Abstract
Introduction
By age 60, 60% of adults with Down syndrome (DS) have dementia. Detecting dementia in persons with intellectual disability (ID) can be challenging because their underlying cognitive impairment can confound presentation of dementia symptoms and because adults with ID may have difficulty reporting symptoms. The National Task Group Early Detection Screen for Dementia (NTG‐EDSD) was developed to aid detection of report of cognitive impairment in adults with ID. We implemented an educational curriculum using the NTG‐EDSD and evaluated the impact of the intervention on professional caregivers’ self‐assessed capacity to identify persons with ID and dementia.
Methods
We held five in‐person training sessions for professional caregivers of persons with ID, partnering with various managed care organizations and social services agencies. We assessed knowledge and attitudes at baseline; immediately after training; and 1 week, 1 month, and 6 months after training.
Results
A total of 154 direct care workers, case managers, health‐care providers, and other social services staff attended the trainings. Satisfaction with the NTG‐EDSD training was high; 94% of attendees agreed or strongly agreed that they could use the NTG‐EDSD with their clients. After training, attendees reported a marked increase in confidence in their ability to track various health circumstances and detect functional decline in their clients, although some gains were not sustained over time. As a result of the training, one managed care organization made the NTG‐EDSD a standard part of its assessment of adults with DS starting at age 40.
Discussion
Social services and health‐care professionals can learn to document signs of cognitive decline in adults with ID using the NTG‐EDSD. Attendees were highly satisfied with the training, experienced an increase in confidence in their care of persons with ID, and found the NTG‐ EDSD feasible to use. Because not all gains were sustained over time, booster trainings may be necessary.
Keywords: Alzheimer's disease, dementia, Down syndrome, intellectual disability, medical education, National Task Group Early Detection Screen for Dementia, screening
1. BACKGROUND
There are >7 million individuals with intellectual disability (ID) in the United States.
Down syndrome (DS) is the most common cause of ID, affecting an estimated 250,000 to 400,000 individuals in the United States. 1 , 2 The life expectancy of persons with ID, including DS, has increased over the past few decades, from 25 years in 1983 to 60 years in 2021. 2 With an increase in life expectancy, there is also an increased risk for developing age‐related conditions such as dementia.
Among adults with ID, those with DS are at highest risk for dementia due to Alzheimer's disease (AD). 3 By age 60, at least half of people with DS have dementia, most commonly due to AD. 4 , 5 The lifetime risk of AD in people with DS is >90% 3 , 4 and is a leading cause of death for persons with DS over age 35 years. 6 In persons with DS, the first biomarkers of AD tend to emerge ≈30 years of age, and changes in cognition emerge ≈40 years of age. 3 By age 50, mild cognitive impairment (MCI) due to AD and AD dementia are present in 50% to 70% of individuals with DS. 3 Though the prevalence of dementia in older adults with ID not due to DS is less well studied, the incidence may be as much as five times higher than in older adults without ID. 19
Identifying dementia in persons with ID is challenging because of pre‐existing cognitive impairment, communication difficulties, and lack of baseline data. 9 Personality and behavior changes can be early signs of dementia in people with DS. 7 , 8 , 9 The earlier the change in cognition, behavior, and functioning is recognized in persons with ID, the greater the opportunity for families and professionals to allocate necessary resources; access available treatment; and plan for future programming, services, and supports. 10
Standardized screening instruments for cognitive impairment used with the general population are not suitable for use in persons with profound to moderate ID because the instruments do not consider pre‐existing cognitive impairment. Persons with ID could perform at or below criteria indicating cognitive impairment prior to the onset of neurodegenerative changes. 11 Instead, cognitive decline for persons with ID can be identified by first establishing a baseline for behavior, cognition, and functioning and then gathering accurate information at multiple time points. This information is used to measure and evaluate changes in the context of the person's lifelong abilities and premorbid level of functioning. 4 , 11
The National Task Group (NTG) on Intellectual Disabilities and Dementia Practices developed the National Task Group Early Detection Screen for Dementia (NTG‐EDSD), an informant‐based questionnaire to aid in the screening and early detection of cognitive impairment in adults with ID. 10 It captures early and subtle changes in function and also considers pre‐existing cognitive impairment. 10 The NTG‐EDSD is not an assessment or diagnostic instrument, but a screening tool that provides information to facilitate conversations between caregivers and health‐care providers. The tool was adapted from the Dementia Screening Questionnaire for Individuals with Intellectual Disability (DSQIID), a validated dementia assessment instrument with very good sensitivity (92%) and specificity (97%) in persons with ID. 10 , 12
A systematic review analyzed 42 studies evaluating 18 informant‐based dementia assessment instruments and provided evidence‐based recommendations for instruments most suitable in clinical practice and research. 21 NTG‐EDSD was reported to have superior quality of evidence for content validity. 21 It had high evidence of sufficient relevance and comprehensiveness, and moderate evidence of sufficient comprehensibility. However, internal consistency has not been evaluated for the NTG‐EDSD. 21
The NTG‐EDSD is a resource for lay‐persons and professionals to record cognitive, behavioral, and functional changes known to be associated with dementia. 10 It gathers information on relevant demographics, ratings of health, mental health, and life stressors, surveys of multiple areas related to functioning, and reviews chronic medical conditions. It also includes items that may identify early signs of dementia. The NTG recommends that the NTG‐EDSD be used annually or as indicated for adults with DS beginning at age 40, and with other at‐risk persons with ID when cognitive changes are identified. 10 The NTG‐EDSD is designed to be completed by a person who is familiar with the individual and who does not have formal training in dementia assessment and diagnosis. 10 The tool is available online (http://www.the‐ntg.org/ntg‐edsd) for no cost and in multiple languages.
