Abstract
The conjugative 450-kb megaplasmid pHG1 is essential for the anaerobic growth of Alcaligenes eutrophus H16 in the presence of nitrate as the terminal electron acceptor. We identified two megaplasmid-borne genes (nrdD and nrdG) which are indispensable under these conditions. Sequence alignment identified significant similarity of the 76.2-kDa gene product NrdD and the 30.9-kDa gene product NrdG with anaerobic class III ribonucleotide reductases and their corresponding activases. Deletion of nrdD and nrdG in A. eutrophus abolished anaerobic growth and led to the formation of nondividing filamentous cells, a typical feature of bacteria whose DNA synthesis is blocked. Enzyme activity of NrdD-like ribonucleotide reductases is dependent on a stable radical at a glycine residue in a conserved C-terminal motif. A mutant of A. eutrophus with a G650A exchange in NrdD showed the DNA-deficient phenotype as the deletion strain, suggesting that G650 forms the glycyl radical. Analysis of transcriptional and translational fusions indicate that nrdD and nrdG are cotranscribed and that the translation efficiency of nrdD is 40-fold higher than that of nrdG. Thus, the two proteins NrdD and NrdG are not synthesized at a stoichiometric level.
Reduction of ribonucleotides, mediated by ribonucleotide reductases (RNRs), is an elementary process for all living organisms which provides the four 2′-deoxyribonucleotides for DNA synthesis and repair. Three classes of RNRs are known which use similar radical mechanism for catalysis (reviewed in references 21, 39, and 40). Regulatory feedback mechanisms keep a balanced level of deoxyribonucleotide inside the cell. A major difference between the various classes of RNRs is the nature of the free radical and the way it is formed.
Class I RNRs occur in all higher organisms and certain aerobic bacteria. These enzymes contain a stable tyrosyl radical which is generated by formation of an oxygen-linked diiron center (12). This reaction is strictly dependent on the presence of molecular oxygen (28, 37). The majority of prokaryotes harbor class II RNRs which are active under both aerobic and anaerobic conditions and use adenosylcobalamin as a cofactor for radical production (27, 55). RNRs of the third class function exclusively in the absence of oxygen. These enzymes contain a stable, but oxygen-sensitive glycyl radical which is introduced by an activase (52). In Escherichia coli, NADPH and flavodoxin are used to reduce the [4Fe-4S] cluster of the activase, which reductively cleaves S-adenosylmethionine to generate the radical (1, 15, 35).
The best-characterized member of class III RNRs is the NrdD protein of E. coli (34, 54). Biochemical data are also available for the corresponding protein from phage T4 (61, 62). Evidence for the existence of a class III RNR has also been presented for Lactococcus lactis (20) and Methanobacterium thermoautotrophicum (17). Furthermore, genome sequences suggest the occurrence of class III RNRs in Haemophilus influenzae (10), Methanococcus jannaschii (3), and Pyrococcus horikoshii (22).
In this report we show that Alcaligenes eutrophus H16, a strictly respiratory member of the β-subgroup of proteobacteria, contains an RNR belonging to class III, which is essential for the organism during anaerobic growth with nitrate as the electron acceptor. The enzyme is dispensable in aerobically grown cells. The two genes encoding the class III RNR and its activase are located on a 450-kb megaplasmid which contains genes for denitrification (41), hydrogen metabolism (13), and autotrophic carbon dioxide fixation (19). Sequence comparison suggests that the enzyme from A. eutrophus is closer related to class III RNRs from archaebacterial species than to the eubacterial counterparts.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The bacterial strains used here are listed in Table 1. A. eutrophus H16 is the wild type, harboring megaplasmid pHG1. Strain HF210 is a megaplasmid-free derivative of strain H16. Strains HF413 and HF456 were derived from the wild type by mutagenesis. E. coli XL1-Blue was used as a host in standard cloning procedures. E. coli S17-1 served as the donor in conjugative plasmid transfer. E. coli strains were grown in Luria-Bertani broth at 37°C. A. eutrophus strains were cultivated in mineral salts medium at 30°C (44) with 0.4% (wt/vol) fructose as the carbon source and 0.2% (wt/vol) ammonium chloride as the nitrogen source (FN-medium). For anaerobic growth under denitrifying conditions the cells were cultivated in 150-ml glass flasks sealed with a rubber septum and containing 100 ml of FN-medium supplemented with 0.2% (wt/vol) potassium nitrate. The gas phase consisted of dinitrogen. Solid media contained 1.5% (wt/vol) agar. Antibiotics were added as follows: for A. eutrophus, kanamycin (400 μg/ml) and tetracyclin (10 μg/ml), and for E. coli, ampicillin (50 μg/ml), kanamycin (30 μg/ml), and tetracyclin (10 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| A. eutrophus strains | ||
| H16 | Wild type, pHG1 | DSM 428, ATCC 17699c |
| HF210 | pHG1-free derivative | 24 |
| HF413 | ΔnrdDG | This study |
| HF456 | NrdD G650A | This study |
| E. coli strains | ||
| S17-1 | pro thi recA hsdS RP4 tra functions | 48 |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac (F′ proAB lacIqZΔM15 Tn10) | Stratagene |
| Plasmids | ||
| pVDZ′2 | TcrlacZ′ mob | 6 |
| pBluescript KS(+) | Apr, lacZ′ flori T7 and T3 promoter | Stratagene |
| pUC18 | Apr, lacPOZ′ | 60 |
| pEDY305 | Tcr, lacZ RP4 oriT | 46 |
| pPHU234/pPHU235 | Tcr, lacZ′ | 18 |
| pLO1/pLO2 | Kmr, sacB RP4 oriT ColE1 ori | 26 |
| pPX41 | Apr, E. coli nrdDG | 53 |
| pGE26 | nosZ, norB, nrdDG | 63 |
| pGE151 | Tcr, lacZ′ RP4 oriT | 25 |
| pGE291 | 6.2-kb EcoRI-HindIII fragment of pGE26 in pVDZ′2 (nrdDG) | This study |
| pGE305 | 5.0-kb XbaI-HindIII fragmentb in pVDZ′2 | This study |
| pGE306 | 4.5-kb XbaI-HindIII fragmentb in pVDZ′2 | This study |
| pGE307 | 4.0-kb XbaI-HindIII fragmentb in pVDZ′2 | This study |
| pGE311 | 4.4-kb EcoRI-BamHI fragmentb in pVDZ′2 | This study |
| pGE312 | 3.2-kb EcoRI-BamHI fragmentb in pVDZ′2 | This study |
| pGE343 | 4.7-kb EcoRI-BamHI fragmentb in pVDZ′2 | This study |
| pGE344 | 5.2-kb EcoRI-BamHI fragmentb in pVDZ′2 | This study |
| pGE384 | 124-bp Ecl136II-BamHI fragment of pCH604 in pEDY305 (nrdDP-lacZ) | This study |
| pGE385 | 2.65-kb Ecl136II-SmaI fragment of pCH604 in pEDY305 (nrdGP-lacZ) | This study |
| pGE386 | 460-bp XbaI-HincII fragment of pCH604 in pPHU234 [Φ(nrdD-lacZ)] | This study |
| pGE387 | 2.6-kb XbaI-SmaI fragment of pCH604 in pPHU235 [Φ(nrdG-lacZ)] | This study |
| pGE388 | Derivative of pGE387 containing a 1.7-kb XhoI deletion [Φ(ΔDnrdG-lacZ)] | This study |
| pGE391 | 6.5-kb PstI fragment of pPX41 in pGE151 (E. coli nrdDG) | This study |
| pCH447 | 6.2-kb EcoRI-HindIII fragment of pGE26 in pBluescript KS(+) (nrdDG) | This study |
| pCH604 | 5.0-kb XbaI-HindIII fragmentb of pCH447 pBluescript KS(+) (nrdDG) | This study |
| pCH605 | 3.05-kb XbaI-XhoI fragment in pLO1 (3.2-kb deletion of nrdDG) | This study |
| pCH606 | 608-bp XhoI-MscI fragment in pLO2 (NrdD G650A) | This study |
Ap, ampicillin; Km, kanamycin; Tc, tetracycline; nrdD, anaerobic RNR; nrdG, activase of NrdD.
