INTRODUCTION
Approximately 18% of healthy women tested have positive IgG anti-nuclear antibodies (ANA), and most never develop a clinical autoimmune disease [1]. In healthy individuals, most autoantibodies are naturally occurring and non-pathogenic; however, in patients with systemic lupus erythematosus (SLE or lupus), pathogenic autoantibodies are produced [e.g., lupus nephritis is partially mediated by anti-double-stranded DNA (dsDNA) antibodies] many years before clinical diagnosis [2]. However, the mechanisms underlying the stimulus for natural versus pathogenic autoantibody production remains undefined.
Translocation of Lactobacillus reuteri exacerbates disease progression in lupus-prone mice via toll-like receptor (TLR) 7 [3]. Translocation of commensal Enterococcus gallinarum from gut to the liver induced type I interferon (IFN) and anti-dsDNA antibody [4]. Previous studies from others and us indicate a possible role of plasma microbial translocation likely through a compromised mucosal barrier, in autoantibody production in SLE [4, 5]. Staphylococcus aureus is a commensal bacterium that mainly colonizes nasal, skin, and other mucosal membranes [6]. The percentage of S. aureus nasal colonization was similar in healthy controls and those with SLE (~20%), but greater in patients with skin lesions (~50%) and associated with renal disease and autoantibody positivity in patients [7, 8]. Chronic exposure to type I IFNs disrupts the skin barrier of lupus patients [8], which may promote systemic microbial translocation (e.g., S. aureus). Previously, we intraperitoneally (i.p.) injected non-vital S. aureus inducing anti-dsDNA autoantibody production, but not kidney disease in C57BL/6 mice [9]. We thus pursued studies presented in this manuscript to define mechanisms by which S. aureus induces autoantibody production.
Peptidoglycan (PGN) is a part of the bacterial cell wall and highly antigenic due to conserved structural molecular motifs unique to a bacterium [10]. PGNs from different bacteria can uniquely modulate immune activities [11]. Physiologically, without an obvious infection, PGN can be detected in the circulation of healthy individuals [12, 13].
Healthy individuals all have low levels of natural non-pathogenic autoantibodies that differ from pathogenic autoantibodies [14]. The mechanisms underlying natural autoantibodies versus induced pathogenic autoantibodies is incompletely understood. Here, we found that S. aureus PGN induces a sustained anti-dsDNA autoantibody response in mice with immune complex glomerulonephritis, whereas B. subtilis PGN induces a short-term anti-dsDNA response with no development of renal disease.
MATERIALS AND METHODS
Mice
Female C57BL/6 and MRL/lpr mice (Jackson Laboratories, Bar Harbor, ME, USA) were housed at the Medical University of South Carolina vivarium (MUSC). Mice were i.p. injected with PBS, S. aureus PGN, or B. subtilis PGN (100 μg/time) (InvivoGen, San Diego, CA). C57BL/6 mice (6-week-old) were treated with PGNs and PBS twice per week for 8 weeks; then the treatment was stopped for another 8 weeks. MRL/lpr lupus-prone mice (6-week-old) were treated with PBS and PGNs twice per week for 12 weeks.
ELISA
The methods were described previously [15]. Calf thymus dsDNA (5 μg/mL, Sigma-Aldrich, St. Louis, MO, USA) and peroxidase-labeled goat anti-mouse IgG (γ) (SeraCare, Milford, MA, USA) or anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (SouthernBiotech, Birmingham, AL, USA) was used. Serum IgG ANA levels were quantified using lysates of Hep-2 laryngeal carcinoma cells (ATCC, Manassas, VA, USA).
Kidney pathology
At the end of the study in MRL/lpr mice (18-week-old), one kidney was frozen, sectioned, and stained with anti-mouse IgG or complement C3 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The fluorescent intensities of IgG and C3 were assessed using a microscope (Zeiss Axio Vet. A1) and Image J with 10 random high-power fields (x400) taken from each section. The other kidney was fixed with 10% formalin for H&E staining, examined in a blinded fashion, and graded for glomerular inflammation, proliferation, crescent formation, and necrosis from 0 to 3+ (0: none; 1+: mild; 2+: moderate; and 3+: severe) [16]. Scores from 0 to 3+ were assigned for each of these features in 30 different areas. The median of these areas yielded the final renal pathology score (the maximum score of 18). Crescent formation and necrosis scoring were doubled to reflect the enhanced severity of these lesions.
Flow cytometry analysis of splenic anti-dsDNA IgG+ B cells
The antibodies include anti-CD3-PerCP-Cy5.5, anti-CD4-BV510, anti-CD8a-APC-vio770, anti-F4/80-APC, anti-CD19-BV421, anti-IgG-FITC, biotinylated dsDNA (130 ng/μL), and Streptavidin-PerCP. Cells were analyzed using a BD FACSVerse and FlowJo software. All antibodies are listed in Table S1.
Autoantibody microarray
Human plasma and mouse serum samples (1:50 dilution) were analyzed for 125 autoantibodies using autoantibody microarrays in the Genomics and Microarray Core at the University of Texas Southwestern Medical Center, as described previously [9].
B cell receptor (BCR) sequencing of splenic anti-dsDNA IgG+ B cells
Splenic anti-dsDNA IgG+ B cells were sorted using flow cytometry. Total RNA was extracted [9]. Purified libraries were quantified based on a 1:10,000 (a serial 100-fold dilution) using the Ion Library TaqMan Quantitation kit (ThermoFisher, Waltham, MA). Following the Ion AmpliSeq Mouse BCR IGH-SR (RNA) protocol, equal amounts of sample were loaded on the Ion Chef System for processing, and 530 chip preparations were sequenced on the Ion Gene Studio S5 Prime (Mayo Medical, Henderson, NC, USA).
B cell class switch recombination (CSR)
Unmanipulated splenic B cells were isolated from 8- to 12-week-old female C57BL/6 mice using the Mouse B Cell negative Isolation Kit (STEMCELL Technologies, Cambridge, MA). B cells were labeled with 10 μmol CFSE [17] and stimulated with medium or PGNs (10 μg/mL) in the presence or absence of anti-TLR2 neutralizing antibody or isotype control antibody (100 ng/mL, InvivoGen), or a positive control [LPS; 1 μg/mL of E. coli 055:B5 (Sigma-Aldrich, St. Louis, MO, USA) plus 2 ng/mL of TGF-β1 (R&D Systems, Minneapolis, MN, USA)] for 72 h. Cells were stained with Ghost red 780, B220, and IgG2b, and analyzed for CSR (CFSElowIgG2b+Ghost–B220+) using flow cytometry.
PGN-induced TLR2 activity.
Different concentrations of PGNs were added to mTLR2-HEK blue Reporter cell line (50,000 cells/well) (InvivoGen) for 20 hours in vitro. SEAP was determined using a spectrophotometer.
Human participants
We recruited unrelated controls (n = 25) or lupus patients (n = 32) as defined by the updated American College of Rheumatology classification criteria [18]. All participants were premenopausal females (age ≥18 years). Pregnant or breastfeeding, recent severe illness, contraindications for blood withdrawals, or received antibiotics within the past 90 days were exclusionary. The clinical information is shown in Table S2. Patients were subdivided based on their serum levels of albumin (normal: 3.5–5.0 g/dL versus low: < 3.5 g/dL), urinary protein (normal: < 11.9 mg/dL versus high: ≥ 11.9 mg/dL), and ratio of urinary protein versus creatinine (normal: < 300 versus high ≥ 300).
Plasma levels of S. aureus DNA translocation
The method for determining plasma total bacterial DNA levels by qPCR was described previously [19]. The PCR primers for S. aureus were forward: 5′-TTCGCTACTAGTTGCTTA-3′ and reverse: 5′-GCACTATATACTGTTGGATC-3′.
Statistical analysis
We applied non-parametric Mann-Whitney U tests to compare differences between two groups, and Spearman’s correlation tests to evaluate associations. All tests were two-sided, and P ≤ 0.05 was considered significant.
RESULTS
S. aureus PGN induced a prolonged anti-dsDNA autoantibody response, whereas B. subtilis PGN induced short term production of anti-dsDNA autoantibodies.
