Abstract
Despite some impressive clinical results with immune checkpoint inhibitors, the majority of patients with cancer do not respond to these agents, in part due to immunosuppressive mechanisms in the tumor microenvironment. High levels of adenosine in tumors can suppress immune cell function, and strategies to target the pathway involved in its production have emerged. CD73 is a key enzyme involved in adenosine production. This led us to identify a novel humanized antagonistic CD73 antibody, mAb19, with distinct binding properties. mAb19 potently inhibits the enzymatic activity of CD73 in vitro, resulting in an inhibition of adenosine formation and enhanced T-cell activation. We then investigated the therapeutic potential of combining CD73 antagonism with other immune modulatory and chemotherapeutic agents. Combination of mAb19 with a PD-1 inhibitor increased T-cell activation in vitro. Interestingly, this effect could be further enhanced with an agonist of the adenosine receptor ADORA3. Adenosine levels were found to be elevated upon doxorubicin treatment in vivo, which could be blocked by CD73 inhibition. Combining CD73 antagonism with doxorubicin resulted in superior responses in vivo. Furthermore, a retrospective analysis of rectal cancer patient samples demonstrated an upregulation of the adenosine pathway upon chemoradiation, providing further rationale for combining CD73 inhibition with chemotherapeutic agents.
This study demonstrates the ability of a novel CD73 antibody to enhance T-cell function through the potent suppression of adenosine levels. In addition, the data highlight combination opportunities with standard of care therapies as well as with an ADORA3 receptor agonist to treat patients with solid tumors.
Introduction
Cancer immunotherapies have changed the treatment landscape for cancer patients, where immune checkpoint inhibitors targeting PD-1 and CTLA-4 can lead to rapid, deep, and durable responses in patients with various cancers (1). However, only a subset of patients respond to such therapies. Lack of response may be due to additional nonoverlapping immunosuppressive mechanisms which are utilized by tumors to evade immune surveillance. One such immune evasion mechanism is thought to be elevated adenosine levels in the tumor microenvironment.
Adenosine has broad immunosuppressive properties, for example, it potently suppresses T-cell functions such as proliferation, cytotoxicity, and cytokine production in vitro (2–4). Adenosine also suppresses the differentiation and maturation of dendritic cells leading to impaired T-cell responses (5). In vivo, genetic deletion of the major immunosuppressive adenosine receptor ADORA2A resulted in tumor rejection in mice (6). In addition, adenosine plays a stimulatory role on immunosuppressive cell types. Adenosine acts as a positive signal for regulatory T cell (Treg) proliferation and activation (7), for the expansion of immunosuppressive myeloid-derived suppressor cells, and the differentiation of macrophages into the immune-suppressive anti-inflammatory (M2) phenotype (8, 9).
CD73 (gene name NT5E) is a cell surface enzyme (ecto-5′-nucleotidase) which converts AMP to adenosine, and thus plays a central role in establishing an immunosuppressive environment in the tumor. There is rapid turnover of cancer cells within the tumor, and these dying cancer cells release ATP. Such extracellular ATP is converted to AMP by the cell surface enzyme CD39, enabling adenosine generation by CD73. As a consequence, adenosine has been found at micromolar concentrations in the tumor microenvironment (10). Several studies have provided evidence of a central role for CD73 in immunosuppression. Blockade of CD73 using the CD73 inhibitor APCP reduced adenosine levels in vitro (11). Enhanced T-cell function was observed using CD73 inhibition or genetic ablation of CD73 in vitro (11–13). In vivo, CD73-deficient mice showed delayed tumor growth (14), and mice treated with CD73 inhibitors showed antitumor effects with tumor regressions upon combination with PD-1 blockade (12, 13). Thus, targeting CD73 may be an attractive option to overcome immunosuppression in the tumor microenvironment.
A number of molecules targeting CD73 are currently being tested in the clinic (15). Oleclumab (MEDI9447) is the most advanced CD73 antagonist with ongoing clinical trials in breast cancer (NCT03616886, NCT03742102), pancreatic cancer (NCT03611556), and prostate cancer (NCT04089553) in combination with durvalumab, the ADORA2A antagonist AZD4635 or chemotherapy. A phase I study of oleclumab in combination with durvalumab showed partial responses in 1 of 21 patients with colorectal cancer and 2 of 20 patients with pancreatic cancer (16). Oleclumab has been shown to inhibit the enzymatic function of CD73 with an optimal inhibition at a 1:1 ratio of antibody to CD73 (17). At higher antibody concentrations, the authors described a loss of inhibition consistent with a loss of bivalent binding (17). We describe here a novel CD73 antibody which demonstrates enzymatic inhibition at antibody concentrations exceeding the 1:1 ratio of antibody to CD73, resulting in a distinct pharmacological profile. X-ray crystallography indicates a complex composition of a CD73 dimer and two Fab molecules, and this mode of binding translates to a potent inhibition of the adenosine pathway. In addition, we provide rationale for combining CD73 inhibition with stimulation of the ADORA3 receptor, or with chemotherapy.
Materials and Methods
Cell lines and cell culture
COLO201 cells (ATCC, CCL-224), NCI-H460 cells (ATCC, HTB-177), and 4T1 cells (ATCC, CRL-2539) were cultivated in RPMI1640 (ATCC, 30-2001), Calu-6 (DSMZ, ACC 734) in EMEM (Sigma-Aldrich, M5650), A549 (ATCC, CCL-185) in F12 K Nut Mix (Gibco, 21127-022), and MC-38 (obtained from Jeffrey Schlom, NIH) in DMEM (Sigma-Aldrich, D6429). Media of COLO201, NCI-H460, 4T1, Calu-6, A549, and MC-38 cells contained 10% FBS (Thermo Fisher Scientific, SH30071.03, heat inactivated). SK-ES-1 cells (ATCC, HTB-86) were cultivated in McCoy's 5a (Gibco, 36600-021) containing 15% FBS. Cell culture was performed at 37°C in a humidified incubator containing 5% CO2. Cell lines are authenticated by short tandem repeat analysis, regularly tested for Mycoplasma contamination and cultured for up to 20 passages.
Generation of isogenic cell lines
Lentiviral constructs were packaged as recommended by the supplier (abm LV003). NCI-H460_CD73(high) cells were generated using the Lenti-SFFV-IRES_dTomato-P2A-Puromycin vector. NCI-H460_CD73(low) cells were generated using a lentiviral system (pLenti-SFFV-dTomato-P2A-Puromycin-miRE) as described previously (18) containing short hairpin RNA targeting CD73.
FACS analysis of cell lines
Fc receptors were blocked (BioLegend, 422301) and cells incubated with CD73 antibody (clone AD2, Miltenyi Biotec, 130-095-183, APC-labeled) or isotype control (clone IS5-21F5, Miltenyi Biotec, 130-092-214). Cells were washed [PBS + 0.5% BSA (Gibco, 041-94553M) + 0.02% So-Azide (BioUltra, 10%; Morphisto, 13553.01000)], measured on a BD FACS Canto II Flow Cytometer and analyzed using FlowJo software.
Isolation of peripheral blood mononuclear cells
Primary human mononuclear cells (PBMC) were purchased from Stemcell Technologies or isolated from heparinized whole blood or leukapheresis samples of healthy donors (Red Cross Austria) using standard density gradient separation with Lymphoprep (Axis-Shield, 111454).
Antibodies
mAb19 (patent WO2019224025A3, Seq ID 115 and 116) was generated as described in the Supplementary Data. mAb19 and the corresponding isotype control (anti-TNP) were expressed in CHO-3E7 cells using previously described protocols (19).
The CD73 comparator antibody and the PD-1 antagonist MK-3475 were produced as described above according to sequences in patent US20160129108 and WO2012/135408, respectively.
CD73 enzymatic activity assay
As detailed in the Supplementary Data, recombinant CD73 was incubated with CD73 antagonist or isotype control. The substrate (200 μmol/L ATP and 600 μmol/L AMP) was added for 30 minutes, CellTiterGlo reagent was added and luminescence was measured using EnVision 2104 Multilabel Reader.
Protein expression
The Fab fragment of mAb19 was prepared by cleaving full-length mAb19 with Endo-Lys C (Roche, 11047825001) using an adapted protocol, as described in the Supplementary Data.
CD73 (encoding residues 27-549) was cloned into vector pET45 (Novagen; ref. 20) and expressed as described in the Supplementary Data.
Structural analysis
CD73 protein was mixed with mAb19 Fab and purified by size-exclusion chromatography. Crystals of the complex were obtained by vapor diffusion sitting drop method as described in the Supplementary Data. Statistics for the data collection and refinement are summarized in Supplementary Table S1. Data have been deposited in the RCSB Protein Data Bank (PDB; http://www.rcsb.org; accession number 7BBJ).
