Abstract
Human pluripotent stem cells (hPSCs) are currently evaluated for clinical applications due to their proliferation and differentiation capacities, raising the need to both assess and enhance, the safety of hPSC-based treatments. Distinct molecular features contribute to the tumorigenicity of hPSCs, manifested in the formation of teratoma tumors upon transplantation in vivo. Prolonged in vitro culturing of hPSCs can enhance selection for specific genetic aberrations, either at the chromosome or gene level. Some of these aberrations are tightly linked to human tumor pathology and increase the tumorigenic aggressiveness of the abnormal cells. In this perspective, we describe major tumor-associated risk factors entailed in hPSC-based therapy, and present precautionary and safety measures relevant for the development and application of such therapies.
Keywords: human pluripotent stem cells, teratoma, teratocarcinoma, cancer-related mutations, aneuploidy, tumorigenicity
Graphical Abstract
Significance Statement.
This perspective describes major tumor-associated risk factors entailed in human pluripotent stem cell-based therapy and presents precautionary and safety measures relevant for the development and application of such therapies.
Introduction
Human pluripotent stem cells (hPSCs) hold a pivotal role in cell therapy and regenerative medicine, due to their capacity to differentiate into all cell types of the human body and their ability to self-renew in an undifferentiated state.1 While the features of both pluripotency and self-renewal represent the major clinical potential of hPSCs, they also hide within them contributing factors to a third feature of hPSCs, in vivo tumorigenicity.2
hPSCs can be isolated from human embryos, establishing human embryonic stem cells (hESCs),3 or reprogrammed from somatic cells, generating human induced pluripotent stem cells (hiPSCs).4 When these cells differentiate in culture, they can generate a large array of immature and mature cell types. However, when undifferentiated hPSCs are transplanted in vivo into mice, they differentiate while forming teratoma tumors.2,5 Teratomas are benign tumors, characterized by a heterogeneous composition of haphazardly differentiated cells, representing all 3 embryonic germ layers. In humans, naturally occurring teratomas belong to the class I of human germ-cell tumors, and their etiology is attributed to the misplacement or dysregulation of germ cells and their derivatives.6 Teratocarcinomas are immature tumors, defined by the presence of undifferentiated embryonal carcinoma-like cells in addition to teratoma-like structures, and are considered malignant compared to mature benign teratomas.7
The readiness of hPSCs to form differentiated teratomas upon their transplantation in vivo, permits unique opportunities to study early developmental processes,8 as well as features of tumorigenicity. Their tumorigenesis is also one of the major hurdles for the various clinical applications of hPSCs. This perspective summarizes the current knowledge about the factors mediating the tumorigenic potential of hPSCs, and highlights risks and precautions that should be considered during the propagation and differentiation of these cells, before being considered for transplantation to patients.
hPSCs-Derived Teratomas
Normal hPSCs share common features with cancer tumors and cancer-derived cell lines, such as similarities in their gene expression profile,9,10 cell cycle progression,11 and telomerase activity.12 Key pluripotency genes, such as NANOG, POU5F1, and LIN28, were shown to have an active role in various cancers,13-15 and many epigenetic features such as DNA-methylation and histone marks are common to hPSCs and cancer cells.16,17 Unlike cancer initiating cells, the capacity of hPSCs to form teratomas does not depend on the acquisition of genetic aberrations. Unlike cancer tumors, teratomas do not grow clonally, exemplified by the facts that at least 20% of the injected cells contribute to the differentiated tumor,8 and that different tumor-initiating cells can contribute to the same tissue or structure within the tumor.18
When compared to spontaneous in vitro differentiation of hPSCs into embryoid bodies, during in vivo differentiation into teratoma the cells present enhanced proliferation.21 Teratomas are vascularized by the host’s endothelium in an HIF1α-dependent manner, although HIF1α-null hPSCs generate massive teratomas despite their reduced vascularization.19 Transcriptional differences between embryoid bodies and teratomas highlight several onco-fetal genes, that are highly expressed in hPSCs, repressed during in vitro differentiation, but are retained when the cells differentiate in vivo.20 The oncofetal gene BIRC5, also known as SURVIVIN, is an anti-apoptotic gene whose inhibition was shown to specifically eliminate hPSCs in vitro, and BIRC5 inhibition induces apoptosis in hPSC-derived teratomas.20
Chromatin modifiers are also implicated in the regulation of teratoma formation. Expression of the histone de-acetylases HDAC1 and HDAC2, was shown to correlate with the maturity of human patient teratomas,21 and the chemical inhibition of the histone de-methylase LSD1 in vivo was shown to prevent teratoma formation from hPSCs.22 Interestingly, HDAC1/2 and LSD1, are both involved in regulation of the pluripotent state,23 and form a protein complex together with the gene ZMYM2. Genetic ablation of ZMYM2 was shown to hinder exit from pluripotency and prevent the formation of hPSCs-derived teratoma, while ZMYM2-null hPSCs over-expressed pluripotency genes and maintained a high proliferative rate in vitro.24
The mechanism of teratoma formation remains to be accurately defined. Teratoma formation naturally depends on the survival of hPSCs and their differentiated derivatives, and it is mediated by the pluripotency gene network, while the extent of tumorigenesis and the maturity of the tumors can be manipulated genetically and pharmacologically.
