Abstract
Mesenchymal stromal cells (MSC) have excellent clinical potential and numerous properties that ease its clinical translation. Mitochondria play a crucial role in energy metabolism, essential for cellular activities, such as proliferation, differentiation, and migration. However, mitochondrial dysfunction can occur due to diseases and pathological conditions. Research on mitochondrial transfer from MSCs to recipient cells has gained prominence. Numerous studies have demonstrated that mitochondrial transfer led to increased adenosine triphosphate (ATP) production, recovered mitochondrial bioenergetics, and rescued injured cells from apoptosis. However, the complex mechanisms that lead to mitochondrial transfer from healthy MSCs to damaged cells remain under investigation, and the factors contributing to mitochondrial bioenergetics recovery in recipient cells remain largely ambiguous. Therefore, this review demonstrates an overview of recent findings in preclinical studies reporting MSC mitochondrial transfer, comprised of information on cell sources, recipient cells, dosage, route of administration, mechanism of transfer, pathological conditions, and therapeutic effects. Further to the above, this research discusses the potential challenges of this therapy in its clinical settings and suggestions to overcome its challenges.
Keywords: mesenchymal stromal cell, mitochondrial transfer, bioenergetics, preclinical model, tunneling nanotube
Graphical Abstract
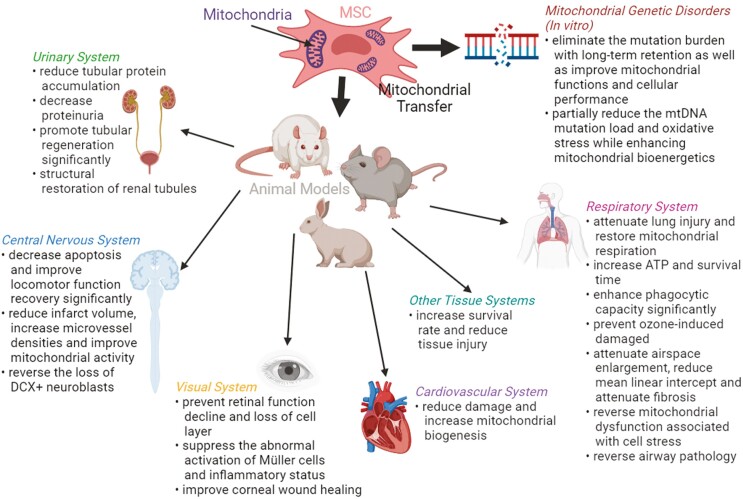
The therapeutic effects of MSC mitochondrial transfer in different tissue systems in preclinical studies as well as the in vitro studies.
Significance Statement.
This article summarizes the preclinical evidence of mesenchymal stromal cell (MSC) mitochondrial transfer in treating a variety of diseases comprehensively. Donor sources, dosage, and routes of administration, as well as the therapeutic effect (short/long term) of MSC/mitochondria transplantation, are discussed in this article. The preclinical evidence for such strategy is convincing, and no safety issues have been raised. Knowledge gaps and challenges toward clinical translation of such strategy have been identified and discussed in this review.
Introduction
Research in mesenchymal stromal cells (MSCs) has become popular in the past 30 years owing to their great clinical potential and ease of clinical translation.1 There are currently over 1000 registered MSC-based clinical trials listed in the ClininalTrials.gov database, and more than 70 000 publications related to MSCs have been released in PubMed. MSCs were first described in 1967 as bone marrow-derived adherent, fibroblast-like clonogenic cells called colony-forming unit-fibroblasts (CFU-F). These cells display strong replication capacity in vitro, wherein they can be differentiated into osteoblasts, chondrocytes, and adipocytes and support hematopoietic stroma when a single CFU-F is transplanted in vivo.2
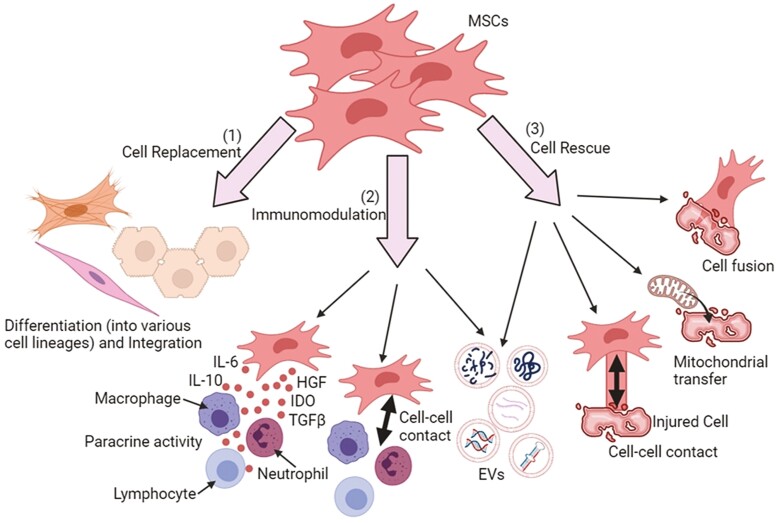
MSCs have numerous properties that help ease their clinical translation, some of which include their ability to be isolated from tissues, such as bone marrow, adipose tissue, and umbilical cord tissue (Wharton’s jelly) with ease, have a high capacity to expand ex vivo, multipotency to differentiate into various cell types, immunomodulatory properties, ability to be manipulated, or genetically modified, as well as possessing immune-evasive or immune-privileged status which allows the usage in allogeneic setting.3 Mechanisms that are fundamental to MSC-based therapy mainly attribute to 3 aspects: cell replacement, where damaged tissues are replaced by MSCs differentiating into various cell lineages and integrating into damaged parts; immunomodulation, where the MSC paracrine secretion regulates immune responses and lastly, cell rescue, where MSCs rescue the damaged tissues via diverse mechanisms, such as secretion of extracellular vesicles (EVs), direct cell–cell contact, cell fusion, as well as restoration of cell bioenergy through mitochondrial transfer.4Figure 1 illustrates a schematic diagram of the mechanisms mentioned above.
Figure 1.
Mechanisms underlying MSC-based therapy. (1) Cell replacement; MSCs can differentiate into various cell lineages and replace the damaged tissues. (2) Immunomodulation; MSCs can regulate immune responses through paracrine activity whereby secretome (ie, soluble factors and extracellular vesicles [EVs]) are released to exert immunomodulatory, pro-mitotic, pro-angiogenic, antiapoptotic, and antioxidative effects. (3) Cell rescue; MSCs can rescue or repair the damaged tissues through cell-cell contact, EV secretion, mitochondrial transfer, and cell fusion. All these biological activities lead to the transfer of cellular components to injured cells.
Abbreviations: IL-6, interleukin-6; IDO, indoleamine 2,3-dioxygenase; HGF, hepatocyte growth factor; TGFβ, transforming growth factor-β, ATP, adenosine triphosphate.
Recently, more evidence demonstrates that mitochondria transfer between cells can rescue and revitalize exhausted cells; this makes mitochondrial transfer a promising approach for treating mitochondrial diseases. Mitochondria are essential in energy metabolism and are crucial for numerous cellular activities, providing the energy that drives the physiological functions of cells via oxidative phosphorylation and production of adenosine triphosphate (ATP).5 Mitochondrial dysfunction is responsible for the pathogenesis of many chronic diseases, such as mitochondrial myopathies, Kearns-Sayre syndrome, Andersen-Tawil syndrome, as metabolic disorders are frequently accompanied by a disruption in mitochondrial function.6 Replacement of nonfunctional mitochondria with healthy mitochondria could potentially reverse mitochondrial dysfunction in certain mitochondrial diseases. The intercellular transfer of healthy mitochondria from MSCs to rescue mammalian cells with dysfunctional mitochondria was first demonstrated in vitro in 2006.7
It has been proposed that intercellular mitochondrial transfer also happens spontaneously under physiological conditions in maintaining tissue homeostasis and development.8 This proposal was based on several observations in vitro as follows. He et al9 showed that bidirectional mitochondrial transfer occurs between cardiomyocytes and cardiofibroblasts, providing structural and functional connectivity for myocardial tissue homeostasis. Moreover, cell-to-cell crosstalk between MSCs and renal tubular cells (RTCs) promotes the differentiation of MSCs toward functional RTCs, suggesting that MSCs could potentially replace the damaged kidney cells and maintain organ homeostasis during organ injury.10 On the other hand, co-cultured mouse cardiomyocytes with human multipotent adipose-derived stem cells (hADSCs) revealed that mitochondrial transfer from stem cells to cardiomyocytes is involved in cardiomyocyte reprogramming back to a progenitor-like state, suggesting that such somatic reprogramming could be essential for tissue homeostasis.11 Unfortunately, there are limited studies on this intercellular mitochondrial transfer under physiologic conditions, which could further clarify its significance in tissue homeostasis.8 This would build on the premise that MSCs from bone marrow or adipose tissue can home efficiently to the affected tissues for tissue maintenance. Interestingly, Sinclair et al12 demonstrated that resident MSCs derived from healthy lung tissues (LT-MSCs) and bronchoalveolar lavage fluid of lung transplant recipients (BAL-MSCs) could also donate cytoplasmic content and mitochondria spontaneously to healthy human bronchial epithelial cells with similar efficiency as bone marrow-derived MSCs via unidirectional transfer. Hence, the mechanism of intercellular mitochondrial transfer between stem cells and specialized cells in maintaining tissue homeostasis would require further studies.
