Abstract
Background
Muscle wasting is prevalent in cancer patients, and early recognition of this phenomenon is important for risk stratification. Recent studies have suggested that the creatinine–cystatin C ratio may correlate with muscle mass in several patient populations. The association between creatinine–cystatin C ratio and survival was assessed in cancer patients.
Methods
A total of 3060 patients who were evaluated for serum creatinine and cystatin C levels at the time of cancer diagnosis were included. The primary outcome was 6‐month mortality. The 1‐year mortality, and length of intensive care unit (ICU) and hospital stay were also evaluated.
Results
The mean age was 61.6 ± 13.5 years, and 1409 patients (46.0%) were female. The median creatinine and cystatin C levels were 0.9 (interquartile range [IQR], 0.6–1.3) mg/dL and 1.0 (IQR, 0.8–1.5) mg/L, respectively, with a creatinine–cystatin C ratio range of 0.12–12.54. In the Cox proportional hazards analysis, an increase in the creatinine–cystatin C ratio was associated with a significant decrease in the 6‐month mortality (per 1 creatinine–cystatin C ratio, hazard ratio [HR] 0.35; 95% confidence interval [CI], 0.28–0.44). When stratified into quartiles, the risk of 6‐month mortality was significantly lower in the highest quartile (HR 0.30; 95% CI, 0.24–0.37) than in the lowest quartile. Analysis of 1‐year mortality outcomes revealed similar findings. These associations were independent of confounding factors. The highest quartile was also associated with shorter lengths of ICU and hospital stay (both P < 0.001).
Conclusions
The creatinine–cystatin C ratio at the time of cancer diagnosis significantly associates with survival and hospitalization in cancer patients.
Keywords: Creatinine, Cystatin C, Muscle mass, Sarcopenia, Cancer, Mortality
Introduction
Muscle wasting is a common condition among patients with cancer. 1 , 2 , 3 , 4 Although its prevalence differs with cancer type and different measurement tools of muscle mass, muscle wasting has been reported in 89% of patients with pancreatic cancer and in 79% of patients with respiratory tract cancer. 5 , 6 Low muscle mass in cancer patients increases the risk of chemotherapy toxicity and post‐operative complications, leading to physical impairment, poor quality of life, and decreased survival. 7 , 8 , 9 Consequently, loss of muscle mass in cancer patients has recently been recognized as a potential treatment target, and early recognition of this phenomenon is considered important for risk stratification and intervention. 10 , 11 , 12 The evaluation of muscle mass requires lean muscle mass quantification using computed tomography (CT) or dual X‐ray absorptiometry. However, these methods are time‐consuming, costly, and not readily available.
Serum creatinine and cystatin C are commonly used kidney function estimation markers. Given that creatinine is mainly released from muscle tissue, serum creatinine level is mainly influenced by skeletal muscle mass. Cystatin C, on the other hand, is produced by all nucleated cells. As serum levels of creatinine depend more on muscle mass than do cystatin C levels, the relative serum concentration of creatinine to cystatin C, defined as the creatinine–cystatin C ratio, has been suggested to correlate with muscle mass in several patient populations, such as critically ill patients and patients with neurodegenerative diseases. 13 , 14 , 15 In addition, a recent evaluation of 182 cancer patients revealed that the creatinine–cystatin C ratio is strongly associated with CT‐assessed muscle mass. 16 Nonetheless, the prognostic value of the creatinine–cystatin C ratio in patients diagnosed with malignant diseases has not been evaluated.
In this study, the association between creatinine–cystatin C ratio and patient survival was assessed in patients diagnosed with cancer. This was done by retrospective evaluation of electronic medical records from two tertiary medical centres.
Methods
Patient selection
Patients who were evaluated for serum creatinine and cystatin C at the time of cancer diagnosis at the Severance Hospital and Gangnam Severance Hospital of the Yonsei University Health System (YUHS) between July 2005 and July 2019 were initially screened. Those who met the following criteria were excluded: (1) age < 18 years, (2) end‐stage kidney disease, and (3) follow‐up duration < 1 month. A total of 3060 patients were included in the final analysis (Figure 1). The present study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of YUHS (4‐2021‐0225). Need for informed consent was waived owing to the retrospective study design.
Figure 1.
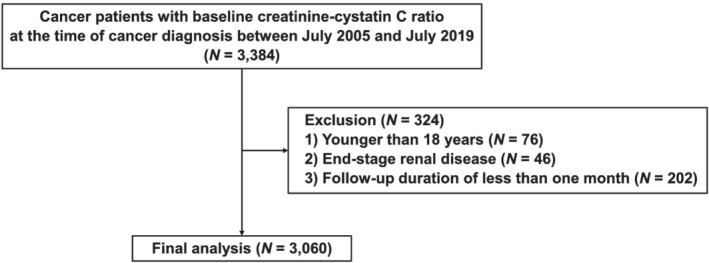
Flow diagram of the study.
Data collection and measurements
Demographic and laboratory data were retrieved from electronic medical records. The time of cancer diagnosis was considered baseline. Baseline demographic and anthropometric data included age, sex, blood pressure, height, weight, the type of cancer diagnosed, cancer stage, and past medical history. Body mass index was calculated as the body weight divided by the height squared (kg/m2). Laboratory evaluations preformed within a 1‐month window period from cancer diagnosis were considered baseline. When multiple laboratory test results were available, the results closest to the date of cancer diagnosis were used. Laboratory data included complete blood cell counts, serum blood urea nitrogen, creatinine, cystatin C, serum albumin, serum sodium, and total bilirubin levels, and prothrombin time. Serum creatinine level was measured using the Jaffe assay, and cystatin C levels were measured by immunonephelometry with calibration against the reference. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation. 17 The ratio of creatinine (mg/dL) to cystatin C (mg/L) was calculated from values measured concomitantly at baseline. The Charlson comorbidity index (CCI) was calculated using medical data recorded at the time of cancer diagnosis. Comorbidities were defined using diagnosis codes from the International Statistical Classification of Diseases and Related Health Problems, 10th revision. Acute kidney injury (AKI) was diagnosed using the AKI Network (AKIN) criteria. 18 AKI was defined as either an absolute increase of 0.3 mg/dL or a 50% increase in serum creatinine level compared with the lowest creatinine level within 3 months before cancer diagnosis. Performance status was evaluated using the Eastern Cooperative Oncology Group (ECOG) scale. 19
Clinical outcomes
Patients were followed up until their last visit at YUHS or death. The primary outcome was 6‐month mortality. The 1‐year mortality, and the length of intensive care unit (ICU) and hospital stay were also evaluated. Survival data were collected from electronic medical records of in‐hospital and outpatient clinics. Survival time was defined as the time interval between cancer diagnosis and either the last visit at YUHS or death. Patients who were lost to follow‐up were treated as censored in the survival analysis.
