Abstract
By transposon Tn917 mutagenesis, two mutants of Staphylococcus xylosus were isolated that showed higher levels of β-galactosidase activity in the presence of glucose than the wild type. Both transposons integrated in a gene, designated glcU, encoding a protein involved in glucose uptake in S. xylosus, which is followed by a glucose dehydrogenase gene (gdh). Glucose-mediated repression of β-galactosidase, α-glucosidase, and β-glucuronidase activities was partially relieved in the mutant strains, while repression by sucrose or fructose remained as strong as in the wild type. In addition to the pleiotropic regulatory effect, integration of the transposons into glcU reduced glucose dehydrogenase activity, suggesting cotranscription of glcU and gdh. Insertional inactivation of the gdh gene and deletion of the glcU gene without affecting gdh expression showed that loss of GlcU function is exclusively responsible for the regulatory defect. Reduced glucose repression is most likely the consequence of impaired glucose uptake in the glcU mutant strains. With cloned glcU, an Escherichia coli mutant deficient in glucose transport could grow with glucose as sole carbon source, provided a functional glucose kinase was present. Therefore, glucose is internalized by glcU in nonphosphorylated form. A gene from Bacillus subtilis, ycxE, that is homologous to glcU, could substitute for glcU in the E. coli glucose growth experiments and restored glucose repression in the S. xylosus glcU mutants. Three more proteins with high levels of similarity to GlcU and YcxE are currently in the databases. It appears that these proteins constitute a novel family whose members are involved in bacterial transport processes. GlcU and YcxE are the first examples whose specificity, glucose, has been determined.
Carbon catabolite repression (CR) is a ubiquitous regulatory process in microorganisms, whereby the availability of a rapidly metabolizable carbon source inhibits expression of genes encoding proteins mainly concerned with the utilization of alternative carbon sources (47). The mechanisms by which CR is achieved are best understood in Escherichia coli and Bacillus subtilis (48). Studies on CR in Bacillus megaterium (26), Lactobacillus pentosus (32), Lactobacillus casei (36), Lactococcus lactis (33), Listeria monocytogenes (2), and Staphylococcus xylosus (12) suggested common regulatory pathways in AT-rich gram-positive bacteria that are distinct from those found in enteric bacteria (48). CR in Bacillus and related organisms is mediated by the catabolite control protein A (CcpA) (23), which binds to operator sites known as catabolite responsive elements (cre) (25). Contradictory in vitro results have been reported regarding the effector(s) stimulating the DNA-binding activity of CcpA (16, 20, 27–29, 35, 42). One of the most important CcpA effectors is a phosphorylated form of HPr, the phosphocarrier protein of the phosphoenolpyruvate-dependent phosphotransferase system (PTS) (41). Phosphorylation of HPr at a serine residue is carried out by HPr kinase (19, 44), which appears to be the key component in signal transduction leading to CR. In B. subtilis, CcpB and Crh, proteins that are similar to CcpA and HPr, respectively, have been implicated in CR (7, 18), but the significance of these findings for other AT-rich gram-positive organisms is not clear at the moment.
Among metabolizable carbohydrates, glucose is preferred by a number of bacteria. To ensure efficient glucose uptake, several glucose transport systems are operative in many of these organisms. For example, studies with PTS-deficient strains of B. subtilis, (10), Streptococcus mutans (6, 9), Streptococcus bovis (46), and Staphylococcus aureus (45) indicated that glucose is internalized by PTS-dependent as well as -independent mechanisms. Apart from a gene encoding a hexose:H+ symporter from B. subtilis (39), no other genes responsible for non-PTS glucose uptake have been identified in these organisms.
We are interested in CR in the AT-rich gram-positive bacterium S. xylosus (49), a nonpathogenic Staphylococcus that is involved in meat fermentations (22). By a transposon mutagenesis aimed at isolating CR mutants of S. xylosus, a gene was identified whose inactivation resulted in a reduction of glucose-mediated CR. The inactivated gene was found to encode a non-PTS glucose uptake protein.
MATERIALS AND METHODS
Bacterial strains and plasmid vectors.
S. xylosus strains used in this study are listed in Table 1. Cloning in E. coli was performed by using DH5α [Φ80dlacZΔM15 Δ(lacZYA-argF) recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 deoR]. Heterologous glcU and ycxE expression was tested in E. coli ZSC112 [ptsG ptsM glkA] (8) by using the glucose kinase (glkA)-containing plasmid pGRB144 (53). B. subtilis 168 (trpC2) served to amplify ycxE. The partial S. xylosus genomic library was constructed with pUC18. With the shuttle vectors pRB473 (5) and pRB474 (13), cloning in S. xylosus and complementation studies were carried out. Allelic replacements were achieved with the aid of the temperature-sensitive shuttle plasmids pBT1 and pBT2 and ermB fragments from Tn551 (4). Plasmid pTV1Ts (57) served for the transposon mutagenesis.
TABLE 1.
S. xylosus strains used
Growth media, DNA manipulations, and transformation.
DNA manipulations, plasmid DNA isolation, Southern blot analysis, transformation of E. coli, and preparation of media and agar plates for bacterial growth were performed according to standard procedures. Plasmid DNA was introduced into S. xylosus by electroporation with glycine-treated electrocompetent cells (4). PCR was carried out with Vent polymerase (New England Biolabs) or rTth DNA Polymerase, XL (Perkin Elmer). S. xylosus was grown in B-medium consisting of 1% peptone, 0.5% yeast extract, 0.5% NaCl, and 0.1% K2HPO4. To test for catabolite repression, sugars were added to a final concentration of 25 mM.
Transposon mutagenesis.
Transposon mutagenesis with Tn917 from pTV1Ts was performed as described previously (14). The six mutants were identified as dark blue colonies on agar plates supplemented with 100 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal)/ml, erythromycin (2.5 μg/ml), and glucose (25 mM) after incubation at 37°C for 24 h.
Primers used for PCR and primer extension.
The following glcU-gdh-specific primers were used (the positions refer to the glcU-gdh sequence from accession no. Y14043): H10, CAGGGATCCCCACTATTACTCTC (142–155); H11, CAGGAATTCGCATGGTCATATCTC (1173–1187); H17, CAGGAATTCGTTATCCTTTACCGCCC (1998–2014); H19, CAGGGTACCGGTAAAGTAGTAGTTATCACAGG (1240–1262); H20, CAGAAGCTTGTTATCCTTTACCGCCCATAAATGC (1990–2014); H30, CAGAAGCTTCCACTATTACTCTC (142–155); H31, CAGGTCGACGCATGGTCATATCTC (1173–1187); H36, CAGGGATCCCACTCTCTATTTGTTTTTCTCCCC (269–292); and H37, GACAAGCTTCCTCCTGCATCAACTGACATTG. Primer H37 hybridized 1.5 kb upstream of glcU, where only partial DNA sequence is available. For the primer extension reaction, the following primer was applied: TCCCTCTAATCTGATTATAAGGACC (384–405).
