Abstract
Background
Although the Pfizer-BioNTech (BNT162b2), Oxford-AstraZeneca (ChAdOx1 nCoV-19), Sinopharm (BBIBP-CorV), and Sputnik V coronavirus disease 2019 (COVID-19) vaccines have been granted emergency approval in many nations, their safety has never been studied and compared in one community-based study. This study aimed to investigate and compare the incidence, nature, severity, and predictors of adverse events following immunization (AEFIs) with COVID-19 vaccines.
Method
This was a prospective observational study conducted in Jordan between 1 January and 21 September 2021. A team of pharmacists and nurses (n = 407) collected the local and systemic AEFIs of four COVID-19 vaccines by prospectively contacting participants registered in the national vaccination program platform. A red-flag technology was inserted to classify and track rare and serious AEFIs.
Results
This study included 658,428 participants who were vaccinated with 1,032,430 doses; 610,591, 279,606, 140,843, and 1390 participants received the first and second doses of the BNT162b2, BBIBP-CorV, ChAdOx1 nCoV-19, and Sputnik V vaccines, respectively. The overall incidence of AEFIs was 28.8%, and the overall rates of systemic, local, and immediate hypersensitivity AEFIs were 22.2%, 18.8%, and 0.5%, respectively. The highest proportions of immediate hypersensitivity AEFIs and systemic AEFIs were reported after administration of the Sputnik V vaccine and ChAdOx1 nCoV-19 first dose, respectively. The most severe AEFIs were reported after ChAdOx1 nCoV-19 first dose and BNT162b2 second dose. The hospitalization and mortality rates after vaccination were 20 in 10,000 and 1 in 10,000, respectively. Based on red-flag tracking, the top three outcome events were lymphadenopathy (157.9/100,000), anxiety disorders (136.6/100,000), and lower respiratory tract infection (100.9/100,000), with Guillain-Barré syndrome (1.8/100,000), vasculitis (3.0/100,000), and myopericarditis (4.8/100,000) being the least common.
Conclusion
The incidence rates of local, systemic, and immediate hypersensitivity AEFIs of four COVID-19 vaccines occur frequently. High incidence rates of rare and serious AEFIs were reported in this study. Younger participants, females, those who had previously had COVID-19, and smokers were more likely to encounter AEFIs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-022-01191-1.
Key Points
| The rates of hospitalization and mortality after vaccination were high in this study. Our findings indicate that swelling in the lymph nodes, as well as erectile dysfunction, are more likely to be seen after the first dose of ChAdOx1 nCoV-19 compared with other vaccines. |
| The findings of this study report that younger participants, females, those who had previously had COVID-19, and smokers were more likely to encounter adverse events following immunization (AEFIs). |
| Sputnik V was significantly associated with higher rates of overall AEFIs and immediate hypersensitivity reactions than other vaccines. |
Introduction
Globally, there are two pathways to tackle a pandemic—reducing contact (e.g. via lockdowns) and via vaccination. The latter is favored by most governments as a medium- to long-term solution because it has a smaller impact on economic outcomes and is more socially acceptable.
Nevertheless, global efforts faced a critical moment with the appearance of new coronavirus disease 2019 (COVID-19) variants. Given their increased transmission and severity [1], Alpha (B.1.1.7), Beta (B.1.351), Delta (B.1.617.2), Gamma (P.1), and Omicron (B.1.1.529) variants posed new challenges. The latter is the most concerning variant given the large number of mutations in its structure, which probably increases the risk of re-infection [2].
Another concern is the safety profile of COVID-19 vaccines. Adverse events following immunization (AEFIs) can be defined as “any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine” [3]. Menni et al. [4] investigated AEFIs for the Pfizer-BioNTech (BNT162b2) and Oxford-AstraZeneca (ChAdOx1 nCoV-19) COVID-19 vaccines in the UK and found that the rates of systemic and local AEFIs were lower than those reported in clinical trials—for example, that reported by Polack and colleagues [5]. The reported AEFIs were more likely to appear in specific groups, such as younger individuals, females, and those with previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Barda et al. [6] found that BNT162b2 may increase the risk of myocarditis, lymphadenopathy, and appendicitis. In the US, an active surveillance system, implemented in late 2020, found that rates of serious AEFIs associated with the second dose of the Pfizer-BioNTech vaccine and the first dose of the Moderna COVID-19 vaccine were 21.2% and 18.8%, respectively [7]. The findings of UK and US studies highlight the necessity for postmarketing surveillance-based studies worldwide.
In this large-scale study, and for the first time, 407 healthcare professionals prospectively observed the adverse events following administration of 1,032,430 doses of an RNA-based vaccine (BNT162b2), inactivated vaccine (BBIBP-CorV), and viral vector vaccines (ChAdOx1 nCoV-19 and Sputnik V). Between December 2020 and April 2021, Jordan, a small country in the Middle East, approved the emergency use of the Pfizer-BioNTech, Sinopharm (BBIBP-CorV), Sputnik V, and Oxford-AstraZeneca vaccines. To July 2022, 10 million doses had been administered in Jordan and more than 44.6% of its population were fully vaccinated [8]. Vaccination in Jordan started among frontliners and elderly subjects, then among young adults. In September 2021, the government initiated a broad vaccination campaign for students aged between 12 and 17 years [8]. Nevertheless, given the scarcity of postmarketing surveillance-based studies that nationally observe and measure the AEFIs of COVID-19 vaccines, approving the emergency use of these vaccines can be a risky step, and, consequently, governmental decisions await clear-cut evidence.
Objective
This study aimed to assess and compare the safety of four types of vaccines (BNT162b2, BBIBP-CorV, ChAdOx1 nCoV-19, and Sputnik V) through evaluating the incidence, types, severity, and predictors of AEFIs in Jordan over 8 months (between 1 January and 1 September 2021).
Methods
Ethics Approval
The study was approved by the Ethics Research Committee of the Jordanian Ministry of Health (REC-MOH-7722).
Subjects and Settings
In early 2021, the National Pharmacovigilance (PV) Committee was established by the Ministry of Health in Jordan and a national vaccination PV register was subsequently created. This register is an online platform encompassing a broad range of information, partly retrieved from the national COVID-19 vaccination program platform, about vaccinated individuals. Any individual intending to receive the vaccine has to register on the national COVID-19 vaccination program platform, filling out personal and medical information then receiving a vaccination appointment message on their mobile phone and eventually receiving a vaccination certificate after receiving the vaccine. This study included all individuals aged > 18 years who received at least one dose of a COVID-19 vaccine and who appeared on the register until 1 September 2021. People diagnosed with mental disorders that could potentially impair their ability to interact with researchers were excluded from the study. Because differentiation between the vaccines’ adverse events and symptoms related to participants’ health conditions was not feasible through telephone calls, having specific health conditions was not an exclusion criterion.