Culture Adaptation and its Implications on the Genomic Stability and Tumorigenicity of hPSCs
The mere propagation of hPSCs in vitro was shown to introduce selective advantage to various genetic and epigenetic aberrations. Such aberrant hPSCs that readily take over the culture, are commonly referred to as “culture adapted” cells.25 The first observations of acquired genomic instability in hPSCs over time, were of large scale chromosomal aberrations, manifested as recurrent full or partial gains, mostly in chromosomes 1, 12, 17, 20, and X.26-30 Smaller copy-number variations (CNVs) were also shown to expand during hPSC culture,31 with interesting observations regarding de novo acquisition and selection of CNVs during the reprogramming of somatic cells into iPSCs.32 The variety of small and large aneuploidies described in hPSCs are not randomly distributed across the genome, and frequently affected loci highlight potential causative genes.33 Some of the most common gains are of the chromosome arm 12p, harboring the pluripotency factor NANOG, and chromosome arms 17q and 20p, harboring the anti-apoptotic genes, BIRC520 and BCL2L1,27,34 respectively.
Next generation sequencing technologies permit a more refined view on the occurrence of point-mutations in hPSCs. Pathogenic single nucleotide variants were first described via whole-exome sequencing (WES) analysis of 22 iPSC lines, revealing an average mutation load of 5 non-synonymous mutations per cell line. About half of these mutations could be detected in low frequencies in the original fibroblasts.35 Moreover, a recent study showed that the reprogramming process positively selects for clones harboring somatic mutations in cancer-associated genes.36 In a comprehensive study that examined the genomic sequence of 140 early-passage hPSC lines, including lines designated for clinical application, they identified recurring dominant-negative mutations in the tumor suppressor gene TP53.37 The mutations found are among the most common mutations in human cancers, and this study was able to show, using RNA-sequencing (RNA-seq) data, the expansion of such mutations with time, not only in cultures of undifferentiated hPSCs but also throughout in vitro differentiation.37 The frequency of cancer-related mutation acquisition was further demonstrated by comparison of early passage WES of 2 of the most commonly used hPSC lines, to published RNA-seq data of the same lines in later passages. In this study, more than 30% of the samples were shown to acquire mainly TP53 mutations, but also less frequent cancer-related mutations in other genes, eg, EGFR and CDK12.38
The dynamics of genomic aberration acquisition is difficult to characterize due to the large number of variables affecting this process (eg, genetic background, culture techniques, and passaging frequency), yet published data can help yield rough estimations. Karyotypic abnormalities were reported to be identified in cells ranging from passage 19 to passage 209,25 and the proportion of a karyotypically abnormal clone was shown to completely take over a normal culture in 5 passages from the time of its initial identification at a low frequency in the population.39
While the most mutated gene in hPSCs seems to be the most pivotal gene in cancer prevention, the most common aneuploidies acquired during the culturing of hPSCs, closely mirror aneuploidies found in a variety of human germ cell tumors.33,40 Indeed, culture adapted cells were shown to form aggressive teratomas and teratocarcinoma-like tumors.41-44 These findings demonstrate the close relationship between culture adaptation and in vivo tumorigenicity, while establishing the relevance of the teratoma assay to assess not only pluripotency, but also tumorigenicity of hPSCs.
