Abstract
Introduction:
Knowledge of the safety of vaccines is crucial, both to prevent and cure them and to decrease the public hesitation in receiving vaccines. Therefore, this study aimed to systematically review the adverse events reported for inactivated vaccines and Novavax.
Methods:
In this systematic review, the databases of PubMed, Scopus, Cochrane, and Web of Science were searched on September 15, 2021. Then we identified the eligible studies using a two-step title/abstract and full-text screening process. Data on the subjects, studies, and types of adverse events were extracted and entered in a word table, including serious, mild, local, and systemic adverse events as well as the timing of side effects’ appearance.
Results:
Adverse effects of inactivated coronavirus vaccines side effects were reported from phases 1, 2, and 3 of the vaccine trials. The most common local side effects included injection site pain and swelling, redness, and pruritus. Meanwhile, fatigue, headache, muscle pain, fever, and gastrointestinal symptoms including abdominal pain and diarrhea were among the most common systemic adverse effects.
Conclusion:
This systematic review indicates that inactivated COVID-19 vaccines, including Sinovac, Sinopharm, and Bharat Biotech, as well as the protein subunit vaccines (Novavax) can be considered as safe choices due to having milder side effects and fewer severe life-threatening adverse events.
Key Words: Adverse Effects, BBV152 COVID-19 vaccine, COVID-19, COVID-19 vaccines, recombinant SARS-CoV-2 vaccine NVX-cov2373, Safety, SARS-CoV-2, sinovac COVID-19 vaccine, Vaccines, Inactivated
1. Introduction:
COVID-19 was first found in Wuhan, China, in December of 2019. It is caused by a new coronavirus, called SARS-COV-2. The virus is similar to the other two coronaviruses, SARS-CoV and MERS-CoV (1, 2). COVID-19 manifestations are coughing, fever or chills, and shortness of breath; thus, it may be challenging to distinguish it from influenza (3-5). COVID-19 spreads more easily through droplets and aerosols than SARS, MERS, and influenza (5). Given these characteristics, it quickly spread from its source to other areas and countries, causing a pandemic across the world. This has been a huge problem for health systems around the world. It is still important to curb thevirus by wearing masks and avoiding public gatherings or closing schools, even though these measures have had a big impact on daily life and the world economy (6). Because there is not yet a standard treatment for COVID-19, vaccines must be administered quickly to stop the global pandemic.
Influenza and poliovirus vaccines have been demonstrated to be safe and efficacious when made from inactivated viruses (7). Having been used for a long time gives them several benefits, such as well-developed manufacturing methods and the ability to easily scale production and storage up and down. Inactivated SARS-CoV-2 vaccines have been shown to generate significant levels of neutralizing antibody titers in mice, rats, guinea pigs, rabbits, and nonhuman primates (8-10). SARS-CoV-2 vaccines have shown strong neutralizing antibody responses and effectiveness against COVID-19 during clinical studies conducted in different countries (11-15). SARS-CoV-2 vaccines manufactured by Sinovac Life Sciences/CoronaVac (China) and Beijing Institute of Biological Products/Sinopharm (China) have received conditional marketing approval from the China National Medical Products Administration and have been placed on WHO's Emergency Use Listing (16, 17) so far. NVX-CoV2373 (Novavax, USA) and ZF2001 (Longcom, China), two COVID-19 protein subunit vaccines, are now undergoing phase-3 clinical studies (18, 19). An early study found that the vaccines, NVX-CoV2373 (Novavax, USA) and ZF2001 (Longcom, China), both of which imitate the receptor-binding domain (RBD) of the SARS-COV-2 S protein, successfully prevent infection and generate high antibody titers against the SARS-COV-2 S protein.
Evidence to assist in selection of vaccines for population-based immunization in diverse areas is limited. To give additional data to optimize the use of the COVID-19 vaccines, we performed a systematic review of the adverse events of the existing inactivated vaccines and Novavax to determine their safety.
2.0. Methods:
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search of relevant records was carried out in the online databases using selected keywords on September 15th, 2021.
2.1. Study objective
The aim of the present study was to evaluate the safety and side effects of the inactivated vaccines of COVID-19 and Novavax.
2.2. Databases
Consistent with the Cochrane handbook of systematic reviews, PubMed, Scopus, Web of Science, and Cochrane were selected databases in this study.
2.3. Search Terms
We designed our search strategy using the keywords from previous articles and the medical subject headings (MeSH) terms. The search was conducted on September 15, 2021. Supplementary material 1 includes the search strategy for all the databases. The following query demonstrates the PubMed search strategy:
((((((COVID-19[Title]) OR (SARS-CoV-2[Title])) OR (SARS-CoV2[Title])) OR (2019-nCoV[Title])) OR (Novel Coronavirus [Title])) AND ((((Vaccine*[Title]) OR (Vaccination[Title])) OR (Vaccinated[Title])) OR (Immunization[Title]))) AND (((((Safety[Title]) OR (Side effect*[Title])) OR (Adverse event*[Title])) OR (Adverse effect*[Title])) OR (Adverse reaction*[Title]))
2.4. Inclusion/exclusion criteria
We included the original studies that assessed the safety and adverse events related to inactivated COVID-19 vaccines and Novavax. The following were the exclusion criteria:
1) Non-original studies, including review articles, meta-analyses, and editorials without original data
2) Abstracts/conference abstracts or unavailability of full texts
3) Protocols of clinical trials or ongoing clinical trials with unpublished results
4) Studies on types of vaccines rather than inactivated vaccines and Novavax or the studies that only reported the efficacy of inactivated vaccines and Novavax without reporting their adverse events
5) Case reports
2.5. Screening and data screening
Records from the start until September 15, 2021, were downloaded into the Endnote software. A two-phase screening was done. Firstly, after removing duplicates, the title and abstract of articles were assessed against the inclusion and exclusion criteria. Then, a full-text screening was completed by two reviewers.