Mitochondrial transfer rescued injured cells with non-functional mitochondria by restoring the mitochondrial function, as indicated by the improved oxidative phosphorylation and increased ATP production.13 Subsequent to a report on how bone marrow-derived MSCs transfer mitochondria can rescue injured lung alveolar epithelial cells in vivo in 2012,14 MSC-based mitochondrial transfer has then been explored as a possible treatment strategy in a variety of mitochondrial disorders, such as respiratory system injury, kidney injury, spinal cord injury, and ischemic stroke.15 In 2015, a protocol to transfer mitochondria artificially from a donor into recipient cells in vitro was published.16 The technique involves forced contact of isolated mitochondria from donor cells with recipient adherent cells via plate centrifugation at 4 °C. This technique was coined as mitoception. The technique has been used to assess the effect of transferring human MSC mitochondria to cancer cells16 and glioblastoma stem cells.17 This review provides an overview of recent findings in the preclinical studies reporting mitochondria transfer from MSCs to treat a myriad of diseases. Additionally, challenges in clinical translation of this relatively new therapeutic strategy and potential approaches for overcoming these issues are discussed.
MSC Sources
MSCs are readily available from various tissue sources, such as bone marrow, adipose tissue, and umbilical cord.18-20Table 1 summarizes the key findings in preclinical studies that reported mitochondrial transfer from MSCs to treat various disease studies (Table 1) applied bone marrow-MSCs (BM-MSCs) derived from humans, rats, and mice. Spees et al7 were the first to demonstrate mitochondrial transfer to A549 cells from human BM-MSCs in vitro. Although BM-MSCs are the most common sources, umbilical cord-derived MSCs (UC-MSCs) can be used as an alternative cell source with higher accessibility and fewer ethical restrictions.21 Other than that, Mahrouf-Yorgov et al22 used human multipotent adipose-derived stem cells (hADSCs) to increase the mitochondrial biogenesis of myocardium in a mouse model of myocardial infarction (MI) model. Paliwal et al23 showed that MSCs isolated from different tissue sources exhibit differential mitochondrial transfer and differential mitochondrial reactive oxygen species (mtROS) reduction in cells under oxidative stress. They found that dental pulp-MSCs (DP-MSCs) and Wharton’s jelly-MSCs (WJ-MSCs) demonstrated higher mitochondrial bioenergetics and more potent respiratory capacities with a lower mitochondrial transfer compared with BM-MSCs and adipose tissue-MSCs (AD-MSCs).
Table 1.
Preclinical studies reported MSC-mediated mitochondrial transfer as the mechanism of action to treat diseases.
| Reference | Donor cell | Target cell | Animal model (pathological condition) | Dosage | Route of administration | Mechanism of mitochondrial transfer | Therapeutic effect |
|---|---|---|---|---|---|---|---|
| 37 | Rat MSCs | Kidney (renal cells) Allogeneic |
Rat with doxorubicin-mediated nephrotoxicity | 8 × 106/mL mitochondria in 500 µL of respiration buffer | Applied directly below the cortex capsule (intraperitoneal administration) | Mitochondria were isolated before being injected into the kidney while the exact mechanism of mitochondrial uptake is yet unknown | - Reduce tubular protein accumulation and decrease proteinuria on day 6 - Decrease cellular oxidative stress and promote tubular regeneration - Increase antioxidant stress enzyme levels |
| 27 | Human UC-MSCs | Lymphoid cells (T cells) Xenogeneic |
Mouse with graft-versus-host disease (GVHD) | 12 × 106 mitocepted human PBMCs (MSC-mitochondria:PBMC ratios of 1:100, 1:25, and 1:10) | Injected through tail vein | No direct evidence (Mitochondrial transfer remains unaffected although inhibitors of TNT formation, gap junctions, hemichannels and macropinocytosis were applied, and no mitochondrial transfer was detected when incubated with conditioned media) | - Increase survival rate associated with a marked reduction in tissue injury (inflammation) on days 8 and 14 |
| 28 | Human BM-MSCs | Lung tissues Xenogeneic |
Mouse with LPS-induced lung injury (ARDS) | MSC-EVs (isolated from 5 × 105 or 1 × 106 MSCs suspended in 50 µl of PBS) | Injected through the tail vein | MSC-EVs | - Attenuate lung injury and restore lung tissue mitochondrial respiration after 24 hours |
| 41 | Human iPSC-MSCs | Retinal ganglion cells Xenogeneic |
Mouse with mitochondrial complex I (NADH: ubiquinone oxidoreductase) deficiency | 1 × 104 iPSC-MSCs in 0.5 μL PBS | Intravitreal injection | Not stated | - Prevent retinal function decline and loss of retinal ganglion cell layer at week 1 - Help to suppress the abnormal activation of Müller cells at week 4 and inflammatory status of the degenerating retina at week 1 |
| 29 | Rat BM-MSCs | Motor neurons of the spinal cord Allogeneic |
Rat with spinal cord injury (SCI) | 10 μL BM-MSCs (1 × 106) and 10 μL mitochondria extracted from 3 × 106 BM-MSCs | Injected into the epicenter of the injured spinal cord using an electrode microneedle | Gap Junctional Intercellular Communication (GJIC) | - Improve locomotor function recovery by week 2 - Decrease apoptosis in the ventral horn of the spinal cord - Reduce the area of lesion cavity, glial scar, and the number of GFAP-positive cells - There is no significant difference in terms of efficacy between BMSCs group and the mitochondria group |
| 43 | Rat BM-MSCs | Cerebrovascular system Allogeneic |
Rat with middle cerebral artery occlusion and reperfusion/injured cerebrovascular system (stroke) | 5 × 105 MSCs in 10 μL of PBS | Intra-arterial injection (common carotid artery) | TNT-like structure | - Significantly reduce infarct volume - Improve motor function (average daily running distance) on day 7 - Display significantly higher microvessel densities in the peri-infarct area (promotion of angiogenesis) - Rescue mitochondrial respiration (improve mitochondrial activity) of brain microvessel |
| 30 | Rat BM-MSCs | Renal proximal tubular epithelial cells Allogeneic |
Rat with streptozotocin-induced diabetic nephropathy | Mitochondria isolated from 1 × 106 MSCs and suspended in 100 µl of PBS | Injected under the renal capsule of the left kidney | Not stated | - Structural restoration of renal tubules by day 3 |
| 44 | Mouse MSCs | Neural stem cells (NSCs) Allogeneic |
Mouse with cisplatin-induced NSC damaged (chemotherapy) | 3 μl of MSC cell suspension (1 × 106 cells per mouse per day) | Administered twice in each nostril | TNT-like structure | - Rescue NSCs from cisplatin-induced cell death in vitro and in vivo - Reverse cisplatin-induced loss of DCX+ neuroblasts 1 month after completion of cisplatin treatment |
| 39 | Human iPSC-MSCs | Lung tissues Xenogeneic |
Mouse with ozone (oxidative stress)-induced mitochondrial dysfunction (COPD) | 1 × 106 iPSC-MSCs | Injected intravenously | TNT-like structure | - Prevent ozone-induced mitochondrial dysfunction, airway hyper-responsiveness (AHR) and inflammation in mouse lungs after 21 hours |
