Abstract
Alzheimer’s disease (AD) is a debilitating neurodegenerative disorder affecting over 5 million people globally and has no established cure. Current AD-related treatments only alleviate cognitive and behavioral symptoms and do not address disease onset or progression, underlining the unmet need to create an effective, innovative AD therapeutic. Extracellular vesicles (EVs) have emerged as a new class of nanotherapeutics. These secreted, lipid-bound cellular signaling carriers show promise for potential clinical applications for neurodegenerative diseases like AD. Additionally, analyzing contents and characteristics of patient-derived EVs may address the unmet need for earlier AD diagnostic techniques, informing physicians of altered genetic expression or cellular communications specific to healthy and diseased physiological states. There are numerous recent advances in regenerative medicine using EVs and include bioengineering perspectives to modify EVs, target glial cells in neurodegenerative diseases like AD, and potentially use EVs to diagnose and treat AD earlier.
Keywords: Alzheimer’s Disease, Extracellular vesicles, Glial cells, Alzheimer’s treatment, Alzheimer’s diagnosis
Graphical Abstract
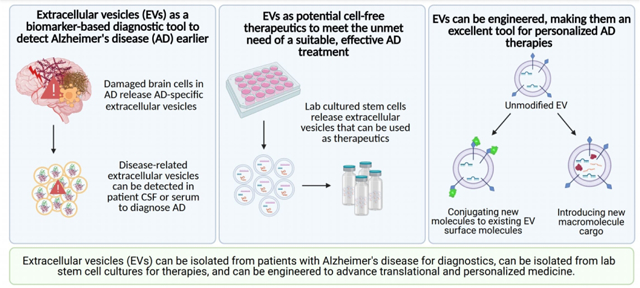
Extracellular vesicles (EVs) are a promising application for targeted molecule delivery. EVs can be engineered to target glial cells, address dysfunctional processes involved in Alzheimer’s disease (AD) progression, and function as informative biomarkers for earlier diagnosis to solve the unmet needs of an effective AD therapy and novel diagnostic method.
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder that affects 5.8 million people globally and accounts for 80% of all dementia cases (Weller & Budson, 2018). The medical expenditures associated with Alzheimer’s disease in the United States are predicted to increase from $305 billion in 2020 to more than $1 trillion in 2050 (Marasco, 2020). Currently, there is no treatment to halt the progression of AD and no systemic biomarkers for early diagnosis of AD (Villar-Vesga et al., 2020). Early detection and intervention of Alzheimer’s disease is key in prevention, lowering the economic burden, and improving patient quality of life.
The hallmark pathological features of AD include increased levels of amyloid-β (Aβ) peptide and hyperphosphorylated tau protein (Dubois et al., 2016). However, there may be a complex interplay of other molecular mechanisms including neurofibrillary tangle (NFT) formation, neurovascular obstruction, astrocyte activation, neuroinflammation, neuronal and glial cell death, and a disrupted blood-brain barrier (BBB) (Y. Liu, Huber, & Wang, 2020; Reza-Zaldivar et al., 2018). Additionally, the presence of Aβ and Tau proteins activates microglia, resident macrophages in the brain, which cannot efficiently clear toxic proteins and dead cells as the disease progresses. This disables the glial cells’ homeostatic functions and promotes free radical production and pro-inflammatory factor secretion (Hemonnot, Hua, Ulmann, & Hirbec, 2019). This combined effect leads to widespread neuronal cell death and brain atrophy.
Approaches to diagnose AD are focused on the dementia stage of the disease when patients have developed cognitive impairments (Crous-Bou, Minguillon, Gramunt, & Molinuevo, 2017). This diagnosis involves taking an extensive psychiatric history, conducting cognitive tests, and performing physical neurological exams on the patient. Finally, there are brain imaging techniques that can confirm an Alzheimer’s diagnosis that rely on the presence of Aβ plaques or hyperphosphorylated tau protein (Martinez et al., 2017; Weller & Budson, 2018). However, amyloidosis, the buildup of amyloid protein, is not present in all patients in preclinical stages of AD and is not the best criteria in early diagnosis of AD (Bondi, Edmonds, & Salmon, 2017).
As of 2021, there is no reliable, effective treatment that prevents or slows neuronal cell death associated with AD. With this progressive neuron degeneration, patients of late-onset AD experience depression, memory loss, disturbed gait, rigidity, and muscle jerks among other symptoms (Bature, Guinn, Pang, & Pappas, 2017). Only five drugs were approved by the US Food and Drug Administration (FDA) for the treatment of AD to alleviate the cognitive and behavioral symptoms, but they fail to slow or stop disease progression (Abeysinghe, Deshapriya, & Udawatte, 2020). In June 2021, a sixth drug was approved by the FDA after nearly 20 years since the last approval. There remains an unmet need for an FDA approved innovative diagnostic to detect AD earlier and a therapeutic that can directly address disease pathology.
Extracellular vesicles (EVs) are 30–150 nm lipid membrane bound vesicles involved in intercellular signaling that are secreted by all cells and carry many proteins, lipids, nucleic acids, mRNA and miRNA cargo of the cells from which they are derived (Kalluri & LeBleu, 2020; Villar-Vesga et al., 2020; Xian et al., 2019). Analyzing EV content is a diagnostic route to gain information about the cellular signaling in diseased states (Figure 1A). In addition to collecting patient derived EVs for diagnosis, lab-generated EVs such as synthetic EVs or modified cell-secreted EVs can be used to target and ameliorate numerous AD-related physiological symptoms, slowing or even stopping disease progression as a treatment method (Figure 1B).
Figure 1.
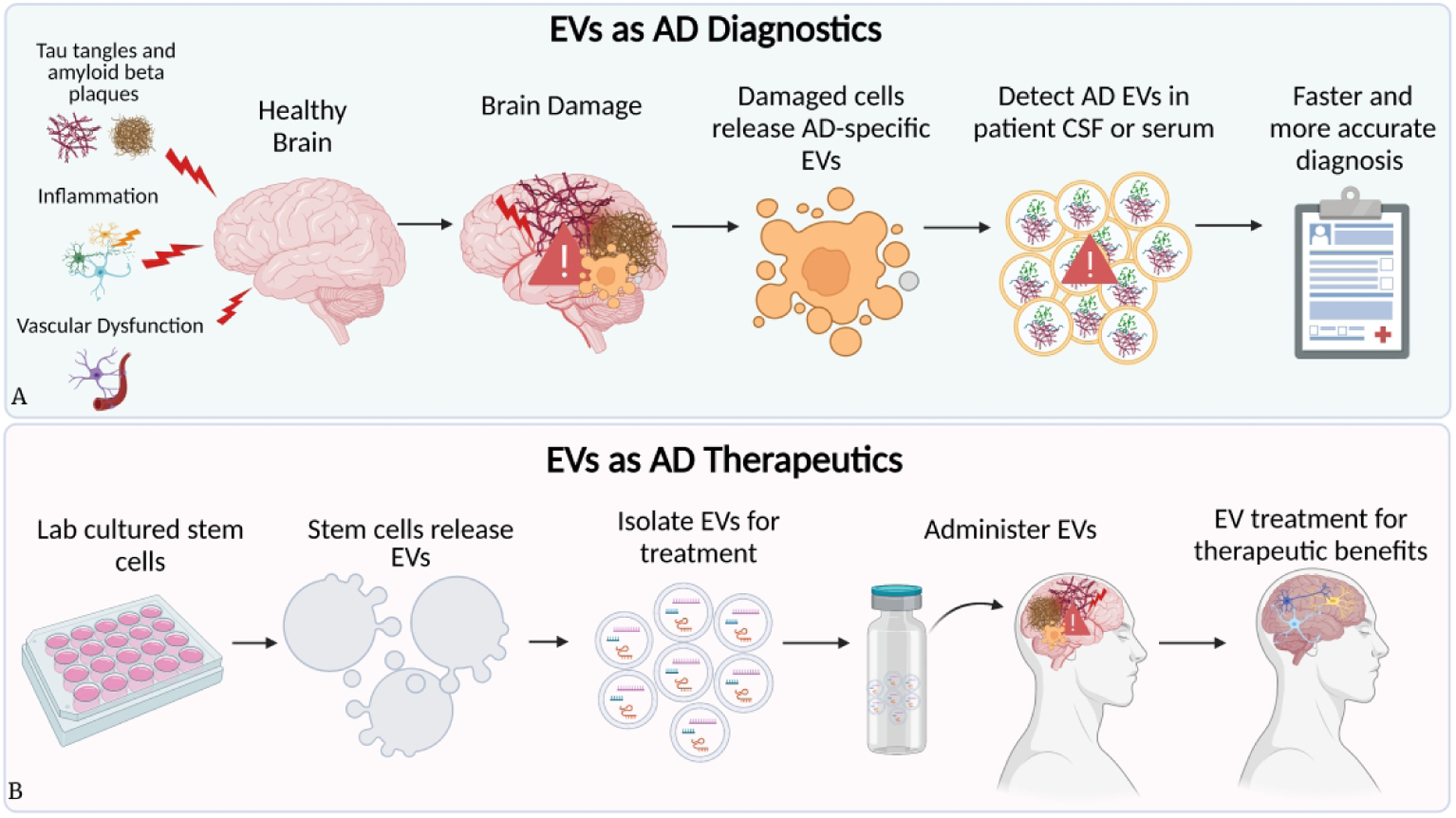
Extracellular vesicles as diagnostics and therapeutics. Diagram representing (A) the utility of EV samples from Alzheimer’s patients in diagnosing, and (B) how EVs can be generated in labs and engineered as a bench-to-bedside clinical therapeutic for Alzheimer’s patients.
The growing field of EV engineering has made numerous advances in EV modification techniques, turning them into effective, cell-free treatments in vitro and in vivo. EVs readily cross the blood brain barrier, can be modified to present desired surface molecules and carry internal cargo, highlighting their potential as a therapeutic tool for Alzheimer’s disease (Kalluri & LeBleu, 2020). EVs can be engineered to potentially target glial cells, address dysfunctional cellular processes involved in AD progression, and function as informative biomarkers for earlier diagnosis.
2. Alzheimer’s Disease (AD) Pathology
The tissue-based pathological hallmark of AD is the deposition of extracellular, insoluble Aβ plaques in the white matter of patients’ brains. However, the sole presence of Aβ plaques is not sufficient for the development of dementia, and cellular changes to neurons characteristic of AD pathology precede Aβ plaque deposition (Walsh & Selkoe, 2020).
There are several hypotheses to the cause of AD onset, including amyloid and tau propagation, neuroinflammation, and neurovascular hypotheses. The amyloid and tau propagation hypotheses implicate amyloid-β deposition and the accumulation of neurofibrillary tangles (NFTs) containing hyperphosphorylated tau protein, respectively, as causes of neuronal death (P. P. Liu, Xie, Meng, & Kang, 2019). The neuroinflammation hypothesis emphasizes the inflammatory factors released by activated microglia and astrocytes in AD brains will lead to neurodegeneration. Finally, the neurovascular hypothesis proposes the damaged cerebral microvasculature found in AD brains leads to brain hypoperfusion and hypoxia (P. P. Liu et al., 2019). The consensus from prior research on AD is that its pathological complexity prevents any one hypothesis from completely describing its underlying mechanisms.
Understanding gene regulation, intra- and extracellular genomes, proteomes, and secretomes associated with cells and EVs is essential to defining diseased and healthy physiological states. It is necessary to investigate the related disease-relevant molecular pathways to interpret the significance of disease biomarker presence in biological fluids and tissues (Croese & Furlan, 2018). For example, AD has multiple proteinaceous and nucleic acid biomarkers associated with its diseased state.
