Since the emergence of the omicron (B.1.1.529) variant of SARS-CoV-2, the burden of COVID-19 disease on health care has decreased, with rates of hospitalisation due to COVID-19 being substantially reduced despite the increased transmissibility of the omicron variant.1 However, caution might still be warranted for patients with immune-mediated inflammatory diseases, because some immunosuppressants used for treatment of these patients have been shown to reduce humoral or cellar immune responses to SARS-CoV-2 vaccination.2, 3 Reassuringly, we previously observed that the incidence and severity of breakthrough infections during the period in which the delta (B.1.617.2) variant of SARS-CoV-2 was dominant were generally similar in patients with immune-mediated inflammatory diseases receiving immunosuppressants and healthy controls, except for those receiving anti-CD20 therapy.4 However, there is still a paucity of clinical data from patients with immune-mediated inflammatory diseases regarding infections caused by the omicron variant. Therefore, we compared the severity of SARS-CoV-2 breakthrough infections during the time period when the omicron (BA.1 and BA.2 lineages) variant was dominant between patients with immune-mediated inflammatory diseases treated with different immunosuppressants and healthy controls, and we assessed how this severity differed from infections when previous SARS-CoV-2 variants were dominant.
In this prespecified substudy, we collected data from participants enrolled in a Dutch prospective cohort study designed to compare the disease severity of COVID-19 between patients with rheumatic immune-mediated inflammatory diseases and healthy controls (Netherlands Trial Register, Trial ID NL8513).5 The research protocol was approved by the medical ethical committee of the VU University Medical Center (registration number 2020.169). All participants gave written informed consent.
All adult patients with immune-mediated inflammatory diseases from the Amsterdam Rheumatology and Immunology Center (ARC) were invited to participate between April 26, 2020, and March 1, 2021. All patients were asked (but not obliged) to recruit their own control participant of the same sex, comparable age (difference of <5 years), and without an immune-mediated inflammatory disease. Online questionnaires were used for collection of demographic and clinical data, and information on SARS-CoV-2 vaccination, as described previously.4, 5 Questionnaires to collect data on SARS-CoV-2 breakthrough infections were sent to participants at fixed timepoints: April 26, Aug 24, and Dec 10, 2021,4 and March 10, 2022. Breakthrough infections were classified as delta or omicron SARS-CoV-2 infections on the basis of publicly available data on the predominant circulating SARS-CoV-2 variant in the Netherlands at the time (appendix p 4).
The primary objective of this substudy was to compare risk of hospitalisation due to SARS-CoV-2 breakthrough infections between patients with immune-mediated inflammatory diseases and healthy controls at the time when omicron was dominant (hereafter referred to as omicron breakthrough infections). Other objectives were to compare the risk of hospitalisation due to omicron breakthrough infections with the risk due to wild-type or alpha (B.1.1.7) variants of SARS-CoV-2 and delta breakthrough infections in patients with immune-mediated inflammatory diseases, and to assess effects of anti-CD20 therapy on the risk of hospitalisation due to omicron breakthrough infections. Additionally, because we previously found significant associations between clinical determinants and the occurrence of delta breakthrough infections,4 we aimed to explore these potential associations for omicron breakthrough infections.
We used descriptive statistics, logistic regression analyses, and Fisher's exact test for analyses, using SPSS (version 27.0; appendix pp 5–6). Additional details of the study protocol and statistical analyses are in the appendix (pp 2–6).
In total, we collected data on omicron breakthrough infections from 1882 patients with immune-mediated inflammatory diseases (1231 [65%] were female and 651 [35%] were male) and 708 healthy controls (480 [68%] were female and 228 [32%] were male). Both cohorts had a mean age of 59 years (SD 12; appendix pp 9, 15).