2.6. Data extraction
This study aimed to evaluate the safety and adverse events related to inactivated COVID-19 vaccines and Novavax. To achieve this goal, data for the following variables were extracted for further analysis: the name of the first author, year of publication, country / ethnic group, type of study, manufacturer, phase of the study, sample population, age, gender, serious side effects, time from injection to adverse effects, local side effects, as well as systemic adverse effects.
2.7. Quality assessment
We assessed the quality of the studies using the Newcastle-Ottawa scale (NOS) checklist, which allocates a score of 0-9 to each study based on selection, comparability, and exposure/outcome(20). Studies that scored 4 or below were considered of poor quality (table 2).
Table 2.
The results of quality assessment of included studies using Newcastle-Ottawa scale (NOS) tool
| The first author (reference) | Selection (Out of 4) | Comparability (Out of 2) |
Exposure/outcome
(Out of 3) |
Total score (Out of 9) |
|---|---|---|---|---|
| Abu-Halaweh, S.(32) | **** | - | ** | 6 |
| Abu-Hammad, O. (29) | **** | - | ** | 6 |
| Al Khames Aga, Q. A. (28) | **** | ** | *** | 9 |
| Ella, R. (48) | **** | ** | *** | 9 |
| Goepfert,P.A. (49) | **** | ** | *** | 9 |
| Guo, W. (27) | **** | ** | *** | 9 |
| Han, B. (51) | **** | ** | *** | 9 |
| Hatmal, MM. (34) | **** | - | ** | 6 |
| Kaya, F. (46) | **** | - | *** | 7 |
| Pichi, F. (37) | **** | - | *** | 7 |
| Pu, J. (36) | **** | ** | *** | 9 |
| Saeed, B. Q. (40) | **** | - | ** | 6 |
| Tanriover, M. D. (41) | **** | ** | *** | 9 |
| Wang, G. (35) | **** | - | *** | 7 |
| Wu, Z. (47) | **** | ** | *** | 9 |
| Xia, S. (30) | **** | ** | *** | 9 |
| Xia, S. (38) | **** | ** | *** | 9 |
| Zhang, M. X. (43) | **** | - | ** | 6 |
| Zhang, Y. (45) | **** | ** | *** | 9 |
3. Results:
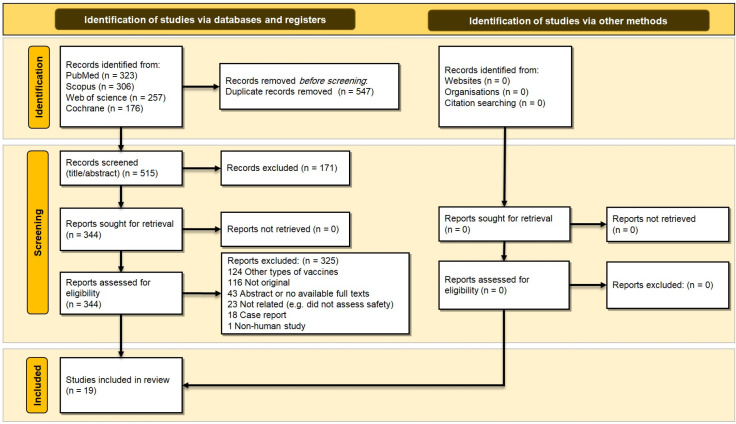
We initially identified a total of 1062 relevant records; however, only 515 remained after duplicate removal. Of the remaining records, 171 and 325 were removed in title/abstract and full-text screenings, respectively. Therefore, 19 studies appeared to be eligible and entered the qualitative synthesis (Figure 1).
Figure 1.
PRISMA 2020 flow diagram of this systematic review
Analysis of the included studies identified inactivated coronavirus vaccines including Bharat Biotech (Covaxin), Sinovac (CoronaVac), and Sinopharm. Sinopharm was the most commonly reported inactivated vaccine. Perfusion S vaccine was also reported in one study. Sinovac (CoronaVac) vaccine had most of its clinical trials in China and a few other countries like Turkey, Brazil, Indonesia, and the Philippines. Bharat Biotech (Covaxin) on the other hand was only clinically trialed in India, while Sinopharm went through trials mostly in China, the USA, and United Arab Emirates. Perfusion S vaccine was also trialed in the US. These studies reported adverse effects from phases 1, 2, and 3 of the vaccine trials. Most of these reported trials involved subjects that were 18 years and above with very few trials conducted in all age groups.
