Abstract
Membrane growth requires lipid supply, which is usually accomplished by lipid synthesis or vesicular trafficking. In the case of autophagosomes, these principles do not apply. Ghanbarpour et al. postulate that autophagosome expansion relies on non-vesicular lipid delivery from the ER, whereby the activity of a lipid transfer protein (LTP) is directly coupled to scramblase activities in the donor and acceptor bilayers1. This new concept opens the possibility that lipid traffic is controlled by scramblases that provide not only specific docking sites for LTPs, thereby directing lipid flow, but also support their activity by overcoming barriers for lipid extraction and deposition.
Keywords: Scramblase, membrane, lipid, autophagosome, lipid transfer protein
Background
Lipid transport within cells is an essential process, not least because lipids must be distributed to various cellular membranes from their principal biosynthetic source, the endoplasmic reticulum (ER). Because lipids are amphipathic, they are generally unable to flip spontaneously across a hydrophobic membrane bilayer or cross the aqueous cytoplasm from one membrane compartment to another at an appreciable rate. Yet a fast and specific distribution of lipids is crucial for cell survival as well as the ability of cells to respond to various stimuli. For this, lipid transport catalysts are needed.
Ghanbarpour et al.1 focus on the biogenesis of autophagosomes, cup-shaped organelles that are formed by the cell to capture cellular material for degradation. The mechanism by which the autophagosome grows from a Golgi-derived seeding vesicle is unclear. However, vesicle-mediated supply of membrane lipids is unlikely, as membrane surface expansion is not accompanied by expansion of luminal volume. Recent work indicates that autophagosome expansion relies on the lipid transfer protein ATG22–4, which operates between the ER and autophagosome membrane, providing a hydrophobic slide for lipid movement, whereby lipid tails engage the slide while their headgroups remain in the aqueous cytoplasm5. However, ATG2-mediated lipid transfer from the cytoplasmic face of the donor to the acceptor bilayer would create imbalances in the transbilayer distribution of lipids, eventually stalling the process unless corrected. The ER is equipped with scramblases, membrane proteins that facilitate bidirectional flip-flop of lipids across a bilayer and which are therefore capable of normalizing the lipid number between the two leaflets of its bilayer6. However, the autophagosome has only a few membrane proteins, including ATG9. Ghanbarpour et al.1 show that ATG9 is a scramblase and identify TMEM41B and/or VMP1 as the corresponding scramblases in the ER.
Main contributions and importance
Ghanbarpour et al. conceptualize the action of the LTP ATG2 in the context of scramblases located in the ER and nascent autophagosome. Here, we highlight their two main contributions.
First, the identification of TMEM41B/VMP1 and ATG9A as scramblases in this1 and related papers7–11 is highly significant. The molecular identity of an ER phospholipid scramblase(s) has been the target of scientific investigations for more than three decades due to the importance of scramblase activity for growth of the ER membrane bilayer12,13. Biochemical reconstitution studies indicated that at least two ER proteins are independently responsible for scrambling, based on selective inhibition by protein modification reagents14,15, but stopped short of identifying the proteins. TMEM41B/VMP1 are therefore the first proteins of the ER to be unambiguously identified as constitutive scramblases via both in vitro and in vivo assays1,8. Found in metazoan cells, they are necessary for autophagy but are unlikely to be the only phospholipid scramblases in the ER. As TMEM41B/VMP1 belong to a protein superfamily sharing the DedA domain, predicted to contain two enigmatic re-entrant loops suggestive of transport function, more proteins from this family might exhibit scramblase activity16–19. Thus, the identification of TMEM41B / VMP1 adds to the collection of known phospholipid scramblases which now includes the ER protein CLPTM1L20, G protein-coupled receptors21, and members of the TMEM16 and Xkr protein families22.