Study Flow
A team of healthcare professionals prospectively collected the AEFIs of four COVID-19 vaccines by way of phone calls. The number of data collectors totaled 407 pharmacists and nurses. Data collectors were trained on vaccine safety and how to collect data via an electronic data reporting form, which included questions about types, severity, date, duration, and outcomes of AEFIs. The principal investigator of this study participated in developing the national vaccination PV register and training the data collectors. Data collectors had full access to the national vaccination PV register, which enabled them to observe information such as participants’ name, sex, age, nationality, and phone number, as well as governorate and number of vaccine doses received. Telephone calls were made to all individuals who had received at least one dose of a COVID-19 vaccine. Each participant received at least one call in which verbal consent was sought, and were called within 2 weeks of vaccination. It was practically impossible to call all participants within the same time frame. The mean time for the calls was 5.42 days after the first dose and 6.91 days after the second dose. Duplicate calls were prevented by allocating a group of participants to each data collector, and then an independent researcher checked responses and filtered the data for any duplicates. Participants were asked whether they encountered any of the electronically listed AEFIs, i.e. systemic (headache, fever, shortness of breath, fatigue, myalgia, arthralgia, nausea, vomiting), local (pain, redness, swelling), or immediate hypersensitivity reaction (urticaria, angioedema, anaphylactic shock) [9]. Participants were also asked whether they experienced any different AEFIs from the listed adverse events (completed a designated separate box in the reporting form). Rare AEFIs was defined as any AEFI that “occurs in <1/1000 but >1/10,000 individuals” [10]. Regarding rare adverse events, participants were asked whether they had a history of the outcome, and if yes, their response was excluded. Participants were also asked to rate the severity of the AEFIs on a 3-point scale: 0–1 indicates low, 2 indicates medium, and 3 indicates high. We adopted Hartwig’s severity assessment in reporting AEFIs [11]. Participants were also asked if they were vaccinated against the seasonal influenza virus. Information technology technicians inserted a tool that automatically marked ‘red flags’ on participants who matched a set of criteria prespecified by the Pharmacovigilance Committee (electronic supplementary material [ESM] P1). Red flags were only taken into account for the analysis if they occurred within 14 days after any dose. Individuals with red flags were contacted again on a weekly basis and their clinical outcomes recorded. The outcomes of the red flags were only documented if confirmed by a medical doctor. Those who did not answer the call were contacted again, up to three further times. Mortality cases were reported either via national records or through relatives. Vaccinated individuals were also able to passively report AEFIs through the national register; however, to avoid data bias, we included only serious or rare AEFIs from passive reporting in the data analysis. The outcomes of the study were:
Incidence of AEFIs.
Types of AEFIs.
Severity of AEFIs.
Incidence of hospitalization after vaccination.
Mortality within 3 months of vaccination.
Comparison between the four vaccines in incidence, type, and severity of AEFIs.
Predictors of AEFIs.
Data Analysis
All data collected from the national vaccinated PV register were exported to a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA), preprocessed and cleaned, then exported to SPSS (IBM Corporation, Armonk, NY, USA) and coded for analysis. Data were entered into SPSS version 26 for statistical analysis. To test differences in age across participants using different vaccines, the one-way analysis of variance (ANOVA) test was used, and to measure differences in categorical variables, the Chi-square test was used. A multivariable logistic regression was constructed to measure predictors for AEFIs. In this model, the dependent variable was the occurrence of AEFI, while independent variables were age (> 65 years vs. < 65 years), previous COVID-19 infection (yes vs. no), sensitive to a drug (yes vs. no), sensitive to food (yes vs. no), sex, influenza vaccination, and smoking (smokers vs. non-smokers). Data are presented as count with proportions for categorical variables and as means with standard deviation for continuous variables. Adjusted odds ratios (aORs) were calculated and displayed with 95% confidence intervals (CIs). For logistic regression, entry was set at 0.05 and removal at 0.1 using the backward Wald method. aORs with 95% CIs were estimated. Checking for collinearity was carried out using tolerance and the variance inflation factor. A p value < 0.05 was considered statistically significant.
Results
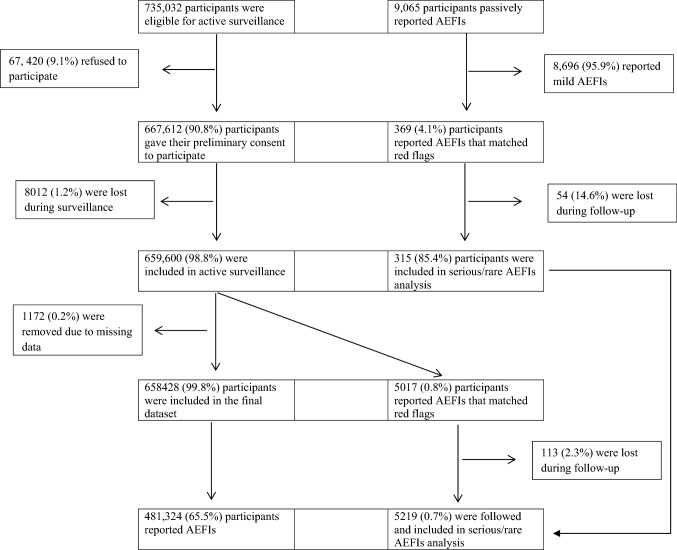
Classification of the study population is illustrated in Fig. 1.
Fig. 1.
Flow chart of the study population. AEFIs adverse events following immunization
Following data cleaning, the study included 1,032,430 vaccine doses administered between 1 January and 31 August 2021, to 658,428 persons, of whom 610,591, 279,606, 140,843, and 1390 were administered both the first and second doses of the BNT162b2, BBIBP-CorV, ChAdOx1 nCoV-19, and Sputnik V vaccines, respectively (Table 1). Participants who had the ChAdOx1 nCoV-19 vaccine were older and were more likely to be female and to have chronic conditions such as heart disease, diabetes, and hypertension than participants who had the other vaccines. The prevalence of smoking was relatively high across all participants, but was highest among those participants who received the Sputnik V vaccine and lowest among those who received the ChAdOx1 nCoV-19 vaccine. Approximately two-thirds (62.9%) and one-fifth (20.9%) of participants who received the Sputnik V vaccine were smokers and had experienced past COVID-19 infection, respectively.
Table 1.