Obtained by exonuclease III deletion from pCH447.
DSM, Deutsche Sammlung für Mikroorganismen; ATCC, American Type Culture Collection.
Plasmids.
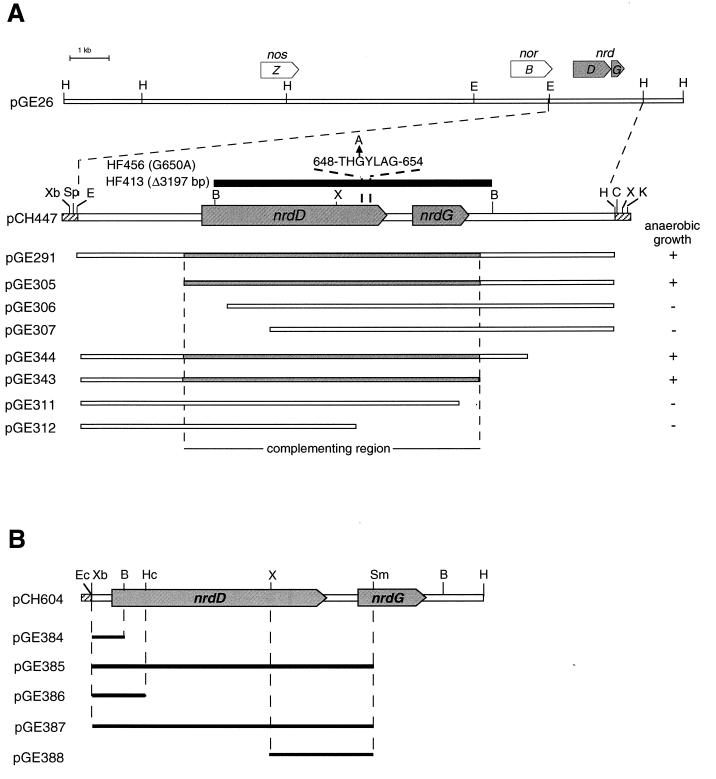
Plasmids used in this study are listed in Table 1. A 6.5-kb PstI fragment from plasmid pPX41 containing nrdDG from E. coli was subcloned into pGE151, yielding plasmid pGE391. In this plasmid, nrdD and nrdG of E. coli are under control of the lac promoter, allowing a constitutive expression in A. eutrophus (25). Cosmid pGE26, isolated from a pHG1 DNA library, contains a 30-kb fragment of megaplasmid pHG1. A 6.2-kb EcoRI-HindIII fragment from pGE26 was cloned into the broad-host-range vector pVDZ′2 and into pBluescript KS(+) yielding plasmids pGE291 and pCH447, respectively (Fig. 1A). Exonuclease III treatment of EcoRI-SpeI-linearized pCH447 resulted in a set of deletion derivatives. Three XbaI-HindIII fragments of 5.0 kb (pCH604), 4.5 kb, and a 4.0 kb were cloned into pVDZ′2, yielding plasmids pGE305, pGE306, and pGE307, respectively (Fig. 1A). Exonuclease III treatment of HindIII-ClaI linearized pCH447 resulted in a second set of deletion derivatives. Four EcoRI-KpnI fragments of 4.4, 3.2, 4.7, and 5.2 kb were first cloned into pUC18 and subsequently transferred as EcoRI-HindIII fragments into pVDZ′2, yielding plasmids pGE311, pGE312, pGE343, and pGE344, respectively (Fig. 1A).
FIG. 1.
Subclones of the nrdDG region. (A) Three of seven subclones of pGE291, generated by bidirectional deletion, restored anaerobic growth (+) of a megaplasmid-free derivative of A. eutrophus. Cloned DNA fragments are indicated by open bars. Highlighted bars depict the region essential for complementation. Identified genes are marked by open arrows: nosZ, nitrous oxide reductase; norB, megaplasmid-encoded copy of nitric oxide reductase; nrdD, anaerobic RNR; nrdG, activase. The deleted DNA fragment in HF413 is indicated as solid bar. The G650A exchange in mutant HF456 is marked by an arrow. Relevant restriction sites: B, BamHI; C, ClaI; E, EcoRI; H, HindIII; K, KpnI; Sp, SpeI; Xb, XbaI; X, XhoI. (B) DNA fragments used for the construction of transcriptional or translational fusions with lacZ as the reporter gene are shown as solid bars. Genes are depicted by open arrows on the restriction map. Relevant restriction sites: B, BamHI; Ec, Ecl136II; Hc, HincII; H, HindIII; Sm, SmaI; Xb, XbaI; X, XhoI.