Postmortem human AD patient brains have shown significant degeneration of microglia (Navarro et al., 2018) and astrocyte senescence (Han, Zhang, Liu, Mi, & Gou, 2020). Astrocytes are the most abundant cells in the brain and are primarily involved in CNS development, homeostasis, and support. Importantly, astrocytes are also activated in response to insult or injury to the brain. Astrogliosis, also known as astrocyte activation, typically results in increased neuroprotection and trophic support for neurons, isolates damaged tissue via glial scar formation, and contributes to tissue regeneration and BBB reconstruction (Rodriguez-Arellano, Parpura, Zorec, & Verkhratsky, 2016). In AD brains, astrocyte dysfunction results in a neurotoxic environment by promoting secondary apoptosis in neighboring cells including neurons. Also, AD astrocytes upregulate expression of GFAP, IL-1 family cytokines, and other inflammatory signals, leading to widespread neuroinflammation (Cui et al., 2019; Park et al., 2018; Reza-Zaldivar et al., 2018). Activated astrocytes have been found to surround senile plaques and NFTs, which are often located near brain vasculature (Kaur, Sharma, & Deshmukh, 2019). Widespread astrocyte apoptosis could act in concert with the aberrant reactivity of astrocytes and microglia associated with AD to exacerbate disease progression.
Microglia, the brain’s resident immune cells, are antigen-presenting cells that play important roles in CNS development, pathogen response, and tissue maintenance (Aguzzi, Barres, & Bennett, 2013). Under normal conditions, astrocyte and microglial activation maintains homeostasis by clearing Aβ plaques and phagocytosing debris to promote neuron survival. However, sustained microglial activation leads to the overexpression of inflammatory cytokines such as IL-1β and TNFα, which lead to cell degeneration, so the microglia and astrocytes can no longer clear plaques or fight neuroinflammation (Kaur et al., 2019). In particular, microglia fail to clear Aβ plaques and are suspected to secrete excessive and cytotoxic cytokines, reactive oxidative species, and degradative enzymes (Efthymiou & Goate, 2017; Keren-Shaul et al., 2017; Lue, Beach, & Walker, 2019).
As a secreted product of microglia known to play a role in many CNS pathways, microglia-derived EVs have been shown to play a role in propagating AD pathology. Microglia in AD brains internalize Aβ and traffic it in EVs for secretion, which promotes formation of soluble Aβ from the extracellular insoluble Aβ42 in plaques (Joshi et al., 2014). Elevated levels of soluble Aβ have been shown to impair synaptic plasticity and produce memory deficits in AD (Baruch et al., 2015). In contrast, microglia EVs in healthy brains attenuate neuron apoptosis, reduce Aβ levels, and regulate neuroinflammatory responses (Murgoci et al., 2018; Y. Song et al., 2019). These studies highlight how EVs are different in cells from disease states as compared to cells from healthy states.
Glial cell-derived EVs have also been shown to interact with neurons and promote the processing of APP in order to alleviate downstream inflammatory effects. In particular, astrocyte EVs were found to deliver a kinase CK1 to neurons through their secreted EVs in order to promote the processing of APP for increased Aβ deposition (Li et al., 2020). For AD specifically, astrocytes from AD patients and an AD mouse model demonstrated an ability to increase astrocyte reactivity and damage vasculature through secreted EVs (Gonzalez-Molina et al., 2021).
From our current understanding of AD pathology, it is becoming increasingly evident that EVs can be engineered to potentially target glial cells, address dysfunctional cellular processes involved in AD progression, and function as informative biomarkers for earlier diagnosis.
3. AD Diagnostics
3.1. Current AD diagnostics
In clinical practice today, both non-invasive imaging techniques and more invasive cerebrospinal fluid (CSF) examinations are used to diagnose AD (Weller & Budson, 2018). A common non-invasive technique is cerebral positron emission tomography (PET) scan with 18F-Florbetapir (Martinez et al., 2017). Florbetapir-PET imaging correlates with the presence and density of Aβ at autopsy, can diagnose AD with 96% sensitivity and 100% specificity, and is a robust tool for non-invasive testing during a patient’s lifetime (Carswell et al., 2018). However, measuring levels of protein amyloidosis through 18F-Florbetapir is not a reliable method to diagnose preclinical AD, a stage where amyloidosis may not be present (Edmonds et al., 2015). A study conducted at UC San Diego’s Neuroscience institute found that 24.2% of the 570 participants showed evidence of neurodegeneration but did not show any evidence of amyloidosis (Edmonds et al., 2015). Some of the participants also showed neurodegeneration first, later followed by amyloidosis. These findings suggest that the traditional imaging techniques that rely on amyloidosis to diagnose AD will not always be effective in preclinical stages of AD (Knopman et al., 2013).
A more invasive but affordable technique used to diagnose AD in clinical practice is withdrawing CSF from the patient, which is measured for the levels of Aβ42, hyperphosphorylated tau and total tau protein content (Weller & Budson, 2018). A comparison study between the PET-scan and CSF withdrawal concluded that there were no differences between the best PET and CSF measurements and no improvement when combining them (Palmqvist et al., 2015). This indicates that although either test can be used to aid the diagnosis of Alzheimer’s disease, neither is better at diagnosing and combining them does not improve early AD detection (Palmqvist et al., 2015).
Examining levels of protein aggregation with CSF and PET imaging do not reliably provide enough information to understand disease progression and the rate of cognitive decline in AD (Franzmeier et al., 2020). Although protein aggregation detection assists in diagnosing AD, analyzing EVs may provide a more holistic understanding of diseased cells. For example, EVs analyzed from postmortem blood and brain samples of AD patients were associated with BBB disruption, astrocyte hyperactivation, neuronal death and calcium dysregulation (Villar-Vesga et al., 2020). Analysis of these EVs revealed proteins involved in functions such as complement activation and blood coagulation (Villar-Vesga et al., 2020). Although EV diagnosing is in early stages of research, the amount of information that can be gathered with patient derived EVs is immensely important for further discovery in Alzheimer’s Disease. There is an urgent need for diagnosing patients earlier in life to prevent symptom onset (Dubois et al., 2016). However, advancements in diagnosis and AD treatment must develop in parallel because if early diagnosis becomes reliable and established, there may not be a suitable treatment method to complement it.
3.1. Extracellular Vesicles (EVs) as AD Diagnostic Biomarkers
EVs are 30–150nm lipid membrane-bound vesicles that are secreted by all cell types (Kalluri & LeBleu, 2020). A growing body of research supports EVs as potent downstream effectors and cellular signaling conveyors because they consist of the native cell’s plasma membrane. EVs can attach to target cells and be internalized to deliver EV cargo, modifying the target cell’s behavior. Formed by the inward budding of lipid membranes, EVs are bound by lipid bilayers and can be shuttled around the cell or secreted extracellularly, communicating targeting destinations via docking proteins such as Rabs, tetraspanins, intracellular adhesion molecules, ceramides, and SNAREs (M. Guo, Yin, Chen, & Lei, 2020).
EVs carry various cargos, such as proteins and functional miRNAs, and exhibit surface markers that can be delivered to and aid in targeting to specific cells, respectively. EVs drive many biological pathways such as immune response modulation and gene expression via intercellular transfer of RNA (Conlan, Pisano, Oliveira, Ferrari, & Mendes Pinto, 2017).
Many teams have found EV-associated proteins in AD patient autopsies and serum, plasmid, and CSF biopsies. It is established that EVs carry Aβ and tau and are thought to spread and exacerbate plaque and NFT formation. Additionally, other EV-associated proteins like flotillin have been detected in amyloid-β plaques in AD brains (Efthalia Angelopoulou, 2020). Furthermore, a recent study analyzing EVs from the CSF of AD patients noted that levels of three proteins (HSPA1A, NPEPPS and PTGFRN) were significantly increased in patients with AD compared to control patients and patients with mild cognitive impairment (MCI) (Muraoka et al., 2020). HSPA1A is a major heat shock protein that prevents environmental stress by preventing tau aggregation, NPEPPS is a protein that is known to protect against tau-induced neurodegeneration, and PTGFRN plays a major role in the gamma secretase complex. Overall, the increased level of these proteins in patient CSF EVs was associated with disease progression (Muraoka et al., 2020).
EVs carry proteins and present surface markers that may serve as diagnostic tools for the other hypotheses of AD, such as the inflammatory and vascular hypotheses, aside from amyloidosis (Figure 1A). However, this theory has yet to be solidified and standardized with widespread data collection. Unique biomarkers are needed to diagnose Alzheimer’s in different stages. EVs specific to AD can be beneficial to track AD progression. Since neurodegenerative diseases often result from the alteration of cellular pathways, miRNAs may play a regulatory role in AD inception and progression. Researchers have already found biomarkers co-localizing with EVs from AD patients, who seem to have lower EV miR-193b levels, among numerous other disease markers found in the CSF and serum (C. G. Liu, Song, Zhang, & Wang, 2014). Recently, a study has found significantly increased levels of miR-23a-3p, miR-223-3p, miR-190a-5p, which are involved in regulating cell proliferation, tumorigenesis, and hypoxic responses, and decreased levels of miR-100-3p, which acts as a tumor suppressor miRNA, in Alzheimer’s patients neural-derived EVs (Serpente et al., 2020). These neural-derived EVs were isolated through immunopurification based on L1CAM or CD81 surface markers from blood plasma, the significance of which is discussed in the conclusion. In the future, patients with AD may be diagnosed accurately at all stages of the disorder using AD specific EVs.
It is established that in Alzheimer’s patients, EVs can carry Aβ and tau and are thought to spread and exacerbate plaque and NFT formation. Alzheimer’s related miRNA research is constantly expanding to reveal novel associated biomarkers for detection of AD. For example, downregulation of tau-regulatory miR-219 has been found to be highly conserved in postmortem AD brains and patients with severe primary age-related tauopathy (Santa-Maria et al., 2015). Manna et al. provide a richly informative review of AD-related miRNA in modulating key processes in AD onset: Aβ accumulation, tau-dependent toxicity, inflammation and neuronal death (Manna et al., 2020). One recent study evaluated miRNA contents in CSF-EVs from sporadic AD patients with early disease onset, and observed altered miR-16-5p, miR-451a, miR-605-5p, and miR-125b-5p expression, which is broadly associated with neuronal apoptosis and proliferation. (McKeever & Agius, 2018). In plasma-derived EVs from patients with AD, dementia, and healthy controls, multiple miRNAs commonly known for their roles in tumor cell invasion and proliferation were found to be differentially regulated in the AD patients compared to dementia patients and healthy controls in addition to pathological proteins including Aβ, α-syn and tau (Gamez-Valero et al., 2019). This study highlights the potential of EV miRNAs in discriminating between two similar but different neurodegenerative dementias. These miRNAs may become known biomarkers of AD if they are found in other AD patients.
Although there is some inconsistency in literature, the most consistently reported EV-associated miRNAs upregulated in AD are miR-9, -135a, -146a, -155, and miR-384, and the most common downregulated miRNAs in AD are miR-193b and the let-7 family (Amakiri, Kubosumi, Tran, & Reddy, 2019; Pluta, 2018; Yang, Liu, Gao, Zhang, & Wang, 2018). These upregulated miRNAs are primarily involved in Aβ regulation while down-regulated miRNAs contribute to oxidative stress. There may be inconsistency due to the source of the miRNAs, which ranged from EVs, CSF, blood, and brain tissue, potentially contributing to the heterogeneity of results. This adds to one of the main challenges facing EV isolations: heterogeneous EV populations. Of note, miR-29a and miR-520d-3p target the mRNAs for BACE1 and amyloid precursor protein (APP), two AD drivers in the amyloid hypothesis of AD (Gayen, Bhomia, Balakathiresan, & Knollmann-Ritschel, 2020). These small RNAs can be considered AD biomarkers and can be used as a starting point for establishing disease-relevant biomarkers.
3.1.1. Extracellular Vesicles and glial cells
EVs and their miRNA cargo provide unique biomarkers for testing the inflammation status of glial cells in neurodegenerative disease. For instance, when astrocytes and other glial cells are activated or shifted to their pro-inflammatory state, they differentially express certain miRNAs. miR-126, miR-130b, miR-139-5p, and miR-141-3p have been shown to be upregulated by IL-1β activated astrocytes and released in EVs (Gayen et al., 2020). In accordance with canonical EV biogenesis pathways, these activated astrocyte-derived EVs have upregulated miRNAs with predicted roles in apoptosis, neuroinflammatory and neurodegenerative signaling pathways. Of note, miR-141-3p is a serum plasma biomarker of AD and chronic inflammatory pain (Gayen et al., 2020). In microglia, miR-9 and miR-124 play important roles in microglial regulation and activation in neuroinflammation and neurodegenerative diseases (Y. Song et al., 2019). These two miRNAs are made by neurons and are horizontally transferred between neurons and microglia via EVs (Veremeyko et al., 2019). Since reactive gliosis and astrogliosis are common in AD brains, these miRNAs may provide information about the altered intercellular communications characteristic of AD related physiological states and serve as biomarkers.