The most common local side effects included pain, redness, pruritus, induration, urticaria, stiffness, and swelling at the site of the injection; while fatigue, body pain, headache, skin rashes, sleepiness, systemic reactogenicity, anorexia, cough, edema, joint pain, chills, muscle pain, fever, malaise, and gastrointestinal symptoms like diarrhea and constipation were the most common systemic side effects (Table 1). The majority of the studies reported no serious side effects for the vaccines. Nevertheless, very few studies reported serious adverse effects like hypertension, chest pain, thrombocytopenia, acute macular neuroretinopathy, limb weakness, limb shaking, menstruation, as well as episcleritis, anterior scleritis, paracentral acute middle maculopathy, and subretinal fluid. There was no documented death in our analysis (Table 1).
Table 1.
Characteristics of various inactivated and protein subunit COVID-19 vaccines and their adverse effects
| ID | First Author | Year | Country | Type | Vaccine name | Phase | Sample | Age | Gender | Serious adverse event | Time | Side effects | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Local | Systemic | ||||||||||||
| 1 | Abu-Halaweh, S. (32) | 2021 | USA | Cohort | Sinopharm | N/A | 513 participants | ≥70 | Male (67%), female (33%) | No serious adverse reaction | N/A | Pain | Fever Headache |
| 2 | Abu-Hammad, O. (29) | 2021 | USA | CS | Sinopharm | N/A | 409 healthcare personnel | N/A | N/A | No serious adverse reaction | N/A | Pain, Redness | Headache |
| 3 | Al Khames Aga, Q. A. (28) | 2021 | USA | CS | Sinopharm | N/A | 340 participants | 18–35, 36–55, >55 | Males (51.61%), female (48.39%) | No serious adverse reaction | 1.733 ± 1.258, 1.405 ± 0.916, 2.080 ± 2.120 duration of signs and symptoms (days) | Pain, Redness, Urticaria, and swelling at the site of the injection | Fatigue, body Pain, Headache, Muscle Pain, Fever, and gastrointestinal effects |
| 4 | Ella, R. (48) | 2021 | India | RCT | Bharat | I/II | 190 participants | 12–65 | Male:(74%), female:(26%) | No serious adverse reaction | First dose: (days 0–7), Second dose: At 7 days after the injection | Pain at the injection site, redness at the injection site, Itching, Stiffness in the upper arm, Weakness in the injection arm | Body ache, Fever, Headache, Malaise, Weakness, Rashes |
| 5 | Goepfert,P.A. (49) | 2021 | USA | RCT | Perfusion S (preS) protein vaccine | I/II | 439 participants | 18-49 & ≥50 |
N/A | No serious adverse reaction | Systemic & local adverse events appeared 7 days after vaccination (first dose) | Pain, swelling & erythema & grade 3 reaction | Myalgia, malaise Fever & headache, |
| 6 | Guo, W. (27) | 2021 | China | RCT | Vero Cells (Sinopharm) | I/II | 784 participants | ≥18 | Female: 58.9%(18-49y), female: 40.55% (≥60 y) | No serious adverse reaction | During 7 days of the first dose | Pain | Fever Headache Fatigue nausea |
| 7 | Han, B. (51) | 2021 | China | RCT | CoronaVac (Sinovac) | I/II | 72 participants for phase I & 480 participant for phase II | 3-17 | Female: (57.7%) (phase I), female: (44.2%) (phase II) |
No vaccine-related serious adverse reaction | During 7 days of the first dose | Pain Swelling Pruritus Erythema |
Fever Headache Cough Anorexia Diarrhea Vomiting Fatigue |
| 8 | Hatmal, MM. (34) | 2021 | USA | CS | Sinopharm (38.2% of participants received Sinopharm 3.46% received Moderna, Sputnik, Covaxin& Johnson & Johnson Other58.34% received AstraZeneca &Pfizer) |
N/A | 2213 participants | N/A | N/A | Among Sinopharm receivers: 2.5% showed severe adverse effects (e.g.,thrombocytopenia, irregular heartbeats, abnormal blood pressure,) | Mostly appeared within 9-12 h after injection | Pain Swelling |
Fatigue Headache Sleepiness Chills Myalgia Joints pain Fever Dizziness Nausea Sweatin |
| 9 | Kaya, F. (46) | 2021 | Turkey | CS | CoronaVac (Sinovac) | III | 329 participants | Mean age: 35.77 ±9.07 |
Male: (51.5%), female: (48.5%) | 33.2% No life-threatening adverse reaction was determined |
During 1.14 ±4 days after injection of the second dose | Pain Redness Swelling |
Nausea Vomiting Fever Headache Fatigue Allergy myalgia |