We first confirmed the optimal concentrations of bacteria and PGN (Figure S1) [9]. Next, we found that 8-week treatment of both PGNs and inactivated S. aureus produced anti-dsDNA IgG antibodies in C57/B6 mice (Figure 1A). After stopping the treatment for 8 weeks, serum anti-dsDNA IgG levels returned to baseline in the B. subtilis PGN and whole S. aureus groups; however, the levels remained elevated in the S. aureus PGN group (2.87-fold higher versus PBS, Figure 1A). Total serum IgGs did not differ, indicating the anti-dsDNA antibodies were not a manifestation of polyclonal activation in autoreactive and non-autoreactive B cells (Figure 1B). S. aureus PGN increased serum IgG ANA levels starting 4 weeks after start of injections and persisting until 8 weeks after stopping the treatment (Figure S2A). Moreover, S. aureus PGN, but not S. aureus lipoteichoic acid (LTA), induced ANA production in mice, indicating not all bacterial cell wall antigens induce an anti-dsDNA response (Figure S2B). Using autoantigen array to measure more than 100 autoantibodies in serum of C57/B6 mice, S. aureus PGN increased a number of IgG autoantibodies, including IgG anti-dsDNA, whereas B. subtilis PGN increased IgM autoantibodies, including IgM anti-dsDNA (Figure S2C). Thus, B. subtilis PGN induced primarily IgM or short-lived IgG autoantibody responses, whereas S. aureus PGN induced stable long-lasting IgG autoantibody responses. Importantly, S. aureus PGN-mediated antibody responses were due to limited B cell clonal responses but not overall polyclonal B cell activation, as total IgG did not differ.
Figure 1.
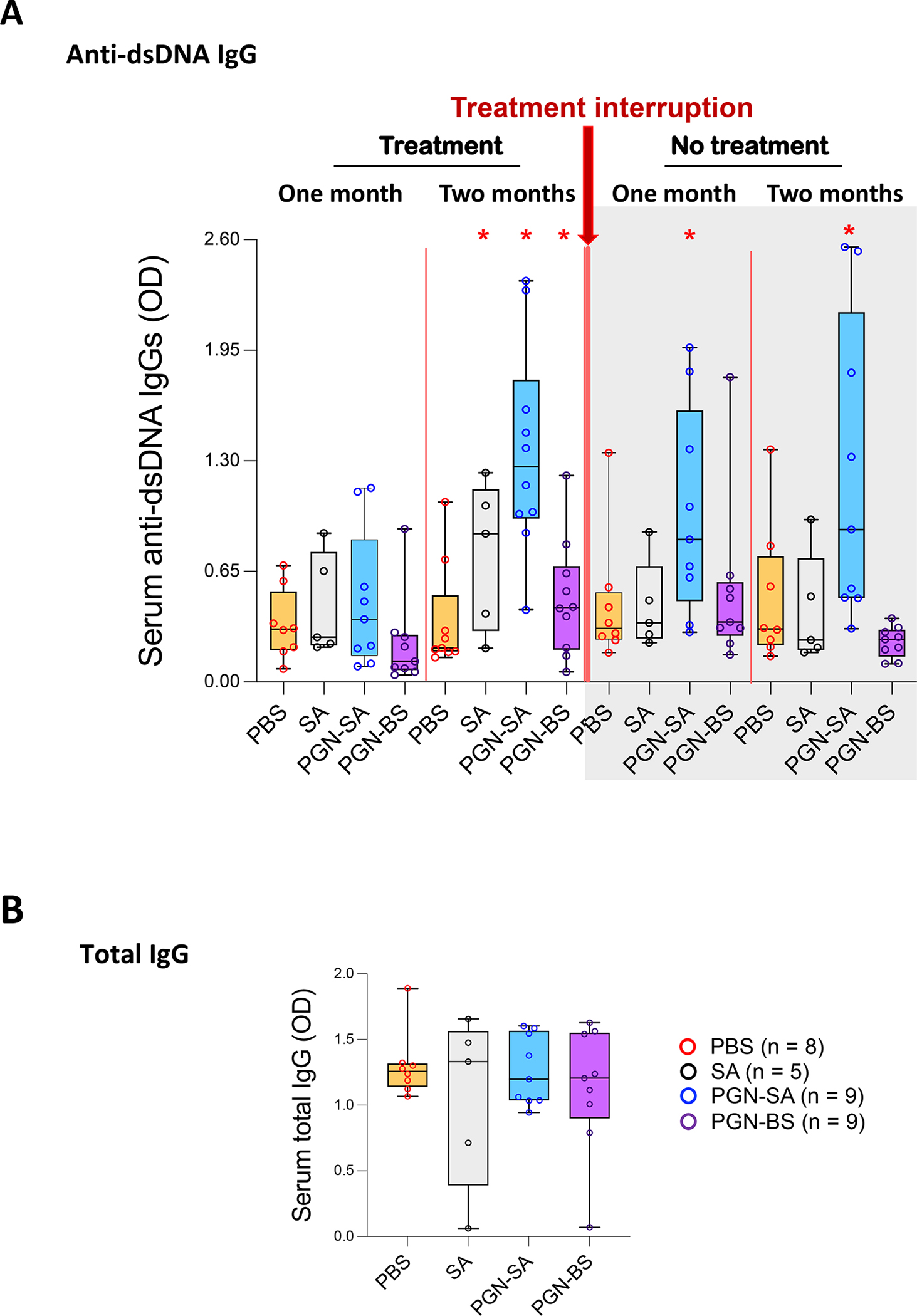
S. aureus PGN but not B. subtilis PGN induces production of stable IgG anti-dsDNA autoantibodies in C57/B6 mice. C57/B6 mice were treated with PBS (0.2 mL), S. aureus (PGN-SA) or B. subtilis PGN (PGN-BS) (100 μg/mouse/injection), or whole inactivated S. aureus (5×107 CFU/mouse/injection) via i.p. injection twice per week for 8 weeks. Then treatment was stopped for 8 weeks. (A) Serum levels of IgG anti-dsDNA and (B) endpoint serum levels of total IgG in mice at 22 weeks of age. Median ± Interquartile range. *P < 0.05 compared to the PBS group.
S. aureus PGN increased pathogenic IgG anti-dsDNA and worsened lupus kidney pathology
IgG anti-dsDNA autoantibodies contribute to kidney damage in SLE [20, 21]. After 12-weeks of treatment in MRL/lpr mice, serum IgG anti-dsDNA levels were greater in both PGN groups than PBS (Figure 2A). Although the percentage of anti-dsDNA IgG+ splenic B cells was higher in the two PGN groups than PBS, no difference was observed between the PGNs (Figure 2B). Serum ANA IgG levels were higher in S. aureus PGN than PBS (Figure S3A), and total serum IgGs were unchanged (Figure 2C).
Figure 2.