The objective of our project was to evaluate the impact of an NTG‐EDSD–based training program on the self‐assessed capacity of ID specialists and health‐care professionals on the use of the NTG‐EDSD to identify and support persons with ID and dementia, their families, and caregivers. We developed an educational program to train direct care workers, case managers, health‐care providers and other social services staff to use the tool. The specific goals of this intervention were to increase the knowledge base of attendees with respect to cognitive impairment in ID, to increase attendees’ confidence in their ability to detect and document the report of cognitive and functional decline in persons with ID, and to promote the use of the NTG‐EDSD.
2. METHODS
2.1. Literature search
We reviewed the literature in PubMed, CINAHL Plus, and Google Scholar for research studies on NTG‐EDSD using search terms: (“Intellectual disability” OR “Down Syndrome”) AND (“Alzheimer's” OR “dementia”) AND (“National Task Group Early Detection Screen for Dementia” OR “EDSD” OR “NTG‐Early Detection Screen for Dementia”). This search identified four relevant studies, each of which assessed a different aspect of the NTG‐EDSD. One study assessed the feasibility of the NTG‐EDSD in the German language. 11 Another study assessed the usefulness, as well as sensitivity and specificity, of the NTG‐EDSD in evaluating dementia status, including MCI, in adults with DS. 11 A pilot study assessed the barriers to implementing screening for dementia using NTG‐EDSD and follow‐up dementia assessments. 20 A systematic review was conducted on informant‐based assessment instruments for dementia in people with ID. 21
RESEARCH IN CONTEXT.
Systematic Review: Literature review identified many studies reporting increased prevalence of Alzheimer's disease biomarkers and dementia symptomatology in persons with Down syndrome (DS). Literature on dementia screening is limited, though there is sound rationale for use of the National Task Group Early Detection Screen for Dementia (NTG‐EDSD).
Interpretation: We developed an educational intervention for using the NTG‐EDSD. Trained health and human service professionals found it feasible to use the NTG‐EDSD as a screening instrument. Attendees’ knowledge and confidence in identifying cognitive and functional decline improved, though some gains were not sustained, suggesting the need for boosters. Training materials are available, allowing for implementation in other settings (https://wai.wisc.edu/dementia‐capable‐wisconsin/).
Future Directions: Next steps could include assessing impact of training on health‐care use and outcomes in adults with ID, and ensuring the tool is applicable to persons from a wide range of ethnic, racial, and socioeconomic backgrounds.
2.2. Intervention
A steering committee, consisting of eight experts in the field of ID from state and local community‐based organizations and health‐care organizations, guided the design of this intervention. The steering committee determined that many professionals working with persons with ID are aware of the greater risk for developing dementia compared to the general population; however, professionals have limited knowledge of how to discern potential indicators of cognitive decline, how to document these changes being observed in persons with ID, and how to advocate for follow‐up evaluation. More specifically, the steering committee felt that professionals working with persons with ID would benefit from having more knowledge about the NTG‐EDSD.
Development of the NTG‐EDSD education session was guided by Adult Learning Theory, which emphasizes the importance of providing background information, conveying significance, tailoring to learners’ professional experiences, and providing opportunities to practice using what is learned. 13 The education session was 3.5 hours. Ninety minutes were devoted to providing background information on the pathological processes of dementia in persons with ID, behavioral and psychological symptoms of dementia in persons with ID, and differences in screening and assessment for dementia in persons with ID compared to the general population. Sixty minutes of the education session included a testimonial on use of the NTG‐EDSD by a professional who regularly uses it in practice, step‐by‐step instructions on use of the tool, and guidance for operationalizing the NTG‐EDSD in their professional roles. Attendees were encouraged to ask questions and engage in dialogue with the presenters and fellow attendees. In the remaining 60 minutes, attendees were provided with the opportunity to apply the NTG‐EDSD to a case study and to receive immediate feedback.
From November 2018 to April 2019, we held five in‐person NTG‐EDSD education sessions throughout Wisconsin with 154 professional caregivers.
2.3. Participants
Professionals who deliver assessment, care, and case management services to persons with ID were recruited by mailing and e‐mailing brochures to programs identified by the steering committee throughout Wisconsin where care and/or coordination is provided to persons with ID. Targeted organizations included: adult family homes, managed care organizations, community‐based organizations focused on persons with ID (e.g., vocational programs), community‐based organizations focused on older adults (e.g., adult day programs), and other county and state organizations. Attendees voluntarily attended one of the training sessions; there were no exclusion criteria. Incentive was provided in the form of continuing education units.
2.4. Outcome measures
Knowledge, self‐efficacy, and attitudes were assessed at baseline (pre‐training); immediately after (post‐) training; and 1 week, 1 month, and 6 months post‐training. Given the lack of literature that exists on the evaluation of the NTG‐EDSD, pre‐training and post‐training surveys assessed knowledge and attitudes using one existing feasibility scale found to have high internal consistency in a previous study of the NTG‐EDSD, as well as questions on confidence of tracking health and function developed for the current project based on the content of the NTG‐EDSD. Both instruments were developed in partnership with members of the NTG. 10 , 14 We do not have psychometric properties to report for the newly developed questions.
