Abstract
Introduction:
Controversies existed regarding the duration of COVID-19 vaccines’ protection and whether receiving the usual vaccine doses would be sufficient for long-term immunity. Therefore, we aimed to systematically review the studies regarding the COVID-19 vaccines’ protection three months after getting fully vaccinated and assess the need for vaccine booster doses.
Methods:
The relevant literature was searched using a combination of keywords on the online databases of PubMed, Scopus, Web of Science, and Cochrane on September 17th, 2021. The records were downloaded and the duplicates were removed. Then, the records were evaluated in a two-step process, consisting of title/abstract and full-text screening processes, and the eligible records were selected for the qualitative synthesis. We only included original studies that evaluated the efficacy and immunity of COVID-19 vaccines three months after full vaccination. This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement to ensure the reliability of results.
Results:
Out of the 797 retrieved records, 12 studies were included, 10 on mRNA-based vaccines and two on inactivated vaccines. The majority of included studies observed acceptable antibody titers in most of the participants even after 6 months; however,it appeared that the titers could also decrease in a considerable portion of people. Due to the reduction in antibody titers and vaccine protection, several studies suggested administering the booster dose, especially for older patients and those with underlying conditions, such as patients with immunodeficiencies.
Conclusion:
Studies indicated that vaccine immunity decreases over time, making people more susceptible to contracting the disease. Besides, new variants are emerging, and the omicron variant is continuing to spread and escape from the immune system, indicating the importance of a booster dose.
Key Words: COVID-19, COVID-19 vaccines, Immunity, SARS-CoV-2, Vaccines, Vaccine-preventable diseases
1. Introduction:
Since the coronavirus disease 2019 (COVID-19) pandemic spread all over the globe, it has been posing a considerable healthcare crisis by affecting more than 250 million individuals and leading to more than 5 million deaths up until now (1). It has also influenced other aspects of life, including economic, technological, and social aspects. Since COVID-19 is highly contagious,substantial effort is required to curtail the pandemic(2). In this regard, vaccines offer a promising opportunity for fighting the pandemic and have shown considerable efficacy against severe COVID-19 infection, hospitalization, and death (3). Despite the emergence of new variants, the most effective approach to curb the pandemic seems to be mass vaccination and reaching herd immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)(4).
The duration of immunity that most vaccines generate against various common infections is limited and developing strong immunity often requires booster doses. The generation of long-term immunity by COVID-19 vaccines and the necessity to administer booster doses for different COVID-19 vaccines is still a matter of debate. Considering this, it is of great importance to define the duration in which the humoral immune responses are efficient enough against COVID-19 infection (3, 5).
Some studies have demonstrated that a few months after the injection of the second dose, the effectiveness of COVID-19 vaccines wanes as antibody levels drop (6, 7). Thus, an additional booster dose may be needed to restore the high level of immunity, especially against new variants, and maintain the equilibrium of the protective humoral immunity and COVID-19 viral load during exposure. Some groups, including the elderly, are at higher risk of profound IgG decrease over time and thus increased probability of being infected with COVID-19(8). However, it is intriguing that even after a few months, the effectiveness against severe disease course and hospitalization is rather sustained (6). In a retrospective cohort study conducted by Tartof et al., participants who were fully vaccinated showed high immunity against all variants of COVID-19 up until six months after vaccination, but the immunity had been decreasing over that time (6). However, they reported no decreased effectiveness against hospital admissions in any age group during the study period.
Thomas et al. found 91% protection from Pfizer/BioNTech vaccine after six months, silencing the concerns and showing its sufficient protection during this time (9). On September 17th, 2021, the United States food and drug administration (FDA) refuted the need for a booster dose six months after the second dose of the Pfizer/BioNTech vaccine for the general population, and only recommended it for people above 65 years of age and some specific groups, but later booster doses were recommended for all the people (10, 11).