Sociodemographic characteristics
| Variable | BNT162b2 | BBIBP-CorV | ChAdOx1 nCoV-19 | Sputnik V | ||||
|---|---|---|---|---|---|---|---|---|
| First dose [n = 418,517] | Second dose [n = 192,074] | First dose [n = 158,424] | Second dose [n = 121,182] | First dose [n = 80,281] | Second dose [n = 60,562] | First dose [n = 1206] | Second dose [n = 184] | |
| Sexa,b,c | ||||||||
| Female | 163,495 (39.1) | 72,724 (37.9) | 56,437 (35.6) | 42,636 (35.2) | 46,644 (58.1) | 27,427 (45.3) | 190 (15.8) | 33 (17.9) |
| Male | 255,022 (60.9) | 119,350 (62.1) | 101,987 (64.4) | 78,546 (64.8) | 33,637 (41.9) | 33,135 (54.7) | 1016 (84.2) | 151 (82.1) |
| Age, years [mean (SD)] | 33.6 (13.7) | 36.6 (13.6) | 36.5 (14.7) | 36.8 (14.8) | 44.2 (10.6) | 44.8 (10.9) | 31.2 (8.3) | 31.7 (8.8) |
| Geographic origin | ||||||||
| Arab | 413,723 (98.9) | 190,362 (99.1) | 154,416 (97.5) | 120,537 (99.5) | 77,274 (96.3) | 59,259 (97.8) | 1047 (86.8) | 173 (94.0) |
| Asian | 2574 (0.6) | 806 (0.4) | 2036 (1.3) | 1022 (0.8) | 1122 (1.4) | 482 (0.8) | 102 (8.4) | 8 (4.3) |
| African | 1331 (0.3) | 596 (0.3) | 1177 (0.7) | 684 (0.6) | 1432 (1.8) | 503 (0.8) | 33 (2.7) | 3 (1.6) |
| European | 384 (0.1) | 203 (0.1) | 459 (0.3) | 336 (0.3) | 266 (0.3) | 174 (0.3) | 14 (1.2) | 0 (0.0) |
| Others | 505 (0.1) | 107 (0.1) | 336 (0.2) | 291 (0.2) | 187 (0.2) | 144 (0.2) | 10 (0.8) | 0 (0.0) |
| Location of residencec | ||||||||
| North of Jordan | 103,154 (24.6) | 44,298 (23.1) | 30,907 (19.5) | 14,022 (11.6) | 14,181 (17.7) | 3699 (6.1) | 77 (6.4) | 16 (8.7) |
| South of Jordan | 55,883 (13.4) | 26,990 (14.1) | 15,609 (9.8) | 6552 (5.4) | 5772 (7.2) | 1896 (3.1) | 18 (1.5) | 2 (1.1) |
| Centre of Jordan | 259,480 (62.0) | 120,786 (62.9) | 111,908 (70.6) | 100,608 (83.0) | 60,328 (75.1) | 54,967 (90.8) | 1111 (92.1) | 166 (90.2) |
| Previous COVID-19 infectiona,b,c | 68,715 (16.4) | 32,796 (17.1) | 32,175 (20.3) | 25,407 (21.0) | 7899 (9.8) | 6151 (10.2) | 252 (20.9) | 27 (14.7) |
| Vaccinated for seasonal influenza | 24,752 (5.9) | 13,170 (6.9) | 11,293 (7.1) | 8930 (7.4) | 5874 (7.3) | 4556 (7.5) | 88 (7.3) | 30 (16.3) |
| Smokera,b,c | 158,026 (37.8) | 78,227 (40.7) | 69,864 (44.1) | 45,882 (37.9) | 28,567 (35.6) | 24,872 (41.1) | 758 (62.9) | 88 (47.8) |
| Sensitive to a drug | 8369 (2.0) | 3737 (1.9) | 7340 (4.6) | 4085 (3.4) | 2187 (2.7) | 1583 (2.6) | 22 (1.8) | 4 (2.2) |
| Sensitive to food | 7051 (1.7) | 9799 (1.6) | 3763 (2.4) | 6240 (2.2) | 1371 (1.7) | 2273 (1.6) | 25 (2.1) | 31 (2.2) |
| Pre-existing chronic conditions | ||||||||
| Cardiovascular diseases | 10,246 (2.4) | 5662 (2.9) | 7333 (4.6) | 6807 (5.6) | 4093 (5.1) | 2557 (4.2) | 21 (1.7) | 6 (3.2) |
| Diabetes mellitus. | 16,163 (3.9) | 4399 (2.3) | 10,001 (6.3) | 4874 (4.0) | 6428 (8.0) | 3522 (5.8) | 28 (2.3) | 4 (2.2) |
| Chronic kidney disease | 1339 (0.3) | 266 (0.1) | 978 (0.6) | 382 (0.3) | 324 (0.4) | 114 (0.2) | 4 (0.3) | 1 (0.5) |
| Chronic liver disease | 612 (0.1) | 215 (0.1) | 379 (0.2) | 136 (0.1) | 184 (0.2) | 64 (0.1) | 0 (0.0) | 0 (0.0) |
| Chronic blood diseases | 1451 (0.3) | 336 (0.2) | 834 (0.5) | 174 (0.1) | 401 (0.5) | 144 (0.2) | 3 (0.2) | 0 (0.0) |
| Chronic digestive system diseases | 1671 (0.4) | 482 (0.3) | 733 (0.5) | 401 (0.3) | 431 (0.5) | 144 (0.2) | 21 (1.7) | 5 (2.7) |
| Nervous system diseases | 1043 (0.2) | 338 (1.8) | 445 (0.3) | 230 (0.2) | 234 (0.3) | 95 (0.2) | 2 (0.2) | 0 (0.0) |
| Bone diseases | 2282 (0.5) | 1055 (0.5) | 1131 (0.8) | 620 (0.5) | 1036 (1.3) | 310 (0.5) | 6 (0.5) | 1 (0.5) |
| Chronic respiratory diseases | 3229 (0.8) | 988 (0.5) | 2019 (1.3) | 701 (0.6) | 734 (0.9) | 196 (0.3) | 15 (1.2) | 3 (1.6) |
| Cancer | 1045 (0.2) | 369 (0.2) | 798 (0.5) | 465 (0.4) | 439 (0.5) | 144 (0.2) | 2 (0.2) | 0 (0.0) |
| HIV | 898 (0.2) | 415 (0.2) | 614 (0.4) | 287 (0.2) | 215 (0.3) | 72 (0.1) | 2 (0.2) | 0 (0.0) |
Data are expressed as n (%) unless otherwise specified
For categorical variables, the Chi-square test was applied, and for continuous variables, the independent t-test was applied.
SD standard deviation, COVID-19 coronavirus disease 2019
aThe difference between the first dose of BNT162b2 and the first dose of BBIBP-CorV was significant (p < 0.05)
bThe difference between the first dose of BBIBP-CorV and the first dose of ChAdOx1 nCoV-19 was significant (p < 0.05)
cThe difference between the first dose of ChAdOx1 nCoV-19 and the first dose of Sputnik V was significant (p < 0.05)
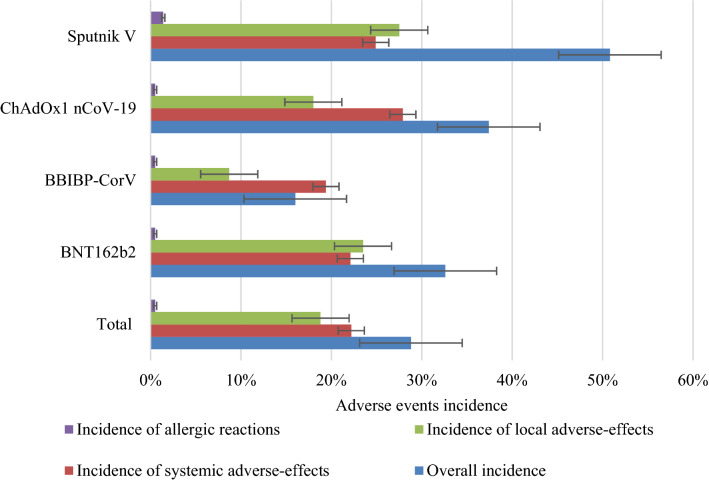
The overall incidence of adverse events experienced after full vaccination was 28.8% (Table 2). The overall rates of systemic, local, and immediate hypersensitivity AEFIs were 22.2%, 18.8%, and 0.5%, respectively. The overall incidence of adverse events caused by the Sputnik V vaccine was significantly higher than those caused by the other vaccines (Fig. 2). Of the 658,428 individuals included in this study, 1008 (20/10,000) participants were hospitalized after vaccination, of whom 488 (48.4%) were hospitalized after the first dose. The highest rates of hospitalization were seen in individuals who received the Sputnik V and ChAdOx1 nCoV-19 vaccines. We documented 93 (1/10,000) mortality cases within 3 months of vaccination, of which, 11 (0.2/10,000) had no pre-existing health conditions. The mean age of patients who deceased and had no pre-existing health conditions was 72.1 (± 12.5) years, while the mean of the elapsed time between vaccination and death among patients who had no pre-existing health conditions was 18.6 (± 11.4) days.