Baseline data included: demographics, professional role, organizational characteristics, experience with NTG‐EDSD, and confidence in documentation procedures. To measure self‐efficacy, attendees answered 17 questions on how confident they were in tracking health circumstances (nine items) and functional decline (eight items) in persons with ID with their current organizational procedures using a 4‐point Likert‐scale (0 = not at all, 1 = a little bit, 2 = quite, 3 = very confident). Feasibility of the NTG‐EDSD among tool users was assessed with 21 questions across four domains: applicability (six items), acceptability (six), practicality (four), and relevance (five). Agreement with each statement was provided using the 5‐point Likert‐scale (0 = strongly disagree, 1 = disagree, 2 = neutral, 3 = agree, 4 = strongly agree) used by the developers. 10
A post‐training survey was administered at the end of the education session. Attendees answered the same 17 questions on their confidence to track health circumstances and functional decline, now using the NTG‐EDSD. This survey also contained questions on satisfaction with training and on understanding of dementia and behavioral and psychological symptoms of dementia in adults with ID.
At 1 week post‐training, staff conducted a structured phone interview that included only open‐ended questions about the use of the NTG‐EDSD tool. Individuals who had not yet used the tool were asked to anticipate the advantages and feasibility or any barriers of routinely using the tool.
One‐ and six‐month post‐training surveys were electronically sent to attendees. Attendees answered questions on confidence in tracking health and function with the NTG‐EDSD. Attendees who had used the tool were asked questions regarding feasibility of and barriers to using the tool. Those who had not used the tool at 1 month were also asked about barriers preventing use of the tool.
2.5. Analysis
This report summarizes results from data collected at baseline and post‐training time points (immediately after training, and 1 week, 1 month, and 6 months after training) for up to 154 training attendees who provided baseline data. Mean scores were calculated and compared across multiple time points for attendees providing survey responses at more than one time point to gauge changes in knowledge and self‐efficacy. Due to the small number of respondents at 6 months post‐training (n = 27 users of the tool, 9 of whom who had used the tool prior to training), we do not present those outcomes.
Overall confidence in tracking health outcomes was determined by taking the sum of ratings when all questions were answered. Responses to pre‐ and post‐training individual questions on confidence in the organization's current documentation procedures for persons with ID and confidence in ability to track health outcomes with the NTG‐EDSD tool were also summarized and evaluated separately for attendees based on their or their organization's prior use of the tool.
A repeated measures analysis of variance was used to assess whether mean ratings for each question differed across time points. Paired t‐tests and Wilcoxon signed rank tests were used to determine significance of differences in responses between two time points for individuals with measures at both time points. Statistical significance was determined with α of 0.05. Bonferroni correction was also considered in evaluating significance, given the larger number of tests performed comparing individual items between time points, using α of 0.05/number of tests in the comparison (17 or 21 individual items) resulting in P‐value range of P ≤ .001 to P ≤ .003. Analyses were performed using SPSS version 25 and SAS version 9.4.
Responses to open‐ended questions from the 1‐week interviews and 1‐ and 6‐month surveys were summarized using a qualitative inductive thematic analysis approach by two or more staff members assigned to the question (authors T.A., T.L., N.S., M.S., S.R.). 23
2.6. Ethics
All procedures were reviewed and determined to be exempt by the institutional review board at the University of Wisconsin–Madison.
3. RESULTS
3.1. Demographics of attendees and organizations represented
Trainings were attended and baseline evaluations provided by 154 professionals. Attendees were predominantly female, White, and not Hispanic or Latino. Training attendees had several years’ experience working in the field of aging, dementia, or ID. Approximately 60% were case managers, case coordinators, or discharge planners (see Table 1).
TABLE 1.
Demographics of attendees of training
| Demographic characteristics of attendees (N = 154) | N (%) or mean (SD) |
|---|---|
| Sex | |
| Male | 9 (5.8%) |
| Female | 144 (93.5%) |
| Sex missing | 1 (0.6%) |
| Ethnicity | |
| Not Hispanic or Latino | 147 (95.5%) |
| Hispanic or Latino | 7 (4.5%) |
| Race a | |
| American Indian or Alaskan Native | 1 (0.6%) |
| Asian/Asian American | 3 (1.9%) |
| Black/African American | 7 (4.5%) |
| Hawaiian Native or Pacific Islander | 1 (0.6%) |
| White | 138 (89.6%) |
| Other | 2 (1.3%) |
| Two or more races | 2 (1.3%) |
| Professional role | |
| Information and referral providers, options counsellors | 3 (2.0%) |
| Case managers, care coordinators, discharge planners | 92 (59.7%) |
| Direct care workers (certified nursing assistants, personal care attendants, companions) | 11 (7.1%) |
| Health‐care providers (physicians, nurse practitioners, nurses) | 20 (13.0%) |
| Health educators, interventionists (providing training to PWD or caregivers) | 10 (6.5%) |
| Other | 18 (11.7%) |
| Years in professional role | 7.8 (8.2) |
| Years in field of aging/dementia | 11.7 (8.0) |
| Years in field of ID | 11.3 (8.6) |
| Prior experience using the NTG‐EDSD tool | 36 (23.4%) |
| Baseline sum in confidence ratings for tracking health outcomes b | 37.7 (10.4) range 0–51 |
Note that total percentage does not equal 100% because attendees could choose more than one category.
N = 140/154 (91%) with complete responses.