Second, Ghanbarpour et al. establish that scramblases in the ER and autophagosome provide docking sites for ATG2, which serves as a bridge over which lipids flow between the two organelles1. The proposed physical association and functional synergy between these two classes of lipid transporters, scramblases and LTPs, is a new paradigm (Figure 1) with the following implications:
Figure 1. Proposed cooperation between scramblases in the ER (TMEM41B, VMP1) and nascent autophagosomal membrane (ATG9), and the bridging lipid transport protein ATG2.
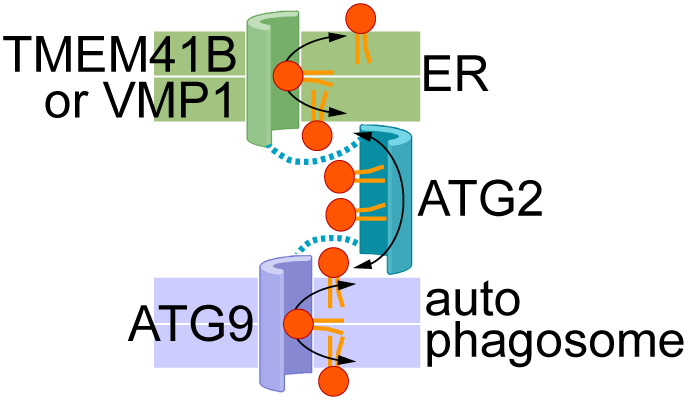
The membrane bilayers are shown as coloured slabs (a white line separates the two halves of each bilayer). Phospholipids are shown generically with a red headgroup and orange acyl chains. Docking of ATG2 to the scramblases is indicated by the dotted lines. The transport proteins are shown according to the credit card model27, with a polar groove in the case of scramblases to accommodate lipid headgroups, and a hydrophobic groove in ATG2 to accommodate lipid tails. Bidirectional flow of lipids is shown by double-headed arrows so that lipids in both leaflets of both membranes are equilibrated by the scramblases and inter-bilayer exchange across the cytoplasm. Lipid transport may be effectively one-directional, with lipids being synthesized in the ER (‘source’) and consumed through expansion of the autophagosomal membrane (‘sink’) (see ‘Open Questions’ section ‘What drives lipids to move towards the acceptor membrane, i.e., the newly forming autophagosome membrane’ for details).
1) Scramblases recruit LTPs to target membranes
ATG2 physically interacts with both sets of scramblases as seen in co-immunoprecipitation experiments. Furthermore, an N-terminal fragment of ATG2 (mini-ATG2), associates with TMEM41B/VMP1-containing liposomes, but not with empty liposomes or those containing ATG9. Thus, the N-terminus of ATG2 docks onto vesicles containing ER scramblases. The generality of LTP-scramblase association is highlighted by recent data7 indicating that VPS13, an LTP in the same family as ATG2, also interacts with scramblases.
2) Scramblases re-equilibrate membrane leaflets after lipids are extracted or inserted by LTPs
The number of lipids on the two sides of a membrane bilayer changes as LTPs extract or introduce lipids at the cytoplasmic face. Development of a lipid number asymmetry could stall lipid flow and membrane expansion. By exchanging lipids across the bilayer, scramblases would prevent build-up of transbilayer lipid number asymmetry, hence facilitating unrestrained lipid flow during membrane expansion.
3) Scramblases channel lipids into or from LTPs
The desorption of lipids from a membrane bilayer is energetically costly23,24. LTPs overcome this energy barrier by directly extracting lipids from membranes into a shielded environment. They also facilitate the reverse process, whereby lipids are deposited into membranes25. Scramblases may promote LTP-mediated lipid extraction/deposition by locally destabilizing/thinning the bilayer19,26. A specific contribution of scramblases to LTP activity would rationalize the need for a direct interaction between these two classes of lipid transporters.
4) Discovery of new scramblases.
The membrane protein interactome of bridging LTPs such as ATG2 and VPS13 may yield the molecular identity of additional scramblases in different subcellular compartments. As noted above, VPS13 also interacts physically with scramblases28,29.
