Abstract
Objective:
There is growing evidence that inflammation is an important mediator of pathophysiology in bipolar disorder. The omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acid (PUFA) metabolic pathways participate in several inflammatory processes and have been linked through epidemiologic and clinical studies to bipolar disorder and its response to treatment. We review the data on PUFAs as biomarkers in bipolar disorder and n-3 PUFA used as treatment for bipolar disorder.
Data Sources:
PubMed and CINAHL were searched for articles on PUFA and bipolar disorder published in the English language through November 6, 2013, with an updated search conducted on August 20, 2015. Keywords searched included omega 3 fatty acids and bipolar disorder, omega 3 fatty acids and bipolar mania, omega 3 fatty acids and bipolar depression, omega 3 fatty acids and mania, omega 3 fatty acids and cyclothymia, omega 3 fatty acids and hypomania, fatty acids and bipolar disorder, essential fatty acids and bipolar disorder, polyunsaturated fatty acids and bipolar disorder, DHA and bipolar disorder, and EPA and bipolar disorder.
Study Selection:
Studies selected measured PUFAs as biomarkers or introduced n-3 PUFA as treatment.
Results:
We identified 17 relevant human clinical articles that either compared PUFA levels between a bipolar disorder group and a control group or used a PUFA intervention to treat depression or mania in bipolar disorder. Human studies suggest low n-3 red blood cell PUFA concentrations and correlations with clinical severity in studies of plasma concentrations in symptomatic bipolar disorder. Results of published n-3 PUFA dietary supplementation trials for bipolar disorder indicate efficacy in treatment for mania or depression in 5 of 5 open-label trials, efficacy in treatment of depression in 1 of 7 randomized controlled trials, and a signal for treatment of depression in 1 meta-analysis.
Conclusions:
Biomarker studies of PUFA and treatment studies of n-3 PUFA in bipolar disorder show promise for indicating a way forward in the study of PUFA in bipolar disorder. Investigation of the intake and metabolism of the n-3 and n-6 PUFA when supplementation is provided in treatment trials might offer clues for identification of when and how PUFA may be important for treatment in bipolar disorder.
Bipolar disorder is a chronic illness with episodic exacerbations affecting 1%–4.4% of the population, which causes significant disability and has a complex, incompletely understood etiology.1 Bipolar I disorder is characterized by manic episodes of elevated mood, energy, and cognition and, in 90% of cases, major depressive episodes of depressed mood, energy, and cognition. Bipolar II disorder is characterized by hypomanic episodes of less severity than manic episodes and major depressive episodes that often are longer and more difficult to treat than bipolar I disorder.2–5 Even with pharmacotherapy, many patients with bipolar disorder continue to have subsyndromal symptoms, which predict relapse and lead to functional impairment.6–10 Improvements in acute and maintenance treatments are needed to prevent relapses and reduce years of disability.
Effective pharmacotherapies for acute episodes and prevention of relapse include medications with differing suggested mechanisms of action and include lithium salts, certain antiepileptic agents (eg, valproic acid, carbamazepine, and lamotrigine), and antipsychotics.11 One suggested common mechanism of mood-stabilizing medications derived from preclinical studies is down-regulation of brain metabolism of arachidonic acid (AA, 20:4n-6), a long-chain omega-6 (n-6) polyunsaturated fatty acid (PUFA).12–14 Arachidonic acid is found in the diet together with its dietary-essential shorter-chain n-6 PUFA precursor, linoleic acid (18:2n-6). Alterations of turnover of lipids and resultant alterations in cell-signaling pathways in brain cell membranes have long been hypothesized to be central to perturbations of neurotransmitter systems in mood disorders and are affected by mood-stabilizing and antidepressant medications in rodent models (Figure 1).12,15,16 Additionally, omega-3 (n-3) PUFA may have a number of effects of interest in bipolar disorder, including increasing cell-membrane fluidity,17 inhibiting proinflammatory cytokines,18–20 and antagonizing phosphoinositide-protein kinase C.21,22
Figure 1. Impact of Psychotropic Medication on Metabolic Pathways of the Polyunsaturated Fatty Acids.
