Abstract
PURPOSE
The development of molecularly targeted tracers is likely to improve the accuracy of diagnostic, screening, and therapeutic tools. Despite the many therapeutic antibodies that are FDA-approved with known toxicity, only a limited number of antibody-dye conjugates have been introduced to the clinic. Thorough evaluation of the safety, stability and pharmacokinetics of antibody conjugates in the clinical setting compared to their parental components could accelerate the clinical approval of antibodies as agents for molecular imaging. Here we investigate the safety and stability of a near-infrared fluorescent dye (IRDye800CW) conjugated panitumumab, an approved therapeutic antibody, and report on the product stability, pharmacokinetics, adverse events, and QTc interval changes in patients.
PROCEDURES
Panitumumab-IRDye800CW was made under good manufacturing practice (GMP) conditions in a single batch on March 26, 2014 and then evaluated over 4.5 years at 0, 3, and 6 months, and then at 6-month intervals thereafter. We conducted early phase trials in head and neck, lung, pancreas and brain cancers with panitumumab-IRDye800CW. Eighty-one patients scheduled to undergo standard of care surgery were infused with doses between 0.06mg/kg to 2.83mg/kg of antibody. Patient ECGs, blood samples, and adverse events were collected over 30-days post-infusion for analysis.
RESULTS
81 patients underwent infusion of the study drug at a range of doses. Six patients (7.4%) experienced an adverse event that was considered potentially related to the drug. The most common event was a prolonged QTc interval which occurred in three patients (3.7%). Panitumumab-IRDye800CW had two OOS results at 42 and 54 months, while meeting all other stability testing criteria.
CONCLUSIONS
Panitumumab-IRDye800CW was safe and stable to administer over a 54-month window with a low rate of adverse events (7.4%) which is consistent with the rate associated with panitumumab alone. This data supports re-purposing therapeutic antibodies as diagnostic imaging agents with limited preclinical toxicology studies.
Keywords: Antibody-dye complex, safety, stability, pharmacokinetics, oncology
INTRODUCTION
As the portfolio of antibody therapeutics grows, diagnostic application of antibodies as molecular targeting agents is recognized as an important diagnostic tool to detect disease in a range of settings such as tumor antigen-specific imaging to guide surgical resection [1], prevent iatrogenic nerve injury intraoperatively [2], and identify metastatic lymph nodes to improve tumor staging and prognosis [3]. However, the more important application will likely be to determine the delivery of these agents and predict potential clinical benefit. Although monoclonal antibody therapies have been successful in extensive preclinical studies, they have a low and unpredictable patient response in the clinic [4–6]. As a result, it is thought that antibodies can be bioconjugated to optical or radiolabels to evaluate the delivery of antibody and check point inhibitor therapies in human subjects and mice models [7–10]. While numerous therapeutic antibodies and optical dyes are well-characterized, the adoption of antibody-dye conjugates is limited due to lack of extensive pre-clinical and clinical testing.
Panitumumab (Vectibix®; Amgen, Thousand Oaks, CA) is a fully-human, anti-EGFR monoclonal IgG2 antibody, initially approved by the FDA in September 2006 for EGFR-expressing metastatic colorectal cancer [11]. Panitumumab-IRDye800CW is a near-infrared fluorescently labelled antibody that binds to the epidermal growth factor receptor (EGFR), a protein of the ErbB family that is overexpressed in, amongst others, head and neck, glioma, pancreatic and lung cancers [12–15]. The attached IRDye800CW is a near-infrared fluorophore ideal for surgical visibility since it has higher tissue penetration depth than fluorophores in the visible range (400–700nm) and with minimal endogenous autofluorescence [16]. IRDye800CW has been demonstrated to have low toxicity and a short half-life when unconjugated [17]. The N-hydroxysuccinimide (NHS) ester reaction binds randomly to lysines throughout the antibody during a standard labeling method that has been performed successfully for chimeric and fully human antibodies with a consistent dye to protein ratio and good imaging results [18–19].
In the current study, we reviewed the product stability of panitumumab-IRDye800CW over 4.5 years, as well as the pharmacokinetics and safety in 81 patients across four cancer types. The purpose is to inform industry and regulatory agencies on the stability and safety of cGMP-produced antibody-dye conjugates for clinical applications.
METHODS
Study design
We conducted four open-label phase I trials in head and neck squamous cell carcinoma (HNSCC; NCT02415881), pancreas (NCT03384238), brain (NCT03510208), and lung (NCT03582124) cancer, approved by the Stanford University Administrative Panel on Human Subjects Research and the FDA. Written informed consent was obtained from all patients.
