Abstract
Pur7 is the product of a gene from the puromycin biosynthetic pur cluster of Streptomyces alboniger. It was expressed in Escherichia coli as a recombinant protein fused to a His tag and then was highly purified through a Ni2+ column. It showed a 3′-amino-3′-dATP pyrophosphohydrolase (nudix) activity which produced 3′-amino-3′-dAMP and pyrophosphate. This is consistent with the presence of a nudix box in its amino acid sequence. As observed with other nudix hydrolases, Pur7 has an alkaline pH optimum and a requirement for Mg2+. Among a large variety of other nucleotides tested, only 3′-amino-3′-dTTP was a Pur7 substrate, although at lower reaction rates than 3′-amino-3′-dATP. These findings suggest that Pur7 has a high specificity for the 3′ amino group at the ribofuranoside moiety of these two substrates. The Km and Vmax values for these dATP and dTTP derivatives were 120 μM and 17 μM/min and 3.45 mM and 12.5 μM/min, respectively. Since it is well known that 3′-amino-3′-dATP is a strong inhibitor of DNA-dependent RNA polymerase, whereas 3′-amino-3′-dAMP is not, Pur7 appears to be similar to other nudix enzymes in terms of being a housecleaning agent that permits puromycin biosynthesis to proceed through nontoxic intermediates. Finally, the identification of this activity has allowed a revision of the previously proposed puromycin biosynthetic pathway.
The nudix hydrolases constitute a large family of enzymes whose members possess a highly conserved structural motif, the nudix box or MutT signature. Its consensus sequence is GX5EX7REUXEEXGU, where U usually is I, L, or V (5). It has been proposed that this motif is the catalytic center of nucleoside triphosphate (NTP)-pyrophosphohydrolases, which produce PPi and the corresponding nucleoside monophosphate (6, 7). Of these hydrolases, the antimutator MutT from Escherichia coli was the prototype (1, 7, 19, 36). Therefore, this group of proteins was identified as the MutT family (5, 12). However, it later became known that not all the enzymes which share this conserved domain have an NTP-pyrophosphohydrolase activity, nor are they involved in preventing mutations. Bessman et al. (5) redefined the family and clarified the field. They proposed the term nudix hydrolases to replace MutT family, due to their common property of hydrolyzing a nucleoside diphosphate linked to another moiety, X (5). So far, the only exception is DIPP from rat, which hydrolyzes diphosphoinositol polyphosphates instead of a nucleoside diphosphate derivative (24). Data bank searches indicated that nudix hydrolases are widely represented from viruses to humans and, at present, some 20 enzymes have been characterized. Interestingly, Sheikh et al. (27) have discovered a nudix hydrolase in the archaeon Methanococcus jannaschii, which indicates the evolutionary conservation of the nudix motif in all kingdoms. It has been proposed that, despite their different substrates and specific functions, the nudix hydrolases have an important common physiological function in sanitizing the cell of toxic endogenous metabolites and in modulating the accumulation of certain intermediates in biochemical pathways. They would thus be “house-cleaning” enzymes, protecting the cell from harmful effects resulting from the unbalanced presence of potentially toxic compounds (5).
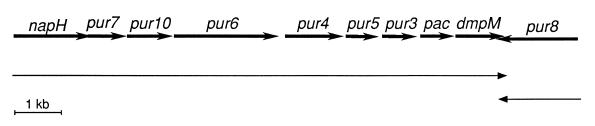
Concerning actinomycetes, 10 putative nudix proteins have been deduced from genome sequencing of different species of Streptomyces. These include one from S. ambofaciens (accession no. Z19590), one from S. lividans (accession no. Z86111), seven from the partial genome sequence of S. coelicolor A3 (accession no. AL021529, AL031035, AL035205, AL022374, AL023797, AL031541, and StI30A [26]), and Pur7 from S. alboniger (32). The latter is the deduced product from an open reading frame within the pur gene cluster for the biosynthesis of the aminonucleoside antibiotic puromycin (32). The pur cluster (Fig. 1) has been cloned and characterized (16, 32), which, in addition to biochemical work on several of the encoded products, permitted us to propose a puromycin biosynthetic pathway (32). Its substrate would be ATP, which is converted into 3′-keto-3′-dATP by Pur10 (23). This intermediate was thought to be converted to 3′-keto-3′-dAMP by Pur7, and this would be converted to 3′-amino-3′-dAMP by the putative aminotransferase Pur4. At this stage, a tyrosinyl moiety would be linked to the 3′-amino group to produce N6,N6,O-tridemethylpuromycin. A puromycin N-acetyltransferase, Pac, would inactivate this intermediate (21, 35) to produce N-acetylpuromycin, which would be dimethylated at N6 by an N-methyltransferase (very likely Pur5) (32). The latter intermediate is O-methylated by DmpM, an O-demethylpuromycin O-methyltransferase (21, 35). This would yield the last precursor, the biologically inactive N-acetylpuromycin, which is secreted and then hydrolyzed by NapH to produce the active antibiotic (17).