Analytical ultracentrifugation
Sedimentation velocity analytical ultracentrifugation (AUC) experiments were conducted using ProteomeLab XL-1 analytical ultracentrifuges (Beckman Coulter) with an An60Ti 8-hole rotor spinning at 40,000 rpm at 20°C with absorbance detection at 280 nm. Samples containing recombinant CD73 and either mAb19 or the CD73 comparator antibody were mixed at a 1:1 molar ratio (0.4 mg/mL final total concentration). g(S) was analyzed using SEDANAL (21).
Adenosine formation assay
Cells were treated with antibody or controls o/n followed by the addition of blocking solution to stop adenosine metabolism (22). Cells were incubated with 300 μmol/L AMP (Sigma-Aldrich, 01930), stop solution was added (15 mmol/L HCL, Merck, 1.09063.1000), and adenosine in the supernatants was quantified by HPLC/MS-MS with ESI+ ionization as described in the Supplementary Data.
CD73 activity present in cell culture supernatants (non–cell-bound CD73) and on the surface of cells (cell-bound CD73) was measured as described in the Supplementary Data.
T-cell assay
As detailed in the Supplementary Data, primary human T cells were prepared and labeled with Cell Trace Violet (Molecular Probes, C34557). T cells were stimulated with anti-CD3 and anti-CD28 antibody and treated o/n with CD73 antagonists or respective anti-TNP isotype control. Cells were cultured for 5 days in the presence of AMP. Flow cytometry and determination of cytokine levels in supernatants was done as described in the Supplementary Data.
Pharmacoscopy
Primary pleural effusion or ascites fluid was taken from patients under an ethically approved clinical study (following the Declaration of Helsinki) at the Medical University of Vienna (MedUni Wien EK# 1868/2018) during routine procedures, after the patient provided written informed consent.
Collection of cells and pharmacoscopy was performed as described previously (23). Cells were isolated and incubated with AIM-V media (Gibco, 12055-091) and mAb19 at 1 μg/mL or controls for the timepoints indicated. Cells were stained with fluorescent-labeled antibodies including anti-EpCAM (APC/PE/PerCP-Cy5.5) and DAPI to identify cancer cells and cell nuclei, respectively. A total of 2 × 2 combined images were taken for each well (4 in total merged, and one TIFF per channel) and analyzed as described previously (24). Statistics were determined by fitting the cell count data to a Poisson regression mixed linear model using experiment and sample, time, and stimulants as independent variables.
Allogeneic dendritic cell/T-cell assay
As detailed in the Supplementary Data, monocytes were enriched from PBMCs and matured into dendritic cells (mDC). mDCs and T cells from different donors were co-cultured and treated with CD73 antagonists, PD-1 antagonist, the ADORA3 receptor agonist CF102 (Sigma-Aldrich, C277), combinations thereof or the relevant isotype controls. After 5 days of cultivation in the presence of AMP human IFNγ levels in supernatants were determined as described in the Supplementary Data.
IHC analysis
Sections of archival formalin-fixed paraffin-embedded (FFPE) tissues from 50 patients with rectal cancer (untreated endoscopy biopsy samples at diagnosis and paired posttreatment surgical samples) were analyzed by IHC for CD73, CD8, and Pan-cytokeratin using the Ventana Benchmark Ultra platform (PR_PATMO_0010) as described previously and in the Supplementary Data (25). A total of 7 samples did not show residual tumor post-chemoradiation and were excluded from the analysis.
RNA analysis
RNA from FFPE tissue samples from patients with rectal cancer was extracted using RNeasy FFPE Kit (Qiagen). mRNA expression was analysed using a custom NanoString nCounter codeset as described in the Supplementary Data.
In vivo microdialysis studies
BALB/c or C57BL/6J female mice bearing MC38, MC38hCD73_65, or 4T1 tumors with an average tumor volume of approximately 200 mm3 were injected intraperitoneally with a dose of 30 mg/kg of CD73 comparator antibody, the corresponding isotype control or doxorubicine (Caelyx, Janssen-Cilag GmbH) at 1.5 mg/kg i.v. (4 mice per group). A total of 48 hours after treatment mice were anesthetized and a microdialysis probe with a CMA nitrocellulose membrane was inserted into the tumor. Dialysate was collected over 160 minutes and adenosine quantified by HPLC/MS-MS, as described in the Supplementary Data.
In vivo efficacy study
As detailed in the Supplementary Data, C57BL/6NTac mice were injected subcutaneously with 1 × 10 E5 human CD73 overexpressing MC38hCD73_65 cells in Matrigel Matrix (Corning). Three days after cell injection mice were treated with doxorubicine, the CD73 comparator antibody, the combination of doxorubicine and the CD73 comparator antibody, or the corresponding isotype control. Doxorubicine was dosed once weekly (every 7 days) at 1.5 mg/kg i.v., the CD73 comparator antibody or isotype control twice weekly (every 3 or 4 days) intraperitoneally at 10 mg/kg. Response (R) was defined for tumor sizes smaller than at the start of treatment at day 1 and is shown for the last day the animal was alive. Mice were handled according to the institutional, governmental and European Union guidelines (Austrian Animal Protection Laws, GV-SOLAS and FELASA guidelines). Animal studies were approved by the internal ethics committee and the local governmental committee.
Results
Discovery of a CD73 antibody with distinct binding properties
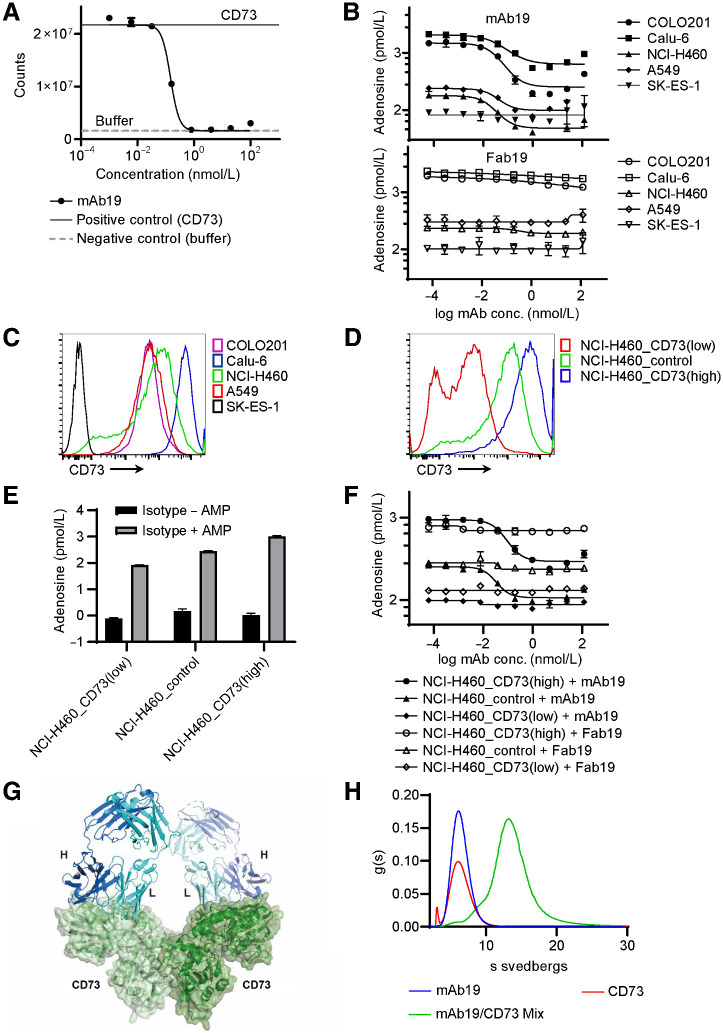
An antibody generation campaign followed by extensive screening led to the identification of clone mAb19, a high-affinity (KD of 74 pmol/L) CD73 antagonist antibody. mAb19 was engineered to contain mutations in the Fc portion to minimize IgG1 effector functions. mAb19 potently inhibited the enzymatic function of CD73 with an IC90 of 0.24 nmol/L up to concentrations of 100 nmol/L, a concentration 90-fold higher than the CD73 concentration in the assay (Fig. 1A). Thus, the antibody shows enzyme inhibition at concentrations exceeding the 1:1 molar ratio of antibody to CD73. Furthermore, mAb19 inhibited the formation of adenosine in a cell-based assay using COLO201, Calu-6, NCI-H460, or A549 cells (Fig. 1B). CD73 was undetectable in SK-ES-1 cells by FACS, adenosine levels were found to be low in these cells and were minimally affected by CD73 blockade using mAb19 (Fig. 1B and C). In contrast to the full-length mAb19, the Fab fragment of mAb19 led to barely detectable CD73 blockade (Fig. 1B). This indicates the requirement of a larger, possibly multivalent, binding partner to inhibit the enzymatic function via steric effects. We next generated isogenic NCI-H460_CD73(high) cells constitutively overexpressing high levels of CD73, and NCI-H460_CD73(low) cells constitutively expressing a small hairpin targeting CD73 resulting in reduced CD73 expression levels (Fig. 1D). Adenosine levels formed by these cell lines in the presence of AMP were found to correlate to CD73 expression levels (Fig. 1E). NCI-H460_CD73(high) cells generated high adenosine levels, which mAb19 was able to inhibit. NCI-H460 Renilla control cells (NCI-H460_control) formed lower levels of adenosine, which could also be inhibited by mAb19. NCI-H460_CD73(low) cells formed very little adenosine, with levels similar to those achieved in NCI-H460_control cells upon CD73 inhibition. mAb19 was not able to reduce the low adenosine levels of the NCI-H460_CD73(low) cells further (Fig. 1F). To gain insight into the mechanism of action of mAb19, we solved the crystal structure of the Fab fragment in complex with CD73 at a resolution of 2.73Å (Fig. 1G). The complex in the asymmetric unit is composed of a CD73 dimer, most similar to a conformation previously referred to as “open II” (PDB ID: 4H2G; Cα RMSD = 0.85; ref. 20). The two Fab molecules cover exclusively the surface of the N-terminal domain composed of residues between amino acids Phe137-Asn211, without affecting the dimerization interface (Supplementary Fig. S1). AUC molecular mass shift was performed to elucidate the complex stoichiometry between the full-length mAb19 and CD73. The data are consistent with a 2:2 complex formation (Fig. 1H), as observed in the co-crystal structure.
