Abstract
Background:
Type 2 diabetes mellitus (T2DM) has been associated with an increased risk of developing several common cancers, but it is unclear whether this association is causal. We aimed to summarize the evidence on T2DM and cancer and evaluate the validity of associations from both observational and Mendelian randomization (MR) studies.
Methods:
We performed an umbrella review of the evidence across meta-analyses of observational studies that examined associations of T2DM with risk of developing or dying from site-specific cancers, and MR studies that explored the potential causal association of T2DM and associated biomarkers with cancer risk.
Results:
We identified eligible observational meta-analyses that assessed associations between T2DM and cancer incidence for 18 cancer sites, cancer mortality for seven sites, and cancer incidence or mortality for four sites. Positive associations between T2DM and six cancers reached strong or highly suggestive evidence. We found eight MR studies assessing the association of genetically predicted T2DM and seven and eight studies assessing the association of genetically predicted fasting insulin or fasting glucose concentrations, respectively, upon site-specific cancers. Positive associations were found between genetically predicted T2DM and fasting insulin and risk of six cancers. There was no association between genetically predicted fasting plasma glucose and cancer except for squamous cell lung carcinoma.
Conclusions:
We found robust observational evidence for the association between T2DM and colorectal, hepatocellular, gallbladder, breast, endometrial, and pancreatic cancers.
Impact:
Potential causal associations were identified for genetically predicted T2DM and fasting insulin concentrations and risk of endometrial, pancreas, kidney, breast, lung, and cervical cancers.
Introduction
The prevalence of diabetes has increased by more than 4-fold since 1980 and in 2014, there were over 420 million individuals living with diabetes (1). Compelling evidence for a causal link between type 2 diabetes mellitus (T2DM) and renal disease (2), coronary heart disease (3) and stroke (2) has led to the development of targeted prevention approaches (4). Type 2 diabetes has also been associated in observational studies with several cancers, including breast, colorectal, endometrial, gallbladder, liver, and pancreatic cancer (5). Cancer is a leading cause of mortality and morbidity, with 18.1 million cases worldwide in 2018 (6) and a recent study estimated 293,000 cancer cases globally could be attributable to diabetes in 2012 (7); accordingly, prevention of type 2 diabetes may also reduce the burden of cancer.
Effective clinical and public health policy can be informed by robust evidence regarding site-specific cancer associations with type 2 diabetes and by identifying potential causal associations and pathophysiological pathways. Hyperinsulinemia and hyperglycemia (8) are leading proposed mechanisms underlying the type 2 diabetes–cancer association; however, these potential mechanisms have not been fully characterized. Observational research into the association between type 2 diabetes and cancer has been extensive but is vulnerable to several biases, including residual confounding, reporting bias (9), and type 2 diabetes classification bias. Our previous umbrella review of meta-analyses of observational studies on type 2 diabetes and cancer concluded that only a minority of reported associations had strongly statistically significant results without hints of bias (5). Subsequently, several meta-analyses on type 2 diabetes and cancer have been published. Thus, we updated our umbrella review of observational evidence investigating type 2 diabetes in relation to cancer incidence or mortality, and extended the analysis to include Mendelian randomization (MR) studies.
Materials and Methods
Eligibility of observational studies
For this update to our previous umbrella review (5), we searched PubMed from January 1, 2014 to June 16, 2020 for systematic reviews or meta-analyses of epidemiological studies using the following algorithm: “(diabetes) AND (cancer OR carcinoma OR neoplasia OR tumor OR neoplasm OR maligna*) AND (meta-analysis OR systematic review).” References from relevant systematic or narrative reviews were manually reviewed. The titles, abstracts, and full texts of the resulting articles were examined in detail by two authors (J. Pearson-Stuttard and A. Kakourou), and discrepancies were resolved by consensus.
We included systematic reviews and meta-analyses of cohort studies or combined cohort and case–control studies in humans, in which type 2 diabetes was the exposure of interest and cancer incidence and/or mortality were the outcomes of interest. We excluded meta-analyses of prognostic studies associating type 2 diabetes and outcomes among patients with cancer. Where meta-analyses did not present study-specific data, such as relative risks (RR), 95% confidence intervals (CI), and number of cases or total population, we extracted these data from the primary studies. When we identified more than one meta-analysis per outcome, the meta-analysis with the highest quality assessment score was selected to avoid duplication; for equivocal quality scores, the larger meta-analysis was retained. The “duplicate” meta-analyses were evaluated separately in a sensitivity analysis. The methodological quality of all the systematic reviews and meta-analyses included in this study was assessed with the AMSTAR tool, an 11-item questionnaire from which positive responses are summed to obtain an overall quality score (high, ≥8; moderate, 4–7; low, <4; ref. 10). The quality evaluation was performed by S. Cividini and reviewed by K.K. Tsilidis.
From each eligible systematic review or meta-analysis, we extracted the name of the first author, year of publication, exposure, outcome, and meta-analytic estimate in duplicate by two study authors (J. Pearson-Stuttard and N. Papadimitriou/G. Monori). From each individual study in a meta-analysis, we extracted the name of first author and publication year, epidemiological design, number of cases and total population, maximally adjusted RR (e.g., hazard ratio or standardized incidence/mortality ratio in prospective or retrospective cohort studies, respectively, and odds ratios in case–control studies) and 95% CIs.
Data analysis of observational studies
The statistical analysis for umbrella reviews has been described previously in detail in the published literature (11–13). Briefly, for each exposure and outcome pair, we calculated the summary effect and the 95% CI using both fixed and random effects inverse variance weighted methods (14). Heterogeneity between studies was assessed with the Cochran's Q test (15) and the I2 metric of inconsistency (16). We calculated 95% prediction intervals for the summary random effect estimates, which further account for between study heterogeneity and represent the range of estimates expected for future studies (17). The small study effects were evaluated by the Egger's regression asymmetry test (P ≤ 0.10) and whether the random effects summary estimate was larger than the point estimate of the largest study (i.e., smallest standard error) in the meta-analysis. For excess significance bias, we compared the observed number of studies with nominally statistically significant results (i.e., P < 0.05) in the published literature to the expected number of studies with significant results (18). The expected number of significant studies in each meta-analysis was calculated from the sum of the statistical power estimates for each component study, calculated with an algorithm from a non-central t distribution (19, 20). The power estimates of each component study depend on the plausible effect size for the tested association, which was assumed to be the effect of the largest study in each meta-analysis (21). Excess significance for individual meta-analyses was determined at P ≤ 0.10 (18).