Abbreviations: ID, intellectual disability; NTG‐EDSD, National Task Group‐Early Detection and Screen for Dementia; PWD, persons with dementia; SD, standard deviation.
Attendees were queried on their organizations’ practices for identifying dementia (Table 2). Nearly two‐thirds of attendees worked for managed care organizations. Approximately 64% of attendees (N = 98) reported that their organizations use the NTG‐EDSD, but only 23% of attendees reported using the tool prior to training and only 20% responded being “quite” or “very” confident in using the tool. Besides the NTG‐EDSD, attendees reported their organizations used a variety of tools to screen for dementia in their ID populations, most commonly the Animal Naming Test 15 , 16 (reported by 11% of attendees) and the Mini‐Cog 17 , 18 (also 11%). Only one‐third to one‐half of attendees worked for organizations that had standard procedures in place, prior to training, for cognitive screening or referrals in response to suspected cognitive impairment in their ID clients. Very few (18%) had systems in place to track whether adults with ID and dementia or caregivers followed through on referrals.
TABLE 2.
Characteristics of organizations, as reported by attendees
| Organizational characteristics for attendees (N = 154) | N attendees (%) |
|---|---|
| Organization category a | |
| Adult family home | 18 (11.7%) |
| Managed care organization | 100 (64.9%) |
| Community‐based organization focused on older adults | 18 (11.7%) |
| Community‐based organization focused on people with ID | 33 (21.4%) |
| Other (e.g., county/state organization, supported living) | 14 (9.1%) |
| Organizational practices a | |
| Use NTG‐EDSD | 98 (63.6%) |
| Conduct a formal screen to detect cognitive changes in clients with ID | 57 (37.0%) |
| Conduct an assessment of caregivers of people with cognitive impairment or dementia to determine their service needs | 76 (49.4%) |
| Have a standard procedure for providing referrals to people with dementia | 63 (40.9%) |
| Have a standard procedure for providing referrals to caregivers | 50 (32.5%) |
| Have a list of dementia‐capable providers and organizations to which people with dementia and their caregivers are referred | 68 (44.2%) |
| Track referrals to determine whether the PWD or their caregivers contact the organization they are referred to | 27 (17.5%) |
Note that total percentage does not equal 100% because some attendees chose more than one category.
Abbreviations: ID, intellectual disability; NTG‐EDSD, National Task Group‐Early Detection and Screen for Dementia; PWD, person with dementia.
3.2. Training satisfaction
Post‐training assessments were completed by 98% of attendees immediately after training (n = 151). Overall, attendees were highly satisfied with the training (Figure 1). Most attendees strongly agreed or agreed that the training improved their knowledge of dementia and of behavioral and psychological symptoms of dementia in persons with ID, and most felt that they could use the NTG‐EDSD tool with clients.
FIGURE 1.
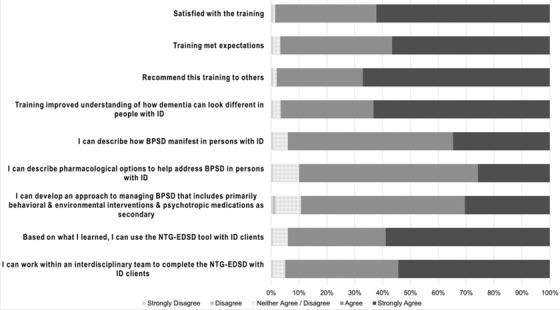
Satisfaction of attendees with training. BPSD, behavioral and psychological symptoms of dementia; ID, intellectual disability; NTG‐EDSD, National Task Group‐Early Detection and Screen for Dementia
3.3. Confidence using the NTG‐EDSD tool
We calculated overall confidence in using the NTG‐EDSD by taking the sum of confidence ratings when all questions were answered. At baseline, attendees reported varied ratings of confidence in tracking health outcomes in persons with ID (mean [standard deviation (SD)] of sum of individual confidence items, 37.7 [10.4], range 0–51). This overall confidence at baseline was not significantly higher for those who had used the tool before training (n = 33) compared to those who had not (n = 107; 38.9 [8.5] versus 37.4 [11.0] without experience, P = .46). Gain in confidence from pre‐ to post‐training was significant for attendees overall (mean [SD] of difference in sum from pre‐ to post‐training, 3.8 [9.4], P < .001). Statistically significant (with Bonferroni correction) increases in individual confidence were noted in 11/17 items (Table 3).
TABLE 3.