Figure 1.
mAb19 is a CD73 antagonist with distinct binding properties. A, The activity of human recombinant CD73 was measured in a luciferase-based assay in the presence of mAb19. Positive control: recombinant CD73; negative control: buffer only. The data shown represent mean ± SD of two biological replicates. B, Inhibition of adenosine formation was determined using COLO201, Calu6, NCI-H460, A549, or SK-ES-1 cells in the presence of AMP and mAb19 (top) or the Fab fragment of mAb19 (Fab19, bottom). Adenosine was quantified by HPLC MS-MS. The data shown represent mean ± SD of two biological replicates. C, Expression of CD73 in COLO201, Calu6, NCI-H460, A549, or SK-ES-1 by FACS. D, Expression of CD73 in NCI-H460_CD73(high), NCI-H460_control, and NCI-H460_CD73(low) cells by FACS. E, Isogenic cell lines NCI-H460_CD73(low), NCI-H460_control and NCI-H460_CD73(high) were treated with isotype control in the presence or absence of AMP. Adenosine was quantified by HPLC MS-MS. The data shown represent mean ± SD of two biological replicates. F, As in B, using the isogenic cell lines NCI-H460_CD73(low), NCI-H460_control, and NCI-H460_CD73(high). G, Crystal structure of CD73 in complex with the Fab fragment of mAb19. The Fab fragment of mAb19 is shown in ribbon representation with the light and heavy chain of the Fab fragment shown in cyan and blue, respectively. The monomers of the CD73 dimer are shown in ribbon representation with transparent surface light and dark green. H, AUC was performed for CD73 only, mAb19 only, or the mixture of CD73 and mAb19.
mAb19 potently inhibits the immunosuppressive adenosine pathway
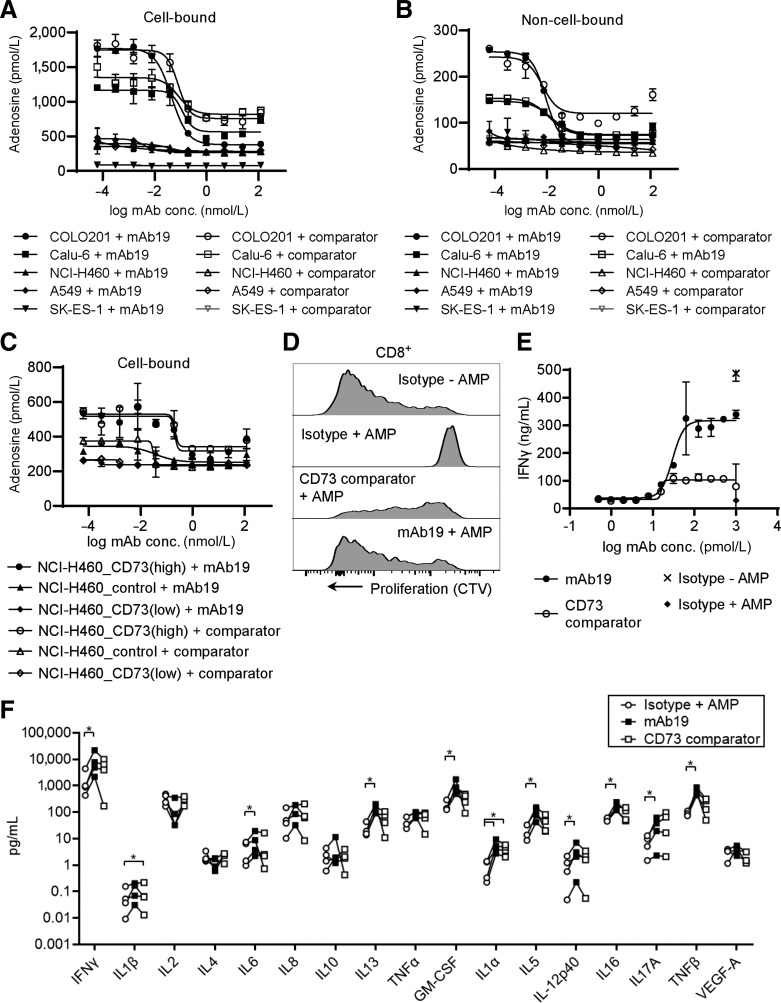
To demonstrate whether the described binding mode of mAb19 translates into different functional properties, we compared activity with another CD73 antibody described in patent US20160129108. This comparator CD73 antibody showed a loss of CD73 inhibition at antibody concentrations above 4 nmol/L, at CD73 concentrations of 1.1 nmol/L (Supplementary Fig. S2A). It formed larger complexes with CD73 when analyzed by AUC molecular mass shift as has been described for oleclumab (ref. 17; Supplementary Fig. S2C). Similar to mAb19, and to what has been described for oleclumab, the Fab of the comparator CD73 antibody showed lower potency of CD73 inhibition compared with the full-length antibody (Supplementary Fig. S2B; ref. 17). Next, we investigated the inhibition of the adenosine pathway by mAb19. The antibody was able to inhibit adenosine generation by CD73 on the cell surface (cell-bound CD73) of human COLO201, Calu-6, NCI-H460, or A549 cells (Fig. 2A). In cell lines with high adenosine levels such as COLO201, the inhibitory activity of mAb19 was more pronounced compared with the comparator CD73 mAb. SK-ES-1 cells showed very low levels of adenosine and thus very little inhibition of adenosine formation upon CD73 blockade. Furthermore, mAb19 inhibited CD73 present in cell culture supernatants (non–cell-bound CD73) of cell lines with sufficiently high adenosine levels (Fig. 2B). Both mAb19 as well as the comparator CD73 antibody showed reduced CD73 inhibition at high antibody concentrations, as described for oleclumab (17). In addition, we tested the inhibition of CD73 on the cell surface of the isogenic cell lines NCI-H460_CD73(high), NCI-H460_control, and NCI-H460_CD73(low). mAb19 inhibited adenosine formation in NCI-H460_CD73(high) and NCI-H460_control cells, whereas no further reduction of adenosine levels was possible in NCI-H460_CD73(low) cells (Fig. 2C). Similar to parental NCI-H460 cells, adenosine levels in the supernatants of the isogenic lines were found to be low and no further inhibition upon CD73 blockade was possible. Furthermore, we tested both mAb19 and the comparator CD73 antibody for their effects on T-cell function. Human T cells isolated from PBMCs were stimulated with anti-CD3 and anti-CD28 antibodies and both proliferation as well as IFNγ release were strongly suppressed by the addition of AMP (Fig. 2D and E). mAb19 was able to revert the suppressive effect of this external AMP on T-cell proliferation and IFNγ release. In comparison with the CD73 comparator antibody, mAb19 induced higher levels of T-cell proliferation and IFNγ release (Fig. 2D and E). We further assessed the cytokine profile after treatment of T cells with mAb19 or the comparator CD73 antibody in the presence of AMP (Fig. 2F). In addition to IFNγ the cytokines IL6, IL8, IL10, IL13, TNFα, GMCSF, IL1α, IL5, IL12p40, IL16, IL-17α, and TNFβ were found at increased levels after mAb19 treatment. The only cytokines found to decrease in the majority of the donors after mAb19 treatment were IL2 and IL4. Treatment with the CD73 comparator mAb led to a differential cytokine profile with lower levels of IFNγ, IL8, IL13, TNFα, GMCSF, IL5, IL12, IL16, and TNFβ compared to mAb19 treatment.