Grading the evidence of observational studies
The strength of observational evidence for type 2 diabetes and cancer was categorized using the aforementioned criteria (11–13). Briefly, a “strong association” referred to meta-analyses with a random effects model P value smaller than 10−6 (a threshold that might substantially reduce false-positive findings; refs. 22–24), more than 1,000 cancer cases, I2 values below 50%, 95% prediction intervals excluding the null value, and no indication of small study effects or excess significance bias. A “highly suggestive association” required a random effects model P value smaller than 10−6, more than 1,000 cancer cases, and nominally significant results in the largest study included (P < 0.05). “Suggestive” associations had a random effects P value smaller than 10−3 and more than 1,000 cases. All other meta-analyses with a nominally significant random effects model P value were classified as “weak association.” The main analysis included meta-analyses of both cohort and case–control studies, but we conducted a sensitivity analysis, including cohort studies only. All statistical analyses were performed using Stata version 13 (25), and all P values were two-tailed.
Eligibility and statistical analysis of MR studies
We searched for MR studies evaluating potential causal associations between type 2 diabetes and cancer. We additionally considered circulating concentrations of fasting insulin, fasting glucose, and glycosylated hemoglobin (HbA1C) as exposures, given their potential role as a mediator or as a primary mechanism in the type 2 diabetes–cancer association (8, 26–33). We used the following search algorithm in PubMed, from inception to June, 16 2020: “(diabetes OR insulin OR glucose OR HbA1c) AND (cancer OR carcinoma OR neoplasia OR tumor OR neoplasm OR maligna*) AND (Mendelian randomization OR Mendelian randomization).” The titles, abstracts, and full texts of the resulting articles were examined in detail by two authors (N. Papadimitriou and G. Markozannes/D. Gill), and discrepancies were resolved by consensus.
We included MR studies that assessed cancer incidence according to genetic instruments for type 2 diabetes, fasting insulin, glucose or HbA1C. From each eligible MR study, we extracted the name of the first author, year of publication, specific exposure studied, choice of genetic instruments, percentage of variance in the exposure explained by the instruments, outcome, sample size (cases and controls), main MR analysis approach, main result and additional sensitivity analyses. This was done in duplicate by two study authors (N. Papadimitriou and G. Markozannes/D. Gill). Type 2 diabetes is a binary trait, therefore only a proportion of the individuals with the genetic variants used to instrument its effects will actually have the condition (34). Furthermore, unlike type 2 diabetes, genetic variants used in MR analysis to proxy its effects may have lifelong cumulative consequences that begin from conception (35). Given the resultant limitations in using MR to estimate the effect of type 2 diabetes on risk of cancer (34, 36), only the statistical significance and direction of associations were assessed (35, 37). Evidence was categorized as either “present” or “not present,” with studies considered as providing evidence of a causal effect if they had a statistically significant effect estimate (P < 0.05) with further evaluation that this finding was not entirely attributable to possible bias related to pleiotropic effects of the genetic variants used as instruments (35).
Results
Description and analysis of observational studies
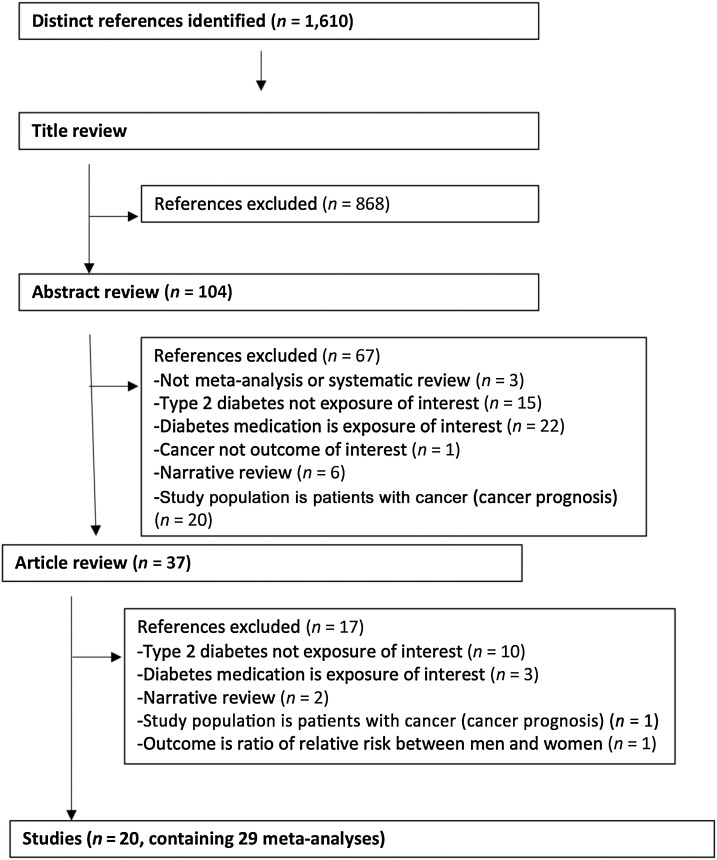
Of the 1,610 articles initially identified in PubMed, 20 studies reporting on 29 meta-analyses met our selection criteria (Fig. 1). When combined with findings from the prior period covered in the previous umbrella review (5), 29 studies met our selection criteria reporting 41 associations for the main (n = 29 meta-analyses) and sensitivity analysis (n = 12 duplicate meta-analyses; Fig. 1). The meta-analyses included associations of type 2 diabetes with risk of incidence (n = 26) or death (n = 9) or incidence or death (n = 6) from oral cancer (38), esophageal (39), gastric (40), colorectal (41), hepatocellular (42, 43), cholangiocarcinoma (44), biliary tract (45), gallbladder (46), pancreatic (47), lung (48), breast (49), endometrial (50), ovarian (51), localized prostate (52), total prostate (53), kidney (54), bladder (55), thyroid (56, 57) cancer, non-Hodgkin's lymphoma (NHL; ref. 58), myeloma (58), leukemia (58), and glioma (59, 60). There were 5 to 57 studies combined per meta-analysis in the main analysis; the median was 11 studies. Only the meta-analyses of cholangiocarcinoma incidence (n = 674) and hepatocellular carcinoma mortality (n = 292) had fewer than 1,000 cases (Supplementary Table S1). The included meta-analysis articles for the main analysis were of moderate study quality base on an average AMSTAR score of 6, ranging from 4 to 8. Respectively, the quality of the studied included in the sensitivity analysis of duplicate meta-analyses was also moderate with an average AMSTAR score of 5.1, ranging from 3 to 7 (Supplementary Table S2).