| 10 | Pichi, F. (37) | 2021 | UAE | CS | Sinopharm | III | 7 participants | Mean age: 41.4 | Male: (42.86%), female:(57.14%) | 9 eyes of 7 patients presented with Ocular Adverse events: (e.g. Uveitis, central serous chorioretinopathy, chronic serous pigment epithelial detachment, blurry vision, sudden paracentralscotoma& hemorrhage), Episcleritis Anterior scleritis Acute macular neuroretinopathy Paracentral acute middle maculopathy Subretinal fluid |
Within 15 days after injection of first dose | N/A | N/A |
| 11 | Pu, J. (36) | 2021 | China | RCT | Sinopharm | I | 294 participants | 18-59 | Male:(45%), female: (55%) | No severe adverse events | day 7 and 28 after booster | Pain, Redness, Swelling, and Itch | Fatigue, Rash, Diarrhea, and Fever |
| 12 | Saeed, B. Q. (40) | 2021 | U | CS | Sinopharm | N/A | 1102 participants | 18-80 | Male: (29%), Female: (71%) |
N/A | N/A | Pain, Redness, Tenderness, Induration, and pruritus at the vaccination site | Fatigue, Fever, Nausea, Diarrhea, Cough, Allergy, Muscle pain, lethargy, Abdominal pain, Back pain, and headache |
| 13 | Tanriover, M. D. (41) | 2021 | Turkey | RCT | CoronaVac | I/II | 11303 participants | 18–59 | Male:(57·4%), Female:(42·6%) |
N/A | day 14 | Pain, Pruritus, Swelling, Induration, Paranesthesia, and Erythema | Allergic reaction, Rash, Fatigue, Headache, Myalgia, Vomiting, Nausea, Chill, Fever, Arthralgia, Cough, and Diarrhea |
| 14 | Wang, G. (35) | 2021 | China | RCT | Sinopharm (Aikewei) | III | 11303 participants | N/A | N/A | Chest distress, palpitation, shortness of breath, limb weakness, limb shaking, general anesthesia, and transient vague |
0–28 days | Local pain, Skin itching, Hand anesthesia, local induration, Muscle soreness, and Local rash | Dizziness, Headache, Fatigue, Cough, Nausea, Dry mouth, Low-grade fever, and Chill |
| 15 | Wu, Z. (47) | 2021 | Hebei, China |
RCT | CoronaVac | I/II | Phase I: 72 participants and phase II: 349 participants |
≥60 | Phase I: (Male: 51.3%, female:48.7%) Phase II: (Male: 48.42%, female: 51.58%) |
Palpitation Hypertension |
days 0 and 28 | Pain Pruritus Erythema Swelling Abdominal pain |
Dizziness Fever Mucocutaneous eruption Oral hypoesthesia Fatigue Diarrhea Muscle pain Rash Hypoesthesia Peripheral edema Cough Headache Nausea Anorexia Abdominal distention Vomiting Drowsiness Joint pains Hypersensitivity |
| 16 | Xia, S. (30) | 2020 | China | RCT | Sinopharm | I/II | Total: 481 participants, phase I: 96 participants were included and phase II: 224 participants | 18-59 | Phase I: (Male: 39.5%, female: 60.5%) Phase II: (Male: 36.6%, female: 63.4%) |
N/A | Days 0 and 28 | Itching, Redness, Swelling, and Pain | Coughing Headache, Fatigue Diarrhea Fever, Nausea, Pruritus, and vomiting |
| 17 | Xia, S. (38) | 2021 | China | RCT | Sinopharm (BBIBP-CorV) | I/II | Phase I: 192 participants and phase II: 448 participants |
18–80 | Phase I (Male: 47%, female: 53% ) Phase II: (male:45%, female:55%) |
N/A | Days 0 and 28 | Pain Swelling Itch Redness Induration |
Fatigue Fever Inappetence Headache Muscle pain Nausea Diarrhea Joint pain |
| 18 | Zhang, M. X. (43) | 2021 | China | CS | CoronaVac | N/A | 1526 participants | 18–60 | Male: (20.7%), female: (79.3%) |
Numbness of limbs, Chest pain, and Menstruation | Days 0 and 28 | Pain, Redness, Swelling, Induration, and Itch |
Muscle pain, Fatigue Stuffy, Rash, Headache, Dizziness, Vomiting, Diarrhea, Appetite impaired, runny nose, Nausea, Fever, Cough, Throat pain, Allergic reaction, urticarial, Lymphadenopathy |
| 19 | Zhang, Y. (45) | 2021 | China | RCT | CoronaVac | I/II | Phase I: 144 participants Phase II: 600 participants |
18–59 | Days 0 and 14 vaccination Male:(44%), female:(56%) Days 0 and 28 vaccinations Male:(49%), female:(51%) |
N/A | Days 0 and 28 | Pain Pain Swelling Redness, Discoloration |
Pruritus, Fatigue Diarrhea, Fever, Headache, Nausea, Cough Hypersensitivity, Muscle pain, and Decreased appetite |
*Time from injection to the appearance of adverse events. RCT: randomized clinical trial; CS: cross sectional.
None of the studies were of poor quality. The mean score of the studies was 7.9 (Table 2). The most commonly encountered problem based on the NOS checklist was the lack of adequate matching of cases and controls, present in some of the observational studies.