Table 2.
Rates of adverse events
| Incidences | All vaccines | BNT162b2 | BBIBP-CorV | ChAdOx1 nCoV-19 | Sputnik V | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total [N = 1,032,430] | First dose [n = 658,427] | Second dose [n = 374,002] | Total [N = 610,591) | First dose [n = 418,517] | Second dose [n = 192,074] | Total [N = 279,606] | First dose [n = 158,424] | Second dose [n = 121,182] | Total [N = 140,843] | First dose [n = 80,281] | Second dose [n = 60,562] | Total [N = 1390] | First dose [n = 1206] | Second dose [n = 184] | |
| Overall incidence | 296,875 (28.8) | 198,511 (30.1) | 98,364 (26.3) | 198,831 (32.6) | 136,812 (32.7) | 62,019 (32.3) | 44,697 (16) | 25,129 (15.9) | 19,568 (16.1) | 52,641 (37.4) | 35,936 (44.8) | 16,705 (27.6) | 706 (50.8) | 634 (52.6) | 72 (39.1) |
| Incidence of systemic adverse effects | 228,874 (22.2) | 147,147 (22.3) | 81,727 (21.9) | 135,006 (22.1) | 91,691 (21.9) | 43,315 (22.6) | 54,164 (19.4) | 30,663 (19.4) | 23,501 (19.4) | 39,358 (27.9) | 24,491 (30.5) | 14,867 (24.5) | 346 (24.9) | 302 (25.0) | 44 (23.9) |
| Incidence of local adverse effects | 193,806 (18.8) | 135,895 (20.6) | 57,911 (15.5) | 143,772 (23.5) | 104,492 (25.0) | 39,280 (20.5) | 24,304 (8.7) | 14,164 (8.9) | 10,140 (8.4) | 25,348 (18.0) | 16,887 (21.0) | 8461 (14.0) | 382 (27.5) | 352 (29.2) | 30 (16.3) |
| Incidence of allergic reactions | 5491 (0.5) | 3411 (0.5) | 2080 (0.6) | 3247 (0.5) | 2043 (0.5) | 1204 (0.6) | 1454 (0.5) | 834 (0.5) | 620 (0.5) | 770 (0.5) | 515 (0.6) | 255 (0.4) | 20 (1.4) | 19 (1.6) | 1 (0.5) |
Data are expressed as n (%)
The overall rate of adverse events caused by the Sputnik V vaccine was significantly higher than those caused by other vaccines
The rate of allergic adverse events caused by the Sputnik V vaccine was significantly higher than those caused by other vaccines (adjusted p < 0.005)
The incidence of systemic adverse events caused by the ChAdOx1 nCoV-19 vaccine first dose was significantly higher than those caused by other vaccines (adjusted p < 0.005)
The rate of local adverse events caused by the BBIBP-CorV vaccine was significantly lower than those caused by other vaccines (adjusted p < 0.005)
Fig. 2.
Rates of adverse events, stratified by the type of adverse event. Rates in each category are reported in the appendix. Error bars account for 95 confidence intervals
Overall, fatigue, fever, and headache were the most frequently documented systemic adverse events (Table 3). Pain at the site of injection and urticaria were the most commonly reported local and immediate hypersensitivity adverse events, respectively. When comparing the ChAdOx1 nCoV-19 vaccine first dose with first doses of the other vaccines, the highest rates of erectile dysfunction (0.5%) and swollen lymph nodes (2.2%) were seen after the ChAdOx1 nCoV-19 vaccine first dose (adjusted p < 0.005).
Table 3.
Incidence of adverse events following immunization of COVID-19 vaccines
| Adverse event | BNT162b2 | BBIBP-CorV | ChAdOx1 nCoV-19 | Sputnik V | ||||
|---|---|---|---|---|---|---|---|---|
| First dose [n = 418,517] | Second dose [n = 192,074] | First dose [n = 158,424] | Second dose [n = 121,182] | First dose [n = 80,281] | Second dose [n = 60,562] | First dose [n = 1206] | Second dose [n = 184] | |
| Adverse event categories | ||||||||
| Systemic adverse effectsb,c,d,e | ||||||||
| Headache | 41,333 (9.9) | 21,873 (11.4) | 9046 (5.7) | 6405 (5.4) | 16,578 (20.6) | 6025 (10.1) | 287 (23.8) | 163 (11.4) |
| Fever | 43,123 (10.3) | 25,934 (13.5) | 6759 (4.3) | 4214 (3.5) | 22,871 (28.5) | 5804 (9.6) | 329 (27.3) | 39 (21.2) |
| Shortness of breath | 3969 (0.9) | 2582 (1.3) | 1211 (0.8) | 900 (0.7) | 1822 (2.3) | 535 (0.9) | 54 (4.3) | 3 (1.6) |
| Drowsiness | 2741 (0.7) | 2650 (1.4) | 852 (0.5) | 695 (0.6) | 3062 (3.8) | 1258 (2.1) | 14 (1.2) | 3 (1.6) |
| Vertigo | 2330 (0.6) | 2061 (1.1) | 513 (0.3) | 485 (0.4) | 988 (1.2) | 602 (1.0) | 16 (1.3) | 1 (0.5) |
| Chills | 1579 (0.4) | 3520 (1.8) | 628 (0.4) | 501 (0.4) | 2206 (2.7) | 974 (1.6) | 18 (1.5) | 5 (2.7) |
| Chest pain | 986 (0.2) | 2018 (1.1) | 392 (0.2) | 210 (0.2) | 1020 (1.3) | 477 (0.8) | 21 (1.7) | 7 (3.8) |
| Back pain | 1761 (0.4) | 4036 (2.1) | 853 (0.5) | 386 (0.3) | 4150 (5.1) | 1175 (1.9) | 38 (3.2) | 5 (2.7) |
| Toothache | 511 (0.1) | 1741 (0.9) | 295 (0.2) | 147 (0.1) | 1448 (1.8) | 528 (0.9) | 14 (1.2) | 6 (3.2) |
| Sore throat | 1468 (0.4) | 1892 (1.0) | 527 (0.3) | 256 (0.2) | 2771 (3.5) | 475 (0.8) | 8 (0.7) | 1 (0.5) |
| Tachycardia | 369 (0.1) | 1203 (0.6) | 98 (0.1) | 49 (0.0) α | 1952 (2.4) | 286 (0.5) | 11 (0.9) | 0 (0.0) |
| Insomnia | 1182 (0.3) | 2503 (1.3) | 688 (0.4) | 354 (0.3) | 4702 (5.9) | 2558 (4.2) | 33 (2.7) | 14 (7.6) |
| Numbness in different parts of the body | 3299 (0.8) | 4802 (2.5) | 472 (0.3) | 257 (0.2) | 3428 (4.2) | 1085 (1.8) | 41 (3.4) | 1 (0.5) |
| Vision loss | 0 (0.0) | 1 (0.0)α | 0 (0.0) | 0 (0.0) | 8 (0.0)a | 0 (0.0) | 1 (0.1) | 0 (0.0) |
| Blurred vision | 328 (0.1) | 255 (0.1) | 199 (0.1) | 175 (0.1) | 856 (1.1) | 462 (0.8) | 6 (0.5) | 3 (1.6)_ |