Figure 2.
mAb19 potently inhibited the CD73/adenosine pathway. A, Inhibition of cell-bound CD73. COLO201, Calu6, NCI-H460, A549 or SK-ES-1 cells were incubated with mAb19 or the CD73 comparator antibody, washed, and adenosine levels after addition of AMP were measured by HPLC/MS-MS. The data shown represent mean ± SD of two biological replicates. B, Inhibition of non–cell-bound CD73. COLO201, Calu6, NCI-H460, A549, or SK-ES-1 cells were incubated with mAb19 or the CD73 comparator antibody. Supernatants were taken and incubated with AMP followed by measurement of adenosine by HPLC/MS-MS. The data shown represent mean ± SD of two biological replicates. C, As in A, using isogenic NCI-H460_CD73(high), NCI-H460_control or NCI-H460_CD73(low) cell lines. D, Isolated human T cells were stimulated with anti-CD3/anti-CD28 and incubated with mAb19 or the CD73 comparator antibody in the presence of AMP. T-cell proliferation was determined by labeling with CTV. E, Isolated human T cells were treated as in D, supernatants were taken and analyzed for IFNγ. The data shown represent mean ± SD of two biological replicates. F, Isolated human T cells from 4 donors were treated as in D, with 1 nmol/L mAb19 or the CD73 comparator antibody for 6 days, supernatants were taken and analyzed for the cytokines indicated. Each circle represents one donor and is the mean ± SD of five biological replicates. Significance is indicated by asterisks; *, P < 0.05.
mAb19 reduces cancer cell burden in a primary human ex vivo pancreatic cancer model
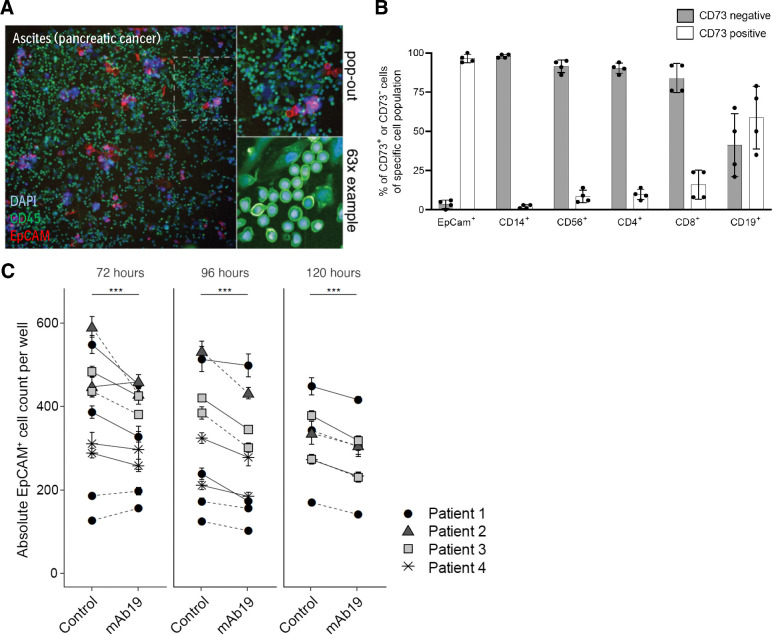
We investigated the effect of mAb19 on cancer cells in malignant pleural effusion or ascites samples from 4 patients diagnosed with pancreatic cancer, as a close-to-clinic physiologically relevant model system. These samples contained both CD45+ immune cells as well as cancer cells identified by surface EpCAM expression (Fig. 3A). The malignant samples each contained a cancer cell population of between 2% and 35% (Supplementary Table S2). The immune cell composition was found to be variable across patients (Supplementary Fig. S3A). CD73 was expressed on the majority of EpCAM+ cancer cells as well as on a fraction of CD56+, CD4+, CD8+, and CD19+ cells (Fig. 3B). Ex vivo, treatment of the primary cancer samples with mAb19 led to a reduction of EpCAM+ cancer cell numbers, determined by image-based phenotypic screening and subsequent single-cell quantification (Fig. 3C). This effect was observed in all four patient samples analyzed as well as in multiple independent repeats. In some samples, the loss of cells can also be seen by eye in the images (Supplementary Fig. S3B).
Figure 3.
mAb19 reduced cancer cell numbers ex vivo in human ascites or pleural effusion samples. A, Example images of an ascites sample from a pancreatic cancer patient. Cells were stained with DAPI, CD45 and EpCAM. B, CD73 expression on EpCAM+, CD14+, CD56+, CD4+, CD8+, and CD19+ cells analyzed by flow cytometry in n = 4 samples, mean with SD over all samples shown. C, Absolute EpCAM+ cell numbers of 1 μg/mL mAb19 versus control (media control in solid lines or 1 μg/mL isotype control in dotted lines) treated ascites or pleural effusion samples from n = 4 patients with pancreatic cancer determined by high-throughput imaging and subsequent single-cell image analysis, incubated for indicated timepoints. Each donor was tested in multiple experimental replicates, each consisting of technical replicates. Mean and SE of EpCAM+ cell counts are shown; significance (generalized linear model) of difference between treatment and control groups at each timepoint is indicated by asterisks; ***, P < 0.001.
Combination of mAb19 with an ADORA3 agonist enhances T-cell activation
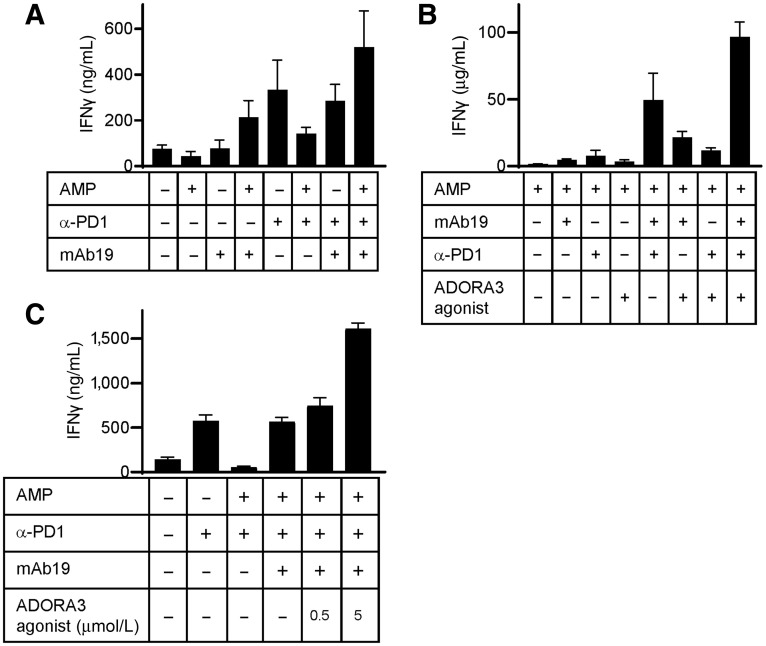
Rational combinations may lead to improved responses of patients with cancer to therapy. We therefore set out to test T-cell targeting agents in combination with CD73 inhibition in an allogeneic dendritic cell/T-cell assay. CD73 inhibition by mAb19 monotherapy in the presence, but not in the absence, of the CD73 substrate AMP led to IFNγ secretion (Fig. 4A). Inhibition of PD-1 by MK-3475 in the same assay resulted in markedly increased IFNγ secretion in a setting without external AMP, and this was strongly suppressed by the addition of AMP. Combining PD-1 inhibition with mAb19 in the presence of AMP was able to restore IFNγ secretion (Fig. 4A). Thus, the maximum benefit of CD73 antagonists is likely achieved in a combination setting targeting multiple immune regulatory mechanisms. We explored additional mechanisms to further enhance T-cell function. Unlike ADORA2A and ADORA2B, the adenosine receptor ADORA3 has been suggested to activate T cells (26, 27). We reasoned that activating the ADORA3 receptor in combination with CD73 inhibition might result in an even stronger modulation of the adenosine pathway and lead to enhanced activation of T-cell function. Combining the ADORA3 receptor agonist CF102 (Chloro-IB-MECA) and mAb19 resulted in an increase of IFNγ secretion compared to single agent treatments (Fig. 4B). IFNγ release induced by the ADORA3 agonist was found to be dose dependent (Fig. 4C). IFNγ release could be further increased by adding the PD-1 inhibitor MK-3475 to the mAb19/ADORA3 receptor agonist combination (Fig. 4B).