Assessing Genetic Integrity and Tumorigenic Potential of hPSCs
Chromosomal aberrations can be detected by various methodologies, among them are G-banding karyotyping, quantitative-PCR, fluorescence in situ hybridization and comparative genome hybridization.45 High-throughput sequencing methods can be also useful for karyotyping, even at relatively low genomic coverage. DNA based assays as whole-genome sequencing (WGS) and WES, although originally designed for sequence variation analysis, can be applied to detect aneuploidies even at very low genomic representation. RNA-seq, commonly used for gene-expression analysis, is also used to detect genomic duplications and deletions, this via algorithms that identify alterations in expression or deviation from conventional allelic ratios.46 However, most current methodologies are limited in their sensitivity to detect low levels of karyotypic mosaicism in hPSC cultures.29,45
The detection of pathogenic point-mutations requires high-resolution DNA sequencing. Sanger sequencing, although limited due to its locus specific experimental design, can be applied to validate with high confidence selected risk loci. Naturally, high-throughput sequencing methods offer the ability for genome-wide analysis of single nucleotide variation, limited only by our ability to interpret variants, and annotate them as benign or pathogenic. WGS offers genome-wide coverage of both coding and non-coding DNA sequences but is somewhat demanding due to the high read coverage required. WES is commonly used, enabling affordable high-fidelity detection of variation in the coding genome, but unable to detect pathogenic variants in non-coding regions as promoters and enhancers. RNA-seq of hPSCs is commonly used research and development because it can be used to detect point-mutations with high confidence,47 while being uniquely sensitive to sequences that are adequately transcribed in the hPSC sample. It should be noted that high-throughput sequencing methods can be subjected to contamination from feeder cells cocultured with hPSCs. Since some murine sequences can resemble human pathogenic variants, they could be misleading, by causing overestimation of the mutational load in the tested hPSC population.48
The genetic makeup of hPSCs can help predict their tumorigenic potential, but should be complemented with methodologies that enable the direct quantification of hPSCs’ tumorigenicity.49 Teratoma assays are currently limited in their ability to quantify the tumorigenicity of individual cell lines, as the field lacks benchmarking of different parameters in the teratoma assay, such as the number of cells injected, the injection site, and host mouse strain used. Nevertheless, teratoma assays can highlight the risk of hPSCs for a malignant transformation, as such a transformation can be efficiently detected by histological analysis of excised tumors, or by algorithms that are capable of quantifying the maturity of teratomas using tumor expression data.49-51
Maximizing the Safety of hPSCs-Based Treatments
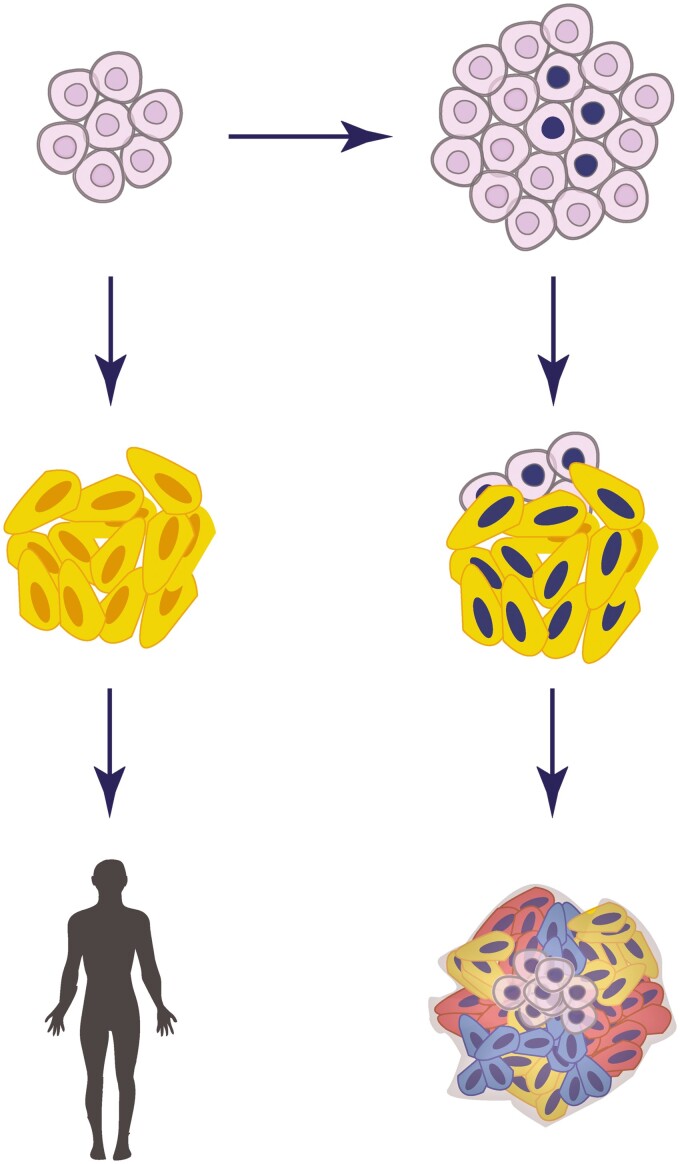
The main risk in cell therapy is that transplanted cells will form tumors in patients with time. Teratoma and even teratocarcinomas or somatic tumors are potential unfortunate outcomes of hPSCs-based treatments (Fig. 1). In the clinical context, hPSCs are ultimately differentiated into specific target cell types. Differentiation efficiency might contribute to the persistence of pluripotent cells throughout differentiation procedures.52 Hence, making sure that the inoculum is not contaminated with residual undifferentiated cells is essential to eliminate the risk of teratoma formation. Several strategies regarding the elimination of hPSCs from differentiated cultures has been previously proposed.53 Immunological targeting of pluripotency surface markers (eg, CLDN6) can permit