To clone ycxE, the following primers deduced from the B. subtilis sequence D50453 (56) were used: BS1, CTAGCATGCACGCTCCTGAAAGC (position 123576–123593); BS2, CTCGTCGACTTTTGCCTGCTCCTTGCCG (position 124657–124677).
Construction of glcU and ycxE expression plasmids.
Plasmid pUD1, containing the glcU-gdh operon on a BamHI-EcoRI fragment, was constructed with primers H10-H17 and plasmid pRB473. Plasmid pGU1, containing glcU alone, was obtained by using primers H10-H11 and pRB473. Combination of glcU and glkA on plasmid pUK1 was achieved by cloning a HindIII-SalI fragment of glcU obtained with primer pair H30-H31 into pGRB144, the glkA-containing plasmid.
For combining ycxE from B. subtilis with glkA from S. xylosus, primers BS1 and BS2 were applied. Cloning of the amplified SphI-SalI ycxE fragment into pGRB144 yielded pYK1. The ycxE-glkA region was moved as a SphI-KpnI fragment into the vegII promoter-containing plasmid pRB474 to yield pYK2. The corresponding ycxE plasmids without glkA, pYE1 and pYE2, were obtained by deleting the SalI-KpnI glkA fragments from pYK1 and pYK2, respectively.
Construction of a gdh deletion mutant.
The construction of the gdh deletion plasmid, pΔGDH, was carried out in two steps. First, the BamHI-EcoRI fragment from pGU1 was cloned into pEC3 (4) in front of the ermB gene. The resulting plasmid was designated pEC-glcU. Secondly, a gdh deletion derivative (′gdh) was synthesized with primer pair H19-H20. In this KpnI-HindIII fragment the first 15 bp of gdh including translation initiation signals were deleted. In a three-fragment cloning, the BamHI-KpnI glcU-ermB fragment, the ′gdh KpnI-HindIII fragment, and the BamHI-HindIII-cut plasmid pBT2 were combined to yield pΔGDH (glcU ermB ′gdh). Allelic replacement of the wild-type gdh gene by ermB ′gdh was carried out as previously described (4). The resulting strain, S. xylosus TX213 (ermB ′gdh), was taken for further studies after the chromosomal organization of the glcU ermB ′gdh region had been confirmed by Southern blot and PCR analyses.
Construction of a glcU deletion mutant.
The construction of a glcU deletion plasmid started with the cloning of an EcoRI-BamHI-gdh fragment amplified by primers H16-H17 into vector pRB473. Into the resulting plasmid, an H36-H37-amplified BamHI-HindIII fragment containing about 1.3 kb upstream of glcU was cloned, yielding plasmid pΔglcU. The plasmid, which contained DNA surrounding glcU, was introduced into wild-type S. xylosus C2a. Spontaneous glcU deletion from the chromosome by gene conversion was detected on B medium agar plates containing X-Gal (100 μg/ml) and glucose (25 mM). Three blue colonies indicating loss of glcU were cured from pΔglcU, and the chromosomal DNA was analyzed for loss of glcU by PCR. One representative of these colonies was designated S. xylosus TX214 (ΔglcU) and was taken for further studies.
Determination of enzyme activities in cell extracts.
For determination of enzymatic activities in S. xylosus, cells were grown in B medium or in B medium supplemented with 25 mM of the appropriate carbon source to an optical density at 578 nm (OD578) of 1.5. Crude extracts were prepared by disrupting the cells with glass beads (53), and determination of the β-galactosidase, β-glucuronidase, and α-glucosidase activities was done as previously described (12). To assay the activity of glucose dehydrogenase, S. xylosus cells were grown in B medium with 25 mM of glucose to an OD578 of 2. Crude extracts were prepared in 75 mM Tris-HCl, pH 8.0. The enzyme activity was assayed spectrophotometrically by monitoring the increase of absorbance at 340 nm, which is indicative of NADH production. The assay was performed at 30°C in 75 mM Tris-HCl (pH 8.0), 0.1 M glucose, 2 mM NAD, and 5 to 500 μg of cellular protein. Protein concentrations were determined by the method of Bradford (3).
Measurements of glucose uptake.
Uptake of glucose in S. xylosus was measured with whole cells grown in B-medium or B-medium supplemented with 25 mM of glucose. Staphylococcal cells were harvested at an OD578 of 1.5, washed in transport buffer (0.1 M morpholinepropanesulfonic acid, 0.5 mM MgSO4, 10 mM NaCl, pH 7.0), and resuspended in the same buffer to a final OD578 of 3.0. After addition of 200 μM [14C]glucose (6.2 mCi/mmol) to 1 ml of prewarmed (30°C) cells, 0.15-ml samples were taken at intervals, collected on membrane filters with a pore size of 0.45 μm, and washed with 5 ml of transport buffer. Filters were dried at 80°C for 1 h. The radioactivity was determined by liquid scintillation counting. Uptake rates are expressed in nanomoles of glucose per minute per milligram of cellular protein. The amount of protein was determined by the method of Bradford (3).
Uptake of glucose in E. coli was carried out accordingly with cells grown in Luria Bertani medium that were adjusted to an OD578 of 15.
RNA preparation and primer extension analysis.
Preparation of RNA and the primer extension reactions were done as described previously (1). The primer used in these experiments contained infrared dye IRD700 at the 5′ end. Reverse transcripts were run on 8% polyacrylamide-urea gels and were detected with a Li-Cor DNA sequencer. The file containing the picture of that gel was imported into Photoshop and printed on glossy paper.
Nucleotide sequence accession number.
The DNA sequence reported here is available from the EMBL database under accession no. Y14043.
RESULTS
Isolation of S. xylosus mutants altered in CR.
In order to isolate CR mutants in S. xylosus, transposon mutagenesis with Tn917 was performed. Expression of the β-galactosidase gene, which is subject to CR (1, 53), served to monitor the appearance of mutants on agar plates containing glucose and X-Gal. While wild-type cells stay light blue for about 48 h, CR mutants should develop a darker color. By using this strategy, six blue colonies were isolated that harbored a copy of Tn917 in their chromosome. The mutant strains were designated S. xylosus TX207 to TX212.
Molecular characterization of the mutants.