4. Discussion:
This study only focused on inactivated COVID-19 vaccines that have been approved by the WHO including Sinovac (CoronaVac), Sinopharm (BBIP-CorV (Vero Cells)), and Bharat Biotech (Covaxin) as well as a protein subunit vaccine (Novavax). Bharat has been trialed 7 times in only one country (India) and it is being used and approved in 12 countries, while Sinovac has been trailed 27 times in 8 countries and approved in 48 countries. Sinopharm, on the other hand, had 19 trials in 10 countries and is widely used in 80 countries (21). Clinical trials have been conducted under unconventional swift circumstances, hence necessitating the extreme care for patient safety to gain the trust of the public and the medical community in procedures used to develop the vaccine, as the safety concerns regarding the COVID-19 vaccines are major obstacles to scaling up the vaccine uptake (22, 23). Reporting these events is of paramount importance in tackling vaccine hesitancy and ultimately curtailing the pandemic (24). A multinational study conducted on adverse effects of the COVID-19 vaccines hypothesizes that there could be a huge variety in side effects due to diversity in age groups and gender as well as high heterogeneity in populations. Consequently, single studies should be interpreted with extreme caution and account for the effect of potential confounders (25). Serious adverse events (SAEs) after vaccine injection include life-threatening events, congenital anomaly or birth defect, death, inpatient hospitalization, significant disruption of the ability to carry out usual life activities, as well as an event that may require medical intervention. On the other hand, common or mild adverse events encompass local and systemic side effects that are considered non-life-threatening and often spontaneously resolve without any medications (26).
4.1 Sinopharm
From our study, this vaccine was found to be the most commonly used inactivated COVID-19 vaccine. This phenomenon has been attributed to the higher number of countries that have approved the Sinopharm vaccine to be used within their boundaries. Furthermore, Sinopharm has been widely reported to be safe with barely any serious side effects except for some mild to moderate complications such as the pain and firmness at the point of injection regardless of the number of doses taken (27-29). Although some studies reported no adverse effects at all, the most common local side effects from this vaccine reported in various studies include redness (erythema), urticaria, swelling, induration, pain, and skin itching at the injection site (30-32). Concerning systemic side effects, fatigue, fever, inappetence, headache, dizziness, muscle and body pain, nausea, vomiting, joint pain as well as gastrointestinal complications like constipation and diarrhea were mostly associated with this type of vaccine(31, 33). Interestingly, a study found that this vaccine could be associated with a sore or dry throat, clogged nose, and runny nose as compared to other types of vaccines (34). Despite most reports suggesting that the Sinopharm vaccine presents mild side effects, the duration of these side effects and the time from injection to the appearance of the side effects varied depending on the dose. The time taken for side effects to appear typically ranges from 9 hours after to about 15 days after the first dose. The second dose and booster doses could take a longer duration up to 28 days for the side effects to appear. However, these adverse effects have been reported to be self-limiting and usually resolve within 1-3 days (27, 28, 30, 32-37). These features exhibited by the Sinopharm vaccine may reflect on the safety profile of the Sinopharm vaccine; however, long-term side effects should also not be ignored (29).
Contrary to the numerous reports of Sinopharm being a quiet vaccine with no serious adverse events (27-30, 32, 38), very few studies have reported serious adverse effects. A study reported ocular complications 5.2 days post-vaccination that presented in the form of uveitis, central serous chorioretinopathy, chronic serous pigment epithelial detachment, blurry vision, sudden paracentralscotoma & hemorrhage, episcleritis, anterior scleritis, acute macular neuroretinopathy, paracentral acute middle maculopathy, and subretinal fluid. Nevertheless, there was no causal relationship established in this study (37). Thrombocytopenia, irregular heartbeats, abnormal blood pressure, chest distress, palpitation, shortness of breath, limb weakness, and limb shaking were also reported as SAEs in some studies (34, 35). This may be similar to the findings of Fan Y et al. in their study describing inactivated vaccines to be associated with serious metabolic, musculoskeletal, immunesystem, and renal disorders (39). However, most of these SAEs appeared to be unrelated to the vaccine and resolved with appropriate treatment with no mortality (27, 35). These adverse effects may be influenced by the age group and gender as suggested by Liet al.(25)and Saeedet al. with females experiencing more SEs in their study(40), but Aga et al. and Guo et al. stipulated that these adverse effects are not dependent on gender, dose, or age(27, 28, 39). Therefore, further multinational studies are recommended to explore the potential side effects of the vaccine in populations with different demographic backgrounds.
4.2 Sinovac (CoronaVac)
Another commonly used inactivated COVID vaccine is Sinovac (CoronaVac) vaccine. Various studies have reported this type of vaccine to induce mild to moderate side effects. All the studies analyzed in this study reported injection site pain to be the most common local side effect of the Sinovacvaccine (41-45). It has been well documented that Sinovac shots are associated with injection site pain, which occurs more often after the second dose as compared to the first dose. Other local adverse events include swelling, discoloration, induration, itching, pruritus, erythema (redness), abdominal pain, and paranesthesia at the injection site (41-47). These local side effects are self-limiting; hence, they resolve in a short period. Though these SEs do not last long, the systemic side effects may take a longer time to resolve. The most common systemic side effects are fever, sleepiness, fatigue, muscle pain, headache, diarrhea, vomiting, allergy, arthralgia, rash, mucocutaneous eruption, oral hypoesthesia, hypoesthesia, peripheral edema, abdominal distention, drowsiness, joint pain, hypersensitivity, runny and stuffy nose, lymphadenopathy, cough, and loss of appetite. Among these symptoms, fatigue is the most frequent systemic adverse reaction to the Sinovac vaccine (33, 41, 43, 44). Additionally, there is no significant variation in symptom presentation among different age groups (42).