Statistical analyses
Continuous variables are expressed as means ± standard deviations or as medians and interquartile ranges (IQRs), and categorical variables are expressed as numbers and percentages. The normality of data distribution was analysed using the Shapiro–Wilk test. P for trend was calculated using linear regression for normally distributed continuous variables, and the Mann–Kendall trend test for non‐normally distributed continuous variables. The χ 2 test for trend was used for trend analyses of categorical variables.
Cumulative survival probabilities were estimated using Kaplan–Meier analysis and log‐rank tests. Cox proportional hazards models were developed to determine the relationship between the creatinine–cystatin C ratio and mortality, and data were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Variables that showed statistical significance in the univariate analysis were included in three models. Model 1 was not adjusted for any covariates. Model 2 included baseline age, sex, AKI, cancer type, cancer stage (IV vs. I–III), cancer treatment modality, CCI, and ECOG score. Model 3 was further adjusted for systolic blood pressure and relevant laboratory parameters. The creatinine–cystatin C ratio was assessed in two forms: as a continuous variable and in categorical quartile groups. The relationship between serum creatinine alone and patient outcomes was also assessed.
In order to minimize the chances of age being a bias factor, additional evaluations were made after 1:1 propensity score matching the patients of the lowest and highest creatinine–cystatin C ratio quartiles for age. The propensity score was determined using logistic regression with the greedy nearest neighbour matching technique without replacement. A calliper of 0.2 times the standard deviation was used. Furthermore, to lower the chances of chemotherapy administration being a bias factor, subgroup analyses were performed regarding chemotherapy administration in patients with gastrointestinal malignancies.
Sensitivity analyses were performed to confirm the findings of the primary analysis. The association between creatinine–cystatin C ratio and 6‐month and 1‐year survival was assessed in patients with an eGFR of <60 mL/min/1.73 m2. Evaluations were also performed separately in patients with haematologic and non‐haematologic malignancies. Considering that sex differences could account for differences in the creatinine–cystatin C ratio, assessments were also done in patients with gynaecologic malignancies and in patients with prostate or testicular cancer. Malignancies of the vulva, cervix, endometrium, uterus, fallopian tube, and the ovaries were considered as gynaecologic origin. Separate evaluations were also done in patients with available ECOG scores.
To assess whether the addition of the creatinine–cystatin C ratio to conventional parameters improves patient outcome prediction, the C‐statistics of the creatinine–cystatin C ratio was further evaluated. Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indices were also evaluated to determine reclassification and discrimination. Bootstrap estimation was performed to calculate 95% CIs of the NRI.
The MICE (multivariate imputation by chained equations) method of multiple multivariate imputations in STATA was used for missing data imputation implying the missing at random assumptions (Supporting Information, Table S1). Five complete data sets were created to account for missing values, and to achieve maximum accuracy, each set with missing values was suitably imputed in the multivariable Cox regression analyses. The estimates of the variables were averaged to give a single mean estimate, with standard errors adjusted according to Rubin's rules. Statistical significance was defined as P < 0.05.
Data were analysed using STATA Version 15 (STATA Corp., College Station, TX, USA) and R language (Version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
The baseline characteristics of patients are presented in Table 1. The mean age was 61.6 ± 13.5 years and 1409 patients (46.0%) were females. The most common type of cancer was gastrointestinal (30.3%), followed by genitourinary (19.2%), gynaecologic (13.2%), lung (13.0%), and haematologic (10.0%). AKI was present in 849 (27.7%) patients. The median baseline creatinine and cystatin C values for all cancer patients at baseline were 0.9 (IQR, 0.6–1.3) mg/dL and 1.0 (IQR, 0.8–1.5) mg/L, respectively, with a creatinine–cystatin C ratio range of 0.12–12.54. When the patients were categorized into quartiles according to baseline creatinine–cystatin C ratio (Q1, Q2, Q3, and Q4; Table 1), the mean age, proportion of females, ECOG score, and CCI score decreased with an increasing creatinine–cystatin C ratio (P < 0.001). In addition, there was a decrease in the proportion of patients with gastrointestinal cancer and stage IV cancer, whereas an increase was observed in the proportion of patients with genitourinary cancers, stage I–III cancer, and in patients treated with chemotherapy (P < 0.001), with an increasing creatinine–cystatin C ratio. Regarding laboratory parameters, serum haemoglobin and serum albumin levels increased (P < 0.001), while eGFR and serum cystatin C levels decreased in the higher creatinine–cystatin C ratio quartiles (P < 0.001).
Table 1.