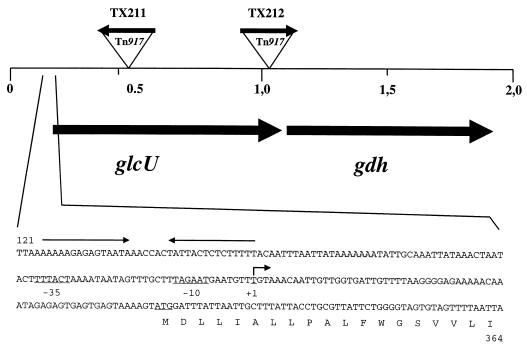
Southern blot analysis using a Tn917-specific probe revealed that the transposon integrated at five different locations within a common genomic region of about 1 kb. Determination of the orientation of Tn917 in the genome of the mutants yielded opposite orientations in TX211 and TX212 (Fig. 1). Therefore, these strains were taken as representatives for further analysis. Chromosomal DNA from the neighborhood of the transposon insertions in strains TX211 and TX212 including the Tn917 ermB gene was cloned in E. coli with the erythromycin resistance as selection marker. With the genomic DNA from the TX211-derived fragment as a hybridization probe, a 3.6-kb HindIII fragment was identified in the wild-type strain covering the region where the Tn917 insertions occurred in the mutants. After cloning the HindIII fragment in E. coli, a nucleotide sequence of 2.2 kb immediately adjacent to the transposon insertion sites was determined. In addition, the exact positions of the transposons in strains TX211 and TX212 were determined by DNA sequencing.
FIG. 1.
Genetic organization of the glcU gdh region of S. xylosus. The region that has been sequenced (accession no. Y14043) is shown. The size and orientation of the genes were deduced from the nucleotide sequence. The location and orientation of Tn917 in the S. xylosus mutants TX211 and TX212 are indicated. The nucleotide sequence of the glcU promoter region is also shown. Numbering refers to the complete DNA sequence (Y14043). The transcriptional start site and an inverted repeat structure are indicated by arrows.
Nucleotide sequence of the mutated region.
The nucleotide sequence is composed of 2,193 bp harboring two large open reading frames (Fig. 1). The first specifies a protein of 288 amino acids with a calculated molecular mass of 30.6 kDa. Structural predictions (24) and hydropathy analysis (31) suggested that the orf1-encoded protein constitutes an integral membrane protein. Since it turned out to encode a glucose uptake protein, the gene was designated glcU. Similarity searches in databases identified two proteins from B. subtilis and B. megaterium, which share 55 and 56% identical residues with GlcU, respectively. The function of both proteins, encoded by ycxE in B. subtilis (30, 56) and orf2 in B. megaterium (34), remains to be elucidated. In addition, two putative membrane proteins, one from Lactobacillus helveticus (AJ002481) and the other from Streptococcus pyogenes (U17382), whose functions have not been determined, show identities of 40 and 32%, respectively, with GlcU from S. xylosus.
The deduced product of the second gene, a protein of 263 amino acids with a molecular mass of 28.6 kDa, showed similarity to various bacterial dehydrogenases and reductases. Since the identity with glucose-1-dehydrogenases from B. megaterium and B. subtilis was especially striking (56% identity), the gene was designated gdh. Interestingly, two gdh genes in B. subtilis and B. megaterium are encoded downstream of the glcU homologs, ycxE and orf2, mentioned above (30, 34, 56). Therefore, the genetic organization of this locus is conserved among the two bacilli and S. xylosus.
Activities of catabolic enzymes in the wild type and the glcU mutant strains.
The isolation of the glcU mutants TX211 and TX212 as blue colonies from X-Gal agar plates containing glucose suggested that glucose-mediated repression of β-galactosidase activity is altered. To determine whether repression by other carbohydrates and repression of other enzymes are also affected by the mutations, the β-galactosidase, β-glucuronidase, and α-glucosidase activities of the wild type and the glcU mutant strains were compared in cultures containing glucose, fructose, sucrose, or no additional sugar. As shown in Table 2, inactivation of glcU resulted in a partial loss of glucose repression of all tested enzymes but left sucrose- or fructose-mediated repression at the wild-type level. The effect was most pronounced for the β-galactosidase activity, where the 17-fold repression in the wild type was reduced to an only 3-fold reduction. It appears that loss of GlcU function in S. xylosus partially relieves catabolic enzymes from glucose repression.
TABLE 2.
Catabolic enzyme activities in S. xylosus C2a, glcU mutant strains TX211, TX212, and TX214, and gdh mutant strain TX213
| Enzyme | Growth conditiona | Enzyme activity in strain (nmol of nitrophenol released min/mg of protein)b
|
||||
|---|---|---|---|---|---|---|
| C2a (wild type) | TX211 (glcU::Tn917) | TX212 (glcU::Tn917) | TX213 (gdh::ermB) | TX214 (ΔglcU) | ||
| β-galactosidase | B + lactose | 105 | 106 | 97 | 106 | 95 |
| B + lactose + glucose | 6 | 28 | 32 | 10 | 35 | |
| B + lactose + sucrose | 34 | 39 | 31 | ndc | nd | |
| B + lactose + fructose | 41 | 48 | 44 | nd | nd | |
| β-glucuronidase | B | 23 | 21 | 20 | 19 | 18 |
| B + glucose | 5 | 9 | 8 | 5 | 8 | |
| B + sucrose | 9 | 8 | 9 | nd | nd | |
| B + fructose | 9 | 9 | 9 | nd | nd | |
| α-glucosidase | B | 11 | 10 | 11 | 12 | 13 |
| B + glucose | 1 | 4 | 5 | 2 | 4 | |
| B + sucrose | 3 | 4 | 3 | nd | nd | |
| B + fructose | 4 | 3 | 4 | nd | nd | |
Cells were grown in complex B medium (B) containing 25 mM of the indicated sugars or without additional carbohydrate; they were harvested at an OD578 of 1.5 and disrupted with glass beads. Extracts prepared from 40 ml of cells were used for determination of the enzyme activities.
Enzymatic activities were determined by using p-nitrophenyl-β-d-galactopyranoside (β-galactosidase), p-nitrophenyl-β-d-glucuronide (β-glucuronidase), and p-nitrophenyl-α-d-glucopyranoside (α-glucosidase) as substrates. Values of at least three independent experiments are shown. Standard deviations were in the range of ±15%.
nd, not determined.
Glucose dehydrogenase activity in the wild type and the glcU mutant strains.
The genetic organization of the glcU-gdh region (Fig. 1) suggested that the two genes could form an operon. Therefore, integration of Tn917 into glcU should reduce gdh expression, perhaps depending on the orientation of the transposon relative to the gdh gene. As summarized in Table 3, Tn917 integration exerts a strong polar effect on gdh expression. When Tn917 and glcU-gdh transcription proceed opposite to each other (TX211), glucose dehydrogenase activity was 30-fold reduced compared to that of the wild type. The same orientation of Tn917 and glcU-gdh transcription (TX212) still resulted in a threefold drop in glucose dehydrogenase activity. These results strongly indicate that the gdh gene does not possess its own promoter and that gdh expression is dependent on readthrough transcription initiated beyond the transposon insertion sites. Therefore, glcU and gdh most likely form an operon in S. xylosus, a situation also encountered at the respective locus, ycxE gdh, in B. subtilis (37, 43).