Plasmid pGE384 is a derivative of the mobilizable, broad-host-range promoter assay vector pEDY305 carrying the 5′ nrdD spanning DNA region inserted upstream of the promoterless lacZ gene. This plasmid was generated by inserting a 124-bp Ecl136II-BamHI fragment of pCH604 between the ScaI and BglII sites of the pEDY305 polylinker (Fig. 1B). The corresponding plasmid for the nrdG promoter region, pGE385, contained a 2.65-kb Ecl136II-SmaI fragment of pCH604 in the ScaI site of the pEDY305 polylinker. Plasmid pGE386 is a derivative of pPHU234 which carries a 460-bp XbaI-HincII fragment of pCH604 inserted between the XbaI and ScaI sites of pPHU234, resulting in a translational fusion of nrdD and lacZ (Fig. 1B). A corresponding nrdG translational fusion (pGE387) was constructed by insertion of a 2.6-kb XbaI-SmaI fragment of pCH604 into the XbaI and ScaI sites of pPHU235. Plasmids pPHU234 and pPHU235 are conjugative broad-host-range vectors with different translational phasing of the polylinker upstream of the lacZ gene (18). pGE388 is a derivative of pGE387 carrying a 1.7-kb XhoI deletion which removed the 5′ nrdD untranslated region and most of the nrdD gene. The transcriptional and translational fusions were verified by restriction analysis.
A deletion was introduced into nrdDG by digestion of a 6.2-kb EcoRI-HindIII fragment of pCH447 with BamHI (Fig. 1A). The religated fragment was cloned into pBluescript KS(+) and subsequently transferred into pLO1 by digestion with XbaI and XhoI (pCH605). The G650A exchange in NrdD (Fig. 1A) was obtained by overlap-extension PCR mutagenesis (16) by using pCH604 as the template and the following four primers (mutation sites are underlined): PXHO (5′-CAACCTCGAGGCTACCCCAG-3′), PMSC (5′-CGCTGCATGGCCAGGCAAGG-3′), G650A1 (5′-GCCGGCGAGGTAGTCATGTG-3′), and G650A2 (5′-CCCACACA TGACTACCTCGC-3′) (Fig. 2). The resulting 608-bp XhoI-MscI fragment was transferred into the SalI-Ecl136II-linearized vector pLO2 to give pCH606. The suicide vectors pLO1 and pLO2 contain the conditionally lethal sacB gene from Bacillus subtilis, which allows selection of A. eutrophus mutants generated by allelic exchange as described previously (26). Mutations were checked by DNA sequencing.
FIG. 2.
Nucleotide and derived amino acid sequences of the complementing region. The deduced amino acid sequences of NrdD and NrdG are shown below the nucleotide sequence. A potential Fnr-binding site (FNR) and a possible hairpin structure (TER) are emphasized by inverted arrows. Potential ribosome binding sites are underlined. Primers used for the construction of the G650A mutant are depicted above and below the sequence: 1, PXHO; 2, G650A2; 3, G650A1; 4, PMSC.
DNA techniques.
Standard DNA techniques were used (42). Plasmid DNA was isolated with tip-20 columns (Qiagen) according to the manufacturer’s instructions. DNA sequencing was done with an automated DNA sequencer (LiCOR) by using a cycle sequencing kit (Amersham) and fluorescent primers (MWG-Biotech).
Enzyme assays.
Anaerobic ribonucleotide reductase activity was determined by using the assay which had been introduced for the anaerobic ribonucleotide reductase of E. coli (34). Soluble extracts from anaerobically grown A. eutrophus cells were prepared as described previously (59) by sonication under an atmosphere of argon. Protein was determined according to the method of Lowry et al. (30). All components were degassed for 45 min prior to use. The assay mixture contained 1 to 1.5 mg of protein in 50 mM Tris-HCl (pH 7.5), 30 mM KCl, 5 mM dithiothreitol, 1 mM NADPH, 20 μM 5′-deazaflavine, and 0.5 mM S-adenosylmethionine. The total volume was 100 μl. The reaction mixture was preincubated for 60 min under illumination. The reductase reaction was started by the addition of 5 mM MgCl2, 5 mM sodium formate, and 1 mM [3H]CTP. After 20 min, the reaction was stopped by the addition of 0.5 ml of HClO4, and the amount of dCTP formed was determined (8). One unit of enzyme activity is expressed as the formation of 1 nmol of dCTP per min. β-Galactosidase was assayed according to the method of Miller (33), except that the optical cell density was measured at 436 nm.
Nucleotide sequence.
The nucleotide sequences for the nrdD and nrdG genes have been deposited in the EMBL database under accession number AJ012479.
RESULTS
Cloning and sequence analysis of a gene locus essential for anaerobic growth of A. eutrophus.
Curing of megaplasmid pHG1 of A. eutrophus H16 led to the loss of anaerobic growth ability with nitrate as the terminal electron acceptor (41). This was unexpected since essential enzymes for denitrification, such as the reductases for nitrate, nitrite, and nitric oxide, are encoded on the chromosome of this organism (5, 38, 59). A second functionally equivalent copy of a nitric oxide reductase gene (norB [5]) has previously been identified to be closely linked to the gene for nitrous oxide reductase (nosZ [63]) on a megaplasmid-borne DNA insert cloned in cosmid pGE26. Subcloning of the 30-kb DNA fragment revealed that the loss of the two denitrification-specific genes did not account for the failure of megaplasmid-free derivatives to grow anaerobically (Fig. 1). However, a third locus (nrd), clearly distinct from nosZ and norB, proved to be essential for anaerobic growth.
nrdD and nrdG encode an anaerobic ribonucleotide reductase and its activase.