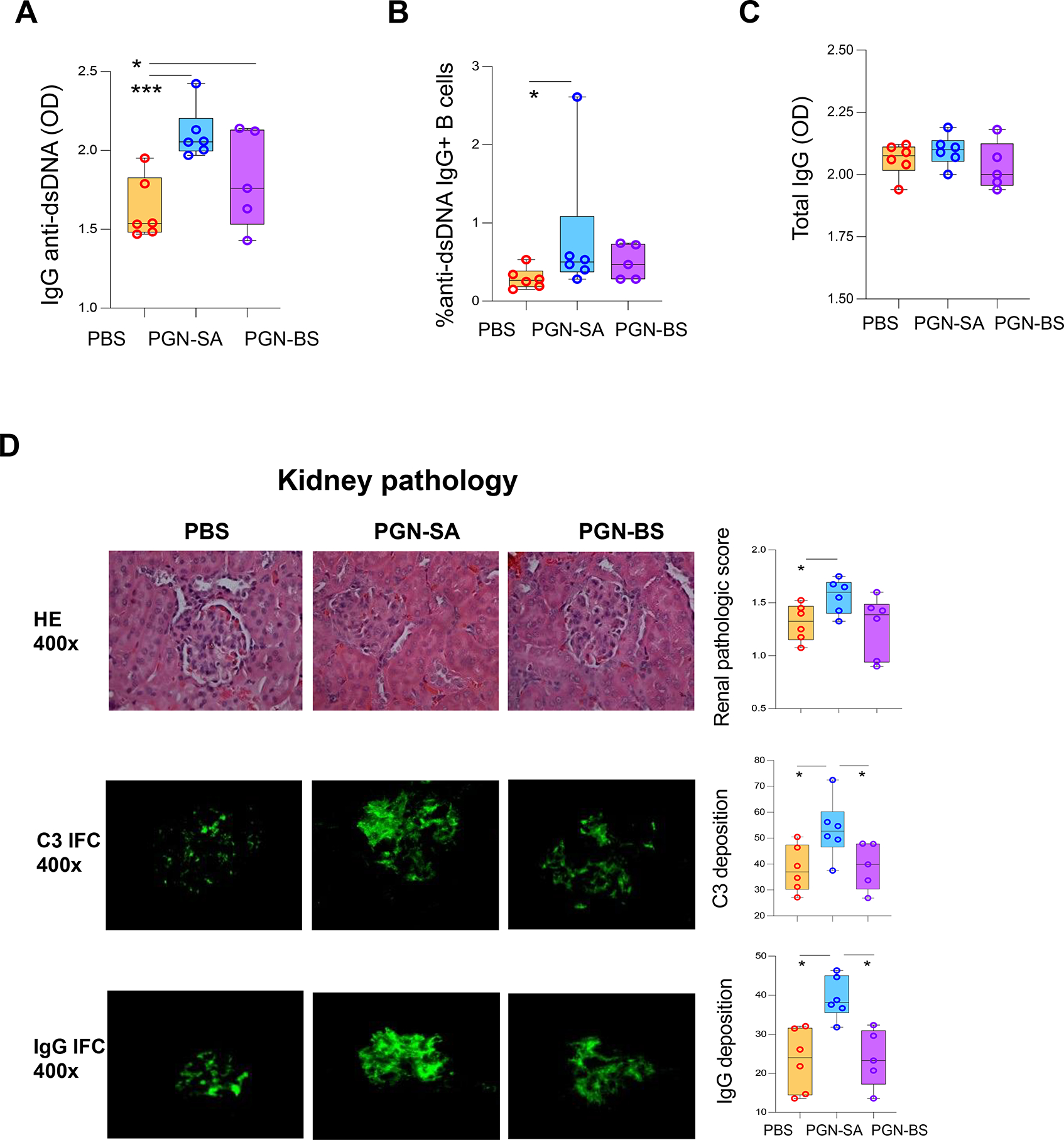
S. aureus PGN accelerates autoantibody production and kidney pathology in MRL/lpr mice. MRL/Lpr mice were treated with PBS, S. aureus PGN (PGN-SA), or B. subtilis PGN (PGN-BS) (100 μg/mouse/injection) via i.p. injection twice per week for 12 weeks. Mice were euthanized and blood and tissue samples were collected at 18 weeks of age. (A) Serum levels of IgG anti-dsDNA. (B) Percent of anti-dsDNA IgG+ B cells in spleen (gating on B cells). (C) Serum levels of total IgG at the end of the study. (D) Representative kidney H&E imaging and collective glomerular lesion index. Representative kidney C3 and total IgG staining and collective quantification of C3 and IgG deposition.
The C3 and total IgG deposition and glomerular lesion score for kidney pathology were higher in S. aureus PGN than the control (Figure 2D), suggesting autoantibody and complement interaction. At the end of study, the ratio of urinary albumin versus creatinine showed a strong trend towards an increase in S. aureus PGN versus PBS, but was not significant (Figure S3B). Notably, the two PGN groups had similar levels of serum anti-dsDNA IgG, different from the response in C57/B6 mice; only S. aureus PGN, however led to increased kidney pathology in MRL/lpr mice. This finding suggests a qualitative difference in anti-dsDNA antibodies induced by the two PGNs, which may reflect higher affinity maturation or more of an extrafollicular response rather than a germinal center response. We did not detect significant differences in interstitial inflammation between the PGN groups.
S. aureus PGN drives specific clonal expansion, somatic mutation, and CSR of splenic anti-dsDNA autoreactive B cells
Differences in autoantibody affinity and pathogenicity reflect variation in the sequences of the variable region of immunoglobulin heavy chain, often in the complementarity determining region (CDR) 3, primarily due to antigen driven somatic mutation [22]. To investigate the mechanisms for the differences in antibody responses of the two PGNs, we sorted splenic anti-dsDNA IgG B cells (Figure S4) from C57BL/6 mice after 8 weeks of treatment, and conducted BCR sequencing to assess usage of immunoglobulin heavy-chain variable region genes (IGHV) (Figure 3). IGHV1-45 and IGHV3-74 were the predominant IGHVs in anti-dsDNA IgG-producing splenic B cells after both PGN treatments (Figure 3A). S. aureus PGN induced more limited clonal expansion with B. subtilis PGN inducing a wider array of clonal expansions (Figure 3B). We did not study MRL mice responses due to their spontaneous autoantibody production that cound confound interpretation of sequence differences.
