Summary
Plants produce myriad aroma compounds—odorous molecules that are key factors in countless aspects of the plant's life cycle, including pollinator attraction and communication within and between plants. For humans, aroma compounds convey accurate information on food type, and are vital for assessing the environment. The phenylpropanoid pathway is the origin of notable aroma compounds, such as raspberry ketone and vanillin. In the last decade, great strides have been made in elucidating this pathway with the identification of numerous aroma‐related biosynthetic enzymes and factors regulating metabolic shunts. These scientific achievements, together with public acknowledgment of aroma compounds' medicinal benefits and growing consumer demand for natural products, are driving the development of novel biological sources for wide‐scale, eco‐friendly, and inexpensive production. Microbes and plants that are readily amenable to metabolic engineering are garnering attention as suitable platforms for achieving this goal. In this review, we discuss the importance of aroma compounds from the perspectives of humans, pollinators and plant–plant interactions. Focusing on vanillin and raspberry ketone, which are of high interest to the industry, we present key knowledge on the biosynthesis and regulation of phenylalanine‐derived aroma compounds, describe advances in the adoption of microbes and plants as platforms for their production, and propose routes for improvement.
Keywords: aroma compounds, heterologous systems, metabolic engineering, microbes, phenylalanine, phenylpropanoids
Introduction
Aroma compounds are volatile molecules produced by plants, microbes, and animals that are detected by the olfactory system and therefore have an odour (smell) (Schwab et al., 2008). Volatile molecules are characterized by low molecular weight, high vapour pressure at ambient temperature and lipophilicity—physical properties that enable them to cross biological barriers and be carried by air to olfactory systems (Pichersky et al., 2006; Rowe and Shepherd, 2016). The word ‘aroma’ originates from the Greek word arōmat which means ‘spice’, and is defined as ‘a distinctive, pervasive, and usually pleasant or savory smell’ in the Merriam‐Webster dictionary. As sessile organisms, plants use aroma compounds as a means of overcoming the limitations of a stationary existence. Their significance is manifested in their involvement in countless processes throughout the plant's life cycle: from adaption to changing environments and defence against herbivores and pathogens, to plant–plant, plant–microbiome, and plant–pollinator interactions (Ameye et al., 2018; Kong et al., 2020; Muhlemann et al., 2014; Ninkovic et al., 2019). To date, myriad aroma compounds have been identified and can be classified as terpenoids, phenylalanine (Phe) derivatives, fatty acid derivatives, and nitrogen/sulphur‐containing compounds (Knudsen et al., 2006). Phe‐derived aroma compounds are highly abundant in plants, and many of the aroma compounds desired by man, such as eugenol (clove), raspberry ketone (RK) (raspberry), and vanillin (vanilla) belong to this class (Table 1). Due to their cardinal importance to plants, animals, and humans alike, the in planta biosynthesis of Phe‐derived aroma compounds has been extensively studied in a wide range of species, and numerous genes and transcription factors involved in their production and emission have been functionally characterized (Akhtar and Pichersky, 2013; Farhi et al., 2010; Klempien et al., 2012; Liao et al., 2020a; Liu et al., 2017; Mageroy et al., 2012; Orlova et al., 2006; Spitzer‐Rimon et al., 2010, 2012). Today, the production of aroma compounds is a multibillion dollar industry, estimated at more than 5 billion USD (Ahuja and Sonal, 2020). Although some of these may be chemically mass‐produced, there is growing consumer demand for biologically produced aroma compounds. Nevertheless, host plants are usually not good sources for the mass production of aroma compounds as they are found in minute quantities, are highly affected by environmental changes, and require laborious extraction processes.
Table 1.