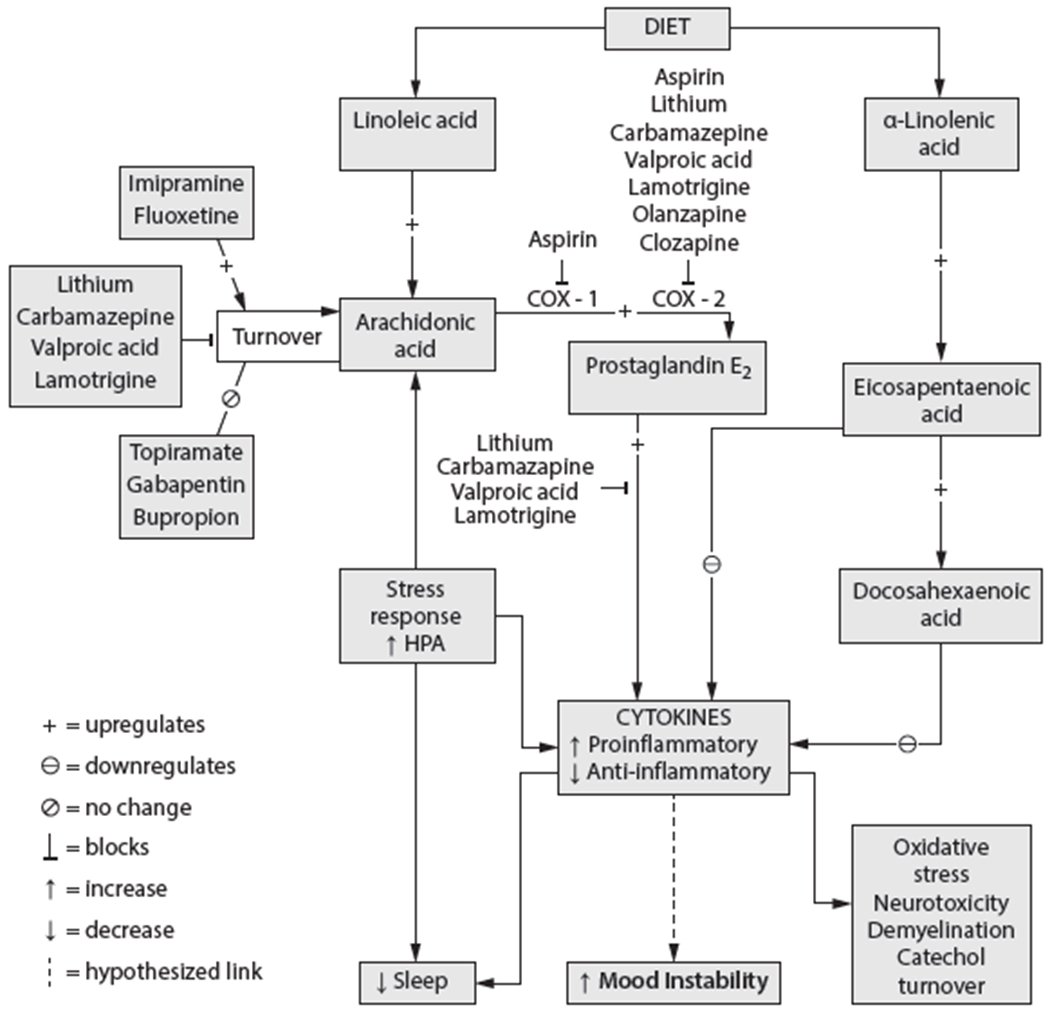
Abbreviations: COX = cyclooxygenase, HPA = hypothalamic pituitary adrenal.
Treatment trials using n-3 PUFA as dietary supplements have had mixed results in bipolar disorder. A review23 in 2008 indicated effective treatment detected in 4 of 7 studies, and those that were effective used a combination of n-3 agents. A Cochrane review24 conducted the same year reviewed 5 studies and concluded that the evidence supported effective treatment for bipolar disorder depression but not mania. We reviewed the evidence from human biomarker studies for altered PUFA levels in bipolar disorder and results of treatment trials with n-3 PUFA supplementation.
METHODS
We used PubMed and CINAHL to search the psychiatric literature for human clinical and biomarker trials published in the English language through November 6, 2013, with an updated search conducted on August 20, 2015. The following words were searched: omega 3 fatty acids and bipolar disorder, omega 3 fatty acids and bipolar mania, omega 3 fatty acids and bipolar depression, omega 3 fatty acids and mania, omega 3 fatty acids and cyclothymia, omega 3 fatty acids and hypomania, fatty acids and bipolar disorder, essential fatty acids and bipolar disorder, polyunsaturated fatty acids and bipolar disorder, DHA and bipolar disorder, and EPA and bipolar disorder.
We identified 17 relevant human clinical articles that either compared PUFA levels between a bipolar disorder group and a control group or used a PUFA intervention to treat depression or mania in bipolar disorder. We included 7 biomarker trials, 1 meta-analysis of randomized controlled trials (RCTs), 7 RCTs, 1 trial that was not randomized, and 4 open-label studies. We use a narrative rather than systematic approach in this review.
RESULTS
Clinical and Epidemiologic Investigation of PUFA in Bipolar Disorder
Clinical studies of blood AA and other PUFA levels in bipolar disorder patients report conflicting findings. Chiu et al25 found lower levels of AA and docosahexaenoic acid (DHA, 22:6n-3) in erythrocyte membranes of subjects with manic bipolar disorder (n = 20) than in control subjects (n = 20) (Table 1).
Table 1.
Trials Measuring Polyunsaturated Fatty Acid Concentrations
| Study | RBC/Plasma | Medication | Group and Mood State | Bipolar Disorder Versus Healthy Control |
Correlation With Clinical Variables | |||
|---|---|---|---|---|---|---|---|---|
| Difference in AA | Difference in DHA | Difference in EPA | Difference in an n-6:n-3 Ratio | |||||
| Chiu et al, 200325 | RBC | Yes (n = 15), No (n = 5) | Manic, n = 20 Healthy control, n = 20 |
↓ | ↓ | ⦸ | ⦸ | ⦸ |
| Sublette et al, 200726 | Plasma | No | Manic, n = 10 Healthy control, n = 10 |
⦸ | ⦸ | ⦸ | ⦸ | ▯ |
| Clayton et al, 200827 | RBC | Yes | Bipolar disorder, n = 15 Healthy control, n = 15 |
NA | ⦸ | ⦸ | ⦸ | ▯ |
| McNamara et al, 201028 | RBC | No | Bipolar disorder, n = 20 MDD, n = 20 Healthy control = 20 |
⦸ | ↓ | ⦸ | ↑ | ⦸ |
| Evans et al, 201229 | Plasma | Yes | Bipolar disorder, n = 40 Healthy control, n = 18 |
⦸ | ⦸ | ⦸ | NA | ▯ |
| Pomponi et al, 201330 | Plasma | Yes | Depressed, n = 21 Manic, n = 9 Euthymic, n = 12 Healthy control, n = 57 |
↑ | ↓ | ↑ | NA | ⦸ |
| Saunders et al, 201531 | Plasma | Yes | Bipolar disorder mixed and depressed, n = 27 Healthy control, n = 31 |
⦸ | ⦸ | (↓)* | ⦸ | ▯ |
P = .002, not significant after correction for multiple testing.