Figure 1.
Flow diagram of selection process of meta-analyses of type 2 diabetes and cancer in observational studies.
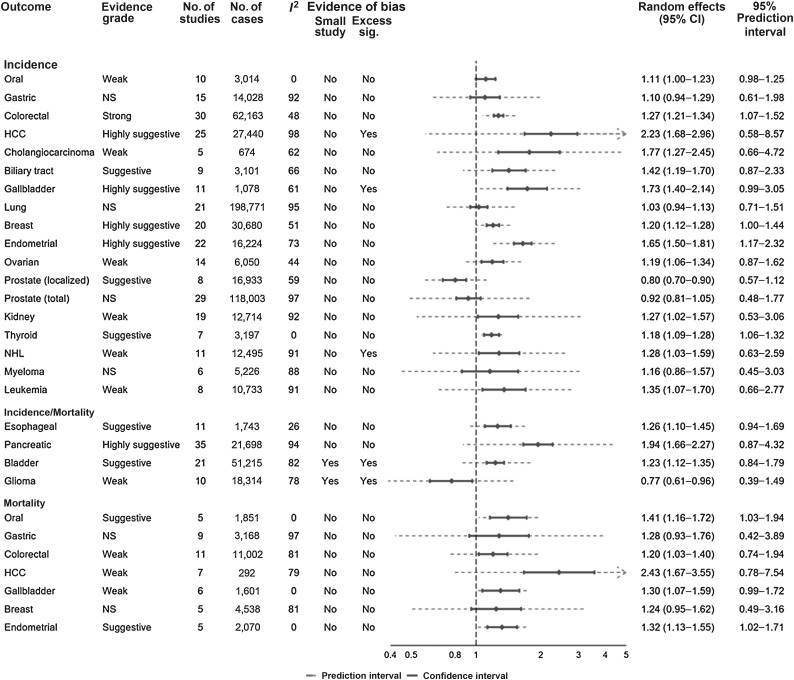
Out of the 29 meta-analyses in the main analysis, the summary random effects estimates were significant at P ≤ 0.05 in 23 meta-analyses (79%). When we used P ≤ 10−6 as a threshold for significance, only six (gallbladder, breast, hepatocellular, colorectal, endometrial and pancreatic cancer incidence) meta-analyses produced significant summary results (Fig. 2), all with increased risks of cancer in individuals with type 2 diabetes. Most (n = 22, 76%) of the largest study effects in each meta-analysis were nominally significant at P≤0.05. The effects of the largest studies were more conservative than the summary effects of the meta-analysis in 14 (48%) of the 29 meta-analyses. The results from random effects and fixed effect models were similar in all studies except three (breast cancer mortality, myeloma incidence) where the P value was significant in the fixed effects, but not in the random effects model, and glioma incidence/mortality where the P value was significant in random effects but not in the fixed effect model (Supplementary Table S1).
Figure 2.
Summary random effects estimates with 95% confidence and prediction intervals from 29 meta-analyses of type 2 diabetes and cancer incidence, mortality, or both.
The Q test showed significant heterogeneity (P ≤ 0.10) for 23 (79%) meta-analyses and 21 (72%) had an I2 statistic >50% (Fig. 2). There was moderate to high heterogeneity (I2 = 50%–75%) in six meta-analyses and high heterogeneity (I2 > 75%) in 15 meta-analyses (incidence of bladder, gastric, hepatocellular, kidney, lung, leukemia, multiple myeloma, NHL, pancreatic, and total prostate cancer; and mortality from breast, colorectal, gastric and hepatocellular cancer and incidence/mortality in glioma). When we calculated 95% prediction intervals, the null value was excluded only for colorectal, endometrial, thyroid cancer incidence, and oral and endometrial cancer mortality (Supplementary Table S1).
Small study effects according to the Egger's test (P < 0.10) were present for the meta-analyses of lung, pancreatic, and localized prostate cancer (incident only), and glioma and bladder cancer (incidence and mortality combined). However, only the bladder cancer and glioma incidence/mortality meta-analyses had adequate information (n = 21 and 10 studies, respectively) for the Egger's test (21). Three (10%) meta-analyses (on incidence of hepatocellular and NHL, and bladder cancer incidence/mortality) had evidence of a significant excess of “positive” studies when the plausible effect was assumed to be equal to the effect of the largest study in each meta-analysis (Supplementary Table S1).
Evidence grading of observational studies
The association between type 2 diabetes and colorectal cancer incidence was the only site supported by strong evidence (summary random effects RR, 1.27; 95% CI, 1.21–1.34), with strongly statistically significant results and no suggestion of bias (Supplementary Table S1; Fig. 2). Highly suggestive evidence was detected for type 2 diabetes and greater risk of hepatocellular, gallbladder, pancreatic, breast, and endometrial cancer incidence. Suggestive evidence was found for the positive association between type 2 diabetes and biliary tract and thyroid cancer (incidence), endometrial and oral cancer (mortality), and bladder and esophageal cancers (incidence/mortality), and for the inverse association for localized prostate cancer. Associations with thyroid cancer incidence and oral and endometrial cancer-related mortality satisfied all criteria for a “strong” grading except for the random effects P value, which was about 10−4 and not at 10−6. The remaining 10 associations under study were only supported by weak evidence.
Sensitivity analyses of the 12 duplicate meta-analyses yielded broadly similar findings (Supplementary Table S3). Most notably, duplicate meta-analyses for breast and thyroid cancer incidence had suggestive and not significant associations, respectively, compared with highly suggestive and suggestive associations, respectively, in the main analysis. When including cohort studies only, the evidence grade remained the same except for breast cancer incidence (from highly suggestive to suggestive) and glioma incidence/mortality (weak to not significant; Supplementary Table S4).