flow-cytometric removal of pluripotent cells, or their specific ablation using cytotoxic antibodies.54 Alternatively, some small molecules (eg, PluriSIns) can specifically abolish pluripotent cells, without affecting differentiated cells in culture.53,55,56 Moreover, transgenics-based methods are available, eg, the integration of a “suicide gene” into the cell’s genome (eg, the herpes simplex’s thymidine kinase gene, that induces cell death upon ganciclovir treatment57). Such a gene can be conditionally expressed under a pluripotency-specific promoter and can thus specifically eliminate pluripotent cells in vitro or in vivo upon induction. In addition, a transcriptional link between the suicide gene and a cell-division gene can improve the system by protecting the suicide system from inactivation in dividing cells.58 The process of transgene introduction into cells entails additional passaging and selection and overall increases the risk of unwanted perturbations, making pharmacological and immunological approaches potentially preferable.
Figure 1.
Schematic representation of the potential consequences of hPSCs-based therapy.
The top panel represents the expansion of culture-adapted cells during in vitro propagation of hPSCs. The left and right panels represent potential consequences of cell therapy using normal and aberrant hPSCs, respectively. The middle panels describe the potential consequence of incomplete differentiation in the cell inoculum designated for treatment.
The major cause of cancer is the acquisition of mutations that occur during cell proliferation and are subjected to positive selection in vivo. As similar selective pressures are present during hPSCs culturing and human tumor pathology, it is highly probable that mutations rising in culture will contribute to malignant transformation of the transplanted cells in vivo. Aberrant clones are initially present at very low percentages within hPSCs populations, making early detection challenging, especially since high-resolution genome-scale inspections are desired. The variety of tumorigenicity risk factors and suggested methodologies that can help eliminate these risks are summarized in Table 1.
Table 1.
Potential tumorigenicity-associated risk factors in hPSCs-based therapy and applicable methodologies for their detection or prevention.
| Potential risk factor for tumor formation | Applicable detection or prevention methods |
|---|---|
| Large to medium chromosomal aberrations | G-banding, spectral karyotype (SKY), Array-CGH, e-Karyotype, WES. |
| Small copy-number variation | SNP-array, quantitative-PCR, FISH, eSNP-karyotype, WES |
| Pathogenic point mutations | Sanger sequencing, RNA-seq, WES, WGS |
| Cell line specific tumorigenic or malignant propensity | Teratoma assay |
| Residual hPSCs in the transplant | Chemical ablation, immunological targeting, genetic elimination |
Concluding Remarks
Safe hPSC-based treatments rely heavily on good practices in hPSCs maintenance and quality control, from the earliest days of cell line derivation. Maintenance of hPSCs should minimally include routine screening for common aberrant variants, eg, chromosomal aberrations, CNVs, and integrity of cancer-related genes (mainly TP53). Additionally, hPSCs culturing procedures (eg, media composition, passaging, and banking) should be optimized to reduce selective bottlenecks and minimize the number of cell divisions in culture. The expansion of hPSCs at industrial scales is especially challenging, but with the advancement of knowledge and technology, the efficiency and safety of large scale propagations are constantly increasing.59 It is noteworthy that genomic instability in culture is not restricted to hPSCs and occurs also during the propagation of adult stem cells.40,60,61 Thus, extensive propagation of hPSC-derived progenitors should require similar quality control measures.