FIG. 1.
Gene organization of the pur cluster of S. alboniger. This figure is modified from Tercero et al. (32). Size of the genes is indicated by thick arrows. Transcripts are indicated below by thin arrows. The first 8 nucleotides of pur7 overlap with napH, which encodes an N-acetylpuromycin-N-acetylhydrolase (17). pur10, pac, dmpM, and pur8 encode NAD-dependent ATP dehydrogenase (23), puromycin-N-acetyltransferase (14), acetyl-O-demethylpuromycin-O-methyltransferase (15), and a transmembrane protein which confers resistance to puromycin (33), respectively. pur6, pur4, pur5, and pur3 encode putative tyrosinyltransferase, aminotransferase, methyltransferase, and monophosphatase activities, respectively (32).
In this work, we show that Pur7 is indeed a nudix hydrolase which has a 3′-amino-3′-dATP pyrophosphohydrolase activity. Therefore, the role of Pur7 proposed earlier (32) is apparently wrong, and this study identifies what is likely the correct role. The Pur7 reaction appears to be a key step in the biosynthesis of puromycin because it inactivates the highly toxic intermediate 3′-amino-3′-dATP through its conversion into an inactive 3′-amino-3′-dAMP.
MATERIALS AND METHODS
Strains and plasmids.
E. coli DH5 (10), MK601 (mut+), and MK602 (mut mutant) (11), and BL21(DE3)pLysS (31) are described in the indicated references. Plasmids used were pBluescript SK(−) (Stratagene) and pRSETb (13). Cells were grown in Luria-Bertani medium (20). General DNA methodology was followed as previously described (25). When required, ampicillin and chloramphenicol were used at concentrations of 100 and 34 μg/ml, respectively.
Chemicals.
2′-Amino-2′-dATP, 3′-amino-3′-dTTP, 3′-azido-3′-dTTP, 3′-fluoro-3′-dTTP, 8-oxo-dGTP, and dPTP (6-[2-deoxy-β-d-ribofuranosyl]-3,4-dihydro-8H-pyrimido-[4,5-C][1,2]oxazin-7-one-5′-triphosphate) were purchased from Amersham Pharmacia Biotech. Unless otherwise indicated, other biochemicals and enzymes were purchased from Sigma. 3′-Amino-3′-dAMP was obtained by treating 3′-amino-3′-dATP with apyrase for 20 min at 30°C in a reaction mixture (20 μl) containing 20 nmol of 3′-amino-3′-dATP, 37.5 mM Tris-HCl (pH 6.5), 3 mM MgCl2, and 1 U of apyrase (see below).
Subcloning and expression of pur7.
A 1-kb BsaAI-NruI fragment from plasmid pPS6.3 (pBluescript including a ClaI-EcoRI fragment of 6.3 kb from the pur cluster) (9) was isolated and cloned into the EcoRV site of Bluescript SK(−). From the resulting construct (pUR7), a 0.8-kb NcoI-ScaI fragment containing pur7 was cloned into the NcoI-HindIII sites of pRSETb. The NcoI restriction site includes the proposed ATG initiator for pur7 (32). The resulting plasmid (pUR7.EX) was transformed into E. coli BL21(DE3)pLysS cells for expression of pur7 as generally described previously (13). These cells were grown at 37°C in 1 liter of Luria-Bertani medium supplemented with ampicillin and chloramphenicol to an A660 of 0.6 and induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.4 mM. The cells were further grown for 2 h and then harvested, washed at 4°C with an isotonic saline solution, and kept at −70°C. To extract the protein, the cells were resuspended in 20 ml of 50 mM Na2HPO4 (pH 8.0), 300 mM NaCl, 1 mM MgCl2, and 5 mM β-mercaptoethanol and broken by three cycles of freezing and thawing, each followed by sonication for 1 min at 15 μm. The crude extract was centrifugated at 15,000 × g for 20 min to remove cell debris, and the supernatant was collected.