Confidence in tracking health outcomes in clients
| Confidence (0 = not at all, 1 = a little bit, 2 = quite, 3 = very confident) | Pre‐training mean (SD) | Post‐training mean (SD) | Diff mean (SD) | P‐value |
|---|---|---|---|---|
| Tracking health circumstances in ID clients | ||||
| 1. Intellectual disability**, *** (N = 147) | 2.10 (0.87) | 2.33 (0.67) | 0.22 (0.91) | .003 |
| 2. Diagnosed intellectual conditions (e.g., autism, Down syndrome)** (N = 149) | 2.10 (0.95) | 2.32 (0.67) | 0.22 (0.96) | .006 |
| 3. Changes in physical health (N = 148) | 2.39 (0.70) | 2.47 (0.62) | 0.08 (0.75) | .193 |
| 4. Changes in mental health* (N = 147) | 2.24 (0.73) | 2.39 (0.65) | 0.15 (0.84) | .033 |
| 5. Current health conditions (e.g., vision impairment, deafness, chronic health conditions) (N = 147) | 2.35 (0.78) | 2.42 (0.66) | 0.07 (0.82) | .314 |
| 6. Current living arrangements (N = 147) | 2.56 (0.70) | 2.59 (0.62) | 0.03 (0.73) | .577 |
| 7. Significant life events (e.g., death of someone close, change in living arrangements)**, *** (N = 149) | 2.30 (0.79) | 2.54 (0.62) | 0.25 (0.77) | <.001 |
| 8. Diagnostic history of mild cognitive impairment (MCI) or dementia**, *** (N = 145) | 2.03 (0.76) | 2.32 (0.68) | 0.29 (0.87) | <.001 |
| 9. Current medications (N = 141) | 2.46 (0.73) | 2.41 (0.68) | ‐0.05 (0.81) | .428 |
| Tracking functional decline in ID clients | ||||
| 10. Activities of Daily Living (e.g., washing, dressing, eating, using the bathroom)**, *** (N = 150) | 2.39 (0.71) | 2.56 (0.57) | 0.17 (0.68) | .002 |
| 11. Language & communication (e.g., conversation, reading, writing)**, *** (N = 150) | 2.16 (0.81) | 2.49 (0.59) | 0.33 (0.77) | <.001 |
| 12. Sleep‐wake change patterns (e.g., sleeping more or less, waking / wandering at night)**, *** (N = 150) | 1.82 (0.91) | 2.38 (0.70) | 0.56 (0.91) | <.001 |
| 13. Ambulation (e.g., unsteady walk, falls, loses balance)**, *** (N = 149) | 2.39 (0.74) | 2.56 (0.57) | 0.17 (0.69) | .003 |
| 14. Memory (e.g., recognition of familiar persons, finding their way in familiar settings)**, *** (N = 149) | 2.11 (0.79) | 2.48 (0.59) | 0.37 (0.82) | <.001 |
| 15. Behavior & affect (e.g., withdrawal from social activities, repetitive behavior)**, *** (N = 149) | 2.20 (0.81) | 2.48 (0.59) | 0.27 (0.75) | <.001 |
| 16. Patients’ self‐reported problems (e.g., changes in abilities to do things, thinking, and interests)**, ***(N = 145) | 2.07 (0.83) | 2.42 (0.63) | 0.35 (0.85) | <.001 |
| 17. Significant changes observed by others (e.g., gait, personality, attentiveness, weight)**, *** (N = 146) | 2.21 (0.75) | 2.52 (0.61) | 0.31 (0.78) | <.001 |
Note: Pre‐training results are confidence in ability to track outcomes using organization's current procedures. Post‐training results were collected immediately after training and represent confidence in ability to track outcomes using the NTG‐EDSD tool.
Statistically significant at *P ≤ .05; **P ≤ .01; ***P ≤ .003.
Abbreviations: ID, intellectual disability; NTG‐EDSD, National Task Group‐Early Detection and Screen for Dementia; SD, standard deviation.
The largest improvements appeared in confidence of documenting changes in functional decline, such as sleep–wake change patterns, behavior and affect changes, and language and communication. These areas showed strong improvements for attendees regardless of whether they had prior experience using the tool or worked for an organization already using the tool (data not shown). Confidence gains in tracking history of MCI were also noted, more prominently among those who did not work for an organization using the tool (n = 50, +0.50, P < .001) compared to those whose organization did use the tool (n = 95, +0.18, P = .052).
Surveys at 1 month after training were completed by 60% of baseline attendees and included confidence data for 52 attendees, 27 of whom had used the tool since training; 59% of those (16/27) had newly used the tool since training. Attendees completing the 1‐month survey were fairly similar in characteristics to attendees at baseline; 61% worked for a managed care organization, 69% worked for an organization using the NTG‐EDSD, and 29% had used the tool prior to training. Overall confidence at baseline was also similar in this subgroup who completed the 1‐month survey, although their gain in confidence immediately after training was somewhat lower (6.3% vs. 9.8% gain in mean confidence score). Confidence ratings at 1 month after training were also significantly lower, before Bonferroni correction, in 13/17 questions compared to post‐training ratings, in 3/17 questions compared to pre‐training ratings, and otherwise statistically very similar to pre‐training ratings (data not shown). Questions on detecting changes in mental health, current medications, language and communication, sleep–wake patterns, behavior and affect, and patients’ self‐reported problems showed declines approaching significance, even with correction. In other words, confidence ratings at 1 month appeared to decline from post‐training increases seen and to return to baseline levels in this subgroup of attendees.
3.4. Feasibility of the NTG‐EDSD tool
The NTG‐EDSD tool was used one or more times in the 6 months after training cumulatively by 41 of the training attendees, as reported at one or more of the post‐training periods; 11 attendees used the tool by 1 week, 31 users by 1 month, and 41 users by 6 months. Attendees reporting use of the tool at 1 month were more likely to work for an organization already using the tool (85%). Among the 41 attendees using the tool at any time after training, 68% (28) reported new use of the tool. Those who were new users reported more professional experience at baseline and were less likely to work for a managed care organization (50%); 64% worked for an organization already using the tool.
Training had a positive impact on reported feasibility of using the tool. Attendees who newly used the tool after training had higher feasibility ratings on 17/21 questions compared to responses among those who had used the tool and rated feasibility prior to training (data not shown).