TABLE 3.
Glucose dehydrogenase activities in the wild-type S. xylosus C2a, glcU mutant strains TX211, TX212, and TX214 and gdh mutant strain TX208
| Straina | Glucose dehydrogenase activity (nmol of NADH produced/min/mg of protein)b |
|---|---|
| S. xylosus C2a | 182 |
| S. xylosus TX211 (glcU::Tn917) | 6 |
| S. xylosus TX212 (glcU::Tn917) | 64 |
| S. xylosus TX213 (gdh::ermB) | <1 |
| S. xylosus TX214 (ΔglcU) | 195 |
Cells were grown in complex B medium with 25 mM glucose; they were harvested at an OD578 of 1.5 and disrupted with glass beads. Extracts prepared from 40 ml of cells were used for determination of glucose dehydrogenase activities.
Glucose dehydrogenase activity was determined by monitoring the increase of absorbance at 340 nm as a measure of NADH production. Values (at least three independent experiments) are equivalent to nanomoles of glucose oxidized by glucose dehydrogenase. Standard deviations were in the range of ±12%.
Catabolic enzyme activities in a glucose dehydrogenase-deficient S. xylosus strain.
The virtually identical relief of glucose-mediated repression of catabolic enzymes in the strains S. xylosus TX211 and TX212, in which glucose dehydrogenase activity differed about 10-fold (Table 3), suggested that the regulatory phenotype in the mutant strains is the result of glcU inactivation rather than of reduced gdh expression. To rule out that lowered glucose dehydrogenase activity is responsible for the phenotype, a gdh insertion mutant was constructed as described in Materials and Methods. The resulting strain, designated S. xylosus TX213, was tested for β-galactosidase, β-glucuronidase, and α-glucosidase activities in the presence and absence of glucose in the medium. As summarized in Table 2, inactivation of the gdh gene did not result in a relief of glucose-mediated repression of these activities.
In addition to the catabolic enzyme activities, glucose dehydrogenase activity was determined in the gdh mutant strain TX213. No activity was detectable under these growth and assay conditions (Table 3), suggesting that the inactivated gdh gene is the only one in S. xylosus.
Catabolic enzyme activities in a glcU deletion strain expressing wild-type levels of glucose dehydrogenase activity.
To rule out the possibility that only the concomitant loss of GlcU and glucose dehydrogenase function affects regulation, a glcU deletion was introduced into the chromosome of S. xylosus as described in Materials and Methods. In the resulting strain, S. xylosus TX214, the glucose dehydrogenase activity was found to be at wild-type level (Table 3), showing that the glcU deletion was nonpolar on gdh expression. Subsequent assays of three catabolic enzyme activities yielded no difference from the S. xylosus TX211 and TX212 values (Table 2). Therefore, the partial loss of glucose repression observed in the transposon mutants is exclusively due to GlcU deficiency. Glucose dehydrogenase does not participate in this process. The regulatory phenotype in all glcU mutant strains could be complemented by cloned glcU on plasmid pGU1 (data not shown).
Glucose uptake in glcU mutant strains.
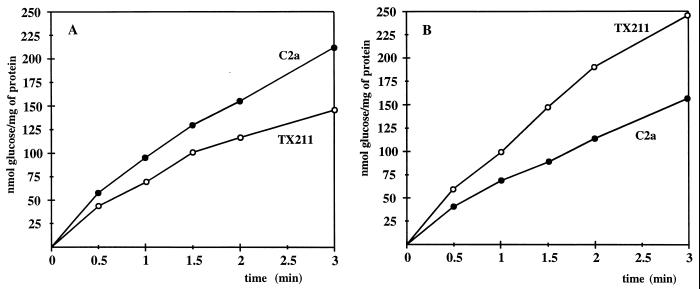
Considering the structural prediction for GlcU to contain membrane-spanning segments and the glucose-dependent regulatory phenotype in the absence of GlcU function, we reasoned that GlcU could be a protein responsible for PTS-independent glucose uptake in S. xylosus. Therefore, transport of glucose and the nonmetabolizable analogue 2-deoxyglucose was examined in the wild type and the glcU mutant strains TX211, TX212, and TX214. Since glucose uptake was identical in all GlcU-deficient strains, the values for S. xylosus TX211 are shown as a representative example (Fig. 2A). With glucose as the substrate in the assays and cells grown in the absence of glucose, a clear reduction of the uptake rate was detectable in the glcU mutant strain. The high residual uptake activity is certainly due to the PTS and, perhaps, to additional glucose transport systems. Attempts are under way to isolate a PTS mutant of S. xylosus, in which GlcU function should be more pronounced. In contrast to glucose uptake, no difference in transport activity could be detected with 2-deoxyglucose as substrate (data not shown).
FIG. 2.
Glucose uptake in S. xylosus wild type C2a and in the glcU mutant TX211. (A) Glucose uptake in cells grown in complex medium without glucose. The cells were grown in B medium without addition of glucose. Glucose uptake was determined by using 200 μM [14C]glucose (6.2 mCi/mmol). The values represent measurements of three cultures. Standard deviations were in the range of ±15%. (B) Glucose uptake in cells grown in complex medium with glucose. The cells were grown in B medium with 25 mM glucose. Glucose uptake was determined by using 200 μM [14C]glucose (6.2 mCi/mmol). The values represent measurements of three cultures. Standard deviations were in the range of ±17%.
Surprisingly, the glcU mutant strain TX211 showed a higher glucose uptake activity than the wild type, when the strains were grown in the presence of glucose (Fig. 2B). The addition of glucose to the growth medium yielded opposite effects in both strains. While glucose uptake in the wild type was 1.4-fold reduced (Fig. 2A), it was 1.6-fold higher in the glcU mutant TX211 (Fig. 2B). On the other hand, determination of the glucose concentration in the medium after 6 h of growth yielded 3.1 mM for the wild-type culture but 6.6 mM in the TX211 spent medium, clearly showing that TX211 took up less glucose. These conflicting results may reflect the influence of accumulated glucose or metabolites on the determination of glucose uptake rates. It has been shown in yeast that intracellular glucose can reduce apparent glucose transport rates in uptake experiments by up to 50% (51). As the S. xylosus strains grow with excess glucose (25 mM), they may indeed accumulate glucose or metabolites under these conditions. Loss of GlcU function could lead to reduced accumulation and, consequently, to the overestimation of glucose uptake in the mutant strains. In addition to these problems, regulation of glucose transport, which could be influenced by a functional GlcU, impedes the interpretation of the glucose uptake assays in glucose-grown cells.