Purification of recombinant Pur7.
To purify the His-tagged recombinant Pur7 protein, 4 ml of Ni2+-nitrilotriacetic acid (NTA) agarose (Qiagen) was added to 15 ml of supernatant and mixed gently by shaking at 4°C for 60 min (22). The lysate-Ni2+-NTA mixture was loaded in a column and washed successively with a mixture of 2 volumes of 50 mM Na2HPO4 (pH 8.0), 300 mM NaCl, 1 mM MgCl2, and 5 mM β-mercaptoethanol; 2 volumes of a mixture of 50 mM Na2HPO4 (pH 8.0), 300 mM NaCl, 20 mM imidazole, 1 mM MgCl2, and 5 mM β-mercaptoethanol; and 2 volumes of a mixture of 50 mM Na2HPO4 (pH 8.0), 300 mM NaCl, 50 mM imidazole, 1 mM MgCl2, and 5 mM β-mercaptoethanol. Elution of Pur7 was achieved with 1 volume of a mixture of 50 mM Na2HPO4 (pH 8.0), 300 mM NaCl, 500 mM imidazole, 1 mM MgCl2, and 5 mM β-mercaptoethanol. The final eluate was dialyzed three times for 1 h against 100 volumes of the buffer used for the Pur7 reaction (see below). The purification process was monitored by subjecting samples from every step to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis followed by staining with Coomassie blue. Protein was quantitated by using the Bio-Rad protein assay kit.
Microsequencing of the purified recombinant Pur7.
The 22-kDa band obtained after the last step of purification was isolated from the gel and treated with trypsin, and the resultant peptides were separated and collected by using a Smart μHPLC device (Pharmacia). Two of the peptides were sequenced by tandem mass spectrometry by the nanospray ionization method, using a LCQ quadrupole ion trap (Finnigan, ThermoQuest, San Jose, Calif.).
Enzyme assays.
To assay Pur7 activity, unless otherwise indicated, reaction mixtures (50 μl) contained 500 μM substrate, 50 mM Tris-HCl (pH 9.5), 5 mM MgCl2, 1 mM dithiothreitol (DTT), and the indicated amount of enzyme. These were found to be the optimal conditions. Reactions took place for 15 min at 30°C, and they were terminated by the addition of a mixture (50 μl) containing one part 7% HClO4 and four parts 20% Norit A. The final mixture was incubated on ice for 5 min. Samples (50 μl) from the supernatant were collected, and PPi was hydrolyzed with either 1 N HCl or inorganic pyrophosphatase. In the first case, 250 μl of 1 N HCl was added and the samples were boiled for 15 min. In the second case, 1 U of inorganic pyrophosphatase (Boehringer Mannheim) was added to the reaction mixtures. The concentration of inorganic orthophosphate was determined as described previously (3) and then modified (6). One unit of Pur7 was defined as the enzyme required to catalyze the hydrolysis of 1 μmol of 3′-amino-3′-dATP/min.
TLC.
The standard 3′-amino-3′-dAMP was obtained by treating 3′-amino-3′-dATP with apyrase (see above). This standard (Rf, 0.58) was run alongside a reaction mixture sample during thin-layer chromatography (TLC) on polyethyleneimine cellulose plates with 1 M LiCl as the ascending solvent. The 3′-amino-3′-dAMP spots were detected by UV absorption.
RESULTS
Assay for mutator phenotype.
To determine if, like other nudix hydrolases, Pur7 could complement the mutT mutator phenotype in E. coli, E. coli MK601 and MK602 were transformed with plasmids pUR7.EX and pRSETb, and mutation frequencies were determined as described previously (11). The result of this experiment showed that this phenotype was not complemented (data not shown), which indicated that it should participate in a different cellular function.
Expression and purification of Pur7.