Baseline characteristics according to creatinine–cystatin C ratio quartiles
| Variables | Total (n = 3060) | Quartiles of creatinine–cystatin C ratio | P for trend | |||
|---|---|---|---|---|---|---|
| Q1 (n = 765) | Q2 (n = 765) | Q3 (n = 765) | Q4 (n = 765) | |||
| Creatinine–cystatin C ratio | 0.82 (0.12–12.54) | 0.52 (0.12–0.63) | 0.72 (0.63–0.82) | 0.92 (0.82–1.05) | 1.31 (1.05–12.54) | |
| Demographic and anthropometric data | ||||||
| Age, years | 61.6 ± 13.5 | 64.7 ± 13.3 | 62.6 ± 13.5 | 60.9 ± 13.3 | 58.3 ± 13.1 | <0.001 |
| Female, % | 1409 (46.0) | 459 (60.0) | 384 (50.2) | 317 (41.4) | 249 (32.5) | <0.001 |
| Mean arterial pressure, mmHg | 88.8 ± 14.1 | 87.6 ± 13.8 | 88.3 ± 13.8 | 89.1 ± 14.1 | 90.1 ± 14.7 | <0.001 |
| Body mass index, kg/m2 | 23.6 ± 10.0 | 22.8 ± 10.6 | 24.2 ± 15.0 | 23.7 ± 6.9 | 23.9 ± 3.3 | <0.001 |
| ECOG score | 0.9 ± 1.0 | 1.0 ± 1.0 | 0.9 ± 1.0 | 0.9 ± 1.0 | 0.8 ± 1.0 | <0.001 |
| Cancer type | ||||||
| Haematologic | 305 (10.0) | 85 (11.1) | 74 (9.7) | 67 (8.8) | 79 (10.3) | 0.50 |
| Gastrointestinal | 927 (30.3) | 300 (39.2) | 249 (32.5) | 227 (29.7) | 151 (19.7) | <0.001 |
| Lung | 399 (13.0) | 96 (12.5) | 118 (15.4) | 99 (12.9) | 86 (11.2) | 0.24 |
| Breast | 113 (3.7) | 48 (6.3) | 30 (3.9) | 17 (2.2) | 18 (2.4) | <0.001 |
| Genitourinary | 588 (19.2) | 84 (11.0) | 84 (11.0) | 158 (20.7) | 262 (34.2) | <0.001 |
| Gynaecologic | 405 (13.2) | 73 (9.5) | 118 (15.4) | 116 (15.2) | 98 (12.8) | 0.08 |
| Neurologic | 48 (1.6) | 9 (1.2) | 17 (2.2) | 9 (1.2) | 13 (1.7) | 0.80 |
| Thyroid | 60 (2.0) | 20 (2.6) | 20 (2.6) | 9 (1.2) | 11 (1.4) | 0.03 |
| Head and neck | 45 (1.5) | 13 (1.7) | 9 (1.2) | 13 (1.7) | 10 (1.3) | 0.74 |
| Other | 170 (5.6) | 37 (4.8) | 46 (6.0) | 50 (6.5) | 37 (4.8) | 0.89 |
| Cancer stage a | ||||||
| Stage I–III | 1421 (51.6) | 242 (35.6) | 315 (45.6) | 391 (56.0) | 473 (69.0) | <0.001 |
| Stage IV | 1334 (48.4) | 438 (64.4) | 376 (54.4) | 307 (44.0) | 213 (31.0) | <0.001 |
| Cancer treatment | ||||||
| Surgery | 896 (29.3) | 90 (11.8) | 137 (17.9) | 208 (27.2) | 304 (39.7) | <0.001 |
| Chemotherapy | 739 (24.2) | 155 (20.3) | 235 (30.7) | 250 (32.7) | 256 (33.5) | <0.001 |
| Radiotherapy | 164 (5.4) | 19 (2.5) | 41 (5.4) | 51 (6.7) | 53 (6.9) | <0.001 |
| Comorbidities | ||||||
| Charlson comorbidity index | 5.6 ± 3.3 | 6.5 ± 3.2 | 5.9 ± 3.3 | 5.4 ± 3.3 | 4.5 ± 3.0 | <0.001 |
| Hypertension | 1225 (40.0) | 310 (40.5) | 295 (38.6) | 311 (40.7) | 309 (40.4) | 0.94 |
| Diabetes mellitus | 539 (17.6) | 139 (18.2) | 135 (17.6) | 141 (18.4) | 124 (16.2) | 0.19 |
| Acute kidney injury | 849 (27.7) | 156 (20.4) | 143 (18.7) | 173 (22.6) | 377 (49.3) | <0.001 |
| Laboratory parameters | ||||||
| Haemoglobin, g/dL | 11.1 ± 2.2 | 10.5 ± 1.9 | 10.9 ± 2.1 | 11.3 ± 2.2 | 11.5 ± 2.3 | <0.001 |
| White blood cell, × 103/μL | 10.1 ± 10.9 | 10.7 ± 8.5 | 10.0 ± 10.2 | 9.0 ± 6.5 | 10.6 ± 15.9 | 0.44 |
| Platelet count, × 103/μL | 240 ± 132 | 227 ± 157 | 252 ± 135 | 244 ± 118 | 238 ± 114 | <0.001 |
| BUN, mg/dL | 23.3 ± 19.8 | 23.8 ± 18.2 | 21.4 ± 16.7 | 21.8 ± 17.6 | 26.0 ± 25.1 | 0.44 |
| Creatinine, mg/dL | 0.9 (0.6–1.3) | 0.6 (0.4–0.9) | 0.8 (0.6–1.0) | 0.9 (0.7–1.3) | 1.3 (1.0–2.2) | <0.001 |
| Cystatin C, mg/L | 1.0 (0.8–1.5) | 1.2 (0.9–1.7) | 1.1 (0.8–1.4) | 0.9 (0.8–1.4) | 0.9 (0.7–1.3) | <0.001 |
| eGFR, mL/min/1.73 m2 | 77.6 ± 33.1 | 94.3 ± 30.3 | 84.0 ± 27.6 | 76.7 ± 30.3 | 55.6 ± 31.1 | <0.001 |
| Albumin, g/dL | 3.3 ± 0.7 | 3.0 ± 0.6 | 3.3 ± 0.7 | 3.5 ± 0.7 | 3.5 ± 0.7 | <0.001 |
| Serum sodium, mmol/L | 137 ± 6 | 136 ± 6 | 137 ± 6 | 137 ± 6 | 138 ± 5 | <0.001 |
| Total bilirubin, mg/dL | 0.6 (0.4–1.0) | 0.7 (0.4–1.5) | 0.5 (0.4–0.9) | 0.6 (0.4–0.9) | 0.6 (0.4–0.8) | <0.001 |
| Prothrombin time, INR | 1.2 ± 0.4 | 1.2 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.4 | <0.001 |
Note: All continuous variables are expressed as mean and standard deviation. All categorical variables are expressed as number and percentage. The median values of creatinine, cystatin C, and total bilirubin are shown with interquartile ranges in parentheses. The median values of creatinine–cystatin C ratio for each quartile are shown with the range in parentheses.