| 22 | Human multipotent adipose-derived stem cells | Myocardium Xenogeneic |
Mouse with MI | 20 μL HBSS solution containing ~4.0 × 105 naïve MSCs |
Injected into the myocardium surrounding the infarcted site (intracardiac delivery) | Not stated | - Reduce damage (protect from cell death), upregulate HO-1, and increase mitochondrial biogenesis after 24 hours |
| 38 | Human BM-MSCs | Alveolar macrophages Xenogeneic |
Mouse with Escherichia coli pneumonia (ARDS) | 1 × 106 MSCs | Injected intravenously through a tail vein in 100 µL of PBS or intranasally in 35 µL of PBS | TNT-like structure | - Enhance phagocytic capacity after 24 hours - Both routes showed similar efficacy |
| 42 | Human iPSC-MSCs | Corneal epithelial cells Xenogeneic |
Rabbit with corneal alkali burn | 1 × 105 MSCs seeded per scaffold | MSCs + matrix (acellular porcine cornea matrix) transplanted onto the corneal surface and sutured with 10-0 nylon | TNT-like structure | - Improve corneal wound healing after 48 hours |
| 24 | Human iPSC-MSCs and BM-MSCs | Airway epithelial cells Xenogeneic |
Rat with cigarette smoke-induced lung damage (COPD) | Two doses of 3 × 106 human BM-MSCs or iPSC-MSCs in PBS | Injected intravenously through the tail vein | TNT-like structure | - Successfully attenuate airspace enlargement (reduce alveolar wall destruction), reduce mean linear intercept, and attenuate fibrosis by day 56 |
| 40 | Mouse BM-MSCs | Bronchial epithelial cells Allogeneic |
Mouse with rotenone (Rot)-induced airway (bronchial epithelial cells) injury and allergic airway inflammation (AAI)(asthma) | 1 × 106 MSCs with fluorescence labeled mitochondria | Intratracheal (in 50 μL of media) administration for Rot-induced airway injury and intranasal (in 30 μL of media) administration for AAI | TNT-like structure | - Reduce caspase-3 and caspase-9 expression and lead to a consequent decrease in bronchial epithelial apoptosis and inflammation, reverse mitochondrial dysfunction and restore bioenergetics (increase in ATP levels in the lung), decrease cytochrome c in a cytosolic extract of the lung, and recover mitochondrial complex I and IV activities after 6 hours (airway injury model); - Attenuate AHR, decrease pro-inflammatory cytokines and restore ATP level, attenuate structural changes in the lungs by day 8 (AAI model) |
| 14 | Mouse BM-MSCs | Lung tissues (alveolar epithelium) Allogeneic |
Mouse with LPS-induced acute lung injury (ALI) | 2 × 105 BM-MSCs in 40 µl PBS | Intranasal instillation | Alveolus-attached BM-MSCs form Cx43-expressing nanotubes and microvesicles in a Ca2+-dependent manner | - Increase alveolar ATP within 5-8 hours - Reduce leukocytosis and albumin leakage in the bronchoalveolar lavage (BAL) - Increase survival time |
Abbreviations: MSC, mesenchymal stem cell; iPSC, induced pluripotent stem cell; UC, umbilical cord; PBMC, peripheral blood mononuclear cell; TNT, tunneling nanotube; BM, bone marrow; LPS, lipopolysaccharide; ARDS, acute respiratory distress syndrome; PBS, phosphate-buffered saline; GFAP, glial fibrillary acidic protein; DCX, doublecortin; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; HBSS, Hank’s balanced salt solution; HO-1, Heme oxygenase-1; ATP, adenosine triphosphate; Cx43, connexin 43; Ca2+, calcium ion.
Controlled differentiation of induced pluripotent stem cells (iPSCs) into MSCs gives rise to a new source of MSCs. The advantages of using iPSC-MSCs compared with adult MSCs are that they have higher proliferation capacity (unlimited supply) and allow for reprogramming of aged adult cells from geriatric patients to “younger” stem cells for autologous therapy.24 Li et al showed that treatment with iPSC-MSCs produces a higher retention rate and better mitochondrial transfer than BM-MSCs in the rescue of cigarette smoke-induced mitochondrial damage.
Autologous MSC therapy has some drawbacks, such as difficulty obtaining sufficient MSCs from some patients and having decreased or altered biological activity when isolated from aged patients and patients with systemic diseases. These shortcomings will result in unsatisfactory treatment outcomes.25 Hence, allogeneic MSC (allo-MSC) therapy has gained traction, free from these shortcomings. Allo-MSCs can be off-the-shelf and readily available to patients, making them ideal for acute diseases such as stroke and myocardial infarction (MI). Allo-MSCs also display immunosuppressive properties and low immunogenicity; thus, they are not rejected after implantation.25 Allo-MSCs and xenogeneic MSCs (xeno-MSCs) have been used in several studies and generated promising therapeutic outcomes without triggering host immune responses as they are immune privileged. Lin et al26 showed that the same amount of xenogeneic iPSC-MSCs was not inferior to allogeneic AD-MSCs in protecting against acute lung ischemia-reperfusion injury in the rat. These results indicated that the recipient’s setting would not influence the therapeutic outcome of MSC therapy, as both allo- and xeno-MSCs did not trigger the host immune response after implantation and displayed similar efficacy.
Dosage and Route of Administration
In the studies, the dose of MSCs, isolated mitochondria, and MSC-EVs applied ranges between 1 × 104 and 3 × 106, 1 × 106 and 8 × 106, and 5 × 105 to 1 × 108, respectively. These doses varied based on the different disease models, administration routes, and MSC sources. A dose-dependent curve of mitochondrial transfer was observed when co-culturing donor MSCs at an increasing MSC:PBMC (peripheral blood mononuclear cell) ratio (ie, 1:100, 1:25, and 1:10).27
In terms of therapeutic efficacy dose comparison between these factors,28 found no significant difference using EVs generated from 5 × 105 or 1 × 106 BM-MSCs in the treatment of lipopolysaccharide (LPS)-induced lung injury (decreasing the bronchoalveolar lavage fluid (BALF) total protein), restoring mitochondrial respiration and ATP production. Li et al29 reported that 1 × 106 BM-MSCs were comparable to mitochondria extracted from 3 × 106 BM-MSCs in the drastic reduction of injured neurons of spinal cord injury (SCI) from apoptosis. In another study, the number of apoptotic Streptozotocin (STZ) induced-renal proximal tubular epithelial cells (PTECs) reduced significantly with the addition of 1 × 104 MSCs in vitro and was comparable to the effect with the addition of mitochondria isolated from 1 × 106 MSCs.30 In a recent study, injection of 1 × 108 MSC-EVs at 3 sites around infarct regions in mouse MI models increased tissue ATP levels, improved left ventricular ejection fraction and reduced left vertical remodeling.31 Treatment with isolated mitochondria that contained the same amount of mitochondrial proteins (1.0 µg/mL) showed no such effects. Furthermore, they demonstrated that mitochondria inside EVs were more resistant to calcium ions overload and oxidative stress than the isolated mitochondria.