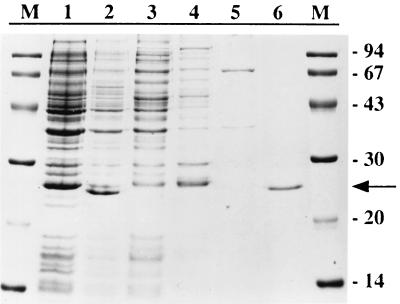
Expression in E. coli of pur7, subcloned as described in Materials and Methods, gives rise to a major band on an SDS-polyacrylamide gel, corresponding to a 22-kDa protein (Fig. 2). This molecular mass is consistent with the expected size (21.5 kDa) for the predicted Pur7 protein plus the extra residues corresponding to the tail used for its purification. Most of this band appeared in the soluble fraction and was not detected in the strain containing pRSETb without the pur7 insert. The recombinant Pur7 was highly purified by means of a Ni2+-NTA agarose column. Two of the peptides resulting from its tryptic digestion had the sequences DLYDDD (the first six residues of a longer peptide) and IFVQRR, which corresponded, respectively, to the amino-terminal-end fusion and the residues at positions 65 to 70 of the deduced amino acid sequence of Pur7. This indicated that the purified band was indeed Pur7.
FIG. 2.
Purification of recombinant Pur7. A Coomassie-stained SDS–11% polyacrylamide gel is shown. Lanes: 1, crude extract of E. coli BL21(DE3)pLysS(pRSETb); 2, crude extract of E. coli BL21(DE3)pLysS(pUR7.EX) 2 h after addition of IPTG; 3, first eluate from the Ni2+-NTA agarose column; 4, eluate with sodium phosphate buffer; 5, eluate with 20 mM imidazole-containing buffer; 6, eluate with 500 mM imidazole-containing buffer; M, molecular mass markers (sizes are in kilodaltons). The arrow indicates recombinant Pur7.
Enzymatic activity of recombinant Pur7 protein.
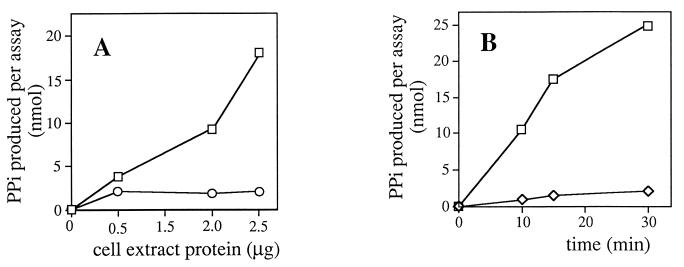
Supernatants from cell extracts of E. coli(pUR7.EX) contained a 3′-amino-3′-dATP pyrophosphohydrolase activity which was absent in the supernatants from the control E. coli BL21(DE3)pLysS(pRSETb) (Fig. 3A). Pyrophosphate was also released from 3′-amino-3′-dATP by purified recombinant Pur7 (Fig. 3B). Purification increased by 90-fold the specific activity of Pur7 (Table 1). TLC showed that the migration of the reaction product and that of 3′-amino-3′-dAMP were identical (data not shown).
FIG. 3.
3′-Amino-3′-dATP pyrophosphohydrolase activity. (A) Reactions were carried out with supernatants from cell extracts of E. coli BL21(DE3)pLysS transformed with either pRSETb (○) or pUR7.EX (□). (B) Reactions were carried out with purified Pur7 (1 mU) in the presence (□) or absence (◊) of inorganic pyrophosphatase. The latter, as a negative control, shows that Pur7 does not release orthophosphate. Other details are described in Materials and Methods.
TABLE 1.
Effects of purification of recombinant Pur7
| Treatment of Pur7 | Protein (mg) | Activity (U) | Specific activity (U/mg) | Fold purification | Yield (%) |
|---|---|---|---|---|---|
| None (crude extract) | 124 | 25.3 | 0.2 | 1 | 100 |
| Ni2+ purification | 1.8 | 32.9 | 18.3 | 91.5 | 130 |
Substrate specificity.
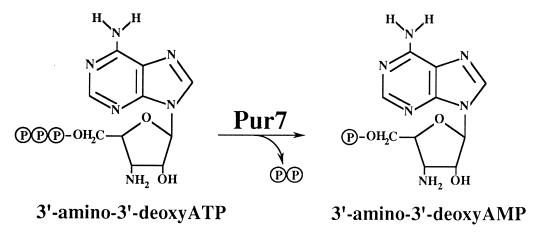
The substrate specificity of Pur7 is summarized in Table 2. Under the experimental conditions used, only 3′-amino-3′-dTTP, in addition to 3′-amino-3′-dATP, was a substrate for Pur7, although at lower rates than the latter. These findings suggest that the reaction catalyzed by Pur7 in S. alboniger is that indicated in Fig. 4 (see Discussion).
TABLE 2.