Data on change in feasibility ratings for those who had used and rated the tool before training, available for 13 respondents at 1 month (Table 4) and nine respondents at 6 months, also showed improvements after training and practice (6‐month data not shown due to the low number of respondents). Taken as a whole, results suggest that training supported improvement in attendees’ perceptions and promoted use of the tool with ID clients.
TABLE 4.
Feasibility of using the NTG‐EDSD tool among prior users (n = 13)
| Feasibility questions about the NTG‐EDSD tool (0 = strongly disagree, 1 = disagree, 2 = neutral, 3 = agree, 4 = strongly agree) | Pre‐training mean (SD) | 1 month post‐training mean (SD) | P‐value |
|---|---|---|---|
| Applicability | |||
| 1. The questions allow an accurate representation of the person | 2.69 (0.75) | 3.15 (0.55) | .008 |
| 2. The response format allows an accurate representation of the person | 2.38 (0.87) | 3.08 (0.64) | .002 |
| 3. I have sufficient experience with persons with ID to complete questionnaire | 2.77 (0.83) | 3.23 (0.83) | .027 |
| 4. I have sufficient information about the person with ID to complete questionnaire | 2.85 (0.69) | 3.08 (0.95) | .337 |
| 5. I have sufficient medical knowledge to complete questionnaire | 2.31 (0.95) | 2.62 (0.87) | .303 |
| 6. The effort needed to complete questionnaire is adequate | 2.54 (0.88) | 3.00 (0.82) | .082 |
| Acceptability | |||
| 7. Questions violate privacy | 1.08 (0.86) | 0.92 (0.64) | .613 |
| 8. Questions are comprehensible | 2.38 (1.04) | 2.92 (0.64) | .012 |
| 9. Instruction for using the tool is comprehensible | 2.46 (0.88) | 3.08 (0.49) | .005 |
| 10. Instruction for using the tool is sufficient | 2.38 (0.96) | 3.00 (0.58) | .014 |
| 11. Questions are unambiguous | 2.00 (0.95) | 2.00 (1.22) | .776 |
| 12. Layout is suitable | 2.77 (0.83) | 2.77 (0.60) | 1.00 |
| Practicality | |||
| 13. Tool is complicated | 1.77 (1.01) | 1.15 (0.99) | .040 |
| 14. Amount of time needed for completion is adequate | 2.31 (1.03) | 2.77 (0.60) | .111 |
| 15. Amount of time needed for reading instruction is adequate | 2.38 (0.77) | 2.69 (0.63) | .104 |
| 16. Using the questionnaire for periodic reassessments would be realizable | 2.62 (0.77) | 3.00 (0.58) | .054 |
| Relevance | |||
| 17. Aspects are missing | 1.92 (0.95) | 1.46 (0.66) | .082 |
| 18. There are unnecessary aspects | 1.31 (0.75) | 1.46 (0.77) | .613 |
| 19. The purpose of the questionnaire is clear | 2.38 (0.77) | 3.23 (0.44) | .001 |
| 20. The significance of the questions in relation to the purpose is clear | 2.46 (1.05) | 2.85 (0.90) | .175 |
| 21. Using the questionnaire for periodic reassessments would be meaningful | 2.69 (0.63) | 3.38 (0.51) | <.001 |
Abbreviations: ID, intellectual disability; NTG‐EDSD, National Task Group‐Early Detection and Screen for Dementia; SD, standard deviation.
3.5. Experiences using NTG‐EDSD Tool
3.5.1. One week after training
One week after training, 44% of individuals participated in the phone interview to report on experiences using the tool. Those completing 1‐week assessments had on average 1 year more experience in their professional role and were more likely to work at an adult family home, but were otherwise similar to attendees at baseline. Attendees reported that barriers to use of the NTG‐EDSD included the lack of new enrollments or current clients appropriate for the use of the tool, delays with organizational permission to use the tool, and lack of time to use the tool. Attendees also noted it was challenging to use with those persons who live alone, or with minimal supports, likely because the tool can be difficult to use to full effect without the information provided by others familiar with the individual with ID. Use of the tool without prior formal training was noted as having been overwhelming; some attendees referred to needing additional training to support training others in their organization.
Attendees noted that the tool is easy to use, helpful for tracking small changes both cognitively and physically, and helpful for sharing information with health‐care providers. The tool facilitated conversations with parents/guardians and professional staff.
3.5.2. One month and 6 months after training
For half of the respondents, the barrier that prevented use of the tool in the 1 month after training was lack of opportunity, because some respondents had low caseloads. Conversely, others noted a lack of time to use the comprehensive tool given their high caseloads. Some attendees indicated having been trained only to supervise and train staff who would use the tool. Other barriers included delays with ongoing implementation planning by organizations, including addressing differences between recommendations noted during training and an agency's current criteria for tool use. A need for additional training was also noted as a barrier and challenge to using the tool. At 6 months, most individuals noted the tool had been incorporated into their biannual or annual review process, or more often when changes in their clients are noted.
4. DISCUSSION
We found that attendees of an educational program on how to use the NTG‐EDSD to detect dementia in persons with ID reported high levels of satisfaction and experienced an increase in confidence in their ability to track cognitive and functional decline in their clients with ID. There was a decline in attendees’ confidence at 1 month after the training, with return to baseline in some, suggesting that there could be a need for online supports or booster training. Attendees having a delayed opportunity to apply what was learned after training may have led to lack of confidence in use of the tool. The complexity of using the tool in the real world, especially if needed information is lacking, may have also led to a decline in confidence. In general, attendees’ assessment of the feasibility of using the NTG‐EDSD improved from pre‐ to post‐training. One managed care organization whose staff attended the training made the NTG‐EDSD a standard part of its assessment of adults with DS starting at age 40—that is, training may have an effect at a systems level.