Complementation of an E. coli mutant deficient in glucose uptake.
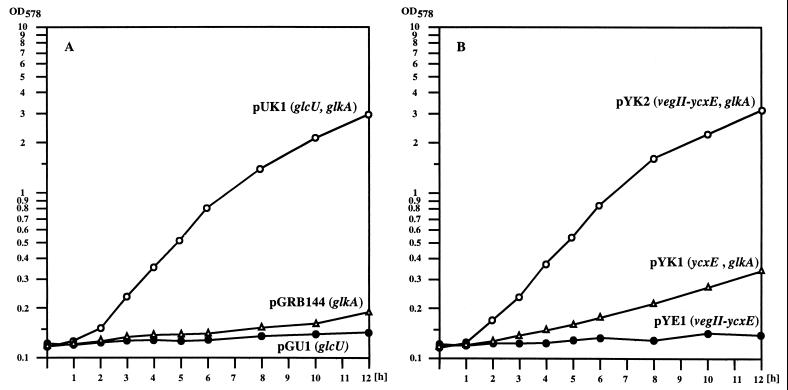
To provide additional evidence that GlcU is capable of taking up glucose, we tried to complement the glucose transport deficiency of the E. coli mutant strain ZSC112 (8). This strain carries mutations in ptsG and ptsM, which specify the major glucose permeases of E. coli, and a mutation in the glucose kinase gene glk. To enable ZSC112 to grow with glucose as the sole carbon source, genes mediating glucose transport and phosphorylation must be provided. With either glcU cloned on plasmid pGU1 or glkA cloned on plasmid pGRB144, ZSC112 could not grow in minimal medium containing glucose (Fig. 3A). When glcU was combined with the glucose kinase gene glkA from S. xylosus on plasmid pUK1, the strain grew well with glucose as carbon source (Fig. 3A). Therefore, glcU mediates glucose uptake in E. coli substantiating its participation in this process in S. xylosus. The dependency of the E. coli mutant on a functional glucose kinase, when GlcU is responsible for glucose uptake, demonstrates that glucose is internalized by GlcU in nonphosphorylated form.
FIG. 3.
Growth of E. coli ZSC112 (ptsG ptsM glk) in glucose-containing minimal medium. (A) Growth of ZSC112 with cloned glcU from S. xylosus. The plasmid-containing strains were grown in M9 minimal medium containing 10 mM glucose and ampicillin (100 μg/ml). (B) Growth of ZSC112 with cloned ycxE from B. subtilis. The plasmid-containing strains were grown in M9 minimal medium containing 10 mM glucose and ampicillin (100 μg/ml).
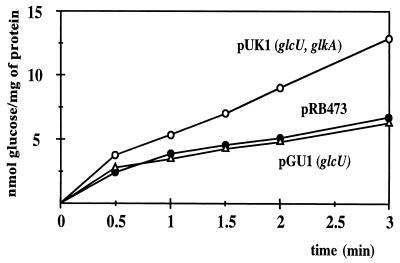
Glucose uptake in the E. coli mutant ZSC112 could be demonstrated provided that glcU was cloned together with glkA (Fig. 4, pUK1). With glcU alone, uptake slightly exceeded that of the background within the first 30 s of the assays. At later time points, it was indistinguishable from the values without cloned genes (Fig. 4). The relatively low transport activity may be due to low expression or limited stability of the membrane protein in the heterologous host. Glucose uptake with cloned glucose kinase was not altered compared to that of the background (data not shown).
FIG. 4.
Glucose uptake in E. coli ZSC112 harboring cloned glcU from S. xylosus. The cells were grown in Luria Bertani medium. Glucose uptake was determined by using 200 μM [14C]glucose (6.2 mCi/mmol). The values represent measurements of three cultures. Standard deviations were in the range of ±13%.
Determination of the transcriptional start site of glcU.
To determine the transcriptional start site of glcU, RNA was isolated from wild-type cells grown with or without glucose and primer extension reactions were carried out with a glcU-specific primer. As shown in Fig. 5, transcription start sites were located 66 bp upstream from the glcU start codon (Fig. 1). The reverse transcript was stronger in glucose-grown cells but was also detectable without the addition of glucose to the growth medium. Inspection of the DNA sequence around the promoter region revealed an inverted repeat located about 40 bp upstream of the glcU promoter. It remains to be determined whether this repeat serves as a site for glucose-specific regulation or as a terminator for upstream genes.
FIG. 5.
Primer extension analysis of glcU transcription. RNA was prepared from S. xylosus C2a grown without and with glucose. Primer extension products were separated along with DNA sequencing reactions on an 8% polyacrylamide-urea gel. Lane 1, Primer extension reaction with 15 μg of RNA from cells grown without glucose; lane 2, Primer extension reaction with 15 μg of RNA from cells grown with 25 mM glucose.
Identification of the glcU ortholog from B. subtilis.
As mentioned above, B. subtilis contains a gene, designated ycxE (30), encoding a product with high identity (55%) to GlcU in front of a glucose dehydrogenase gene. We were therefore interested to determine whether ycxE would also specify a glucose uptake protein. To that end, a ycxE fragment was amplified from chromosomal B. subtilis DNA by PCR and cloned into the glkA-containing plasmid pGRB144, yielding pYK1. Since the B. subtilis ycxE-gdh operon has been described to be expressed from a promoter recognized by the alternative sigma factor ςG (43), a promoter, vegII from B. subtilis, which is also active in E. coli (40) was placed in front of ycxE to yield pYK2. Growth experiments with E. coli ZSC112 harboring plasmid pYK1 or pYK2, showed that ycxE with its own promoter did not enable the strain to grow efficiently with glucose as carbon source (Fig. 3B, pYK1). Expression of ycxE driven by vegII, however, resulted in growth (Fig. 3B, pYK2) comparable to that mediated by S. xylosus glcU. Therefore, the B. subtilis ycxE gene specifies, like glcU, a glucose uptake protein.
The identification of the ycxE gene product as a glucose uptake protein prompted us to determine whether ycxE could also substitute for glcU in glucose-mediated regulation in S. xylosus. To avoid possible complications with overexpressed glkA, this gene was deleted from plasmids pYK1 and pYK2, yielding pYE1 and pYE2, respectively. After transformation of the glcU mutant TX211 with the pYE plasmids, β-galactosidase, α-glucosidase, and β-glucuronidase activities were measured in cells grown in the presence or absence of glucose. While ycxE expressed from vegII on plasmid pYE2 restored glucose repression in the glcU mutant, plasmid pYE1 harboring ycxE only with its own promoter had no effect (data not shown). Apparently, the ςG-specific B. subtilis promoter of ycxE is not active in S. xylosus.