Abbreviations: AA = arachidonic acid, DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, MDD = major depressive disorder, NA = not applicable, n-3=omega-3, n-6=omega-6, RBC=red blood cell.
Symbols: ↑ = increase in bipolar disorder vs healthy control, ↓ = decrease in bipolar disorder vs healthy control, ⦸ = no difference in bipolar disorder and health control, ▯ = no difference.
However, no overall difference was found in total n-3 or n-6 levels, in levels of eicosapentaenoic acid (EPA, 20:5n-3) or of its n-3 precursor of DHA, or in the AA:EPA or the n-6:n-3 ratio.25 DHA and AA membrane concentrations were not correlated with current age, age at onset of disease, or number of episodes or length of illness, nor was a significant difference seen between medicated (n = 15) and nonmedicated patients (n = 5) or between nonsmokers (n = 13) and smokers (n = 7). All samples were taken after fasting.
Clayton et al27 found lower fasting red blood cell EPA and DHA levels in 15 adolescents with bipolar disorder when they were compared to 15 healthy control subjects; however, after adjustment was made for lower dietary intake as measured by a food frequency questionnaire, the group difference was negated. Red blood cell DHA was negatively correlated with depression and aggression, and plasma EPA was positively correlated with mania and externalizing behaviors. There was no correlation between AA, EPA, or DHA and lithium or valproic acid medication. Additionally, no n-3 level correlated with smoking, alcohol use, or body mass index.
McNamara et al28 found no difference in erythrocyte membrane EPA or AA levels and no association with symptom severity in unmedicated bipolar disorder patients with manic and depressive symptoms (n = 20) compared to healthy controls (n = 20) (Table 1). They did report lower DHA and EPA + DHA levels and higher AA:DHA, AA:EPA + DHA, and n-6:n-3 ratios in patients than in controls.
Similarly, Sublette et al26 found no group difference between unmedicated manic patients (n = 10) and healthy controls (n = 10) in fasting plasma unesterified or esterified concentrations of AA, AA metabolites, DHA, or EPA (Table 1). Severity of manic symptoms correlated with the ratio of unesterified AA to EPA, and lower unesterified AA levels correlated with more severe manic symptoms.
Evans et al29 found that nonfasting plasma concentrations of linoleic acid, γ-linolenic acid, dihomo-γ-linolenic acid, AA, α-linolenic acid (18:3n-3), EPA, and DHA did not differ between 40 bipolar disorder subjects and 18 healthy controls in a study investigating these 7 PUFAs and 2 enzymes (fatty acid desaturase 1 and 2) in the n-3 and n-6 pathways. This study showed a negative correlation between linoleic acid and depression severity, a positive correlation between γ-linolenic acid and depression severity, and a positive correlation between depression severity and fatty acid desaturase 2, which metabolizes linoleic acid and α-linolenic acid.
Pomponi et al30 studied fasting plasma concentrations of 15 fatty acids in bipolar disorder and healthy control subjects enrolled in a study of Mediterranean diet. Approximately 30% of subjects studied were in euthymic phase, approximately 50% in depressive phase, and approximately 15% in manic or hypomanic phase. The majority were receiving mood-stabilizing medication at the time of the study. Results from this study showed elevated levels of linoleic acid, AA, α-linolenic acid, and EPA in the bipolar disorder group, but lower levels of DHA in the bipolar disorder group when compared to healthy controls. Subgroup analysis indicated no effect from smoking or disease phase.
In the Saunders et al trial,31 plasma concentrations of n-3 and n-6 including linoleic acid, AA, α-linolenic acid, EPA, and DHA were measured in 27 bipolar disorder subjects in episode and 31 healthy controls. No difference was found in AA or DHA, and a lower level of EPA in bipolar disorder was suggested but not statistically significant when multiple testing was accounted for. A positive correlation was demonstrated between the unesterified:esterified ratio of EPA and mania and suicidality, and panic attacks and suicidality correlated negatively with EPA.
In summary, results to date demonstrate mixed findings regarding peripheral markers of the AA cascade and of n-3 PUFAs in bipolar disorder.
Treatment Trials of PUFA Supplementation in Bipolar Disorder
Open-label trials.