Notably, Sinovac injection is associated with no life-threatening adverse events. The evidence from phases 1 and 2 trials reported no vaccine-related mortality or grade 4 adverse events so far (41, 46). However, sporadic serious adverse events were reported in separate studies conducted by Zhanget al. and Wuet al. (43, 47). They reported pneumonia, numbness of limbs, chest pain, palpitation, hypertension, and menstruation as serious side effects that occurred in people after Sinovac vaccination; however, these SAEs appeared to be vaccine-unrelated (42, 47). Adverse events of the Sinovac vaccine are expected to occur within 7 days after the first dose of the vaccine but it may take up to 28 days as reported by some studies (42, 45). Additionally, the second dose may elicit side effects from 1- 4 days post-vaccination. The safety profile of this vaccine from clinical trials in phases 1 and 2 postulates that it is safe and well-tolerated in various age groups.
4.3 Bharat Biotech
The Bharat Biotech vaccine was found to be the least used inactivated corona vaccine. Its clinical trials are comparable to the extent to which it is being used, but this may not imply its efficacy and safety profile. From the phase 1 and 2 trials, this vaccine has been reported to be well-tolerated by all the subjects with no significant differences in safety for various age groups (48). Ella et al. reported that the most common adverse events that were deemed mild to moderate included pain at the injection site, redness at the injection site, itching, stiffness in the upper arm, and weakness in the injection arm as local side effects. Furthermore, they reported the systemic side effects to entail body ache, fever, headache, malaise, weakness, and rashes (48). The time of SEs appearance was not significantly different from other vaccines like Sinopharm, Sinovac, Moderna, and AstraZeneca (34). In the first shot, the Covaxin produces SEs within 0-7 days after injection, while the second dose was reported to elicit SEs 7 days after receiving the vaccine. The Covaxin produced no severe or life-threatening adverse effects in trials (48). However, these findings may as well require multi-site surveillance studies to ascertain the long-term effects and population heterogeneity effects since the current data may be insufficient to make a definitive conclusion.
4.4 Novavax
The Novavax vaccine, which is a recombinant protein subunit vaccine, was reported by a single study in our analysis. Despite concerns about the reactogenicity and side effects caused by the adjuvant in these vaccines, their desirable safety profiles due to the absence of live viral components, the addition of adjuvant to increase immunogenicity, and the ease of scalability of the recombinant protein serve as advantages for this type of vaccine. As a result, vaccine developers are drawn to this sort of vaccine. Goepfertet al. reported no vaccine-related unintended adverse events or severe adverse events of special interest in phase 1 and 2 trials of the Perfusion S (preS) protein vaccine. Solicited side effects may appear after 7 days of taking the first dose. Local side effects include pain, swelling, erythema, and grade 3 reaction; while systemic side effects include myalgia, malaise, fever, and headache. It was observed that systemic adverse events usually appeared on the second day after the second dose of the Perfusion S (preS) protein vaccine. Furthermore, immune responses in the elderly seem to be lower than younger age group independent of the number of doses (49). Emerging clinical evidence from investigations of the NVX-CoV2373 vaccine (Novavax), a recombinant nanoparticle vaccine, revealed that it is safe and associated with a significant immune response in healthy adult participants. The majority of the time, reactogenicityis modest and brief. The frequency of significant adverse events seems to be minimal and comparable in all age groups (50).
4.5 Limitations and Recommendations
The results of the present review may be limited in various aspects, as there is no specific system for the registration of COVID-19 vaccine side effects in different countries. It is also limited in the aspect of data retrieval, since some studies were incomplete and their results had not been published yet. Additionally, study subjects in the analyzed articles may have given biased reports of their adverse effects. This may be due to the difference in theirlevel of education. Consequently, the included studies may have had reporting bias; hence, there is an influence on the reliability of reported side effects. There are no scales for the severity of common side effects like pain and fatigue; hence, these reported side effects are patient-dependent, so mild ones may be neglected. Also, we have no scale for comparison of these effects between studies and some reported adverse effects (e.g cardiovascular events) may be due to the co-existence of patients’ underlying diseases and vaccination. Considering that the COVID-19 vaccine rollout is relatively new, all of the adverse effects may not be well-known and the long-term effects are still undetermined. Consequently, there is not sufficient evidence to affirm that these complications are solely due to the vaccines. Therefore, multinational studies are recommended to address the influence of demographic heterogeneity on the manifestation of vaccines’ adverse effects as well as to determine the long-term adverse events of the inactivated COVID vaccines.
5. Conclusion
This systematic review investigated the adverse effects of WHO-approved inactivated vaccines including Sinovac (CoronaVac), Sinopharm (BBIP-CorV), and Bharat Biotech (Covaxin) as well as a protein subunit vaccine (Novavax). Sinopharm followed by Sinovac, and Bharat were the most common inactivated vaccines trialed and used globally. The most common local side effects are pain, redness, and swelling at the injection site; while fatigue, body pain, headache, muscle pain, fever, and malaise were the most common systemic side effects. Almost all these local and systemic adverse effects were self-limiting. Therefore, they resolved within minutes to days post-vaccination. Although few SAEs were reported after injecting inactivated and protein subunit vaccines, particularly with the Sinopharm vaccine, no statistically significant relation was found between the vaccines and these side effects or any related mortality. Hence, inactivated vaccines seem to be a safe choice due to their mild side effects and few life-threatening adverse events.