Concerns still exist regarding vaccines’ duration of immunity and the need for booster doses, especially for other types of vaccines (12). Considering that new variants of COVID-19 may continue to emerge all around the world and disrupt the efforts that have been done so far to control the pandemic, it is of great importance to determine which vaccines require a booster dose for maintaining immunity against COVID-19. A systematic evaluation of this matter elucidates the path for designing new vaccination strategies. Therefore, we aimed to systematically review the studies regarding the COVID-19 vaccines’ protection three or more months after getting fully vaccinated and assess if vaccine booster doses are required.
2. Methods:
This study is a comprehensive review of the literature to describe COVID-19 vaccines’ protection over time. We also investigated the need for booster doses. In order to ensure solidity and reliability of the outcomes, this review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
2.1. Data sources
We executed a comprehensive and systematic search in the online databases of PubMed, Scopus, Web of Science, and Cochrane on September 17th, 2021. Keywords were selected using the medical subject headings (MeSH) and previous studies. We provided the search terms for all the databases in Supplementary material 1. The search terms for PubMed were as follows:
“COVID-19” OR “SARS-CoV-2” OR “SARS-CoV2” OR “2019-nCoV” OR “Novel Coronavirus” [Title/ Abstract]
“Vaccine” OR “Vaccination” OR “Vaccinated” OR “Immunization” [Title/ Abstract]
“Immunity duration” OR “Immunity period” OR “Protection duration” OR “Duration”OR“Month” OR “Year”[Title/ Abstract]
[A] AND [B] AND [C]
2.2. Study selection
The retrieved records were imported to an EndNote file and the duplicates were removed. In a biphasic approach, threeindependent researchers screened and selected eligible studies. In the first phase, the retrieved records were reviewed and screened based on the relevancy of titles and abstracts. The full texts of the remaining articles were assessed based on eligibility criteria in the second phase to select the most appropriate articles. Original articles discussing the efficacy of COVID-19 vaccines at least three months after full vaccination (second dose in most cases, first dose in case of single-dose vaccines) were included in our study.
Publications subject to one or more of the following exclusion criteria were excluded from our study:
Non-original studies, such as review articles
Case reports and case series
Abstract papers, conference abstracts, and other studies without available full texts
Ongoing clinical trials without yet published results
Preclinical studies and studies on subjects other than humans, such as pure laboratory or animal studies
Studies evaluating vaccine effectiveness in periods shorter than three months after becoming fully vaccinated against COVID-19. Three months was chosen as the cut-off point because full vaccinations usually provide adequate protection in the first three months (13-15).
2.3. Data extraction
Two researchers extracted the following information from the eligible studies included in the review (each recorded the data of half of the studies):first author (reference) ID, country and year of study, type of study, study population, sex percentage and mean age of the population, vaccine type, time passing from vaccination,changes in antibody levels, vaccine efficacy against infection, and disease severity parameters as well as mortality, authors’ opinion about booster dose, and summary of other notable findings. These data were transferred into a word table, and then another independent researcher reviewed the extracted results to re-check and verify them.
2.4. Quality/risk of bias assessment
We utilized the Newcastle-Ottawa Scale (NOS) risk assessment tool to evaluate bias risk of the included studies. This scale adds up to a total score of nine in three categories. These categories consist of selection, comparability, and exposure/outcome and receive maximum scores of four, two, and three, respectively (Table 2).
Table 2.