Some open-label treatment trials of n-3 PUFA supplementation in bipolar disorder have been suggestive of treatment effects (Table 2). Hirashima et al17 in 2004 reported no significant change in depression symptoms (P = .88) or manic symptoms (P = .35) from baseline to week 4 in women treated with high-dose (5–5.2 g/d of EPA + 3–3.4 g/d of DHA + 1.7 g/d of other fatty acids; n = 6) or low-dose (1.3 g/dEPA + 0.7 g/d DHA; n = 6) n-3 PUFA compared to placebo (n = 12), but although a significant increase in membrane fluidity was detected by T2 median times on magnetic resonance spectroscopy, relative to the comparison group, no difference was found between the 2 dosing groups.
Table 2.
Nonrandomized and Open-Label Studies of PUFA Supplementation in Bipolar Disorder
| Study | Design | Dose | Duration | No. of Patients | Outcomes | Results |
|---|---|---|---|---|---|---|
| Hirashima et al, 200417 | Nonrandomized open label | 5–5.2 g/d EPA and 3–3.4 g/d DHA and 1.7 g/d of other fatty acids, or 1.3 g/d EPA and 0.7 g/d DHA | 4 wk | 24 (women) | YMRS HDRS MRI T2 values |
Significant decrease in T2 values No significant decrease in YMRS or in HDRS |
| Osher et al, 200533 | Open label | 2 g/d EPA | 6 mo | 12 | HDRS | 7 Patients achieved a 50% or greater reduction in HDRS within 1 mo |
| Sagduyu et al, 200534 | Add-on open label | 1–2 g/d omega-3 fatty acids | 37 | Frequency of irritability Likert scale Irritability component of YMRS |
Significant decrease in frequency and severity of irritability | |
| Wozniak et al, 200735 | Open label | 1,290–4,300 mg combined EPA and DHA | 8 wk | 20 (pediatric) | YMRS CDRS BPRS CGI-I RBC membrane levels of EPA and DHA |
Statistically significant reduction in YMRS scores RBC membrane levels of EPA and DHA increased in treated subjects Significant reduction in CDRS and overall BPRS 40% Patients were rated as much or very much improved on CGI-I |
| Clayton et al, 200936 | Open label | 360 mg/d EPA and 1,560 mg/d DHA | 6 wk | 18 (pediatric) | YMRS HDRS C-GAS CBCL-PR RBC percentage of EPA and DHA |
Significant decrease in YMRS scores Significant decrease in HDRS Significant improvement of C-GAS Significant improvement of CBCL-PR Significant increase in the RBC membrane percentage of EPA and DHA |
Abbreviations: BPRS = Brief Psychiatric Rating Scale, CBCL-PR = Child Behavior Checklist-Parent Report, CDRS = Child Depression Rating Scale, C-GAS = Global Assessment Scale for Children, CGI-I = Clinical Global Impressions-Improvement scale, DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, HDRS = Hamilton Depression Rating Scale, MRI = magnetic resonance imaging, PUFA = polyunsaturated fatty acid, RBC = red blood cell, YMRS = Young Mania Rating Scale.
Osher et al33 reported on bipolar I disorder outpatients (n = 12) with depressive symptoms who were treated open label with 2 g/d of EPA for 6 months; 58% achieved a 50% or greater reduction in the Hamilton Depression Rating Scale score within 1 month, and no patient developed hypomanic or manic symptoms during the trial.
Sagduyu et al34, in an open-label add-on study of the effect of n-3 PUFAs (mean ± SD dose = 2,878 ± 2,012 mg) in patients with bipolar disorder (n = 37), showed a significant decrease in percentage of irritable days (P < .001) and a significant decrease in severity of irritability (P < .001).
Wozniak et al35 reported on a prospective open-label trial of n-3 PUFA monotherapy (mean ± SD dose at end point = 2,602 ± 1,014 mg/d, range, 3–10 capsules of 375-mg of EPA and 55-mg of DHA) in the treatment of pediatric patients with bipolar I (n = 13) or II (n = 5) disorder or bipolar disorder not otherwise specified (NOS) (n = 2). All subjects were in a manic state upon entry. Taking mood stabilizers, anticonvulsants, antidepressants, or neuroleptic pharmacotherapies was not allowed during the study, but a psychostimulant was permitted if the treating clinician judged it necessary for the patient. Over the 8-week study, subjects showed statistically significant but modest reduction in mania scores (P = .001) and depression scores (Child Depression Rating Scale) (P = .002) and a significant reduction in overall Brief Psychiatric Rating Scale scores (P = .002) from baseline to end point.35 Also, 40% of patients were rated as much or very much improved at study end point on the Clinical Global Impressions Improvement scale.
Clayton et al36 described an open-label study in 18 youth with bipolar I (n = 7) and II (n = 6) disorder and bipolar disorder NOS (n = 5) who received a total of 360 mg EPA and 1,560 mg of DHA per day for 6 weeks. They found significant decreases in mania scores (P = .004) and depression scores (P = .002) and significant improvements of global functioning (P = .001) and parent report of total internalizing and externalizing behaviors (P = .01).
Randomized, double-blind, placebo-controlled trials.
While results from open trials have been promising, results from randomized, double-blind, placebo-controlled trials of n-3 PUFA supplementation have been more varied (Table 3).
Table 3.