Figure 3.
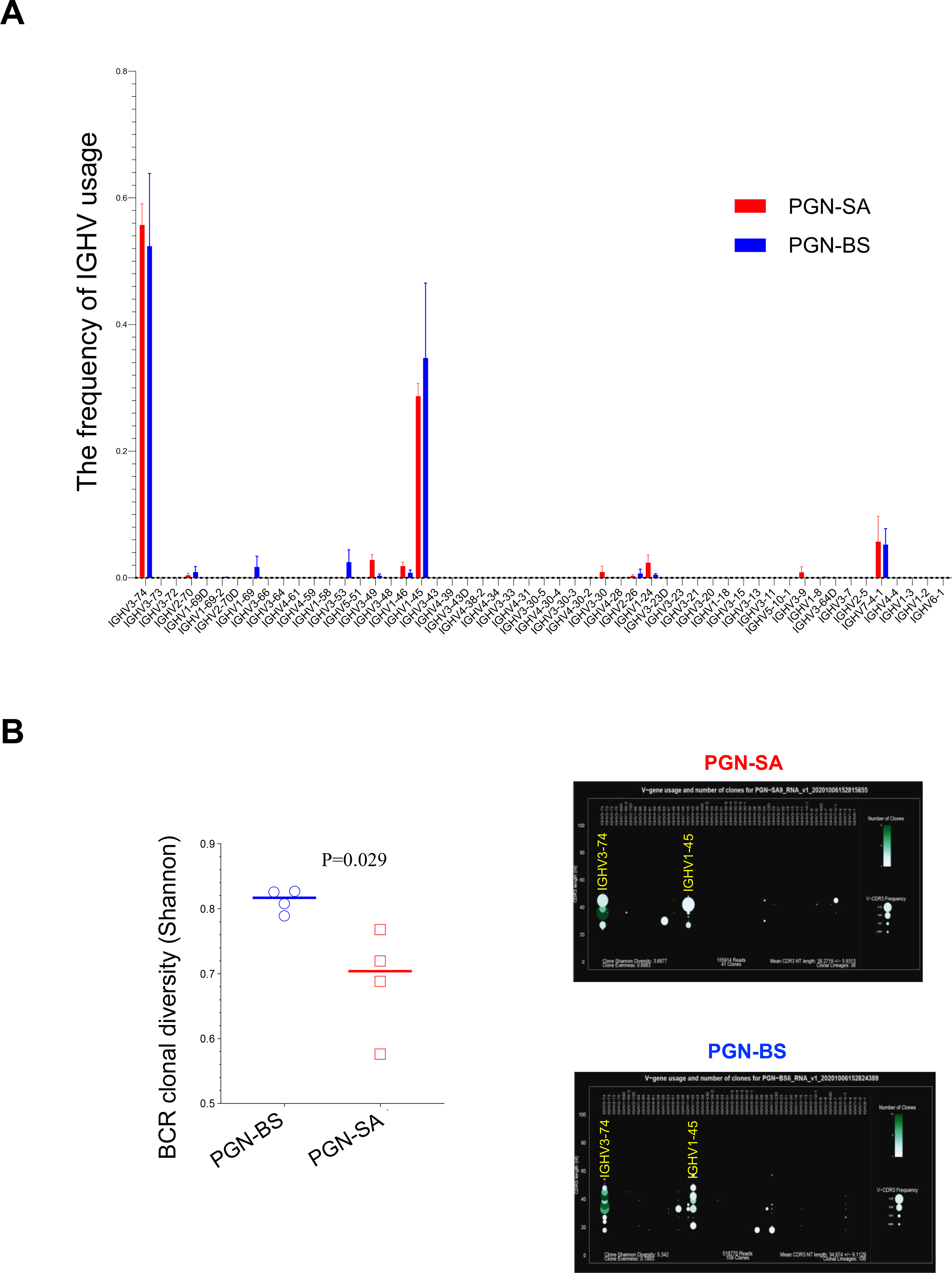
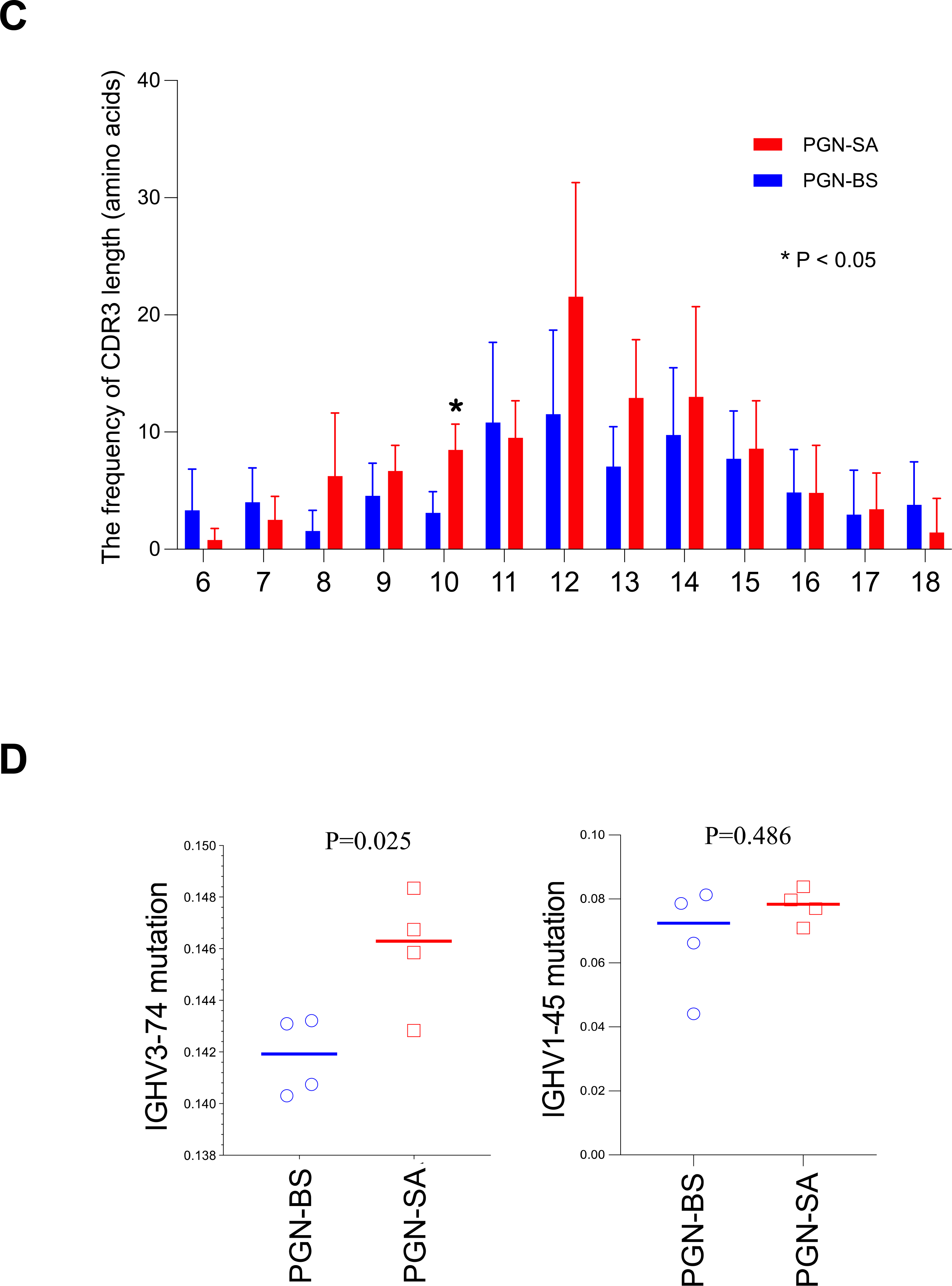
Characteristics of BCR repertoire from anti-dsDNA IgG+ B cells from spleen. C57/B6 mice were treated with S. aureus or B. subtilis PGNs via i.p. injection twice per week for 8 weeks. At the end of study, B cells from mice at 14 weeks of age were isolated from spleen and anti-dsDNA IgG+ B cells were sorted using flow cytometry. RNA was extracted, and BCR sequencing was conducted. BCR clonality was analyzed in C57/B6 mice treated with S. aureus (SA) and B. subtilis (BS) PGN (n = 4 per group). (A) The frequency of IGHV usage. (B) BCR clonal diversity. (C) The frequency of CDR3 length. (D) Somatic mutation of IGHV3-74 and IGHV1-45. Statistical analysis used non-parametric Mann-Whitney U tests.
The BCR diversity was lower in S. aureus PGN than B. subtilis PGN treated mice (Figure 3B); CDR3 amino acid length was generally similar between PGNs, except the CDR3 length of 10 amino acids was more common in S. aureus PGN (Figure 3C). The somatic mutation rate of IGHV3-74, but not IGHV1-45, was higher in S. aureus PGN versus B. subtilis PGN (Figure 3D). Prior sequencing of anti-dsDNA antibodies from lupus prone mice identified a higher presence of arginine in the CDR3 variable region [23]. Although there was a trend towards increased CDR3 arginines in the S. aureus PGN group, it did not reach significance likely due to the small sample size.
To study CSR, we found that S. aureus PGN predominantly induced IgG2b anti-dsDNA in C57BL/6 mice in vivo (Figure 4A). Further, S. aureus PGN, but not B. subtilis PGN, promoted B-cell CSR to IgG2b (the percentage of IgG2b+ proliferating B cells [24]) via TLR2 in isolated splenic B cells from untreated C57/B6 mice in vitro (Figure 4B). To denature any contaminating proteins, S. aureus PGN was heat inactivated at 56°C for 30 min, CSR IgG2b induction was no different compared to untreated PGN (Figure S5). Both bacterial PGNs induced similar TLR2 activation (Figure 4C).
Figure 4.