List of chemical structures, aromas, and plant hosts of Phe‐derived aroma compounds
| Compound | Chemical structure | Aroma | Plants |
|---|---|---|---|
| C6–C1 benzenoids | |||
| Benzaldehyde |
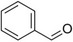
|
Almond | Prunus amygdalus |
| Benzyl acetate |
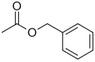
|
Fresh, jasmine and pear‐like | Jasminum |
| Benzyl alcohol |
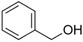
|
Mild aromatic | Jasminum |
| Benzyl benzoate |
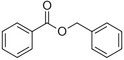
|
Light, balsamic, reminiscent of almond | Myroxylon balsamum |
| Cinnamaldehyde |
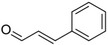
|
Characteristic cinnamon, pungent‐spicy | Cinnamomum verum |
| Cinnamyl alcohol |
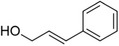
|
Warm‐balsamic, floral, sweet | Cinnamomum verum, Myroxylon balsamum |
| Guaiacol |
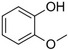
|
Smokey | Guaiacum |
| Methyl benzoate |
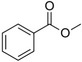
|
Strong sweet‐floral with a fruity undertone | Antirrhinum majus, Petunia |
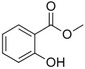
| Methyl salicylate |
|
Sweet wintergreen mint, reminiscent of root beer | Betula lenta, Gauitheria procumbens |
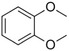
| Veratrole |
|
Creamy‐sweet | Silene latifolia |
| C6–C2 phenylpropanoid‐related | |||
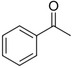
| Acetophenone |
|
Resembling oranges | Camellia sinensis |
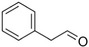
| Phenylacetaldehyde |
|
Green floral, rose‐like | Rosa |
| Phenylacetonitrile |
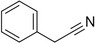
|
Aromatic | Brassicales |
| 1‐Phenylethanol |
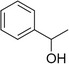
|
Mild flowery, gardenia–hyacinth | Camellia sinensis |
| 2‐Phenylethanol |
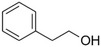
|
Mild rose‐like, honey | Rosa |
| Phenylethyl acetate |
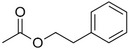
|
Rose | Rosa |
| Phenylethyl benzoate |
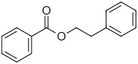
|
Rose, balsamic | Cinnamomum zeylanicum |
| C6–C3 phenylpropanoids | |||
| Anethole |
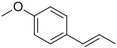
|
Anise | Pimpinella anisum |
| Chavicol |
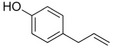
|
Herbal | Ocimum basilicum |
| Estragole |
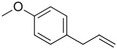
|
Sweet, reminiscent of anise | Ocimum basilicum, Malus domestica |
| Eugenol |
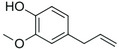
|
Clove | Syzygium aromaticum |
| Isoeugenol |
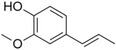
|
Clove | Syzygium aromaticum |
| Methyl eugenol |
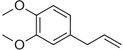
|
Mild‐spicy, carnation | Croton malambo |
| Methyl isoeugenol |
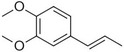
|
Delicate clove–carnation | Daucus carota |
| Vanillin |
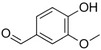
|
Vanilla, creamy | Vanilla planifolia |
| C6–C4 phenylbutanones | |||
| Raspberry ketone |
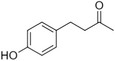
|
raspberry | Rubus idaeus |
| Zingerone |
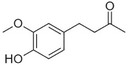
|
Pungent odour, ginger | Zingiber officinale |
Owing to their economic, agricultural, and industrial value, enhancement of the production of specialized metabolites in general, and aroma compounds in particular, by host plants and in heterologous systems has garnered massive attention in the last decade, manifested by a plethora of research papers and reviews tackling this topic. The goal of the present review is to give a broader perspective on plant Phe‐derived aroma compounds, from their interactions with other organisms to their production in plants and heterologous systems. The first part of the review presents plant aroma compounds' importance for humans, pollinators, and plant–plant interactions. The second part focuses on the biosynthesis of Phe‐derived aroma compounds and regulation of their fluxes in plants. The final section discusses aroma compound production in plants and heterologous systems, focusing on the most popular Phe‐derived compounds for consumers, RK, and vanillin.