DISCUSSION
The screening for transposon-generated CR mutants in S. xylosus led to the identification of an operon encoding a glucose uptake protein, GlcU, and a glucose dehydrogenase. GlcU and the glucose kinase GlkA (53), constitute a glucose utilization system enabling S. xylosus to catabolize glucose independently from the PTS.
In B. subtilis and B. megaterium, operons are present containing a glcU homolog in front of a glucose dehydrogenase gene (34, 56). Growth experiments with the B. subtilis GlcU counterpart, YcxE, in E. coli demonstrated that the YcxE protein also mediates glucose uptake. Since the corresponding B. megaterium protein shows a high degree of identity (78%) to YcxE, it is reasonable to assume the same function for that protein. Therefore, the three genes, glcU from S. xylosus, ycxE from B. subtilis, and orf2 from B. megaterium constitute a novel group of orthologous genes encoding glucose uptake proteins.
The physiological roles of these proteins, however, appear to be different in Bacillus and S. xylosus. The Bacillus glcU ortholog and the following gdh gene are transcribed 3 h after the onset of sporulation in the forespore by ςG-containing RNA polymerase (37), and glucose dehydrogenase activity is detected in forespores and in mature spores (17). Therefore, the ycxE gene product may also be present in spores and could perhaps play a role in glucose uptake in the germination process (52, 54). It does not seem to contribute to glucose uptake during vegetative growth. On the other hand, GlcU serves in S. xylosus, additionally to the PTS, to take up glucose during growth. Coexpression of glcU and gdh suggests that GlcU also recruits glucose for glucose dehydrogenase. Production of gluconate by that enzyme would open an alternative route to obtaining energy from glucose. As this possibility did not influence CR, glucose dehydrogenase may be more important under physiological conditions that are different from those in our study.
Besides the glcU orthologs, two genes that are clearly homologous to glcU are currently in the databases (AJ002481 and U17382). Both are found in gram-positive bacteria, S. pyogenes and L. helveticus, respectively, and both specify membrane proteins consistent with a function in transport processes. Due to the limited similarity to GlcU (40 and 32%, respectively) it appears difficult to predict the substrate for these putative uptake proteins, and experimental data are currently not available. Therefore, the function of these two proteins remains to be determined. So far, the family of glcU-related genes consists of only five members. The rapid progress in whole genome sequencing will most likely reveal new homologs and may eventually answer the question whether this group of genes remains restricted to gram-positive bacteria.
While the participation of GlcU in glucose uptake of S. xylosus is clear, the mechanism by which GlcU allows glucose to enter the cells remains to be elucidated. The activity of GlcU in uptake assays could only be demonstrated when glucose was metabolizable. In S. xylosus, GlcU-mediated transport of 2-deoxyglucose was not detectable, and in E. coli uptake of glucose by GlcU was apparently dependent on a functional glucose kinase. These results are indicative of sugar uptake by facilitated diffusion. By this process, sugars are taken up without the consumption of energy, but the carbohydrates cannot be accumulated against a concentration gradient. In conventional, long-term uptake assays, the activity of facilitated diffusion systems is only detectable with metabolizable substrates and shows a pronounced dependence on the respective sugar kinases (11). The fast equilibration of external and internal sugar concentrations mediated by facilitators may be detected by short-term uptake assays. In addition, influx counterflow is observed in sugar-preloaded cells (11, 38, 55). Attempts to demonstrate that GlcU indeed constitutes a glucose facilitator have so far not been successful. Despite this failure, we still favor the idea that GlcU is one of the few bacterial examples of facilitated diffusion systems (38, 46, 50, 55). Clearly, more work will be needed to elucidate the mechanism of GlcU-mediated glucose uptake.
Inactivation of glcU in S. xylosus resulted in a partial loss of glucose-mediated repression of α-glucosidase, β-glucuronidase, and β-galactosidase activities (Table 2). Since repression of α-glucosidase expression in glucose-grown cells is exclusively exerted by CcpA (12), GlcU is obviously required for full glucose-mediated CcpA activity. To account for this observation, we suggest the following. When GlcU is inactive, reduced glucose uptake leads to diminished accumulation of glycolytic intermediates and eventually to a less active HPr kinase. Consequently, activation of CcpA by HPr-ser-P in the presence of glucose is reduced but not totally lost. If one considers glucose-6-phosphate as an alternative effector for CcpA (15, 20), the consequences for CcpA activation in the absence of GlcU would be the same. In any case, the influence of GlcU on glucose-mediated CR should depend on a functional glucose kinase. And indeed, a glucose kinase mutant of S. xylosus, which has been described previously (53), has virtually the same regulatory phenotype as the glcU mutant strain. The isolation and subsequent inactivation of the HPr kinase gene will be needed to distinguish the in vivo significance of HPr-ser-P and glucose-6-phosphate as effectors for CcpA in S. xylosus.
The question arises why our β-galactosidase expression screen to detect mutants defective in CR appeared to be biased towards glcU or, as in a previous study, the glucose kinase gene glkA (53), genes that both affect PTS-independent glucose utilization. Initially, we expected to isolate the ccpA and the HPr kinase gene. During the analysis of ccpA, which had been detected by a PCR approach (13), and the lactose operon (1), it became clear that CR of the lac operon is not exclusively due to CcpA. Plating the ccpA mutant on β-galactosidase screening plates resulted in small colonies that were less colored than the wild type, instead of the expected dark blue clones. Apparently, the growth defect of the ccpA mutant (13) prevented detection of the regulatory phenotype. One is tempted to speculate that the HPr kinase mutant may exhibit a similar growth defect. Another surprise was the failure to detect PTS genes. Whether this observation really indicates that non-PTS glucose transport dominates PTS-mediated glucose transport under these conditions remains an interesting question for future studies.
In conclusion, the current work has led to the identification of a novel group of proteins responsible for PTS-independent uptake of glucose and, most likely, other compounds. Detailed biochemical work will be necessary to elucidate the mechanism by which these proteins recognize and take up their substrates.
ACKNOWLEDGMENTS
We thank F. Götz, in whose laboratory the work has been carried out, for continuous interest and support and P. L. Hyunh for excellent technical assistance. We also thank G. Sprenger for helpful advice in uptake assays.
The work was supported by the European Community Biotech Programme (BIO2-CT92-0137) and by the Deutsche Forschungsgemeinschaft (Br 947/3-1).