| Uncontrolled laughing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.0)a | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Urinary incontinence | 2011 (0.5) | 674 (0.4) | 221 (0.1) | 133 (0.1) | 3963 (4.9) | 1320 (2.2) | 19 (1.6) | 8 (4.3) |
| Cough | 2866 (0.7) | 1472 (0.8) | 206 (0.1) | 145 (0.1) | 2460 (3.1) | 1885 (3.1) | 3 (0.2) | 0 (0.0) |
| Brain fog | 449 (0.1) | 152 (0.1) | 122 (0.1) | 46 (0.0)a | 5103 (6.4) | 2993 (4.9) | 47 (3.9) | 3 (1.6) |
| Hair loss | 166 (0.0)a | 43 (0.0)a | 71 (0.0)a | 68 (0.0)a | 432 (0.5) | 177 (0.3) | 2 (0.2) | 0 (0.0) |
| Epistaxis | 368 (0.1) | 196 (0.1) | 53 (0.0)a | 38 (0.0)a | 2946 (3.7) | 882 (1.5) | 14 (1.2) | 2 (1.1) |
| Rash | 3274 (0.8) | 2454 (1.2) | 396 (0.2) | 142 (0.1) | 3660 (4.6) | 1108 (1.8) | 15 (1.2) | 1 (0.5) |
| Diarrhea | 1755 (0.4) | 625 (0.3) | 462 (0.3) | 156 (0.1) | 2980 (3.7) | 1650 (2.7) | 5 (0.4) | 3 (1.6) |
| Swollen testicles | 22 (0.0)a | 39 (0.0)a | 0 (0.0) | 1 (0.0)a | 65 (0.1) | 17 (0.0)a | 2 (0.2) | 0 (0.0) |
| Erectile dysfunction | 8 (0.0)a | 106 (0.1)a | 1 (0.0)a | 2 (0.0)a | 176 (0.5) | 46 (0.1) | 1 (0.1) | 0 (0.0) |
| Lack of appetite | 269 (0.1) | 330 (0.2) | 189 (0.1) | 145 (0.1) | 634 (0.8) | 485 (0.8) | 5 (0.4) | 3 (1.6) |
| Bruising throughout the body | 144 (0.0)a | 256 (0.1) | 32 (0.0)a | 5 (0.0)a | 852 (1.1) | 241 (0.4) | 17 (1.4) | 0 (0.0) |
| Gastric pain | 1656 (0.4) | 1896 (1.0) | 158 (0.1) | 142 (0.1) | 2115 (2.6) | 1053 (1.7) | 8 (0.7) | 0 (.0) |
| Severe eye pain | 1330 (0.3) | 1251 (0.7) | 428 (0.3) | 134 (0.1) | 1063 (1.3) | 263 (0.4) | 3 (0.2) | 1 (0.5) |
| Syncope | 98 (0.0)a | 118 (0.1) | 3 (0.0)a | 0 (0.0) | 156 (0.2) | 14 (0.0)a | 1 (0.1) | 0 (0.0) |
| Sudden weight gain | 43 (0.0)a | 5 (0.0)a | 1(0.0)a | 3 (0.0)a | 53 (0.1) | 9 (0.0)a | 0 (0.0) | 0 (0.0) |
| Sudden weight loss | 0 (0.0) | 7 0 (0.0)a | 0 (0.0) | 0 (0.0) | 119 (0.1) | 23 (0.0)a | 0 (0.0) | 1 (0.5) |
| Swelling in the lower limbs | 186 (0.0)a | 141 (0.1) | 22 (0.0)a | 38 (0.0)a | 269 (0.3) | 135 (0.2) | 14 (1.2) | 1 (0.5) |
| Swelling in lymph nodes in the armpit | 563 (0.1) | 457 (0.2) | 36 (0.0)a | 0 (0.0)a | 1765 (2.2) | 441 (0.7) | 3 (0.2) | 0 (0.0) |
| Swelling in the cervical lymph nodes | 325 (0.1) | 387 (0.2) | 18 (0.0)a | 0 (0.0)a | 523 (0.7) | 115 (0.2) | 0 (0.0) | 3 (1.6) |
| Menstrual cycle irregularity | 756 (0.4) | 1423 (1.9) | 174 (0.3) | 111 (0.3) | 967 (2.1) | 155 (0.6) | 1 (0.5) | 2 (6.1) |
| Fatigue | 60,409 (14.4) | 35,081 (18.3) | 11,659 (7.4) | 9462 (7.8) | 24,293 (30.3) | 9576 (15.8) | 399 (34.1) | 43 (23.8) |
| Myalgia | 17,849 (4.3) | 12,336 (6.4) | 3186 (2.0) | 2680 (2.2) | 11,758 (14.6) | 3279 (5.4) | 166 (13.8) | 22 (12.0) |
| Arthralgia | 22,831 (5.5) | 16,902 (8.8) | 4243 (2.7) | 3574 (2.9) | 16,337 (20.3) | 4514 (7.5) | 251 (20.8) | 21 (11.4) |
| Nausea and vomiting | 6745 (1.6) | 4159 (2.2) | 1355 (0.9) | 1005 (0.8) | 2525 (3.1) | 699 (1.2) | 75 (6.2) | 1 (0.5) |
| Local adverse effects | ||||||||
| Pain at the site of injection | 100,909 (24.1) | 38,401 (20.0) | 13384 (8.4) | 9854 (8.1) | 15972 (19.9) | 8179 (13.5) | 340 (28.2) | 29 (15.8) |
| Redness at the site of injection | 7115 (1.7) | 2857 (1.5) | 1383 (0.9) | 521 (0.4) | 2181 (2.7) | 633 (1.0) | 37 (3.1) | 4 (2.2) |
| Swelling at the site of injection | 12,706 (3.0) | 5134 (2.7) | 1566 (1.0) | 764 (0.6) | 2794 (3.5) | 880 (1.5) | 63 (5.2) | 9 (4.9) |
| Immediate hypersensitivity reaction | ||||||||
| Urticaria | 1183 (0.3) | 683 (0.4) | 499 (0.3) | 352 (0.3) | 352 (0.4) | 155 (0.3) | 7 (0.6) | 1 (0.5) |
| Angioedema | 1176 (0.3) | 730 (0.4) | 467 (0.3) | 353 (0.3) | 205 (0.3) | 115 (0.2) | 12 (1.0) | 0 (0.0) |
| Anaphylactic shock | 256 (0.1) | 156 (0.1) | 124 (0.1) | 181 (0.1) | 40 (0.0) α | 56 (0.0)a | 5 (0.4) | 6 (0.4) |
Data are expressed as n (%)
COVID-19 coronavirus disease 2019
Denominators of erectile dysfunction experienced after receiving each vaccine is the number of males who received that vaccine
Denominators of the delayed period experienced after receiving each vaccine is the number of females who received that vaccine
aIndicates percentages < 0.1%
bThe difference between the first BNT162b2 dose and the first BBIBP-CorV dose was significant (p < 0.05)
cThe difference between the first BBIBP-CorV dose and the first ChAdOx1 nCoV-19 dose was significant (p < 0.05)
dThe difference between the first ChAdOx1 nCoV-19 dose and the first Sputnik V dose was significant (p < 0.05)
eThe difference between the first and second doses of ChAdOx1 nCoV-19 was significant (p < 0.05)
The outcomes of the red flags follow-up are summarized in Table 4. We documented 18 disorders likely associated with COVID-19 vaccination. The number of mortality cases linked with these disorders was 11, of which 6 and 3 were associated with myocardial infarction and venous thrombosis, respectively; these deceased patients had no pre-existing health conditions. The top three outcome events were lymphadenopathy (157.9/100,000), anxiety disorders (136.6/100,000), and lower respiratory tract infection (100.9/100,000), with Guillain–Barré syndrome (1.8/100,000), vasculitis (3.0/100,000), and myopericarditis (4.8/100,000) being the least common.