Nucleotide sequence analysis of the complementing DNA segment revealed two open reading frames nrdD and nrdG within a region of 3.3 kb (Fig. 2). nrdD predicts a protein of 676 amino acids (76.2 kDa) and is separated from nrdG by 271 bp. nrdG has the coding capacity for a protein of 256 amino acids (30.9 kDa). A potential hairpin-like structure (ΔG0′ = −123.9 kJ) at bp positions 3236 to 3266 (Fig. 2) points to a transcription termination signal immediately downstream of nrdG. The predicted gene product of nrdD shows homology to class III ribonucleotide reductases (Fig. 3), with overall identities of 19% (phage T4 [56]), 23% (E. coli [53]), 24% (H. influenzae [10]), 25% (M. jannaschii [3], M. thermoautotrophicum [50]), and 27% (P. horikoshii [22]). An intein present in NrdD of M. jannaschii (36) has been removed from the sequence to promote alignment. It is interesting to note that the archaebacterial NrdD proteins show the highest similarity to NrdD from A. eutrophus. Sequence comparison revealed that three cysteine residues (C186, C392, and C646) are conserved in all NrdD-like proteins available in the database. The highly conserved glycine G650 (marked in boldface in Fig. 3), which corresponds to G681 in the E. coli sequence, is the most likely candidate for carrying the stable glycyl radical in the RNR of A. eutrophus. An adjacently positioned tyrosine residue was identified in all NrdD proteins. Furthermore, the alignment uncovered a consensus motif AHxxGxIxxH (underlined in Fig. 3).
FIG. 3.
Alignment of NrdD from A. eutrophus with class III RNRs. The NrdD sequences are from A. eutrophus (A_EUT), M. jannaschii (M_JAN; National Center for Biotechnology Information [NCB] accession number 1591520), M. thermoautotrophicum (M_THE; NCB accession number 2622659), E. coli (E_COL; NCB accession number 1790686), H. influenzae (H_INF; NCB accession number 1573024), P. horikoshii (P_HOR; NCB accession number 3130259), and phage T4 (PH_T4; NCB accession number P07071). Residues conserved in all sequences are marked by asterisks. A consensus motif is underlined. Three conserved cysteine residues are boxed. The potential radical sites are indicated in boldface.
Sequence comparison of the A. eutrophus nrdG showed typical features of class III RNR-associated activases (Fig. 4). The highest identity (26%) was found to NrdG of M. thermoautotrophicum (50). A cysteine motif CxxxCxxC, present in all NrdG proteins accessible so far, may participate in the coordination of a [4Fe-4S] cluster. In E. coli, a [4Fe-4S] cluster bridges the two NrdG subunits in the homodimer (34, 35). It is interesting to note that the NrdG homolog of M. jannaschii has been annotated as a pyruvate formate lyase-specific activase, albeit genome sequence analysis of this archaeon lacks a pyruvate formate lyase but does predict the existence of a class III RNR (3). Hence, we have added this protein to the list of RNR-specific activases (Fig. 4).
FIG. 4.
Alignment of NrdG from A. eutrophus with class III RNR activase proteins. The NrdG sequences are from A. eutrophus (A_EUT), M. thermoautotrophicum (M_THE; NCB accession number 2621339), M. jannaschii (M_JAN; NCB accession number 2826326), P. horikoshii (P_HOR; NCB accession number 3130260); E. coli (E_COL; NCB accession number 1790685), H. influenzae (H_INF; NCB accession number 1574712), and phage T4 (PH_T4; NCB accession number P07075). Amino acids conserved in all sequences are marked by asterisks. Cysteine residues which may participate in coordination of an Fe-S-cluster are boxed.
RNR mutants.
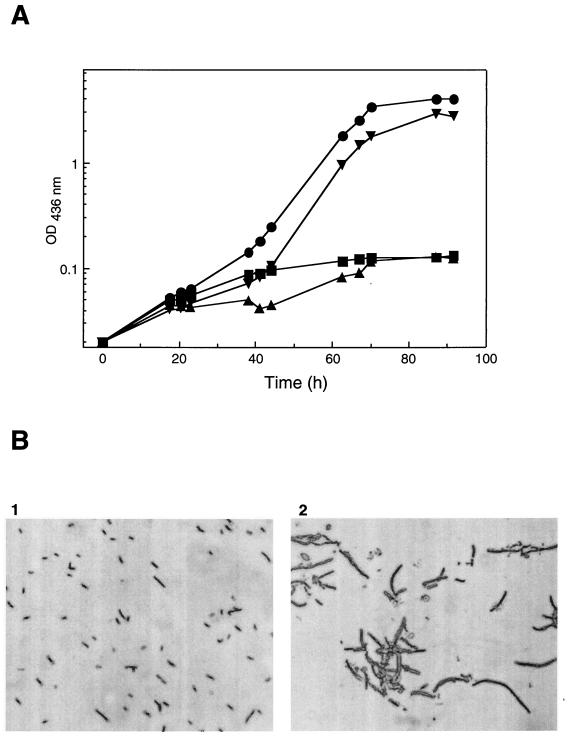
Deletion of a 3.3-kb DNA segment from the nrd locus of A. eutrophus yielded mutant HF413 (Fig. 1A). HF413 was unimpaired in aerobic growth (data not shown), but under anoxic conditions the cell density increased only slightly (Fig. 5A), and the formation of long cell filaments was observed (Fig. 5B). This morphological change is indicative for inhibited cell division caused by depletion of deoxyribonucleotides under anaerobiosis. Normal growth and cell morphology of HF413 resumed upon introduction of plasmid pGE291, which harbors the nrdDG genes of A. eutrophus (Fig. 5A). Heterologous complementation of HF413 with the nrdDG genes of E. coli on plasmid pGE391 was not successful (data not shown). A second NrdD deficient mutant was constructed by replacing the conserved G650 with an Ala residue by using site-directed mutagenesis (Fig. 1A). The resulting mutant HF456 behaved exactly like the deletion strain (Fig. 5A), thus supporting the notion that G650 is essential for the function of NrdD in A. eutrophus.
FIG. 5.
Phenotype and cell-morphology of RNR mutants. (A) Strains were grown anaerobically in FN-medium supplemented with 0.2% sodium nitrate. Results for A. eutrophus H16 (●), nrdDG deletion mutant HF413 (▴), complemented mutant HF413(pGE291) (▾), and NrdD G650A exchange mutant HF456 (■) are as indicated. (B) Samples were taken from anoxic cultures after 70 h and examined by light microscopy. Panels: 1, wild-type A. eutrophus H16; 2, nrdD-nrdG deletion mutant HF413.
RNR activity.