MR studies
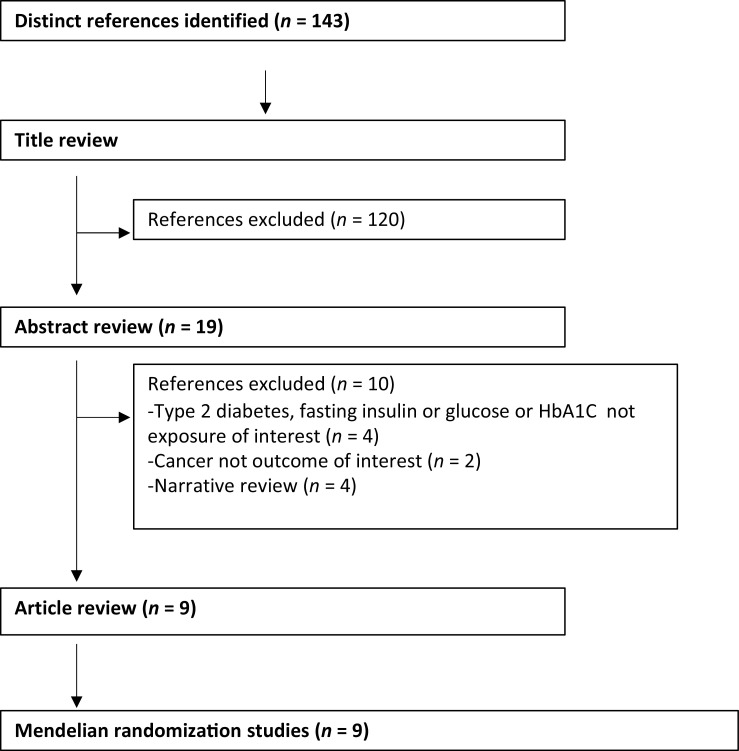
Of the 143 articles initially identified in the PubMed search (Fig. 3), eight studies and 31 MR analyses assessed a potential causal effect of genetically predicted type 2 diabetes with risk of pancreatic (61–63), liver (63), endometrial (63, 64), renal cell (63, 65), glioma (66), thyroid (63, 67), breast (63, 68), prostate (63, 68), cervix (63), biliary tract (63), ovarian (63), leukemia (63), head and neck (63), bladder (63), lung (63), stomach (63), NHL (63), colorectal (63), testicular (63), multiple myeloma (63), melanoma (63), brain (63), and esophageal cancers (63). Seven studies and 30 MR analyses assessed a potential causal effect of genetically predicted fasting insulin concentrations with risk of pancreatic (61–63), lung (69), breast (63, 70), endometrial (63, 64), renal cell (63, 65), glioma (66), liver (63), thyroid (63), cervix (63), biliary tract (63), ovarian (63), leukemia (63), head and neck (63), bladder (63), lung (63), stomach (63), NHL (63), colorectal (63), testicular (63), prostate (63) multiple myeloma (63), melanoma (63), brain (63), and esophageal cancer (63). There were eight studies and 32 MR analyses assessing a potential causal effect of genetically predicted fasting glucose concentrations with risk of pancreatic (61–63), lung (69), breast (63, 68, 70), endometrial (63, 64), renal cell (63, 65), glioma (66), prostate (68), thyroid (63), liver (63), thyroid (63), cervix (63), biliary tract (63), ovarian (63), leukemia (63), head and neck (63), bladder (63), lung (63), stomach (63), NHL (63), colorectal (63), testicular (63), prostate (63) multiple myeloma (63), melanoma (63), brain (63), and esophageal cancer (63). Finally there was one study that assessed the potential role of genetically predicted HbA1C concentrations in risk of breast (68) and prostate (68) cancer development (Supplementary Table S5). The methodological approaches used varied between studies, although consideration was consistently offered to potential bias arising from the pleiotropic effect of genetic variants through pathways unrelated to the exposure under consideration.
Figure 3.
Flow diagram of selection process of Mendelian randomization studies.
Potentially causal positive associations were identified between genetically predicted type 2 diabetes and risk of pancreatic, kidney, endometrial, and cervical cancers, whereas inverse associations were observed with risk of esophageal carcinoma and melanoma. However, a larger MR study found no association between type 2 diabetes and renal cell carcinoma. Positive associations were observed for genetically predicted fasting insulin concentrations and risk of pancreatic, endometrial, kidney, breast and lung cancer. Genetically predicted fasting glucose or HbA1C concentrations were not associated with cancer risk with the exception of a positive association identified between fasting glucose and squamous cell lung cancer only. Consistent results were achieved in sensitivity analyses performed to investigate possible bias related to pleiotropic variants in the main analysis.
Triangulation of evidence
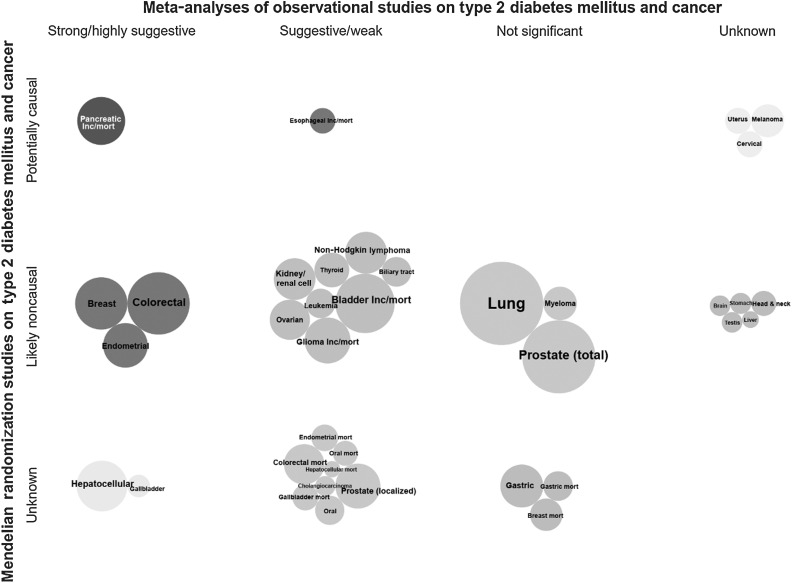
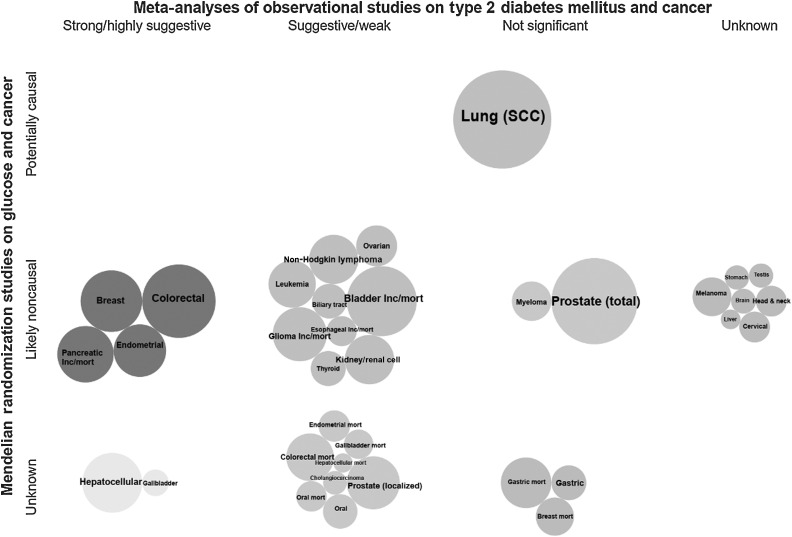
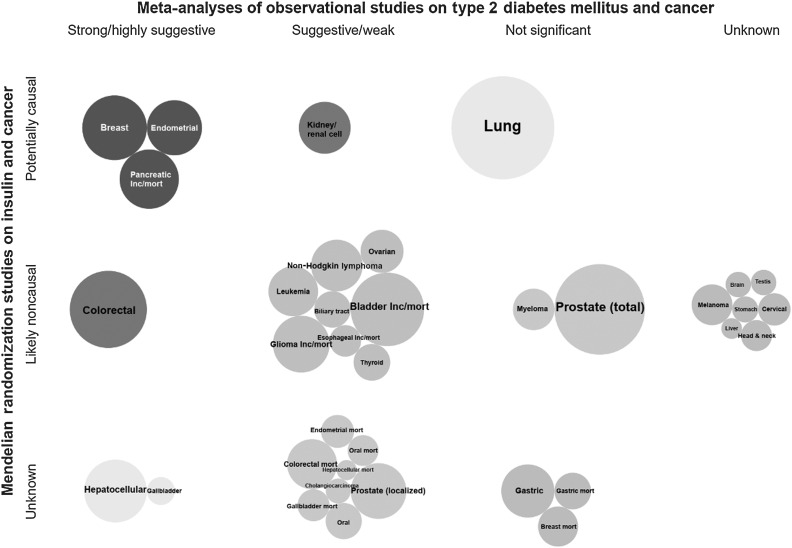
Triangulation of the evidence from both observational and MR studies suggests a mixed picture (Figs. 4–6). Among the six cancer sites (i.e., colorectal, hepatocellular, gallbladder, breast, endometrial, and pancreatic cancers) that showed strong or highly suggestive observational evidence for a positive association with T2DM, there was corroborating MR evidence for genetically predicted T2DM and/or insulin concentrations and risk of pancreatic, endometrial, and breast cancers. Genetically predicted fasting glucose or HbA1C concentrations were not associated with any major cancer site. The associations of genetically predicted T2DM and/or insulin concentrations with risk of colorectal, hepatocellular, and gallbladder cancers did not reach statistical