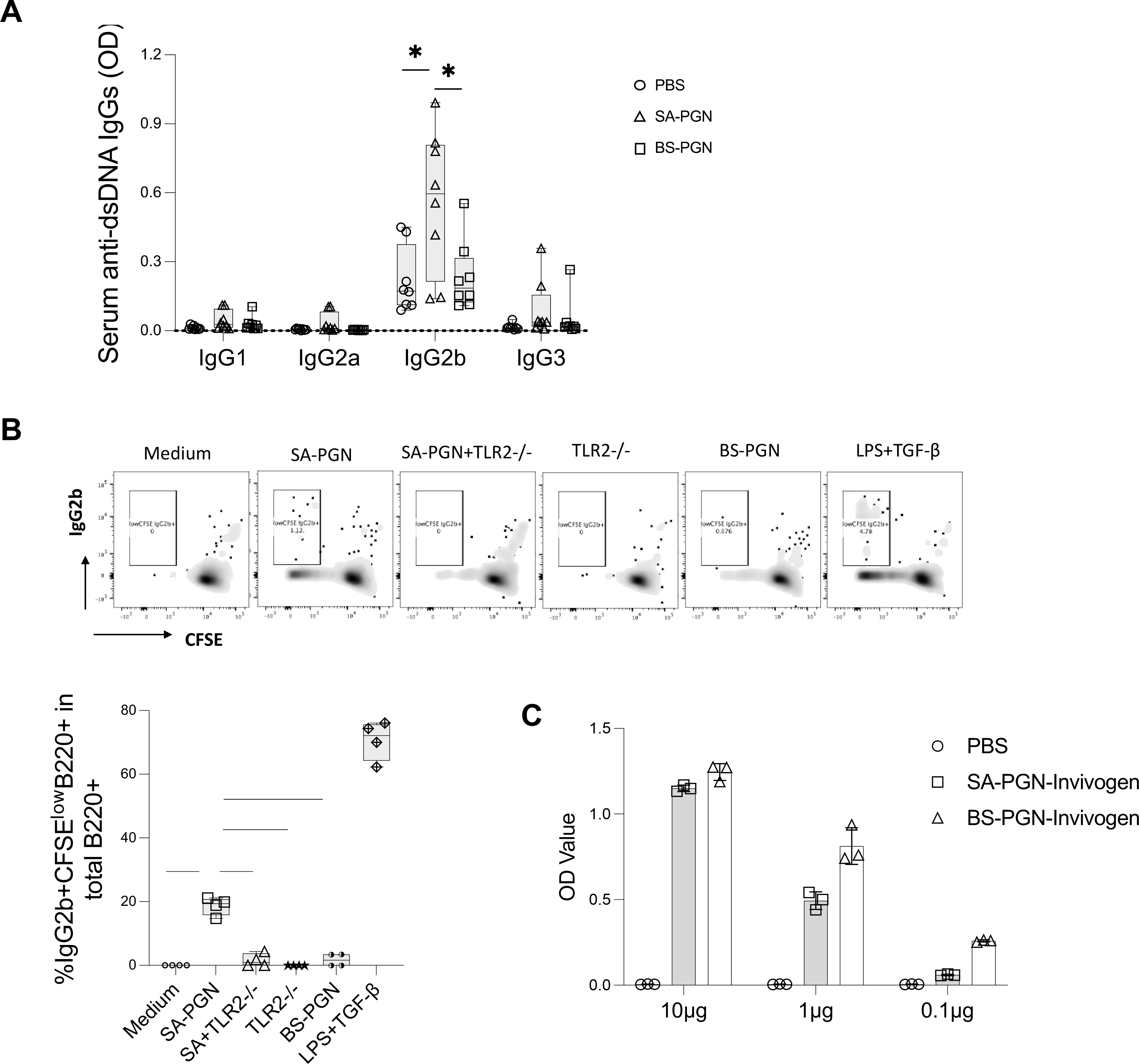
IgG2b CSR mediated by S. aureus PGN. (A) C57/B6 mice were treated with S. aureus or B. subtilis PGNs via i.p. injection twice per week for 8 weeks. At the end of study, subclasses of serum anti-dsDNA IgG were evaluated in mice at 14 weeks of age. (B) B cells were isolated from spleen of untreated C57/B6 mice, cultured with basal medium, LPS plus TGF-β1 (a positive control), S. aureus (SA) or B. subtilis (BS) PGN in the presence of absence of TLR2 mAb or isotype Ab for 72 h. The percentages of proliferating IgG2b+ B cells (%CFSElowIgG2b+ in IgG2b+B220+ cells) were calculated. (C) The TLR2 induced activity by S. aureus and B. subtilis PGN (P = 0.25). Statistical analysis used non-parametric Mann-Whitney U tests.
Plasma levels of S. aureus DNA translocation increase in patients with SLE and correlate with lupus-specific autoantibody levels
Next, we assessed plasma levels of S. aureus DNA (copy number/mL) and the ratio of S. aureus DNA versus total bacterial rDNA, and found that both were higher in patients than controls (Figure 5A). Plasma S. aureus DNA translocation correlated with plasma levels of various lupus-related autoantibodies, including anti-dsDNA IgG (Figure 5B). Based on clinical assessments of renal disease, S. aureus DNA translocation was increased in patients with low serum albumin, high serum creatine, and high urinary protein levels, versus controls (Figure S6A). Translocation of S. aureus DNA did not differ based on lupus treatment regimens (Figure S6B). At the time of sample collection, none of the patients had ongoing active lupus skin disease.
Figure 5.
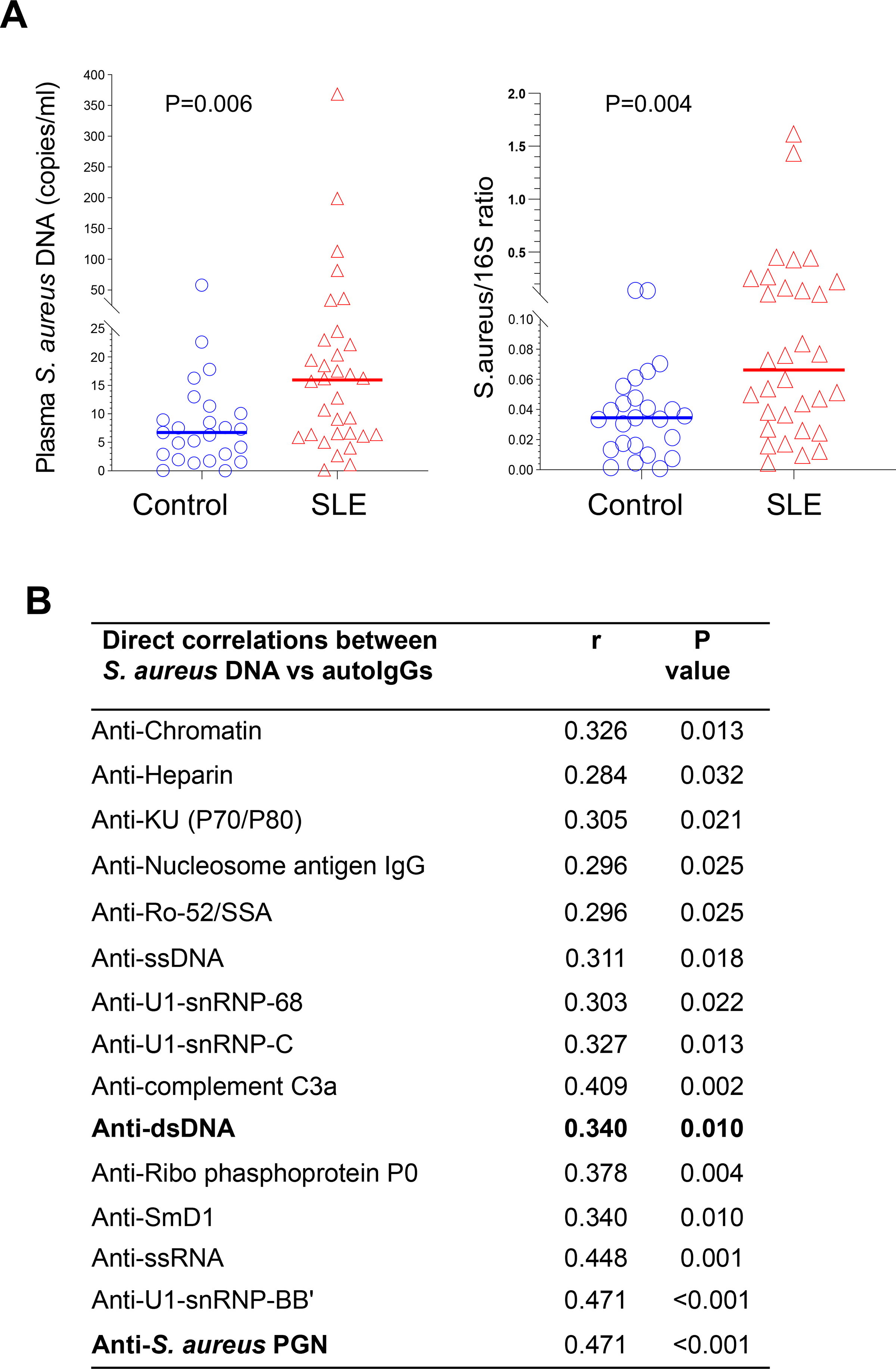
Increased plasma S. aureus DNA levels in patients with SLE and their correlation with lupus-related autoantibody levels. (A) Presence of plasma S. aureus DNA (absolute copy number per mL blood or ratio of S. aureus DNA versus total 16S rDNA) measured by qPCR in extracted microbial DNA from plasma samples of patients with SLE (n = 32) and healthy controls (n = 25). (B) Spearman correlation coefficient (r) and P values of S. aureus DNA copy number per mL blood and levels of each lupus-related autoantibody. Autoantibody levels were evaluated in plasma samples by ELISA.
DISCUSSION
In this study, administration of B. subtilis PGN induced a short-term anti-dsDNA response, whereas S. aureus PGN induced a sustained anti-dsDNA response that accelerated renal disease in MRL/lpr mice. S. aureus PGN induced anti-dsDNA IgG B cells by specific clonal expansion, incleased somatically mutated, and inducing class switching predominantly to IgG2b. Plasma translocation of S. aureus DNA in patients was greater than matched controls, which correlated with plasma anti-dsDNA IgG levels, suggesting similarity between the murine and the human responses. The higher plasma S. aureus DNA in patients, we postulate, is one factor that induces autoantibody production and disease development in susceptible individuals.