Table 4.
Rare events likely associated with COVID-19 vaccinesa
| Diagnosis | No. of persons with signs and symptomsb | No. of events | No. of events per 100,000 persons | Clinical outcome | |||
|---|---|---|---|---|---|---|---|
| Recovered | Partially recovered | Died | Under investigation | ||||
| Cardiovascular/vascular | |||||||
| Myocardial infarction | 657,222 | 267 | 40.6 | 122 | 131 | 6 | 8 |
| Deep vein thrombosis/ulnar artery thrombosis/vaccine-induced thrombotic thrombocytopenia | 657,956 | 216 | 32.8 | 174 | 36 | 3 | 3 |
| Myopericarditis | 657,766 | 32 | 4.8 | 11 | 16 | 0 | 5 |
| Arrhythmia/tachycardia/bradycardia | 657,565 | 49 | 7.4 | 16 | 24 | 0 | 9 |
| Hypertension | 651,165 | 563 | 86.5 | 345 | 217 | 0 | 1 |
| Hypotension | 654,996 | 428 | 65.3 | 362 | 60 | 0 | 2 |
| Vasculitis | 657,684 | 20 | 3.0 | 8 | 9 | 0 | 3 |
| Neurological and psychiatric | |||||||
| Mood and anxiety disorders | 657,366 | 896 | 136.6 | 248 | 599 | 0 | 49 |
| Epileptic seizures | 657,956 | 21 | 3.2 | 6 | 14 | 0 | 1 |
| Guillain–Barré syndrome | 658,362 | 12 | 1.8 | 6 | 6 | 0 | 0 |
| Nephrology | |||||||
| Acute kidney injury/IgA nephropathy | 655,783 | 129 | 19.7 | 89 | 35 | 1 | 5 |
| Respiratory | |||||||
| Pulmonary embolism | 657,362 | 165 | 25.1 | 104 | 36 | 0 | 25 |
| Endocrine | |||||||
| Hyperglycemia | 625,808 | 316 | 50.5 | 269 | 41 | 0 | 6 |
| Hypoglycemia | 624,682 | 164 | 26.3 | 98 | 63 | 0 | 3 |
| Infections | 0 | ||||||
| Herpes zoster infection | 656,987 | 149 | 22.7 | 106 | 38 | 0 | 5 |
| Herpes simplex infection | 657,169 | 98 | 14.9 | 73 | 23 | 0 | 2 |
| Lower respiratory tract infection | 652,431 | 658 | 100.9 | 510 | 134 | 1 | 14 |
| Hematologic and lymphatic disorders | |||||||
| Lymphadenopathy | 655,720 | 1036 | 157.9 | 721 | 287 | 0 | 28 |
COVID-19 coronavirus disease 2019, Ig immunoglobulin
aOccurred within 14 days of receiving the first or second dose, based on following up red flags on the adverse events application. Follow-up spanned for 1 month. Cases varied in severity
bEach adverse event was analyzed separately; any individual with a history of the outcome event was excluded from the event analysis
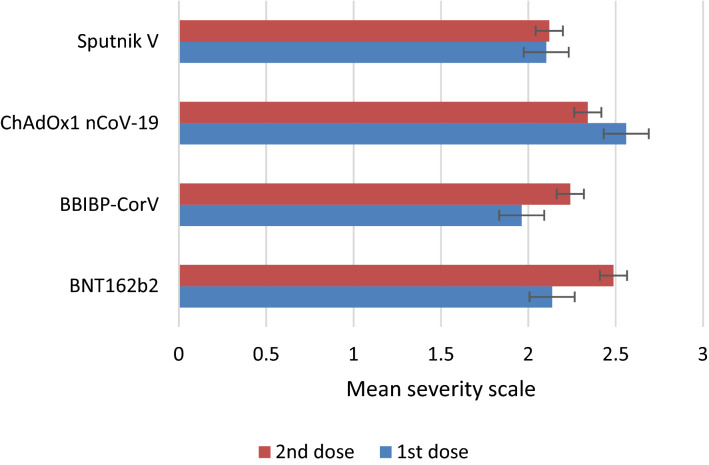
When comparing the mean severity scale of adverse events caused by COVID-19 vaccines, we found that the ChAdOx1 nCoV-19 first dose caused significantly more severe adverse events than the first and second doses of the other vaccines (adjusted p < 0.005), with the exception of the BNT162b2 second dose, which caused adverse events with similar severity to that of the ChAdOx1 nCoV-19 first dose (adjusted p > 0.05) (Fig. 3). We also found that individuals who were not vaccinated against seasonal influenza experienced more severe adverse events after COVID-19 vaccination (adjusted p < 0.005).
Fig. 3.
Mean severity scale of adverse events stratified by first and second doses of COVID-19 vaccines. COVID-19 coronavirus disease 2019
The logistic regression model showed that individuals aged > 65 years (aOR 0.437, 95% CI 0.425–0.449) and males (aOR 0.500, 95% CI 0.459–0.505) were less likely to experience adverse events after COVID-19 vaccination (see the ESM). In contrast, individuals previously infected with COVID-19 (aOR 1.181, 95% CI 1.168–1.195), vaccinated against seasonal influenza (aOR 1.143, 95% CI 1.121–1.166), sensitive to a drug (aOR 1.557, 95% CI 1.518–1.597), or sensitive to food (aOR 1.774, 95% CI 1.720–1.830) were more likely to encounter adverse events after COVID-19 vaccination. Additionally, smoking (aOR 1.139, 95% CI 1.126–1.153) was a significant predictor for adverse events after COVID-19 vaccination. The results of logistic regression models for each vaccine are listed in Tables 4–7 of the ESM. Smoking was the only predictor that showed consistent results across all vaccines. Predictors for adverse events reported by individuals who received the BNT162b2 vaccine, except smoking, were all different from the other vaccines.