The results of Newcastle-Ottawa scale (NOS) risk of bias assessment
|
The first author
(Reference) |
Selection
(out of 4) |
Comparability
(out of 2) |
Exposure/ Outcome
(out of 3) |
Total score
(out of 9) |
|---|---|---|---|---|
| A.Erice(19) | *** | - | ** | 5 |
| J.Favresse(20) | *** | * | ** | 6 |
| W.Gou(21) | *** | - | *** | 6 |
| J.F. Hedges(24) | **** | * | ** | 7 |
| P.Naaber(16) | **** | * | ** | 7 |
| M.Pouquet(26) | *** | * | *** | 7 |
| E. Terpos(18) | **** | * | * | 6 |
| S. J. Thomas(9) | **** | * | ** | 7 |
| M. Tré-Hardy(17) | *** | * | *** | 7 |
| I.Vicenti(27) | **** | * | *** | 8 |
| I.Waldhorn(22) | **** | * | *** | 8 |
| K. Zakaria(25) | **** | * | ** | 7 |
3. Results:
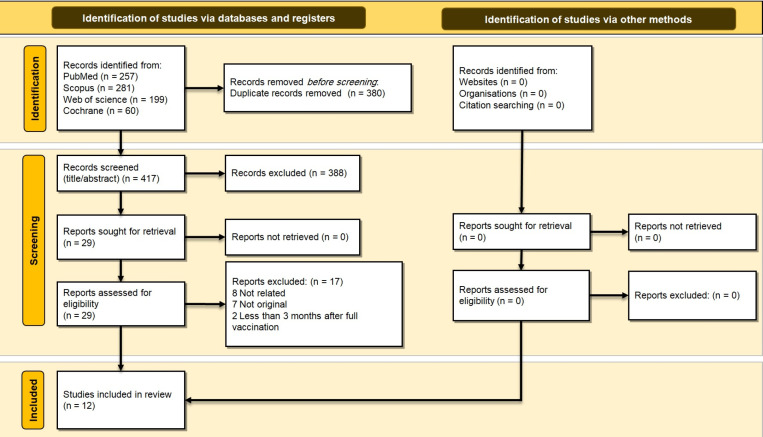
In this study, by applying systematic search strategies, 797 relevant records were identified and retrieved from PubMed, Scopus, Web of Science, and Cochrane. After a primary review of retrieved articles, 380 duplicates were removed, and the title and abstract of the remaining 417 articles were evaluated. By applying the selection criteria, 388 articles were excluded, and only 29 articles were screened by their full texts. After the review of full texts, 17 articles were excluded. Finally, 12 articles met the inclusion criteria and were included in the final review (Figure 1).
Figure 1.
PRISMA 2020 flow diagram for this systematic review
Table 1 summarizes the results of the studies. The studies were conducted in various countries with 15 countries involved overall; one study was multinational and included six countries (USA, Turkey, Germany, South Africa, Brazil, and Argentina), and the other studies were conducted in Belgium (n=2), USA, China, Estonia, France, Spain, Israel, Greece, Italy, and Kazakhstan (each n=1).The vaccine types in included studies were mRNA-based (n=10), and inactivated virus (n=2)vaccines. The interval between the administration of the second dose of the vaccine and the antibody titer assessment varied between 4 weeks to 6 months. The majority of included studies observed acceptable antibody titers in most of the participants even after 6 months (16, 17); however, the titers decreased in a considerable portion of the people(18). Due to the reduction in the antibody titer over time, several studies suggested administering the booster dose, especially for older patients and those with underlying conditions, such as patients with immunodeficiencies(17-19).Table 2 demonstrates the results of the quality assessment. All the studies had acceptable quality assessment scores, but they mostly lacked adequate matching for confounders.
Table 1.