The results of this study point to the existence of two separate RNRs in A. eutrophus, one instrumental under anaerobic conditions and a second essential for aerobic growth. Attempts to determine anaerobic RNR activity in crude extracts from anaerobically grown cells of the wild-type H16 by using the protocol designed for E. coli (34) yielded an enzymatic activity of 0.01 U per min per mg of protein. This corresponds to 10% of the activity determined in anaerobic extracts of E. coli (14). Replacement of the argon atmosphere by air, however, resulted in a significant increase of RNR activity up to 0.8 U per min per mg of protein. This result reflects high level of class I RNR in anaerobically cultivated cells of A. eutrophus and differs from the behavior of E. coli, which contains only traces of class I RNR during anaerobic growth (4, 14). This interfering activity does not permit a reliable assay for class III RNR in crude extracts of A. eutrophus.
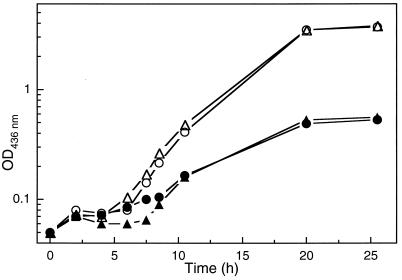
More evidence for the existence of class I RNR in A. eutrophus was obtained by the application of an inhibitor. The addition of 5 mM hydroxyurea to aerobically growing cells led to an increase of the doubling time from 2 to 7 h in both the wild type and the NrdD deficient mutant HF413 (Fig. 6). Hydroxyurea is an efficient radical scavenger and a well-known inhibitor, particularly of class I RNRs (7, 11). The sensitive response of A. eutrophus supports the notion that the organism contains a class I RNR in addition to the class III enzyme.
FIG. 6.
Aerobic growth of A. eutrophus in the presence of hydroxyurea. The wild type (○ and ●) and the nrdDG deletion mutant HF413 (▵ and ▴) were grown aerobically in FN-medium. Solid symbols indicate growth in the presence of 5 mM hydroxyurea.
nrdD and nrdG of A. eutrophus form an operon.
A sequence motif 5′-TTGCG N4 GTCAA-3′ was identified 79 bp upstream of the nrdD-translational start (Fig. 2) which resembles the binding site of the anaerobic transcriptional activator Fnr from E. coli (51). Transcription and translation of nrdD and nrdG were studied with the aid of reporter gene fusions (Fig. 1B) cloned on a broad-host-range plasmid. The recombinant plasmids were introduced into A. eutrophus H16 by conjugation. The level of transcription and translation was monitored by β-galactosidase activity (Table 2). We observed that, under oxic conditions, there was no transcription of nrdD and nrdG. In the absence of oxygen, β-galactosidase activities of the nrdD-transcriptional fusions (pGE384) and the nrdG-transcriptional fusions (pGE385) were almost identical, which suggests that nrdD and nrdG are cotranscribed from a common promoter located upstream of nrdD. This assumption is supported by the result obtained with the translational fusion in pGE388 (Table 2), which showed no β-galactosidase activity due to the absence of the nrdD promoter region (Fig. 1B). Substantially diverging levels of translation were observed when we compared the activities obtained with pGE386 and pGE387. The expression of the reductase NrdD is 40-fold higher than the expression of the activase NrdG. This result agrees with the observation that the putative ribosome binding site upstream of nrdD matches more closely the E. coli consensus ribosome binding site than the putative ribosome binding site upstream of nrdG (Fig. 2).
TABLE 2.
Expression of nrdD and nrdG
| Plasmid | lacZ fusion | β-Galactosidase (U)a
|
|
|---|---|---|---|
| Aerobic | Anaerobic | ||
| pGE384 | nrdDp-lacZ | 29 | 4,156 |
| pGE385 | nrdDGp-lacZ | 17 | 4,628 |
| pGE386 | Φ(nrdD-lacZ) | 0 | 5,986 |
| pGE387 | Φ(nrdG-lacZ) | 0 | 157 |
| pGE388 | Φ(ΔnrdD-nrdG-lacZ) | 0 | 0 |
The activity of β-galactosidase is expressed in arbitrary units. The data represent the means of two independent experiments. Activities were assayed from transconjugant wild-type cells grown to optical densities (measured at 436 nm) of 1.0 to 1.2.
DISCUSSION
A. eutrophus H16 harbors a 450-kb megaplasmid pHG1 which carries genetic determinants for the expression of two alternative metabolic pathways: energy generation from the oxidation of molecular hydrogen (13) and anaerobic respiration via denitrification (41). In contrast to hydrogen oxidation, which is encoded entirely on pHG1, genes for denitrification are dispersed on the chromosome and on the megaplasmid of A. eutrophus. A megaplasmid-free derivative of the wild type fails to denitrify and forms long filamentous cells. In this report we have shown that this phenotype is due to the absence of the megaplasmid-borne genes nrdD and nrdG, which encode an anaerobic class III RNR and its corresponding activase. This is the first example of a plasmid-encoded RNR. Introduction of the two genes into a megaplasmid-free recipient restored anaerobic growth and hence denitrification of the cells. It was shown before that transfer of the megaplasmid to taxonomically related bacteria lacking hydrogen oxidation and denitrification capacities yield transconjugants which have gained these metabolic activities (47). In fact, we could now demonstrate that transfer of nrdD and nrdG into the nondenitrifying strain Alcaligenes hydrogenophilus restores anaerobic growth on nitrate (47). This result shows that this host is missing housekeeping functions for anaerobic growth but harbors genes required for denitrification.
Aerobic growth of A. eutrophus was very sensitive to hydroxyurea, indicating that an oxygen-dependent class I RNR is instrumental during the aerobic growth of A. eutrophus. This enzyme appears to be also formed during anaerobic growth, which strongly interferes with the assay for anaerobic class III RNR activity in crude extracts. Thus, purification of the class III RNR is necessary before reliable statements concerning the enzymatic properties can be made. This result contrasts the situation in E. coli, which contains only residual amounts of the class I RNR in extracts from anaerobically cultivated cells (7, 11). Moreover, the assay employed in this study has been specifically designed for the class III enzyme of E. coli and may not meet special requirements of the corresponding enzyme from A. eutrophus. In particular, it is unknown whether NrdDG of A. eutrophus depends also on formate as the electron donor.