Aroma compounds' interactions with organisms
Aroma and humans
Aroma compounds are involved in our everyday lives. Smelling allows us to evaluate our surroundings and is used for hazard avoidance, reproduction, and social interactions. Food is a major aspect of the human–volatile relationship as aroma compounds convey the most accurate information on a food's availability and quality (Fig. 1a). Perhaps, the most important aspect is the contribution of aroma compounds to foods' flavour, and it is no wonder humans have been using them for this purpose since ancient times (Schwab et al., 2008). For example, residues of vanillin were identified in ceramic juglets in Tel Megiddo, Israel, dating back ca. 3500 years (Linares et al., 2019). The connection between food and aroma goes even deeper as aroma compounds can shape humans' food preference and even influence their eating behaviour (Rolls et al., 1996; Small and Green, 2011). For example, aroma intensity of vanilla in a custard dessert affected food intake, with higher intensity resulting in smaller bite size (de Wijk et al., 2012). In addition, aroma compounds are suggested to possess health benefits; Phe‐derived compounds like vanillin and cinnamaldehyde have demonstrated anticarcinogenic properties, and RK aids in weight loss (Ahn et al., 2015; Ayseli and Ipek Ayseli, 2016; King et al., 2007; Morimoto et al., 2005). Due to their immense contribution, Phe‐derived compounds, for example, eugenol (clove), cinnamaldehyde (cinnamon), phenylacetaldehyde (rose), benzaldehyde (almond), RK (raspberry) and of course vanillin (vanilla), are highly desired in the food and beverage and pharma industries. The specific rise in demand for ‘natural’ aroma compounds of biological origin has led several large food companies to pledge complete removal of artificial flavours from their products (Bomgardner, 2016). This has ignited extensive research into finding platforms for the production of these compounds (see Microbes and plants as platforms for aroma compound production). In addition, in crops that are consumed fresh, such as tomato, basil, and strawberry (Fan et al., 2021; Tieman et al., 2017; Walters et al., 2021), much effort is being made to identify flavour‐contributing Phe‐derived aroma compounds and the levels that appeal to consumers. Integrating knowledge on the interplay between humans and aroma molecules may enable generating healthier and tastier foods and crops to improve humans' well‐being.
Figure 1.
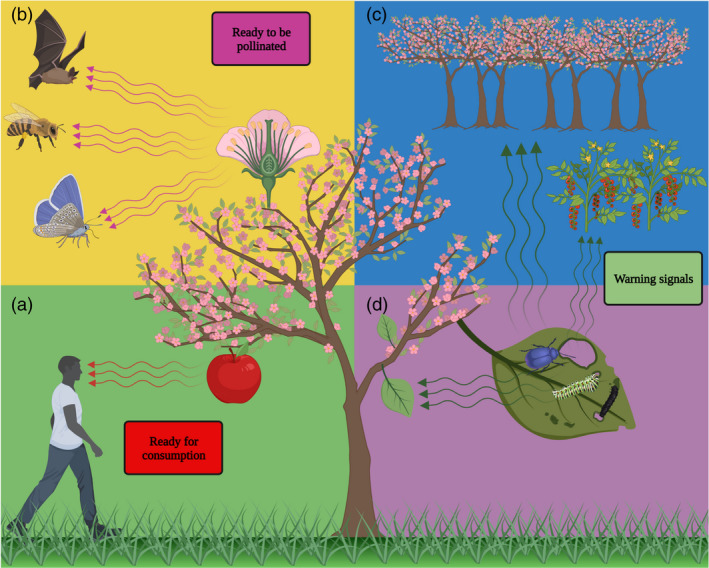
Schematic presentation of interactions between plant‐derived aroma compounds and humans and pollinators, and within and between plants. (a) Ripe fruit releases aroma compounds perceived by the olfactory system. (b) Flowers that are ready for pollination release aroma compounds that make them more conspicuous, attracting, and guiding potential pollinators. (c–d) herbivore infestation may elicit the release of airborne signalling volatiles from damaged tissue that act in planta (c) or warn neighbouring plants (d).