Summary of findings based on each study
| First author (reference) | Type of study | Study population (N) | Male (%) | Mean age (SD) | Type | Time after vaccination | Changes in antibody level s | Vaccine efficacy against | Author’s opinion about booster dose | Summary of findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection | Disease severity | Mortality | ||||||||||
| A.Erice(19) Spain, 2021 |
Observational study | Adults | 62% | 46.0 years (SD 11.4 years) | mRNA vaccine | Serum samples were obtained a mean of 40.1 days (SD 2.8 days) and 88.8 days (SD 2.8 days) after the second dose of BNT162b2 | Median [IQR] anti-RBD titres 1.5 months after vaccination were 9,356 [5,844 - 16,876] AU/mL; three months after vaccination, median anti-RBD titres had declined to 3,952 [2,190 - 8,561] AU/mL (p <0.001) | Advanced severe COVID-19 has been reported in fully vaccinated individuals a median of 39.5 days after the second dose of BNT162b2 | A low anti-RBD antibody titer is one aspect related tothe advanced SARS-CoV-2 infection after complete vaccination with BNT162b2 | |||
| J.Favresse(20) Belgium, 2021 |
Ongoing multicenter, prospective, and interventional study | Healthcare professionals | 22.5 | 43 | mRNA COVID-19 vaccine | 3 months | The maximal antibody response was reached between days 28 and 42 (2204 versus 1,863; P = 0.20), with a 48.8–57.7-fold increase compared to day 14 (i.e. 38.2 U/mL) | As calculated by the one-compartmental model, the estimated half-life of antibodies observed from data collected until 90 days after vaccination for seronegative members was 55 days (95% CI: 37–107 days) | ||||
| W.Gou(21) China, 2021 |
Clinical trial | Healthy adults aged ≥18 | 41.1 and 59.5 in twoage groups | The mean (standard deviation) age was 43.1 (9.6) years in participants aged 18–59 years and 66.7 (4.3) in those aged ≥60 years (79.2% aged 60–69 years) | Inactivated | 90 days | Geometric mean titerof neutralizing antibody on day 90 after the third injection ranged from 87 to 129, respectively, among participants receiving three doses of vaccines | The initial results of the Phase 1/2 trial among adults, including those aged 60 years or older, showed that the inactivated vaccine against SARS-CoV-2 was safe and immunogenic. | ||||
| J.F. Hedges(24) USA, 2021 |
Cohort | 41.8 | mRNA | 6 months | The neutralization titers had declined 6 months after vaccination,similar to 6 months after natural infection. | The antibody responses induced by vaccination were significantly higher than those induced by natural infection. Therefore, the study suggests that vaccination is still vital, even for those naturally infected or diagnosed with COVID-19. | ||||||
| P.Naaber(16) Estonia, 2021 |
Longitudinal observational | Healthcare workers | 42 | 42.5 | mRNA | 6 months | In the first serum sample, the median anti-S-RBD IgG reached 540.0 AU/mL (IQR 64.5-1102.0). In the following tests, a progressive decay of antibodies was seen, up to the value of 55.7 AU/mL (IQR 26.2-84.7) at the 6-month follow-up | This study may allow to define a protective antibody threshold, below which the risk of break-through infections significantly increases and which could, hence, guide the time point when to offer a booster dose. | The study approves the persistence of anti-S-RBD neutralizing antibodies through 6 months after the vaccination. | |||
| M.Pouquet(26) France, 2021 |
Longitudinal survey | Health care workers | RNA-based vaccines | 6 months | ||||||||
| E. Terpos(18) | Prospective study | Health care workers | 32.9 | 48 | mRNA | 3 months | Three months after the second vaccination (i.e., on D111), the decline in NAb titers was even more prominent with a median inhibition of 92.7% (SD 11.8) | The longitudinal study is continuing in order to determine the time point of NAbs decrease below the positivity threshold, and the fading of protective immunity against COVID-19; when a booster vaccine dose might be necessary. | Both NAbs and anti-S-RBD antibodies, the maximum levels are seen at day 36. A statistically significant decrease in both types of antibodies was observed after day36 up to day111 | |||
| S. J. Thomas(9) 6 countries, USA, Turkey, Germany, South Africa, Brazil, Argentina; 2021 |
Clinical trial | Adolescents and adults | 50.9 | 51 | mRNA | 6 months | Vaccine efficacy of 91.1% | BNT162b2 effectively prevents COVID-19 for up to 6 months after the second dose across various populations, despite the emergence of SARS-CoV-2 variants, including the beta variant, and the vaccine continues to show a promising safety profile. | ||||