A high degree of similarity between NrdD from E. coli, H. influenzae, and phage T4 made it difficult to identify particular residues of potential structural or functional relevance in class III RNRs. A specific role was assigned to G681 of E. coli NrdD and G580 of phage T7 NrdD which carry the stable radical (54, 35). A glycine residue is conserved at the C terminus of all NrdD proteins described so far. Mutational exchange of the corresponding G650 to alanine in NrdD of A. eutrophus abolished anaerobic growth of the mutant strain. We therefore conclude that G650 is the site of the radical in NrdD of A. eutrophus. Three cysteine residues are conserved at similar positions in all NrdD sequences available so far. These residues may play a role in substrate reduction, as reported for a similar set of cysteines in class I and class II RNRs (2, 31, 49, 58). This assumption is confirmed by the recently published crystal structure of the phage T4 NrdD (29). Cysteine residues C286 and C392 of the A. eutrophus NrdD correlate with cysteine residues C79 and C290 in NrdD of phage T4, which reside in the active site of the enzyme (29). A common reaction mechanism, which involves three cysteine residues, has been proposed for all classes of RNRs (9). However, residue N311 in NrdD of phage T4 has been found to reside in place of the third conserved cysteine of class I RNRs (29). N311 is also conserved in all class III RNRs, including NrdD of A. eutrophus. In view of the complex reaction and allosteric regulation of class III RNRs, it seems surprising that only a few additional residues are conserved in the NrdD proteins. Particularly worth mentioning are two elements: AHxxGxIxxH and a tyrosine residue adjacent to the postulated radical site. The former motif seems to be involved in binding the phosphate of the substrate (29). Interestingly, two conserved CxxC motifs (residues 543 to 546 and residues 561 to 564 in NrdD of phage T4) are missing in NrdD of A. eutrophus. These residues are supposed to be involved in radical generation in NrdD of phage T4 (29).
Comparison of NrdG sequences revealed the presence of a conserved CxxxCxxC motif which may bridge two NrdG monomers via an Fe-S cluster, as has been shown for NrdG of E. coli (34, 52). Moreover, a pair of glycines is located at a defined distance to the cysteine cluster within the primary NrdG sequences. Both motifs are also present in pyruvate formate lyase (Pfl) activases and in the PqqE, NifB, and MoaA proteins (57). No specific physiological function has been assigned to PqqE, NifB, and MoaA, which are all involved in cofactor synthesis (32).
In vivo assays of promoter activity revealed that nrdD and nrdG are cotranscribed from a promoter upstream of nrdD, suggesting an arrangement in an operon. An operon-like structure was also proposed for the nrdD and nrdG genes of E. coli (52). Both gene products assemble into a heterotetramer at α2β2 stoichiometry, which resembles the composition of class I RNRs (34). Translational fusions with nrdD and nrdG of A. eutrophus showed that the expression of NrdD is 40-fold higher than the expression of NrdG. This result suggests that in this organism the two proteins are expressed in nonstoichiometric ratios. Since the glycyl radical is recycled after substrate reduction, a permanently formed reductase-activase complex is not necessarily required for catalysis. This view is supported by the fact that pyruvate formate lyase of E. coli is expressed to a significantly higher extent than its activase (43). Both types of activases use a [4Fe-4S] cluster to derive a 5′-deoxyadenosylradical from S-adenosylmethionine for the activation of their target proteins (23, 35). It is interesting that NrdG of A. eutrophus shows a higher degree of similarity to the pyruvate formate lyase-related activase than to NrdG of E. coli (data not shown), thus supporting the view of a common, highly related class of proteins which act as a functional module in combination with various enzyme systems.
ACKNOWLEDGMENTS
We are grateful to Peter Reichard and Rolf Eliasson for their advice with the ribonucleotide reductase assay, which was performed at the Karolinska Institute at Stockholm. We thank Albert Jordan for providing plasmids and Thomas Eitinger for critical reading of the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
REFERENCES
- 1.Bianchi V, Eliasson R, Fontecave M, Mulliez E, Hoover D M, Matthews R G, Reichard P. Flavodoxin is required for the activation of the anaerobic ribonucleotide reductase. Biochem Biophys Res Commun. 1993;197:792–797. doi: 10.1006/bbrc.1993.2548. [DOI] [PubMed] [Google Scholar]
- 2.Booker S, Licht S, Broderick J, Stubbe J. Coenzyme B12-dependent ribonucleotide reductase: evidence for the participation of five cystein residues in ribonucleotide reduction. Biochemistry. 1994;33:12676–12685. doi: 10.1021/bi00208a019. [DOI] [PubMed] [Google Scholar]
- 3.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G C, Blake J A, FitzGerald L M, Clayon R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J-F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Weidmann J F, Fuhrmann J L, Nguyen D, Utterback T R, Kelley J M, Peterson J D, Sadow P W, Hanna M C, Cotton M D, Roberts K M, Hurst M A, Kaine B P, Borodovsky M, Klenk H-P, Fraser C M, Smith H O, Woese C R, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 4.Casado C, Llagostera M, Barbé J. Expression of nrdA and nrdB genes of Escherichia coli is decreased under anaerobiosis. FEMS Microbiol Lett. 1991;67:153–157. doi: 10.1016/0378-1097(91)90346-c. [DOI] [PubMed] [Google Scholar]
- 5.Cramm R, Siddiqui R A, Friedrich B. Two isofunctional nitric oxide reductases in Alcaligenes eutrophus H16. J Bacteriol. 1997;179:6769–6777. doi: 10.1128/jb.179.21.6769-6777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deretic V, Chandrasekharappa S, Gill J F, Chatterjee D K, Chachrabarty A M. A set of cassettes and improved vectors for genetic and biochemical characterization of Pseudomonas genes. Gene. 1987;57:61–72. doi: 10.1016/0378-1119(87)90177-6. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenberg A, Reichard P. Electron spin resonance of the iron-containing protein B2 from ribonucleotide reductase. J Biol Chem. 1972;247:3485–3488. [PubMed] [Google Scholar]