Adults with primary or recurrent HNSCC, pancreatic, brain or lung cancer scheduled for standard-of-care surgery were eligible. Qualifying patients had >12 weeks life expectancy, a Karnofsky performance status of 70%, or a level 1 ECOG/Zubrod. Exclusion criteria included abnormal magnesium or potassium levels, previous infusion reactions to monoclonal antibodies, QT interval prolongation (greater than 440ms in males and 450ms in females) on baseline electrocardiogram (ECG), substantial liver or cardiovascular disease. Patients taking Class IA or Class III antiarrhythmic agents were also not eligible.
A 100mg loading dose of unconjugated panitumumab was given prior to the antibody-dye conjugate infusion to assess infusion reactions to the unlabeled antibody in 12 HNSCC patients, one brain cancer patient, and all 11 pancreatic cancer patients. Doses between 0.06–2.84mg/kg were given to patients; the specific range of doses given in each cancer cohort is reported in Table 1. Patients enrolled after 6/3/2019 (n=3, lung; n=4, brain; n=5, HNSCC) were infused with the second batch of panitumumab-IRDye800CW.
Table 1:
Demographics and characteristics of study patients.
| Head and Neck Cancer (n=56) | Lung Cancer (n=6) | Pancreatic Cancer (n=11) | Brain Cancer (n=8) | Totals (n=81) | |
|---|---|---|---|---|---|
|
| |||||
| Age, years (range) | 61 (32–85) | 60 (32–71) | 66 (40–82) | 59 (42–72) | 61 (32–85) |
| Female (%) | 19 (33.9%) | 4 (66.7%) | 1 (9.1%) | 5 (62.5%) | 29 (35.8%) |
| Weight, kg (range) | 73 (41–101) | 82 (52–138) | 75 (62–101) | 73 (41–91) | 74 (41–138) |
| Tumor staging | |||||
| Stage 1 | 5 (8.9%) | - | 1 (9.1%) | - | 6 (7.4%) |
| Stage 2 | 11 (19.6%) | 4 (66.7%) | 3 (27.3%) | - | 18 (22.2%) |
| Stage 3 | 12 (21.4%) | 1 (16.7%) | 5 (45.5%) | 1 (12.5%) | 19 (23.5%) |
| Stage 4 | 25 (42.8%) | - | 2 (18.2%) | 7 (87.5%) | 34 (41.9%) |
| Stage unknown | 3 (5.3%) | 1 (16.7%) | - | - | 4 (4.9%) |
| Prior chemotherapy | 4 (7.1%) | 1 (16.7%) | 5 (45.5%) | 2 (25%) | 12 (14.8%) |
| Prior radiation | 14 (25%) | 1 (16.7%) | 2 (18.2%) | 2 (25%) | 19 (23.5%) |
| Average antibodya dose, mg/kg (range) | 0.9 (0.06–2.4) | 0.7 (0.4 –1.1) | 2.0 (1.5–2.8) | 1.1 (0.6–1.7) | 1.1 (0.06–2.8) |
| Average antibody-dye conjugate dose, mg/kg (range) | 0.7 (0.06–1.2) | 0.7 (0.4–1.1) | 0.7 (0.3–1.2) | 1 (0.6–1.4) | 0.7 (0.06–1.4) |
Includes loading dose.
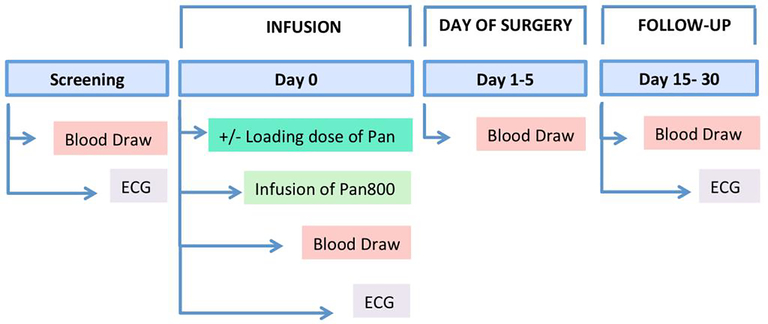
After screening, and eligibility confirmation, patients received an intravenous infusion of the study drugs on Day 0. Before and after study drug infusion, a blood sample and an ECG were obtained. Standard-of-care surgery followed 1–5 days after study drug infusion whereby another blood sample was collected before surgery. To aid with surgical workflow, we kept patient scheduling open for 1–5 days. HNSCC, pancreas, and brain cancer patients were followed for 30 days after study drug infusion; lung cancer patients for 21 days. Each patient had a final ECG and blood draw recorded at follow-up (Figure 1).