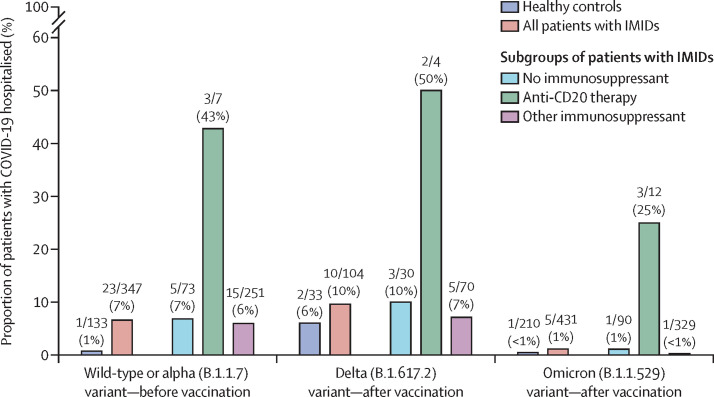
SARS-CoV-2 omicron breakthrough infections were detected in 431 (23%) of 1882 patients and 210 (30%) of 708 controls, of whom the majority had received a SARS-CoV-2 vaccine dose within 3 months of infection (304 [71%] of 431 patients with immune-mediated inflammatory diseases and 142 [68%] of 210 healthy controls; appendix p 10). We previously observed that, in patients with immune-mediated inflammatory diseases, wild-type or alpha infections occurred in 347 (11%) of 3080 unvaccinated individuals and delta breakthrough infections occurred in 104 (5%) of 2206 vaccinated patients (appendix p 11).4, 5 Symptomology of SARS-CoV-2 infections was mostly similar across SARS-CoV-2 variants and between patients and controls (appendix p 16). Hospitalisation after omicron breakthrough infection was required in five (1%) of 431 patients with immune-mediated inflammatory diseases and in one (<1%) of 210 healthy controls (appendix p 10). One patient with an immune-mediated inflammatory disease who had an omicron breakthrough infection and was treated with anti-CD20 therapy died. Of the five hospitalised patients with immune-mediated inflammatory diseases, three were on anti-CD20 therapy (appendix p 12). Rates of hospitalisation were significantly higher in patients receiving anti-CD20 therapy than in patients treated with other immunosuppressants (p<0·0001).
Regression analyses showed that the risk of hospitalisation after omicron breakthrough infections was similar in patients with immune-mediated inflammatory diseases and healthy controls (adjusted odds ratio [aOR] 1·48 [95% CI 0·16–13·28]; appendix p 13). In patients with immune-mediated inflammatory diseases, omicron breakthrough infections were associated with lower risk of hospitalisation than were wild-type or alpha infections in unvaccinated individuals (0·16 [0·06–0·45]) and delta infections in vaccinated individuals (0·08 [95% CI 0·023–0·28]; appendix p 13). Rates of hospitalisation due to COVID-19 disease, stratified by dominant SARS-CoV-2 variant (wild-type, delta, and omicron), and divided into different treatment groups are shown in the figure . Notably, rates of hospitalisation were consistently higher in patients receiving anti-CD20 therapy than in patients receiving no or other immunosuppressants or in healthy controls.
Figure.
Rates of hospitalisation due to COVID-19, in healthy controls and patients with IMIDs, by treatment type and by dominant SARS-CoV-2 variant
Other immunosuppressants includes all patients who are on immunosuppressants other than anti-CD20 therapy. Data above bars are n/N (%). IMID=immune-mediate inflammatory diseases.
Finally, exploratory analyses showed that older age (aOR 0·96 for each increasing year [95% CI 0·95–0·97; p<0·0001), having a immune-mediated inflammatory disease (0·69 [0·56–0·85]), a history of SARS-CoV-2 with wild-type or alpha variant (0·53 [0·40–0·72) or delta variant (0·24 [0·13–0·43]), and a third vaccine dose (0·66 [0·50–0·87]) were associated with reduced likelihood of omicron breakthrough infections, and we found no difference by sex, vaccine type, or by presence of comorbidities (specifically diabetes, obesity, cardiovascular disease, and pulmonary disease; appendix p 13). Interaction terms were not significantly associated with occurrence of omicron breakthrough infections (appendix p 14).
To our knowledge, this is the first prospective study to compare the disease severity, as measured through risk of hospitalisation, of SARS-CoV-2 breakthrough infections during the time period that the omicron variant was dominant between patients with rheumatic immune-mediated inflammatory diseases and healthy controls. We found that patients with immune-mediated inflammatory diseases are less frequently hospitalised when infected with the SARS-CoV-2 omicron variant than with previous variants, which is in line with data from studies in the general population6 and patients with cancer.7 We also observed that hospitalisation rates after SARS-CoV-2 omicron breakthrough infections were similar for most patients and healthy controls, although we found that a higher proportion of patients receiving anti-CD20 therapy were hospitalised than among those treated with other immunosuppressants. These results are consistent with a Canadian population-based study that found high effectiveness of SARS-CoV-2 vaccination against severe alpha and delta SARS-CoV-2 infections in patients with immune-mediated inflammatory diseases,8 and a case series that found that severe SARS-CoV-2 breakthrough infections mostly occurred in patients receiving anti-CD20 therapy or mycophenolate mofetil.9 Anti-CD20 therapy and mycophenolate mofetil severely impair antibody production after SARS-CoV-2 vaccination, even after multiple vaccine doses,3 whereas most other immunosuppressants have no effects or less pronounced effects on humoral immunity.2, 3 Therefore, collectively, these data highlight the importance of humoral immunity in protection against severe SARS-CoV-2 infections, even for variants with a lower pathogenicity such as the omicron variant. Although our cohort was small, the repeated signals that we observed for risks of anti-CD20 therapy for previous SARS-CoV-2 variants are in line with the medical literature, so we argue that continued caution with prescription of anti-CD20 therapy might be warranted for the duration of the ongoing COVID-19 pandemic.
