ABSTRACT
Exosomes are a subtype of extracellular vesicles (EVs), released by all cell types, that originate from the invagination of the endosomal limiting membrane. These EVs can transport biological information in the form of proteins and RNA and have been the focus of intensive research over the last decade. It is becoming apparent that EVs can have important roles in health and disease. EVs are also promising noninvasive biomarkers of disease (liquid biopsies) and valuable vectors for innovative therapies. However, little is known about the mechanisms that regulate the loading of cytosolic proteins into exosomes. We recently showed that soluble proteins containing amino acid sequences biochemically related to the KFERQ motif are loaded into nascent exosomes at the endosomal limiting membrane, in a process mediated by LAMP2A. Because of the subcellular localization and machinery involved, this mechanism has many similarities with chaperone-mediated autophagy (CMA) and endosomal microautophagy (e-Mi), but also some important differences. In this punctum we will focus on the mechanistic details of exosomal LAMP2A loading of cargo (e-LLoC) as well as on its implications for intercellular and interorgan communication.
KEYWORDS: eLLoC, LAMP2A, exosomes, intercellular communication, inter-organ communication, chaperones, autophagy, endosomes, lysosome
Exosomes are nanosized vesicles of 40–160 nm in diameter that are secreted by most cell types and found in most, if not all, biological fluids. Exosomes contain lipids, metabolites, proteins and RNA and can travel and transfer information from one cell to another. In contrast to microvesicles, formed by the outward budding of the plasma membrane, exosomes are formed by the inward budding of the endosomal limiting membrane to produce endosomes containing intralumenal vesicles (ILVs), referred to as multivesicular bodies (MVBs). The fusion of MVBs with lysosomes for degradation of its contents by lysosomal hydrolases is often the final step of the endocytic pathway. However, at least a subset of MVBs can fuse with the plasma membrane leading to the release of ILVs to the extracellular space in the form of exosomes. After secretion, exosomes can dock and fuse with, or be internalized by, other cells to deliver their cargo.
Currently, many aspects of ILV biogenesis are still not completely understood. For example, while it is generally accepted that ILV formation is mediated by the endosomal sorting complex required for transport (ESCRT) machinery, pivotal experiments showed that ESCRT-depleted cells can still
generate ILVs. Moreover, the cargo repertoire of exosomes does not necessarily reflect the cytosolic contents of the originating cell, suggesting the existence of a mechanism for cargo sorting at the endosomal membrane. A number of alternative mechanisms and players have been reported to assist in ILV formation and cargo sorting. These include the sphingolipid ceramide, the tetraspanin CD63, the toll-like receptor trafficking chaperone UNC93B1 and the SDC (syndecan)-SDCBP/syntenin-PDCD6IP/Alix pathway. Recently we elucidated a new mechanism for the sorting of proteins into exosomes (Figure 1), involving LAMP2A (lysosomal associated membrane protein 2A) [1].
Figure 1.
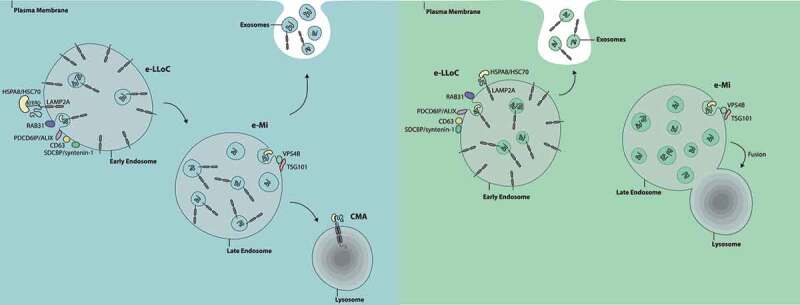
In e-LLoC, LAMP2A participates in the loading of KFERQ-containing proteins into ILVs at the EE limiting membrane. HSPA8, CD63, PDCD6IP/ALIX, SDCBP/syntenin-1, RAB31 and the lipid ceramide participate in this mechanism as well. In contrast, e-Mi occurs in LE, is independent of LAMP2A and involves the ESCRT machinery (TSG101 and VSP4B), while CMA involves the translocation of substrates across the lysosomal membrane. It is possible that e-LLoC and e-Mi occur at different maturation stages of the endocytic pathway, e-LLoC at EE and e-Mi at LE (left panel). Alternatively, ESCRT and non-ESCRT machinery may be asymmetrically distributed (right panel). In this case, endosomes enriched in non-ESCRT machinery would be e-LLoC active, while endosomes enriched in ESCRT machinery would be e-Mi active. It is also possible that e-LLoC endosomes are more likely to fuse with the plasma membrane and release exosomes, while e-Mi endosomes are more prone to fuse with lysosomes for degradation.