Figure 4.
Combination therapy for mAb19 has the potential to improve T-cell activation. A, Allogeneic human T cells were cocultured with mature dendritic cells and treated with 1 μg/mL mAb19, 10 μg/mL MK-3475 (α-PD1), the combination thereof or the corresponding isotype control in the presence or absence of AMP. After 5 days supernatants were analyzed for IFNγ. Shown are mean values of five biological replicates of one representative donor pair. B, As in A, with treatment of the cells with 1 μg/mL mAb19, 10 μg/mL MK-3475 (α-PD1), 5 μmol/L ADORA3 receptor agonist CF102, combinations thereof or the corresponding controls in the presence of AMP. C, As in A, with treatment of the cells with 1 μg/mL mAb19, 10 μg/mL MK-3475 (α-PD1), 0.5 or 5 μmol/L ADORA3 receptor agonist CF102, combinations thereof or the corresponding isotype controls in the presence or absence of AMP.
Elevated extracellular adenosine levels in mouse tumors upon chemotherapy treatment can be reversed by blocking CD73
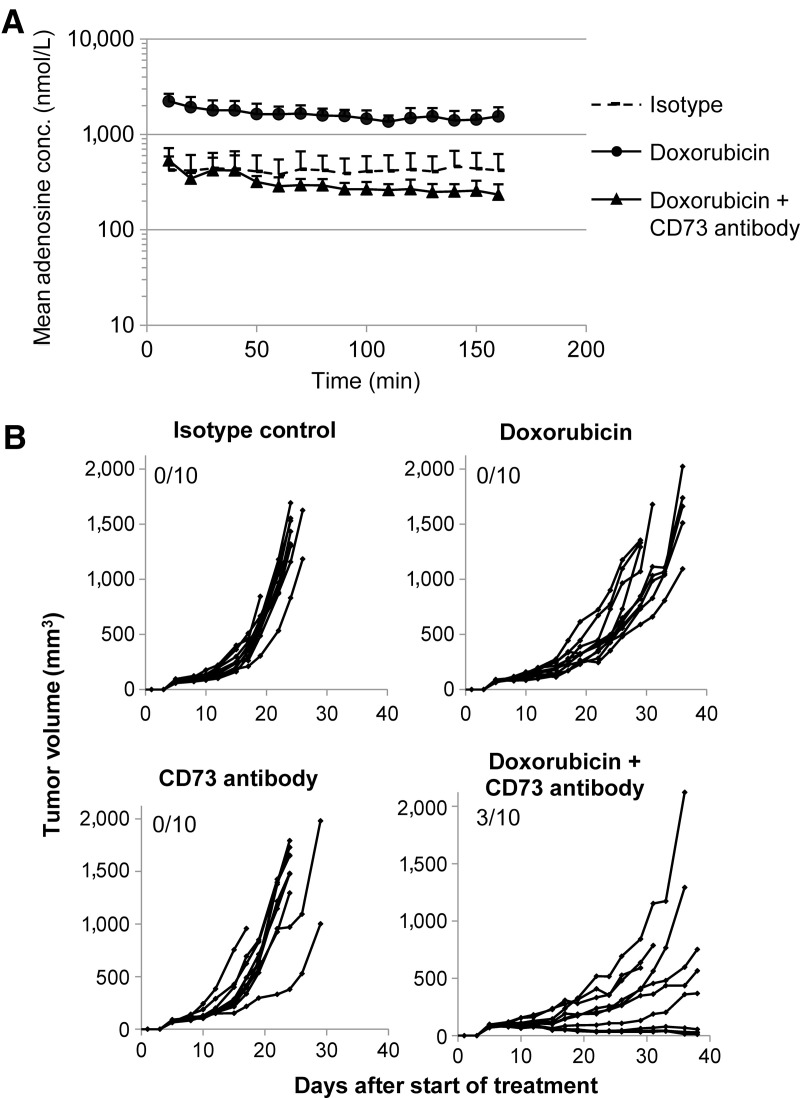
Chemotherapy is standard-of-care treatment for many cancer indications. Dying cancer cells release ATP into the extracellular space (28), and this might be a source of immunosuppressive adenosine through the enzymatic activity of CD39 and CD73. Because mAb19 showed limited cross-reactivity to mouse CD73 (Supplementary Fig. S2D), we used the mouse cross-reactive CD73 comparator antibody to study CD73 inhibition in vivo. We applied microdialysis as a method to determine extracellular adenosine concentrations in situ in syngeneic mouse tumors (Supplementary Fig. S4A). Treatment of mice carrying human CD73-overexpressing MC38hCD73_65 tumors with the chemotherapeutic doxorubicin led to 3- to 5-fold elevated intratumoral adenosine concentrations compared with control treatment. Upon simultaneous dosing with doxorubicin and the mouse cross-reactive comparator CD73 antibody, intratumoral adenosine concentrations were restored to baseline levels or below (Fig. 5A), providing evidence that CD73 inhibition can block accumulation of adenosine in the tumor microenvironment after chemotherapy treatment.
Figure 5.
Elevated intratumoral adenosine levels in vivo upon treatment with chemotherapy can be reversed by co-administration of a CD73 antibody. A, Modulation of extracellular tumoral adenosine levels as direct target engagement PD biomarker can be measured in vivo by microdialysis. C57BL/6NTac female mice were injected with MC38hCD73_65 cells subcutaneously. Tumor-bearing mice were treated with doxorubicine (1.5 mg/kg; i.v.), doxorubicin (1.5 mg/kg; i.v.) plus CD73 comparator antibody (30 mg/kg; i.p.) or with the corresponding isotype control. A total of 48 hours after treatment, intratumoral adenosine levels were measured by microdialysis. B, C57BL/6NTac female mice were injected with MC38hCD73_65 cells subcutaneously. Three days after tumor cell injection, treatment with the CD73 comparator antibody (every 3 or 4 days at a dose of 10 mg/kg; i.p.), doxorubicin (every 7 days at a dose of 1.5 mg/kg; i.v.), the combination thereof or with isotype control was started. Tumor diameters were measured three times per week. Shown are tumor volumes of individual tumors as well as the number of complete responses (left top corner of each graph).
CD73 inhibition enhances the effect of chemotherapy
We next tested antitumor efficacy of combining doxorubicin with CD73 inhibition. Whereas doxorubicin treatment alone at a suboptimal dose, as well as CD73 monotherapy treatment, did not show tumor regressions in the syngeneic mouse tumor model MC38hCD73_63, regressions were observed upon combination treatment (Fig. 5B). Thus, combining doxorubicin treatment with CD73 inhibition is superior to single-agent treatment in vivo.
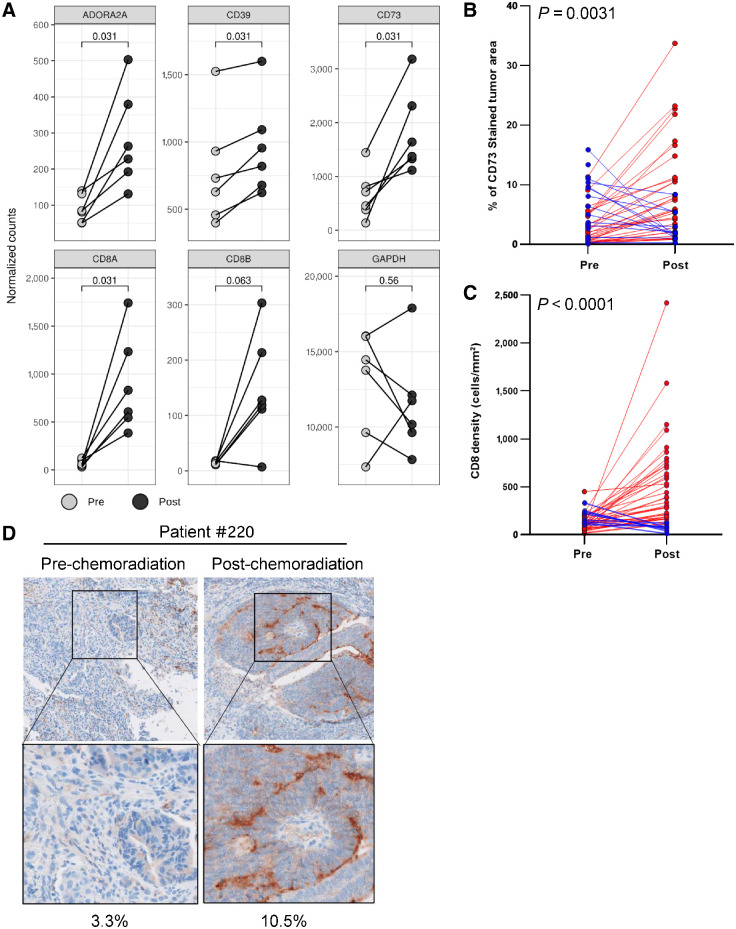
To understand the effect of cell killing agents such as chemotherapy on CD73 and the adenosine pathway in human cancers, we analysed biopsies from patients with rectal cancer pre- and post-chemoradiation for expression of adenosine pathway genes. There was a significant upregulation of CD73 RNA post-chemoradiation (Fig. 6A). Analysis of additional genes of the adenosine pathway also showed an upregulation of their expression, including CD39, the gene encoding the enzyme upstream of CD73, ADORA2A, and ENT1, a transporter mediating the uptake of adenosine from the extracellular space into the cell (Fig. 6A; Supplementary Fig. S4B). We further detected an upregulation of CD8A and CD8B at the RNA level (Fig. 6A). Consistently, IHC staining for CD73 and CD8 showed a significant increase in protein expression after chemoradiation and was detected in the majority of patients (Fig. 6B–D; Supplementary Table S3). Thus, chemoradiation led to an upregulation of multiple adenosine pathway genes in rectal cancer.