Recent studies have identified several groups of miRNAs that serve as glial cell signatures. Following isolation, microglia-derived EVs were found to possess a miRNA signature of six vesicular miRNAs with validated neuroprotective functions (Lemaire et al., 2019). EVs obtained from stimulated glial cells were determined to contain altered miRNAs compared to pro-regenerative microglia (Prada et al., 2018). Screening found three miRNAs of interest, miR-146a-5p, miR-181a, and miR-223, all of which have validated dendritic targets and are upregulated in EVs secreted by inflammatory microglia (Prada et al., 2018). This indicates that glial cell-derived EVs possess certain miRNA signatures indicative of the state of their parent cell. Identification of altered miRNA profiles can provide important tools for the use of EVs as AD biomarkers.
Astrocyte-derived EVs also play a key role in the central nervous system and Alzheimer’s disease progression. In AD, astrocytes are known to engulf a significant portion of aggregated protein like Aβ42 protofibrils and release these proteins in their EVs, causing severe toxicity in nearby neurons (Upadhya, Zingg, Shetty, & Shetty, 2020).
In a study done by Goetzl and team, the protein composition of astrocyte-derived EVs (ADEVs) was analyzed in Alzheimer’s patients (Goetzl et al., 2016). AD patients had a higher level of BACE-1 (β-site amyloid precursor protein-cleaving enzyme 1) and sAPPβ (soluble amyloid precursor protein β) in their ADEVs compared to control samples. These ADEVs from AD patients also had a lower level of GDNF (glial-derived neurotrophic factor). Altered levels of proteins in astrocyte-derived EVs may directly influence neuronal health and drive AD progression.
EVs released from astrocytes do not contain the exact miRNA profile of astrocytes themselves. Astrocytes can modulate the miRNA profiles of EVs that they release to influence functions in other cell types. Moreover, certain miRNAs found in astrocyte-derived EVs have been implicated in disease states. For instance, miR-297a has been shown to be upregulated in post-ischemic cortex and miR-432 could be a biomarker for schizophrenia (Jovicic & Gitler, 2017). Overall, astrocyte-derived EVs remain an enticing platform for further research to find a diagnostic biomarker for Alzheimer’s disease.
3.1.2. Using extracellular vesicles as a diagnostic tool: final thoughts
EV-associated biomarkers have potential to be used as diagnostics in the future. Patient care can be drastically improved and streamlined if identified biomarkers can be used to indicate predispositions to develop AD or other neurodegenerative diseases later in life. For example, a miRNA panel in patient serum or CSF samples could be used to improve early-stage AD diagnosis (Manna et al., 2020). Recently, the first study using single molecule array antibody sandwich technology found that blood phosphorylated tau levels could be used to predict tau and Aβ pathology and differentiate AD patients from other neurodegenerative disorders (Karikari et al., 2020). EV-associated biomarkers have been found in AD patient autopsies and serum, plasmid and CSF samples (C. G. Liu et al., 2014). However, work is needed to achieve a high level of biomarker knowledge and clinical significance. Additionally, it is imperative to identify cell type specific markers on EVs for reliable detection methods, diagnosis standardization, and EV isolation techniques. For example, one group recently found the L1CAM neuronal-derived EV marker was not associated with neuronal EVs (Norman et al., 2021). Although this is a single paper, the larger impact indicates that it will be useful for EV researchers to conduct similar studies on EVs from other cell types to validate their markers. This is discussed further in the conclusion section. Taken together, these findings support studying differential gene expression and regulatory networks to understand biomarker identification to determine predisposition to AD and neurodegenerative disease states and develop evidence-based therapies.
4. AD Therapeutics
4.1. Current AD therapeutics
Only six drugs are currently FDA-approved for the treatment of Alzheimer’s disease and no new drug has been approved by the FDA since 2003 with the exception of the recently approved drug Aducanumab (Cummings, 2021). To date, no approved drug addresses AD pathology, amyloid plaques, or neuroinflammation at all, and all fail to slow or stop disease progression. Donepezil (Aricept), galantamine (Razadyne) and rivastigmine (Exelon), are cholinesterase inhibitors; they prevent the breakdown of acetylcholine, a neurotransmitter important for learning and memory (Yiannopoulou & Papageorgiou, 2020). Cholinesterase inhibitors have been effective as cognitive enhancing agents and show improvement for measures of cognition, global function and stabilization of activities of daily living as compared to placebo treatments (Cummings, 2021). However, cholinesterase inhibitors only benefit patients with cognitive symptoms and do not help patients with mild and asymptomatic forms of Alzheimer’s (Cummings, 2021). The fourth drug, memantine (Namenda) is an NMDA receptor antagonist that protects cells from excess stimulation of NMDA receptors by glutamate, preventing cell death. The fifth drug is a combination of donepezil and memantine and has the brand name Namzaric (Yiannopoulou & Papageorgiou, 2020). Lastly, accelerated approval was given to Aducanumab, which seeks to reduce Aβ plaques to alleviate AD-related symptoms. However, all six FDA approved drugs fail to address the neuropsychiatric symptoms of AD like agitation, psychosis, apathy, etc. and none directly target AD mechanism and progression, leaving a need for new AD therapeutics (Cummings, 2021).
Currently, there are 121 agents in clinical trials for AD. None of these trials include drug therapy for depression seen in AD patients. Of the 121 trials proposed, 80.2% are disease-modifying agents, 9.9% are cognitive enhancing agents and 9.9% are agents for neuropsychiatric symptoms (Cummings, 2021). Targeting behaviors specific to AD will be difficult in these clinical trials due to the absence of known symptom-specific biology for the neuropsychiatric symptoms of AD (Cummings, 2021). There is a need for more sensitive outcome measures, better trial participant characterization, and the use of biomarkers to improve the success of drugs in the pipeline for FDA approval (Cummings, 2021).
4.2. EV Engineering
EVs are versatile in their use and show dual promise as biomarkers for AD diagnostics and potential therapeutics. EVs can be easily modified or loaded with macromolecule cargo, both of which fall into the field of EV engineering. EVs derived from different cell types and loaded with cargo targeting disease-specific proteins and cells have provided therapeutic benefits in CNS disease and injury, including spinal cord injury, multiple sclerosis, and Alzheimer’s disease (Alvarez-Erviti et al., 2011; Didiot et al., 2016; S. Guo et al., 2019; Ren et al., 2019; Shamili et al., 2018; T. Tian et al., 2018).
4.2.1. EV modifications
EV lipid membrane bilayers can accommodate transmembrane proteins and lipid- or sugar-modified peptides but can also be modified in other newly established ways. For example, aptamers have emerged as a promising targeting ligand for nanodelivery. These single-stranded DNAs or RNAs fold to create 3D structures that can be attached to ligands on EV surfaces. Once the EVs arrive at their target location, they can either get undergo endocytosis or purely interact with a surface receptor and induce a response in the cell. Future EV therapeutics for neurodegenerative diseases like AD may employ aptamers as surface markers or payloads to improve targeting or effective cellular signaling. Though EVs often bear some markers of their parent cell, they typically carry EV-specific surface proteins and cargo (Z. Song et al., 2020). Harnessing common EV biomarkers, such as transmembrane proteins, may provide a platform to conjugate functional ligands or molecules that have high affinity to target cell surface markers (Figure 1). Table 1 addresses several studies that used modified EV surfaces to increase therapeutic efficacy. To address AD physiology at the cellular level, it will be advantageous to leverage bioengineered surface ligands to home directly to astrocytes and other glia.
Table 1.
Reviewing recent studies utilizing EVs engineered with specific surface modifications.
| EV Surface Modification: | Example: | EV Cell Source: | Major Findings: | Reference: |
|---|---|---|---|---|
| Aptamer | Covalently conjugated IJM-3064 aptamer | Mouse bone marrow mesenchy mal stem cells (BM-MSCs) | EVs with a surface-bound aptamer targeting myelin significantly reduced inflammatory cell infiltration into the CNS and demyelination in a mouse model of multiple sclerosis. | (Shamili et al., 2018) |
| Targeting Peptide | CNS-specific RVG peptide via DOPE-NHS linker | Mouse BM-MSCs | In AD mice, RVG-EVs improved targeting to cortex and hippocampus, rescued learning, and memory difficulties, and were associated with decreased amyloid-β plaque deposition and inflammatory cytokines. | (Cui et al., 2019) |
| EV Surface and Cargo Modifications: | Example: | |||
|
Surface: Chimeric protein Cargo: siRNA |
Surface: DARPin G3 protein with LAMP-2b attachment Cargo: siRNA against TPD52 |
Human embryonic kidney cells (HEK293T) | In vitro: EVs engineered with DARPin G3 were used to target HER2/neu positive breast cancer cells. siRNA against oncogene TPD52 were loaded into these engineered EVs and successfully downregulated TPD52 gene expression in HER2 positive cell lines. | (Limoni, Moghada m, Moazzeni, Gomari, & Salimi, 2019) |
|
Surface: Targeting peptide Cargo: Aptamer |
Surface: Lamp2b fused to RVG peptide Cargo: Aptamer specific for asynuclein protein |
Human embryonic kidney cells (HEK293T) | Aptamer-loaded RVG-EVs significantly reduced α-synuclein pathological aggregates and rescued neuronal death in vivo in a mouse model of Parkinson’s disease, a common neurodegenerative disease. | (Ren et al., 2019) |
|
Surface: Targeting Peptide Cargo: Protein |
Surface: Integrin-targeting c(RGDyK) peptide Cargo: Curcumin |
Mouse BM-MSCs | EVs targeted to reactive cerebral vascular endothelial cells intravenously injected into mouse models of ischemic stroke reduced inflammation and inhibited apoptosis in lesion sites. | (T. Tian et al., 2018) |
As many past clinical trials have used more broad, nonspecific treatments to alleviate symptoms of AD with little success, more focus has been placed in recent years on developing targeted treatments for both decreased off-target effects and increased desired effects. One method for modifying EVs involves fusion of vesicle or liposome membranes to EVs to combine the surface proteins of the respective membranes. To date, EV surface engineering via membrane fusion has not been researched in the context of central nervous system diseases. However, protocols for membrane fusion between EVs and liposomes have been established for other indications (Sato et al., 2016). This allows for novel engineering of EVs by essentially combining the plasma membranes of brain-specific cell types or artificial liposomes to increase the targeting efficiency of therapeutic EVs.
Besides chemical and membrane fusion methods to bioengineer the surface of EVs, a more bottom-up approach is to create synthetic EVs. Many groups are actively creating synthetic EVs, also known as EV mimetics, to have fine control over the surface ligand expression. For example, microfluidics approaches have resulted in the development of biomimetic nanovesicles with higher uptake and immunomodulatory properties compared to liposomes (Molinaro et al., 2018). Extrusion approaches have also been applied to cancer immunotherapy to created hybrid nanovesicles that modulate macrophage responses (Rao et al., 2020). These techniques can be applied to AD to create well-defined EV mimetics for more targeted treatments.
EVs naturally contain a mixture of small RNA and peptide species, and the specific contents are a function of the originating cell type and the physiological context. MiRNAs play critical roles in the CNS, and are involved in neurogenesis, glial cell regulation, development, immunomodulation, and spinal cord regeneration (Reza-Zaldivar et al., 2018). miRNAs have wide-ranging abilities, such as regulating gene expression, cellular functions, and inflammatory response (Jiang, Vader, & Schiffelers, 2017; Reza-Zaldivar et al., 2018). Using EVs to deliver therapeutic miRNAs to target cells like astrocytes and microglia may alter their gene expression, potentially changing the cell’s diseased state to a healthy one. This miRNA delivery may be able to induce astrocytes to clear plaques, or induce a reduction in neuroinflammation, slowing AD progression. Table 2 highlights several recent studies that loaded macromolecules into EVs for in vivo treatment. EVs can be engineered to carry desired cargo in several methods, including electroporation, diffusion, or genetically modifying the parent cell to synthesize specific macromolecules that become enveloped in the secreted EVs (Figure 3). The composition, electrical charge, and size of cargo should be carefully evaluated to choose the most efficient loading method.