| M. Tré-Hardy(17) Belgium, 2021 |
Prospective study | Health care workers | 25.4 | 50.1 | mRNA | 5 months | Antibody values went from 400 [400-400] AU/mL at 3 months after first injection to 221.0 [202.3-241.2] AU/mL at 6 months after first injection, and from 400 [400-400] AU/mL at to 400 [365.0-400] AU/mL at | Introducing a booster dose, under certain circumstances, could have a significant impact in terms of public health | All applicants still had detectable SARS-CoV-2 IgG antibodies up to 5 months after complete vaccination. | |||
| I.Vicenti(27) Italy, 2021 |
Longitudinal study | Health care workers (HCWs) | 39.1 | mRNA | 3 months | Previously infected vaccinated HCWs (n=23): 546 Uninfected vaccinated HCWs (n=13): 20 |
In uninfected HCWs completing the two-dose vaccine program, a third mRNA vaccine dose is a sensible option to counteract the substantial NtAb decline occurring at a significantly higher rate compared with previously infected, vaccinated HCWs | Median NtAb at V2_90 (90±2 days after the second dose) was still significantly higher than median NtAb at V_0 (before receiving the first dose) both in HCWs with past mild disease (p=0.01) and in those experiencing asymptomatic infection (p=0.001). |
||||
| I.Waldhorn(22) Israel, 2021 |
Prospective follow-up report of the primary study | Cancer patients with solid tumors | 55 | 66 | mRNA | 166 ± 29 days | Both cohorts depicted a drastic decline in serology titer over time,but the titer remained above the threshold value | There was no notable difference in the median absolute serology titer between the seropositive individuals within the two cohorts (patients vs. controls). | ||||
| K. Zakaria(25) Kazakhstan, 2021 |
Clinical trial | Adults aged 18 years and older | 77.3 | 28 | Inactivated whole-virion | 6 months | An increase in the titers of neutralizing antibody was statistically significant, reaching Geometric Mean Titer of 5.1 (95% CI 3·5–7·6) on day 21 and Geometric Mean Titer of 100 (95% CI 77–129) on day 42. On day180 after the first immunization, the Geometric Mean Titer dropped to 7 (95% CI 5–7) | In both trials, specific antibodies were detected in MNA and ELISA on study day 180, but the titers dropped in comparison today 42. | ||||
SD: standard deviation; IQR: interquartile range; CI: confidence interval; S-RBD: spike protein receptor-binding domain; Ig: Immunoglobulin;NAb/NtAb: neutralizing antibody; MNA:microneutralization assay; ELISA:enzyme-linked immunosorbent assay.
4. Discussion
COVID-19 pandemic is a serious global challenge due to its high prevalence and the emergence of new variants.Vaccination is one of the best solutions to mitigate the immense burden of the virus, in addition to the social distancing, using face masks, and observing health protocols.We reviewed 12 articles concerning COVID-19 vaccination, elicited antibody response, duration of triggered immunity, and the necessity of the booster dose. In 10 studies, the vaccine type was mRNA-based, and in two studies, it was inactivated vaccine. In seven studies, participants were healthcare workers, adults, and individuals with cancer. The majority of articles mainly discussed the importance of booster doses, since antibody titers decline over time.
Favresse et al. reported that antibody titers significantly decreased three months after vaccination with BNT162b2 in seronegative and seropositive healthcare workers (20).Consistently, the study by Erice et al. showed a reduction in anti-SARS-CoV-2 receptor-binding domain antibody (anti-RBD antibody) titers in healthy individuals three months after the second dose; indicating that a booster dose could be beneficial(19). Terpos et al. also reported a decline in effective antibody titers (anti-S-RBD antibody and neutralizing antibody) six months after vaccination(18). These findings emphasize the beneficial effects of a booster dose against COVID-19; particularly, in reducing the rate of hospitalization and mortality, which are specifically important in the elderly and people with underlying diseases.
A study by Gou et al. on efficacy of inactivated vaccines evaluated 2 age groups, one of which mostly consisted of the elderly. This study demonstrated the value of a booster dose, as there is always concern over elderly people's morbidity and mortality (21),yet more studies are required in order to assess the antibody alteration in elderly population.