Figure 1: Study overview.
Following screening and eligibility confirmation, patients were infused with the study drug on Day 0, with or without loading dose. Prior to and after infusion of the study drug, a blood sample for pharmacokinetics and an ECG was collected. Blood samples for pharmacokinetics were collected at the following time points: day of surgery, follow-up, on Day 15, 21 and/or 30. On final follow-up, ECG was collected.
Panitumumab-IRDye800CW conjugation
The first batch of panitumumab-IRDye800CW was produced under cGMP condition through NCI’s NeXT program on March 26, 2014 and the second batch of panitumumab-IRDye800CW was produced by LI-COR Biosciences Inc, under cGMP conditions, on July 27, 2018. Both batches were produced by conjugating panitumumab (Vectibix®; Amgen, Thousand Oaks, CA) to IRDye800CW-NHS ester (LI-COR Biosciences Inc.; Lincoln, NE) via a 2-hour incubation at 20°C in the dark with a final dye:protein ratio of 2.0:1 and 2.4:1, a final concentration of 5.24mg/mL and 4.53mg/mL, and a conjugation yield of 85% and 88% for the first and second batch respectively. Quality control release testing included appearance, SDS-PAGE/Coomassie stain, protein concentration, dye:protein, Biacore receptor binding, free dye content, sterility, endotoxin content, pH, particulates. The purity of drug was evaluated with HPLC-SEC, which has been previously published [20]. Sterile vials were transported to the Stanford University Medical Center Investigational Pharmacy and stored in temperature-controlled and dark conditions.
Adverse Events and QTc Intervals
Adverse events were categorized per the National Cancer Institute Common Terminology Criteria (CTCAE) v4.03 for patients included prior to November 27, 2017 (n=21, HNSCC only) or v5.0. for patients included after November 27, 2017 (n=60). Grade 1 adverse events were mild, grade 2 was moderate, grade 3 was severe, and grade 4 was immediately life-threatening or fatal. Adverse event data was collected until final follow-up on Day 21 (lung) or Day 30 (HNSCC, brain and pancreas). Patients were evaluated with general physical exam and Karnofsky performance status at enrollment, the day of surgery, and in follow-up visits. Laboratory testing, including metabolic panel, serum chemistry, complete blood count, prothrombin/partial thromboplastin times, was performed at the time of screening, day of surgery, and follow-up appointments. On the screening and surgery days, thyroid stimulating hormone levels were also collected. ECGs were performed at the time of screening, pre- and post-infusion of panitumumab-IRDye800CW, and at the final follow-up visit. QTc prolongation is a common adverse drug reaction that are associated with life-threatening proarrythmias, were obtained from ECGs in accordance with the FDA Guidance E14 which requires routine assessment of this [21].
Adverse events were unrelated if drug administration and event had no temporal relationship, and/or the event is clearly due to an accident, other therapies, or the patient’s medical condition. A possibly related event showed some temporal relationship to the drug administration, and is unlikely caused by the participant’s other medical conditions. A strong temporal relationship between the event and the drug administration, and/or an event that is inexplicable by the participant’s condition or other therapies is considered probably related. A definitely related adverse event is similar to a probably related event, except the event also follows a known response from the drug.
Pharmacokinetics
Conjugate stability in plasma was assessed by SDS-PAGE. 2.5μL of plasma was run on NuPage 4–12% Bis-Tris gels (Invitrogen; Carlsbad, CA). After imaging on the Odyssey CLx (LI-COR Biosciences; Lincoln, NE) at 800nm, the free dye percentage was calculated by dividing the free dye signal at 1 kDa by the signal of the total plasma sample per lane, as described previously [22].
To quantify panitumumab-IRDye800CW in circulation, plasma samples were diluted 1:16, and plated in triplicate on 96-well half-well black clear-bottom plates (Catalog No. 3881; Corning Inc., Corning, NY) alongside a standard curve of panitumumab-IRDye800CW (0, 0.1, 1, 10, 25, 50, 100mg/L), as previously described [23]. Fluorescence values of the standard curve and plasma samples were read on a Tecan Spark multimode plate reader (Tecan Group Ltd.; Männedorf, Zurich, Switzerland) at 775nm. Fluorescence measurements from the Tecan were multiplied by the conjugate percentage, the inverse of free dye percentage determined through the SDS-PAGE analysis.