Discussion
Types and Rates of Adverse Events Following Immunization (AEFIs)
Although the safety of COVID-19 vaccines has been assessed in clinical trials [5, 12–14], concerns about their safety profiles are widespread. Therefore, active post-authorization surveillance is a necessity, particularly in nations where fears from vaccination complications are highly prevalent.
Overall, a broad spectrum of significant reactogenicity was reported after administration of COVID-19 vaccines. Fatigue, fever, headache, and pain at the site of injection were the most frequently reported AEFIs. Swelling in lymph nodes in the armpits occurred more frequently than expected after administration of the ChAdOx1 nCoV-19 first dose. Noticeably, 176 (0.5%) of 80,281 participants who received the ChAdOx1 nCoV-19 first dose reported AEFIs related to erectile function. While a recent study found that the incidence of urologic AEFIs following administration of the BNT162b2 vaccine was 0.7%, erectile impairment was not among these events [15]. To date, the impact of the vaccines on fertility, sperm production, or sexual function has not been assessed or compared across vaccines [16].
The overall rates of local and systemic AEFIs reported in our study were lower than those of UK and US community-based studies [4, 7], in which all AEFIs were reported passively through applications. We found significant differences in AEFI rates across COVID-19 vaccines. Similar to the published literature [4], the first dose of ChAdOx1 nCoV-19 caused a significantly higher rate of systemic AEFIs than other vaccines. Additionally, the lowest rate of local AEFIs was reported in participants who received the BBIBP-CorV vaccine, which was in line with interim analysis of clinical trials [14]. Although interim analysis of the phase III trial of the Sputnik V vaccine showed a similar safety profile to the other vaccines [12], we found that the Sputnik V vaccine was significantly associated with higher rates of overall AEFIs and immediate hypersensitivity reactions than the other vaccines.
Hospitalization and Mortality Following Immunization
In this study, the incidence rates for hospitalization and death after vaccination were 0.15% and 0.01%, respectively. The concerning issue is that 11 (11.8%) of the 93 death cases had no known pre-existing health conditions. According to physicians and clinicians, the death causes were myocardial infarction (n = 6), heart-related thrombosis (n = 3), acute kidney injury (n = 1), and lower respiratory tract infection (n = 1). While few studies have reported death cases after vaccination, the incidence rate of death after vaccination among adults and adolescents in the US was 0.002% and 0.003%, respectively [17, 18]. Due to differences in methodological approaches followed in data collection, and variation in vaccines administered to the Jordanian and US populations, a realistic comparison between the findings of these studies is implausible. In addition, the causal relation between vaccination and death was not studied. Accordingly, these findings offer insights to likely undiscovered areas in COVID-19 vaccination research.
Characteristics of Rare AEFIs
While most of the rare AEFIs discovered in our study have been previously reported in the published literature, they involved a broad spectrum of the body systems and occurred more frequently than expected. The incidence of lymphadenopathy (157.9/100,000 persons) observed in our study was higher than that of an Israeli study (80.5/100,000 persons) [6]. The absence of a red-flag tool that enables investigators to follow serious events related to COVID-19 vaccination in previous community-based studies might have contributed to these outcomes. In addition, most post-authorization safety studies are based on passive reporting of AEFIs, which may not reflect true incidence rates or reliable association with vaccination. Our findings indicated a potential influence of COVID-19 vaccines on blood pressure, which is consistent with previous reports [19, 20]. In addition, we observed 20 (3/100,000 persons) vasculitis cases after COVID-19 vaccination, which was consistent with a series of reports and clinical cases [21–23]. This may indicate that some components of COVID-19 vaccines might have triggered immune responses and led to vasculitis, especially that no primary infections were reported in our patients before vaccination.
Severity of AEFIs
The severity of reactogenicity was at its highest level after the ChAdOx1 nCoV-19 first dose and BNT162b2 second dose. Due to severe AEFIs encountered after its first dose, many healthcare professionals did not recommend ChAdOx1 nCoV-19 for others [24]. The reason for the severe AEFIs following the BNT162b2 second dose could be attributed to the high incidence of systemic AEFIs and immediate hypersensitivity reactions compared with the first dose [4]. Although we found a significant association between vaccination against seasonal influenza and the occurrence of AEFIs after COVID-19 vaccination, those vaccinated against seasonal influenza experienced less severe AEFIs following COVID-19 vaccination. This was partially consistent with a randomized controlled phase IV trial that assessed the safety of concomitant administration of COVID-19 vaccines with seasonal influenza vaccines [25]. Another trial found no significant difference in the severity of AEFIs between individuals who received a COVID-19 vaccine with a seasonal influenza vaccine and those who received a COVID-19 vaccine alone [9]. The impact of concomitant administration of COVID-19 vaccines with seasonal influenza vaccines on reactogenicity should be investigated in the context of not only reporting AEFIs but also the potential mechanism of interaction between the two types of vaccines.
Determinants of AEFIs
The predictors for AEFIs were assessed in our study using logistic regression. We observed that AEFIs were more likely to be encountered by people younger than 65 years of age and females. These findings were in line with previous studies that measured adverse events following administration of the BNT162b2, BBIBP-CorV, and ChAdOx1 nCoV-19 vaccines [4, 26, 27]. Unlike in the case of the BNT162b2 vaccine, elderly subjects and males were more likely to experience AEFIs with the ChAdOx1 nCoV-19 and BBIBP-CorV vaccines. Furthermore, differences in the incidence of AEFIs across both females and males were documented for other vaccines, such as the influenza and Japanese encephalitis vaccines, which may indicate that females have more intense immune response than males [28, 29]. We also found that individuals who were previously infected with COVID-19, or smokers, were more likely to experience AEFIs. Previous COVID-19 infection was found to be a driver for AEFIs [30, 31]. Our findings regarding the link between smoking and AEFIs have not been previously reported in COVID-19 studies. Nonetheless, Mehwish and colleagues [32] tested the link between smoking and hepatitis B vaccine response and found a significant difference in immune activation across smokers and non-smokers. Hence, further studies are needed to establish causality between smoking and AEFIs.
Limitations and Future Work
To our knowledge, this is the first active surveillance-based study that provided a cross-vaccine comparison between four different types of COVID-19 vaccines. Furthermore, this is the first study to use a red-flags tool for rare and serious AEFIs tracking up to 3 months after vaccination. However, the findings of this study need to be addressed in the context of its limitations. First, although this was an active surveillance-based study, most of the data presented were self-reported, which could introduce bias to the findings. For example, we listed rare AEFIs and excluded participants with a history of the outcome; however, the medical history was provided by participants themselves. Furthermore, vaccinees might be more likely to report some events more than others based on their understanding of the observers’ questions. Second, given the nature of our study, our findings failed to provide evidence of causality between hospitalization or mortality and COVID-19 vaccination. Third, we could not rule out the possibility of underreporting, especially the outcomes of rare and serious AEFIs, due to loss of contact with participants. Fourth, bias could be induced given that ethnic groups were not equally represented in this study. Finally, the study sample might not be representative of the whole population in Jordan.