The PGN structure is comprised of repeating disaccharide backbones of N-acetylglucosamine and β-(1–4)-N-acetylmuramic acid that are cross-linked by peptide stem chains attached to residues of β-(1–4)-N-acetylmuramic acid [25]. Among bacterial species, PGNs have different lengths of sugar polymer. Bacillus PGN has ~50 to 250 disaccharides, whereas S. aureus PGN has ~18 disaccharides [26]. This difference in length/structure may contribute to the distinct effects of two PGNs we observed.
Anti-dsDNA antibodies can induce kidney damage in SLE, though they are not the only factor [27, 28]. Levels of IgG anti-dsDNA rise in serum before renal flares in some lupus patients [29]. Importantly, each IgG isotype differs in their biological properties. Human IgG1 and IgG3, but not IgG2 and IgG4, activate complement [30]. In mice, IgG2a, IgG2b, and IgG3 can form immune complexes with complement and contribute to nephritis. These IgG subclasses are often detected in the glomeruli of murine lupus [31–33]. Thus, IgG subclasses of autoantibodies may impact lupus nephritis, though a variety of factors interplay including genetic susceptibility and the kidney response to immune complex deposition. C57/B6 mice are more resistant to induction of lupus nephritis than other strains and the target organ susceptibility to immune insult can differ. This perhaps explains why renal disease was not seen in the B6 mice but was evident in the MRL/lpr mice. The predominant IgG subclasses in lupus patients with renal flares were IgG1 anti-DNA in the serum and IgG2 anti-nucleosome antibodies in kidney biopsies [29, 34]; autoantibodies deposited in the kidney and blood may have different antigenic specificities.
Autoreactive B cells can be activated via polyclonal nonspecific activation or antigen-specific activation. Only a few studies report the IGHV usages of anti-dsDNA antibodies [35, 36]. The IGHV1-3, IGHV3-23, and IGHV3-74 genes were dominant in human anti-dsDNA antibodies [36], and the IGHV J558 and 7183 genes were dominant in MRL/lpr mice anti-dsDNA antibodies [35]. Monoclonal anti-ds DNA autoantibodies derived from lupus mouse spleens show evidence of antigen driven somatic mutation and enrichment in VHCDR3 arginine [35]. Recent studies in both humans and mice indicate that most autoantibodies in lupus may be derived extrafollicularly with help from peripheral helper T cells [37]. Thus, likely genetic and local B cell zone factors (e.g., TLR ligands) influence B cell maturation and responses.
To understand the mechanism of the differential responses to the two PGNs, we analyzed BCR sequences of anti-dsDNA IgG+ splenic B cells, and found that IGHV1-45 and IGHV3-74 were predominant for both PGNs. The total serum IgG did not differ. Thus, neither bacterial PGNs promoted B-cell overall polyclonal activation. S. aureus PGN promoted a more limited clonal expansion of anti-dsDNA IgG-producing B cells, and while B. subtilis PGN induced a more diverse clonal expansion. The most intriguing and perhaps important difference between the two PGNs was that anti-dsDNA antibody production by B subtilis PGN was transient and stopped when PGN injections ended. In contrast, S aureus PGN induced anti-dsDNA antibody production was sustained long after PGN stopped, suggesting induction of an antigen driven autoantibody response and a break in tolerance. What makes an antibody pathogenic is not completely understood. The antibody affinity, the CDR3 region and the Fcg region of an antibody all may contribute to the pathogenicity of the antibody. We performed BCR sequencing of isolated anti-dsDNA antibody producing B cells from the spleens between the two PGN groups and there were differences in IgG subtype and in the VH CDR3 region (Figure 3). We did find IGHV3-74 mutation was higher in the S. aureus PGN group compared to the B. subtilis control group (Figure 3D), but did not find evidence of enhanced VHCDR3 charge. Moreover, we do not know the half-life of the autoantibodies induced by S. aureus PGN. The anti-dsDNA response is present for a longer period of time following injection of S. aureus PGN than B subtilis. This could relate to either a longer antibody half-life versus prolonged production of anti-dsDNA antibodies by antibody producing cells. It is more likely that the induced response is prolonged rather than the antibodies have a longer half-life.
Our study has several limitations. The main limitation is the potential contamination in the S. aureus PGN preparation that could contain other S. aureus cell wall components (e.g., surface protein A, Staphylococcal protein A [a known B cell superantigen], LTA, lipoproteins/peptides). Thus, the TLR2 stimulating capacity of S. aureus PGN may come from non-PGN cell wall contaminants [26, 38, 39]. As bacterial DNA alone does not induce autoantibody production, except when administered in Freund’s adjuvant [40, 41], contaminating DNA likely did not contribute to this effect. There is strong evidence supporting a role for S. aureus PGN on autoantibody production: 1) a TLR2 inhibitor blocked the PGN effects; 2) CSR induction did not differ in heat inactivation and untreated S. aureus PGNs; 3) PGNs can be detected in the circulation in healthy humans [12, 13]; 4) S. aureus LTA predominately induces pro-inflammatory cytokines in myeloid cells where PGN has limited effects [42–44]. Further LTA i.p. injection did not induce autoantibodies in C57/B6 mice; 5) structural difference between S. aureus and Bacillus PGNs impacts immune responses [26]; and 6) Staph Protein A and Staphylococcus Enterotoxin B reduce disease severity in lupus-prone mice [45, 46]. Additionally, only 20% of patients had skin lesions in the current study. However, S aureus is not limited to the skin but can be found in the other mucosal areas. Importantly, levels of S. aureus DNA translocation were increased in patients with high urinary protein levels compared to controls, suggesting an association between S. aureus translocation and renal involvement in SLE.
Finally, the serum IgG anti-DNA levels were higher in the S aureus group, but the number of B cells producing anti-dsDNA were the same in the two PGNs; S. aureus PGN-mediated CSR was via TLR2, and TLR2 induced activity was similar between the two PGNs. These results suggest that S. aureus PGN induces both higher quantity and quality of IgG anti-dsDNA per B cell. S. aureus PGN-mediated specific B cell clone selection may be due to different coreceptor usage (e.g., TLR2-TLR1, TLR2-TLR6) or other mechanisms, deserving further investigations. Nonetheless, this study provides novel insight into the potential role of one bacterial cell wall component that is present systemically in humans in inducing pathogenic autoantibodies. Therapeutics aimed at blocking TLR2 or staph colonization may have a role in treating lupus.
Supplementary Material
Supplementary Figure 1. Dose titration for production of anti-dsDNA IgG and TLR2 activation of PGNs. C57/B6 mice were treated with PBS and different doses of S. aureus PGN via i.p. injection for 8 weeks. Serum levels of anti-dsDNA IgG (A) and body weight (B) were assessed.
Supplementary Figure 2. S. aureus PGN but not B. subtilis PGN induced production of stable IgG ANA autoantibodies in C57/B6 mice. C57/B6 mice were treated with PBS (0.2 mL), S. aureus or B. subtilis PGN (100μg/mouse/injection), or whole inactivated S. aureus (5×107 CFU/mouse/injection), twice per week for 8 weeks. Then treatment was stopped for 8 weeks. Blood was collected in mice at 22 weeks of age. (A) Serum levels of IgG ANA levels. Median ± Interquartile range. (B) B6 mice received PBS, LTA (100 μg/mouse/time), or PGN i.p. (100 μg/mouse/time), twice per week for one and two months. Plasma ANA IgG levels were evaluated by ELISA. (C) Serum levels of IgG or IgM anti-dsDNA antibodies after 8 weeks of treatment with PGNs or PBS. Mean fluorescent intensity (MFI) of IgG or IgM anti-dsDNA. Statistical analysis involved non-parametric Mann-Whitney U tests.
Supplementary Figure 3. S. aureus PGN but not B. subtilis PGN induced production of IgG ANA in MRL/lpr mice. MRL/lpr mice were treated with PBS, S. aureus PGN, or B. subtilis PGN for 12 weeks. The serum and urinary markers were assessed at the end of the study in mice at 18 weeks of age. (A) Serum levels of IgG ANA. (B) Urinary albumin versus creatinine BUN (albumin/cr) and serum urea and creatinine in treated MRL/Lpr mice. Statistical analysis used non-parametric Mann-Whitney U tests.