Numerous routes of administration were applied in these studies, such as intraperitoneal, intravenous, intravitreal, intra-arterial, intranasal, intracardiac, intratracheal, and more. Several studies involved direct injection of MSCs or MSC-derived mitochondria into the injured part (local delivery), and others involved transfer via blood vessels or veins (systemic delivery). Local delivery has been widely utilized in animal models, especially small animals, as it is easy to operate in laboratories.32 The critical advantage of this strategy is that it retains high cell concentration in the targeted site without significant washout, and permits large cells, such as MSCs, to be injected into the desired part. However, there is a risk of perforation around the injection site since it is invasive.33 While systemic delivery is an attractive, non-invasive strategy that permits repeated injection of large numbers of cells,34 Wolf et al35 demonstrated that intravenous delivery of MSCs in the pig model restored myocardial function after myocardial infarction. However, cells ranging from 20 to 50 µm remained within the systemic vasculature, suggesting a higher risk of vascular occlusion from systemic delivery.36
In a model of doxorubicin (DOX)-mediated nephrotoxicity in rats, Kubat et al37 administered isolated mitochondria intraperitoneally below the cortex capsule of each kidney, and similarly, Konari et al30 administered isolated mitochondria under the renal capsule of the left kidney of rats with Streptozotocin (STZ)-induced diabetic nephropathy. On the other hand, for diseases related to the respiratory system, the routes of administration are generally intravenous, intranasal, and intratracheal. For example, in an acute respiratory distress syndrome (ARDS) model, MSC-EVs were injected intravenously through the tail vein of mice.28 While in another study, MSCs were instilled intranasally in mice to promote the regeneration of lipopolysaccharide (LPS)-induced injured lung tissues.14 For Escherichia coli pneumonia MSCs were injected intravenously and intranasally in mice to transfer their mitochondria to alveolar macrophages, where both the routes of administration did not affect the efficacy.38 Moreover, in the mouse chronic obstructive pulmonary disease (COPD) model, MSCs were injected intravenously and transferred their mitochondria to rescue the lung tissues (airway smooth muscle cells) with ozone-induced mitochondrial dysfunction39 or cigarette smoke-induced damage in airway epithelial cells of rats.24 Ahmad et al40 administered MSCs intratracheally to bronchial epithelial cells of mice in Rotenone (Rot)-induced airway injury model and intranasally to bronchial epithelial cells in ovalbumin-induced allergic airway inflammation (AAI) model.
In a mouse model of graft-versus-host disease (GVHD), Court et al27 injected mitocepted human PBMCs intravenously through the tail vein and the transferred mitochondria increase the mRNA transcripts expression involved in T-cell activation and differentiation in PBMCs. The mitocepted PBMCs reduced the degree of tissue inflammation and tissue injury in the main target organs of GVHD mouse model as compared to the non-treated PBMCs. In a model of mitochondrial complex I deficient retina (Ndufs4 knockout mice model), Jiang et al41 injected MSCs into the vitreous cavity of one eye (intravitreal) and demonstrated that the mitochondria from MSCs were detected in the retina starting from 96 h post-injection. Examples of other disease models where MSC mitochondrial transfer was shown or assumed to occur and found to be beneficial are: Jiang et al42 transplanted MSCs+matrix (scaffold) onto the burned area of the corneal surface in a rabbits corneal alkali burn model; Li et al29 injected MSCs and mitochondria separately into the rats’ epicenter of spinal cord in a spinal cord injury (SCI) model; Liu et al43 injected MSCs into the common carotid artery (intra-arterial) of rats and transfer to the cerebrovascular system in an ischemic stroke model while, Boukelmoune et al44 administered MSCs twice in each nostril (intranasal) of mouse and transfer to neural stem cells (NSCs) in a cisplatin-induced NSC damaged model; Mahrouf-Yorgov et al22 injected human multipotent adipose-derived stem cells (hADSC) into the infracted site of the myocardium (intracardiac) in a mouse myocardial infarction (MI) model. In these studies, cells or mitochondria are delivered to the injured sites via (i) direct injection, (ii) injection to the adjacent tissues, or (iii) the circulatory system to promote tissue regeneration.
Mechanism of Mitochondrial Transfer
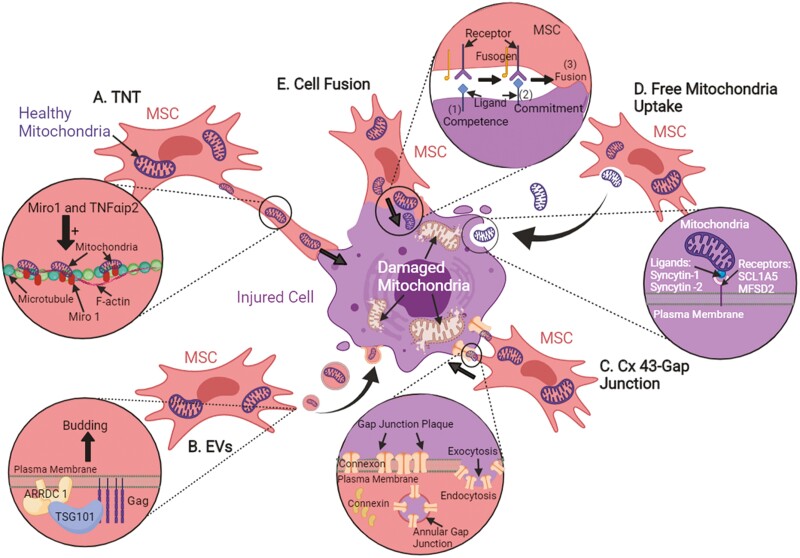
Intercellular mitochondrial transfer can occur via tunneling nanotubes (TNTs), extracellular vesicles (EVs), gap junctions, uptake of isolated mitochondria, and cell fusion. Fig. 2 illustrates a schematic diagram of the abovementioned transfer mechanisms.
Figure 2.
The possible intercellular mitochondrial transfer mechanisms from MSCs to rescue injured cells. (A) The structural components of TNTs formed between MSCs and injured cells contain F-actin and microtubules. (B) Mitochondria can be enveloped in vesicles and transferred to injured cells together with other cytosolic contents. (C) Direct cell-to-cell contact can transfer mitochondria through the formation of gap junction, where gap junctions are transmembrane complexes of connexin proteins. (D) Free mitochondria alone can be internalized by recipient cells without carrier, yet, the exact uptake mechanism remains unknown. (E) Cell fusion is another transfer mechanism, where cytosolic content and organelles such as mitochondria can be shared or exchanged between cells.
Tunneling Nanotubes (TNTs)
TNTs are spontaneous membranous tubes that interconnect cells and are discovered to be heterogeneous in morphology and composition, varying between and within cell systems.45 Two (2) types of TNTs were observed in WJ-MSC cultures with varying thickness and composition. Type I TNTs are thinner tubes with no organelles inside, whereas type II TNTs are thicker tubes with polyribosomes, cisterns of rough endoplasmic reticulum, vesicles, and mitochondria inside.46 Another study showed that the structural components of TNTs formed between BM-MSCs and human umbilical vein endothelial cells (HUVECs) contained F-actin and microtubules.47 Nuclear factor-kappa light chain enhancer of activated B cells (NF-κB) signaling pathway was reported to be involved predominantly in TNT development for MSC-mediated mitochondrial transfer, where inhibition of this NF-κB pathway reduces the formation of TNT, suggesting that Rotenone/NF-κB/TNF alpha-induced protein-2 (Rot/NF-κB/TNFαip2) signaling pathway is mainly involved in TNT development. The transcription factor NF-κB will be activated and phosphorylated when the corneal epithelial cells are treated with Rot. Activation of NF-κB increased the expression of TNFαip2 protein, which triggered F-actin polymerization that subsequently upregulates TNT formation.42 Moreover, Ahmad et al40 showed that mitochondrial movement from MSCs to recipient cells is regulated by Miro1, a calcium-sensitive mitochondrial Rho-GTPase. The overexpression of Miro1 enhanced mitochondrial transfer and therapeutic efficacy, while the knockdown of Miro1 inhibited mitochondrial transfer and led to the loss of therapeutic efficacy.
Extracellular Vesicles
Extracellular vesicles (EVs) are spheroidal structures surrounded by a phospholipid bilayer membrane that envelopes a broad array of protein, nucleic acid, chemical, and structural contents derived from the cell of origin.48-50 They can be classified as exosomes (30-100 nm), shedding vesicles (100 nm to 1 µm), or apoptotic bodies (>1 µm) according to their origins.51 One of the mechanisms of microvesicle (MV) biogenesis found in mammalian cells is the recruitment of TSG101 protein to the cell surface by arrestin-domain containing protein 1 (ARRDC 1), where the formation and release of ARRDC 1-mediated microvesicles (ARMMs) at the plasma membrane is mediated by the recruitment of tumor susceptibility gene 101 (TSG101) protein.28 Silva et al52 showed that MSC-EVs contain mitochondria, which can easily be internalized by recipient cells and intercalated into the endogenous mitochondrial network to restore mitochondrial biogenesis and attenuate mitochondrial dysfunction. In addition, Islam et al14 reported that mitochondrial transfer occurs through TNTs and connexin 43 (Cx43)-expressing microvesicles in a Ca2+-dependent manner. The released microvesicles moved away from the cell at 1.8 ± 0.5 µm min–1.