Abbreviations: BUN, blood urea nitrogen; ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate; INR, international normalized ratio.
Cancer staging was excluded in patients with haematologic cancers.
Patient outcomes
A total of 793 (25.9%) and 907 (29.6%) patients died within 6 months and 1 year after cancer diagnosis, respectively. The length of ICU and hospital stay within the first 6 months of cancer diagnosis was 5.3 ± 13.0 days and 29.8 ± 31.6 days, respectively. Trends towards lower 6‐month and 1‐year mortality and shorter length of ICU and hospital stay in the higher creatinine–cystatin C ratio quartiles were significant (all P < 0.01; Table 2).
Table 2.
Patient outcomes according to creatinine–cystatin C ratio quartiles
| Variables | Total (n = 3060) | Quartiles of creatinine–cystatin C ratio | P for trend | |||
|---|---|---|---|---|---|---|
| Q1 (n = 765) | Q2 (n = 765) | Q3 (n = 765) | Q4 (n = 765) | |||
| 6‐month mortality, n (%) | 793 (25.9) | 327 (42.7) | 206 (26.9) | 140 (18.3) | 120 (15.7) | <0.001 |
| 1‐year mortality, n (%) | 907 (29.6) | 352 (46.0) | 235 (30.7) | 174 (22.7) | 146 (19.1) | <0.001 |
| Cumulative length of ICU stay a , days | 5.3 ± 13.0 | 8.3 ± 19.3 | 3.5 ± 5.7 | 4.3 ± 7.5 | 4.2 ± 11.1 | 0.002 |
| Cumulative length of hospital stay a , days | 29.8 ± 31.6 | 37.4 ± 36.1 | 27.6 ± 28.1 | 28.0 ± 29.0 | 26.1 ± 31.3 | <0.001 |
Note: All continuous variables are expressed as mean and standard deviation. All categorical variables are expressed as number and percentage.
Abbreviation: ICU, intensive care unit.
Cumulative lengths of ICU and hospital stays within 6 months after cancer diagnosis. Evaluation was done among patients who survived beyond 6 months of cancer diagnosis.
Association between creatinine–cystatin C ratio and patient outcomes
The Kaplan–Meier plots revealed that cumulative 6‐month and 1‐year survival probabilities were significantly lower for patients in Q1, the lowest creatinine–cystatin C ratio group, compared with those of patients in other quartiles (P < 0.001 by log‐rank test). Cumulative 6‐month and 1‐year survival probabilities for each quartile sequentially improved in quartiles with increasing creatinine–cystatin C ratios (Figure 2). When the relationship between creatinine–cystatin C ratio and mortality was further assessed using multivariate Cox proportional hazards regression analyses, HR for 6‐month and 1‐year mortality was 0.35 (95% CI, 0.28–0.44) and 0.39 (95% CI, 0.32–0.48) per 1 increase in creatinine–cystatin C ratio, respectively (Table 3). When the creatinine–cystatin C ratio was assessed as a categorical variable, HRs for 6‐month and 1‐year mortality were significantly lower in groups with higher creatinine–cystatin C ratios than those in Q1. The HRs for the quartiles decreased successively with increasing creatinine–cystatin C ratio in the quartiles. For Q4, the HR for 6‐month and 1‐year mortality was 0.30 (95% CI, 0.24–0.37) and 0.33 (95% CI, 0.27–0.40) when compared with Q1, respectively. This observed association between creatinine–cystatin C ratio and mortality was maintained even after significant adjustments for potential confounding factors.
Figure 2.
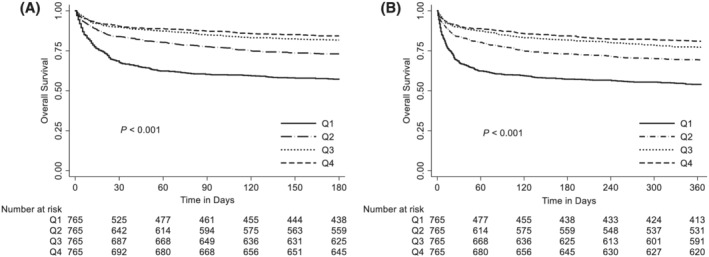
Cumulative survival probability within (A) 6 months and (B) 1 year of cancer diagnosis according to creatinine–cystatin C ratio quartiles. Kaplan–Meier curves of 6‐month and 1‐year survival stratified to creatinine–cystatin C ratio quartiles.
Table 3.
Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine–cystatin C ratio quartiles
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| 6‐month mortality | ||||||
| Creatinine–cystatin C ratio (per 1 increase) | 0.35 (0.28–0.44) | <0.001 | 0.42 (0.33–0.53) | <0.001 | 0.61 (0.49–0.75) | <0.001 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.55 (0.46–0.66) | <0.001 | 0.59 (0.50–0.71) | <0.001 | 0.71 (0.59–0.85) | <0.001 |
| Q3 | 0.35 (0.29–0.43) | <0.001 | 0.40 (0.32–0.49) | <0.001 | 0.52 (0.42–0.65) | <0.001 |
| Q4 | 0.30 (0.24–0.37) | <0.001 | 0.34 (0.27–0.43) | <0.001 | 0.50 (0.40–0.64) | <0.001 |
| 1‐year mortality | ||||||
| Creatinine–cystatin C ratio (per 1 increase) | 0.39 (0.32–0.48) | <0.001 | 0.46 (0.37–0.58) | <0.001 | 0.63 (0.52–0.76) | <0.001 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.58 (0.49–0.68) | <0.001 | 0.62 (0.52–0.74) | <0.001 | 0.72 (0.61–0.86) | <0.001 |
| Q3 | 0.40 (0.33–0.48) | <0.001 | 0.45 (0.38–0.55) | <0.001 | 0.57 (0.47–0.70) | <0.001 |
| Q4 | 0.33 (0.27–0.40) | <0.001 | 0.38 (0.31–0.47) | <0.001 | 0.54 (0.43–0.67) | <0.001 |
Note: Model 1: Unadjusted model. Model 2: Adjusted for age, sex, acute kidney injury, cancer type, cancer stage (IV vs. I–III), cancer treatment, Charlson comorbidity index, and Eastern Cooperative Oncology Group score. Model 3: Model 2, with additional adjustments for systolic blood pressure, haemoglobin, white blood cell count, serum albumin, and prothrombin time.