Given the high prevalence of dementia in persons with DS and other causes of ID, there is an urgent need for ways to effectively detect cognitive decline in this population. Wide adoption of the NTG‐EDSD could help address this need. The NTG‐EDSD has the advantage of having been developed by a large panel of experts following an approach with a sound rationale, namely combining baseline assessment of cognition and functioning with periodic reassessment. Those who intend to use the NTG‐EDSD may benefit from a formal educational intervention, such as the one we describe here.
We are aware of a few reports on using the NTG‐EDSD; however, a study has not been done to assess knowledge and self‐efficacy of professionals in identifying and documenting cognitive and functional decline in persons with ID when using the NTG‐EDSD. One study assessed the feasibility of 221 paid caregivers’ use of a German‐language version of the NTD‐EDSD in persons with ID. 14 Participants completed the NTG‐EDSD for one person with ID whom they had known for at least 6 months. Eighty‐three percent of respondents found the NTG‐EDSD to be “useful” or “very useful” for detecting cognitive impairment in persons with ID. Their ratings of feasibility of using the tool were high, especially among participants with prior experience caring for persons with dementia.
Another study assessed the accuracy of NTG‐EDSD in a group of 185 adults with DS. 22 Informants were interviewed with the NTG‐EDSD, and results were compared to an independent dementia status rating based on consensus review of detailed assessments of cognition, functional abilities, and health status, including physician examination. The NTG‐EDSD was found to be a useful tool for evaluating dementia status; however, estimates of sensitivity and specificity indicated that NTG‐EDSD findings need to be supplemented by additional sources to achieve an acceptable level of screening accuracy.
A pilot study assessed barriers to screening and follow‐up assessment for dementia among older adults with ID without DS. 20 Sixty‐three participants’ caregivers completed the NTG‐EDSD with the requirement that the participant be known to the caregiver for at least 6 months. A focus group of care coordinators found turnover of direct care staff and inability to contact families made it difficult to identify an informant who knew the participant well enough to reliably and accurately answer the NTG‐EDSD questions. Coordinators reported that conducting the dementia screening or further evaluation was not always possible if there were more urgent needs to address. One of the most challenging issues reported by care coordinators was that there were few available providers skilled in or comfortable with assessing the presence of dementia for people with ID in their communities.
Limitations of our study include: (1) the lack of a control group prevented us from testing hypotheses about the effectiveness of the educational intervention; (2) all outcome measures were self‐report; that is, we did not collect outcomes necessary to demonstrate increased skills in the detection of cognitive decline; (3) we did not collect data regarding the cost of the educational intervention, including the time attendees spent in training and therefore not providing direct care; (4) attendees were mostly White, non‐Hispanic, and female, thereby limiting generalizability of the intervention to professionals from other backgrounds; (5) fewer attendees provided responses at 6 months, which may have biased the results, including a possible avoidance of response among those not finding the tool feasible. However, we note that individuals responding to each of the post‐training assessments were fairly similar to the overall group of training attendees. Some trainees also reported not being a direct user of the tool or that adoption of the tool was in process by their organization, preventing report on use.
In conclusion, we have demonstrated the feasibility of an educational intervention to train health and social services workers in the use of the NTG‐EDSD to help detect cognitive decline in persons with ID. Next steps could include measuring health‐care outcomes in persons with ID; ensuring that the tool is applicable to persons from a wide range of ethnic, racial, and socioeconomic backgrounds; and designing interventions for family caregivers in the detection of dementia in persons with ID.
CONFLICTS OF INTEREST
Dr. Walaszek declares the following interests: He receives royalties from American Psychiatric Association Publishing; he has received honoraria from Aurora Advocate Healthcare, University of Nebraska, and Wisconsin Association of Medical Directors for presentations given. Dr. Endicott has received an honorarium from UnityPoint Health–Meriter and the Wisconsin Nurses Association APRN Forum for presentations given. Dr. Carlsson receives grant funding from NIH, NIH/Lilly, NIH/Eisai, and the Department of Veterans Affairs. The other authors do not have any interests to disclose. Author disclosures are available in the supporting information.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
We would like to thank the members of our project Steering Committee, including Maria Stanley, MD; Marcia Stickel, RN, BA, BSN; Melissa Schoenbrodt; Brenda Bauer; Jeremy Gundlach; Afra Smith; and Dave Verban. (One of the co‐authors, JK, was also on the Steering Committee.) We would also like to thank the following people who aided with scheduling the training sessions: Carla Lundeen, Jenny Hayden, and Holly Onsager. Finally, we would like to thank Matthew Janicki, PhD, for his review of this manuscript. This manuscript was supported in part by a cooperative agreement (“Dementia Capable Wisconsin: Creating New Partnerships in Dementia Care.” No. 90ALGG0004‐01‐00) from the Administration on Aging (AoA), Administration for Community Living (ACL), U.S. Department of Health and Human Services (DHHS). Grantees carrying out projects under government sponsorship are encouraged to express freely their findings and conclusions. Therefore, points of view or opinions do not necessarily represent official AoA, ACL, or DHHS policy. Bader Philanthropies, Inc. and the State of Wisconsin Department of Health Services provided support to the Wisconsin Alzheimer's Institute.