- 8.Eliasson R, Pontis E, Fontecave M, Gerez C, Harder J, Jörnvall H, Krook M, Reichard P. Characterisation of components of the anaerobic ribonucleotide reductase system from Escherichia coli. J Biol Chem. 1992;267:25541–25547. [PubMed] [Google Scholar]
- 9.Eliasson R, Reichard P, Mulliez E, Ollagnier S, Fontecave M, Liepinsh E, Otting G. The mechanism of the anaerobic Escherichia coli ribonucleotide reductase investigated with nuclear magnetic resonance spectroscopy. Biochem Biophys Res Commun. 1995;214:28–35. doi: 10.1006/bbrc.1995.2252. [DOI] [PubMed] [Google Scholar]
- 10.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodeck A, Kelley J M, Weidmann J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Fontecave M, Eliasson R, Reichard P. Oxygen-sensitive ribonucleoside triphosphate reductase is present in anaerobic Escherichia coli. Proc Natl Acad Sci USA. 1989;86:2147–2151. doi: 10.1073/pnas.86.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontecave M, Nordlund P, Eklund H, Reichard P. The redox centres of ribonucleotide reductase of Escherichia coli. Adv Enzymol Relat Areas Mol Biol. 1992;65:147–183. doi: 10.1002/9780470123119.ch4. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich B, Schwartz E. Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu Rev Microbiol. 1993;47:351–383. doi: 10.1146/annurev.mi.47.100193.002031. [DOI] [PubMed] [Google Scholar]
- 14.Garriga X, Eliasson R, Torrents E, Jordan A, Barbé J, Gibert I, Reichard P. nrdD and nrdG genes are essential for strict anaerobic growth of Escherichia coli. Biochem Biophys Res Commun. 1996;229:189–192. doi: 10.1006/bbrc.1996.1778. [DOI] [PubMed] [Google Scholar]
- 15.Harder J. Ribonucleotide reductases and their occurrence in microorganisms: a link to RNA/DNA transition. FEMS Microbiol Rev. 1993;12:273–292. doi: 10.1111/j.1574-6976.1993.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 16.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 17.Hogenkamp H P C, Follmann H, Thauer R K. Ribonucleotide reductase in cell extracts of Methanobacterium thermoautotrophicum. FEBS Lett. 1987;219:197–201. [Google Scholar]
- 18.Hübner P, Willison J C, Vignais P M, Blickle T. Expression of regulatory nif genes in Rhodobacter capsulatus. J Bacteriol. 1991;173:2993–2999. doi: 10.1128/jb.173.9.2993-2999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husemann M, Klintworth R, Büttcher V, Salnikow J, Weissenborn C, Bowien B. Chromosomally and plasmid-encoded gene clusters for CO2-fixation (cfx genes) in Alcaligenes eutrophus. Mol Gen Genet. 1988;214:112–120. [Google Scholar]
- 20.Jordan A, Pontis E, Åslund F, Hellman U, Gibert I, Reichard P. The ribonucleotide reductase system of Lactococcus lactis. J Biol Chem. 1996;271:8779–8785. doi: 10.1074/jbc.271.15.8779. [DOI] [PubMed] [Google Scholar]
- 21.Jordan A, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 1998;67:71–98. doi: 10.1146/annurev.biochem.67.1.71. [DOI] [PubMed] [Google Scholar]
- 22.Kawarabayasi Y, Sawada M, Horikawa H, Haikawa Y, Hino Y, Yamamoto S, Sekine M, Baba S, Kosugi H, Hosoyama A, Nagai Y, Sakai M, Ogura K, Otuka R, Nakazawa H, Takamiya M, Ohfuku Y, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K, Nakamura Y, Robb T F, Horikoshi K, Masuchi Y, Shizuya H, Kikuchi H. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 1988;5:55–76. doi: 10.1093/dnares/5.2.55. [DOI] [PubMed] [Google Scholar]
- 23.Knappe J, Sawers G. A radical-chemical route to acetyl-CoA: the anaerobically induced pyruvate formate-lyase system of Escherichia coli. FEMS Microbiol Rev. 1990;75:383–398. doi: 10.1111/j.1574-6968.1990.tb04108.x. [DOI] [PubMed] [Google Scholar]
- 24.Kortlüke C, Friedrich B. Maturation of membrane-bound hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1992;174:6290–6293. doi: 10.1128/jb.174.19.6290-6293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kortlüke C, Horstmann K, Schwartz E, Rhode M, Binsack R, Friedrich B. A gene complex coding for the membrane-bound hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1992;174:6277–6289. doi: 10.1128/jb.174.19.6277-6289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenz O, Schwartz E, Dernedde J, Eitinger M, Friedrich B. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J Bacteriol. 1994;176:4385–4393. doi: 10.1128/jb.176.14.4385-4393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licht S, Gerfen G J, Stubbe J. Thiyl radicals in ribonucleotide reductases. Science. 1996;271:477–481. doi: 10.1126/science.271.5248.477. [DOI] [PubMed] [Google Scholar]
- 28.Ling J S, Sahlin M, Sjöberg B-M, Loehr T M, Sanders-Loehr J. Dioxygen is the source of the μ-oxo brige in iron ribonucleotide reductase. J Biol Chem. 1994;269:5595–5601. [PubMed] [Google Scholar]
- 29.Logan D T, Andersson J, Sjöberg B-M, Nordlund P. A glycyl radical site in the crystal structure of a class III ribonucleotide reductase. Science. 1999;283:1499–1504. doi: 10.1126/science.283.5407.1499. [DOI] [PubMed] [Google Scholar]
- 30.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:264–275. [PubMed] [Google Scholar]
- 31.Mao S S, Holler T P, Yu G X, Bollinger J M, Booker S, Johnston M I, Stubbe J. A model for the role of multiple cysteine residues involved in ribonucleotide reduction—amazing and still confusing. Biochemistry. 1992;31:9733–9743. doi: 10.1021/bi00155a029. [DOI] [PubMed] [Google Scholar]
- 32.Menéndez C, Iglio G, Henninger H, Brandsch R. A pAO1-encoded molybdopterin cofactor gene (moaA) of Arthrobacter nicotinovorans: characterisation and site-directed mutagenesis of the encoded protein. Arch Microbiol. 1995;164:142–151. doi: 10.1007/BF02525320. [DOI] [PubMed] [Google Scholar]
- 33.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 34.Ollagnier S, Mulliez E, Gaillard J, Eliasson R, Fontecave M, Reichard P. The anaerobic Escherichia coli ribonucleotide reductase. J Biol Chem. 1996;271:9410–9416. doi: 10.1074/jbc.271.16.9410. [DOI] [PubMed] [Google Scholar]