Substrate specificity of recombinant Pur7
| Substratea | Specific activity (U/mg of protein) |
|---|---|
| 3′-Amino-3′-dATP | 54.6 |
| 3′-Amino-3′-dTTP | 20.2 |
| 2′-Amino-dATP | <0.1 |
| 8-Oxo-dGTP | <0.1 |
| dPTP | <0.1 |
| 3′-Azido-3′-dTTP | <0.1 |
| 3′-Fluoro-3′-dTTP | <0.1 |
| 3′-Deoxy-ATP (cordycepin triphosphate) | <0.1 |
| Nucleoside triphosphates | <0.1 |
| 2′-Deoxy-nucleoside triphosphates | <0.1 |
Substrate concentrations were 2 mM except in the cases of 3′-amino-3′-dATP and 3′-amino-3′-dTTP, for which concentrations were 1 mM.
FIG. 4.
Reaction catalyzed by Pur7.
Requirements of the reaction.
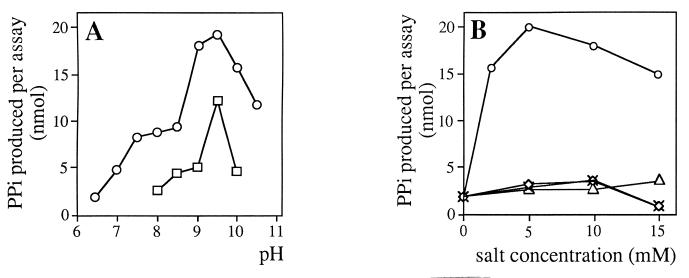
The reaction conditions used are indicated under Materials and Methods. Optimal enzymatic activity was at pH 9.5 (Fig. 5). Curiously, reaction rates were lower in glycine than in Tris-HCl buffer. In addition, Pur7 requires Mg2+, which could not be replaced by other divalent cations (Ca2+ or Mn2+), or the monovalent cation NH4+. Furthermore, maximal activity was reduced by approximately 30% in the absence of DTT, 1 mM being the optimal concentration (data not shown).
FIG. 5.
Effect of pH and cations on the reaction catalyzed by Pur7. Assays were performed as described in Material and Methods, except that the pH (A) or cations (B) of the reactions, which contained 1 mU of Pur7, were changed as indicated. In panel A, buffers used were 50 mM Tris-HCl (○) or 80 mM glycine (□). In panel B, salts used were MgCl2 (○), CaCl2 (◊), NH4Cl (×), or MnCl2 (▵).
Kinetic parameters.
The Lineweaver-Burk plots of the initial reaction rates (up to 15 min) against the substrate concentrations (3′-amino-3′-dATP or 3′-amino-3′-dTTP) were all linear. The calculated Km and Vmax for 3′-amino-3′-dATP were 120 μM and 17 μmol/min, respectively, and for 3′-amino-3′-dTTP the values were 3.45 mM and 12.5 μmol/min, respectively (data not shown).
DISCUSSION
pur7 was previously identified as an open reading frame included in the puromycin biosynthetic gene cluster (pur) from S. alboniger (Fig. 1) (32). The deduced protein has a highly conserved structural motif, the nudix box, which allowed its inclusion in the family of nudix hydrolases, as defined by Bessman et al. (5). Despite its location within the pur cluster, which suggested a role in the biosynthesis of the antibiotic puromycin, we initially tested whether, like other members of this family, pur7 could complement the mutT mutator phenotype in E. coli. The fact that it failed to complement this phenotype indicated a different role in the cell, most likely in the biosynthesis of puromycin. In this respect, it is now known that the antimutator nucleoside triphosphatase activity is shared by only some of the nudix hydrolases (reference 27 and references therein). In the present work, we have shown that the recombinant Pur7, expressed and purified from E. coli as a His-tagged fused protein, has a 3′-amino-3′-dNTP pyrophosphohydrolase activity and that in S. alboniger, 3′-amino-3′-dATP seems to be its substrate. 3′-Amino-3′-dTTP was also hydrolyzed, although at lower rates than 3′-amino-3′-dATP. This pyrophosphohydrolyzing activity is consistent with the existence of a nudix motif in the amino acid sequence of Pur7. Parameters of the reaction catalyzed by Pur7, i.e., an optimal alkaline pH or the requirement for a divalent cation, are coincident with the optimal reaction conditions found for other nudix hydrolases. Interestingly, none of the other similar nucleoside triphosphates tested, which lacked a 3′-amino group at the ribofuranoside moiety, was a substrate for Pur7. These results suggest, therefore, that this residue is important for substrate specificity and that 3′-keto-3′-dATP is not a substrate.