Conclusions
The local and systemic adverse events of four COVID-19 vaccines occurred less frequently than expected. Nonetheless, the incidence rates of rare and serious AEFIs were higher than seen in the published literature. Younger participants, females, those who had previously had COVID-19, and smokers were more likely to encounter AEFIs. There were some significant differences in the nature, incidence, and severity of AEFIs across the four vaccines. For example, the ChAdOx1 nCoV-19 vaccine first dose was significantly associated with a high proportion of erectile dysfunction and more severe AEFIs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all participants who volunteered to take part in this study, as well as all sub-investigators, study coordinators and site staff who contributed to the conduct of the study. The authors wish to acknowledge the Ministry of Health colleagues from the COVID-19 Vaccines Pharmacovigilance Committee, IT Department, and Project Management for their contributions to the overall project. The authors also wish to acknowledge EMPHNET for their technical assistance, digital solutions, and logistic and operational support related to this work.
Declarations
Availability of data and materials
Data are available upon reasonable request.
Funding
This study was funded by the Smart Labs Group and The Deanship of Scientific Research and Graduate Studies at the University of Petra.
Conflict of interest
Derar H. Abdel-Qader, Hasan Abdel-Qader, Jennifer Silverthorne, Chuenjid Kongkaew, Ahmad Z. Al Meslamani, Wail Hayajneh, Osama M. Abu Ata, Walid Shnaigat, Salah AbuRuz, Mohannad Al Nsour, Abdallah Alhariri, Khaldoun Shnewer, Mohammad Da’ssan, Nathir M. Obeidat, Khaldoon E. Nusair, Mothafer S. Jalamdeh, Feras Hawari, Khaldoun Khader, Tareq Hakim, Fatima A. Hammad, Mustafa Al Qudah, and Mohammad Asad declare they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
This study was approved by the Ethics Research Committee of the Jordanian Ministry of Health (REC-MOH-7722).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
None.
Authors’ contributions
DAQ, HAQ, JS, CK, AZM: Concept development, study design, training of data collectors, data collection, project administration, methodology, data analysis, manuscript writing. WH, OAA, WS, SAR, MAN: Conceptualization, data collection, methodology, manuscript drafting. AA, KS, MD, NMO, KEN: Review and editing, methodology, supervision, data analysis. MSJ, FH, KK, TH, FA, MQ, MA: Data analysis, proofreading, editing, manuscript drafting, statistical procedures.
References
- 1.Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385(2):187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed 18 Feb 2022.
- 3.World Health Organization. Adverse Events Following Immunization (AEFI). 2020. https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/aefi. Accessed 21 Apr 2022.
- 4.Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuters. COVID-19 Tracker—Jordan. https://graphics.reuters.com/world-coronavirus-tracker-and-maps/countries-and-territories/jordan/. Accessed 31 Jul 2022.
- 9.Toback S, Galiza E, Cosgrove C, Galloway J, Goodman AL, Swift PA, et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):167–179. doi: 10.1016/S2213-2600(21)00409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’alò GL, Zorzoli E, Capanna A, Gervasi G, Terracciano E, Zaratti L, et al. Frequently asked questions on seven rare adverse events following immunization. J Prev Med Hyg. 2017;58(1):E13–26. [PMC free article] [PubMed] [Google Scholar]
- 11.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232. [PubMed] [Google Scholar]
- 12.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385(25):2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2022;22(2):196–208. doi: 10.1016/S1473-3099(21)00462-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Souders C, Carmel M, Anger JT. Low rates of urologic side effects following coronavirus disease vaccination: an analysis of the food and drug administration vaccine adverse event reporting system. Urology. 2021;153:11–13. doi: 10.1016/j.urology.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo SP, Hsieh T-C, Pastuszak AW, Hotaling JM, Patel DP. Effects of SARS CoV-2, COVID-19, and its vaccines on male sexual health and reproduction: where do we stand? Int J Impot Res. 2022;34:138–144. doi: 10.1038/s41443-021-00483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Selected Adverse Events Reported after COVID-19 Vaccination. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html. Accessed 24 Dec 2021.
- 18.Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D, et al. COVID-19 vaccine safety in adolescents aged 12–17 years—United States, December 14, 2020–July 16, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1053–1058. doi: 10.15585/mmwr.mm7031e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zappa M, Verdecchia P, Spanevello A, Visca D, Angeli F. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine. Eur J Intern Med. 2021;90:111–113. doi: 10.1016/j.ejim.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thunstedt DC, Straube A, Schöberl F. Isolated intracranial hypertension following COVID-19 vaccination: a case report. Cephalalgia Rep. 2021 doi: 10.1177/25158163211044797. [DOI] [Google Scholar]
- 21.Cavalli G, Colafrancesco S, De Luca G, Rizzo N, Priori R, Conti F, et al. Cutaneous vasculitis following COVID-19 vaccination. Lancet Rheumatol. 2021;3(11):e743–e744. doi: 10.1016/S2665-9913(21)00309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mücke VT, Knop V, Mücke MM, Ochsendorf F, Zeuzem S. First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: a case report. BMC Infect Dis. 2021;21(1):958. doi: 10.1186/s12879-021-06655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon Y, Eshete T, Mekasha B, Assefa W. COVID-19 vaccine: side effects after the first dose of the oxford astrazeneca vaccine among health professionals in low-income country: Ethiopia. J Multidiscip Healthc. 2021;14:2577–2585. doi: 10.2147/JMDH.S331140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarus R, Baos S, Cappel-Porter H, Carson-Stevens A, Clout M, Culliford L, et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial. Lancet. 2021;398(10318):2277–2287. doi: 10.1016/S0140-6736(21)02329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side Effects and Perceptions Following Sinopharm COVID-19 Vaccination. medRxiv. 2021. https://www.medrxiv.org/content/early/2021/07/01/2021.06.28.21258847. Accessed 17 Apr 2022. [DOI] [PMC free article] [PubMed]
- 27.El-Shitany NA, Harakeh S, Badr-Eldin SM, Bagher AM, Eid B, Almukadi H, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among saudi residents: a retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–1401. doi: 10.2147/IJGM.S310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein SL, Pekosz A. Sex-based biology and the rational design of influenza vaccination strategies. J Infect Dis. 2014;209(Suppl 3):S114–S119. doi: 10.1093/infdis/jiu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.d’Arminio Monforte A, Tavelli A, Perrone PM, Za A, Razzini K, Tomasoni D, et al. Association between previous infection with SARS CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: data from 3,078 health care workers. EClinicalMedicine. 2021;36:100914. doi: 10.1016/j.eclinm.2021.100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colaneri M, Di Benedetto A, Marvulli LN, Bocchio F, Cutti S, Marena C, et al. Are people previously infected with SARS-CoV-2 likely to experience COVID-19 symptoms again after vaccination? Results from an Italian COVID-19 referral center. Hum Vaccin Immunother. 2022 doi: 10.1080/21645515.2021.1920273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Younas M, Carrat F, Desaint C, Launay O, Corbeau P, Group for the AHV-BT. Immune activation, smoking, and vaccine response. AIDS. 2017;31(1):171–3. 10.1097/QAD.0000000000001311. Accessed 17 Apr 2022. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.