1019023 and NRF-2021R1A2B5B03002614 to Y.T.K.), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute and Korea Dementia Research Center (KDRC) funded by the Ministry of Science, ICT, and Future Planning (MSIP) (HU21C0201 to C.H.J.), the NRF grant of Korea government (MSIT) (NRF-2021R1A2C3004965), the R&D Convergence Program of NST (CAP-16-03-KRIBB) and by the Korea Research Institute of Bioscience and Biotechnology Research Initiative Program (to B.Y.K.), and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2021R1A2C209373411) and KIST Institutional Program (2E31522; Atmospheric Environment Research Program, 2E31700).
Funding Statement
This work was supported by the Korea Institute of Science and Technology [2E31522, 2E31700]; Ministry of Science, ICT and Future Planning [HU21C0201]; National Research Council of Science and Technology [CAP-16-03-KRIBB]; National Research Foundation of Korea [NRF-2021R1A2C3004965]; National Research Foundation of Korea [2021R1A2C209373411]; National Research Foundation of Korea [NRF-2020R1A5A1019023, NRF-2021R1A2B5B03002614].
Disclosure statement
No potential conflicts of interest were disclosed.
Reference
- [1].Ji CH, Kim HY, Lee MJ, et al. The AUTOTAC chemical biology platform for targeted protein degradation via the autophagy-lysosome system. Nat Commun. 2022;13(1):904. PubMed PMID: 35173167. [DOI] [PMC free article] [PubMed] [Google Scholar]