significance in an MR study conducted in the UK Biobank cohort, but this study had relatively small number of cancer cases. There were three additional positive associations between genetically predicted T2DM and/or insulin concentrations with risk of kidney, lung, and cervical cancers, which reached weak, and non-significant observational evidence or no relevant meta-analysis was identified, respectively. Furthermore, inverse associations were observed between genetically predicted T2DM, but not with insulin or glucose concentrations, and risk of esophageal carcinoma and melanoma, which were in disagreement with the observational evidence that showed a suggestive positive association for esophageal cancer and no relevant meta-analysis was identified for melanoma risk.
Figure 4.
Triangulation of evidence from observational and Mendelian randomization studies assessing association between type 2 diabetes and site-specific cancers. Bubble size corresponds to the number of cases in the corresponding meta-analysis (more cases→larger bubble). If no meta-analysis was available but an MR analysis was, then bubble size represents the number of cases in the MR analysis. Unless stated as incidence/mortality (i.e., both), is incidence, *, mortality.
Figure 6.
Triangulation of evidence from observational and Mendelian randomization studies assessing association between fasting glucose and site-specific cancers. Bubble size corresponds to the number of cases in the corresponding meta-analysis (more cases→larger bubble). If no meta-analysis was available but an MR analysis was, then bubble size represents the number of cases in the MR analysis. Unless stated as incidence/mortality (i.e., both), is incidence, *, mortality.
Figure 5.
Triangulation of evidence from observational and Mendelian randomization studies assessing association between fasting insulin and site-specific cancers. Bubble size corresponds to the number of cases in the corresponding meta-analysis (more cases→larger bubble). If no meta-analysis was available but an MR analysis was, then bubble size represents the number of cases in the MR analysis. Unless stated as incidence/mortality (i.e., both), is incidence, *, mortality.
Discussion
Our study provides a comprehensive update of the observational evidence linking type 2 diabetes and cancer risk across 21 different sites and is substantially enhanced by the inclusion of MR studies that address potential causation and mechanisms. The most robust observational evidence was detected for T2DM and increased risk of colorectal, breast, endometrial, gallbladder, hepatocellular, and pancreatic cancers, whereas MR studies supported a causal association between genetically predicted T2DM and/or fasting insulin concentrations and risk of endometrial, breast, and pancreatic cancer, as well as with lung, kidney, and cervical cancer.
This updated umbrella review advances previous work (5); along with several updated analyses, there are several cancer sites included in this analysis not previously included, namely lung cancer incidence, oral cancer and glioma incidence and mortality, and gallbladder, esophageal, pancreatic and bladder cancer-related mortality. Another major advancement of this study is the inclusion of MR studies. The opportunity to draw upon genetic data from large-scale, international consortia in MR studies allows for triangulation of evidence using distinct methodological approaches that make orthogonal underlying assumptions and suffer from distinct sources of bias (71). The MR findings of our study support a potential causal effect of genetically predicted T2DM and/or fasting insulin levels, rather than genetically predicted fasting glucose levels, on risk of breast, endometrial, pancreatic, and kidney cancers. These findings are consistent with experimental and molecular epidemiological data that support a role for insulin signaling in the development of several cancers (72, 73), and may therefore represent an important pathway linking T2DM and cancer.