What is the function of Pur7 in the puromycin biosynthetic pathway? Antibiotic biosynthesis is a complex process which often requires self-protection mechanisms in the producer organism against both the produced antibiotic and some harmful intermediates (8). Tercero et al. (32) proposed that puromycin biosynthesis starts with ATP, which would be dehydrogenated by Pur10 in the first step of the pathway. Indeed, this was later shown to be the case (23). The product of this reaction, 3′-keto-3′-dATP, was initially thought to be the substrate for Pur7 (32). The resulting 3′-keto-3′-dAMP, similar to other substrates for secondary metabolism aminotransferases (2, 18), would be the substrate for the putative aminotransferase Pur4, which would introduce the amino group at the 3′ position (32). However, the results from the present work indicate that this part of the pathway proceeds differently. Firstly, 3′-amino-3′-dATP and, although less well, 3′-amino-3′-dTTP were substrates for Pur7, which hydrolyzes them to produce PPi and the corresponding 3′-amino-3′-dNMP. Secondly, the 3′ amino group of the ribofuranoside moiety appears to be an essential requirement for this activity, since other nucleotides tested, including 3′-dATP (cordycepin 5′-triphosphate), 3′-azido-3′-dTTP, and 3′-fluoro-3′-dTTP, were not substrates for Pur7 (Table 1). In this respect, 3′-keto-3′-dATP, which could not be tested in this work due to its extreme instability (23), should have a stereochemical conformation of the ribofuranoside moiety quite different than that of the 3′ amino derivatives. This could prevent its activity as a Pur7 substrate. Therefore, 3′-keto-3′-dATP should be the substrate for Pur4 to produce 3′-amino-3′-dATP. This intermediate is a strong inhibitor of DNA-dependent RNA polymerase (4, 28, 34) and, consequently, a highly toxic compound for the cell. However, its hydrolysis by Pur7 converts it into 3′-amino-3′-dAMP, which lacks biological activity (29, 30), and allows the pathway to continue. Pur7 appears, therefore, to be a key enzyme in the biosynthesis of the antibiotic puromycin, which, by preventing the accumulation of the toxic intermediate 3′-amino-3′-dATP, permits both puromycin biosynthesis and cell viability. Therefore, Pur7 is, as a member of the nudix hydrolase family, another example of a house-cleaning enzyme (5). This reflects the importance of these enzymes, which, despite their different specific functions, have a common role in the maintenance of cell viability. It is also an example of the involvement of a nudix hydrolase in secondary metabolism, which extends the function of these proteins to apparently nonessential processes. Despite this, some of these processes, as is the case of antibiotic biosynthesis, have been well conserved in nature. Pur7 is, to the best of our knowledge, the first nudix enzyme of actinomycetes to be characterized and the first which has been shown to be implicated in secondary metabolism. Finally, the pyrophosphohydrolysis of 3′-amino-3′-dTTP by Pur7 might be used to synthesize a new hybrid antibiotic, which would contain a dT backbone instead of dA.
ACKNOWLEDGMENTS
We are grateful to A. Martín for expert technical assistance, to E. Fernández for helpful comments during the course of this work, and to J. Vázquez and A. Marina for peptide sequencing.
This research was supported by a grant (BIO4-CT950198) from the Cell Factory Program of the European Union and an institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular “Severo Ochoa.”