Open questions
The proposed interaction between LTPs and scramblases raises several questions.
1) How does the physical interaction between an LTP and scramblases promote function?
In addition to acting as LTP docking sites, and equilibrating phospholipids across the respective bilayers, scramblases may have a non-canonical role in facilitating lipid movement into and out of the LTP, thereby influencing the kinetics of LTP-mediated lipid transport. Specifically, does the geometry of the LTP-scramblase docking site enable lipid hand-off between the two transport systems?
2) What drives lipids to move towards the acceptor membrane, i.e., the newly forming autophagosome membrane?
The LTP-scramblase system allows lipids to flow in both directions (Figure 1), yet unidirectional transport is needed to expand the autophagosome membrane. The observation that newly synthesized phospholipids are preferentially incorporated into autophagosomes11 hints at a possible mechanism by which this could be accomplished. Suppose that TMEM41B/VMP1 proteins are localized to a specialized region of the ER that is enriched in phospholipid biosynthetic enzymes (analogous to the mitochondria-associated membrane30) and surrounded by a lateral diffusion barrier that slows lipid escape into the bulk ER. The resulting build-up of newly synthesized phospholipids in this region would drive their export to the autophagosome via ATG2. According to this scenario, binding of TMEM41B/VMP1 would promote lipid entry into the proximal (ER) end of the ATG2 groove. ATG9 bound at the distal end of the groove would facilitate incorporation of the transported lipids into the autophagosomal membrane and mediate lipid equilibration across the bilayer to allow membrane expansion.
3) Is the coupling between LTPs and scramblases required for autophagosome biogenesis?
An ATG2 variant that cannot interact with ATG9 was sufficient to rescue the phenotype of ATG2 depleted cells1,3. This could mean that the interaction can either be complemented by other factors, the lipid transport efficiency can be affected outside of the experiment’s detection limitation, or the scramblase has no detectable influence on the inter-membrane lipid transport function of ATG2 in resting cells but becomes important under certain physiological conditions.
4) Is the LTP-scramblase interaction model broadly important?
Ghanbarpour et al.1 describe the alliance of an LTP and scramblases in the context of autophagosome biogenesis. Recent work indicates that VPS13 also interacts with scramblases28,29. LTP-scramblase interaction would be important for the expansion of any cellular membrane system that is not reliably served by vesicular transport, and where LTP efficiency is improved by docking onto a scramblase that acts as a cofactor in lipid handling. Ghanbarpour et al.1 speculate that LTP-scramblase partnerships may play a role in the formation of prospores, acrosomes, lipoprotein particles and viral replication centers.
Conclusion
Ghanbarpour et al. provide a new conceptual framework whereby docking of the ends of an LTP onto scramblases serves to maintain a balanced lipid distribution across the bilayers as non-vesicular lipid transport between membranes occurs. Whether the scramblases are simply LTP docking sites or whether they are functionally important when connected to LTPs remains to be seen. It would not be necessary to position a bilayer-normalizing scramblase right at the site of lipid extraction and deposition by an LTP unless the scramblase also facilitates LTP action. Future research will address these questions while evaluating the importance of scramblases for efficient inter-membrane lipid transport in autophagosome biogenesis and beyond.