The cancer sites with strong or highly suggestive observational evidence of a robust association with type 2 diabetes, namely colorectal, breast, endometrial, gallbladder, hepatocellular and pancreatic cancers, are also strongly associated with overweight and obesity (13, 74). Indeed previous work estimated that more than 800,000 new cancer cases each year are attributable to the combination of high BMI and diabetes, with these risk factors both increasing the risk of six site-specific cancers (7). Though some of these observational findings contrast with the null MR findings for genetically predicted type 2 diabetes and risk of colorectal, breast, gallbladder, and hepatocellular cancers; this may be due to the very heterogeneous genetic instruments for type 2 diabetes, which might involve different underlying mechanisms (e.g., beta-cell function, insulin, obesity, etc.; ref. 75) and lead to horizontal pleiotropy, or may be also due to the relatively small number of cancer cases (except for breast cancer) used in some current MR studies. Some of these reasons might also explain the inverse associations observed between genetically predicted T2DM and risk of esophageal carcinoma and melanoma. However, genetically predicted T2DM was associated with risk of pancreatic and endometrial cancers, and genetically predicted fasting insulin concentrations were associated with breast cancer risk. Future MR studies should try to subgroup the T2DM genetic instruments to specific mechanisms of action. Accordingly, further understanding of potential mediation by insulin levels or BMI could allow more precise causal identification, and also risk stratification and screening opportunities in this patient group.
The evidence provided by our study has clinical and public health implications, particularly for endometrial, breast, and pancreatic cancers, where evidence from both observational and MR studies is most robust. The global burden of cancer attributable to all diabetes is expected to increase 30% in women and 20% in men over the next two decades (7). Secondary prevention measures, reducing the risk of complications, are vital to reducing morbidity and mortality in patients with type 2 diabetes. Further research is needed to characterize the mechanisms and/or predictive characteristics of fasting insulin in relation to cancer risk, which could inform the development of clinical guidelines for early screening. In addition, these results highlight the overlapping nature of type 2 diabetes and cancer, which jointly occupy an increasing share of the global disease burden (76). Population-based strategies that target the largest modifiable drivers of type 2 diabetes and cancer (poor diet, obesity, alcohol, tobacco and physical inactivity) through altering the environment to favor affordable, health-promoting behaviors are positioned as an effective and equitable approach (77).
Our study has several strengths. We were deliberately systematic in our search algorithm for all studies of type 2 diabetes and cancer; however, the published meta-analyses of observational studies provided limited granularity by sex, cancer sub-type, or other covariates such as menopausal status and hormone replacement therapy use in women. We applied several statistical criteria and sensitivity analyses to evaluate the strength and validity of the observational evidence, which should not be considered causal criteria, especially when used individually, but we think that they are useful for identifying biases when used together. The MR framework also has several advantages and is complimentary to traditional epidemiology; it is able to overcome the confounding and reverse causation bias that limits the ability to draw causal inference in traditional observational research by using genetic variants that are randomly allocated at conception as instruments to proxy the effect of the exposure under consideration (78).
Limitations of this analysis include the accuracy of diabetes status. Self-reported type 2 diabetes status is 99% specific, but just 66% sensitive compared with medical records (79). Miss-classification bias is likely given that 46% of all estimated diabetes prevalence is in undiagnosed individuals. Clinical diagnosis of diabetes, diabetes duration, long-term glucose control (HbA1c and other related biomarkers), and treatment regimens (80) data are optimal but often unavailable in large cohort studies. Although our literature search was systematic, and our results consistent with duplicate independent meta-analyses, there is a risk of incomplete search. With respect to evidence grading, both asymmetry and excess significance tests offer hints of bias, not definitive proof thereof, but our estimates are likely to be conservative as a negative test result does not exclude the potential for bias. We confined the analysis to type 2 diabetes as there were very few studies of variable quality available considering associations between type 1 diabetes and cancer (81).
The MR approach also has its own limitations. For this analysis, we assessed studies using genetic instruments for type 2 diabetes, fasting insulin, glucose, and HbA1C. Type 2 diabetes is a binary outcome and as such corresponding genetic instruments will only relate to its incidence in a fraction of the considered populations (termed “compliers”). As such, MR effect estimates that consider type 2 diabetes as the exposure can be biased (34, 36), with genetic variants used as instruments to exert effects throughout an individual's life course, whereas type 2 diabetes typically arises in later life; therefore, the relative lack of association between genetically predicted type 2 diabetes with cancer risk must be interpreted with caution. In contrast, fasting insulin is a continuous trait, and it is more plausible that its genetic instruments will uniformly affect fasting insulin levels across individuals in the outcome population. Thus, this “monotonicity” assumption of MR will be held, and resultant effect estimates will be less susceptible to bias (35, 36). These potential sources of bias may in part explain the partial discrepancy in MR results when considering type 2 diabetes versus fasting insulin as the exposure. In addition, MR approaches vary, with no standardized or widely applicable reporting framework currently in use, which may result in more subtle bias related to methodological nuances (82). Importantly, the accumulation of data from future genome-wide association studies will enable investigation into other potential mechanisms underpinning the risk of cancer in patients with diabetes.
Conclusion
There is mounting evidence of a robust association between patients with type 2 diabetes and an increased risk of common cancers (i.e., pancreatic, endometrial and breast cancer, but also colorectal, hepatocellular, gallbladder, and kidney cancers). Understanding the mechanistic pathways underlying this risk is crucial to allow evidence-based prevention policies.
Authors' Disclosures
J. Pearson-Stuttard reports personal fees from Novo Nordisk A/S and Lane Clark & Peacock LLP, and is vice-chairman of the Royal Society for Public Health outside the submitted work. D. Gill reports other from Novo Nordisk outside the submitted work. No disclosures were reported by the other authors.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization. Because all data are from published studies (secondary data) and no patient data are identifiable, no ethics approval was required for this study.
Acknowledgments
No additional acknowledgements above authorship. No specific funding for this study. J. Pearson-Stuttard was supported by a National Institute for Health Research Academic Clinical Fellowship and now via the Wellcome Trust 4i Program at Imperial College London (203928/Z/16/Z).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 1033
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Authors' Contributions
J. Pearson-Stuttard: Conceptualization, formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. N. Papadimitriou: Investigation, methodology, writing–review and editing. G. Markozannes: Investigation, methodology, writing–review and editing. S. Cividini: Formal analysis, investigation, writing–review and editing. A. Kakourou: Investigation, writing–review and editing. D. Gill: Validation, investigation, writing–original draft, writing–review and editing. E.C. Rizos: Investigation, writing–review and editing. G. Monori: Investigation, writing–review and editing. H.A. Ward: Investigation, writing–review and editing. M. Kyrgiou: Writing–review and editing. M.J. Gunter: Conceptualization, writing–review and editing. K.K. Tsilidis: Conceptualization, supervision, methodology, writing–review and editing.