Total mg of drug in each patient was calculated based on gender and weight [23]. Half-life in each individual patient was calculated by taking natural logs of the total amount (mg) of drug in each patient at different time points and plotted on MS Excel to determine the slope (−k), used in the following formula to discern half-life: t1/2 = ln(2)/k. The standard deviation was divided by the mean of the individual half-life calculations for each cohort to determine the percentage error.
Statistical Analysis
Descriptive statistics were performed using GraphPad Prism (Version 6.0c, GraphPad Software, La Jolla, CA), and Microsoft Excel 2017 (Version 15.41, Microsoft, Redmond, WA). Doses given in mg/kg were calculated by dividing total dose (mg) including loading doses and panitumumab-IRDye800CW, by weight (kg). Patients were then split into five cohorts based on dose range: <0.5mg/kg, 0.5–1.0mg/kg, 1.0–1.5mg/kg, 1.5–2.0mg/kg, and 2.0–3.0mg/kg. Microdosed patients (0.06mg/kg) were not included in pharmacokinetics analysis, as values were too low to calculate half-life accurately. A one-way ANOVA was used to compare the QTc interval percentage change across dose cohorts and between screening ECG, post-infusion ECG, final follow-up ECG. Statistical significance was considered p<0.05.
Stability Testing
Panitumumab-IRDye800CW underwent stability testing at the Frederick National Laboratory for Cancer Research (operated by Leidos Biomedical Research, Inc.) at 0, 3, and 6 months after production, and then continued at every six-month interval for a total of 54 months. Tests for visual appearance, protein content (A280), dye:protein ratio (A780 and A280), monomer purity (HPLC-SEC), percentage free dye (HPLC-SEC), identity (SDS-PAGE), and potency (Biacore) were performed at each interval with the results based on predetermined assay specifications set by the laboratory (Table S1). Sterility tests were performed annually. Binding potency was assayed using Biacore with different control lots of unconjugated Vectibix® as they expired or were depleted during the course of testing. Any out-of-specification (OOS) results initiated further investigation and were reported to the FDA.
RESULTS
Patient Characteristics
Between December 2015 and December 2019, 81 patients received panitumumab-IRDye800CW. Patients included a range of cancer types including HNSCC (n=56), pancreas (n=11), brain (n=8), and lung cancer (n=6) (Table 1). Patients were divided into cohorts according to the dose (mg/kg) of study drug they received: 8 patients (9.9%) received <0.5mg/kg; 32 patients (39.5%) received 0.5–1.0mg/kg; 13 patients (16.0%) received 1.0–1.5mg/kg; 10 patients (12.3%) received 1.5–2.0mg/kg dosing range, and 10 (12.3%) received 2.0–3.0mg/kg of panitumumab-IRDye800CW.
Adverse Events and Pharmacokinetics
Six patients (7.4%) experienced an adverse event that could be potentially attributed to the study drugs (Table 2). The most common was QTc interval prolongation immediately after infusion of panitumumab-IRDye800CW compared to pre-infusion measurements. Four patients (4.9%) experienced a grade 1 adverse event that was considered possibly related: in the 0.5–1.0mg/kg cohort one patient presented with a grade 1 prolonged QTc interval, and in the 2.0–3.0mg/kg cohort, there were three grade 1 adverse events, one patient had a prolonged QTc interval, another presyncope, and one reported vomiting (Table 2). Only 1 patient experienced a possibly related grade 2 adverse event, hypertension, in the 1.0–1.5mg/kg cohort. All adverse events occurred on the first day of the study. No patients suffered infusion reactions or dermatologic toxicity as is commonly encountered with multiple or prolonged exposure to panitumumab [24].
Table 2:
Adverse Events
| Dose cohort | Total patients (n=81) | Number of AEsa (n=6) | Gradeb | Toxicity | Serious adverse event | Relationship to Panitumumab-IRDye800CW |
|---|---|---|---|---|---|---|
|
| ||||||
| <0.5 mg/kg | 12 | 1 (8.3%) | 1 | Prolonged QTc | No | Possible |
| 0.5–1.0 mg/kg | 35 | 1 (2.9%) | 1 | Prolonged QTc | No | Probable |
| 1.0–1.5 mg/kg | 12 | 1 (8.3%) | 2 | Hypertension | No | Possible |
| 1.5–2.0 mg/kg | 12 | - | - | - | - | - |
| 2.0–3.0 mg/kg | 10 | 1 (10%) | 1 | Presyncope | No | Possible |
| 1 (10%) | 1 | Prolonged QTc | No | Possible | ||
| 1 (10%) | 1 | Vomiting | No | Possible | ||
AE = adverse event
Grade 1 = mild, Grade 2 = moderate
The average percentage QTc interval change was 5% or less across, with no significant difference between the various doses and average percentage QTc interval change between the cohorts (Figure 2). QTc intervals of all patients returned to baseline at the follow-up ECG (15–30 days post infusion).