In exploratory analyses, we found that a history of SARS-CoV-2 and a third vaccine dose were associated with a reduced risk of acquiring an omicron breakthrough infection, and that these effects were similar between patients with immune-mediated inflammatory diseases and controls. This finding suggests that immunological memory and recall immune responses are of roughly similar effectiveness in preventing detectable SARS-CoV-2 infections in patients with immune-mediated inflammatory diseases and in the general population. However, these results should be interpreted with caution, because we used the occurrence of omicron breakthrough infections rather than their severity in these exploratory analyses, which can be biased by behavioural differences with regards to infection prevention measures between people. For example, we observed that having a rheumatic disease and older age were associated with a lower risk of omicron breakthrough infection, which probably reflects stricter adherence to isolation measures among patients with immune-mediated inflammatory diseases and older people rather than immunological superiority.10 By contrast, behavioural differences with regard to infection prevention measures do not affect the relative risk of severe disease outcomes (ie, hospitalisation, admission to an intensive care unit, or death) in patients with SARS-CoV-2 infections, but absolute numbers of these outcomes depend strongly on the total number of infections and will thus be lower when infections are less common. Therefore, our results emphasise that monitoring both the occurrence and severity of infections is important to account for varying degrees in exposure to SARS-CoV-2.
A limitation of this study is the small number of participants who had severe COVID-19, which precludes accurate risk estimations and detailed analyses, and means that our results should be interpreted with caution. A more detailed discussion on strengths and limitations of this study is in the appendix (pp 7–8).
In summary, we found that risk of hospitalisation due to SARS-CoV-2 omicron breakthrough infections is substantially lower than the risk due to previous SARS-CoV-2 variants, and that risks of hospitalisation are similar for most patients with immune-mediated inflammatory diseases and healthy controls. However, our data suggest that patients receiving anti-CD20 therapy remain at increased risk of COVID-19-related hospitalisation, indicative of more severe disease when infected with the omicron variant than patients treated with other immunosuppressants. This finding emphasises the importance of continued critical evaluation and re-evaluation of the risks and benefits of anti-CD20 therapy in patients with immune-mediated inflammatory diseases during the COVID-19 pandemic.
Acknowledgments
The study was supported by ZonMw and Reade Foundation. The funders had no role in study design, data analyses, or writing of the report. FE and TWK report (governmental) grants from ZonMw (the Netherlands Organization for Health Research and Development) to study immune responses after SARS-CoV-2 vaccination in autoimmune diseases. FE also reports grants from Prinses Beatrix Spierfonds, CSL Behring, Kedrion, Terumo BCT, Grifols, Takeda Pharmaceutical Company, and Guillain-Barré Syndrome-Chronic Inflammatory Demyelinating Polyneuropathy (GBS-CIDP) Foundation; consulting fees from UCB Pharma and CSl Behring; and honoraria from Grifols. All other authors declare no competing interests.
Supplementary Material
References
- 1.Fan Y, Li X, Zhang L, Wan S, Zhang L, Zhou F. SARS-CoV-2 omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7:141. doi: 10.1038/s41392-022-00997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3:e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 2022;4:e338–e350. doi: 10.1016/S2665-9913(22)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boekel L, Stalman EW, Wieske L, et al. Breakthrough SARS-CoV-2 infections with the delta (B.1.617.2) variant in vaccinated patients with immune-mediated inflammatory diseases using immunosuppressants: a substudy of two prospective cohort studies. Lancet Rheumatol. 2022;4:e417–e429. doi: 10.1016/S2665-9913(22)00102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boekel L, Hooijberg F, Vogelzang EH, et al. Antibody development and disease severity of COVID-19 in non-immunised patients with rheumatic immune-mediated inflammatory diseases: data from a prospective cohort study. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mair MJ, Mitterer M, Gattinger P, et al. Enhanced SARS-CoV-2 breakthrough infections in patients with hematologic and solid cancers due to omicron. Cancer Cell. 2022;40:444–446. doi: 10.1016/j.ccell.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widdifield J, Kwong JC, Chen S, et al. Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes among individuals with immune-mediated inflammatory diseases tested between March 1 and Nov 22, 2021, in Ontario, Canada: a population-based analysis. Lancet Rheumatol. 2022;4:e430–e440. doi: 10.1016/S2665-9913(22)00096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liew J, Gianfrancesco M, Harrison C, et al. SARS-CoV-2 breakthrough infections among vaccinated individuals with rheumatic disease: results from the COVID-19 Global Rheumatology Alliance provider registry. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooijberg F, Boekel L, Vogelzang EH, et al. Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population. Lancet Rheumatol. 2020;2:e583–e585. doi: 10.1016/S2665-9913(20)30286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.