REFERENCES
- 1.Abeygunawardana C, Weber D J, Frick D N, Bessman M J, Mildvan A S. Sequence-specific assignments of the backbone 1H, 13C, and 15N resonances of the MutT enzyme by heteronuclear multidimensional NMR. Biochemistry. 1993;32:13071–13080. doi: 10.1021/bi00211a017. [DOI] [PubMed] [Google Scholar]
- 2.Ahlert J, Distler J, Mansouri K, Piepersberg W. Identification of stsC, the gene encoding the l-glutamine:scyllo-inosose aminotransferase from streptomycin-producing streptomycetes. Arch Microbiol. 1997;168:102–113. doi: 10.1007/s002030050475. [DOI] [PubMed] [Google Scholar]
- 3.Ames B N, Dubin D T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960;235:769–775. [PubMed] [Google Scholar]
- 4.Armstrong V W, Eckstein F. Interaction of substrate analogues with Escherichia coli DNA-dependent RNA polymerase. Eur J Biochem. 1976;70:33–38. doi: 10.1111/j.1432-1033.1976.tb10952.x. [DOI] [PubMed] [Google Scholar]
- 5.Bessman M J, Frick D N, O’Handley S F. The MutT proteins or “nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J Biol Chem. 1996;271:25059–25062. doi: 10.1074/jbc.271.41.25059. [DOI] [PubMed] [Google Scholar]
- 6.Bhatnagar S K, Bessman M J. Studies on the mutator gene, mutT of Escherichia coli. Molecular cloning of the gene, purification of the gene product, and identification of a novel nucleoside triphosphatase. J Biol Chem. 1988;263:8953–8957. [PubMed] [Google Scholar]
- 7.Bhatnagar S K, Bullions L C, Bessman M J. Characterization of the mutT nucleoside triphosphatase of Escherichia coli. J Biol Chem. 1991;266:9050–9054. [PubMed] [Google Scholar]
- 8.Cundliffe E. How antibiotic-producing organisms avoid suicide. Annu Rev Microbiol. 1989;43:207–233. doi: 10.1146/annurev.mi.43.100189.001231. [DOI] [PubMed] [Google Scholar]
- 9.Espinosa J C. Caracterización molecular del cluster pur de Streptomyces alboniger y estudio de la biosíntesis de Puromicina. Tesis doctoral. Madrid, Spain: Facultad de Ciencias, Universidad Autónoma de Madrid; 1997. [Google Scholar]
- 10.Hanahan D. Techniques for transformation of E. coli. In: Glover D M, editor. DNA cloning: a practical approach. Oxford, United Kingdom: IRL Press; 1985. pp. 109–136. [Google Scholar]
- 11.Kakuma T, Nishida J, Tsuzuki T, Sekiguchi M. Mouse MTH1 protein with 8-oxo-7,8-dihydro-2′-deoxiguanosine 5′-triphosphatase activity that prevents transversion mutation. J Biol Chem. 1995;270:25942–25948. doi: 10.1074/jbc.270.43.25942. [DOI] [PubMed] [Google Scholar]
- 12.Koonin E V. A highly conserved sequence motif defining the family of MutT-related proteins from eubacteria, eukaryotes and viruses. Nucleic Acids Res. 1993;21:4847. doi: 10.1093/nar/21.20.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroll D J, Abdel-Malek H, Marcell T, Simpson S, Chen C, Gutierrez-Hartmann A, Lustbader J W, Hoeffler J P. A multifunctional prokaryotic protein expression system: overproduction, affinity purification, and selective detection. DNA Cell Biol. 1993;12:441–453. doi: 10.1089/dna.1993.12.441. [DOI] [PubMed] [Google Scholar]
- 14.Lacalle R A, Pulido D, Vara J, Zalacain M, Jiménez A. Molecular analysis of the pac gene encoding a puromycin N-acetyl transferase from Streptomyces alboniger. Gene. 1989;79:375–380. doi: 10.1016/0378-1119(89)90220-5. [DOI] [PubMed] [Google Scholar]
- 15.Lacalle R A, Ruiz D, Jiménez A. Molecular analysis of the dmpM gene encoding an O-demethyl puromycin O-methyltransferase from Streptomyces alboniger. Gene. 1991;109:55–61. doi: 10.1016/0378-1119(91)90588-3. [DOI] [PubMed] [Google Scholar]
- 16.Lacalle R A, Tercero J A, Jiménez A. Cloning of the complete biosynthetic gene cluster for an aminonucleoside antibiotic, puromycin, and its regulated expression in heterologous hosts. EMBO J. 1992;11:785–792. doi: 10.1002/j.1460-2075.1992.tb05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacalle R A, Tercero J A, Vara J, Jimenez A. Identification of the gene encoding an N-acetylpuromycin N-acetylhydrolase in the puromycin biosynthetic gene cluster from Streptomyces alboniger. J Bacteriol. 1993;175:7474–7478. doi: 10.1128/jb.175.22.7474-7478.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H-W, Thorson J S. Pathways and mechanisms in the biogenesis of novel deoxysugars by bacteria. Annu Rev Microbiol. 1994;48:223–256. doi: 10.1146/annurev.mi.48.100194.001255. [DOI] [PubMed] [Google Scholar]
- 19.Maki H, Sekiguchi M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature. 1992;355:273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- 20.Miller J F. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 21.Pattabiraman T N, Pogell B M. Biosynthesis of puromycin in Streptomyces alboniger. Possible precursors of the antibiotic in a commercial sample. Biochim Biophys Acta. 1969;182:245–247. doi: 10.1016/0005-2787(69)90539-5. [DOI] [PubMed] [Google Scholar]
- 22.Qiagen GmbH. March 1997. The QIAexpressionist. A handbook for high-level expression and purification of 6× His-tagged proteins. Qiagen GmbH, Hilden, Germany.