Currently, hPSCs-based clinical trials asses the safety of the treatment through cell transplantation into relevant model animals and the absence of a pathogenic outcome when compared to untreated controls.1 While standardization of the teratoma assay could help benchmark the spectrum of tumorigenic propensities in hPSC lines, the injection of treatment-designated cells into immune deficient mice currently provides the best assessment of in vivo tumorigenicity. Advanced pre-treatment quality control methodologies, combined with pharmacological and biotechnological approaches seem to be the key for the elimination of the tumorigenic potential in hPSC-based treatments.
Acknowledgments
We thank members of the Azrieli Center for Stem Cells and Genetic Research for critical reading of the manuscript. This work was partially supported by the Rosetrees Trust and by the Azrieli Foundation. N.B. is the Herbert Cohn Chair in Cancer Research.
Contributor Information
Elyad Lezmi, The Azrieli Center for Stem Cells and Genetic Research, Department of Genetics, Silberman Institute of Life Sciences, The Hebrew University of Jerusalem, Edmond J. Safra Campus, Givat Ram, Jerusalem, Israel.
Nissim Benvenisty, The Azrieli Center for Stem Cells and Genetic Research, Department of Genetics, Silberman Institute of Life Sciences, The Hebrew University of Jerusalem, Edmond J. Safra Campus, Givat Ram, Jerusalem, Israel.
Conflict of Interest
The authors declared no potential conflicts of interest.
Author Contributions
E.L.: conception and design, manuscript writing, and final approval of manuscript; N.B.: conception and design, manuscript writing, and final approval of manuscript, financial support.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Trounson A, DeWitt ND.. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 2016;17(3):194-200. 10.1038/nrm.2016.10 [DOI] [PubMed] [Google Scholar]
- 2. Ben-David U, Benvenisty N.. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11(4):268-277. 10.1038/nrc3034 [DOI] [PubMed] [Google Scholar]
- 3. Thomson JA, Itskovitz-eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145-1147. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- 4. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 5. Blum B, Benvenisty N.. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100(08):133-158. 10.1016/S0065-230X(08)00005-5 [DOI] [PubMed] [Google Scholar]
- 6. Cunningham JJ, Ulbright TM, Pera MF, Looijenga LHJ.. Lessons from human teratomas to guide development of safe stem cell therapies. Nat Biotechnol. 2012;30(9):849-857. 10.1038/nbt.2329 [DOI] [PubMed] [Google Scholar]
- 7. Damjanov I, Andrews PW.. The terminology of teratocarcinomas and teratomas. Nat Biotechnol. 2007;25(11):12122007-12121212. 10.1038/nbt1107-1212a [DOI] [PubMed] [Google Scholar]
- 8. McDonald D, Wu Y, Dailamy A, et al. Defining the teratoma as a model for multi-lineage human development. Cell. 2020;183(5):1402-1419.e18. 10.1016/j.cell.2020.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sperger JM, Chen X, Draper JS, et al. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci USA. 2003;100(23):13350-13355. 10.1073/pnas.2235735100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40(5):499-507. 10.1038/ng.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227(2):271-278. 10.1006/dbio.2000.9912 [DOI] [PubMed] [Google Scholar]
- 12. Hiyama E, Hiyama K.. Telomere and telomerase in stem cells. Br J Cancer. 2007;96(7):1020-1024. 10.1038/sj.bjc.6603671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeter CR, Liu B, Liu X, et al. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011;30(36):3833-3845. 10.1038/onc.2011.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xin YH, Bian BSJ, Yang XJ, et al. POU5F1 enhances the invasiveness of cancer stem-like cells in lung adenocarcinoma by upregulation of MMP-2 expression. PLoS One. 2013;8(12):2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balzeau J, Menezes MR, Cao S, Hagan JP.. The LIN28/let-7 pathway in cancer. Front Genet. 2017;8(31):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laugesen A, Helin K.. Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell. 2014;14(6):735-751. 10.1016/j.stem.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 17. Shukla V, Vaissière T, Herceg Z.. Histone acetylation and chromatin signature in stem cell identity and cancer. Mutat Res. 2008;637(1-2):1-15. 10.1016/j.mrfmmm.2007.07.012 [DOI] [PubMed] [Google Scholar]