Figure 2: QTc interval changes.
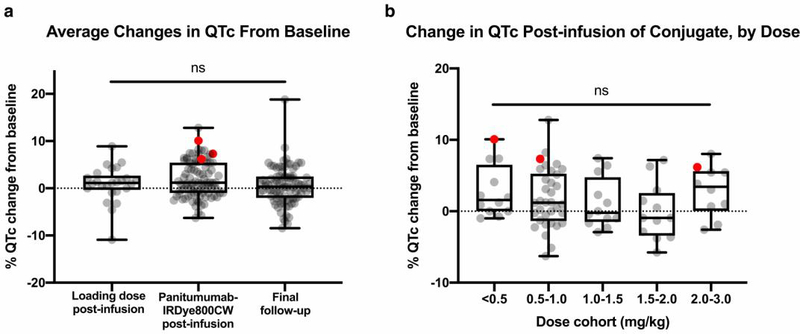
a) Changes in QTc was calculated as a percentage change of the QTc interval from baseline screening ECG and the following time points: after infusion of unconjugated antibody (loading dose) (95% CI [−0.68,2.45]), infusion of antibody-dye conjugate (95% CI [1.04,2.65]), and at final follow-up (95% CI [−0.35,1.32]). Red dots signify the % QTc changes of patients who experienced a prolonged QTc adverse event. b) Changes in QTc was calculated as a percentage change of the QTc interval from baseline screening ECG and post-infusion ECG, separated by dose. The <0.5, 0.5–1.0, 1.0–1.5, 1.5–2.0, and 2.0–3.0 mg/kg cohorts had 95% CIs [0.47, 5.09], [0.39, 3.25], [−1.12, 3.38], [−2.61, 2.45], and [0.39, 5.44], respectively.
Consistent with the known non-linear clearance properties of antibodies, a longer half-life was observed with increasing dose. A half-life of 14.5 hours was observed in the <0.5mg/kg cohort, which increased to 20.9 hours in the 0.5–1.0mg/kg cohort, 24.8 hours for the 1.0–1.5mg/kg cohort, 35.7 in the 1.5–2.0mg/kg cohort, and 33.3 hours in the 2.0–3.0mg/kg cohort (Table 3).
Table 3:
Pharmacokinetics of Panitumumab-IRDye800CW
| Dose cohort | Dose range (mg/kg) | % of therapeutic dosea | Total number of patients | Half-life, average (h) (SD) |
|---|---|---|---|---|
|
| ||||
| <0.5 mg/kg | 0.06–0.49 | 1.0–8.2% | 8 | 14.5 (4.12) |
| 0.5–1.0 mg/kg | 0.52–0.97 | 8.6–16.1% | 32 | 20.9 (5.52) |
| 1.0–1.5 mg/kg | 1.05–1.48 | 17.5–24.7% | 13 | 24.8 (6.9) |
| 1.5–2.0 mg/kg | 1.52–1.98 | 25.3–33.0% | 10 | 35.7 (11.4) |
| 2.0–3.0 mg/kg | 2.09–2.84 | 34.8–47.3% | 10 | 33.3 (5.4) |
Therapeutic dose = 6 mg/kg.
Stability
The percentage of panitumumab-IRDye800CW aggregates increased from 1.02% at 0 months to 7.26% at 54 months (Figure 3B). Percentage free dye fluctuated throughout the 54 months with no discernible trend but remained low (Figure 3C). The dye:protein ratio decreased from 2.0:1 at 0 months to 1.4:1 at 54 months (r2=0.93; p<0.0001), remaining within the reference ratio range of between 1 to 3 (Figure 3D). The protein concentration appeared to increase from 5.24mg/mL to 5.59mg/mL at the end of 54 months of testing (r2=0.68; p<0.00), surpassing the specification limit (5.0±0.5mg/mL) at 42 months (5.53 mg/mL) and 54 months (5.59 mg/mL) (Figure 3E). Binding potency relative to unconjugated panitumumab was found to be fluctuating due to the high inter-assay error (±30%), but this did appear to begin decreasing after 30 months (r2=0.20, p=0.17) (Figure 3F). With the exception of the protein concentration value, all parameters passed stability testing at 54 months. A representative SDS-PAGE gel validating the molecular identity of panitumumab-IRDye800CW has been previously published [23].
Figure 3: Stability testing of panitumumab-IRDye800CW.