- 23.Rubio M A, Espinosa J C, Tercero J A, Jiménez A. The Pur10 protein encoded in the gene cluster for puromycin biosynthesis of Streptomyces alboniger is an NAD-dependent ATP dehydrogenase. FEBS Lett. 1998;437:197–200. doi: 10.1016/s0014-5793(98)01228-9. [DOI] [PubMed] [Google Scholar]
- 24.Safrany S T, Caffrey J J, Yang X, Bembenek M E, Moyer M B, Burkhart W A, Shears S S. A novel context for the “MutT” module, a guardian of cell integrity, in a diphosphoinositol polyphosphate phosphohydrolase. EMBO J. 1998;17:6599–6607. doi: 10.1093/emboj/17.22.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.S. coelicolor Blast Server. 2 June 1999, revision date. [Online.] The Sanger Centre. http://www.sanger.ac.uk/Projects/S_coelicolor/blast_server.shtml. [6 April 1999, last date accessed.]
- 27.Sheikh S, O’Handley S F, Dunn C A, Bessman M J. Identification and characterization of the nudix hydrolase from the archaeon, Methanococcus jannaschii, as a highly specific ADP-ribose pyrophosphatase. J Biol Chem. 1998;273:20924–20928. doi: 10.1074/jbc.273.33.20924. [DOI] [PubMed] [Google Scholar]
- 28.Shigeura H T, Boxer G E. Incorporation of 3′-deoxyadenosine-5′-triphosphate into RNA by RNA polymerase from Micrococcus lysodeykticus. Biochem Biophys Res Commun. 1964;17:758–763. [Google Scholar]
- 29.Shigeura H T, Boxer G E, Meloni M L, Sampson S D. Structure-activity relationship of some purine 3′-deoxyribonucleosides. Biochemistry. 1966;5:994–1004. doi: 10.1021/bi00867a027. [DOI] [PubMed] [Google Scholar]
- 30.Shigeura H T, Gordon C N. The effects of 3′-deoxyadenosine on the synthesis of ribonucleic acid. J Biol Chem. 1965;240:806–810. [PubMed] [Google Scholar]
- 31.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. In: Goeddel D V, editor. Gene expression technology. San Diego, Calif: Academic Press, Inc.; 1990. pp. 60–88. [DOI] [PubMed] [Google Scholar]
- 32.Tercero J A, Espinosa J C, Lacalle R A, Jiménez A. The biosynthetic pathway of the aminonucleoside antibiotic puromycin, as deduced from the molecular analysis of the pur cluster of Streptomyces alboniger. J Biol Chem. 1996;271:1579–1590. doi: 10.1074/jbc.271.3.1579. [DOI] [PubMed] [Google Scholar]
- 33.Tercero J A, Lacalle R A, Jiménez A. The pur8 gene from the pur cluster of Streptomyces alboniger encodes a highly hydrophobic polypeptide which confers resistance to puromycin. Eur J Biochem. 1993;218:963–971. doi: 10.1111/j.1432-1033.1993.tb18454.x. [DOI] [PubMed] [Google Scholar]
- 34.Truman J T, Klenow H. Effect of 3′-amino-3′-deoxyadenosine on nucleic acid synthesis in Ehrlich ascites tumor cells. Mol Pharmacol. 1968;4:77–86. [PubMed] [Google Scholar]
- 35.Vara J, Malpartida F, Hopwood D A, Jiménez A. Cloning and expression of a puromycin N-acetyl transferase gene from Streptomyces alboniger in Streptomyces lividans and Escherichia coli. Gene. 1985;33:197–206. doi: 10.1016/0378-1119(85)90094-0. [DOI] [PubMed] [Google Scholar]
- 36.Weber D J, Abeygunawardana C, Bessman M J, Mildvan A S. Secondary structure of the MutT enzyme as determined by NMR. Biochemistry. 1993;32:13081–13088. doi: 10.1021/bi00211a018. [DOI] [PubMed] [Google Scholar]