Gap Junctions (Direct Cell-to-Cell Contact)
Most studies reported that the mitochondrial transfer is mediated through TNTs formation rather than gap junctions. This may be because TNTs formation can easily be observed in in vitro co-culture systems.53 Gap junctions are transmembrane complexes of connexin proteins that allow intercellular communication where ions and small signaling molecules can be transferred between neighboring cells54,55) revealed that 6 connexin proteins assembled into an oligomer called connexon, and these connexons are transported to and inserted into the plasma membrane. Gap junction plaque can be formed during cell–cell contact where a hemichannel can dock head-on with another hemichannel from an opposing cell and cluster to form plaque. A portion of the entire plaque is internalized and forms an annular gap junction. This annular gap junction may then be degraded via several processes, and the content can be released. Islam et al14 emphasized the positive effect of gap junction protein, Cx43 on mitochondrial transfer by stabilizing the attachment of MSCs to alveolar epithelial cells and promoting the formation of TNTs and MVs. Li et al29 demonstrated that mitochondrial transfer from MSCs to the injured motor neurons occurred via gap junction. In addition, the Western blot assay revealed that heterotypic gap junction by Cx43 and Cx32 might form between MSCs and neurons, where Cx43 was expressed in MSCs but not in motor neurons; meanwhile, Cx32 was expressed in motor neurons but not in MSCs.
Uptake of Isolated Mitochondria (Direct Free Mitochondria Uptake or Endocytosis)
The exact mechanism of isolated mitochondria uptake is unknown, but it was thought that mitochondria could be internalized by recipient cells via macropinocytosis.56 Macropinocytosis is an endocytic process where extracellular contents are engulfed in vesicles known as macropinosomes.57 Díaz-Carballo et al58 demonstrated that free mitochondria uptake relies on the integrity of the outer mitochondrial membrane and the fusion proteins, eg, syncytin-1 and syncytin-2 on it, which may function as ligands in the interaction between free mitochondria and recipient cell. Kubat et al37 injected isolated mitochondria from MSCs into the renal cortex of rats in a DOX-mediated nephrotoxicity model, and tubular regeneration was noted after mitochondria transplantation. Furthermore, Li et al29 injected both MSCs and isolated mitochondria into the injured spinal cord, and immunofluorescence staining showed that mitochondria were localized inside the injured parenchyma and the body of neurons. In a diabetic nephropathy model in rats, Konari et al30 administered isolated mitochondria into the renal capsule of the kidney, and a massive cluster of mitochondria was detected under the renal capsule 1, 3 days after the administration of mitochondria.
Cell Fusion
Another form of intercellular communication is through cell fusion, where the plasma membrane of 2 independent cells combines while nuclear morphology is retained. Cytosolic content and organelles are shared between these 2 cells, especially if permanent fusion occurs. On the other hand, partial fusion involves a direct but temporal exchange of subcellular organelles between cells, such as mitochondria and protein complexes.59 There are 3 steps involved in cell–cell fusion , namely competence, commitment, and fusion. Fusion competence allows cells to differentiate into fusion-competent cells through one or more of these complex processes: triggered and activated by extracellular signals, execution of developmental programs, cell polarization, migration, morphological changes, polarized secretion, and surface display of critical molecules required for the next step. Next, commitment involves cell–cell interactions, induction, and activation of the fusion machinery. Finally, the cells are fused by merging the plasma membranes, the main barriers that define the cells. Cytoplasms are mixed, leading to further signaling and developmental changes.60 Cell fusion is used, yet, rarely for MSC-driven mitochondria sharing, and the underlying mechanisms to remain elusive.11 Acquistapace et al61 showed that MSCs could reprogram adult mouse cardiomyocytes to a rejuvenated progenitor-like state via partial cell fusion and mitochondrial transfer.
Pathological Conditions and Therapeutic Effects
Urinary System
In a doxorubicin (DOX)-mediated nephrotoxicity model, Kubat et al37 monitored the rats until the 9th day and sacrificed them after 10 days of DOX administration. Protein accumulation of tubular cells was reduced (P < .05), and proteinuria level started to decrease on the 6th day (P < .001) after infusion of mitochondria into the renal cortex. Mitochondrial administration also decreased cellular oxidative stress (P < .001) and promoted tubular regeneration (P = .009) after renal injury. Meanwhile, in an STZ-induced diabetic nephropathy model, Konari et al30 monitored the rats for 3 days, and renal tissues were obtained 1 and 3 days after mitochondrial injection. Collagen IV expression in the tubular basement membrane and expression of megalin, as the protein transporter, in the brush border was restored similar to that of the intact and normal proximal tubular epithelial cells (PTECs) due to the structural restoration of renal tubules.
Respiratory System
In an LPS-induced lung injury model of acute respiratory distress syndrome (ARDS), Silva et al28 sacrificed the mice 24 h after LPS instillation, where bronchoalveolar lavage fluid (BALF) and lungs were collected. MSC-EVs administration attenuates lung injury, where either dose of MSC-EVs (isolated from 5 × 105 or 1 × 106 MSCs) had decreased the LPS-stimulated increase in BALF total protein, total and differential cell counts, as well as restored lung tissue mitochondrial respiration and ATP production. Correspondingly, the lungs were recovered after 24 h (LPS instillation) in an LPS-induced acute lung injury (ALI) model, wherein the survival of mice had increased with MSCs instillation.14 Furthermore, in an E. coli pneumonia model of ARDS, Jackson et al38 euthanized the mice 24 or 48 h after infection when BALF or lungs were collected. Mitochondrial transfer from MSCs to alveolar macrophages enhances phagocytic capacity (P = .003) and is involved in the antimicrobial effect of MSCs.
Furthermore, in an ozone-induced mitochondrial dysfunction model of chronic obstructive pulmonary disease (COPD), Li et al39 collected lungs 21 h after ozone exposure for analysis. MSCs were able to prevent but not reverse ozone-induced lung damage. MSCs injected 24 h before ozone exposure showed a significant decrease in lung damage. In contrast, MSCs injected 6 h after ozone exposure showed no difference compared with ozone exposure in the saline-injected group. In another cigarette smoke (CS)-induced lung damage model of COPD, the rats were exposed to 4% CS for 56 days, and they were sacrificed on day 5724). The transplantation of iPSC-MSCs can repair CS-induced lung damage causing alveolar wall destruction, mean linear intercept (Lm) reduction, where mean linear intercept represents the lung’s structural changes, and the attenuation of fibrosis after MSCs instillation (P < .05).
Moreover, Ahmad et al40 studied both Rot-induced airway injury and ovalbumin-induced allergic airway inflammation (AAI) models, where the mice have euthanized 6 and 24 h post-MSC treatment in the Rot-induced airway injury model. Mice were challenged for 7 days, while MSCs were delivered on day 3, and mice were dissected on day 5 for mitochondrial uptake visualization or on day 8 to measure a few parameters in the ovalbumin-induced AAI model. The mitochondrial donation was able to reverse mitochondrial dysfunction associated with cell stress in the airway injury model and reverse airway pathology in the AAI model.
Visual System
In a Ndufs4 knockout model where the mouse was subjected to mitochondrial complex I (NADH: ubiquinone oxidoreductase) deficiency and eventually retinal ganglion cell (RGC) loss and death, Jiang et al41 monitored the mice for 4 weeks, and they were sacrificed at 96 h, 1 week, and 4 weeks after MSC injection. MSCs injection successfully preserved retinal function, delayed the loss of RGC, and helped suppress the abnormal activation of Müller cells and inflammatory response of degenerating retina. While in a corneal alkali burn model, Jiang et al42 euthanized the rabbits 48 h after MSC transplantation. MSC transplantation achieved the best wound healing compared with the Rot-MSC (P < .05) or matrix-only group (P = .06); this suggests that only healthy MSCs can improve corneal wound healing.