Aroma and pollinators
As sessile organisms, plants are dependent on external factors for sexual reproduction; ca. 87.5% of angiosperms are pollinated by animals, including birds, mammals and reptiles, with insects being the dominant taxa (Ollerton, 2017). Flowers advertise themselves to pollinators by various cues, olfactory ones playing a major role (Fig. 1b; Joffard et al., 2020). Phe‐derived volatile compounds are widespread among floral blends, and benzaldehyde, methyl salicylate (MeSa), benzyl alcohol, and 2‐phenylethanol are the most common (Knudsen et al., 2006). These aroma compounds strongly contribute to specificity in plant–pollinator interactions. For example, loss of benzaldehyde emission in Capsella underlies the transition from animal‐mediated pollination to selfing (Jantzen et al., 2019; Sas et al., 2016). In moth‐pollinated petunia, loss of benzaldehyde production led to a shift in pollinator to hummingbirds, which depend largely on their eyesight (Amrad et al., 2016). Directed evolution by bumble bee pollination of Brassica rapa led to increased floral emission of benzyl nitrile (Ramos and Schiestl, 2019), another Phe‐derived volatile (Liao et al., 2020b). From a human perspective, animal pollination is especially important because it is responsible for 35% (by volume) of crop production (Klein et al., 2007). Perils such as global environmental changes present a tangible threat to volatile‐mediated plant–pollinator mutualism (Byers and Chang, 2017; Glenny et al., 2018). A standing question is whether advanced breeding techniques can be harnessed to improve (or at least maintain) pollination efficiencies to overcome future problems (Dudareva et al., 2013). Success has been extremely limited so far, as numerous complexities still need to be addressed. For example, floral bouquets are typically comprised of complex blends of numerous volatiles originating from various biochemical pathways. Increasing the metabolic flux into multiple distinct branches belonging to often competing pathways is extremely complicated, especially when they are negatively correlated, compete for the same substrates, or affect other agriculturally important traits (Iijima et al., 2004). Furthermore, pollinators often perceive blends as separate from the components that make up the blend (Wycke et al., 2020). Understanding pollinator‐specific perceptual characteristics of components and blends is especially important when planning which compound/s to target. Solving these pending problems is a prerequisite for success in this endeavour.
Aroma as defence signals within and between plants
In addition to pollination, plants also make use of volatile compounds as part of their defence mechanism, and they are used in both intra‐ and inter‐plant communication (Fig. 1c,d) (Ninkovic et al., 2020; Vlot et al., 2020). Phe‐derived aroma compounds, such as eugenol, vanillin and guaiacol, have been shown to possess antimicrobial properties (Fitzgerald et al., 2004; Friedman et al., 2002; Walsh et al., 2019; Wang and Fan, 2014). The volatile methyl ester of salicylic acid (SA), MeSA, is a signalling phytohormone that upon conversion to bioactive SA, regulates or activates resistance mechanisms (Park et al., 2007; Shulaev et al., 1997; Vlot et al., 2009). MeSA is a mobile signal that has been shown to travel through the phloem from infected to uninfected tissues, enabling activation of systemic acquired resistance (Park et al., 2007). In addition, MeSA is suggested to act as an airborne signal between neighbouring plants; MeSa produced in response to viral infection of tobacco plants activated defence mechanisms in its healthy, uninfected neighbours (Shulaev et al., 1997). Although some possible pathways have been suggested, the exact mechanisms of volatile signal perception in plants remain to be investigated (Ninkovic et al., 2020). Furthermore, MeSA has been shown to serve indirectly against herbivores by recruiting their natural predators (Ament et al., 2010; Rowen et al., 2017).
Generating plants with modified emission levels of MeSA and other defence volatiles to improve the defence efficiency and priming of plant populations against pathogens and herbivores seems like a logical goal. Nevertheless, tampering with the defence mechanism might be a double‐edged sword because predicting all layers of volatile‐mediated plant–plant and plant–pathogen interactions is extremely complex. For instance, synthetic lures containing MeSA placed in tomato fields improved resistance to both pathogens and herbivores, but increased their susceptibility to colonization by thrips (Rowen et al., 2017). Taken together, although the ability to generate tailor‐made metabolically engineered plants with superior volatile‐based defence mechanisms or alert systems is an attainable goal, a better understanding of the many aspects of plant volatiles' interactions with the environment is required.