Table 2.
Reviewing recent studies utilizing EVs loaded with therapeutic cargo.
| Engineered EV Cargo | Example: | EV Cell Source: | Major Findings: | Reference: |
|---|---|---|---|---|
| Cargo: siRNA | PTEN-siRNA | BM-MSCs | When administered intranasally to rat models of spinal cord injury, MSC-EVs loaded with PTEN-siRNA via simple incubation silenced PTEN expression in spinal cord lesions, regenerated corticospinal axons, and resulted in significant functional and structural recovery. | (S. Guo et al., 2019) |
| Cargo: siRNA | lincRNA-Cox2-siRNA | Mouse primary astrocytes | In vivo: Intranasally delivered lincRNA-Cox2-siRNA loaded EVs could reach the brain and reduce LPS-induced microglial proliferation | (Liao et al., 2020) |
| Cargo: siRNA | LincRNA-Cox2-siRNA | Mouse primary astrocytes | In vivo: EVs via intranasal administration containing lincRNA-Cox2 siRNA were able to restore microglial phagocytic activity in mice administered with morphine. | (Hu et al., 2018) |
| Cargo: hsiRNA | HsiRNA targeting Huntingtin mRNA |
Human primary U87 glioblastoma cells |
In vitro: hsiRNA-loaded EVs were taken up by mouse primary cortical neurons and silenced Huntingtin (Htt) mRNA and HTT protein expression. In vivo: EVs induced bilateral Htt mRNA silencing after a unilateral stereotactic injection into striata of the mouse brain. This suggests that the EVs can travel and unload gene-regulating cargo efficiently. |
(Didiot etal., 2016) |
| Cargo: Protein | Prostaglandin receptor antagonist | Human BM-MSCs | In vivo: EVs suppressed inflammatory cytokines, exhibited sustained inhibition of reactive astrocytes, and rescued the memory and learning deficiencies induced by hippocampal damage in mice. | (S. Y. Chen et al., 2019) |
Figure 3.
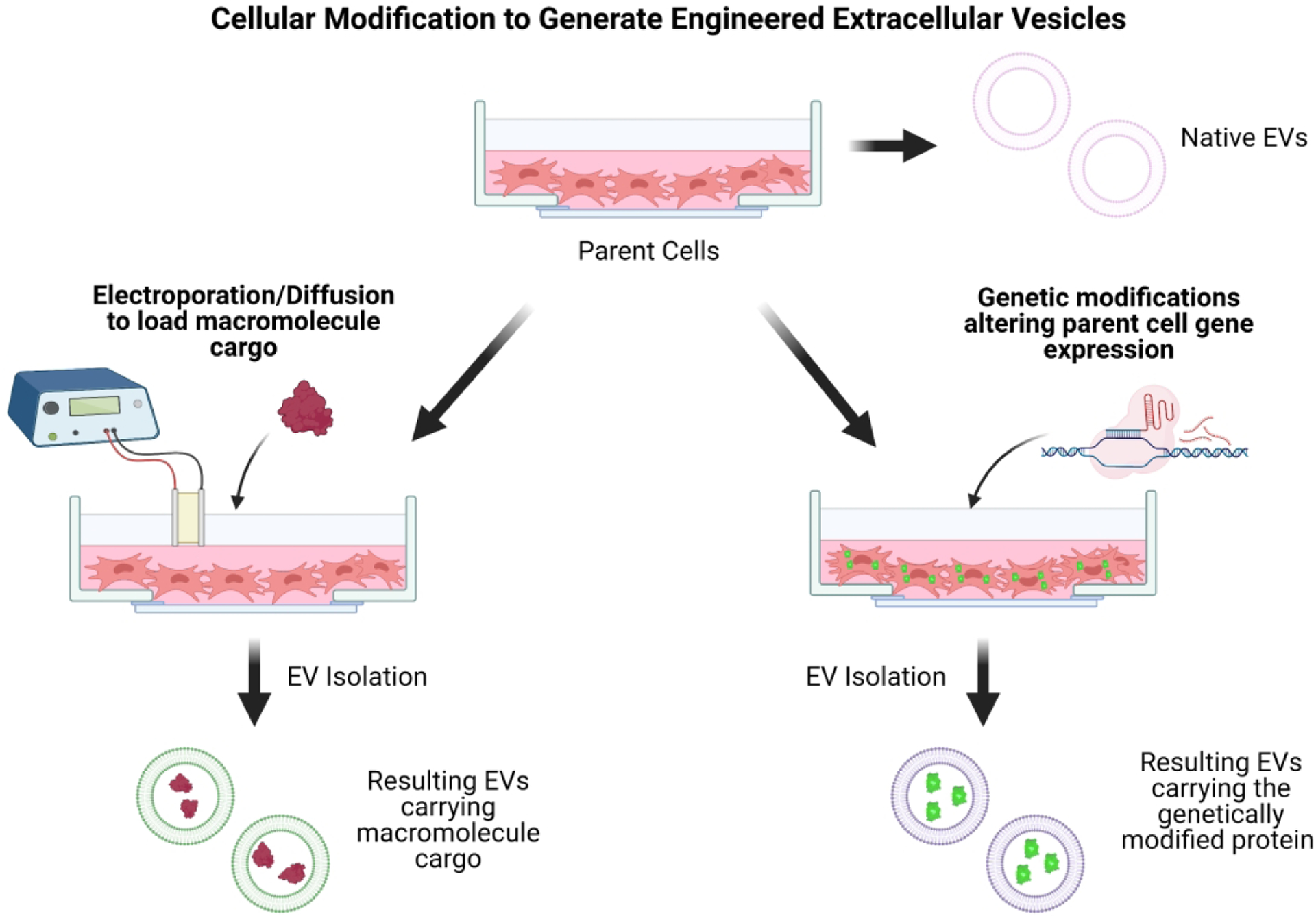
Cellular modification to generate engineered extracellular vesicles. Parent cells that will secrete the desired EVs can be modified genetically or induced through electroporation or diffusion to take up specific cargo to incorporate into EVs.
4.3. Bioengineered EVs as AD Therapeutics
EVs have been demonstrated to ameliorate the common pathologies of AD mentioned previously, including Aβ plaque clearance (Yuyama et al., 2015), downregulating reactive astrogliosis (M. Guo et al., 2020), and preventing apoptosis in neurons and oligodendrocyte precursor cells (Reza-Zaldivar et al., 2018). EVs have also been applied to a variety of in vitro and in vivo models and disease systems as a potential therapeutic, such as Parkinson’s disease, multiple sclerosis, stroke, and ischemia (Qu et al., 2018; Shamili et al., 2018; Y. Song et al., 2019; Zheng, Huang, Ma, Chen, & Gao, 2019). Cell-free approaches are the next step in Alzheimer’s treatment; EVs do not divide or metabolize, eliminating the risk of cancer induction and uncontrolled cell division associated with cell therapies. EVs are easily modified to carry cargo such as macromolecules, drugs, and membrane proteins targeting specific receptors on desired cell types (Figure 2). Additionally, they are nano-sized and easily cross the BBB. Engineered EVs show great potential in therapeutics of neurodegenerative disorders like AD (Figure 1B).
Figure 2.
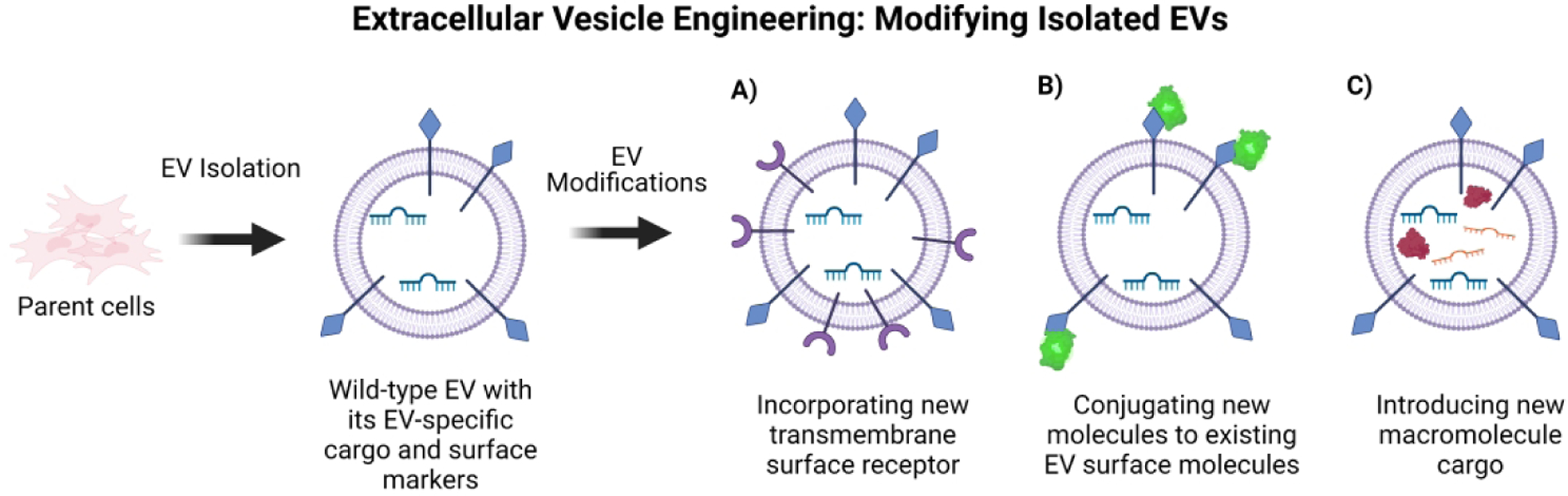
Overview of extracellular vesicle engineering. EVs can be modified once they are collected and purified from parent cells and can include A) new surface markers, B) have new molecules physically conjugated to their existing surface markers, or C) contain new cargo macromolecules.
One significant category of EVs to note in the context of AD are glial cell-derived EVs. These include EVs obtained from either microglia or astrocytes, which subsequently can exhibit various phenotypes depending on their activation state. Significant research has already been conducted investigating the role of native glial cell-derived EVs in the CNS microenvironment. Recently, part of the multifaceted role of microglia in the brain and specifically in AD pathology was attributed to microglial EVs and their role in multi-target signaling (Van den Broek et al., 2020). Because glial cell-derived EVs naturally interact with many of the cell types of interest in the brain, they represent promising candidates for targeting the underlying mechanisms of AD pathology. For example, intranasal delivery of EVs isolated from astrocytes and loaded with lincRNA-Cox2-siRNA were able to reduce LPS-induced microglial proliferation in mice. Astrocyte derived EVs with lincRNA-Cox2-siRNA cargo were also able to restore microglial phagocytic activity in morphine mice (Hu et al., 2018) (Table 2). Engineering both their surface and interior contents would be a novel approach towards the development of cell-free therapeutics for AD treatment.
4.3.1. EV treatment decreases amyloid plaque deposition
Generally regarded as the main driver of AD under the amyloid hypothesis of AD, Aβ protein plaques accumulate extracellularly and lead to neurotoxicity, neuron apoptosis, and DNA damage (G. F. Chen et al., 2017). Aβ generated by APP cleavage has been found in AD patient CSF-derived EVs (Kalluri & LeBleu, 2020; Rajendran et al., 2006). To address Aβ plaques, bioengineered MSC-EVs with a surface RVG peptide have been shown to target activated astrocytes in APP/PS1 mice, a murine model of AD. The EV-treated mice had decreased Aβ plaque deposition and levels of Aβ and pro-inflammatory mediators secreted, and even showed improved cognitive function in memory tasks (Cui et al., 2019). Murine dendritic cell-derived EVs have been designed to target neurons in vitro via surface modification with the neuron-specific RVG peptide. The RVG-EVs, loaded with siRNA, silenced beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) expression in cultured Neuro2A cells and lowered the concentration of Aβ42 in mouse cortical sections (Alvarez-Erviti et al., 2011).