Central Nervous System
In an SCI model, Li et al29 observed the rats for 6 weeks, and they were sacrificed 6 weeks after treatment. Mitochondrial transplantation significantly decreased apoptosis (P < .01) in the early stage of SCI, improved locomotor function recovery (P < .01), and reduced the area of lesion cavity, glial scar, and the number of glial fibrillary acidic protein (GFAP)-positive cells in the late stage of SCI. While in an ischemic stroke model, Liu et al43 observed the rats for 28 days for behavioral tests and to be sacrificed on days 5, 7, and 15 after MSC transplantation. MSCs injection significantly reduced infarct volume, improved motor functions, displayed higher microvessel densities, and improved basic oxygen consumption and maximum mitochondrial oxidative capacity. In another cisplatin-induced NSC damaged model, Boukelmoune et al44 euthanized the mice 1 month after completion of cisplatin treatment where mice were injected with cisplatin for 2 cycles of 5 days, with 5 days of rest in between. MSCs were administered 48 and 96 h after the last cisplatin dose. MSCs rescued NSCs from cisplatin-induced cell death and reversed the loss of doublecortin (DCX) + neuroblasts.
Cardiovascular System
Mahrouf-Yorgov et al22 accessed the mouse ischemic heart area 24 h after MSCs injection in an MI model. MSCs protected cardiac tissue from cell death and increased expression of heme oxygenase-1 (HO-1) and peroxisome proliferator-activated receptor-gamma coactivator 1-α (PGC1-α) and mitochondrial biogenesis. In a simulated ischemia/reperfusion (SI/R) injury model, Han et al62 co-cultured H9c2 cells with BM-MSCs in vitro, and this co-culture decreased apoptosis of H9c2 cells and increased mitochondrial membrane potential (∆ψm).
Other Tissue Systems
In a graft-versus-host disease (GVHD) model, Court et al27 monitored the mouse until the 23rd day as the onset of GVHD progression occurs typically 7 days after PBMC injection. Euthanasia was performed on days 8-9 for immunological analysis and on day 14 for histological analysis, or when total body weight loss was >20% of the original weight. The MitoCepted PBMC-induced group significantly improved mice survival rate, with a median survival of 19 days (P < .05) and reduced tissue injury of the spleen, small intestine, liver, and lung (main organs affected in GVHD). In a cytarabine (Ara-C)-treated chemotherapy stress model in vitro, co-culture of BM-MSCs and HUVECs significantly alleviate apoptosis (P < .01) and promote proliferation (P < .05), as well as significantly increase migration capacity (P < .01) and improve capillary angiogenesis (P < .01).47 In a model where cells were pretreated with ethidium bromide to mutate the mitochondrial DNA (mtDNA), direct co-culture with BM-MSCs produced clones of rescued cells with functional mitochondria.7
Mitochondrial Genetic Disorders
Mitochondrial diseases are usually hereditary disorders where mitochondria fail to produce enough energy for the body to function correctly. Mitochondrial dysfunction can happen in childhood or adulthood due to mutations in either mitochondrial DNA or nuclear mitochondrial genes.63 In a mitochondrial myopathy, encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) model in vitro, by co-culturing WJ-MSCs and MELAS fibroblasts, the mutation burden was eliminated with long-term retention (persisted in continuous culture for up to 28 days) as well as improved mitochondrial functions and cellular performance including respiratory complexes translation, ROS overexpression, cell proliferation, and apoptotic resistance.21 Moreover, Chuang et al64 demonstrated that in a myoclonus epilepsy associated with ragged-red fibers (MERRF) model in vitro, WJ-MSCs were transferred mitochondria with normal mtDNA into the MERRF cybrid. This partially reduced the mtDNA mutation load and oxidative stress while enhancing mitochondrial bioenergetics. These in vitro studies provide evidence of long-term retention of transferred mitochondria from MSCs. However, there is currently no in vivo animal study using MSC mitochondrial transfer to treat mitochondrial genetic disorders. The potential of treating inheritable mitochondrial myopathy remains an exciting exploration that awaits further in vivo proof of concept.
Potential Challenges and Suggestions
Potential Challenges in Translating MSC Mitochondrial Transfer Therapy in Clinical Settings
Despite the widespread research and discussion on MSC therapy over the past decade, some critical questions remain to be answered. Some of these questions point toward optimizing and standardizing MSC source, dose, and route of administration in mitochondrial transfer therapy, presenting undiscovered challenges for medical intervention to treat mitochondrial dysfunction diseases. Very few studies have investigated the mitochondrial donation capacity variance and compared the effect of different MSC sources, doses, and cell delivery techniques on the therapeutic outcomes. In addition, the question of how allogeneic and xenogeneic MSCs or isolated mitochondria transplantation can activate host immune response and cause treatment failure in the long term remains unknown and unexplored to date. Further to the above, while some mechanisms of mitochondrial transfer in the rescue of damaged cells have been established, signals that trigger the activation of this mechanism remain unclear, apart from whether or not the mechanism activated is reliant on donor or recipient cells or the microenvironment. The signaling pathway involved in the mechanism of action and the regulation of these mechanisms also requires further exploration and answering.
Moreover, the current knowledge gap in this field still exists and needs to be closed through further and more in-depth research. For example, what are the long-term effects of mitochondria transferred from MSCs? What are the fates of these mitochondria transferred in injured tissues within weeks or even months?29 Can mitochondria donated from MSCs with lower mitochondrial bioenergetics fulfill the higher bioenergetic needs of recipient cells, such as cardiomyocytes, that contracted tirelessly? Should the MSCs be differentiated before delivery to obtain better therapeutic outcomes? The effects of other mechanisms such as the release of paracrine factors from MSCs or the delivery of other cytosolic contents such as ATP or microRNA via TNTs or EVs cannot be excluded and require further investigations.24 Further understanding of these gaps can facilitate translating MSC mitochondrial transfer therapy into a clinical setting.
Next, the efficacy and safety profile of MSC mitochondrial transfer therapy remain doubtful as there is insufficient preclinical and clinical data on these. How to ensure the quality of mitochondria from the MSCs or isolated from MSCs? How to ensure that sufficient mitochondria can be transferred to the targeted tissues when administered intravenously? What effect does the donor mitochondrial DNA exert on its host long-term? Caicedo et al16 showed that the transfer of MSC mitochondria to cancer cells results in enhanced oxidative phosphorylation activity of cancer cells and favored cancer cell proliferation and invasion. This result indicates that there is a possibility that the MSC mitochondria administered can be uptaken by the cancer cells and enhance the bioenergetics of cancer cells. Volarevic et al65 also showed that MSCs have the potential to be differentiated into undesired tissues, such as bone and cartilage while suppressing the anti-tumor immune response and generating new blood vessels. Other than that, co-culture of iPSC-MSCs with airway smooth muscle cells without cigarette smoke medium treatment can increase cell apoptosis, suggesting that iPSC-MSCs may induce stress in cells at baseline.39
Although the usage of iPSC-MSCs is becoming popular in the field of cell therapy, the undesired differentiation and malignant transformation potentials of iPSC-MSCs are a major safety and ethical issue as there are risks of tumor growth and metastasis in humans.65 Furthermore, ethical concerns regarding the premature clinical translation of MSC therapy on humans in certain applications are still debated. It is interesting to note that while specific Good Manufacturing Practice (GMP) guidelines for cell-based therapeutic products have been released and updated by the European Commission66 and US FDA.67 Yet, the validation strategy is challenging due to the unclear mechanism of action and the lack of reference standards, as well as the effectiveness of the potency assay being affected by many variables, such as the donor diversity and cellular population heterogeneity of the potency assay.68 Cell-derived products such as isolated mitochondria, secretomes, and extracellular vesicles which are currently broadly categorized as biologics may require specific guidelines in their manufacturing and use. Additional concerns such as, “Can immortalized or genetically-enhanced MSCs produce equally safe and efficacious mitochondrial transfer?”69 and “How to improve the isolation techniques to obtain active and intact mitochondria for therapeutic purposes?”29 will need to be answered before clinical translation. It may also be worthy to explore the enhancement of mitochondria bioenergetics and increase the mitochondrial load in EV cargos to maximize the benefit of their transfer to host cells.