In addition, a study by Waldhorn et al. showed a significant decrease in antibody titer over time. Since the study population was cancer patients with an average age of 66, it is possible that immunodeficiency and advanced age are both to blame for the considerable drop (22). However, more studies for assessment of antibody alterations in each group, separately, could be useful in determining the effect of each change in factor on antibody titer over time.
In recent months,the COVID-19 wave attracted global attention due to its new variant (Omicron B.1.1.529). Although this new variant has lower mortality, it is more contagious, spreads faster, and can even result in severe illness. This global issue could be best resolved by the enhancement of the immune system; therefore, the third dose of vaccine is beneficial to accentuate antibody response(23).
Concerningthe immune response, Hedges et al. found that vaccination causes higher levels of antibody in comparison with previous COVID-19 infection. This showed the necessity of vaccination even in individuals with previous COVID-19 infection(24). Besides, it has been shown that a booster dose of the COVID-19 vaccine can elicit a strong antibody response that could protect the individuals from acquiring the disease and severe disease, and subsequently reduce the mortality and morbidity of the disease (11).
Studies suggest that age plays an important role in vaccination. The study of Naaber et al. reported that older people may have a weaker response to COVID-19 vaccines and also may have fewer side effects(16). Terpos et al. also showed that antibody titers decrease more slowly in younger persons; therefore, younger individuals had higher antibody titers compared to older people with the same number of days passing from vaccination(18). Likewise, Erice et al. observed that younger individuals(especially those aged 21 to 30) had higher antibody titers following COVID-19 vaccination(19). Compared to other included studies, Zakaria et al. evaluated the younger study population (mean age: 28) and discovered that antibody titer decreased over time(25). Considering this finding and based on the study by Terpos et al. (18), booster doses continue to play an important role.
Terpos et al. also showed that underlying diseases such as diabetes or autoimmune diseases may affect the antibody titers, leading to lower neutralizing antibody titers(18). These findings showed that the efficacy of vaccines can be influenced by different factors, including age and underlying disease. Therefore, a booster dose would be most beneficial in these vulnerable groups. However, it has been recently recommended for all age groups from all backgrounds (11).
Limitations
This study has several limitations. First, the number of included studies was limited and they did not encompass all types of vaccines, and the publications existed only on mRNA-based and inactivated vaccines. We also could not conduct a meta-analysis due to the limited number of studies and their heterogeneity. Regarding the study populations, 7 out of 12 studies were performed on healthcare workers, an important group vulnerable to the COVID-19. This can be considered both a strength and a limitation, as healthcare workers are a special and vulnerable group and require specific attention, but this means that the number of population-based studies on the general population were limited. On the other hand, only one study targeted another important group, the immunocompromised pateints, and further specific studies on this group are required. Furthermore, although the studies had acceptable quality assessment scores, many of them lacked adequate matching for confounders. A strength of the present review was that included studies were conducted in 15 countries, making the results more reliable worldwide. Overall, we could deduce the benefits of booster doses using the existing evidence.
5. Conclusion
Studies have shown that the immunity due to COVID-19 vaccines diminishes over time. Such decrease is more evident in older people and those with specific underlying diseases, such as immunodeficiencies. Furthermore, new COVID-19 variants, particularly Omicron, are on the rise and it has been documented that they may evade the immunity rendered by vaccines; therefore, immediate efforts are required to refurbish the vaccines to trigger the appropriate antibody responses against these new variants. Moreover, booster doses are recommended to enhance the overall immunity of the general population against COVID-19.
6. Declarations:
6.1. Ethics approval and consent to participate
Not applicable
6.2. Consent to publication
Not applicable
6.3. Availability of data and material
The authors stated that all information provided in this article could be share.