- 18. Blum B, Benvenisty N.. Clonal analysis of human embryonic stem cell differentiation into teratomas. Stem Cells. 2007;25(8):1924-1930. 10.1634/stemcells.2007-0073 [DOI] [PubMed] [Google Scholar]
- 19. Yu JL, Rak JW, Carmeliet P, et al. Heterogeneous vascular dependence of tumor cell populations. Am J Pathol. 2001;158(4):1325-1334. 10.1016/S0002-9440(10)64083-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N.. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol. 2009;27(3):281-287. 10.1038/nbt.1527 [DOI] [PubMed] [Google Scholar]
- 21. Lagger S, Meunier D, Mikula M, et al. Crucial function of histone deacetylase 1 for differentiation of teratomas in mice and humans. EMBO J. 2010;29(23):3992-4007. 10.1038/emboj.2010.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osada N, Kikuchi J, Umehara T, et al. Lysine-specific demethylase 1 inhibitors prevent teratoma development from human induced pluripotent stem cells. Oncotarget. 2018;9(5):6450-6462. 10.18632/oncotarget.24030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin F, Lan R, Zhang X, et al. LSD1 regulates pluripotency of embryonic stem/carcinoma cells through histone deacetylase 1-mediated deacetylation of histone H4 at lysine 16. Mol Cell Biol. 2014;34(2):158-179. 10.1128/MCB.00631-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lezmi E, Weissbein U, Golan-Lev T, et al. The chromatin regulator ZMYM2 restricts human pluripotent stem cell growth and is essential for teratoma formation. Stem Cell Rep. 2020;15(6):1275-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker DEC, Harrison NJ, Maltby E, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25(2):207-215. 10.1038/nbt1285 [DOI] [PubMed] [Google Scholar]
- 26. Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22(1):53-54. 10.1038/nbt922 [DOI] [PubMed] [Google Scholar]
- 27. Amps K, Andrews PW, Anyfantis G, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29(12):1132-1144. 10.1038/nbt.2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayshar Y, Ben-David U, Lavon N, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7(4):521-531. 10.1016/j.stem.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 29. Baker D, Hirst AJ, Gokhale PJ, et al. Detecting genetic mosaicism in cultures of human pluripotent stem cells. Stem Cell Rep. 2016;7(5):998-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Na J, Baker D, Zhang J, Andrews PW, Barbaric I.. Aneuploidy in pluripotent stem cells and implications for cancerous transformation. Prot Cell. 2014;5(8):569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laurent LC, Ulitsky I, Slavin I, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8(1):106-118. 10.1016/j.stem.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hussein SM, Batada NN, Vuoristo S, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471(7336):58-62. 10.1038/nature09871 [DOI] [PubMed] [Google Scholar]
- 33. Halliwell J, Barbaric I, Andrews PW.. Acquired genetic changes in human pluripotent stem cells: origins and consequences. Nat Rev Mol Cell Biol. 2020;21(12):715-728. 10.1038/s41580-020-00292-z [DOI] [PubMed] [Google Scholar]
- 34. Avery S, Hirst AJ, Baker D, et al. BCL-XL mediates the strong selective advantage of a 20q11.21 amplification commonly found in human embryonic stem cell cultures. Stem Cell Rep. 2013;1(5):379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gore A, Li Z, Fung HL, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63-67. 10.1038/nature09805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kosanke M, Osetek K, Haase A, et al. Reprogramming enriches for somatic cell clones with small-scale mutations in cancer-associated genes. Mol Ther. 2021;29(8):2535-2553. 10.1016/j.ymthe.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Merkle FT, Ghosh S, Kamitaki N, et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature. 2017;545(7653):229-233. 10.1038/nature22312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Avior Y, Lezmi E, Eggan K, Benvenisty N.. Cancer-related mutations identified in primed human pluripotent stem cells. Cell Stem Cell. 2021;28(1):10-11. 10.1016/j.stem.2020.11.013 [DOI] [PubMed] [Google Scholar]