Suggestions on Methods to Improve MSC Mitochondrial Therapy
More preclinical and clinical studies must be carried out to better understand the detailed mechanisms of mitochondrial transfer and obtain the optimized and standardized cell preparation for the best therapeutic outcomes. Besides, priming MSCs with growth factors or cytokines can be considered in the MSCs mitochondrial transfer therapy. Our study previously has shown that such priming can increase the mitochondrial quantity and density, leading to higher ATP production (unpublished data). Strategies to increase the release of EVs, incidence of cell fusion, and mitochondrial transfer via gap junction may be explored in the future. In addition, MSC mitochondria may be isolated and purified and then encapsulated in biomaterials to achieve controlled release delivery.70 Methods to isolate large EVs (500-700 nm) such as centrifuging the MSC conditioned media at a low speed (around 100 RCF)28 or filtering at specific ranges (500 < × < 700) nm can also be used. According to Appleby,71 2 main points that must be fulfilled before ethical clinical application of mitochondrial replacement technique (MRT) (assumed to be applied as well to MSC mitochondrial transfer therapy) are: first, MRTs must undergo a comprehensive schedule of preclinical safety assessment before it is approved for human use. Second, any clinical use of MRTs needs to be accompanied by sufficient safeguards to reduce the health risks to future persons as much as practicable.
Conclusion
Mitochondrial transfer of MSC therapy has shown to be a promising therapeutic strategy in mitochondrial dysfunction disease models due to the effectiveness of MSCs or isolated mitochondria transplantation in tissue repair. Nevertheless, further investigations remain crucial to identify the knowledge gaps, troubleshoot the challenges for clinical applications, and resolve ethical concerns regarding this therapy. Despite the numerous issues that require solving, this review provides a better understanding of the current preclinical evidence of MSC mitochondrial transfer for cell rescue, thereby paving the way for clinical translation of MSC therapy.
Acknowledgments
We thank BioRender for providing us with the templates to create the figures.
Contributor Information
Yu Ling Tan, Center for Tissue Engineering and Regenerative Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia.
Sue Ping Eng, NK Biocell Sdn. Bhd., Kuala Lumpur, Malaysia.
Pezhman Hafez, Yakin Splendour Global Holdings Berhad, Kuala Lumpur, Malaysia.
Norwahidah Abdul Karim, Department of Biochemistry, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia.
Jia Xian Law, Center for Tissue Engineering and Regenerative Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia.
Min Hwei Ng, Center for Tissue Engineering and Regenerative Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia.
Funding
This study is supported by grants GUP-2019-085 and FF-2020-189 provided by the Universiti Kebangsaan Malaysia (UKM) and the Faculty of Medicine, UKM.
Conflict of Interest
NK Biocell Sdn. Bhd. and Yakin Splendour Holdings Berhad did not fund the study and do not have any commercial interest in this project. The authors affiliated with these companies were previously academic researchers in the Universiti Kebangsaan Malaysia.
Author Contributions
Y.L.T.: collection and/or assembly of data, data analysis and interpretation, manuscript writing. S.P.E.: conception and design, manuscript writing. P.H.: manuscript writing. N.A.K., J.X.L.: final approval of manuscript. M.H.N.: conception and design, final approval of manuscript
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Pittenger MF, Discher DE, Péault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regener Med. 2019;4(1):22. 10.1038/s41536-019-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP.. Heterotopic transplants of bone marrow. Transplantation. 1968;6(2):230-247. [PubMed] [Google Scholar]
- 3. Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN.. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;7(9):651-663. 10.1002/sctm.18-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan XL, Zhang Y, Li X, Fu QL.. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77(14):2771-2794. 10.1007/s00018-020-03454-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinrich TW, Kam JH, Ferrara BT, et al. A day in the life of mitochondria reveals shifting workloads. Sci Rep. 2019;9(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johannsen DL, Ravussin E.. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009;9(6):780-786. 10.1016/j.coph.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spees JL, Olson SD, Whitney MJ, Prockop DJ.. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103(5):1283-1288. 10.1073/pnas.0510511103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu D, Gao Y, Liu J, et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transd Targeted Ther. 2021;6(1):65. 10.1038/s41392-020-00440-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He K, Shi X, Zhang X, et al. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc Res. 2011;92(1):39-47. 10.1093/cvr/cvr189 [DOI] [PubMed] [Google Scholar]
- 10. Plotnikov EY, Khryapenkova TG, Galkina SI, Sukhikh GT, Zorov DB.. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res. 2010;316(15):2447-2455. 10.1016/j.yexcr.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 11. Acquistapace A, Bru T, Lesault PF, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29(5):812-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sinclair KA, Yerkovich ST, Hopkins PMA, Chambers DC.. Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res Ther. 2016;7(1):91. 10.1186/s13287-016-0354-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rustom A. The missing link: does tunnelling nanotube-based supercellularity provide a new understanding of chronic and lifestyle diseases? Open Biol. 2016;6(6):160057. 10.1098/rsob.160057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759-765. 10.1038/nm.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomzikova MO, James V, Rizvanov AA.. Mitochondria donation by mesenchymal stem cells: current understanding and mitochondria transplantation strategies. Front Cell Dev Biol. 2021;9(April):653322. 10.3389/fcell.2021.653322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caicedo A, Fritz V, Brondello JM, et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep. 2015;5:9073. 10.1038/srep09073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mombo BN, Gerbal-Chaloin S, Bokus A, et al. MitoCeption: transferring isolated human MSC mitochondria to glioblastoma stem cells. J Visualized Exp. 2017; 120:55245. 10.3791/55245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hafez P, Chowdhury SR, Jose S, et al. Development of an in vitro cardiac ischemic model using primary human cardiomyocytes. Cardiovas Eng Technol. 2018;9(3):529-538. 10.1007/s13239-018-0368-8 [DOI] [PubMed] [Google Scholar]
- 19. Lim J, Razi ZRM, Law JX, et al. Mesenchymal stromal cells from the maternal segment of human umbilical cord is ideal for bone regeneration in allogenic setting. Tissue Eng Regener Med. 2018;15(1):75-87. 10.1007/s13770-017-0086-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rashidbenam Z, Jasman MH, Tan GH, et al. Fabrication of adipose-derived stem cell-based self-assembled scaffold under hypoxia and mechanical stimulation for urethral tissue engineering. Int J Mol Sci. 2021;22(7):3350. 10.3390/ijms22073350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin TK, der Chen S, Chuang YC, et al. 2019. Mitochondrial transfer of Wharton’s jelly mesenchymal stem cells eliminates mutation burden and rescues mitochondrial bioenergetics in rotenone-stressed MELAS fibroblasts. Oxid. Med.Cell. Longevity. 2019;2019:9537504. 10.1155/2019/9537504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahrouf-Yorgov M, Augeul L, da Silva CC, et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24(7):1224-1238. 10.1038/cdd.2017.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paliwal S, Chaudhuri R, Agrawal A, Mohanty S.. Human tissue-specific MSCs demonstrate differential mitochondria transfer abilities that may determine their regenerative abilities. Stem Cell Res Ther. 2018;9(1):298. 10.1186/s13287-018-1012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Zhang Y, Yeung SC, et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51(3):455-465. 10.1165/rcmb.2013-0529OC [DOI] [PubMed] [Google Scholar]
- 25. Zhang J, Huang X, Wang H, et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. 2015;6:234. 10.1186/s13287-015-0240-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin KC, Yeh JN, Chen YL, et al. Xenogeneic and allogeneic mesenchymal stem cells effectively protect the lung against ischemia-reperfusion injury through downregulating the inflammatory, oxidative stress, and autophagic signaling pathways in rat. Cell Transplant. 2020;29:963689720954140. 10.1177/0963689720954140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Court AC, Le-Gatt A, Luz-Crawford P, et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21(2):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silva JD, Su Y, Calfee CS, et al. MSC extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. 2021;58(1):2002978. 10.1183/13993003.02978-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H, Wang C, He T, et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019;9(7): 2017-2–035.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Konari N, Nagaishi K, Kikuchi S, Fujimiya M.. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci Rep. 2019;9(1):5184. 10.1038/s41598-019-40163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ikeda G, Santoso MR, Tada Y, et al. Mitochondria-rich extracellular vesicles from autologous stem cell–derived cardiomyocytes restore energetics of ischemic myocardium. J Am Coll Cardiol. 2021;77(8):1073-1088. 10.1016/j.jacc.2020.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Z, Mikrani R, Zubair HM, et al. Systemic and local delivery of mesenchymal stem cells for heart renovation: challenges and innovations. Eur J Pharmacol. 2020;876(June 2019):173049. 10.1016/j.ejphar.2020.173049 [DOI] [PubMed] [Google Scholar]