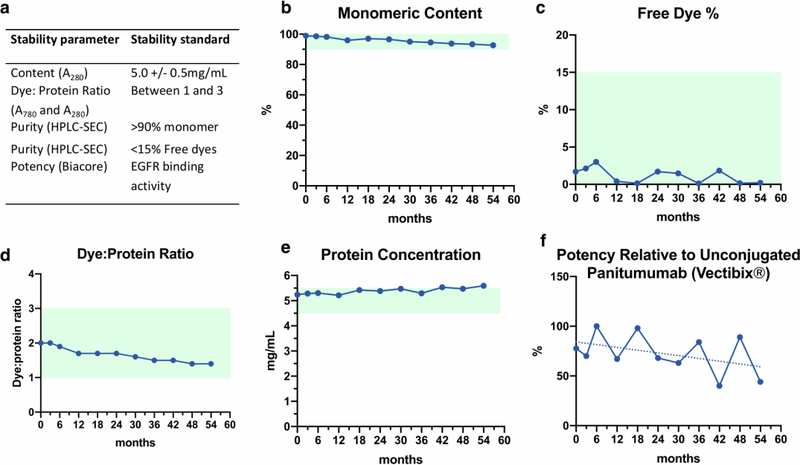
a) Stability parameters and standards. b) Purity measured by HPLC-SEC over 54 months (reference: monomer purity ≥90% of total protein). c) Percentage of free dye measured by HPLC-SEC over 54 months (reference: free dye ≤15%). d) Dye:protein ratio calculated by A780 and A280 (reference dye:protein ratio between 1 and 3). e) Protein concentration measured across 54 months by A280 (reference 5±0.5mg/mL). OOS at 42 (5.53 mg/mL) and 54 months (5.59 mg/mL). f) Binding activity relative to commercial non-conjugated panitumumab (Vectibix®) binding EGFR, as measured by BIAcore assay (r2=0.2, p=0.17).
DISCUSSION
Therapeutic antibodies including panitumumab, cetuximab and bevacizumab have been used as targeting agents for optical molecular imaging in a number of early phase clinical trials and preclinical verifications [19,25,26,27]. However, there is limited data on the long-term stability of these bioconjugates and their safety profile since most of the individual reports only include a dozen or more patients. In this study, we evaluate these parameters for panitumumab-IRDye800CW across 81 patients who received a single dose as part of surgical imaging trials. The doses varied from less than 0.5mg/kg up to almost 3.0mg/kg; the highest dose administered this represented 47.3% of the therapeutic dose for panitumumab (6mg/kg).
Aggregate formation increased over the 54 months of testing, a common phenomenon of protein pharmaceuticals [28–30], which contributed to measurement errors confounding the trends observed in protein concentration, dye:protein ratio, and binding potency. Importantly, aggregates are filtered out of solution before drug infusion into patients. The OOS investigation at 42 months concluded that the apparent increase in protein concentration was due to a measurement effect caused by increased aggregation, causing a higher effective extinction coefficient at 280nm, thus increasing the A280 absorbance measurement. FDA correspondence after the OOS investigation allowed continued use of the product if monomeric content stayed within specification, which it did. Notably, the investigation proposed that future lots be specified at the release value±0.5mg/mL, which, if adhered by this lot, would have rendered no OOS observations. We also observed a decreasing dye:protein ratio, but since the dyes are covalently bound to the antibody, and the percentage free dye remained unchangingly low, our data suggests that the observed decrease is due to fluorophore quenching from increasing aggregates [31,32]. The apparent downward trend of potency after the 30-month testing date was concluded to likely be due to aggregation as well [28–29]. Altogether, we conclude that panitumumab-IRDye800CW has at least the same stability period of the parental compound panitumumab, which is 24 months[31].
We previously demonstrated the superior safety profile of panitumumab-IRDye800CW (n=15) compared with cetuximab-IRDye800CW (n=12), a chimeric anti-EGFR-antibody, in a smaller population of patients [23]. The current study represents a 5-fold increase in sample size and increasing doses up to nearly half of the therapeutic dose as compared to our previous study [23]. However, the percentage of reported adverse events remained the same. We observed that adverse events did not escalate with increasing dose and were not consistent in nature – they included presyncope, vomiting, and hypertension. A prolonged QTc interval was the most common adverse event and was identified in 3.7% of patients (3 out of 81). Treatment trials in patients receiving therapeutic doses of panitumumab (6mg/kg) report a 5-fold higher rate of adverse events over the course of therapy [34]. No patients in our imaging study experienced dermatologic toxicities, which has been reported in 90% of patients receiving multiple doses of panitumumab for treatment [11,23,34]. Since we only gave panitumumab once and at less than half the therapeutic dose of panitumumab, we observed a significantly lower rate of adverse events compared to patients receiving panitumumab in a therapeutic setting.