Abbreviations: CI, confidence interval; HR, hazard ratio.
To account for age as a potential confounding factor, age was matched by 1:1 propensity score matching of the lowest and highest creatinine–cystatin C ratio quartile groups, yielding 622 patients in each group (Table S2). Similar to the results of the primary analysis, 6‐month and 1‐year mortality rates were lower, and ICU and hospital stays were shorter in Q4 than Q1 (all P < 0.05; Table S3). In the Cox proportional hazards models, HR for 6‐month and 1‐year mortality was 0.43 (95% CI, 0.34–0.54) and 0.47 (95% CI, 0.38–0.58) per 1 increase in creatinine–cystatin C ratio, respectively. This relationship was maintained after confounding factor adjustments (Table S4).
Association between creatinine and patient outcomes
When the relationship between creatinine and mortality was assessed, HR for 6‐month and 1‐year mortality was 1.08 (95% CI, 1.05–1.13) and 1.08 (95% CI, 1.04–1.12) per 1 increase in serum creatinine, respectively. However, no significant associations were found in the fully adjusted model (6‐month mortality: HR 0.99, 95% CI 0.94–1.05; 1‐year mortality: HR 0.99, 95% CI 0.94–1.04) (Table S5).
Sensitivity analysis
To confirm that the associations between creatinine–cystatin C ratio and survival were valid regardless of underlying kidney disease, the relationships were re‐evaluated among patients with an eGFR < 60 mL/min/1.73 m2. The HR per 1 increase in the value of creatinine–cystatin C ratio was 0.47 (95% CI, 0.37–0.60) and 0.49 (95% CI, 0.39–0.61) for the 6‐month and 1‐year mortality, respectively. The HRs for mortality decreased successively in the higher creatinine–cystatin C ratio quartiles. For Q4, the HR for 6‐month and 1‐year mortality was 0.25 (95% CI, 0.18–0.36) and 0.26 (95% CI, 0.19–0.36) when compared with Q1, respectively. These associations were maintained even after adjusting for confounding factors (Table S6). The Kaplan–Meier plots revealed that 6‐month and 1‐year cumulative survival probabilities were significantly lower for patients in Q1 than for those in other quartiles (P < 0.001 by log‐rank test). The 6‐month and 1‐year cumulative survival probabilities for each quartile increased sequentially with an increase in the creatinine–cystatin C ratio (Figure S1).
Additional analyses performed in patients with haematologic malignancies revealed similar findings. The HR per 1 increase in creatinine–cystatin C ratio was 0.54 (95% CI, 0.32–0.93) and 0.66 (95% CI, 0.44–1.00) for 6‐month and 1‐year mortality, respectively. The HR for 6‐month and 1‐year mortality for Q4 was 0.45 (95% CI, 0.24–0.84) and 0.54 (95% CI, 0.32–0.94), respectively, when compared with Q1 (Table S7). The Kaplan–Meier plots revealed similar findings, with both the 6‐month (P = 0.01 by log‐rank test) and 1‐year (P = 0.03 by log‐rank test) survival probabilities being the lowest in Q1 (Figure S2). Analyses of patients with non‐haematologic malignancies also revealed comparable results (Table S8 and Figure S3). When Kaplan–Meier analyses were done in patients with gynaecologic malignancies (Figure S4) and in patients with prostate or testicular cancer (Figure S5), the risk of 6‐month and 1‐year mortality was consistently lower in patients allocated to Q1 than other quartiles (all P < 0.001 by log‐rank test).
Analysis made in 2036 (66.5%) patients with available ECOG scores also revealed similar findings. The adjusted HR per 1 increase in creatinine–cystatin C ratio was each 0.71 (95% CI, 0.55–0.90) and 0.74 (95% CI, 0.60–0.91) for 6‐month and 1‐year mortality, respectively (Table S9).
Subgroup analysis
In order to assess the effect of chemotherapy on the relationship between creatinine–cystatin C ratio and outcome, subgroup analyses were performed regarding chemotherapy administration in patients with gastrointestinal cancer. Similar to the results of the main analysis, the risk of 1‐year mortality significantly decreased with increases in the creatinine–cystatin C ratio in gastrointestinal cancer patients. When further subgroup analyses were made regarding whether chemotherapy had been administered, no significant interaction was found between chemotherapy treatment history and creatinine–cystatin C ratio (P for interaction = 0.96; Table S10).
Creatinine–cystatin C ratio and mortality risk reclassification and discrimination
The C‐statistics of the creatinine–cystatin C ratio was 0.642 (95% CI, 0.618–0.660) and 0.628 (95% CI, 0.606–0.650) for 6‐month and 1‐year mortality, respectively. In comparison, the C‐statistics of creatinine alone for 6‐month and 1‐year mortality was 0.530 (95% CI, 0.503–560) and 0.526 (95% CI, 0.501–0.551), respectively. The addition of the creatinine–cystatin C ratio to conventional parameters significantly improved the predictive performance. The 6‐month mortality NRI was 0.214 (95% CI, 0.107–0.255), 0.160 (95% CI, 0.064–0.207), and 0.157 (95% CI, 0.031–0.216) for haemoglobin, serum albumin, and the ECOG score, respectively. In addition, the 6‐month mortality IDI for haemoglobin, serum albumin, and the ECOG score was 0.030 (95% CI, 0.007–0.050), 0.014 (95% CI, 0.004–0.027), and 0.024 (95% CI, 0.006–0.039), respectively (Table S11).