References
- 1. Ghanbarpour A, Valverde DP, Melia TJ, Reinisch KM. 2021. A model for a partnership of lipid transfer proteins and scramblases in membrane expansion and organelle biogenesis Proc Natl Acad Sci U S A 118:e2101562118. 10.1073/pnas.2101562118 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 2. Osawa T, Kotani T, Kawaoka T, Hirata E, Suzuki K, Nakatogawa H, Ohsumi Y, Noda NN. 2019. Atg2 mediates direct lipid transfer between membranes for autophagosome formation Nat Struct Mol Biol 26:281–288. 10.1038/s41594-019-0203-4 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 3. Valverde DP, Yu S, Boggavarapu V, Kumar N, Lees JA, Walz T, Reinisch KM, Melia TJ. 2019. ATG2 transports lipids to promote autophagosome biogenesis J Cell Biol 218:1787–1798. 10.1083/jcb.201811139 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 4. Maeda S, Otomo C, Otomo T. 2019. The autophagic membrane tether ATG2A transfers lipids between membranes eLife 8:e45777. 10.7554/eLife.45777 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 5. Wong LH, Gatta AT, Levine TP. 2019. Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes Nat Rev Mol Cell Biol 20:85–101. 10.1038/s41580-018-0071-5 [DOI] [PubMed] [Google Scholar]
- 6. Pomorski TG, Menon AK. 2016. Lipid somersaults: Uncovering the mechanisms of protein-mediated lipid flipping Prog Lipid Res 64:69–84. 10.1016/j.plipres.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang D, Xu B, Liu L, Wu L, Zhu Y, Ghanbarpour A, Wang Y, Chen FJ, Hu Y, Kang Y, Zhou W, Wang X, Ding W, Li X, Jiang Z, Chen J, Zhang X, Zhou H, Li JZ, Guo C, Zheng W, Zhang X, Li P, Melia T, Reinisch K, Chen XW. 2021. TMEM41B acts as an ER scramblase required for lipoprotein biogenesis and lipid homeostasis Cell Metab 33:1655–1670.e8. 10.1016/j.cmet.2021.05.006 [DOI] [PubMed] [Google Scholar]
- 8. Li YE, Wang Y, Du X, Zhang T, Mak HY, Hancock SE, McEwen H, Pandzic E, Whan RM, Aw YC, Lukmantara IE, Yuan Y, Dong X, Don A, Turner N, Qi S, Yang H. 2021. TMEM41B and VMP1 are scramblases and regulate the distribution of cholesterol and phosphatidylserine J Cell Biol 220:e202103105. 10.1083/jcb.202103105 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 9. Maeda S, Yamamoto H, Kinch LN, Garza CM, Takahashi S, Otomo C, Grishin NV, Forli S, Mizushima N, Otomo T. 2020. Structure, lipid scrambling activity and role in autophagosome formation of ATG9A Nat Struct Mol Biol 27:1194–1201. 10.1038/s41594-020-00520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 10. Matoba K, Kotani T, Tsutsumi A, Tsuji T, Mori T, Noshiro D, Sugita Y, Nomura N, Iwata S, Ohsumi Y, Fujimoto T, Nakatogawa H, Kikkawa M, Noda NN. 2020. Atg9 is a lipid scramblase that mediates autophagosomal membrane expansion Nat Struct Mol Biol 27:1185–1193. 10.1038/s41594-020-00518-w [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 11. Orii M, Tsuji T, Ogasawara Y, Fujimoto T. 2021. Transmembrane phospholipid translocation mediated by Atg9 is involved in autophagosome formation J Cell Biol 220:e202009194. 10.1083/jcb.202009194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bishop WR, Bell RM. 1985. Assembly of the endoplasmic reticulum phospholipid bilayer: the phosphatidylcholine transporter Cell 42:51–60. 10.1016/s0092-8674(85)80100-8 [DOI] [PubMed] [Google Scholar]
- 13. Menon AK, Watkins WE, 3rd, Hrafnsdóttir S. 2000. Specific proteins are required to translocate phosphatidylcholine bidirectionally across the endoplasmic reticulum Curr Biol 10:241–52. 10.1016/s0960-9822(00)00356-0 [DOI] [PubMed] [Google Scholar]
- 14. Chang QI, Gummadi SN, Menon AK. 2004. Chemical modification identifies two populations of glycerophospholipid flippase in rat liver ER Biochemistry 43:10710–8. 10.1021/bi049063a [DOI] [PubMed] [Google Scholar]