The average half-life of panitumumab-IRDye800CW was found to be 24.6 hours, or approximately 1 day, with a range of 8.5–58.1 hours. The half-life was proportional to dose which is supported by previous studies validating the non-linear pharmacokinetics of antibody-dye conjugates [35]. A single dose of less than 50% of the therapeutic dose was delivered, and as a result the half-life was much lower as expected than that reported for therapeutic dose of panitumumab (7.5 days, range 3.6–10.9 days) [11].
CONCLUSION
Panitumumab-IRDye800CW exhibited few adverse events in the clinic with a half-life of approximately one day. GMP production of large batch panitumumab-IRDye800CW was found to be mostly stable over 54 months with out-of-specification results at 42 months and 54 months due to an observed apparent increase in protein content determined to be the result of protein aggregation over time. Stability was found to be non-inferior to panitumumab. FDA-approved therapeutic antibodies have well-characterized safety profiles and current evidence presented here suggest that the process of antibody-dye conjugation does not significantly change the safety profile. Given that we and others have demonstrated a simple production pathway [36], these therapeutic antibody-optical dye bioconjugates could eventually be considered for an accelerated approval process that does not require non-clinical toxicity studies to reduce cost and efficiently bring these agents to the clinic [37,38].
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the support of the NCI NExT Program, NIH R01 CA190306-01, the Stanford Molecular Imaging Scholars (SMIS) program (NIH T32CA118681), and the Netherlands Organization for Scientific Research (Rubicon; 019.171LW.022).
Footnotes
COMPETING INTERESTS
Eben Rosenthal has equipment loans from LI-COR. No other potential conflicts of interest relevant to this article exist.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Keulen S van, Nishio N, Fakurnejad S, et al. (2019) The Clinical Application of Fluorescence-Guided Surgery in Head and Neck Cancer. J Nucl Med doi: 10.2967/jnumed.118.222810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He K, Zhou J, Yang F, et al. (2018) Near-infrared Intraoperative Imaging of Thoracic Sympathetic Nerves: From Preclinical Study to Clinical Trial. Theranostics 8(2):304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishio N, van den Berg NS, van Keulen S, et al. (2019) Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat Commun. 10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haslam A, Prasad V. (2019) Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open. 2(5):e192535–e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventola CL. (2017) Cancer Immunotherapy, Part 3: Challenges and Future Trends. Pharm Ther. 42(8):514–521. [PMC free article] [PubMed] [Google Scholar]
- 6.Sambi M, Bagheri L, Szewczuk MR. (2019) Current Challenges in Cancer Immunotherapy: Multimodal Approaches to Improve Efficacy and Patient Response Rates. Gupta SC, ed. J Oncol. doi: 10.1155/2019/4508794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conner KP, Rock BM, Kwon GK, et al. (2014) Evaluation of Near Infrared Fluorescent Labeling of Monoclonal Antibodies as a Tool for Tissue Distribution. Drug Metab Dispos. 42(11):1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu G, Fakurnejad S, Martin BA, et al. (2020) Predicting Therapeutic Antibody Delivery into Human Head and Neck Cancers. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-19-3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutson S, Raja E, Bomgarden R, et al. (2016) Development and Evaluation of a Fluorescent Antibody-Drug Conjugate for Molecular Imaging and Targeted Therapy of Pancreatic Cancer. PLOS ONE. 11(6):e0157762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensch F, van der Veen EL, Lub-de Hooge MN, et al. (2018) 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 24(12):1852–1858. [DOI] [PubMed] [Google Scholar]
- 11.Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. (2007) FDA drug approval summary: panitumumab (Vectibix). The Oncologist. 12(5):577–583. [DOI] [PubMed] [Google Scholar]
- 12.Bethune G, Bethune D, Ridgway N, Xu Z. (2010) Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2(1):48–51. [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyankrishna S, Grandis JR. (2006) Epidermal Growth Factor Receptor Biology in Head and Neck Cancer. J Clin Oncol. 24(17):2666–2672. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira-Cunha M, Newman WG, Siriwardena AK. (2011) Epidermal Growth Factor Receptor in Pancreatic Cancer. Cancers. 3(2):1513–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saadeh FS, Mahfouz R, Assi HI. (2018) EGFR as a clinical marker in glioblastomas and other gliomas. Int J Biol Markers. 33(1):22–32. [DOI] [PubMed] [Google Scholar]