Supplementary Figure 4. For spleen B cell BCR sequencing, spleen B cells were isolated using MACS at the end of C57/B6 mouse study, cells were stained with dsDNA antigen, and anti-dsDNA IgG+ B cells were sorted using flow cytometry (gating on IgG+ cells).
Supplementary Figure 5. Plasma levels of S. aureus DNA based on markers of renal function and treatment regimens. Plasma levels of S. aureus DNA in different patients with SLE based on levels of serum albumin, BUN, creatinine, urinary protein, and ratio of urinary protein to creatine (A) and different treatment regimens, including glucocorticoids, antimalarials, antithrombotics, and immunosuppressives (B).
Supplementary Figure 6. Comparison of IgG2b CSR induction in response to S. aureus PGN and heat inactivated S. aureus PGN. Unmanipulated B cells were isolated from splenocytes of 8- to 12-week-old female C57BL/6 mice, and stimulated with 10 μg/mL of untreated S. aureus PGN or heat inactivated S. aureus PGN (PGN-SA-HI, 56°C for 30 min) for 72 hours in vitro. The percentages of proliferating IgG2b+ B cells (%CFSElowIgG2b+ in IgG2b+B220+ cells) were calculated. No difference was observed in two groups of PGN.
Supplementary Table 1. Antibodies used in this study.
Supplementary Table 2. Demographic, clinical, and laboratory characteristics.
Acknowledgments.
We thank Crystal Herron for editorial assistance.
Funding, grant/award information.
This work was supported by grants from the National Institutes of Health: P60 AR062755 (Gilkeson, Kamen, and Oates), P30AR072582 (Gilkeson, Kamen, and Oates), AR068406 (Kamen), and UL1 RR029882, UL1 TR001450, and the Medical Research Service at the Ralph H. Johnson VA Medical Center Merit grant VA CSRD MERIT (CX001211, Gilkeson).
Footnotes
Competing interests. The authors declare no competing financial and non-financial interests.
Ethical approval information. The Institutional Review Board at MUSC approved this study for human, and all participants gave informed consent. All animal studies were approved by the Institutional Animal Care and Use Committee at MUSC.
Patient and Public Involvement. It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
Data sharing statement.
Data are available in Mendeley Data (doi:10.17632/m8sz7s7zgz.1).
REFERENCES
- 1.Slight-Webb S, Smith M, Bylinska A, Macwana S, Guthridge C, Lu R, Merrill JT, Chakravarty E, Arriens C, Munroe ME et al. : Autoantibody-positive healthy individuals with lower lupus risk display a unique immune endotype. J Allergy Clin Immunol 2020, 146(6):1419–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB: Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003, 349(16):1526–1533. [DOI] [PubMed] [Google Scholar]
- 3.Zegarra-Ruiz DF, El Beidaq A, Iniguez AJ, Lubrano Di Ricco M, Manfredo Vieira S, Ruff WE, Mubiru D, Fine RL, Sterpka J, Greiling TM et al. : A Diet-Sensitive Commensal Lactobacillus Strain Mediates TLR7-Dependent Systemic Autoimmunity. Cell host & microbe 2019, 25(1):113–127 e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E, Greiling T, Ruff W et al. : Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018, 359(6380):1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogunrinde E, Zhou Z, Luo Z, Alekseyenko A, Li QZ, Macedo D, Kamen DL, Oates JC, Gilkeson GS, Jiang W: A Link Between Plasma Microbial Translocation, Microbiome, and Autoantibody Development in First-Degree Relatives of Systemic Lupus Erythematosus Patients. Arthritis & rheumatology 2019, 71(11):1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham PL 3rd, Lin SX, Larson EL: A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med 2006, 144(5):318–325. [DOI] [PubMed] [Google Scholar]
- 7.Conti F, Ceccarelli F, Iaiani G, Perricone C, Giordano A, Amori L, Miranda F, Massaro L, Pacucci VA, Truglia S et al. : Association between Staphylococcus aureus nasal carriage and disease phenotype in patients affected by systemic lupus erythematosus. Arthritis Res Ther 2016, 18:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirobhushanam S, Parsa N, Reed TJ, Berthier CC, Sarkar MK, Hile GA, Tsoi LC, Banfield J, Dobry C, Horswill AR et al. : Staphylococcus aureus Colonization Is Increased on Lupus Skin Lesions and Is Promoted by IFN-Mediated Barrier Disruption. J Invest Dermatol 2020, 140(5):1066–1074 e1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Z, Li M, Wu Y, Meng Z, Martin L, Zhang L, Ogunrinde E, Zhou Z, Qin S, Wan Z et al. : Systemic translocation of Staphylococcus drives autoantibody production in HIV disease. Microbiome 2019, 7(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthew E Griffin JE, Becker Jessica L., Luo Ji-Dung, Carroll Thomas S., Jha Jyoti K., Fanger Gary R., Hang Howard C.: Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Scinece 2021, 373:1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin ME, Espinosa J, Becker JL, Luo JD, Carroll TS, Jha JK, Fanger GR, Hang HC: Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science 2021, 373(6558):1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z, Wang J, Xu X, Wang H, Qiao Y, Chu WC, Xu S, Chai L, Cottier F, Pavelka N et al. : Antibody neutralization of microbiota-derived circulating peptidoglycan dampens inflammation and ameliorates autoimmunity. Nat Microbiol 2019, 4(5):766–773. [DOI] [PubMed] [Google Scholar]
- 13.Molinaro R, Mukherjee T, Flick R, Philpott DJ, Girardin SE: Trace levels of peptidoglycan in serum underlie the NOD-dependent cytokine response to endoplasmic reticulum stress. J Biol Chem 2019, 294(22):9007–9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling HZ, Xu SZ, Leng RX, Wu J, Pan HF, Fan YG, Wang B, Xia YR, Huang Q, Shuai ZW et al. : Discovery of new serum biomarker panels for systemic lupus erythematosus diagnosis. Rheumatology (Oxford) 2020, 59(6):1416–1425. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XK, Gallant S, Molano I, Moussa OM, Ruiz P, Spyropoulos DD, Watson DK, Gilkeson G: Decreased expression of the Ets family transcription factor Fli-1 markedly prolongs survival and significantly reduces renal disease in MRL/lpr mice. J Immunol 2004, 173(10):6481–6489. [DOI] [PubMed] [Google Scholar]
- 16.Muraoka M, Hasegawa H, Kohno M, Inoue A, Miyazaki T, Terada M, Nose M, Yasukawa M: IK cytokine ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum 2006, 54(11):3591–3600. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Lederman MM, Harding CV, Sieg SF: Presentation of soluble antigens to CD8+ T cells by CpG oligodeoxynucleotide-primed human naive B cells. J Immunol 2011, 186(4):2080–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O et al. : Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012, 64(8):2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang W: A protocol for quantizing total bacterial 16S rDNA in plasma as a marker of microbial translocation in vivo. Cell Mol Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamen DL, Barron M, Parker TM, Shaftman SR, Bruner GR, Aberle T, James JA, Scofield RH, Harley JB, Gilkeson GS: Autoantibody prevalence and lupus characteristics in a unique African American population. Arthritis and rheumatism 2008, 58(5):1237–1247. [DOI] [PubMed] [Google Scholar]
- 21.Langkilde H, Voss A, Heegaard N, Laustrup H: Autoantibodies persist in relatives to systemic lupus erythematosus patients during 12 years follow-up. Lupus 2017, 26(7):723–728. [DOI] [PubMed] [Google Scholar]
- 22.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T: Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102(5):553–563. [DOI] [PubMed] [Google Scholar]
- 23.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG: Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A 1987, 84(24):9150–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins AM: IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol Cell Biol 2016, 94(10):949–954. [DOI] [PubMed] [Google Scholar]
- 25.Humann J, Lenz LL: Bacterial peptidoglycan degrading enzymes and their impact on host muropeptide detection. J Innate Immun 2009, 1(2):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf AJ, Underhill DM: Peptidoglycan recognition by the innate immune system. Nat Rev Immunol 2018, 18(4):243–254. [DOI] [PubMed] [Google Scholar]