Figure 6.
Pathway upregulation after chemoradiation in rectal cancer. A, Tumor biopsies were collected from patients with rectal cancer before chemoradiation as well as a surgery sample after chemoradiation. Six paired samples pre- and post-chemoradiation were analyzed by NanoString for genes involved in the adenosine pathway with GAPDH as control. Significance was calculated using a Wilcoxon paired t test. Pre: pre-chemoradiation; Post: post-chemoradiation. A total of 50 paired samples were analyzed for CD73 (B) or CD8 (C) by IHC. Significance was calculated using a Wilcoxon paired t test. Samples with an increase in signal post-chemoradiation are shown in red, samples with a decrease in signal post-chemoradiation are shown in blue. D, CD73 analysis by IHC for patient 220 pre- and post-chemoradiation. Percent CD73-positive tumor area is indicated below.
Discussion
Here, we describe a novel antagonistic CD73 antibody, mAb19, that forms 2:2 complexes with CD73. CD73 complex formation for other CD73 antibodies is different, with larger complexes formed in the case of oleclumab, consistent with interdimer bridges between multiple CD73 dimers (17, 29, 30). As a consequence, mAb19 showed sustained inhibition of CD73 at concentrations up to 100 nmol/L, exceeding a 1:1 ratio of antibody to CD73. Oleclumab, for example, has been reported to lose CD73 inhibition at antibody concentrations exceeding a 1:1 ratio due to the loss of bivalent binding (17).
The distinct mode of binding of mAb19 to CD73 was found to translate into potent inhibition of the adenosine pathway. mAb19 showed more pronounced blockade of adenosine generation in cell lines with high levels of adenosine and T-cell activation in the presence of AMP compared with the CD73 comparator antibody. The full-length antibody of mAb19 is required for inhibition of CD73, similar to what has been described for oleclumab (17). The homogeneous complexes and the resulting sterical inhibition of CD73 most likely contribute to the stronger inhibition of the adenosine pathway by mAb19, when compared with the CD73 comparator antibody. In isogenic NCI-H460 cell lines with different CD73 expression levels high CD73 levels correlated with high levels of adenosine formed in the presence of the CD73 substrate AMP. Blockade of CD73 using mAb19 inhibited the formation of adenosine, indicating CD73 dependence for adenosine generation.
CD73 occurs in a membrane-bound as well as a soluble form, both of which are enzymatically active (31). In this study, we describe CD73 on the surface of cells as “cell-bound CD73” and CD73 present in cell culture supernatants as “non-cell-bound CD73.” Cell culture supernatants likely contain soluble CD73 (sCD73), and may also contain CD73 bound to exosomes (32). Both membrane bound as well as soluble CD73 are expected to be present in the tumor microenvironment, membrane-bound CD73 on the cell surface of a number of different cell types or on exosomes, and sCD73 in the interstitial space of the tumor. Both forms have the capacity to generate immunosuppressive adenosine; however, it has not yet been elucidated which form of CD73 is the major contributor to the immunosuppression exerted by adenosine in the tumor microenvironment. As such, it is an advantage to maximally inhibit both cell-bound as well as non–cell-bound CD73, as was achieved with mAb19.
A unique aspect of this study was the demonstration of pharmacologic impact of a CD73 inhibitor in a human ex vivo primary solid tumor model system. Here, the ex vivo treatment of pleural effusion or ascites samples taken from patients diagnosed with pancreatic cancer with mAb19 consistently resulted in a decrease of EpCAM+ cancer cell numbers, which may be due to mAb19-mediated cancer cell death. The incomplete antitumor efficacy of CD73 antagonists alone seen in these studies is consistent with the notion that the maximum benefit of CD73 antagonists is achieved by the rational combination with other drugs. The mechanism leading to the reduction of cancer cell number still needs to be further elucidated. Human ex vivo models are an attractive alternative to mouse tumor models (33). There are limitations of mouse tumor models, in particular the fact that the tumor microenvironment including infiltrating immune cells are of mouse origin. Findings in mouse models may thus not be directly translatable to human tumors.
Patients with cancer are frequently treated with combinations of drugs in order to achieve better antitumor responses. We therefore undertook a broad approach to identify combination partners for CD73 inhibitors like mAb19. For combinations targeting immune cells, we made use of an allogeneic dendritic cell/T-cell assay, which is suitable for evaluating T-cell responses upon treatment with molecules targeting this cell type. As this assay is based on immune cells only, testing of combinations targeting both T cells as well as tumor cells was performed in syngeneic mouse tumor models. PD-1 inhibition is an approved immunotherapy for several tumor indications, and CD73 inhibition is being evaluated in combination with blockade of PD-1 in the clinic. We demonstrated the benefit of combining CD73 and PD-1 inhibition in an allogeneic dendritic cell/T-cell assay. Interestingly, AMP strongly suppressed the immune stimulatory effect of PD-1 blockade in this assay, demonstrating the multi-layered immune suppressive network that may also be expected in clinical settings. A weak response of patients to PD-1 inhibition might be linked to high intratumoral adenosine levels and a combination with CD73 inhibition might be particularly effective in such a setting.
We also investigated modulation of two targets within the adenosine pathway, CD73 inhibition in combination with activation of the adenosine receptor ADORA3. Adenosine signals via the G protein–coupled adenosine receptors ADORA1, ADORA2A, ADORA2B, and ADORA3. ADORA2A and ADORA2B receptors signal via Gs, resulting in an increase of intracellular cyclic AMP which translates into an immunosuppressive effect of adenosine. ADORA1 and ADORA3 are different in that signaling is mediated by Gi/o, leading to a decrease of intracellular cyclic AMP levels (34). Combining CD73 inhibition with stimulation of the ADORA3 receptor using the agonist CF102 led to stronger T-cell responses compared to CD73 inhibition alone. This effect may be explained by a stimulatory function of the ADORA3 receptor on T cells, as has been suggested by some studies (26, 27). By inhibiting CD73 upstream of all four adenosine receptors, we aim to block the inhibitory signal of the ADORA2A/2B receptors to increase T-cell function. However, blockade of adenosine formation by CD73 inhibition upstream of ADORA3 might lead to decreased T-cell activities. Therefore, combining CD73 inhibition with ADORA3 receptor stimulation might be more effective to stimulate T cells. In a phase II study of the ADORA3 agonist CF102 in hepatocellular carcinoma a subgroup of Child Pugh B7 patients showed clinical effects (35). CF102 is planned to be tested in a phase III clinical study in this patient population. It is of note that the pursued mechanism of CF102 in these clinical studies was a direct (cytotoxic) effect on cancer cells by activating ADORA3 (36, 37). It may be of interest to interrogate the effect of CF102 on immune cells and investigate a combination of CF102 with CD73 and PD-1 antagonists to maximally stimulate T-cell function.