- 16.Adams KE, Ke S, Kwon S, et al. (2007) Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J Biomed Opt. 12(2):024017. [DOI] [PubMed] [Google Scholar]
- 17.Marshall MV, Draney D, Sevick-Muraca EM, Olive DM. (2010) Single-Dose Intravenous Toxicity Study of IRDye 800CW in Sprague-Dawley Rats. Mol Imaging Biol. 12(6):583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ter Weele EJ, Terwisscha van Scheltinga AGT, Linssen MD, et al. (2016) Development, preclinical safety, formulation, and stability of clinical grade bevacizumab-800CW, a new near infrared fluorescent imaging agent for first in human use. Eur J Pharm Biopharm. 104:226–234. [DOI] [PubMed] [Google Scholar]
- 19.Zinn KR, Korb M, Samuel S, et al. (2015) IND-Directed Safety and Biodistribution Study of Intravenously Injected Cetuximab-IRDye800 in Cynomolgus Macaques. Mol Imaging Biol. 17(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharyya S, Patel N, Wei L, et al. (2014) Synthesis and biological evaluation of panitumumab-IRDye800 conjugate as a fluorescence imaging probe for EGFR-expressing cancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.[Internet] U.S. Food and Drug Administration. (2019) E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. [PubMed] [Google Scholar]
- 22.Aldrich MB, Wang X, Hart A, et al. (2011) Assessment of Free Dye in Solutions of Dual-Labeled Antibody Conjugates for In Vivo Molecular Imaging. Mol Imaging Biol. 13(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao RW, Teraphongphom N, de Boer E, et al. (2018) Safety of panitumumab-IRDye800CW and cetuximab-IRDye800CW for fluorescence-guided surgical navigation in head and neck cancers. Theranostics. 8(9):2488–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. (2007) FDA Drug Approval Summary: Panitumumab (Vectibix™). The Oncologist. 12(5):577–583. [DOI] [PubMed] [Google Scholar]
- 25.Day KE, Beck LN, Deep NL, Kovar J, Zinn KR, Rosenthal EL. (2013) Fluorescently labeled therapeutic antibodies for detection of microscopic melanoma. The Laryngoscope. 123(11):2681–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamberts LE, Koch M, Jong JS de, et al. (2017) Tumor-Specific Uptake of Fluorescent Bevacizumab–IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin Cancer Res. 23(11):2730–2741. [DOI] [PubMed] [Google Scholar]
- 27.Marston JC, Kennedy GD, Lapi SE, et al. (2019) Panitumumab-IRDye800CW for Fluorescence-Guided Surgical Resection of Colorectal Cancer. J Surg Res. 239:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vázquez-Rey M, Lang DA. (2011) Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng. 108(7):1494–1508. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg AS. (2006) Effects of protein aggregates: An immunologic perspective. AAPS J. 8(3):E501–E507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts CJ. (2014) Protein Aggregation and Its Impact on Product Quality. Curr Opin Biotechnol. 0:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabó Á, Szendi-Szatmári T, Ujlaky-Nagy L, et al. (2018) The Effect of Fluorophore Conjugation on Antibody Affinity and the Photophysical Properties of Dyes. Biophys J. 114(3):688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schobel U, Egelhaaf H-J, Fröhlich D, Brecht A, Oelkrug D, Gauglitz G. (2000) Mechanisms of Fluorescence Quenching in Donor—Acceptor Labeled Antibody—Antigen Conjugates. J Fluoresc. 10(2):147–147. [Google Scholar]
- 33.Amgen Inc. (2009) Vectibix (panitumumab) [package insert]. Thousand Oaks, CA. [Google Scholar]
- 34.Fakih M, Vincent M. (2010) Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr Oncol. 17(Suppl 1):S18–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal EL, Warram JM, de Boer E, et al. (2015) Safety and Tumor-specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 21(16):3658–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linssen MD, Weele EJ ter, Allersma DP, et al. (2019) Roadmap for the Development and Clinical Translation of Optical Tracers Cetuximab-800CW and Trastuzumab-800CW. J Nucl Med. 60(3):418–423. [DOI] [PubMed] [Google Scholar]
- 37.Scheuer W, van Dam GM, Dobosz M, Schwaiger M, Ntziachristos V. (2012) Drug-based optical agents: infiltrating clinics at lower risk. Sci Transl Med. 4(134):134ps11. [DOI] [PubMed] [Google Scholar]
- 38.Tummers WS, Warram JM, Tipirneni KE, et al. (2017) Regulatory Aspects of Optical Methods and Exogenous Targets for Cancer Detection. Cancer Res. 77(9):2197–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.