Randomized Controlled Trials of PUFA Supplementation in Bipolar Disorder
| Study | Design | Dose | Duration, wk | No. of Patients | Outcomes | Results |
|---|---|---|---|---|---|---|
| Stoll et al, 199937 | RCT DB, PC |
EPA 6.2 and DHA 3.4 g/d vs placebo caps | 16 | 44 | HDRS YMRS |
Significant reduction in HDRS scores in omega-3 group, but not YMRS scores |
| Chiu et al, 200538 | RCT DB, PC |
EPA 440 mg and DHA 240 mg/d vs olive oil placebo caps | 4 | 15 | YMRS | Reduced scores in both groups, but no significant difference between groups |
| Keck et al, 200639 | RCT DB, PC |
EPA 6 g/d vs placebo caps | 16 | 121 | IDS-C YMRS |
No significant difference between groups in any outcome |
| Frangou et al, 200640 | RCT DB, PC |
EPA 1 or 2 g/d vs placebo caps | 12 | 75 | HDRS YMRS |
Significant reduction in both EPA groups as compared to placebo in HDRS scores, but not on YMRS scores |
| Frangou et al, 200741 | RCT DB, PC |
EPA 2 g/d vs liquid paraffin | 12 | 14 | HDRS | No significant difference between groups |
| Gracious et al, 201042 | RCT DB, PC |
Flaxseed oil (ALA) 6.6 g/d vs placebo | 16 | 51 | CDRS-R YMRS |
No significant difference between groups |
| Murphy et al, 201243 | RCT DB, PC |
EPA 3 g/d + cytidine 2 g/d vs EPA 3 g/d + placebo vs placebo + placebo | 16 | 45 | MADRS YMRS |
No significant difference between groups |
Abbreviations: ALA = α-linolenic acid, CDRS-R = Child Depression Rating Scale-Revised, DB = double-blind, DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, HDRS = Hamilton Depression Rating Scale, IDS-C = Inventory of Depressive Symptomatology, MADRS = Montgomery-Asberg Depression Rating Scale, PC = placebo-controlled, PUFA = polyunsaturated fatty acid, RCT = randomized controlled trial, YMRS = Young Mania Rating Scale.
In a 4-month, double-blind, placebo-controlled parallel group trial by Stoll et al,37 44 outpatients with bipolar I or II disorder were randomized to receive either n-3 PUFA (EPA 6.2 g and DHA 3.4 g/d) as ethyl esters or olive oil placebo, in addition to their ongoing usual treatment. Participants in the n-3 group showed a significantly longer period of remission than those in the placebo group, and significant improvement of active treatment over placebo in global improvement, functional improvement, and depression, but not mania. This study was stopped early due to early separation of the groups on the main outcome, which was remission, and to lack of further availability of the compound. Subsequent studies summarized below did not replicate this effect, but none used the same high dose (9.6 g/d) or design.
Chiu et al38 reported no superiority of n-3 (EPA 440 mg and DHA 240 mg) as ethyl esters as compared to olive oil placebo augmentation of 20 mg/kg/d of valproate in treating acute mania in a double-blind, placebo-controlled, randomized study. Newly hospitalized patients in the acute manic phase of DSM-IV bipolar disorder (N = 15) were given a fixed dose of valproate 20 mg/kg/d and were randomized into treatment groups receiving n-3 or olive oil placebo. Both groups improved significantly on the Young Mania Rating Scale, but there was no between-group difference in improvement or time to improvement.
In a double-blind, randomized, placebo-controlled augmentation trial of 4 months’ duration by Keck et al,39 116 patients with bipolar I or II disorder or bipolar disorder NOS were given n-3 PUFA (EPA 6 g/d) as ethyl esters or placebo as adjunctive treatment to mood stabilizers for bipolar depression and rapid cycling bipolar disorder. Results showed no significant change in depressive (P = .82) or manic symptoms (P = .32) or in manic switches (P = .43).
Frangou et al40 reported that outpatients (n = 75) with bipolar I or II disorder with mild depression, in a 12-week, double-blind, randomized, placebo-controlled trial of ethyl EPA 1 g/d or 2 g/d as adjunctive treatment, showed a significant benefit with active treatment over control for depressive symptoms (P = .03) and global improvement (P = .04) scores. Frangou et al41 followed up with another moderator study showing that 12 weeks of randomized, double-blind treatment with ethyl EPA or placebo added to lithium was associated with increased N-acetylaspartate (NAA) levels in the brain, as indicated by magnetic resonance spectroscopy, in moderately depressed women with bipolar I disorder (n = 14), but there was no difference in change in depression symptoms between EPA and placebo groups.
Murphy et al43 did not detect a treatment effect in a 16-week, double-blind, placebo-controlled add-on trial comparing adjunctive n-3 PUFA (EPA 3.36 g plus DHA 2.24 g from fish oil per day) with and without an enhancer, cytidine, in 45 outpatients with a diagnosis of bipolar I disorder.
In a pediatric population studied by Gracious et al,42 a 16-week randomized, double-blind, placebo-controlled trial in symptomatic youth aged 6–17 years with bipolar I or II disorder, given supplementation with flax oil containing α-linolenic acid, showed no benefit.
A meta-analysis of English-language RCTs of n-3 supplementation for treatment of bipolar disorder conducted for longer than 4 weeks was conducted by Sarris et al.44 The study included all of the RCTs mentioned above except Murphy et al.43 This study concluded that n-3 supplementation for bipolar disorder depression was effective, with a pooled standard difference in means of 0.338 (95% CI, 0.035 to 0.641), but not for bipolar disorder mania, with a pooled standard difference in means of 0.198 (95% CI, −0.037 to 0.433). Five of 6 of the studies were rated high quality, and minor heterogeneity was found between bipolar disorder depression studies (I2 = 30%, P = .213) and substantial homogeneity in bipolar disorder mania studies (I2 = 0%, P = .98).