Finally, we investigated combining CD73 inhibition with cell killing agents like chemotherapy, as such agents are standard of care treatments for many patients with cancer. Chemotherapy and radiotherapy are known to have immunosuppressive effects (38, 39). We hypothesized that cell death induced by chemotherapy treatment might result in release of ATP from dying cells. This might lead to increased conversion of ATP to AMP and CD73-mediated formation of adenosine, disturbing intratumoral adenosine homoeostastis and resulting in elevated levels of immunosuppressive adenosine in the extracellular space. Using microdialysis in tumor-bearing mice in situ, we were able to demonstrate that doxorubicin treatment led to increased extracellular adenosine concentrations in the tumors. Concomitant treatment with doxorubicin and the mouse cross-reactive comparator CD73 antibody restored baseline intratumoral adenosine levels. This provides supporting evidence that formation of immunosuppressive adenosine in the tumor microenvironment can be inhibited by treatment with a CD73 antibody in a situation where intratumoral adenosine homoeostasis is disrupted, for example, under chemotherapy treatment. Our data also provide rationale for combining CD73 inhibition with chemotherapy, and indeed, combining CD73 blockade with doxorubicin in a syngeneic mouse tumor model showed increased antitumor efficacy. Similar in vivo data have been described before for combinations of chemotherapy or radiotherapy with blockade of the adenosine pathway. These studies made use of inhibitors of the adenosine receptors ADORA2A or ADORA2B, of the cell surface enzyme CD39, or of CD73 using the tool antibody TY/23 which differs from CD73 antibodies in the clinic by its functional Fc effector region (29, 40–44). Indeed, clinical trials combining CD73 inhibition with chemotherapy are under way (15).
Adenosine cannot easily be quantified in biological samples due to its extremely short half-life and rapid degradation in ex vivo samples (45). Therefore, we analyzed markers of the adenosine pathway in human rectal cancer samples by NanoString and IHC instead. We showed an upregulated expression of several adenosine pathway genes in rectal cancer samples after chemoradiation. This may be an immune evasion mechanism of the tumor following chemoradiation. This is in line with a number of studies describing an upregulation or activation of CD73 after chemotherapy or radiotherapy treatment (42, 46, 47). In addition, we observed an increase of CD8+ T-cell numbers in the tumors after chemoradiation, as has been described previously (48–50). We hypothesize that while chemoradiation induces an infiltration of CD8+ T cells into the tumor, these T cells may not be functional due to immunosuppression by the adenosine pathway. In this case, a combination of cancer cell killing agents like chemotherapy or radiotherapy with CD73 inhibition might be beneficial to restore T-cell functionality.
In summary, we describe here a novel CD73 antibody, mAb19, with unique pharmacologic properties. mAb19 inhibited immunosuppression associated with the adenosine pathway and is therefore a promising molecule for clinical studies. In addition, we highlight the potential for improved efficacy by combination with an ADORA3 receptor agonist or with cell killing agents like chemotherapy.
Authors' Disclosures
M. Wurm reports grants from Austrian Research Promotion Agency (FFG) during the conduct of the study; personal fees from Boehringer Ingelheim RCV, GmbH & Co KG outside the submitted work; in addition, M. Wurm has a patent for WO2019224025A3 pending. O. Schaaf reports grants from Austrian Research Promotion Agency (FFG) during the conduct of the study; personal fees from Boehringer Ingelheim RCV, GmbH & Co KG outside the submitted work; in addition, O. Schaaf has a patent for WO2019224025A3 pending. K. Reutner reports grants from Austrian Research Promotion Agency (FFG) during the conduct of the study; personal fees from Boehringer Ingelheim RCV, GmbH & Co KG outside the submitted work. R. Ganesan reports personal fees from Boehringer Ingelheim outside the submitted work; in addition, R. Ganesan has a patent for WO2019224025A3 pending. S. Mostböck reports grants from Austrian Research Promotion Agency (FFG) during the conduct of the study; personal fees from Boehringer Ingelheim RCV, GmbH & Co KG outside the submitted work; in addition, S. Mostböck has a patent for WO2019224025A3 pending. C. Pelster reports grants from Austrian Research Promotion Agency (FFG) during the conduct of the study; personal fees from Boehringer Ingelheim RCV, GmbH & Co KG outside the submitted work. J. Böttcher reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Boehringer Ingelheim outside the submitted work; in addition, J. Böttcher has a patent for WO2019224025A3 licensed to Boehringer Ingelheim. B. de Andrade Pereira reports personal fees from Boehringer Ingelheim Pharma GmbH & Co.KG outside the submitted work; in addition, B. de Andrade Pereira has a patent for WO2019224025A3 pending. C. Taubert is an employee of Allcyte GmbH. I. Alt is an employee of Allcyte GmbH. A. Auguste reports grants from Austrian Research Promotion Agency (FFG) during the conduct of the study; personal fees from Boehringer Ingelheim RCV, Gmbh & Co KG (or other BI affiliation) outside the submitted work. K.B. Stadermann reports personal fees from Boehringer Ingelheim Pharma GmbH & Co. KG and Evonik Nutrition & Care GmbH outside the submitted work. J. Capdevila reports personal fees from Lilly, Bayer, ITM, Ipsen, Adacap, and Eisai outside the submitted work. P.G. Nuciforo reports grants from Boehringer Ingelheim during the conduct of the study; personal fees from Targos, Novartis, MSD Oncology, and grants from Bayer outside the submitted work. P.J. Adam reports grants from Austrian Research Promotion Agency (FFG) during the conduct of the study; personal fees from Boehringer Ingelheim RCV GmbH & Co KG outside the submitted work. A.B. Vogt reports grants from Austrian Research Promotion Agency (FFG) during the conduct of the study; personal fees from Boehringer Ingelheim RCV, GmbH & Co KG outside the submitted work. I. Hofmann reports grants from Austrian Research Promotion Agency (FFG) during the conduct of the study; personal fees from Boehringer Ingelheim RCV, GmbH & Co KG outside the submitted work; in addition, I. Hofmann has a patent for WO2019224025A3 pending. No disclosures were reported by the other authors.
Acknowledgments
We would like to thank Sabine Lang, Irene Schweiger, Ilse Apfler, Abdallah Souabni, Michaela Streicher, Susanne Wollner-Gaida, Teresa Zanin, Harald Studensky, Sandra Döbel, Alexander Weiss-Puxbaum, Patrick Werni, Julia Martin, Ulrike Hagel, Lidia Alonso, and Jorge Hernando for excellent technical support. We thank Simon Plyte and Markus Zettl for excellent contributions to identifying mAb19. We thank Inigo Tirapu, Sarah Low, and Guangwei Yang for valuable discussions on the article. We would like to thank Gregory Vladimer for excellent contributions to the work on ex vivo human samples. Furthermore, we would like to thank Loïc Cerf and Imane Nafia from Explicyte (France) for the excellent support and execution of the microdialysis studies.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 2095
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Authors' Contributions
M. Wurm: Conceptualization, validation, visualization, methodology, writing–original draft, writing–review and editing. O. Schaaf: Conceptualization, validation, visualization, methodology, writing–original draft, writing–review and editing. K. Reutner: Validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. R. Ganesan: Conceptualization, supervision, validation, writing–review and editing. S. Mostböck: Conceptualization, formal analysis, supervision, validation, visualization, writing–review and editing. C. Pelster: Validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. J. Böttcher: Visualization, writing–original draft, writing–review and editing. B. de Andrade Pereira: Visualization, writing–review and editing. C. Taubert: Investigation, visualization, writing–original draft, writing–review and editing. I. Alt: Investigation. G. Serna: Software, validation, methodology, writing–original draft, writing–review and editing. A. Auguste: Formal analysis, validation, visualization, writing–original draft. K.B. Stadermann: Formal analysis, visualization, writing–original draft. D. Delic: Writing-original draft. F. Han: Resources, writing–original draft. J. Capdevila: Resources, writing–original draft. P.G. Nuciforo: Resources, writing–review and editing. R. Kroe-Barrett: Conceptualization, supervision, validation, writing–review and editing. P.J. Adam: Conceptualization, writing–original draft, writing–review and editing. A.B. Vogt: Conceptualization, supervision, writing–original draft, writing–review and editing. I. Hofmann: Conceptualization, supervision, validation, methodology, writing–original draft, writing–review and editing.