Walaszek A, Albrecht T, LeCaire T, et al. Training professional caregivers to screen for report of cognitive changes in persons with intellectual disability. Alzheimer's Dement. 2022;8:e12345. 10.1002/trc2.12345
REFERENCES
- 1. Presson A, Partyka G, Jensen K, et al. Current estimate of Down syndrome population prevalence in the United States. J Pediatr. 2013;163(4):1163‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Down Syndrome Society. Down Syndrome Facts, http://www.ndss.org/down‐syndrome/down‐syndrome‐facts 2021.
- 3. Fortea J, Vilaplana E, Carmona‐Iragui M, et al. Clinical and biomarker changes of Alzheimer's disease in adults with Down syndrome: a cross‐ sectional study. Lancet. 2020;395(10242):1988‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCarron M, McCallion P, Reilly E, Dunne P, Carroll R, Mulryan N. A prospective 20‐year longitudinal follow‐up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2017;61(9):843‐852. [DOI] [PubMed] [Google Scholar]
- 5. Sinai A, Moktysz C, Bernal J, et al. Predictors of age of diagnosis and survival of Alzheimer's disease in Down syndrome. J Alzheimers Dis. 2018;61(2):717‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hithersay R, Startin C, Hamburg S. Association of dementia with mortality among adults with Down syndrome older than 35 years. JAMA Neurol. 2019;76:152‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ball SL, Holland AJ, Hon J, Huppert FA, Treppner P, Watson PC. Personality and behavior changes mark the early stages of Alzheimer's disease in adults with Down's syndrome: findings from a prospective population‐based study. Int J Geriatr Psychiatry. 2006;21:661‐673. [DOI] [PubMed] [Google Scholar]
- 8. Janicki MP, Henderson CM, Rubin IL. Neurodevelopmental conditions and aging: report on the Atlanta Study Group Charrette on Neurodevelopmental Conditions and Aging. Disabil Health J. 2008;1:116‐124. [DOI] [PubMed] [Google Scholar]
- 9. Strydom A, Shooshtari S, Lee L, et al. Dementia in older adults with intellectual disabilities: epidemiology, presentation, and diagnosis. J Policy Pract Intellect Disabil. 2010;7:96‐110. [Google Scholar]
- 10. Esralew L, Janicki MP, Disipio M, Jokinen N, Keller SM, Members of the National Task Group Section on Early Detection and Screening. National Task Group Early Detection Screen for Dementia: Manual. Retrieved from www.aadmd.org/ntg/screening 2013.
- 11. Silverman W, Krinsky‐McHale S, Lai F, et al. Evaluation of the National Task Group‐Early Detection Screen for Dementia: sensitivity to ‘mild cognitive impairment in adults with Down syndrome. J Appl Res Intellect Disabil. 2021;34:905‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deb S, MA, H , Prior L, Bhaumik S. Dementia screening questionnaire for individuals with intellectual disabilities. Br J Psychiatry. 2007;190:440‐444. [DOI] [PubMed] [Google Scholar]
- 13. Merriam S. S. Andragogy and self‐directed learning: pillars of adult learning theory. New Dir Adult Contin Educ. 2001;89:3‐14. [Google Scholar]
- 14. Zeilinger E, C, G , Janicki M, Esralew L, Weber G. Practical applications of the NTG‐EDSD for screening adults with intellectual disability for dementia: a German‐language version feasibility study. J Intellect Dev Disabil. 2016;41(1):42‐49. [Google Scholar]
- 15. Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta‐analysis. Neuropsychologia. 2004;42(9):1212‐1222. [DOI] [PubMed] [Google Scholar]
- 16. McDonnell M, Dill L, Panos S, et al. Verbal fluency as a screening tool for mild cognitive impairment. Int Psychogeriatr. 2020;32(9):1055‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borson S, Scanlan JM, Chen P, Ganguli M. The Mini‐Cog as a screen for dementia: validation in a population‐based sample. J Am Geriatr Soc. 2003;51(10):1451‐1454. [DOI] [PubMed] [Google Scholar]
- 18. Fage BA, Chan CC, Gill SS, et al. Mini‐Cog for the diagnosis of Alzheimer's disease dementia and other dementias within a community setting. Cochrane Database Syst Rev. 2015;(2):CD010860. [DOI] [PubMed] [Google Scholar]
- 19. Strydom A, Chan T, King M, Hassiotis A, Livingston G. Incidence of dementia in older adults with intellectual disabilities. Res Dev Disabil. 2013;34(6):1881‐1885. [DOI] [PubMed] [Google Scholar]
- 20. Holingue C, Wise E, Caoili A, Klein A, Kalb L, Beasley J. Screening for dementia among adults with intellectual disability: outcomes from a pilot study. J Ment Health Res Intellect Disabil. 2022;15(1):20‐36. [Google Scholar]
- 21. Zeilinger E, Zrnic Novakovic I, Komenda S, et al. Informant‐based assessment instruments for dementia in people with intellectual disability: a systemic review and standardised evaluation. Res Dev Disabil. 2022;121:104148. [DOI] [PubMed] [Google Scholar]
- 22. Silverman W, Krinsky‐McHale S, Lai F, et al. Evaluation of the national task group‐early detection screen for dementia: sensitivity to ‘mild cognitive impairment’ in adults with down syndrome. J Appl Res Intellect Disabil. 2021;34:905‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107‐115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