- 35.Ollagnier S, Mulliez E, Schmidt P P, Eliasson R, Gaillard J, Deronzier C, Bergman T, Gräslund A, Reichard P, Fontecave M. Activation of the anaerobic ribonucleotide reductase from Escherichia coli. J Biol Chem. 1997;272:24216–24223. doi: 10.1074/jbc.272.39.24216. [DOI] [PubMed] [Google Scholar]
- 36.Perler F B. InBase, the New England Biolabs Intein Database. Nucleic Acids Res. 1999;27:346–347. doi: 10.1093/nar/27.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersson L, Gräslund A, Ehrenberg A, Sjöberg B-M, Reichard P. The iron center in ribonucleotide reductase of Escherichia coli. J Biol Chem. 1980;255:6706–6712. [PubMed] [Google Scholar]
- 38.Rees E, Siddiqui R A, Köster F, Schneider B, Friedrich B. Structural gene (nirS) for the cytochrome cd1 nitrite reductase of Alcaligenes eutrophus H16. Appl Environ Microbiol. 1997;63:800–802. doi: 10.1128/aem.63.2.800-802.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichard P. From RNA to DNA, why so many ribonucleotide reductases? Science. 1993;260:1773–1777. doi: 10.1126/science.8511586. [DOI] [PubMed] [Google Scholar]
- 40.Reichard P. The evolution of ribonucleotide reduction. Trends Biochem Sci. 1997;22:81–85. doi: 10.1016/s0968-0004(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 41.Römermann D, Friedrich B. Dentrification by Alcaligenes eutrophus is plasmid dependent. J Bacteriol. 1985;162:852–854. doi: 10.1128/jb.162.2.852-854.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 43.Sauter M, Sawers R G. Transcriptional analysis of the gene encoding pyruvate formate-lyase-activating enzyme of Escherichia coli. Mol Microbiol. 1990;4:355–363. doi: 10.1111/j.1365-2958.1990.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 44.Schlegel H G, Kaltwasser H, Gottschalk G. Ein Submersverfahren zur Kultur wasserstoffoxidierender Bakterien: wachstumsphysiologische Untersuchungen. Arch Microbiol. 1961;38:209–222. [PubMed] [Google Scholar]
- 45.Schneider B, Nies A, Friedrich B. Transfer and expression of lithoautotrophy and denitrification in a host lacking these metabolic activities. Appl Env Microbiol. 1988;54:3173–3176. doi: 10.1128/aem.54.12.3173-3176.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz E, Gerischer U, Friedrich B. Transcriptional regulation of Alcaligenes eutrophus hydrogenase genes. J Bacteriol. 1998;180:3197–3204. doi: 10.1128/jb.180.12.3197-3204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siedow, A., and B. Friedrich. Unpublished data.
- 48.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 49.Sjöberg B-M. Structure of ribonucleotide reductase from Escherichia coli. In: Eckstein F, Lilley D M F, editors. Nucleic acids and molecular biology. Heidelberg, Germany: Springer-Verlag; 1995. pp. 192–221. [Google Scholar]
- 50.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H M, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicare R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Safer H, Patwell D, Prabhakar S, McDougall S, Shimer G, Goyal A, Pietrovski S, Church G M, Daniels C J, Mao J-I, Rice P, Nolling J, Reeve J N. Complete genome sequence of Methanobacterium thermoautotrophicum delta H: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spiro S, Guest J. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990;75:399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- 52.Sun X, Eliasson R, Pontis E, Andersson J, Buist G, Sjöberg B-M, Reichard P. Generation of the glycyl radical of the anaerobic Escherichia coli ribonucleotide reductase requires a specific activating enzyme. J Biol Chem. 1995;270:2443–2446. doi: 10.1074/jbc.270.6.2443. [DOI] [PubMed] [Google Scholar]
- 53.Sun X, Harder J, Krock M, Jörnvall H, Sjöberg B-M, Reichard P. A possible glycine radical in anaerobic ribonucleotide reductase from Escherichia coli: nucleotide sequence of the cloned nrdD gene. Proc Natl Acad Sci USA. 1993;90:577–581. doi: 10.1073/pnas.90.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun X, Ollagnier S, Schmidt P P, Atta M, Mulliez E, Lepape L, Eliasson R, Gräslund A, Fontecave M, Reichard P, Sjöberg B-M. The free radical of the anaerobic ribonucleotide reductase from Escherichia coli is at glycine 681. J Biol Chem. 1996;271:6827–6831. [PubMed] [Google Scholar]
- 55.Tamao Y, Blakley R L. Direct spectrophotometric observation of an intermediate formed from deoxyadenosylcobalamin in ribonucleotide reduction. Biochemistry. 1973;12:24–34. doi: 10.1021/bi00725a005. [DOI] [PubMed] [Google Scholar]
- 56.Tomaschewski J, Ruger W. Nucleotide sequence and primary structures of gene products coded for by the T4 genome between map positions 48.266 kb and 39.166 kb. Nucleic Acids Res. 1987;15:3632–3633. doi: 10.1093/nar/15.8.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toyama H, Chistoserdova L, Lidstrom M E. Sequence analysis of pqq genes required for biosynthesis of pyrroloquinoline quinone in Methylobacterium extorquens AM1 and the purification of a biosynthetic intermediate. Microbiology. 1997;143:595–602. doi: 10.1099/00221287-143-2-595. [DOI] [PubMed] [Google Scholar]
- 58.Uhlin U, Eklund H. Structure of ribonucleotide reductase protein R1. Nature. 1994;370:533–539. doi: 10.1038/370533a0. [DOI] [PubMed] [Google Scholar]
- 59.Warnecke-Eberz U, Friedrich B. Three nitrate reductase activities in Alcaligenes eutrophus. Arch Microbiol. 1992;159:405–409. [Google Scholar]
- 60.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 61.Young P, Öhmann M, Sjöberg B-M. Bacteriophage T4 gene 55.9 encodes an activity required for anaerobic ribonucleotide reduction. J Biol Chem. 1994;269:27815–27818. [PubMed] [Google Scholar]
- 62.Young P, Öhmann M, Xu M Q, Shub D A, Sjöberg B-M. Intron-containing T4 bacteriophage gene sunY encodes an anaerobic ribonucleotide reductase. J Biol Chem. 1994;269:20229–20232. [PubMed] [Google Scholar]
- 63.Zumft W G, Dreusch A, Löchelt S, Cuypers H, Schneider B, Friedrich B. Derived amino acid sequences of the nosZ gene (respiratory N2O reductase) from Alcaligenes eutrophus and Pseudomonas aeruginosa reveal potential copper-binding residues. Eur J Biochem. 1992;208:31–40. doi: 10.1111/j.1432-1033.1992.tb17156.x. [DOI] [PubMed] [Google Scholar]