- 15. Vehring S, Pakkiri L, Schröer A, Alder-Baerens N, Herrmann A, Menon AK, Pomorski T. 2007. Flip-flop of fluorescently labeled phospholipids in proteoliposomes reconstituted with Saccharomyces cerevisiae microsomal proteins Eukaryot Cell 6:1625–34. 10.1128/EC.00198-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okawa F, Hama Y, Zhang S, Morishita H, Yamamoto H, Levine TP, Mizushima N. 2021. Evolution and insights into the structure and function of the DedA superfamily containing TMEM41B and VMP1 J Cell Sci 134:jcs255877. 10.1242/jcs.255877 [DOI] [PubMed] [Google Scholar]
- 17. Hama Y, Morishita H, Mizushima N. 2022. Regulation of ER-derived membrane dynamics by the DedA domain-containing proteins VMP1 and TMEM41B EMBO Rep 23:e53894. 10.15252/embr.202153894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mesdaghi S, Murphy DL, Sánchez Rodríguez F, Burgos-Mármol JJ, Rigden DJ. 2020. In silico prediction of structure and function for a large family of transmembrane proteins that includes human Tmem41b [version 2; peer review: 2 approved, 1 approved with reservations] F1000Res 9:1395. 10.12688/f1000research.27676.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reinisch KM, Chen XW, Melia TJ. 2021. "VTT"-domain proteins VMP1 and TMEM41B function in lipid homeostasis globally and locally as ER scramblases Contact (Thousand Oaks) 4. 10.1177/25152564211024494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Menon AK, Maki Y, Liu YS, Iwasaki Y, Fujita M, Guerrero PA, Silva DV, Seeberger PH, Murakami Y, Kinoshita T. 2022. Genome-wide CRISPR screen reveals CLPTM1L as a lipid scramblase required for efficient glycosylphosphatidylinositol biosynthesis Proc Natl Acad Sci U S A 119:e2115083119. 10.1073/pnas.2115083119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khelashvili G, Menon AK. 2022. Phospholipid Scrambling by G Protein-Coupled Receptors Annu Rev Biophys 51:39–61. 10.1146/annurev-biophys-090821-083030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagata S, Suzuki J, Segawa K, Fujii T. 2016. Exposure of phosphatidylserine on the cell surface Cell Death Differ 23:952–61. 10.1038/cdd.2016.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLean LR, Phillips MC. 1981. Mechanism of cholesterol and phosphatidylcholine exchange or transfer between unilamellar vesicles Biochemistry 20:2893–900. 10.1021/bi00513a028 [DOI] [PubMed] [Google Scholar]
- 24. Dittman JS, Menon AK. 2017. Speed Limits for Nonvesicular Intracellular Sterol Transport Trends Biochem Sci 42:90–97. 10.1016/j.tibs.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khelashvili G, Chauhan N, Pandey K, Eliezer D, Menon AK. 2019. Exchange of water for sterol underlies sterol egress from a StARkin domain eLife 8:e53444. 10.7554/eLife.53444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrungaro C, Kornmann B. 2019. Lipid exchange at ER-mitochondria contact sites: a puzzle falling into place with quite a few pieces missing Curr Opin Cell Biol 57:71–76. 10.1016/j.ceb.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 27. Pomorski T, Menon AK. 2006. Lipid flippases and their biological functions Cell Mol Life Sci 63:2908–21. 10.1007/s00018-006-6167-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adlakha J, Hong Z, Li P, Reinisch KM. 2022. Structural and biochemical insights into lipid transport by VPS13 proteins J Cell Biol 221:e202202030. 10.1083/jcb.202202030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bean BDM, Dziurdzik SK, Kolehmainen KL, Fowler CMS, Kwong WK, Grad LI, Davey M, Schluter C. 2018. Competitive organelle-specific adaptors recruit Vps13 to membrane contact sites J Cell Biol 217:3593–3607. 10.1083/jcb.201804111 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 30. Vance JE. 1980. Phospholipid synthesis in a membrane fraction associated with mitochondria J Biol Chem 265:7248–56. [PubMed] [Google Scholar]