Discussion
In this study, creatinine–cystatin C ratio measured at the time of cancer diagnosis was associated with both 6‐month and 1‐year survival, as well as lengths of ICU and hospital stays. The HRs for 6‐month and 1‐year mortality decreased gradually with increasing creatinine–cystatin C ratio values. In addition, ICU and hospital stay durations were shorter in those in the higher creatinine–cystatin C ratio groups. The highest creatinine–cystatin C ratio quartile exhibited approximately 30% lower risk of both 6‐month and 1‐year mortality, when compared with the lowest quartile. This association was maintained even after adjusting for potential confounding factors, including demographic factors, comorbidities, cancer type, stage, treatment modality, performance status, and clinically relevant laboratory parameters.
Creatinine–cystatin C ratio, measured at the time of cancer diagnosis, was clearly associated with cancer patient outcomes. An increase of 1 unit in the creatinine–cystatin C ratio value was related to a 65% decrease in risk of 6‐month mortality. This association between creatinine–cystatin C ratio and outcome has been previously noticed in several other patient groups. In AKI patients undergoing kidney replacement therapy and cardiac surgery patients, the creatinine–cystatin C ratio effectively predicted mortality. 20 , 21 In addition, creatinine–cystatin C ratio was a significant predictor of hospitalization among patients with chronic obstructive pulmonary disease 22 and an independent predictor of functional outcomes in neurocritically ill patients. 23 The findings of this study show that the outcome predictive quality of creatinine–cystatin C ratio found in these patient populations could also be applicable to patients with cancer. In cancer patients, prognosis is usually determined by cancer‐specific factors such as cancer type, stage, grade, and genetic traits. However, general features that affect overall health, such as performance and nutritional status, are also factors that significantly affect outcomes in patients with cancer. 24 , 25 While considerable advances have been made regarding prognosis stratification using cancer‐specific factors, practical and effective tools for assessing general health among cancer patients are lacking. In this study, the creatinine–cystatin C ratio showed a clear association with mortality as well as hospital duration regardless of age, sex, cancer type, stage, applied treatment modalities, and performance status. Collectively, creatinine–cystatin C ratio may be considered as a universally applicable, easy to obtain, and efficient prognosis assessment method in patients with cancer.
On the other hand, creatinine–cystatin C ratio, in addition to being a prognosis predictive indicator, may also be a surrogate reflecting treatment‐related toxicity. The observed decreases in cumulative lengths of ICU and hospital stays with increases in creatinine–cystatin C ratio could also be suggesting a close connection between creatinine–cystatin C ratio and frailty. Patients with sarcopenia, as indicated by low serum creatinine levels, are candidates of miscalculations in chemotherapy doses. They are prone to receiving higher doses of chemotherapeutic agents than needed due to kidney function overestimation. 26 Such increases in risk of chemotherapy‐related toxicities could have partly contributed to the poorer survival in patients with lower creatinine–cystatin C ratios. Future prospective investigations would be needed to further determine the cause–effect relationship of the creatinine–cystatin C ratio and outcomes.
Serum creatinine and cystatin C are widely used laboratory values for determining kidney function. Typically, with kidney function impairment, the amount of creatinine and cystatin C cleared through the kidney decreases, increasing their serum levels. However, under kidney injury conditions, creatinine and cystatin C also exhibit variable clearance kinetics, subsequently causing their levels to vary. This variability in serum levels of patients with decreased kidney function could affect the relationship between creatinine–cystatin C ratio and outcome in patients. 27 Nonetheless, the creatinine–cystatin C ratio and mortality association was significant even after adjusting for underlying AKI, suggesting that the relationship with prognosis is independent of kidney injury status. Moreover, in a sensitivity analysis of cancer patients with eGFR < 60 mL/min/1.73 m2, mortality risk gradually reduced in patients with increased creatinine–cystatin C ratio. These findings support the possibility that creatinine–cystatin C ratio could be considered, irrespective of kidney function status, when assessing prognosis of cancer patients.
One of the possible explanations for the relationship between creatinine–cystatin C ratio and prognosis in patients with cancer is that the creatinine–cystatin C ratio represents muscle mass, 13 which is a well‐known risk factor affecting outcomes in various patient populations. Survival rates have been reported to be low in chronic lung disease patients and patients receiving intensive care with reduced muscle mass. 13 , 14 , 28 A recent study showing that creatinine–cystatin C ratio correlated significantly with CT and bioelectrical impedance analysis assessed muscle mass in 44 patients with cancer further supports this hypothesis. 29 In addition to its correlation with muscle mass, serum creatinine levels are low in individuals with high white blood cell counts, 30 and increased cystatin C levels have been observed in chronic inflammatory states. 31 , 32 Therefore, creatinine–cystatin C ratio could also be a representation of inflammatory status. Given that cancer cell‐driven inflammatory cytokines promote neoplastic spread and metastasis, 33 the creatinine–cystatin C ratio and prognosis relationship may be mediated through inflammation. Another possible explanation for the creatinine–cystatin C ratio and outcome association could be related to the fact that cystatin C may reflect tumour burden. 34 , 35 , 36 High levels of cystatin C were found in tissue samples from colon cancer 34 and breast cancer, 35 whereas low levels of cystatin C were expressed in benign tissues. 37 The elevated cystatin C expression in these cancers regulates cathepsin B, a lysosomal cysteine protease, which promotes cancer invasion and basement membrane destruction. 38
This study has several limitations. First, due to the retrospective nature of the study, the independent association between creatinine–cystatin C ratio and survival should be interpreted with caution. Differences in treatment modalities applied to the patients could have introduced effects that were unaccounted for. Second, nutritional and inflammatory status were not considered. Given that cancer patients are often in a state of malnutrition and serum creatinine levels could be affected by nutritional status, further evaluations on nutritional and inflammatory state‐related factors such as weight loss and serum C‐reactive protein levels could help better understand the association between creatinine–cystatin C ratio and outcomes in patients with cancer. Finally, most participants in this study were Asian. Given that serum creatinine concentrations may vary across different ethnicities, 39 further validation of the prognostic significance of the creatinine–cystatin C ratio in patients with cancer belonging to other ethnicities would be needed.