References
- 1. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Genuth S, Eastman R, Kahn R, Klein R, Lachin J, Lebovitz H, et al. Implications of the United kingdom prospective diabetes study. Diabetes Care 2003;26:S28–32. [DOI] [PubMed] [Google Scholar]
- 3. Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS ONE 2013;8:e65174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute for Health and Care Excellence. NICE Guidelines Clinical Guidelines 66—TYPE 2 diabetes. Available from:https://www.nice.org.uk/guidance/cg66. Accessed 5 Aug2015. [Google Scholar]
- 5. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 6. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 7. Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 2018;6:e6–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giovannucci E. Insulin and colon cancer. Cancer Causes Control 1995;6:164–79. [DOI] [PubMed] [Google Scholar]
- 9. Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan A-W, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One 2008;3:e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JP, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta-analyses. Alzheimers Dement 2017;13:406–18. [DOI] [PubMed] [Google Scholar]
- 12. Markozannes G, Tzoulaki I, Karli D, Evangelou E, Ntzani E, Gunter MJ, et al. Diet, body size, physical activity and risk of prostate cancer: an umbrella review of the evidence. Eur J Cancer 2016;69:61–9. [DOI] [PubMed] [Google Scholar]
- 13. Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 2017;356:j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 15. Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. [Google Scholar]
- 16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 18. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials 2007;4:245–53. [DOI] [PubMed] [Google Scholar]
- 19. Tsilidis KK, Panagiotou OA, Sena ES, Aretouli E, Evangelou E, Howells DW, et al. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol 2013;11:e1001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsilidis KK, Papatheodorou SI, Evangelou E, Ioannidis JP. Evaluation of excess statistical significance in meta-analyses of 98 biomarker associations with cancer risk. J Natl Cancer Inst 2012;104:1867–78. [DOI] [PubMed] [Google Scholar]
- 21. Ioannidis JPA. Clarifications on the application and interpretation of the test for excess significance and its extensions. J Math Psych 2013;57:184–7. [Google Scholar]
- 22. Ioannidis JP, Tarone R, McLaughlin JK. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology 2011;22:450–6. [DOI] [PubMed] [Google Scholar]
- 23. Sterne JA, Davey Smith G. Sifting the evidence-what's wrong with significance tests? BMJ 2001;322:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson VE. Revised standards for statistical evidence. Proc Natl Acad Sci U S A 2013;110:19313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP. 2013. [Google Scholar]
- 26. Bruning PF, Bonfrèr JMG, van Noord PAH, Hart AAM, de Jong-Bakker M, Nooijen WJ. Insulin resistance and breast-cancer risk. Int J Cancer 1992;52:511–6. [DOI] [PubMed] [Google Scholar]
- 27. Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst 1999;91:542–7. [DOI] [PubMed] [Google Scholar]
- 28. Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, Swanson GM, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer 1999;80:1830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol 2005;6:103–11. [DOI] [PubMed] [Google Scholar]
- 30. Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, et al. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–37. [DOI] [PubMed] [Google Scholar]
- 31. Gapstur S, Gann P, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA 2000;283:2552–8. [DOI] [PubMed] [Google Scholar]
- 32. Seow A, Yuan J-M, Koh W-P, Lee H-P, Yu MC. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese health study. J Natl Cancer Inst 2006;98:135–8. [DOI] [PubMed] [Google Scholar]
- 33. Jee S, Ohrr H, Sull J, Yun J, Ji M, Samet J. Fasting serum glucose level and cancer risk in korean men and women. JAMA 2005;293:194–202. [DOI] [PubMed] [Google Scholar]
- 34. Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol 2018;33:947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res 2007;16:309–30. [DOI] [PubMed] [Google Scholar]
- 36. Burgess S, Small Dylan S. Predicting the direction of causal effect based on an instrumental variable analysis: a cautionary tale. J Causal Inference 2016;4:49–59. [Google Scholar]
- 37. VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P. Methodological challenges in mendelian randomization. Epidemiology 2014;25:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gong Y, Wei B, Yu L, Pan W. Type 2 diabetes mellitus and risk of oral cancer and precancerous lesions: a meta-analysis of observational studies. Oral Oncol 2015;51:332–40. [DOI] [PubMed] [Google Scholar]
- 39. Huang W, Ren H, Ben Q, Cai Q, Zhu W, Li Z. Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control 2012;23:263–72. [DOI] [PubMed] [Google Scholar]
- 40. Miao ZF, Xu H, Xu YY, Wang ZN, Zhao TT, Song YX, et al. Diabetes mellitus and the risk of gastric cancer: a meta-analysis of cohort studies. Oncotarget 2017;8:44881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol 2011;26:863–76. [DOI] [PubMed] [Google Scholar]
- 42. Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev 2012;28:109–22. [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Wang B, Yan S, Shen F, Cao H, Fan J, et al. Type 2 diabetes and gender differences in liver cancer by considering different confounding factors: a meta-analysis of cohort studies. Ann Epidemiol 2016;26:764–72. [DOI] [PubMed] [Google Scholar]
- 44. Jing W, Jin G, Zhou X, Zhou Y, Zhang Y, Shao C, et al. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev 2012;21:24–31. [DOI] [PubMed] [Google Scholar]
- 45. Ren HB, Yu T, Liu C, Li YQ. Diabetes mellitus and increased risk of biliary tract cancer: systematic review and meta-analysis. Cancer Causes Control 2011;22:837–47. [DOI] [PubMed] [Google Scholar]
- 46. Gu J, Yan S, Wang B, Shen F, Cao H, Fan J, et al. Type 2 diabetes mellitus and risk of gallbladder cancer: a systematic review and meta-analysis of observational studies. Diabetes Metab Res Rev 2016;32:63–72. [DOI] [PubMed] [Google Scholar]
- 47. Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer 2011;47:1928–37. [DOI] [PubMed] [Google Scholar]