DISCUSSION
Here, we have reviewed trials measuring plasma and red blood cell fatty acid concentrations in patients with bipolar disorder and the clinical trials testing use of n-3 PUFA diets for treatment.
Over 90% of PUFAs in the mammalian brain are composed of the long-chain PUFAs AA and DHA.45 Arachidonic acid and DHA can be derived from the diet or can be synthesized in the liver from their respective shorter-chain PUFAs, linoleic acid and α-linolenic acid, which are nutritionally essential. Arachidonic acid, DHA, and their metabolites function as intracellular second messengers and as modulators of neuroinflammation, neurotransmission, gene transcription, and other important brain process.15
In the reviewed studies investigating the relations between peripheral markers of PUFA and bipolar disorder, DHA was found to be lower in both the Chiu et al25 and McNamara et al32 studies, which measured concentration in erythrocyte membranes of symptomatic bipolar disorder compared with healthy controls. In contrast, the plasma concentrations of any n-3 or n-6 PUFA differed between groups in only 1 of 4 studies; however, plasma concentrations were correlated with symptom severity in 3 of the 4 studies. An explanation for the differing results may lie in the measurement methods, as some studies measured concentrations in erythrocyte membrane and some measured plasma concentrations as well as concurrent drugs taken. Circulating plasma concentrations are more reflective of current dietary PUFA intake status, and somewhat reflect the synthesis patterns from liver. Circulating plasma unesterified PUFA is available to cross the blood-brain barrier, while red blood cell membrane concentrations are reflective of the steady state, and perhaps reflective of the membrane concentration in the brain, though that has yet to be determined.46,47 It is because of these differing methods of measurement that are not biologically equivalent that we are unable to use meta-analytic techniques to combine the data to clarify the findings.
One confounding factor unable to be accounted for in 5 of 7 of these studies of PUFA concentrations, measured by either red blood cell membrane concentration or plasma concentration, may be the role of background dietary intake of PUFAs. Dietary PUFA composition can influence blood and brain PUFA concentrations. One study, Pomponi et al,30 included only subjects (bipolar disorder and healthy control) who were enrolled in a study of Mediterranean diet. Clayton et al27 measured self-reported dietary intake and accounted for that in the group comparison. Epidemiologic studies reported that high consumption of n-3 PUFA-rich seafood was associated with significantly lower rates of bipolar disorder.48,49 Because the PUFA precursors linoleic acid and α-linolenic acid are derived entirely from the diet and are nutritionally essential, it is important that clinical studies of PUFA levels or PUFA supplementation record diets in patients and controls. The divergent findings suggest a need for careful attention to dietary fatty acid intake and for systematic assessment of peripheral markers and perhaps cerebrospinal fluid markers in certain cases. This inquiry might identify the specific bioactive lipid mediators and signaling pathways (Figure 1) involved in the pathophysiology of bipolar disorder, allowing for the design of targeted dietary and medication strategies. Additional limitations in these studies are relatively small sample sizes, differing mood states of bipolar disorder participants at the time of biological sampling, and concurrent use of psychiatric medications that alter PUFA metabolism.12
Many factors may contribute to the varied results in human clinical trials of PUFA as treatment for bipolar disorder. While subjects in open-label studies improved almost uniformly, the randomized controlled trials revealed that, often, treatments did not separate from placebo in response of manic symptoms. In 2 of 7 trials, 1 involving only EPA and 1 with EPA and DHA, treatment did separate from placebo for depressive symptoms. Interpretation of the literature and of the varied responses seen in randomized controlled trials is confounded by factors including heterogeneity of diagnosis, design of trials, compliance to study drug, composition and dose of supplements, and concurrent pharmacotherapy.50–55 However, the meta-analysis by Sarris et al44 indicates that pooled results with different n-3 augmentation strategies show a signal for treatment of bipolar disorder depression. Although the analysis by Sarris et al44 indicated that publication bias is unlikely, there may be unpublished trials with unfavorable results.
In addition to the methodological differences and complex diagnostic problems in psychiatry, we also must understand the biological underpinnings of the intervention in a physiological, biochemical, and molecular context. In this article, we review both biomarker studies and intervention studies. While a limitation of this review is the narrative rather than systematic approach, this allowed a more inclusive discussion of both types of studies to bring the discussions together. While measurement and dietary factors may be confounding factors, biomarker studies have shown differences in PUFAs compared to controls and correlation with bipolar disorder disease severity, and RCTs have had mixed results. It is possible that in intervention studies, a subset of participants had deficits in n-3 PUFA concentrations and responded to supplementation while others did not. Additionally, in several biomarker studies, DHA levels were low. If reduced conversion to α-linolenic acid and EPA to DHA is a factor, perhaps the metabolism of EPA supplement to DHA is altered as well. In the open-label study by Wozniak et al,35 plasma and red blood cell levels were measured, and both DHA and EPA increased with a combined treatment. Further studies investigating the plasma and red blood cell membrane levels before and after treatment may be useful.