4.3.2. EVs attenuate neuroinflammation and target reactive astrogliosis
Activated astrocytes and microglia in AD upregulate and secrete high levels of pro-inflammatory proteins and cytokines, leading to chronic neuroinflammation. MSC-EVs have been shown to inhibit in vivo astrocyte activation in mice (Cui et al., 2019). Intraventricularly injected MSC-EVs in mice attenuated an induced inflammatory hippocampal brain response and decreased TNFα and IL-1β expression (Xian et al., 2019). The same injected MSC-EVs targeted astrocytes, exerted therapeutic effects at the cellular level and led to improved scores on a mouse memory task compared to control mice (Xian et al., 2019). In a murine model of inflammatory brain injury, astrocyte derived EVs have been shown to regulate peripheral acute cytokine responses to brain lesions and promote leukocyte transmigration into the brain (Dickens et al., 2017; Venturini et al., 2019). Microglia-derived EVs have also been validated in a mouse model to exert neuroprotective effects following neuronal uptake in an ischemia-reperfusion injury model (Y. Song et al., 2019). Using EVs from astrocytes and microglia may provide an advantage in targeting engineered EVs to their target cells smoothly or allow them to be taken up selectively as opposed to other cell types, increasing drug efficacy. In mouse models of multiple sclerosis, oligodendrocyte-derived EVs have been shown to suppress the overactive immune cells and downregulate monocyte gene expression of pro-inflammatory cytokines such as IL-1β, which are commonly associated with AD and reactive gliosis (Casella et al., 2020). Placenta-derived mesenchymal stromal/stem cell (PMSC) derived EVs have been used to promote neuron regeneration and exert neuroprotective and immunomodulatory effects in the CNS (Kumar et al., 2019; Xian et al., 2019). In the context of AD, EV treatment may attenuate neuroinflammation, potentially decreasing reactive gliosis that exacerbates disease progression. Instead of constant immunosuppression, researchers seeking translational work must consider that successful EV treatment research trials need to show a delicate balance of maintaining homeostatic regulation of neuroinflammation.
4.3.3. EVs are associated with angiogenesis and improved neurovasculature
Neurovascular dysfunction is ubiquitous in the AD brain and has even been proposed to be a “vascular hypothesis of AD” (Govindpani et al., 2019). The BBB and its many endothelial cells are in direct contact with astrocytes and microglia, which support neurons. The BBB and brain blood vessels are incredibly important to AD pathology; early qualitative studies reported distorted arterioles and capillaries in AD brains, and many AD patients exhibited a general reduction in total mean arterial blood flow in major cerebral arteries (Govindpani et al., 2019). The interaction between EVs surface molecules and adapter molecules on the surface of endothelial cells has been shown to regulate EV internalization by the endothelial cells (Matsumoto, Stewart, Banks, & Zhang, 2017). Vascular dysfunction is a common problem in AD and other neurodegenerative diseases. MSC-EVs have been demonstrated to promote in vivo neurovascular plasticity in rats post-stroke induction (Xin et al., 2013). In an ex vivo rat aortic ring assay, PMSC-EVs significantly promoted angiogenesis and vessel sprouting (Hao et al., 2020). These results show potential in using MSC-derived EVs for vascular regeneration, which may ameliorate the distorted blood vessels in AD brains.
In terms of glial cell-derived EVs, microglia have been shown to promote angiogenesis in an EV-dependent manner. In a mouse ischemia model, polarized BV2 microglia secreted EVs to restore damaged vasculature following stroke, potentially through the increased miR-26a content in the secreted microglia EVs (Y. Tian et al., 2019). Native, unmodified EVs can be further improved by surface engineering for targeting applications or exogenous cargo loading for increased therapeutics effects such as angiogenesis. As EV engineering techniques continue to progress and protocols for EV yields are optimized, there will be increasing opportunities for exploring the potential of engineering glial EVs in targeting processes such as neurovascularization.
4.3.4. EVs repair disrupted blood-brain barrier
Another hallmark of AD is a permeable BBB, indicating partial disruption or dysfunction of the barrier’s selective permeability (S. Y. Chen et al., 2019; Matsumoto et al., 2017). This detrimental mechanical change allows previously prohibited proteins, cells, and other substrates into the vulnerable peri-neural space. It is thought that this mechanical breakdown is a precursor to increased neuroinflammation, because reactive T cells and other immune cells are now free to invade the brain (Govindpani et al., 2019). In the 5XFAD mouse model of familial AD, neural stem cell derived EVs (NSC-EVs) repaired the disrupted BBB in vitro (Y. Liu et al., 2020). In the study, sodium fluorescein and Evans Blue dye were injected intraperitoneally in wild-type and NSC-EV administered mice. Immediately after, cardiac blood samples were collected, mice were perfused, and brain cross sections were examined. The AD mice given NSC-EVs lacked dye in the blood samples and showed healed BBBs comparable to age-matched wild-type mice (Y. Liu et al., 2020). In vivo, bone marrow MSC-EVs loaded with prostaglandin antagonists administered to mice attenuated inflammation, inhibited reactive astrogliosis, and increased BBB integrity (S. Y. Chen et al., 2019). The pathological migration of immune-reactive cells across the BBB may play a role in AD-related neurodegeneration, but using EVs to repair the permeable BBB may be a potential therapeutic application.
4.3.5. EVs rescue AD-related loss in memory and motor function
AD-related dementia is thought to arise largely from neuronal and glial cell death. EVs secreted from PMSCs have a neuroprotective and antioxidant effect on neurons and can increase the number of neurites and overall neuron number in vitro (Kumar et al., 2019). Additionally, neurons, microglia, and astrocytes have been shown to secrete EVs containing inflammatory cytokines after induced nervous tissue damage (Bianco et al., 2009; Kalani, Tyagi, & Tyagi, 2014). These secreted EVs may be useful for diagnostic applications; collecting patient derived EVs and analyzing their secretomes may indicate EV changes between healthy and diseased states.
A hallmark symptom of AD is memory loss, which is predominantly caused by neuronal and glial cell death in the hippocampus and other brain areas (Bature et al., 2017; Reza-Zaldivar et al., 2018). Memory loss often leads to frustration and conflict between patients and their caregivers (Mortazavizadeh, Maercker, Roth, Savaskan, & Forstmeier, 2020). In vivo EV treatments in murine models have shown promising results in memory and learning rescue and enhancement.
For example, unmodified human umbilical cord mesenchymal stromal cell (hUCMSC) derived EVs have been demonstrated to restore learning and memory impairment in a mouse model of status epilepticus (Xian et al., 2019). MSCs exert therapeutic effects through paracrine signaling, and within those bioactive factors, EVs may be key to those processes (Kalluri & LeBleu, 2020). In APP/PS1 Alzheimer’s mice, MSC-derived EVs engineered with an RVG surface peptide rescued memory deficits compared to the control AD mice (Cui et al., 2019). Since the hippocampus is commonly affected in AD patient brains, another team induced hippocampal damage in mice and recorded their baseline and post-damage memory task scores. Then, injected BMSC-EVs loaded with a prostaglandin receptor antagonist were able to suppress inflammatory cytokines and rescued the memory and learning deficiencies caused by hippocampal damage (S. Y. Chen et al., 2019). MSC-EVs applied to neurons reduced apoptosis at lesion site, enhanced neurogenesis, and ameliorated neurological deficits (Harris et al., 2018; Harting et al., 2018; Reza-Zaldivar et al., 2018). In APP/PS1 mice, a model of AD, MSC-EVs bioengineered to target activated astrocytes inhibited astrocyte activation and rescued mouse memory deficits (Cui et al., 2019). Furthermore, there was a reduction in plaque deposition, Aβ levels, and normalized inflammatory cytokine levels such as TNFα and IL-β in the mouse brains (Cui et al., 2019). Though these results are optimistic, the differences between murine and human brains are extensive and cannot be seamlessly translated. More work is needed, but future clinical trials may examine the potential memory benefits of EVs in patients with Alzheimer’s or dementia.
Regarding motor loss due to neuronal death in AD, MSC-EVs have been demonstrated to improve motor function and attenuate myelin loss and Purkinje cell apoptosis in mice (You et al., 2020). This group performed tail vein injection of MSC-EVs in mice modeling Machado-Joseph disease, a dominant hereditary ataxia condition (Bondi et al., 2017). EV therapy for motor dysfunction has yet to be tested in animal models of AD and humans.
In these many ways we see the incredible potential of EVs as a powerful and multipotent cell-free AD therapeutic. Taken together, these findings suggest the use of EVs as a cell-free therapeutic and the necessity of investigating EVs from a variety of parent cell lines. We propose the use of bioengineered EVs as the next step in AD research by targeting AD biomarkers in astrocytes and microglia (Figure 1B).
4.4. EVs in Clinical trials
Though the body of in vitro and in vivo EV research is promising and growing rapidly, we must critically examine the therapeutic potential of EV therapy in humans. There are several completed and active EV trials pledging to study various diseases. For therapeutic EV treatments, EVs can be left unmodified to examine their abilities in their natural state. Alternatively, the field of EV bioengineering has led to promising results in human trials and in vitro and in vivo studies.
Only a few clinical trials in recent years have explored the therapeutic and diagnostic applications of EVs in neurodegenerative disease. NCT01860118 studied EV biomarkers from the blood and urine of patients with Parkinson’s disease. Though the results are not yet announced, this study is an example for other neurodegenerative diseases to utilize EV biomarkers in studying disease states. Investigating progression or notable changes in EV proteomes in patients over time may provide useful information on how aging or prolonged drug treatment may alter EV content.
NCT04388982 is an actively recruiting clinical trial to evaluate the safety and efficacy of allogenic adipose MSC-derived EVs in Alzheimer’s patients. They plan to measure patient cognition and quality of life, as well as changing levels of amyloid-β in serum and CSF. Investigating these unmodified EVs might shed light on their intrinsic ability to reduce neuronal apoptosis and exhibit neuroprotective effects. NCT04040348 plans to examine the therapeutic effects of MSC infusions in patients with mild to moderate Alzheimer’s disease. They will assess cognitive function, depressive symptoms, quality of life for the participant and caregiver, and levels of serum biomarkers such as ApoE and Tau protein. Although MSCs are promising in their anti-inflammatory and neuroprotective powers, the team must consider how to identify and ameliorate stem cell “trapping” in the lungs and vasculature, or potential cell proliferation which could lead to tumorigenesis. MSC-EVs therefore pose a promising, cell-free, alternate therapy that avoids issues related to stem cell treatment. NCT04575337 is an actively recruiting clinical trial to collect, detect, and screen biomarkers at multiple stages of Alzheimer’s disease and compare them to age-matched elderly patients with normal cognitive function. This panel of biomarkers could be used to diagnose early-stage AD or establish a risk-prediction model for AD to create prophylactic measures. Since EVs are released from the CNS and in AD patients are known to carry AD biomarkers such as certain miRNAs and proteins, further trials may compare this study’s results with patient-derived EV cargo sequencing and characterization.
5. Risks and Challenges
As with any clinical ventures we must critically examine any potential risks and challenges. The first posed challenge is ensuring the engineered EVs will cross the BBB in humans. It is well-established that EVs easily cross the BBB without altering or losing their internal contents or surface markers (Kalluri & LeBleu, 2020). It has been found that upon IL-1β intracerebral injection in mice, astrocytes released increased numbers of EVs that crossed the BBB in vivo and went to the liver, lung, and spleen (Dickens et al., 2017). A subsequent challenge concerning the clinical applications of EVs is developing a robust biodistribution validation method for humans instead of rodents. This validation method must accurately and reliably detect and report that the EVs have arrived at their target location and maintain their bioengineered identity. This method may also be useful in testing the half-life or duration of EVs in patient brains, potentially using real-time imaging for fluorescently tagged EVs.