REFERENCES
- 1.Bassias J, Brückner R. Regulation of lactose utilization genes in Staphylococcus xylosus. J Bacteriol. 1998;180:2273–2279. doi: 10.1128/jb.180.9.2273-2279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behari J, Youngman P. A homolog of CcpA mediates catabolite control in Listeria monocytogenes but not carbon source regulation of virulence genes. J Bacteriol. 1998;180:6316–6324. doi: 10.1128/jb.180.23.6316-6324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Brückner R. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett. 1997;151:1–8. doi: 10.1111/j.1574-6968.1997.tb10387.x. [DOI] [PubMed] [Google Scholar]
- 5.Brückner R, Wagner E, Götz F. Characterization of a sucrase gene from Staphylococcus xylosus. J Bacteriol. 1993;175:851–857. doi: 10.1128/jb.175.3.851-857.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley N D, Hamilton I R. Vesicles prepared from Streptococcus mutans demonstrate the presence of a second glucose transport system. Microbiology. 1994;140:2639–2648. doi: 10.1099/00221287-140-10-2639. [DOI] [PubMed] [Google Scholar]
- 7.Chauvaux S, Paulsen I T, Saier M H., Jr CcpB, a novel transcription factor implicated in catabolite repression in Bacillus subtilis. J Bacteriol. 1998;180:491–497. doi: 10.1128/jb.180.3.491-497.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis S J, Epstein W. Phosphorylation of d-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosetransferase, and glucokinase. J Bacteriol. 1975;122:1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvitkovitch D G, Boyd D A, Thevenot T, Hamilton I R. Glucose transport by a mutant of Streptococcus mutans unable to accumulate sugars via the phosphoenolpyruvate phosphotransferase system. J Bacteriol. 1995;177:2251–2258. doi: 10.1128/jb.177.9.2251-2258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahl M K. Enzyme IIglc contributes to trehalose metabolism in Bacillus subtilis. FEMS Microbiol Lett. 1997;148:233–238. [Google Scholar]
- 11.DiMarco A A, Romano A H. d-Glucose transport system of Zymomonas mobilis. J Bacteriol. 1985;49:151–157. doi: 10.1128/aem.49.1.151-157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egeter O, Brückner R. Catabolite repression mediated by the catabolite control protein CcpA in Staphylococcus xylosus. Mol Microbiol. 1996;21:739–749. doi: 10.1046/j.1365-2958.1996.301398.x. [DOI] [PubMed] [Google Scholar]
- 13.Egeter O, Brückner R. Characterization of a genetic locus essential for maltose-maltotriose utilization in Staphylococcus xylosus. J Bacteriol. 1995;177:2408–2415. doi: 10.1128/jb.177.9.2408-2415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiegler H, Brückner R. Identification of the serine acetyltransferase gene of Staphylococcus xylosus. FEMS Microbiol Lett. 1997;148:181–187. doi: 10.1111/j.1574-6968.1997.tb10286.x. [DOI] [PubMed] [Google Scholar]
- 15.Fujita Y, Miwa Y. Catabolite repression of the Bacillus subtilis gnt operon mediated by the CcpA protein. J Bacteriol. 1994;176:511–513. doi: 10.1128/jb.176.2.511-513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita Y, Miwa Y, Galinier A, Deutscher J. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol Microbiol. 1995;17:953–960. doi: 10.1111/j.1365-2958.1995.mmi_17050953.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujita Y, Ramaley R, Freese E. Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis. J Bacteriol. 1977;132:282–293. doi: 10.1128/jb.132.1.282-293.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galinier A, Haiech J, Kilhoffer M C, Jaquinod M, Stülke J, Deutscher J, Martin-Verstraete I. The Bacillus subtilis crh gene encodes a HPr-like protein involved in carbon catabolite repression. Proc Natl Acad Sci USA. 1997;94:8439–8444. doi: 10.1073/pnas.94.16.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galinier A, Kravanja M, Engelmann R, Hengstenberg W, Kilhoffer M C, Deutscher J, Haiech J. New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression. Proc Natl Acad Sci USA. 1998;95:1823–1828. doi: 10.1073/pnas.95.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gösseringer R, Küster E, Galinier A, Deutscher J, Hillen W. Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J Mol Biol. 1997;266:665–676. doi: 10.1006/jmbi.1996.0820. [DOI] [PubMed] [Google Scholar]
- 21.Götz F, Zabielski J, Philipson L, Lindberg M. DNA homology between the arsenate resistance plasmid pSX267 from Staphylococcus xylosus and the penicillinase plasmid pI258 from Staphylococcus aureus. Plasmid. 1983;9:126–137. doi: 10.1016/0147-619x(83)90015-x. [DOI] [PubMed] [Google Scholar]
- 22.Hammes W P. Bacterial starter cultures in food production. Food Biotechnol. 1990;4:383–397. [Google Scholar]
- 23.Henkin T M, Grundy F J, Nicholson W L, Chambliss G H. Catabolite repression of alpha-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacl and galR repressors. Mol Microbiol. 1991;5:575–584. doi: 10.1111/j.1365-2958.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann K, Stoffel W. TMbase—a database of membrane spanning segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 25.Hueck C J, Hillen W, Saier M H., Jr Analysis of a cis-active sequence mediating catabolite repression in gram-positive bacteria. Res Microbiol. 1994;145:503–518. doi: 10.1016/0923-2508(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 26.Hueck C J, Kraus A, Schmiedel D, Hillen W. Cloning, expression and functional analyses of the catabolite control protein CcpA from Bacillus megaterium. Mol Microbiol. 1995;16:855–864. doi: 10.1111/j.1365-2958.1995.tb02313.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim J H, Chambliss G H. Contacts between Bacillus subtilis catabolite regulatory protein CcpA and amyO target site. Nucleic Acids Res. 1997;25:3490–3496. doi: 10.1093/nar/25.17.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J H, Guvener Z T, Cho J Y, Chung K C, Chambliss G H. Specificity of DNA binding activity of the Bacillus subtilis catabolite control protein CcpA. J Bacteriol. 1995;177:5129–5134. doi: 10.1128/jb.177.17.5129-5134.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J H, Voskuil M I, Chambliss G H. NADP, corepressor for the Bacillus catabolite control protein ccpA. Proc Natl Acad Sci USA. 1998;95:9590–9595. doi: 10.1073/pnas.95.16.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 31.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 32.Lokman B C, Heerikhuisen M, Leer R J, van den Broek A, Borsboom Y, Chaillou S, Postma P W, Pouwels P H. Regulation of expression of the Lactobacillus pentosus xylAB operon. J Bacteriol. 1997;179:5391–5397. doi: 10.1128/jb.179.17.5391-5397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luesink J L, van Herpen R E M A, Grossiord B P, Kuipers O P, M d V W. Transcriptional activation of the glycolytic las operon and catabolite repression of the gal operon in Lactococcus lactis are mediated by the catabolite control protein CcpA. Mol Microbiol. 1998;30:789–798. doi: 10.1046/j.1365-2958.1998.01111.x. [DOI] [PubMed] [Google Scholar]