In conclusion, biomarker studies of PUFA and treatment studies of n-3 PUFA in bipolar disorder show promise for indicating a way forward in the study of PUFA in bipolar disorder. Investigation of the intake and metabolism of the n-3 and n-6 PUFAs when supplementation is provided in treatment trials might offer clues for identification of when and how PUFA may be important for treatment in bipolar disorder.
Clinical Points.
The literature on treatment of bipolar disorder with omega-3 fatty acids is confusing and has not been reviewed in conjunction with studies that report fatty acid levels or concentrations.
Many patients ask about using omega-3 treatments for bipolar disorder, and data support potential treatment for depression but not mania.
Acknowledgments:
The authors would like to acknowledge Aubrey Reider, BA, (Penn State College of Medicine, Hershey, Pennsylvania) for technical assistance with the figure. Ms Reider has no conflicts of interest to report.
Funding/support:
The project described was supported by the National Center for Research Resources, grant KL2 RR033180 (Dr Saunders), and is now at the National Center for Advancing Translational Sciences, grant KL2 TR000126, National Institute on Aging, National Institutes of Health (NIH), Bethesda, Maryland. The contribution of Dr Rapoport was supported entirely by the Intramural Program of the National Institute on Aging, NIH, Bethesda, Maryland. The contribution of Dr Ramsden was supported by the National Institute on Alcohol Abuse and Alcoholism, NIH, Bethesda, Maryland.
Potential conflicts of interest:
Dr Saunders has been a consultant for Projects In Knowledge, CME. Dr Gelenberg has received an investigator-initiated grant through Penn State from Pfizer; has served as a consultant to Zynx, Allergan, Forest, and ZARS Pharma; and is a major stock owner of Healthcare Technology Systems. Drs Ramsden, Sherazy, Davis, and Rapoport have nothing to disclose.
Role of the sponsor:
The sponsors of this research did not have direct influence over the collection, analysis, or interpretation of data.
Footnotes
Drug names: carbamazepine (Epitol, Tegretol, and others), lamotrigine (Lamictal and others), lithium (Lithobid and others), valproic acid (Depakene and others).
Disclaimers: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Neither Dr Gelenberg, JCP’s editor in chief, nor Drs Saunders and Davis, editorial board members, were involved in the editorial review or decision to publish this article.
REFERENCES
- 1.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2nd ed. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 3.Ghaemi SN, Bauer M, Cassidy F, et al. ; ISBD Diagnostic Guidelines Task Force. Diagnostic guidelines for bipolar disorder: a summary of the International Society for Bipolar Disorders Diagnostic Guidelines Task Force Report. Bipolar Disord. 2008;10(1 pt 2):117–128. [DOI] [PubMed] [Google Scholar]
- 4.Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(5):453–473. [DOI] [PubMed] [Google Scholar]
- 5.Vieta E, Suppes T. Bipolar II disorder: arguments for and against a distinct diagnostic entity. Bipolar Disord. 2008;10(1 pt 2):163–178. [DOI] [PubMed] [Google Scholar]
- 6.Judd LL, Akiskal HS, Schettler PJ, et al. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005:62(12):1322–1330. [DOI] [PubMed] [Google Scholar]
- 7.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–537. [DOI] [PubMed] [Google Scholar]
- 8.Bauer M, Glenn T, Alda M, et al. Comparison of pre-episode and pre-remission states using mood ratings from patients with bipolar disorder. Pharmacopsychiatry. 2011;44(suppl 1):S49–S53. [DOI] [PubMed] [Google Scholar]
- 9.Bauer M, Glenn T, Rasgon N, et al. Decreasing the minimum length criterion for an episode of hypomania: evaluation using self-reported data from patients with bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261(5):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer M, Glenn T, Grof P, et al. Subsyndromal mood symptoms: a useful concept for maintenance studies of bipolar disorder? Psychopathology. 2010;43(1):1–7. [DOI] [PubMed] [Google Scholar]
- 11.Ng F, Mammen OK, Wilting I, et al. ; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559–595. [DOI] [PubMed] [Google Scholar]
- 12.Rapoport SI. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS Chem Neurosci. 2014;5(6):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapoport SI, Basselin M, Kim HW, et al. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Brain Res Rev. 2009:61(2):185–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapoport SI, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry. 2002;59(7):592–596. [DOI] [PubMed] [Google Scholar]
- 15.Hibbeln JR, Palmer JW, Davis JM. Are disturbances in lipid-protein interactions by phospholipase-A2 a predisposing factor in affective illness? Biol Psychiatry. 1989;25(7):945–961. [DOI] [PubMed] [Google Scholar]
- 16.Allison JH, Stewart MA. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971;233(43):267–268. [DOI] [PubMed] [Google Scholar]
- 17.Hirashima F, Parow AM, Stoll AL, et al. Omega-3 fatty acid treatment and T(2) whole brain relaxation times in bipolar disorder. Am J Psychiatry. 2004;161(10):1922–1924. [DOI] [PubMed] [Google Scholar]
- 18.Gravaghi C, La Perle KM, Ogrodwski P, et al. Cox-2 expression, PGE(2) and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem. 2011;22(4):360–365. [DOI] [PubMed] [Google Scholar]
- 19.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91(2):439–446. [DOI] [PubMed] [Google Scholar]