- 48. Lee JY, Jeon I, Lee JM, Yoon JM, Park SM. Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer 2013;49:2411–23. [DOI] [PubMed] [Google Scholar]
- 49. Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007;121:856–62. [DOI] [PubMed] [Google Scholar]
- 50. Liao C, Zhang D, Mungo C, Tompkins DA, Zeidan AM. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol Oncol 2014;135:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang L, Wang L, Zhang J, Wang B, Liu H. Association between diabetes mellitus and subsequent ovarian cancer in women: a systematic review and meta-analysis of cohort studies. Medicine 2017;96:e6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu H, Jiang HW, Ding GX, Zhang H, Zhang LM, Mao SH, et al. Diabetes mellitus and prostate cancer risk of different grade or stage: a systematic review and meta-analysis. Diabetes Res Clin Pract 2013;99:241–9. [DOI] [PubMed] [Google Scholar]
- 53. Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis 2013;16:151–8. [DOI] [PubMed] [Google Scholar]
- 54. Bao C, Yang X, Xu W, Luo H, Xu Z, Su C, et al. Diabetes mellitus and incidence and mortality of kidney cancer: a meta-analysis. J Diabetes Complications 2013;27:357–64. [DOI] [PubMed] [Google Scholar]
- 55. Xu Y, Huo R, Chen X, Yu X. Diabetes mellitus and the risk of bladder cancer: a PRISMA-compliant meta-analysis of cohort studies. Medicine 2017;96:e8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schmid D, Behrens G, Jochem C, Keimling M, Leitzmann M. Physical activity, diabetes, and risk of thyroid cancer: a systematic review and meta-analysis. Eur J Epidemiol 2013;28:945–58. [DOI] [PubMed] [Google Scholar]
- 57. Yeo Y, Ma SH, Hwang Y, Horn-Ross PL, Hsing A, Lee KE, et al. Diabetes mellitus and risk of thyroid cancer: a meta-analysis. PLoS One 2014;9:e98135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Castillo JJ, Mull N, Reagan JL, Nemr S, Mitri J. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood 2012;119:4845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao L, Zheng Z, Huang P. Diabetes mellitus and the risk of glioma: a meta-analysis. Oncotarget 2016;7:4483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Y, Sun Y, Tang J, Zhou W, Liu X, Bi Y, et al. Does diabetes decrease the risk of glioma? A systematic review and meta-analysis of observational studies. Ann Epidemiol 2019;30:22–9. [DOI] [PubMed] [Google Scholar]
- 61. Carreras-Torres R, Johansson M, Gaborieau V, Haycock PC, Wade KH, Relton CL, et al. The role of obesity, type 2 diabetes, and metabolic factors in pancreatic cancer: a mendelian randomization study. J Natl Cancer Inst 2017;109:djx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lu Y, Gentiluomo M, Lorenzo-Bermejo J, Morelli L, Obazee O, Campa D, et al. Mendelian randomisation study of the effects of known and putative risk factors on pancreatic cancer. J Med Genet 2020;57:820–8. [DOI] [PubMed] [Google Scholar]
- 63. Yuan S, Kar S, Carter P, Vithayathil M, Mason AM, Burgess S, et al. Is type 2 diabetes causally associated with cancer risk? Evidence from a two-sample mendelian randomization study. Diabetes 2020;69:1588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nead KT, Sharp SJ, Thompson DJ, Painter JN, Savage DB, Semple RK, et al. Evidence of a causal association between insulinemia and endometrial cancer: a mendelian randomization analysis. J Natl Cancer Inst 2015;107:djv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Johansson M, Carreras-Torres R, Scelo G, Purdue MP, Mariosa D, Muller DC, et al. The influence of obesity-related factors in the etiology of renal cell carcinoma-A mendelian randomization study. PLoS Med 2019;16:e1002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Disney-Hogg L, Sud A, Law PJ, Cornish AJ, Kinnersley B, Ostrom QT, et al. Influence of obesity-related risk factors in the aetiology of glioma. Br J Cancer 2018;118:1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fussey JM, Beaumont RN, Wood AR, Vaidya B, Smith J, Tyrrell J. Does obesity cause thyroid cancer? A mendelian randomization study. J Clin Endocrinol Metab 2020;105:e2398–e2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Au Yeung SL, Schooling CM. Impact of glycemic traits, type 2 diabetes and metformin use on breast and prostate cancer risk: a Mendelian randomization study. BMJ Open Diabetes Res Care 2019;7:e000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carreras-Torres R, Johansson M, Haycock PC, Wade KH, Relton CL, Martin RM, et al. Obesity, metabolic factors and risk of different histological types of lung cancer: a Mendelian randomization study. PLoS ONE 2017;12:e0177875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shu X, Wu L, Khankari NK, Shu XO, Wang TJ, Michailidou K, et al. Associations of obesity and circulating insulin and glucose with breast cancer risk: a Mendelian randomization analysis. Int J Epidemiol 2019;48:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol 2016;45:1866–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Murphy N, Jenab M, Gunter MJ. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol 2018;15:659–70. [DOI] [PubMed] [Google Scholar]
- 73. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915–28. [DOI] [PubMed] [Google Scholar]
- 74. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Udler MS, Kim J, von Grotthuss M, Bonàs-Guarch S, Cole JB, Chiou J, et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med 2018;15:e1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Capewell S, Capewell A. An effectiveness hierarchy of preventive interventions: neglected paradigm or self-evident truth? J Public Health 2018;40:350–8. [DOI] [PubMed] [Google Scholar]
- 78. Smith GD, Ebrahim S. “ Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 79. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004;57:1096–103. [DOI] [PubMed] [Google Scholar]
- 80. International Diabetes Federation. IDF diabetes atlas. 6th ed. Brussels: International Diabetes Federation; 2013. [Google Scholar]
- 81. Sona MF, Myung SK, Park K, Jargalsaikhan G. Type 1 diabetes mellitus and risk of cancer: a meta-analysis of observational studies. Jpn J Clin Oncol 2018;48:426–33. [DOI] [PubMed] [Google Scholar]
- 82. Tan VY, Yarmolinsky J, Lawlor DA, Timpson NJ. Letter regarding article, “Associations of obesity and circulating insulin and glucose with breast cancer risk: a Mendelian randomization analysis.” Int J Epidemiol 2019;48:1014–5. [DOI] [PMC free article] [PubMed] [Google Scholar]