To provide a complete picture of EV therapy’s impact on patients, long-term regulation and re-administration of EVs and their downstream effects must be examined. Although it is established that EVs can migrate to certain target sites in vivo and are often selectively taken up, critics and researchers alike seek to evaluate long-term therapeutic effects. EVs are often lauded for having a longer half-life than MSCs (Sarko & McKinney, 2017). With the advent of EV nanoparticle tracking analyses, many teams are currently studying EV biodistribution. In mice, EVs administered intravenously and modified with fluorescently tagged CD63 membrane protein have shown via blood kinetic analysis to be cleared rapidly from circulation, having a half-life below ten minutes (Lazaro-Ibanez et al., 2021). Beyond this recent study, detailed analyses of EV half-life in vivo have not been robustly established (Verweij et al., 2019). Adding to the uncertainty, EV secretion and degradation patterns are not well understood, and some researchers have proposed that modifying EVs may jeopardize their biodistribution and half-life (Kwok et al., 2021; Lazaro-Ibanez et al., 2021).
Generating sufficient EV numbers for human dosages is limited by the notoriously heterogeneous nature of EVs obtained through isolation. Homogeneous EV populations are essential for creating standardized therapies. The EV production team must go through a series of arduous and time-intensive steps: generate and isolate EVs, identify and sequester the desired EVs in the population, and then obtain enough to fill a dose. EV heterogeneity is not only an issue for treatment, but also for diagnostic purposes. EVs have shown potential to be used as biomarkers for disease and healthy states. However, it may be challenging to standardize certain EVs as definite biomarkers because in each sample the EV phenotypic profile varies (Hornung, Dutta, & Bitan, 2020). New technologies such as single-EV sequencing and high-sensitivity flow cytometry allow detection of individual EVs to characterize their proteomes (Vagner et al., 2019). As the field continues to progress, individual EVs may be more easily detected and segregated according to specific desired characteristics. Currently, there is still an unmet need for standardized, reproducible isolation methods and biomarker determination. (Hornung et al., 2020)
One clinical concern for AD is the potential neurotoxicity or off-target physiological effects from EV therapy. First, EVs must be rigorously tested in vitro and in vivo in animal models prior to a human clinical trial. Many teams have observed no neurotoxicity from autologous EVs after they were re-administered into the animals (Xian et al., 2019; Xin et al., 2013; You et al., 2020). To study Parkinson’s disease, murine blood derived EVs were collected and incubated with dopamine, then injected intravenously back into the donor mice. The team found that the dopamine loaded EVs led to lower toxicity against human neuroblastoma cells and did not trigger significant cell death in vivo (Qu et al., 2018). In a clinical trial using dendritic cell-derived EVs as a maintenance immunotherapy for non-small-cell lung cancer, EVs showed minimal toxicity and were deemed a well-tolerated phase I therapeutic. Only one patient out of the 22-patient cohort developed hepatotoxicity (Besse et al., 2016). Though these studies are reassuring, more work is needed to thoroughly test each new EV therapy.
On the cellular level, one large concern is if astrocytes and microglia will preferentially uptake EVs and yield desired downstream effects. Although the process is not well understood, there have been several studies showing astrocytes and microglia taking up natural and engineered EVs, showing functional changes in response (S. Y. Chen et al., 2019; Cui et al., 2019; Gayen et al., 2020; Lin et al., 2018; Venturini et al., 2019; Xian et al., 2019). Future studies must design effective EVs that target astrocyte and microglial biomarkers that will instruct the cells to reduce neuroinflammation, clear plaques, and/or recruit other cells to aid.
Regarding EVs for diagnosing AD, It is important to note that sometimes patients never develop AD even if certain associated biomarkers are present or detected postmortem, such as amyloid plaques and tau (Walsh & Selkoe, 2020). This reinforces the fact that every patient is unique; their EV biomarkers will reflect that but must be standardized across numerous patients for diagnostic and therapeutic purposes. Additionally, researchers may benefit from tailoring engineered therapeutic EVs to individuals based on that person’s EV profile. Gradually, as engineered EVs demonstrate efficacy in treating AD pathophysiology, EV therapeutics may become standardized and able to treat larger patient populations.
Conclusion
Alzheimer’s disease is one of the hardest to diagnose and treat in proper time. The diagnosing techniques for AD rely heavily on protein aggregation in the patients, which may or may not be present at early stages of the disease. For AD treatment, only six drugs are approved by the Food and Drug Administration in the United States and none of them address the mechanisms that cause the disease in the first place (Cummings, 2021; Yiannopoulou & Papageorgiou, 2020). Currently there is no cure for Alzheimer’s, only treatments to alleviate symptoms (Cummings, 2021; Villar-Vesga et al., 2020). While diagnoses and treatments stay difficult to implement and innovate, the economic burden of AD increases each year and is estimated to be approximately $1 trillion by 2050 (Marasco, 2020). There is an unmet economical and scientific need to provide novel and creative approaches to solve AD.
Extracellular vesicles (EVs) pose a minimally invasive and cell free treatment that may be able to resolve many pathophysiological drivers of AD and slow or even stop disease progression and neuronal cell death (Harris et al., 2018; Harting et al., 2018; Kumar et al., 2019; Reza-Zaldivar et al., 2018). EVs can be applied for both AD diagnosis and treatment. Similar EV isolations in patients with epilepsy, traumatic brain injury and neurogenerative disorders show promise in diagnosing CNS disorders (Armstrong & Wildman, 2018; Karttunen et al., 2019; Saadatpour et al., 2016).
In the field of EV therapeutics, there is a pressing need for identifying cell type-specific markers for distinct EV populations. It is well known that EVs represent a largely heterogeneous population and often do not possess single markers for conclusive identification. Identifying EV markers in the context of various diseases or indications could accelerate the development of novel treatments by increasing the efficiency of testing both in vitro and in vivo. This is especially important when considering some of the recent debate regarding previously well-established EV markers. A Nature Methods paper recently showed that cell adhesion molecule L1CAM was not a marker of neuronal EVs (Norman et al., 2021). This was controversial in the EV field, because prior to this revelation, Kapogiannis et al had shown in multiple studies that L1CAM-enriched EVs were associated with numerous neuronal markers such as tau and BDNF, so they posited that L1CAM could be used as a “liquid biopsy” for neuronal metabolism or as a biomarker for neurodegenerative diseases (Mustapic et al., 2017; Pulliam, Sun, Mustapic, Chawla, & Kapogiannis, 2019). Though this one new study has challenged the longstanding perception of L1CAM’s application in AD diagnosis and treatment, it may support finding a different marker for neuronal EVs, and potentially also call into question the validity of currently used EV markers. This recent example serves to highlight the need for additional EV-associated biomarkers for further advancements in EV research.
In the near future, surface markers and internal cargo of patient derived EVs could be analyzed and compared to established data, potentially diagnosing AD earlier. Bioengineered EVs targeting astrocytes and microglia may fight AD plaque accumulation or neuroinflammation, alongside addressing other issues such as vascular dysfunction (Casella et al., 2020; Govindpani et al., 2019; Hao et al., 2020; Kumar et al., 2019; Xian et al., 2019). Future EV treatments will be cell-free, eliminating risks commonly associated with stem cell therapies, such as tumorigenesis, unplanned cell division, and unwanted immune reactivity. Future clinical trials can test these nanoparticle therapies in in AD and other CNS disorders. Continued research is essential to improve EV purification, yield, tracking, targeting and validation methods.
Sidebar 1: EVs as a novel treatment approach and personalized therapy
Early intervention, with novel diagnostic biomarkers and treatments, can have lasting implications on patient quality of life. Signs of AD dementia in patients include memory loss, difficulty completing familiar tasks, confusion with time or place, trouble speaking, issues with visual images and spatial relationships, changes in mood and personality, and other signs (Breijyeh & Karaman, 2020). Early intervention may prevent progression to dementia, improve patient quality of life and relieve caregiver burden. EVs engineered in the lab are a novel therapeutic approach to AD. Due to their engineering and modification potential, therapeutic EVs provide the possibility of individualized therapy for Alzheimer’s patients. Personalized therapeutic approaches are more robust in targeting the specific disease mechanisms the patient suffers with, making them a more desirable therapeutic compared to traditional pharmaceuticals.
Sidebar 2: Stages of AD and the need for earlier diagnosis using biomarkers
Historically, Alzheimer’s disease had been classified by the dementia stage of the disease. Today we know that dementia is only a part of the disease, and AD often begins with mild cognitive impairment (MCI) (Lopez et al., 2018). In some individuals, cognitive decline begins earlier than when they receive the MCI diagnosis. Currently, there are no systemic biomarkers to detect all stages of AD successfully and no established serum- or CSF-derived biomarkers to predict whether the MCI diagnosis will lead to dementia (Villar-Vesga et al., 2020). Novel diagnostic biomarkers, such as extracellular vesicles (EVs) released by diseased patient cells, have the potential to detect all stages of Alzheimer’s and exponentially improve patient outcomes. Extracellular vesicles (EVs) isolated from patient serum may provide information on individual-level differences and solidify our understanding of AD disease mechanisms.
Acknowledgements
The authors would like to acknowledge Connor Dougherty for helpful discussions on this subject.
Funding Information
This study was supported in part by the following funding: National Center for Advancing Translational Sciences (TL1 TR001861), National Institutes of Health (5R01NS100761-02, R03HD091601-01), Shriners Hospitals for Children (84705-NCA-19, 85108-NCA-19, 87200-NCA-19), the National Institute on Aging of the National Institutes of Health under Award No. P30AG010129 through the UC Davis Alzheimer’s Disease Center Pilot Program, and the UC Davis School of Veterinary Medicine Lodric Maddox Graduate Fellowship (UL1 TR001860).
Footnotes
Research Resources
Original figures were created in BioRender.
The authors declare no conflicts of interest.
Contributor Information
Sabrina Valentina Lazar, Department of Surgery, University of California, Davis.
Sirjan Mor, Department of Surgery, University of California, Davis.
David Wang, Department of Surgery, Department of Biomedical Engineering, University of California, Davis.
Leora Goldbloom-Helzner, Department of Surgery, Department of Biomedical Engineering, University of California, Davis.
Kaitlin Clark, Department of Surgery, University of California, Davis.
Dake Hao, Department of Surgery, Shriners Hospitals for Children Northern California – Institute for Pediatric Regenerative Medicine, University of California, Davis.
Diana Lee Farmer, Department of Surgery, Shriners Hospitals for Children Northern California – Institute for Pediatric Regenerative Medicine, University of California, Davis.
Aijun Wang, Department of Surgery, Department of Biomedical Engineering, Shriners Hospitals for Children Northern California – Institute for Pediatric Regenerative Medicine, University of California, Davis.