6.4. Competing interests
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
6.5. Funding and support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
6.6. Authors' contributions
1. The conception and design of the study:Esmaeil Mehraeen, SeyedAhmad SeyedAlinaghi
2. Acquisition of data: Amirali Karimi, Alireza Shojaei
3. Analysis and interpretation of data: Ava Amiri, Sara Mahdiabadi
4. Drafting the article: Amirata Fakhfouri, Armin Razi, Hengameh Mojdeganlou, Paniz Mojdeganlou, Alireza Barzegary, Zahra Pashaei, Amir Masoud Afsahi, Parnian Shobeiri, Omid Dadras
5. Revising it critically for important intellectual content: SeyedAhmad SeyedAlinaghi, Omid Dadras, Esmaeil Mehraeen
6. Final approval of the version to be submitted: all authors
6.7. Acknowledgments
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, Iranian Research Center for HIV/AIDS, Tehran University of Medical Sciences, and Walailak University.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. [Available from: https://covid19.who.int.
- 2.Mehraeen E, Oliaei S, SeyedAlinaghi S, Karimi A, Mirzapour P, Afsahi AM, et al. COVID-19 in Pediatrics: A Systematic Review of Current Knowledge and Practice. Infect Disord Drug Targets. 2022;22(5):e290921196908. doi: 10.2174/1871526521666210929121705. [DOI] [PubMed] [Google Scholar]
- 3.Mehraeen E, SeyedAlinaghi S, Karimi A. Can children of the Sputnik V vaccine recipients become symptomatic? Human vaccines & immunotherapeutics. 2021;17(10):3500–1. doi: 10.1080/21645515.2021.1933689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehraeen E, Dadras O, Afsahi AM, Karimi A, Pour MM, Mirzapour P, et al. Vaccines for COVID-19: A Systematic Review of Feasibility and Effectiveness. Infect Disord Drug Targets. 2022;22(2):e230921196758. doi: 10.2174/1871526521666210923144837. [DOI] [PubMed] [Google Scholar]
- 5.Sheikhbahaei E, Mirghaderi SP, Moharrami A, Habibi D, Motififard M, Mortazavi SMJ. Incidence of Symptomatic COVID-19 in Unvaccinated Patients Within One Month After Elective Total Joint Arthroplasty: A Multicenter Study. Arthroplast Today. 2022;14:110–5. doi: 10.1016/j.artd.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet (London, England). 2021;398(10309):1407–16. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glück V, Grobecker S, Köstler J, Tydykov L, Bertok M, Weidlich T, et al. Immunity after COVID-19 and vaccination: follow-up study over 1 year among medical personnel. Infection. 2022;50(2):439–46. doi: 10.1007/s15010-021-01703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achiron A, Mandel M, Dreyer-Alster S, Harari G, Gurevich M. Humoral SARS-COV-2 IgG decay within 6 months in COVID-19 healthy vaccinees: The need for a booster vaccine dose? Eur J Intern Med. 2021;94:105–7. doi: 10.1016/j.ejim.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;385(19):1761–73. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA. Vaccines and Related Biological Products Advisory Committee Briefing Document - US Food and Drug Administration. 2021. [Available from: https://www.fda.gov/media/152161/download.