- 33. Charwat S, Gyöngyösi M, Lang I, et al. Role of adult bone marrow stem cells in the repair of ischemic myocardium: current state of the art. Exp Hematol. 2008;36(6):672-680. 10.1016/j.exphem.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 34. Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863-868. 10.1161/01.CIR.0000084828.50310.6A [DOI] [PubMed] [Google Scholar]
- 35. Wolf D, Reinhard A, Seckinger A, et al. Dose-dependent effects of intravenous allogeneic mesenchymal stem cells in the infarcted porcine heart. Stem Cells Dev. 2009;18(2):321-329. 10.1089/scd.2008.0019 [DOI] [PubMed] [Google Scholar]
- 36. Walczak P, Zhang J, Gilad AA, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39(5):1569-1574. 10.1161/STROKEAHA.107.502047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kubat GB, Ozler M, Ulger O, et al. The effects of mesenchymal stem cell mitochondrial transplantation on doxorubicin-mediated nephrotoxicity in rats. J Biochem Mol Toxicol. 2021;35(1):e22612. 10.1002/jbt.22612 [DOI] [PubMed] [Google Scholar]
- 38. Jackson M, Morrison TJ, Doherty DF, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34(8):2210-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X, Michaeloudes C, Zhang Y, et al. Mesenchymal stem cells alleviate oxidative stress–induced mitochondrial dysfunction in the airways. J Allergy Clin Immunol. 2018;141(5): 1634-1645.e5. [DOI] [PubMed] [Google Scholar]
- 40. Ahmad T, Mukherjee S, Pattnaik B, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang D, Xiong G, Feng H, et al. Donation of mitochondria by iPSC-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect-induced degeneration. Theranostics. 2019;9(8):2395-2410. 10.7150/thno.29422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang D, Gao F, Zhang Y, et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016;7(11):e2467. 10.1038/cddis.2016.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu K, Guo L, Zhou Z, Pan M, Yan C.. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc Res. 2019;123(January):74-80. 10.1016/j.mvr.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 44. Boukelmoune N, Chiu GS, Kavelaars A, Heijnen CJ.. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol Commun. 2018;6(1):139. 10.1186/s40478-018-0644-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Austefjord MW, Gerdes HH, Wang X.. Tunneling nanotubes: diversity in morphology and structure. Commun Integr Biol. 2014;7(1):e27934. 10.4161/cib.27934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sanchez V, Villalba N, Fiore L, et al. Characterization of tunneling nanotubes in Wharton’s jelly mesenchymal stem cells. an intercellular exchange of components between neighboring cells. Stem Cell Rev Rep. 2017;13(4):491-498. 10.1007/s12015-017-9730-8 [DOI] [PubMed] [Google Scholar]
- 47. Feng Y, Zhu R, Shen J, et al. Human bone marrow mesenchymal stem cells rescue endothelial cells experiencing chemotherapy stress by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev. 2019;28(10):674-682. 10.1089/scd.2018.0248 [DOI] [PubMed] [Google Scholar]
- 48. Foo JB, Looi QH, Chong PP, et al. Comparing the therapeutic potential of stem cells and their secretory products in regenerative medicine. Stem Cells Int. 2021;2021:1-30. 10.1155/2021/2616807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liau LL, Al-Masawa ME, Koh B, et al. 2020. The potential of mesenchymal stromal cell as therapy in neonatal diseases. Front Pediat. 2020;8:591693. 10.3389/fped.2020.591693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pitt JM, Kroemer G, Zitvogel L.. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Investig. 2016;126(4):1139-1143. 10.1172/jci87316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mittelbrunn M, Sánchez-Madrid F.. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13(5):328-335. 10.1038/nrm3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q.. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci USA. 2012;109(11):4146-4151. 10.1073/pnas.1200448109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Paliwal S, Chaudhuri R, Agrawal A, Mohanty S.. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci. 2018;25(1):31. 10.1186/s12929-018-0429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Falk MM, Bell CL, Kells Andrews RM, Murray SA.. Molecular mechanisms regulating formation, trafficking and processing of annular gap junctions. BMC Cell Biol. 2016;17(Suppl 1):22. 10.1186/s12860-016-0087-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kotini M, Barriga EH, Leslie J, et al. Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat Commun. 2018;9(1):3846. 10.1038/s41467-018-06368-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kitani T, Kami D, Matoba S, Gojo S.. Internalization of isolated functional mitochondria: Involvement of macropinocytosis. J Cell Mol Med. 2014;18(8):1694-1703. 10.1111/jcmm.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rao AD, McArthur GA.. Chapter 11 - Targeting Metabolic Vulnerabilities in RAS-Mutant Cells. In: Azmi AS, ed. Conquering RAS. Academic Press; 2017:193–212. 10.1016/B978-0-12-803505-4.00011-4 [DOI] [Google Scholar]
- 58. Díaz-Carballo D, Klein J, Acikelli AH, et al. Cytotoxic stress induces transfer of mitochondria-associated human endogenous retroviral RNA and proteins between cancer cells. Oncotarget. 2017;8(56):95945-95964. 10.18632/oncotarget.21606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Murray LMA, Krasnodembskaya AD.. Concise review: intercellular communication via organelle transfer in the biology and therapeutic applications of stem cells. Stem Cells. 2019;37(1):14-25. 10.1002/stem.2922 [DOI] [PubMed] [Google Scholar]
- 60. Aguilar PS, Baylies MK, Fleissner A, et al. Genetic basis of cell–cell fusion mechanisms. Trends Genet. 2013;29(7):427-437. 10.1016/j.tig.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mohammadalipour A, Dumbali SP, Wenzel PL.. Mitochondrial transfer and regulators of mesenchymal stromal cell function and therapeutic efficacy. Front Cell Dev Biol. 2020;8:603292. 10.3389/fcell.2020.603292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Han H, Hu J, Yan Q, et al. Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol Med Rep. 2016;13(2):1517-1524. 10.3892/mmr.2015.4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alston CL, Rocha MC, Lax NZ, Turnbull DM, Taylor RW.. The genetics and pathology of mitochondrial disease. J Pathol. 2017;241(2):236-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chuang YC, Liou CW, der Chen S, et al. Mitochondrial transfer from Wharton’s jelly mesenchymal stem cell to MERRF cybrid reduces oxidative stress and improves mitochondrial bioenergetics. Oxid Med Cell Longevity. 2017;2017:5691215. 10.1155/2017/5691215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Volarevic V, Markovic BS, Gazdic M, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36-45. 10.7150/ijms.21666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. European Commission. 2017. Guidelines on good manufacturing practice specific to advanced therapy medicinal products. https://www.gmp-compliance.org/files/guidemgr/2017_11_22_guidelines_gmp_for_atmps.pdf
- 67. US FDA. 2020. Regulatory considerations for human cells, tissues, and cellular and tissue-based products: minimal manipulation and homologous use guidance for industry and food and drug administration staff contains nonbinding recommendations regulatory considerations. Guidance for Industry and Food and Drug Administration Staff. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal. [Google Scholar]
- 68. Viganò M, Budelli S, Lavazza C, et al. Tips and tricks for validation of quality control analytical methods in good manufacturing practice mesenchymal stromal cell production. Stem Cells Int. 2018;2018: 3038565. 10.1155/2018/3038565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Han D, Zheng X, Wang X, Jin T, Cui L, Chen, Z.. 2020. Mesenchymal stem/stromal cell-mediated mitochondrial transfer and the therapeutic potential in treatment of neurological diseases. Stem Cells Int. 2020;2020:8838046. 10.1155/2020/8838046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Westensee IN, Brodszkij E, Qian X, et al. Mitochondria encapsulation in hydrogel-based artificial cells as ATP producing subunits. Small. 2021;17(24):e2007959. 10.1002/smll.202007959 [DOI] [PubMed] [Google Scholar]
- 71. Appleby JB. The ethical challenges of the clinical introduction of mitochondrial replacement techniques. Med Health Care Philos. 2015;18(4):501-514. 10.1007/s11019-015-9656-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.