References
- Abeysinghe A, Deshapriya R, & Udawatte C (2020). Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions. Life Sciences, 256, 117996. doi: 10.1016/j.lfs.2020.117996 [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, & Bennett ML (2013). Microglia: scapegoat, saboteur, or something else? Science, 339(6116), 156–161. doi: 10.1126/science.1227901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, & Wood MJ (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29(4), 341–345. doi: 10.1038/nbt.1807 [DOI] [PubMed] [Google Scholar]
- Amakiri N, Kubosumi A, Tran J, & Reddy PH (2019). Amyloid Beta and MicroRNAs in Alzheimer’s Disease. Frontiers in Neuroscience, 13, 430. doi: 10.3389/fnins.2019.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D, & Wildman DE (2018). Extracellular Vesicles and the Promise of Continuous Liquid Biopsies. J Pathol Transl Med, 52(1), 1–8. doi: 10.4132/jptm.2017.05.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, … Schwartz M (2015). Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun, 6, 7967. doi: 10.1038/ncomms8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bature F, Guinn BA, Pang D, & Pappas Y (2017). Signs and symptoms preceding the diagnosis of Alzheimer’s disease: a systematic scoping review of literature from 1937 to 2016. BMJ Open, 7(8), e015746. doi: 10.1136/bmjopen-2016-015746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, … Chaput N (2016). Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology, 5(4), e1071008. doi: 10.1080/2162402X.2015.1071008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, … Verderio C (2009). Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO Journal, 28(8), 1043–1054. doi: 10.1038/emboj.2009.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, & Salmon DP (2017). Alzheimer’s Disease: Past, Present, and Future. Journal of the International Neuropsychological Society, 23(9–10), 818–831. doi: 10.1017/S135561771700100X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breijyeh Z, & Karaman R (2020). Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules, 25(24). doi: 10.3390/molecules25245789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell CJ, Win Z, Muckle K, Kennedy A, Waldman A, Dawe G, … Perry RJ (2018). Clinical utility of amyloid PET imaging with (18)F-florbetapir: a retrospective study of 100 patients. J Neurol Neurosurg Psychiatry, 89(3), 294–299. doi: 10.1136/jnnp-2017-316194 [DOI] [PubMed] [Google Scholar]
- Casella G, Rasouli J, Boehm A, Zhang W, Xiao D, Ishikawa LLW, … Rostami A (2020). Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Science Translational Medicine, 12(568). doi: 10.1126/scitranslmed.aba0599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K, & Xu HE (2017). Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacologica Sinica, 38(9), 1205–1235. doi: 10.1038/aps.2017.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Lin MC, Tsai JS, He PL, Luo WT, Herschman H, & Li HJ (2019). EP4 Antagonist-Elicited Extracellular Vesicles from Mesenchymal Stem Cells Rescue Cognition/Learning Deficiencies by Restoring Brain Cellular Functions. Stem Cells Transl Med, 8(7), 707–723. doi: 10.1002/sctm.18-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan RS, Pisano S, Oliveira MI, Ferrari M, & Mendes Pinto I (2017). Exosomes as Reconfigurable Therapeutic Systems. Trends in Molecular Medicine, 23(7), 636–650. doi: 10.1016/j.molmed.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croese T, & Furlan R (2018). Extracellular vesicles in neurodegenerative diseases. Molecular Aspects of Medicine, 60, 52–61. doi: 10.1016/j.mam.2017.11.006 [DOI] [PubMed] [Google Scholar]
- Crous-Bou M, Minguillon C, Gramunt N, & Molinuevo JL (2017). Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimer’s Research & Therapy, 9(1), 71. doi: 10.1186/s13195-017-0297-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J, … Liu JR (2019). RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immunity & Ageing, 16, 10. doi: 10.1186/s12979-019-0150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J (2021). New approaches to symptomatic treatments for Alzheimer’s disease. Molecular Neurodegeneration, 16(1), 2. doi: 10.1186/s13024-021-00424-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens AM, Tovar YRLB, Yoo SW, Trout AL, Bae M, Kanmogne M, … Haughey NJ (2017). Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal, 10(473). doi: 10.1126/scisignal.aai7696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, … Khvorova A (2016). Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Molecular Therapy, 24(10), 1836–1847. doi: 10.1038/mt.2016.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, … Washington Dc, U. S. A. (2016). Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement, 12(3), 292–323. doi: 10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW, & Alzheimer’s Disease Neuroimaging I (2015). Subtle Cognitive Decline and Biomarker Staging in Preclinical Alzheimer’s Disease. Journal of Alzheimer’s Disease, 47(1), 231–242. doi: 10.3233/JAD-150128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efthalia Angelopoulou YNP, Shaikh Mohd. Farooq, Piperi Christina. (2020). Flotillin: A Promising Biomarker for Alzheimer’s Disease. Journal of Personalized Medicine, 10(2). doi: doi. org/10.3390/jpm10020020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efthymiou AG, & Goate AM (2017). Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Molecular Neurodegeneration, 12(1), 43. doi: 10.1186/s13024-017-0184-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier N, Koutsouleris N, Benzinger T, Goate A, Karch CM, Fagan AM, … Ewers M (2020). Predicting sporadic Alzheimer’s disease progression via inherited Alzheimer’s disease-informed machine-learning. Alzheimers Dement, 16(3), 501–511. doi: 10.1002/alz.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamez-Valero A, Campdelacreu J, Vilas D, Ispierto L, Rene R, Alvarez R, … Beyer K (2019). Exploratory study on microRNA profiles from plasma-derived extracellular vesicles in Alzheimer’s disease and dementia with Lewy bodies. Transl Neurodegener, 8, 31. doi: 10.1186/s40035-019-0169-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayen M, Bhomia M, Balakathiresan N, & Knollmann-Ritschel B (2020). Exosomal MicroRNAs Released by Activated Astrocytes as Potential Neuroinflammatory Biomarkers. International Journal of Molecular Sciences, 21(7). doi: 10.3390/ijms21072312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, … B. L. Miller (2016). Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB Journal, 30(11), 3853–3859. doi: 10.1096/fj.201600756R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Molina LA, Villar-Vesga J, Henao-Restrepo J, Villegas A, Lopera F, Cardona-Gomez GP, & Posada-Duque R (2021). Extracellular Vesicles From 3xTg-AD Mouse and Alzheimer’s Disease Patient Astrocytes Impair Neuroglial and Vascular Components. Frontiers in Aging Neuroscience, 13, 593927. doi: 10.3389/fnagi.2021.593927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindpani K, McNamara LG, Smith NR, Vinnakota C, Waldvogel HJ, Faull RL, & Kwakowsky A (2019). Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J Clin Med, 8(5). doi: 10.3390/jcm8050651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Yin Z, Chen F, & Lei P (2020). Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer’s disease. Alzheimer’s Research & Therapy, 12(1), 109. doi: 10.1186/s13195-020-00670-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I, … Levenberg S (2019). Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano, 13(9), 10015–10028. doi: 10.1021/acsnano.9b01892 [DOI] [PubMed] [Google Scholar]
- Han X, Zhang T, Liu H, Mi Y, & Gou X (2020). Astrocyte Senescence and Alzheimer’s Disease: A Review. Frontiers in Aging Neuroscience, 12, 148. doi: 10.3389/fnagi.2020.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao D, Swindell HS, Ramasubramanian L, Liu R, Lam KS, Farmer DL, & Wang A (2020). Extracellular Matrix Mimicking Nanofibrous Scaffolds Modified With Mesenchymal Stem Cell-Derived Extracellular Vesicles for Improved Vascularization. Front Bioeng Biotechnol, 8, 633. doi: 10.3389/fbioe.2020.00633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris VK, Stark J, Vyshkina T, Blackshear L, Joo G, Stefanova V, … Sadiq SA (2018). Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. EBioMedicine, 29, 23–30. doi: 10.1016/j.ebiom.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, … Olson SD (2018). Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells, 36(1), 79–90. doi: 10.1002/stem.2730 [DOI] [PubMed] [Google Scholar]
- Hemonnot AL, Hua J, Ulmann L, & Hirbec H (2019). Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Frontiers in Aging Neuroscience, 11, 233. doi: 10.3389/fnagi.2019.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung S, Dutta S, & Bitan G (2020). CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Frontiers in Molecular Neuroscience, 13, 38. doi: 10.3389/fnmol.2020.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Liao K, Niu F, Yang L, Dallon BW, Callen S, … Buch S (2018). Astrocyte EV-Induced lincRNA-Cox2 Regulates Microglial Phagocytosis: Implications for Morphine-Mediated Neurodegeneration. Mol Ther Nucleic Acids, 13, 450–463. doi: 10.1016/j.omtn.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Vader P, & Schiffelers RM (2017). Extracellular vesicles for nucleic acid delivery: progress and prospects for safe RNA-based gene therapy. Gene Therapy, 24(3), 157–166. doi: 10.1038/gt.2017.8 [DOI] [PubMed] [Google Scholar]
- Joshi P, Turola E, Ruiz A, Bergami A, Libera DD, Benussi L, … Verderio C (2014). Microglia convert aggregated amyloid-beta into neurotoxic forms through the shedding of microvesicles. Cell Death Differ, 21(4), 582–593. doi: 10.1038/cdd.2013.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicic A, & Gitler AD (2017). Distinct repertoires of microRNAs present in mouse astrocytes compared to astrocyte-secreted exosomes. PloS One, 12(2), e0171418. doi: 10.1371/journal.pone.0171418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A, Tyagi A, & Tyagi N (2014). Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Molecular Neurobiology, 49(1), 590–600. doi: 10.1007/s12035-013-8544-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, & LeBleu VS (2020). The biology, function, and biomedical applications of exosomes. Science, 367(6478). doi: 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, … Blennow K (2020). Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurology, 19(5), 422–433. doi: 10.1016/S1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- Karttunen J, Heiskanen M, Navarro-Ferrandis V, Das Gupta S, Lipponen A, Puhakka N, … Pitkanen A (2019). Precipitation-based extracellular vesicle isolation from rat plasma co-precipitate vesicle-free microRNAs. J Extracell Vesicles, 8(1), 1555410. doi: 10.1080/20013078.2018.1555410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, Sharma V, & Deshmukh R (2019). Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology, 27(4), 663–677. doi: 10.1007/s10787-019-00580-x [DOI] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, … Amit I (2017). A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell, 169(7), 1276–1290 e1217. doi: 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Knopman DS, Jack CR Jr., Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, … Petersen RC (2013). Selective worsening of brain injury biomarker abnormalities in cognitively normal elderly persons with beta-amyloidosis. JAMA Neurol, 70(8), 1030–1038. doi: 10.1001/jamaneurol.2013.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Becker JC, Gao K, Carney RP, Lankford L, Keller BA, … Wang A (2019). Neuroprotective effect of placenta-derived mesenchymal stromal cells: role of exosomes. FASEB Journal, 33(5), 5836–5849. doi: 10.1096/fj.201800972R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok ZH, Zhang B, Chew XH, Chan JJ, Teh V, Yang H, … Tay Y (2021). Systematic Analysis of Intronic miRNAs Reveals Cooperativity within the Multicomponent FTX Locus to Promote Colon Cancer Development. Cancer Research, 81(5), 1308–1320. doi: 10.1158/0008-5472.CAN-20-1406 [DOI] [PubMed] [Google Scholar]
- Lazaro-Ibanez E, Faruqu FN, Saleh AF, Silva AM, Tzu-Wen Wang J, Rak J, … Dekker N (2021). Selection of Fluorescent, Bioluminescent, and Radioactive Tracers to Accurately Reflect Extracellular Vesicle Biodistribution in Vivo. ACS Nano, 15(2), 3212–3227. doi: 10.1021/acsnano.0c09873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire Q, Raffo-Romero A, Arab T, Van Camp C, Drago F, Forte S, … Lefebvre C (2019). Isolation of microglia-derived extracellular vesicles: towards miRNA signatures and neuroprotection. Journal of Nanobiotechnology, 17(1). doi: ARTN11910.1186/s12951-019-0551-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Moniruzzaman M, Dastgheyb RM, Yoo SW, Wang M, Hao H, … Haughey NJ (2020). Astrocytes deliver CK1 to neurons via extracellular vesicles in response to inflammation promoting the translation and amyloidogenic processing of APP. J Extracell Vesicles, 10(2), e12035. doi: 10.1002/jev2.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K, Niu F, Dagur RS, He M, Tian C, & Hu G (2020). Intranasal Delivery of lincRNA-Cox2 siRNA Loaded Extracellular Vesicles Decreases Lipopolysaccharide-Induced Microglial Proliferation in Mice. Journal of Neuroimmune Pharmacology, 15(3), 390–399. doi: 10.1007/s11481-019-09864-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoni SK, Moghadam MF, Moazzeni SM, Gomari H, & Salimi F (2019). Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl Biochem Biotechnol, 187(1), 352–364. doi: 10.1007/s12010-018-2813-4 [DOI] [PubMed] [Google Scholar]
- Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, … Tsai LH (2018). APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron, 98(6), 1141–1154 e1147. doi: 10.1016/j.neuron.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CG, Song J, Zhang YQ, & Wang PC (2014). MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol Med Rep, 10(5), 2395–2400. doi: 10.3892/mmr.2014.2484 [DOI] [PubMed] [Google Scholar]
- Liu PP, Xie Y, Meng XY, & Kang JS (2019). History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther, 4, 29. doi: 10.1038/s41392-019-0063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huber CC, & Wang H (2020). Disrupted blood-brain barrier in 5xFAD mouse model of Alzheimer’s disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes. Biochem Biophys Res Commun. doi: 10.1016/j.bbrc.2020.02.074 [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Chang Y, Klunk WE, Mathis C, Price J, … Kuller LH (2018). Amyloid deposition and brain structure as long-term predictors of MCI, dementia, and mortality. Neurology, 90(21), e1920–e1928. doi: 10.1212/WNL.0000000000005549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Beach TG, & Walker DG (2019). Alzheimer’s Disease Research Using Human Microglia. Cells, 8(8). doi: 10.3390/cells8080838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna I, De Benedittis S, Quattrone A, Maisano D, Iaccino E, & Quattrone A (2020). Exosomal miRNAs as Potential Diagnostic Biomarkers in Alzheimer’s Disease. Pharmaceuticals (Basel, Switzerland), 13(9). doi: 10.3390/ph13090243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco RA (2020). Current and evolving treatment strategies for the Alzheimer disease continuum. American Journal of Managed Care, 26(8 Suppl), S167–S176. doi: 10.37765/ajmc.2020.88481 [DOI] [PubMed] [Google Scholar]
- Martinez G, Vernooij RW, Fuentes Padilla P, Zamora J, Bonfill Cosp X, & Flicker L (2017). 18F PET with florbetapir for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews, 11, CD012216. doi: 10.1002/14651858.CD012216.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto J, Stewart T, Banks WA, & Zhang J (2017). The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Current Pharmaceutical Design, 23(40), 6206–6214. doi: 10.2174/1381612823666170913164738 [DOI] [PubMed] [Google Scholar]
- McKeever A, & Agius M (2018). Dementia risk assessment and risk reduction using cardiovascular risk factors. Psychiatr Danub, 30(Suppl 7), 469–474. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30439828 [PubMed] [Google Scholar]
- Molinaro R, Evangelopoulos M, Hoffman JR, Corbo C, Taraballi F, Martinez JO, … Tasciotti E (2018). Design and Development of Biomimetic Nanovesicles Using a Microfluidic Approach. Adv Mater, 30(15), e1702749. doi: 10.1002/adma.201702749 [DOI] [PubMed] [Google Scholar]
- Mortazavizadeh Z, Maercker A, Roth T, Savaskan E, & Forstmeier S (2020). Quality of the caregiving relationship and quality of life in mild Alzheimer’s dementia. Psychogeriatrics, 20(5), 568–577. doi: 10.1111/psyg.12546 [DOI] [PubMed] [Google Scholar]
- Muraoka S, Jedrychowski MP, Yanamandra K, Ikezu S, Gygi SP, & Ikezu T (2020). Proteomic Profiling of Extracellular Vesicles Derived from Cerebrospinal Fluid of Alzheimer’s Disease Patients: A Pilot Study. Cells, 9(9). doi: 10.3390/cells9091959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgoci AN, Cizkova D, Majerova P, Petrovova E, Medvecky L, Fournier I, & Salzet M (2018). Brain-Cortex Microglia-Derived Exosomes: Nanoparticles for Glioma Therapy. Chemphyschem, 19(10), 1205–1214. doi: 10.1002/cphc.201701198 [DOI] [PubMed] [Google Scholar]
- Mustapic M, Eitan E, Werner JK Jr., Berkowitz ST, Lazaropoulos MP, Tran J, … Kapogiannis D (2017). Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Frontiers in Neuroscience, 11, 278. doi: 10.3389/fnins.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro V, Sanchez-Mejias E, Jimenez S, Munoz-Castro C, Sanchez-Varo R, Davila JC, … Vitorica J (2018). Microglia in Alzheimer’s Disease: Activated, Dysfunctional or Degenerative. Frontiers in Aging Neuroscience, 10, 140. doi: 10.3389/fnagi.2018.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman M, Ter-Ovanesyan D, Trieu W, Lazarovits R, Kowal EJK, Lee JH, … Walt DR (2021). L1CAM is not associated with extracellular vesicles in human cerebrospinal fluid or plasma. Nature Methods, 18(6), 631–634. doi: 10.1038/s41592-021-01174-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Alzheimer’s Disease Neuroimaging I, Minthon L, … Swedish Bio, F. S. G. (2015). Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology, 85(14), 1240–1249. doi: 10.1212/WNL.0000000000001991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wetzel I, Marriott I, Dreau D, D’Avanzo C, Kim DY, … Cho H (2018). A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nature Neuroscience, 21(7), 941–951. doi: 10.1038/s41593-018-0175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta R, Ułamek-Kozioł M, Januszewski S, & Czuczwar SJ (2018). Exosomes as possible spread factor and potential biomarkers in Alzheimer’s disease: current concepts. Biomarkers in Medicine, 12(9), 1025–1033. [Google Scholar]
- Prada I, Gabrielli M, Turola E, Iorio A, D’Arrigo G, Parolisi R, … Verderio C (2018). Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathologica, 135(4), 529–550. doi: 10.1007/s00401-017-1803-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Sun B, Mustapic M, Chawla S, & Kapogiannis D (2019). Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. Journal of Neurovirology, 25(5), 702–709. doi: 10.1007/s13365-018-0695-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, … Sun X (2018). Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. Journal of Controlled Release, 287, 156–166. doi: 10.1016/j.jconrel.2018.08.035 [DOI] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, & Simons K (2006). Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America, 103(30), 11172–11177. doi: 10.1073/pnas.0603838103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L, Wu L, Liu Z, Tian R, Yu G, Zhou Z, … Chen X (2020). Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun, 11(1), 4909. doi: 10.1038/s41467-020-18626-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Zhao Y, Xue F, Zheng Y, Huang H, Wang W, … Zhang J (2019). Exosomal DNA Aptamer Targeting alpha-Synuclein Aggregates Reduced Neuropathological Deficits in a Mouse Parkinson’s Disease Model. Mol Ther Nucleic Acids, 17, 726–740. doi: 10.1016/j.omtn.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza-Zaldivar EE, Hernandez-Sapiens MA, Minjarez B, Gutierrez-Mercado YK, Marquez-Aguirre AL, & Canales-Aguirre AA (2018). Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer’s Disease. Frontiers in Cellular Neuroscience, 12, 317. doi: 10.3389/fncel.2018.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Arellano JJ, Parpura V, Zorec R, & Verkhratsky A (2016). Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience, 323, 170–182. doi: 10.1016/j.neuroscience.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Saadatpour L, Fadaee E, Fadaei S, Nassiri Mansour R, Mohammadi M, Mousavi SM, … Mirzaei H (2016). Glioblastoma: exosome and microRNA as novel diagnosis biomarkers. Cancer Gene Therapy, 23(12), 415–418. doi: 10.1038/cgt.2016.48 [DOI] [PubMed] [Google Scholar]
- Santa-Maria I, Alaniz ME, Renwick N, Cela C, Fulga TA, Van Vactor D, … Crary JF (2015). Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. Journal of Clinical Investigation, 125(2), 681–686. doi: 10.1172/JCI78421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarko DK, & McKinney CE (2017). Exosomes: Origins and Therapeutic Potential for Neurodegenerative Disease. Frontiers in Neuroscience, 11, 82. doi: 10.3389/fnins.2017.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, … Akiyoshi K (2016). Engineering hybrid exosomes by membrane fusion with liposomes. Scientific Reports, 6, 21933. doi: 10.1038/srep21933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpente M, Fenoglio C, D’Anca M, Arcaro M, Sorrentino F, Visconte C, … Galimberti D (2020). MiRNA Profiling in Plasma Neural-Derived Small Extracellular Vesicles from Patients with Alzheimer’s Disease. Cells, 9(6). doi: 10.3390/cells9061443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamili FH, Bayegi HR, Salmasi Z, Sadri K, Mahmoudi M, Kalantari M, … Abnous K (2018). Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int J Pharm, 549(1–2), 218–229. doi: 10.1016/j.ijpharm.2018.07.067 [DOI] [PubMed] [Google Scholar]
- Song Y, Li Z, He T, Qu M, Jiang L, Li W, … Yang GY (2019). M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics, 9(10), 2910–2923. doi: 10.7150/thno.30879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Xu Y, Deng W, Zhang L, Zhu H, Yu P, … Qin C (2020). Brain Derived Exosomes Are a Double-Edged Sword in Alzheimer’s Disease. Frontiers in Molecular Neuroscience, 13, 79. doi: 10.3389/fnmol.2020.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, … Gao J (2018). Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials, 150, 137–149. doi: 10.1016/j.biomaterials.2017.10.012 [DOI] [PubMed] [Google Scholar]
- Tian Y, Zhu P, Liu S, Jin Z, Li D, Zhao H, … Dong Z (2019). IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv Clin Exp Med, 28(4), 421–430. doi: 10.17219/acem/91826 [DOI] [PubMed] [Google Scholar]
- Upadhya R, Zingg W, Shetty S, & Shetty AK (2020). Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. Journal of Controlled Release, 323, 225–239. doi: 10.1016/j.jconrel.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner T, Chin A, Mariscal J, Bannykh S, Engman DM, & Di Vizio D (2019). Protein Composition Reflects Extracellular Vesicle Heterogeneity. Proteomics, 19(8), e1800167. doi: 10.1002/pmic.201800167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broek B, Pintelon I, Hamad I, Kessels S, Haidar M, Hellings N, … Irobi J (2020). Microglial derived extracellular vesicles activate autophagy and mediate multi-target signaling to maintain cellular homeostasis. J Extracell Vesicles, 10(1), e12022. doi: 10.1002/jev2.12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini A, Passalacqua M, Pelassa S, Pastorino F, Tedesco M, Cortese K, … Cervetto C (2019). Exosomes From Astrocyte Processes: Signaling to Neurons. Frontiers in Pharmacology, 10, 1452. doi: 10.3389/fphar.2019.01452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veremeyko T, Kuznetsova IS, Dukhinova M, Y. A,WY, Kopeikina E, Barteneva NS, & Ponomarev ED (2019). Neuronal extracellular microRNAs miR-124 and miR-9 mediate cell-cell communication between neurons and microglia. Journal of Neuroscience Research, 97(2), 162–184. doi: 10.1002/jnr.24344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij FJ, Revenu C, Arras G, Dingli F, Loew D, Pegtel DM, … van Niel G (2019). Live Tracking of Inter-organ Communication by Endogenous Exosomes In Vivo. Developmental Cell, 48(4), 573–589 e574. doi: 10.1016/j.devcel.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Villar-Vesga J, Henao-Restrepo J, Voshart DC, Aguillon D, Villegas A, Castano D, … Posada-Duque R (2020). Differential Profile of Systemic Extracellular Vesicles From Sporadic and Familial Alzheimer’s Disease Leads to Neuroglial and Endothelial Cell Degeneration. Frontiers in Aging Neuroscience, 12, 587989. doi: 10.3389/fnagi.2020.587989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, & Selkoe DJ (2020). Amyloid beta-protein and beyond: the path forward in Alzheimer’s disease. Current Opinion in Neurobiology, 61, 116–124. doi: 10.1016/j.conb.2020.02.003 [DOI] [PubMed] [Google Scholar]
- Weller J, & Budson A (2018). Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res, 7. doi: 10.12688/f1000research.14506.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, … Long Q (2019). Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics, 9(20), 5956–5975. doi: 10.7150/thno.33872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, & Chopp M (2013). Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Journal of Cerebral Blood Flow and Metabolism, 33(11), 1711–1715. doi: 10.1038/jcbfm.2013.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Liu CG, Gao SC, Zhang Y, & Wang PC (2018). The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomedical and Environmental Sciences, 31(2), 87–96. doi: 10.3967/bes2018.011 [DOI] [PubMed] [Google Scholar]
- Yiannopoulou KG, & Papageorgiou SG (2020). Current and Future Treatments in Alzheimer Disease: An Update. J Cent Nerv Syst Dis, 12, 1179573520907397. doi: 10.1177/1179573520907397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You HJ, Fang SB, Wu TT, Zhang H, Feng YK, Li XJ, … Pei Z (2020). Mesenchymal stem cell-derived exosomes improve motor function and attenuate neuropathology in a mouse model of Machado-Joseph disease. Stem Cell Research & Therapy, 11(1), 222. doi: 10.1186/s13287-020-01727-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, … Igarashi Y (2015). A potential function for neuronal exosomes: sequestering intracerebral amyloid-beta peptide. FEBS Letters, 589(1), 84–88. doi: 10.1016/j.febslet.2014.11.027 [DOI] [PubMed] [Google Scholar]
- Zheng M, Huang M, Ma X, Chen H, & Gao X (2019). Harnessing Exosomes for the Development of Brain Drug Delivery Systems. Bioconjugate Chemistry, 30(4), 994–1005. doi: 10.1021/acs.bioconjchem.9b00085 [DOI] [PubMed] [Google Scholar]