- 20.Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38(4):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNamara RK, Ostrander M, Abplanalp W, et al. Modulation of phosphoinositide-protein kinase C signal transduction by omega-3 fatty acids: implications for the pathophysiology and treatment of recurrent neuropsychiatric illness. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4–5):237–257. [DOI] [PubMed] [Google Scholar]
- 22.Seung Kim HF, Weeber EJ,Sweatt JD, et al. Inhibitory effects of omega-3 fatty acids on protein kinase C activity in vitro. Mol Psychiatry. 2001;6(2):246–248. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull T, Cullen-Drill M, Smaldone A. Efficacy of omega-3 fatty acid supplementation on improvement of bipolar symptoms: a systematic review. Arch Psychiatr Nurs. 2008;22(5):305–311. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery P, Richardson AJ. Omega-3 fatty acids for bipolar disorder. Cochrane Database Syst Rev. 2008;(2):CD005169. [DOI] [PubMed] [Google Scholar]
- 25.Chiu CC, Huang SY, Su KP, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13(2):99–103. [DOI] [PubMed] [Google Scholar]
- 26.Sublette ME, Bosetti F, DeMar JC, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9(7):759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayton EH, Hanstock TL, Hirneth SJ,et al. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43(11):1031–1038. [DOI] [PubMed] [Google Scholar]
- 28.McNamara RK, Jandacek R, Rider T, et al. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126(1–2):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans SJ, Kamali M, Prossin AR, et al. Association of plasma ω-3 and ω-6 lipids with burden of disease measures in bipolar subjects. J Psychiatr Res. 2012;46(11):1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pomponi M, Janiri L, La Torre G, et al. Plasma levels of n-3 fatty acids in bipolar patients: deficit restricted to DHA. J Psychiatr Res. 2013;47(3):337–342. [DOI] [PubMed] [Google Scholar]
- 31.Saunders EFH, Reider A, Singh G, et al. Low unesterified: esterified eicosapentaenoic acid (EPA) plasma concentration ratio is associated with bipolar disorder episodes, and omega-3 plasma concentrations are altered by treatment. Bipolar Disord. 2015;17(7):729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNamara RK, Jandacek R, Rider T, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160(3):285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osher Y, Bersudsky Y, Belmaker RH. Omega-3 eicosapentaenoic acid in bipolar depression: report of a small open-label study. J Clin Psychiatry. 2005;66(6):726–729. [DOI] [PubMed] [Google Scholar]
- 34.Sagduyu K, Dokucu ME, Eddy BA, et al. Omega-3 fatty acids decreased irritability of patients with bipolar disorder in an add-on, open label study. Nutr J. 2005;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wozniak J, Biederman J, Mick E, et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17(6–7):440–447. [DOI] [PubMed] [Google Scholar]
- 36.Clayton EH, Hanstock TL, Hirneth SJ, et al. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009:63(8):1037–1040. [DOI] [PubMed] [Google Scholar]
- 37.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):407–412. [DOI] [PubMed] [Google Scholar]
- 38.Chiu CC, Huang SY, Chen CC, et al. Omega-3 fatty acids are more beneficial in the depressive phase than in the manic phase in patients with bipolar I disorder. J Clin Psychiatry. 2005:66(12):1613–1614. [DOI] [PubMed] [Google Scholar]
- 39.Keck PE Jr, Mintz J, McElroy SL, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006:60(9):1020–1022. [DOI] [PubMed] [Google Scholar]
- 40.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188(1):46–50. [DOI] [PubMed] [Google Scholar]
- 41.Frangou S, Lewis M, Wollard J, et al. Preliminary in vivo evidence of increased N-acetylaspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol. 2007;21(4):435–439. [DOI] [PubMed] [Google Scholar]
- 42.Gracious BL, Chirieac MC, Costescu S, et al. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord. 2010;12(2):142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy BL, Stoll AL, Harris PQ, et al. Omega-3 fatty acid treatment, with or without cytidine, fails to show therapeutic properties in bipolar disorder: a double-blind, randomized add-on clinical trial. J Clin Psychopharmacol. 2012;32(5):699–703. [DOI] [PubMed] [Google Scholar]
- 44.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81–86. [DOI] [PubMed] [Google Scholar]
- 45.Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968;9(5):570–579. [PubMed] [Google Scholar]
- 46.Smith QR, Nagura H. Fatty acid uptake and incorporation in brain: studies with the perfusion model .J Mol Neurosci. 2001;16(2–3):167–172, discussion 215–221. [DOI] [PubMed] [Google Scholar]
- 47.Robinson PJ, Noronha J, DeGeorge JJ, et al. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Brain Res Rev. 1992;17(3):187–214. [DOI] [PubMed] [Google Scholar]
- 48.Hibbeln JR, Nieminen LR, Blasbalg TL, et al. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83(suppl 6):1483S–1493S. [DOI] [PubMed] [Google Scholar]
- 49.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160(12):2222–2227. [DOI] [PubMed] [Google Scholar]
- 50.Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009;28(5):525–542. [DOI] [PubMed] [Google Scholar]
- 52.Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17(12):1144–1149, discussion 1163–1167. [DOI] [PubMed] [Google Scholar]
- 53.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91(3):757–770. [DOI] [PubMed] [Google Scholar]
- 54.Sublette ME, Ellis SP, Geant AL, et al. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72(12):1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry. 2012;17(12):1161–1163, author reply 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]