6. Declarations:
6.1. Ethics approval and consent to participate
Not applicable
6.2. Consent to publication
Not applicable
6.3. Availability of data and material
The authors stated that all information provided in this article could be shared.
6.4. Competing interests
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
6.5. Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
6.6. Authors' contributions
1. The conception and design of the study: Esmaeil Mehraeen, SeyedAhmad SeyedAlinaghi
2. Acquisition of data: Amirali Karimi, Marcarious M. Tantuoyir
3. Analysis and interpretation of data: Arian Afzalian, Newsha Nazarian, Hengameh Mojdeganlou
4. Drafting the article: Pegah Mirzapour, Ahmadreza Shamsabadi, Mohsen Dashti, Afsaneh Ghasemzadeh, Farzin Vahedi, Parnian Shobeiri, Zahra Pashaei, Omid Dadras
5. Revising it critically for important intellectual content: SeyedAhmad SeyedAlinaghi, Esmaeil Mehraeen, Omid Dadras
6. Final approval of the version to be submitted: all authors
6.7. Acknowledgments
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, Iranian Research Center for HIV/AIDS, Tehran University of Medical Sciences, and Walailak University.
References
- 1.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clinical microbiology and infection. 2020;26(6):729–34. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dadras O, Alinaghi SAS, Karimi A, MohsseniPour M, Barzegary A, Vahedi F, et al. Effects of COVID-19 prevention procedures on other common infections: a systematic review. European journal of medical research. 2021;26(1):67. doi: 10.1186/s40001-021-00539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin C, Liu F, Yen T-C, Lan X. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. European journal of nuclear medicine and molecular imaging. 2020;47(5):1281–6. doi: 10.1007/s00259-020-04734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanie Jahromi MS, Aghaei K, Taheri L, Kalani N, Hatami N, Rahmanian Z. Intensive Care Unit of COVID-19 during the Different Waves of Outbreaks in Jahrom, South of Iran. Journal of Medicinal and Chemical Sciences. 2022;5(5):734–42. [Google Scholar]
- 5.Sheikhbahaei E, Mirghaderi SP, Moharrami A, Habibi D, Motififard M, Mortazavi SMJ. Incidence of Symptomatic COVID-19 in Unvaccinated Patients Within One Month After Elective Total Joint Arthroplasty: A Multicenter Study. Arthroplast Today. 2022;14:110–5. doi: 10.1016/j.artd.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilder-Smith A, Freedman DO. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. Journal of travel medicine. 2020 doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P. Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring. Vaccine. 2009;27(15):2114–20. doi: 10.1016/j.vaccine.2009.01.125. [DOI] [PubMed] [Google Scholar]
- 8.Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z-J, Zhang H-J, Lu J, Xu K-W, Peng C, Guo J, et al. Low toxicity and high immunogenicity of an inactivated vaccine candidate against COVID-19 in different animal models. Emerging microbes & infections. 2020;9(1):2606–18. doi: 10.1080/22221751.2020.1852059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713–21. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious Diseases. 2021;21(6):803–12. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. The Lancet Infectious Diseases. 2021;21(5):637–46. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Zhao S, Ou J, Zhang J, Lan W, Guan W, et al. COVID-19: coronavirus vaccine development updates. Frontiers in immunology. 2020;11:3435. doi: 10.3389/fimmu.2020.602256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. Jama. 2020;324(10):951–60. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. The Lancet Infectious Diseases. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudharsanan N, Favaretti C, Hachaturyan V, Bärnighausen T, Vandormael A. The effect of framing and communicating COVID-19 vaccine side-effect risks on vaccine intentions for adults in the UK and the USA: A structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22(1):592. doi: 10.1186/s13063-021-05484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman Y, Palgi Y, Goodwin R, Ben-Ezra M, Greenblatt-Kimron L. A storm in a teacup: older adults' low prevalence of COVID-19 vaccine side-effects and their link with vaccination anxiety. International psychogeriatrics. 2021;33(12):1335–7. doi: 10.1017/S1041610221001071. [DOI] [PubMed] [Google Scholar]
- 18.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. New England Journal of Medicine. 2020;383(24):2320–32. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang S, Li Y, Dai L, Wang J, He P, Li C, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. The Lancet Infectious Diseases. 2021;21(8):1107–19. doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meira CM Jr, Meneguelli KS, Leopoldo MPG, Florindo AA. Anxiety and Leisure-Domain Physical Activity Frequency, Duration, and Intensity During Covid-19 Pandemic. Frontiers in psychology. 2020:11:603770. doi: 10.3389/fpsyg.2020.603770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(WHO) WHO. 10 Vaccines Approved for Use by WHO 2021. [[updated 27 December 2021; cited 2021 28 December]]. Available from: https://covid19.trackvaccines.org/agency/who.