In conclusion, the creatinine–cystatin C ratio at the time of cancer diagnosis was significantly associated with survival and hospitalization in patients with cancer. This relationship remained valid regardless of cancer type, stage, and treatment modality. Creatinine–cystatin C ratio could be considered as a potentially useful prognostic factor in patients diagnosed with cancer. However, further validations would be needed for its generalized application.
Conflict of interests
The authors have no conflict of interests.
Funding
None.
Supporting information
Table S1. Types of missing data
Table S2. Baseline characteristics of patients in the lowest and highest creatinine‐cystatin C ratio quartile after propensity score matching for age
Table S3. Patient outcomes of patients in the lowest and highest creatinine‐cystatin C ratio quartile after propensity score matching for age
Table S4. Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality of patients in the lowest and highest creatinine‐cystatin C ratio quartile after propensity score matching for age
Table S5. Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine quartiles
Table S6. Sensitivity analysis: Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine‐cystatin C ratio quartiles in patients with eGFR <60 mL/min/1.73 m2
Table S7. Sensitivity analysis: Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine‐cystatin C ratio quartiles in patients with hematologic malignancies
Table S8. Sensitivity analysis: Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine‐cystatin C ratio quartiles in patients with non‐hematologic malignancies
Table S9. Sensitivity analysis: Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine‐cystatin C ratio quartiles in patients with available ECOG scores
Table S10. Effect of creatinine‐cystatin C ratio (per 1 increase) on the risk of 1‐year mortality in patients who did and did not receive chemotherapy in patients with gastrointestinal malignancies
Table S11. Improvement of reclassification and discrimination of mortality risk with addition of the creatinine‐cystatin C ratio to baseline hemoglobin, serum albumin and ECOG
Figure S1. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with eGFR <60 mL/min/1.73 m2
Figure S2. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with hematologic malignancies
Figure S3. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with non‐hematologic malignancies
Figure S4. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with gynecologic malignancies
Figure S5. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with prostate or testicular cancer
Acknowledgements
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 40
Jung C.‐Y., Kim H. W., Han S. H., Yoo T.‐H., Kang S.‐W., and Park J. T. (2022) Creatinine–cystatin C ratio and mortality in cancer patients: a retrospective cohort study, Journal of Cachexia, Sarcopenia and Muscle, 13, 2064–2072, 10.1002/jcsm.13006
References
- 1. Fearon K, Arends J, Baracos VE. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–99. [DOI] [PubMed] [Google Scholar]
- 2. Baracos VE. Cancer‐associated cachexia and underlying biological mechanisms. Annu Rev Nutr 2006;26:435–461. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt SF, Rohm M, Herzig S, Diaz MB. Cancer cachexia: more than skeletal muscle wasting. Trends Cancer 2018;4:849–860. [DOI] [PubMed] [Google Scholar]
- 4. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon K. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;18:17105. [DOI] [PubMed] [Google Scholar]
- 5. Rosa‐Caldwell ME, Fix DK, Washington TA, Greene NP. Muscle alterations in the development and progression of cancer‐induced muscle atrophy: a review. J Appl Physiol 2020;128:25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun L, Quan XQ, Yu S. An epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutr Cancer 2015;67:1056–1062. [DOI] [PubMed] [Google Scholar]
- 7. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 8. Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 2017;28:2107–2118. [DOI] [PubMed] [Google Scholar]
- 9. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta‐analysis. Ann Surg 2018;268:58–69. [DOI] [PubMed] [Google Scholar]
- 10. Roeland EJ, Bohlke K, Baracos VE, Bruera E, Fabbro ED, Dixon S, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol 2020;38:2438–2453. [DOI] [PubMed] [Google Scholar]
- 11. Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle 2013;4:95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aversa Z, Costelli P, Muscaritoli M. Cancer‐induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol 2017;9:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kashani KB, Frazee EN, Kukrálová L, Sarvottam K, Herasevich V, Young PM, et al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med 2017;45:e23–e29. [DOI] [PubMed] [Google Scholar]
- 14. Barreto EF, Poyant JO, Coville HH, Dierkhising RA, Kennedy CC, Gajic O, et al. Validation of the sarcopenia index to assess muscle mass in the critically ill: a novel application of kidney function markers. Clin Nutr 2019;38:1362–1367. [DOI] [PubMed] [Google Scholar]
- 15. Tetsuka S, Morita M, Ikeguchi K, Nakano I. Creatinine/cystatin C ratio as a surrogate marker of residual muscle mass in amyotrophic lateral sclerosis. Neurol Clin Neurosci 2013;1:32–37. [Google Scholar]
- 16. Fu X, Tian Z, Wen S, Sun H, Thapa S, Xiong H, et al. A new index based on serum creatinine and cystatin C is useful for assessing sarcopenia in patients with advanced cancer. Nutrition 2021;82:111032. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zubrod CG, Schneiderman M, Frei E III, Brindley C, Gold GL, Shnider B, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chron Dis 1960;11:7–33. [Google Scholar]
- 20. Jung CY, Joo YS, Kim HW, Han SH, Yoo TH, Kang SW, et al. Creatinine‐cystatin C ratio and mortality in patients receiving intensive care and continuous kidney replacement therapy: a retrospective cohort study. Am J Kidney Dis 2021;77:509–516. [DOI] [PubMed] [Google Scholar]
- 21. Herou E, Dardashti A, Nozohoor S, Zindovic I, Ederoth P, Grubb A, et al. The mortality increase in cardiac surgery patients associated with shrunken pore syndrome correlates with the eGFR cystatin C/eGFR creatinine ratio. Scand J Clin Lab Invest 2019;79:167–173. [DOI] [PubMed] [Google Scholar]