- 34.Mitamura T, Ebora R V, Nakai T, Makino Y, Negoro S, Urabe I, Okada H. Structure of isoenzyme genes of glucose dehydrogenase from Bacillus megaterium IAM1030. J Ferment Bioeng. 1990;70:363–369. [Google Scholar]
- 35.Miwa Y, Nagura K, Eguchi S, Fukuda H, Deutscher J, Fujita Y. Catabolite repression of the Bacillus subtilis gnt operon exerted by two catabolite-responsive elements. Mol Microbiol. 1997;23:1203–1213. doi: 10.1046/j.1365-2958.1997.2921662.x. [DOI] [PubMed] [Google Scholar]
- 36.Monedero V, Gosalbes M J, Pérez-Martínez G. Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J Bacteriol. 1997;179:6657–6664. doi: 10.1128/jb.179.21.6657-6664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakatani Y, Nicholson W, Neitzke W L, Setlow P, Freese E. Sigma-G RNA polymerase controls forespore-specific expression of the glucose dehydrogenase operon in Bacillus subtilis. Nucleic Acids Res. 1988;17:999–1017. doi: 10.1093/nar/17.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker C, Barnell W O, Snoep J L, Ingram L O, Conway T. Characterization of the Zymomonas mobilis glucose facilitator gene product (glf) in recombinant Escherichia coli: examination of transport mechanism, kinetics and the role of glucokinase in glucose transport. Mol Microbiol. 1995;15:795–802. doi: 10.1111/j.1365-2958.1995.tb02350.x. [DOI] [PubMed] [Google Scholar]
- 39.Paulsen I T, Chauvaux S, Choi P, Saier M H., Jr Characterization of glucose-specific catabolite-resistant mutants of Bacillus subtilis: identification of a novel hexose:H+ symporter. J Bacteriol. 1998;180:498–504. doi: 10.1128/jb.180.3.498-504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peschke U, Beuck V, Bujard H, Gentz R, Le Grice S. Efficient utilization of Escherichia coli transcriptional signals in Bacillus subtilis. J Mol Biol. 1985;186:547–555. doi: 10.1016/0022-2836(85)90129-9. [DOI] [PubMed] [Google Scholar]
- 41.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;6:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramseier T M, Reizer J, Küster E, Hillen W, Saier M H. In vitro binding of the CcpA protein of Bacillus megaterium to cis-acting catabolite responsive elements (CREs) of gram-positive bacteria. FEMS Microbiol Lett. 1995;129:207–213. doi: 10.1111/j.1574-6968.1995.tb07581.x. [DOI] [PubMed] [Google Scholar]
- 43.Rather P N, Moran C P., Jr Compartment-specific transcription in Bacillus subtilis: identification of the promoter for gdh. J Bacteriol. 1988;170:5086–5092. doi: 10.1128/jb.170.11.5086-5092.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reizer J, Hoischen C, Titgemeyer F, Rivolta C, Rabus R, Stülke J, Karamata D, Saier M H J, Hillen W. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol Microbiol. 1998;27:1157–1169. doi: 10.1046/j.1365-2958.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 45.Reizer J, Sutrina S L, Saier M H, Stewart G C, Peterkofsky A, Reddy P. Mechanistic and physiological consequences of HPr(ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J. 1989;8:2111–2120. doi: 10.1002/j.1460-2075.1989.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell J B. Low-affinity, high-capacity system of glucose transport in the ruminal bacterium Streptococcus bovis: evidence for a mechanism of facilitated diffusion. Appl Environ Microbiol. 1990;56:3304–3307. doi: 10.1128/aem.56.11.3304-3307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saier M H., Jr A multiplicity of potential carbon catabolite repression mechanisms in prokaryotic and eukaryotic microorganisms. New Biol. 1991;3:1137–1147. [PubMed] [Google Scholar]
- 48.Saier M H, Jr, Chauvaux S, Deutscher J, Reizer J, Ye J J. Protein phosphorylation and regulation of carbon metabolism in gram-negative versus gram-positive bacteria. Trends Biochem Sci. 1995;20:267–271. doi: 10.1016/s0968-0004(00)89041-6. [DOI] [PubMed] [Google Scholar]
- 49.Schleifer K H, Kloos W E. Isolation and characterization from human skin. I. Amended descriptions of Staphylococcus epidermidis and Staphylococcus saprophyticus and description of three new species: Staphylococcus cohnii, Staphylococcus haemolyticus and Staphylococcus xylosus. J Syst Bacteriol. 1975;174:3042–3048. [Google Scholar]
- 50.Sweet G, Gandor C, Voegele R, Wittekindt N, Beuerle J, Truniger V, Lin E C, Boos W. Glycerol facilitator of Escherichia coli: cloning of glpF and identification of the glpF product. J Bacteriol. 1990;172:424–430. doi: 10.1128/jb.172.1.424-430.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teusink B, Diderich J A, Westerhof H V, van Dam K, Walsh M C. Intracellular glucose concentration in derepressed yeast cells consuming glucose is high enough to reduce the glucose transport rate by 50% J Bacteriol. 1998;180:556–562. doi: 10.1128/jb.180.3.556-562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vary J C, Halvorson H O. Initiation of bacterial spore germination. J Bacteriol. 1968;95:1327–1334. doi: 10.1128/jb.95.4.1327-1334.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner E, Marcandier S, Egeter O, Deutscher J, Götz F, Brückner R. Glucose kinase-dependent catabolite repression in Staphylococcus xylosus. J Bacteriol. 1995;177:6144–6152. doi: 10.1128/jb.177.21.6144-6152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wax R, Freese E. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of l-alanine. J Bacteriol. 1968;95:433–438. doi: 10.1128/jb.95.2.433-438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weisser P, Krämer R, Sahm H, Sprenger G A. Functional expression of the glucose transporter of Zymomonas mobilis leads to restoration of glucose and fructose uptake in Escherichia coli mutants and provides evidence for its facilitator action. J Bacteriol. 1995;177:3351–3354. doi: 10.1128/jb.177.11.3351-3354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamane K, Kumano M, Kurita K. The 25 degrees–36 degrees region of the Bacillus subtilis chromosome: determination of the sequence of a 146 kb segment and identification of 113 genes. Microbiology. 1996;142:3047–3056. doi: 10.1099/13500872-142-11-3047. [DOI] [PubMed] [Google Scholar]
- 57.Youngman P, Poth H, Green B, York K, Olmedo G, Smith K. Methods for genetic manipulation, cloning, and functional analysis of sporulation genes in Bacillus subtilis. In: Smith I, Slepecky A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C: American Society for Microbiology; 1989. pp. 65–87. [Google Scholar]