- 22.Panda DS, Giri RK, Nagarajappa AK, Basha S. Covid-19 vaccine, acceptance, and concern of safety from public perspective in the state of Odisha, India. Human vaccines & immunotherapeutics. 2021;17(10):3333–7. doi: 10.1080/21645515.2021.1924017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joffe S, Babiker A, Ellenberg SS, Fix A, Griffin MR, Hunsberger S, et al. Data and Safety Monitoring of COVID-19 Vaccine Clinical Trials. The Journal of infectious diseases. 2021;224(12):1995–2000. doi: 10.1093/infdis/jiab263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riad A, Schünemann H, Attia S, Peričić TP, Žuljević MF, Jürisson M, et al. COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines' Side Effects. International journal of environmental research and public health. 2021;18(15) doi: 10.3390/ijerph18157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Ostropolets A, Makadia R, Shoaibi A, Rao G, Sena AG, et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. BMJ (Clinical research ed). 2021;373 doi: 10.1136/bmj.n1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karayeva E, Kim HW, Bandy U, Clyne A, Marak TP. Monitoring Vaccine Adverse Event Reporting System (VAERS) Reports Related to COVID-19 Vaccination Efforts in Rhode Island. Rhode Island medical journal (2013). 2021;104(7):64–6. [PubMed] [Google Scholar]
- 27.Guo W, Duan K, Zhang Y, Yuan Z, Zhang YB, Wang Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: A randomized, double-blind, placebo-controlled, phase 1/2 trial. EClinicalMedicine. 2021;38:101010. doi: 10.1016/j.eclinm.2021.101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Khames Aga QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahham AT, et al. Safety of COVID-19 vaccines. Journal of medical virology. 2021;93(12):6588–94. doi: 10.1002/jmv.27214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Hammad O, Alduraidi H, Abu-Hammad S, Alnazzawi A, Babkair H, Abu-Hammad A, et al. Side Effects Reported by Jordanian Healthcare Workers Who Received COVID-19 Vaccines. Vaccines. 2021;9(6) doi: 10.3390/vaccines9060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. Jama. 2020;324(10):951–60. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niebel D, Novak N, Wilhelmi J, Ziob J, Wilsmann-Theis D, Bieber T, et al. Cutaneous Adverse Reactions to COVID-19 Vaccines: Insights from an Immuno-Dermatological Perspective. Vaccines. 2021;9(9):944. doi: 10.3390/vaccines9090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Halaweh S, Alqassieh R, Suleiman A, Al-Sabbagh MQ, AbuHalaweh M, AlKhader D, et al. Qualitative Assessment of Early Adverse Effects of Pfizer-BioNTech and Sinopharm COVID-19 Vaccines by Telephone Interviews. Vaccines. 2021;9(9) doi: 10.3390/vaccines9090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, et al. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J Clin Biochem. 2021;36(4):427–39. doi: 10.1007/s12291-021-00968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM, et al. Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects. Vaccines. 2021;9(6):556. doi: 10.3390/vaccines9060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, Zhu L, Zhu Y, Ye Q, Yu X, Fu M, et al. Safety survey by clinical pharmacists on COVID-19 vaccination from a single center in China. Human vaccines & immunotherapeutics. 2021;17(9):2863–7. doi: 10.1080/21645515.2021.1913964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pu J, Yu Q, Yin Z, Zhang Y, Li X, Yin Q, et al. The safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in Chinese adults aged 18-59 years: A phase I randomized, double-blinded, controlled trial. Vaccine. 2021;39(20):2746–54. doi: 10.1016/j.vaccine.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG. Association of Ocular Adverse Events With Inactivated COVID-19 Vaccination in Patients in Abu Dhabi. JAMA ophthalmology. 2021;139(10):1131–5. doi: 10.1001/jamaophthalmol.2021.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. The Lancet Infectious diseases. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan YJ, Chan KH, Hung IF. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines. 2021;9(9):989. doi: 10.3390/vaccines9090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID-19 vaccination. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2021;111:219–26. doi: 10.1016/j.ijid.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet (London, England). 2021;398(10296):213–22. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.SeyedAlinaghi S, Mirzapour P, Dadras O, Pashaei Z, Karimi A, MohsseniPour M, et al. Characterization of SARS-CoV-2 different variants and related morbidity and mortality: a systematic review. European journal of medical research. 2021;26(1):51. doi: 10.1186/s40001-021-00524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, et al. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert review of vaccines. 2021;20(7):891–8. doi: 10.1080/14760584.2021.1925112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang ZN, Zhao YY, Li L, Gao HD, Cai Q, Sun XX, et al. [Evaluation of safety of two inactivated COVID-19 vaccines in a large-scale emergency use] Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2021;42:1–6. doi: 10.3760/cma.j.cn112338-20210325-00249. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious diseases. 2021;21(2):181–92. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaya F, Pirincci E. Determining the frequency of serious adverse reactions of inactive SARS-COV-2 vaccine. Work (Reading, Mass). 2021;69(3):735–9. doi: 10.3233/WOR-210473. [DOI] [PubMed] [Google Scholar]
- 47.Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious diseases. 2021;21(6):803–12. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. The Lancet Infectious diseases. 2021;21(7):950–61. doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goepfert PA, Fu B, Chabanon AL, Bonaparte MI, Davis MG, Essink BJ, et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1-2, dose-ranging study. The Lancet Infectious diseases. 2021;21(9):1257–70. doi: 10.1016/S1473-3099(21)00147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. The New England journal of medicine. 2021;385(13):1172–83. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. The Lancet Infectious diseases. 2021;21(12):1645–53. doi: 10.1016/S1473-3099(21)00319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]