References
- 1. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways. Am J Clin Oncol 2016;39:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, et al. CD73 on tumor cells impairs antitumor t-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res 2010;70:2245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mora-García M de L, García-Rocha R, Morales-Ramírez O, Montesinos JJ, Weiss-Steider B, Hernández-Montes J, et al. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J Transl Med 2016;14:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, et al. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood 2003;101:3985–90. [DOI] [PubMed] [Google Scholar]
- 6. Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MKK, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 2006;103:13132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol 2012;3:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antonioli L, Blandizzi C, Pacher P, Haskó G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 2013;13:842–57. [DOI] [PubMed] [Google Scholar]
- 9. Paul AB, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol 2012;33:231–7. [DOI] [PubMed] [Google Scholar]
- 10. Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res 1997;57:2602–5. [PubMed] [Google Scholar]
- 11. Häusler SFM, del Barrio IM, Strohschein J, Chandran PA, Engel JB, Hönig A, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother 2011;60:1405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin R, Liu L, Xing Y, Meng T, Ma L, Pei J, et al. Dual mechanisms of novel CD73-targeted antibody and antibody–drug conjugate in inhibiting lung tumor growth and promoting antitumor immune-effector function. Mol Cancer Ther 2020;19:2340–52. [DOI] [PubMed] [Google Scholar]
- 13. Hay CM, Sult E, Huang Q, Mulgrew K, Fuhrmann SR, McGlinchey KA, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology 2016;5:e1208875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest 2011;121:2371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghalamfarsa G, Kazemi MH, Mohseni SR, Masjedi A, Hojjat-Farsangi M, Azizi G, et al. CD73 as a potential opportunity for cancer immunotherapy. Expert Opin Ther Tar 2018;23:127–42. [DOI] [PubMed] [Google Scholar]
- 16. Overman MJ, LoRusso P, Strickler JH, Patel SP, Clarke SJ, Noonan AM, et al. Safety, efficacy and pharmacodynamics (PD) of MEDI9447 (oleclumab) alone or in combination with durvalumab in advanced colorectal cancer (CRC) or pancreatic cancer (panc). J Clin Oncol 36: 15s, 2018. (suppl; abstr 4123). [Google Scholar]
- 17. Geoghegan JC, Diedrich G, Lu X, Rosenthal K, Sachsenmeier KF, Wu H, et al. Inhibition of CD73 AMP hydrolysis by a therapeutic antibody with a dual, non-competitive mechanism of action. MAbs 2016;8:454–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fellmann C, Hoffmann T, Sridhar V, Hopfgartner B, Muhar M, Roth M, et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep 2013;5:1704–13. [DOI] [PubMed] [Google Scholar]
- 19. Ganesan R, Raymond EL, Mennerich D, Woska JR, Caviness G, Grimaldi C, et al. Generation and functional characterization of anti-human and anti-mouse IL-36R antagonist monoclonal antibodies. MAbs 2017;9:1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knapp K, Zebisch M, Pippel J, El-Tayeb A, Müller CE, Sträter N. Crystal structure of the human ecto-5′-nucleotidase (CD73): insights into the regulation of purinergic signaling. Structure 2012;20:2161–73. [DOI] [PubMed] [Google Scholar]
- 21. Stafford WF, Sherwood PJ. Analysis of heterologous interacting systems by sedimentation velocity: curve fitting algorithms for estimation of sedimentation coefficients, equilibrium and kinetic constants. Biophys Chem 2004;108:231–43. [DOI] [PubMed] [Google Scholar]
- 22. Ramakers B, Pickkers P, Deussen A, Rongen G, Broek P, Hoeven JG, et al. Measurement of the endogenous adenosine concentration in humans in vivo: methodological considerations. Curr Drug Metab 2008;9:679–85. [DOI] [PubMed] [Google Scholar]
- 23. Snijder B, Vladimer GI, Krall N, Miura K, Schmolke A-S, Kornauth C, et al. Image-based ex-vivo drug screening for patients with aggressive haematological malignancies: interim results from a single-arm, open-label, pilot study. Lancet Haematol 2017;4:e595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidl C, Vladimer GI, Rendeiro AF, Schnabl S, Krausgruber T, Taubert C, et al. Combined chemosensitivity and chromatin profiling prioritizes drug combinations in CLL. Nat Chem Biol 2019;15:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serna G, Ruiz-Pace F, Hernando J, Alonso L, Fasani R, Landolfi S, et al. Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer. Ann Oncol 2020;31:1366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morello S, Sorrentino R, Montinaro A, Luciano A, Maiolino P, Ngkelo A, et al. NK1.1 cells and CD8 T cells mediate the antitumor activity of Cl-IB-MECA in a mouse melanoma model. Neoplasia 2011;13:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montinaro A, Forte G, Sorrentino R, Luciano A, Palma G, Arra C, et al. Adoptive immunotherapy with Cl-IB-MECA-treated CD8+ T cells reduces melanoma growth in mice. PLoS One 2012;7:e45401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyd-Tressler A, Penuela S, Laird DW, Dubyak GR. Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism. J Biol Chem 2014;289:27246–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perrot I, Michaud H-A, Giraudon-Paoli M, Augier S, Docquier A, Gros L, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep 2019;27:2411–25. [DOI] [PubMed] [Google Scholar]
- 30. Stefano JE, Lord DM, Zhou Y, Jaworski J, Hopke J, Travaline T, et al. A highly potent CD73 biparatopic antibody blocks organization of the enzyme active site through dual mechanisms. J Biol Chem 2020;295:18379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maksimow M, Kyhälä L, Nieminen A, Kylänpää L, Aalto K, Elima K, et al. Early prediction of persistent organ failure by soluble CD73 in patients with acute pancreatitis. Crit Care Med 2014;42:2556–64. [DOI] [PubMed] [Google Scholar]
- 32. Morandi F, Marimpietri D, Horenstein AL, Bolzoni M, Toscani D, Costa F, et al. Microvesicles released from multiple myeloma cells are equipped with ectoenzymes belonging to canonical and non-canonical adenosinergic pathways and produce adenosine from ATP and NAD. Oncoimmunology 2018;7:e1458809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krall N, Superti-Furga G, Vladimer GI. Patient-derived model systems and the development of next-generation anticancer therapeutics. Curr Opin Chem Biol 2020;56:72–8. [DOI] [PubMed] [Google Scholar]
- 34. Allard B, Turcotte M, Stagg J. Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin Ther Targets 2014;18:863–81. [DOI] [PubMed] [Google Scholar]
- 35. Stemmer SM, Manojlovic NS, Marinca MV, Petrov P, Cherciu N, Ganea D, et al. A phase II, randomized, double-blind, placebo-controlled trial evaluating efficacy and safety of namodenoson (CF102), an A 3 adenosine receptor agonist (A 3 AR), as a second-line treatment in patients with Child-Pugh B (CPB) advanced hepatocellular carcinoma (HCC). J Clin Oncol 37: 15s, 2019. (suppl; abstr 2503). [Google Scholar]
- 36. Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, et al. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-kappaB signal transduction pathways. Int J Oncol 2008;33:287–95. [PubMed] [Google Scholar]
- 37. Stemmer SM, Benjaminov O, Medalia G, Ciuraru NB, Silverman MH, Bar-Yehuda S, et al. CF102 for the treatment of hepatocellular carcinoma: a phase I/II, open-label, dose-escalation study. Oncol 2013;18:25–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harris J, Sengar D, Stewart T, Hyslop D. The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer 1976;37:1058–69. [DOI] [PubMed] [Google Scholar]
- 39. Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor microenvironment as a “Game Changer” in cancer radiotherapy. Int J Mol Sci 2019;20:3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ho P, Hsieh M-Y, Hotson A, Miller R, McCaffery I, Willingham S. Abstract 5598: adenosine signaling through A2AR limits the efficacy of anti-CTLA4 and chemotherapy in preclinical models. 2017. [Google Scholar]
- 41. Mittal D, Sinha D, Barkauskas D, Young A, Kalimutho M, Stannard K, et al. Adenosine 2B receptor expression on cancer cells promotes metastasis. Cancer Res 2016;76:4372–82. [DOI] [PubMed] [Google Scholar]
- 42. Li H, Lv M, Qiao B, Li X. Blockade pf CD73/adenosine axis improves the therapeutic efficacy of docetaxel in epithelial ovarian cancer. Arch Gynecol Obstet 2019;299:1737–46. [DOI] [PubMed] [Google Scholar]
- 43. Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013;110:11091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wennerberg E, Kawashima N, Demaria S. Adenosine regulates radiation therapy-induced anti-tumor immunity. J Immunother Cancer 2015;3:P378. [Google Scholar]
- 45. Moser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol 1989;256:C799–806. [DOI] [PubMed] [Google Scholar]
- 46. Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, et al. Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A 2018;115:E1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wirsdörfer F, de Leve S, Cappuccini F, Eldh T, Meyer AV, Gau E, et al. Extracellular adenosine production by ecto-5′-nucleotidase (CD73) enhances radiation-induced lung fibrosis. Cancer Res 2016;76:3045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shinto E, Hase K, Hashiguchi Y, Sekizawa A, Ueno H, Shikina A, et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol 2014;21:414–21. [DOI] [PubMed] [Google Scholar]
- 49. Lim YJ, Koh J, Kim S, Jeon S-R, Chie EK, Kim K, et al. Chemoradiation-induced alteration of programmed death-ligand 1 and CD8+ tumor-infiltrating lymphocytes identified patients with poor prognosis in rectal cancer: a matched comparison analysis. Int J Radiat Oncol Biology Phys 2017;99:1216–24. [DOI] [PubMed] [Google Scholar]
- 50. Matsutani S, Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, et al. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci 2018;109:966–79. [DOI] [PMC free article] [PubMed] [Google Scholar]