Up to this point, LAMP2A was considered to be involved exclusively in CMA, by mediating substrate translocation across the lysosomal membrane in a process that is also dependent on the chaperone HSPA8/HSC70. However, based on quantitative analysis of LC-MS/MS data we showed that exosomes are, indeed, enriched in KFERQ-containing proteins. Moreover, the majority of proteins that are downregulated upon LAMP2A KO contain at least one KFERQ-like sequence. Interestingly, HSPA8 is also decreased in exosomes isolated from LAMP2A KO cells, suggesting that the presence of LAMP2A is essential to target the HSPA8-KFERQ-protein complexes to exosomes. This hypothesis is consistent with the data showing that HSPA8 chemical inhibition is sufficient to hinder protein loading into exosomes. We further showed that this sorting process occurs in endosomes, early in the endocytic pathway, participating in the loading of proteins into ILVs at the endosome limiting membrane. This loading is independent of the ESCRT-machinery components TSG101 and VPS4B because depletion of these proteins does not remove KFERQ-motif containing proteins from exosomes. However, depletion of ESCRT-independent machinery such as CD63, PDCD6IP/ALIX, SDCBP/syntenin-1 and RAB31, as well as the lipid ceramide does have this effect. These data set e-LLoC apart from e-Mi. In fact, in e-Mi, LAMP2A is not necessary, whereas both TSG101 and VSP4B are needed. In addition, in vitro assays and confocal imaging show that e-LLoC occurs in early endosomes (EE), while e-Mi participates in the loading of proteins in late endosomes (LE).
Why there is such a diversity of mechanisms in endosome formation and cargo sorting remains unclear. One possible explanation as to why e-LLoC occurs in EE is that the ability to generate ILVs is greater in EE than in LE, because the proteins associated with ILV biogenesis become depleted in the limiting membrane of LE. For example, CD63 is ~7x enriched in the ILVs of LE as compared to the endosomal limiting membrane. Furthermore, it is also likely that the machinery associated with e-MI, including the ESCRT machinery, is active in later endosomal compartments, whereas this is not the case for LAMP2A and other ESCRT-independent machinery. It is also possible that the recruitment of ESCRT components and non-ESCRT machinery occurs at different stages of MVB maturation. However, further research is needed to clarify this. Alternatively, ESCRT components and non-ESCRT machinery could be delivered to different endocytic compartments. Consistent with this hypothesis, our data showed that endosomes enriched in LAMP2A (when compared with endosomes enriched in LAMP2B, the product of a different isoform of the LAMP2 gene), contain different endosomal components. In fact, the ESCRT machinery preferentially localizes to LAMP2B-enriched endosomes while non-ESCRT machinery segregates preferentially to LAMP2A-enriched endosomes.
These lines of evidence support a model in which endosomal compartments are not a homogeneous entity and that different subsets may contain distinct molecular components, affecting the final contents of ILVs and, as a consequence, of exosomes. However, whether the presence of heterogenous MVBs reflects the existence of different MVB subpopulations or distinct maturation stages of the same population is still unclear (Figure 1). In addition, it also possible that MVB heterogenicity can indicate that some endosomes might be more likely to fuse with the plasma membrane to release exosomes, while others are more prone to fuse with the lysosome for degradation. In this case, LAMP2A-enriched endosomes could preferentially give rise to exosomes whereas LAMP2A-depleted ones would follow the canonical pathway of lysosome fusion (Figure 1).
Our study opens new avenues for research, both in the nature of endosomes and in the mechanisms regulating exosomes biogenesis, in particular in the sorting of proteins into these EVs. The biological consequences of e-LLoC are still difficult to anticipate but are likely significant. For example, we showed that the hypoxia master regulator HIF1A (hypoxia inducible factor 1 subunit alpha) is loaded into exosomes by the action of its KFERQ-like motif. We further showed that HIF1A can travel in exosomes and transfer a hypoxia response from hypoxic to normoxic cells, both in vitro and in the zebrafish.
To summarize, our data support a model in which e-LLoC is restricted to a subpopulation of endosomes and, by extension, of exosomes. These endosomes are enriched in LAMP2A and other endosomal components such as CD63, PDCD6IP/Alix and SDCBP/syntenin-1, but depleted of ESCRT components such as TSG101 and VPS4B. This mechanism is likely to affect intercellular and interorgan communication.
Funding Statement
This work is supported by Portuguese Foundation for Science and Technology (JVF grant: SFRH/BPD/121271/2016, ARS grant: PD/BD/106052/2015, “Programa Operacional Regional de Lisboa - FEDER/ Project 02/SAICT/2020/072552” and iNOVA4Health – UIDB/04462/2020 and UIDP/04462/2020, and by the Associated Laboratory LS4FUTURE (LA/P/0087/2020), two programs financially supported by Fundação para a Ciência e Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior.
Disclosure statement
The patent “A method for selective loading of proteins into exosomes and products 1003 thereof”, submitted by IBET (Instituto de Biologia Experimental e Tecnologica), Lisbon, 1004 Portugal, PCT/IB2020/051341, by inventors Joao Vasco Ferreira, Ana da Rosa Soares and 1005 Paulo Pereira. All authors declare they have no other competing interests.
Reference
- [1].Ferreira JV, da Rosa Soares A, Ramalho J, et al. LAMP2A regulates the loading of proteins into exosomes. Sci Adv. 2022;8(12):eabm1140. [DOI] [PMC free article] [PubMed] [Google Scholar]