- 27.Yung S, Chan TM: Mechanisms of Kidney Injury in Lupus Nephritis - the Role of Anti-dsDNA Antibodies. Front Immunol 2015, 6:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamura M, Kanayama Y, Amastu K, Negoro N, Kohda S, Takeda T, Inoue T: Significance of enzyme linked immunosorbent assay (ELISA) for antibodies to double stranded and single stranded DNA in patients with lupus nephritis: correlation with severity of renal histology. Annals of the rheumatic diseases 1993, 52(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bootsma H, Spronk PE, Ter Borg EJ, Hummel EJ, de Boer G, Limburg PC, Kallenberg CG: The predictive value of fluctuations in IgM and IgG class anti-dsDNA antibodies for relapses in systemic lupus erythematosus. A prospective long-term observation. Annals of the rheumatic diseases 1997, 56(11):661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozsnyay Z, Sarmay G, Walker M, Maslanka K, Valasek Z, Jefferis R, Gergely J: Distinctive role of IgG1 and IgG3 isotypes in Fc gamma R-mediated functions. Immunology 1989, 66(4):491–498. [PMC free article] [PubMed] [Google Scholar]
- 31.Baudino L, Nimmerjahn F, Azeredo da Silveira S, Martinez-Soria E, Saito T, Carroll M, Ravetch JV, Verbeek JS, Izui S: Differential contribution of three activating IgG Fc receptors (FcgammaRI, FcgammaRIII, and FcgammaRIV) to IgG2a- and IgG2b-induced autoimmune hemolytic anemia in mice. J Immunol 2008, 180(3):1948–1953. [DOI] [PubMed] [Google Scholar]
- 32.Baudino L, Azeredo da Silveira S, Nakata M, Izui S: Molecular and cellular basis for pathogenicity of autoantibodies: lessons from murine monoclonal autoantibodies. Springer Semin Immunopathol 2006, 28(2):175–184. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Lu M, Zhai S, Wu K, Peng L, Yang J, Xia Y: ALW peptide ameliorates lupus nephritis in MRL/lpr mice. Arthritis research & therapy 2019, 21(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bijl M, Dijstelbloem HM, Oost WW, Bootsma H, Derksen RH, Aten J, Limburg PC, Kallenberg CG: IgG subclass distribution of autoantibodies differs between renal and extra-renal relapses in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2002, 41(1):62–67. [DOI] [PubMed] [Google Scholar]
- 35.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M: Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med 1990, 171(1):265–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JJ, Colella AD, Beroukas D, Chataway TK, Gordon TP: Precipitating anti-dsDNA peptide repertoires in lupus. Clin Exp Immunol 2018, 194(3):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, Donlin LT, Henderson LA, Wei K, Mizoguchi F et al. : Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542(7639):110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekeredjian-Ding I, Inamura S, Giese T, Moll H, Endres S, Sing A, Zahringer U, Hartmann G: Staphylococcus aureus protein A triggers T cell-independent B cell proliferation by sensitizing B cells for TLR2 ligands. J Immunol 2007, 178(5):2803–2812. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Nooh MM, Kotb M, Re F: Commercial peptidoglycan preparations are contaminated with superantigen-like activity that stimulates IL-17 production. J Leukoc Biol 2008, 83(2):409–418. [DOI] [PubMed] [Google Scholar]
- 40.Gilkeson GS, Pippen AM, Pisetsky DS: Induction of cross-reactive anti-dsDNA antibodies in preautoimmune NZB/NZW mice by immunization with bacterial DNA. The Journal of clinical investigation 1995, 95(3):1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilkeson GS, Ruiz P, Pippen AM, Alexander AL, Lefkowith JB, Pisetsky DS: Modulation of renal disease in autoimmune NZB/NZW mice by immunization with bacterial DNA. J Exp Med 1996, 183(4):1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Standiford TJ, Arenberg DA, Danforth JM, Kunkel SL, VanOtteren GM, Strieter RM: Lipoteichoic acid induces secretion of interleukin-8 from human blood monocytes: a cellular and molecular analysis. Infect Immun 1994, 62(1):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danforth JM, Strieter RM, Kunkel SL, Arenberg DA, VanOtteren GM, Standiford TJ: Macrophage inflammatory protein-1 alpha expression in vivo and in vitro: the role of lipoteichoic acid. Clinical immunology and immunopathology 1995, 74(1):77–83. [DOI] [PubMed] [Google Scholar]
- 44.Skinner NA, MacIsaac CM, Hamilton JA, Visvanathan K: Regulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16+ monocytes in response to sepsis-related antigens. Clin Exp Immunol 2005, 141(2):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun N, Erley C, Klein R, Kotter I, Saal J, Risler T: Immunoadsorption onto protein A induces remission in severe systemic lupus erythematosus. Nephrol Dial Transplant 2000, 15(9):1367–1372. [DOI] [PubMed] [Google Scholar]
- 46.Kim C, Siminovitch KA, Ochi A: Reduction of lupus nephritis in MRL/lpr mice by a bacterial superantigen treatment. J Exp Med 1991, 174(6):1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Dose titration for production of anti-dsDNA IgG and TLR2 activation of PGNs. C57/B6 mice were treated with PBS and different doses of S. aureus PGN via i.p. injection for 8 weeks. Serum levels of anti-dsDNA IgG (A) and body weight (B) were assessed.
Supplementary Figure 2. S. aureus PGN but not B. subtilis PGN induced production of stable IgG ANA autoantibodies in C57/B6 mice. C57/B6 mice were treated with PBS (0.2 mL), S. aureus or B. subtilis PGN (100μg/mouse/injection), or whole inactivated S. aureus (5×107 CFU/mouse/injection), twice per week for 8 weeks. Then treatment was stopped for 8 weeks. Blood was collected in mice at 22 weeks of age. (A) Serum levels of IgG ANA levels. Median ± Interquartile range. (B) B6 mice received PBS, LTA (100 μg/mouse/time), or PGN i.p. (100 μg/mouse/time), twice per week for one and two months. Plasma ANA IgG levels were evaluated by ELISA. (C) Serum levels of IgG or IgM anti-dsDNA antibodies after 8 weeks of treatment with PGNs or PBS. Mean fluorescent intensity (MFI) of IgG or IgM anti-dsDNA. Statistical analysis involved non-parametric Mann-Whitney U tests.
Supplementary Figure 3. S. aureus PGN but not B. subtilis PGN induced production of IgG ANA in MRL/lpr mice. MRL/lpr mice were treated with PBS, S. aureus PGN, or B. subtilis PGN for 12 weeks. The serum and urinary markers were assessed at the end of the study in mice at 18 weeks of age. (A) Serum levels of IgG ANA. (B) Urinary albumin versus creatinine BUN (albumin/cr) and serum urea and creatinine in treated MRL/Lpr mice. Statistical analysis used non-parametric Mann-Whitney U tests.
Supplementary Figure 4. For spleen B cell BCR sequencing, spleen B cells were isolated using MACS at the end of C57/B6 mouse study, cells were stained with dsDNA antigen, and anti-dsDNA IgG+ B cells were sorted using flow cytometry (gating on IgG+ cells).
Supplementary Figure 5. Plasma levels of S. aureus DNA based on markers of renal function and treatment regimens. Plasma levels of S. aureus DNA in different patients with SLE based on levels of serum albumin, BUN, creatinine, urinary protein, and ratio of urinary protein to creatine (A) and different treatment regimens, including glucocorticoids, antimalarials, antithrombotics, and immunosuppressives (B).
Supplementary Figure 6. Comparison of IgG2b CSR induction in response to S. aureus PGN and heat inactivated S. aureus PGN. Unmanipulated B cells were isolated from splenocytes of 8- to 12-week-old female C57BL/6 mice, and stimulated with 10 μg/mL of untreated S. aureus PGN or heat inactivated S. aureus PGN (PGN-SA-HI, 56°C for 30 min) for 72 hours in vitro. The percentages of proliferating IgG2b+ B cells (%CFSElowIgG2b+ in IgG2b+B220+ cells) were calculated. No difference was observed in two groups of PGN.
Supplementary Table 1. Antibodies used in this study.
Supplementary Table 2. Demographic, clinical, and laboratory characteristics.
Data Availability Statement
Data are available in Mendeley Data (doi:10.17632/m8sz7s7zgz.1).