- 11.Fast HE, Zell E, Murthy BP, Murthy N, Meng L, Scharf LG, et al. Booster and Additional Primary Dose COVID-19 Vaccinations Among Adults Aged ≥65 Years - United States, August 13, 2021-November 19, 2021. MMWR Morbidity and mortality weekly report. 2021;70(50):1735–9. doi: 10.15585/mmwr.mm7050e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juno JA, Wheatley AK. Boosting immunity to COVID-19 vaccines. Nature medicine. 2021;27(11):1874–5. doi: 10.1038/s41591-021-01560-x. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595(7867):426–31. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiefer MK, Allen KD, Russo JR, Ma'ayeh M, Gee SE, Kniss D, et al. Decline in Sars-CoV-2 antibodies over 6-month follow-up in obstetrical healthcare workers. Am J Reprod Immunol. 2021;86(6):e13490. doi: 10.1111/aji.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris RJ, Whitaker HJ, Andrews NJ, Aiano F, Amin-Chowdhury Z, Flood J, et al. Serological surveillance of SARS-CoV-2: Six-month trends and antibody response in a cohort of public health workers. J Infect. 2021;82(5):162–9. doi: 10.1016/j.jinf.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tré-Hardy M, Cupaiolo R, Wilmet A, Antoine-Moussiaux T, Della Vecchia A, Horeanga A, et al. Immunogenicity of mRNA-1273 COVID vaccine after 6 months surveillance in health care workers; a third dose is necessary. J Infect. 2021;83(5):559–64. doi: 10.1016/j.jinf.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terpos E, Trougakos IP, Karalis V, Ntanasis-Stathopoulos I, Gumeni S, Apostolakou F, et al. Kinetics of Anti-SARS-CoV-2 Antibody Responses 3 Months Post Complete Vaccination with BNT162b2; A Prospective Study in 283 Health Workers. Cells. 2021;10(8):1942. doi: 10.3390/cells10081942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erice A, Varillas-Delgado D, Caballero C. Decline of antibody titres 3 months after two doses of BNT162b2 in non-immunocompromised adults. Clin Microbiol Infect. 2022;28(1):139.e1. doi: 10.1016/j.cmi.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favresse J, Bayart JL, Mullier F, Elsen M, Eucher C, Van Eeckhoudt S, et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerging microbes & infections. 2021;10(1):1495–8. doi: 10.1080/22221751.2021.1953403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo W, Duan K, Zhang Y, Yuan Z, Zhang YB, Wang Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: A randomized, double-blind, placebo-controlled, phase 1/2 trial. EClinicalMedicine. 2021;38:101010. doi: 10.1016/j.eclinm.2021.101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldhorn I, Holland R, Goshen-Lago T, Shirman Y, Szwarcwort-Cohen M, Reiner-Benaim A, et al. Six Month Efficacy and Toxicity Profile of BNT162b2 Vaccine in Cancer Patients with Solid Tumors. Cancer discovery. 2021 doi: 10.1158/2159-8290.CD-21-1072. [DOI] [PubMed] [Google Scholar]
- 23.Mohapatra RK, Tiwari R, Sarangi AK, Islam MR, Chakraborty C, Dhama K. Omicron (B 1 1 529) variant of SARS-CoV-2: Concerns, challenges, and recent updates. J Med Virol. 2022;94(6):2336–42. doi: 10.1002/jmv.27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedges JF, Thompson MA, Snyder DT, Robison A, Taylor MP, Jutila MA. Titers, Prevalence, and Duration of SARS-CoV-2 Antibodies in a Local COVID-19 Outbreak and Following Vaccination. Vaccines (Basel). 2021;9(6):587. doi: 10.3390/vaccines9060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakarya K, Kutumbetov L, Orynbayev M, Abduraimov Y, Sultankulova K, Kassenov M, et al. Safety and immunogenicity of a QazCovid-in® inactivated whole-virion vaccine against COVID-19 in healthy adults: A single-centre, randomised, single-blind, placebo-controlled phase 1 and an open-label phase 2 clinical trials with a 6 months follow-up in Kazakhstan. EClinicalMedicine. 2021;39:101078. doi: 10.1016/j.eclinm.2021.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pouquet M, Decarreaux D, Prévot-Monsacré P, Hervé C, Werner A, Grosgogeat B, et al. Nationwide Seroprevalence of SARS-CoV-2 IgG Antibodies among Four Groups of Primary Health-Care Workers and Their Household Contacts 6 Months after the Initiation of the COVID-19 Vaccination Campaign in France: SeroPRIM Study Protocol. Pathogens. 2021;10(7):911. doi: 10.3390/pathogens10070911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vicenti I, Basso M, Gatti F, Scaggiante R, Boccuto A, Zago D, et al. Faster decay of neutralizing antibodies in never infected than previously infected healthcare workers three months after the second BNT162b2 mRNA COVID-19 vaccine dose. Int J Infect Dis. 2021;112:40–4. doi: 10.1016/j.ijid.2021.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]