- 39. Olariu V, Harrison NJ, Coca D, et al. Modeling the evolution of culture-adapted human embryonic stem cells. Stem Cell Res. 2010;4(1):50-56. 10.1016/j.scr.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 40. Ben-David U, Mayshar Y, Benvenisty N.. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell. 2011;9(2):97-102. 10.1016/j.stem.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 41. Blum B, Benvenisty N.. The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle. 2009;8(23):3822-3830. 10.4161/cc.8.23.10067 [DOI] [PubMed] [Google Scholar]
- 42. Andrews PW, Matin MM, Bahrami AR, et al. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem Soc Trans. 2005;33(6):1526-1530. 10.1042/BST20051526 [DOI] [PubMed] [Google Scholar]
- 43. Werbowetski-Ogilvie TE, Bossé M, Stewart M, et al. Characterization of human embryonic stem cells with features of neoplastic progression. Nat Biotechnol. 2009;27(1):91-97. 10.1038/nbt.1516 [DOI] [PubMed] [Google Scholar]
- 44. Ben-David U, Arad G, Weissbein U, et al. Aneuploidy induces profound changes in gene expression, proliferation and tumorigenicity of human pluripotent stem cells. Nat Commun. 2014;5(1):1-11. [DOI] [PubMed] [Google Scholar]
- 45. Ben-David U, Mayshar Y, Benvenisty N.. Virtual karyotyping of pluripotent stem cells on the basis of their global gene expression profiles. Nat Protoc. 2013;8(5):989-997. 10.1038/nprot.2013.051 [DOI] [PubMed] [Google Scholar]
- 46. Weissbein U, Schachter M, Egli D, Benvenisty N.. Analysis of chromosomal aberrations and recombination by allelic bias in RNA-Seq. Nat Commun. 2016;7(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lezmi E, Benvenisty N.. Identification of cancer-related mutations in human pluripotent stem cells using RNA-seq analysis. Nat Protoc. 2021;16(9):4522-4537. 10.1038/s41596-021-00591-5 [DOI] [PubMed] [Google Scholar]
- 48. Stirparo GG, Smith A, Guo G.. Cancer-related mutations are not enriched in naive human pluripotent stem cells. Cell Stem Cell 2021;28(1):164-169.e2. 10.1016/j.stem.2020.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Allison TF, Andrews PW, Avior Y, et al. Assessment of established techniques to determine developmental and malignant potential of human pluripotent stem cells. Nat Commun. 2018;9(1):1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Damjanov I, Andrews PW.. Teratomas produced from human pluripotent stem cells xenografted into immunodeficient mice-a histopathology atlas. Int J Dev Biol. 2016;60(10-11-12):337. 10.1387/ijdb.160274id [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Avior Y, Biancotti JC, Benvenisty N.. TeratoScore: assessing the differentiation potential of human pluripotent stem cells by quantitative expression analysis of teratomas. Stem Cell Rep. 2015;4(6):967-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hentze H, Soong PL, Wang ST, et al. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009;2(3):198-210. 10.1016/j.scr.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 53. Ben-David U, Benvenisty N.. Chemical ablation of tumor-initiating human pluripotent stem cells. Nat Protoc. 2014;9(3):729-740. 10.1038/nprot.2014.050 [DOI] [PubMed] [Google Scholar]
- 54. Ben-David U, Nudel N, Benvenisty N.. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun. 2013;4(1):1992. 10.1038/ncomms2992 [DOI] [PubMed] [Google Scholar]
- 55. Lee MO, Moon SH, Jeong HC, et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci USA. 2013;110(35):3281-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ben-David U, Gan QF, Golan-Lev T, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12(2):167-179. 10.1016/j.stem.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 57. Schuldiner M, Itskovitz-Eldor J, Benvenisty N.. Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells. 2003;21(3):257-265. 10.1634/stemcells.21-3-257 [DOI] [PubMed] [Google Scholar]
- 58. Liang Q, Monetti C, Shutova MV, et al. Linking a cell-division gene and a suicide gene to define and improve cell therapy safety. Nature 2018;563(7733):701-704. [DOI] [PubMed] [Google Scholar]
- 59. Manstein F, Triebert W, Ullmann K, et al. High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Stem Cells Transl Med. 2021;10(7):1063-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weissbein U, Ben-David U, Benvenisty N.. Virtual karyotyping reveals greater chromosomal stability in neural cells derived by transdifferentiation than those from stem cells. Cell Stem Cell. 2014;15(6):687-691. 10.1016/j.stem.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 61. Blokzijl F, De Ligt J, Jager M, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 2016;538(7624):260-264. 10.1038/nature19768 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.