- 22. Amado CA, García‐Unzueta MT, Lavin BA, Guerra AR, Agüero J, Ramos L, et al. The ratio serum creatinine/serum cystatin C (a surrogate marker of muscle mass) as a predictor of hospitalization in chronic obstructive pulmonary disease outpatients. Respiration 2019;97:302–309. [DOI] [PubMed] [Google Scholar]
- 23. Wang S, Xie L, Xu J, Hu Y, Wu Y, Lin Z, et al. Predictive value of serum creatinine/cystatin C in neurocritically ill patients. Brain Behav 2019;9:e01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin L, Watanabe S, Fainsinger R, Lau F, Ghosh S, Quan H, et al. Prognostic factors in patients with advanced cancer: use of the patient‐generated subjective global assessment in survival prediction. J Clin Oncol 2010;28:4376–4383. [DOI] [PubMed] [Google Scholar]
- 25. Laird BJ, Kaasa S, McMillan DC, Fallon MT, Hjermstad MJ, Fayers P, et al. Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation‐based prognostic system. Clin Cancer Res 2013;19:5456–5464. [DOI] [PubMed] [Google Scholar]
- 26. Bretagne M, Jouinot A, Durand JP, Huillard O, Boudou Rouquette P, Tlemsani C, et al. Estimation of glomerular filtration rate in cancer patients with abnormal body composition and relation with carboplatin toxicity. Cancer Chemother Pharmacol 2017;80:45–53. [DOI] [PubMed] [Google Scholar]
- 27. Ravn B, Prowle JR, Mårtensson J, Martling CR, Bell M. Superiority of serum cystatin C over creatinine in prediction of long‐term prognosis at discharge from ICU. Crit Care Med 2017;45:e932–e940. [DOI] [PubMed] [Google Scholar]
- 28. Mador MJ. Muscle mass, not body weight, predicts outcome in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:787–789. [DOI] [PubMed] [Google Scholar]
- 29. Ulmann G, Kaï J, Durand JP, Neveux N, Jouinot A, De Bandt JP, et al. Creatinine‐to‐cystatin C ratio and bioelectrical impedance analysis for the assessment of low lean body mass in cancer patients: comparison to L3‐computed tomography scan. Nutrition 2021;81:110895. [DOI] [PubMed] [Google Scholar]
- 30. Reddan DN, Klassen PS, Szczech LA, Coladonato JA, O'Shea S, Owen WF Jr, et al. White blood cells as a novel mortality predictor in hemodialysis patients. Nephrol Dial Transplant 2003;18:1167–1173. [DOI] [PubMed] [Google Scholar]
- 31. Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004;65:1416–1421. [DOI] [PubMed] [Google Scholar]
- 32. Keller CR, Odden MC, Fried LF, Newman AB, Angleman S, Green CA, et al. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int 2008;71:239–244. [DOI] [PubMed] [Google Scholar]
- 33. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:P883–P899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kos J, Krasovec M, Cimerman N, Nielsen HJ, Christensen IJ, Brunner N. Custeine proteinase inhibitors stefin‐A, stefin‐B, and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res 2020;6:505–511. [PubMed] [Google Scholar]
- 35. Kwon WS, Kim TS, Nahn CH, Moon Y, Kim JJ. Aberrant cystatin‐C expression in blood from patients with breast cancer is a suitable marker for monitoring tumor burden. Oncol Lett 2018;16:5583–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang Y, Zhang J, Zhang C, Hong L, Jiang Y, Ling L, et al. The role of cystatin C as a proteasome inhibitor in multiple myeloma. Hematology 2020;25:457–463. [DOI] [PubMed] [Google Scholar]
- 37. Nishikawa H, Ozaki Y, Nakanishi T, Blomgren K, Tada T, Arakawa A, et al. The role of cathepsin B and cystatin C in the mechanisms of invasion by ovarian cancer. Gynecol Oncol 2004;92:881–886. [DOI] [PubMed] [Google Scholar]
- 38. Corticchiato O, Cajot JF, Abrahamson M, Chan SJ, Keppler D, Sordat B. Cystatin C and cathepsin B in human colon carcinoma: expression by cell lines and matrix degradation. Int J Cancer 1992;52:645–652. [DOI] [PubMed] [Google Scholar]
- 39. Hsu J, Johansen KL, Hsu C, Kaysen GA, Chertow GM. Higher serum creatinine concentrations in Black patients with chronic kidney disease: beyond nutritional status and body composition. Clin J Am Soc Nephrol 2008;3:992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Types of missing data
Table S2. Baseline characteristics of patients in the lowest and highest creatinine‐cystatin C ratio quartile after propensity score matching for age
Table S3. Patient outcomes of patients in the lowest and highest creatinine‐cystatin C ratio quartile after propensity score matching for age
Table S4. Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality of patients in the lowest and highest creatinine‐cystatin C ratio quartile after propensity score matching for age
Table S5. Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine quartiles
Table S6. Sensitivity analysis: Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine‐cystatin C ratio quartiles in patients with eGFR <60 mL/min/1.73 m2
Table S7. Sensitivity analysis: Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine‐cystatin C ratio quartiles in patients with hematologic malignancies
Table S8. Sensitivity analysis: Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine‐cystatin C ratio quartiles in patients with non‐hematologic malignancies
Table S9. Sensitivity analysis: Hazard ratios and 95% confidence intervals for 6‐month and 1‐year mortality based on creatinine‐cystatin C ratio quartiles in patients with available ECOG scores
Table S10. Effect of creatinine‐cystatin C ratio (per 1 increase) on the risk of 1‐year mortality in patients who did and did not receive chemotherapy in patients with gastrointestinal malignancies
Table S11. Improvement of reclassification and discrimination of mortality risk with addition of the creatinine‐cystatin C ratio to baseline hemoglobin, serum albumin and ECOG
Figure S1. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with eGFR <60 mL/min/1.73 m2
Figure S2. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with hematologic malignancies
Figure S3. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with non‐hematologic malignancies
Figure S4. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with gynecologic malignancies
Figure S5. Cumulative survival probability within (A) 6‐months, and (B) 1‐year of cancer diagnosis according to creatinine‐cystatin C ratio quartiles in patients with prostate or testicular cancer