Thus, future goals of harnessing Phe‐derived aroma compounds for human needs are not an easy task, because these volatiles are entangled in many of the plant's interactions with the environment that are vital to humans, pollinators, and plants. Hence, the engineering approach to reach these goals needs to be more ‘holistic’ and less ‘atomistic’, due the many layers of aroma function. For instance, MeSA and guaiacol are disliked by consumers and in tomatoes, reducing their levels has been suggested for flavour improvement (Tieman et al., 2017). Yet, lowering their levels might compromise the plants and their population defence and priming mechanisms, rendering the plants more susceptible to pathogens and herbivores. MeSA is also a prevalent compound in floral bouquets with a proven role in pollinator attraction (Hoballah et al., 2005; Knudsen et al., 2006), hence perturbing its synthesis might also have adverse effects on pollination efficiency. Taken together, it is clear that future strategies for metabolic engineering of aroma compounds in plants should ideally consider integration of flavour, pathogen and herbivore resistance, pollination efficiency and more.
Phe‐derived aroma compound production and regulation in plants
Phenylpropanoid pathway
The phenylpropanoid pathway produces myriad metabolites involved in numerous processes of utmost importance in the plant's life cycle, such as lignin, flavonoids, and aroma compounds (Table 1; Vogt, 2010). These metabolites are produced via an intricate and strongly interlinked network of enzymes and regulators. The precursors of the pathway are the aromatic amino acids (AAAs). Biosynthesis of AAAs begins in the plastid‐localized shikimate pathway. In the first step, the enzyme 3‐deoxy‐D‐arabino‐heptulosonate‐7‐phosphate (DAHPS) condenses the pentose‐phosphate‐pathway‐derived erythrose‐4‐phosphate (E4P) and glycolysis‐derived phosphoenolpyruvate (PEP) (Fig. 2). Further enzymatic steps lead to the formation of the pathway's final product—chorismate. Chorismate mutase 1 (CM1) subsequently converts chorismate to prephenate, from which Phe may be biosynthesized in plants mainly via the arogenate pathway (Lynch and Dudareva, 2020). However, Phe biosynthesis is not limited to the plastids; as in microbes, it may also occur in the cytosol via the phenylpyruvate pathway with the aid of chorismate mutase 2 (CM2) (Qian et al., 2019). Phenylpropanoid production begins with Phe being directed to two branches mediated by competing enzymes, yielding four aroma compound classes based on their carbon backbone: benzenoids (C6–C1), phenylpropanoid‐related compounds (C6–C2), phenylpropenes (C6–C3), and phenylbutanones (C6–C4) (Fig. 2). Metabolic flow towards benzenoids, phenylpropenes, and phenylbutanones is mediated by the action of L‐phenylalanine ammonia‐lyase (PAL), which in the committed step deaminates Phe to trans‐cinnamic acid (CA). This constitutes a main transition point of carbon from primary (amino acid) metabolism to phenylpropanoid‐specialized metabolism. Benzenoids production occurs via the peroxisome‐localized β‐oxidative and/or cytosolic non‐β‐oxidative pathways (Widhalm and Dudareva, 2015). CA conversion to p‐coumaric acid by cinnamate 4‐hydroxylase (C4H) followed by multiple enzymes, including CoA ligases like 4‐coumarate‐CoA ligase (4CL), directs metabolism toward phenylpropenes, phenylbutanones, lignins, and flavonoids. Recently, a bifunctional Phe/L‐tyrosine ammonia‐lyase (PTAL), which also converts tyrosine to p‐coumaric acid, was identified in Brachypodium distachyon (Barros et al., 2016; Barros and Dixon, 2020). In addition to route through PAL, Phe may be channelled towards the production of C6–C2 phenylpropanoid‐related aroma compounds via different pathways (Fig. 2), as revealed in tomato, petunia, rose and poplar (Farhi et al., 2010; Günther et al., 2019; Hirata et al., 2016; Kaminaga et al., 2006; Tieman et al., 2006).
Figure 2.
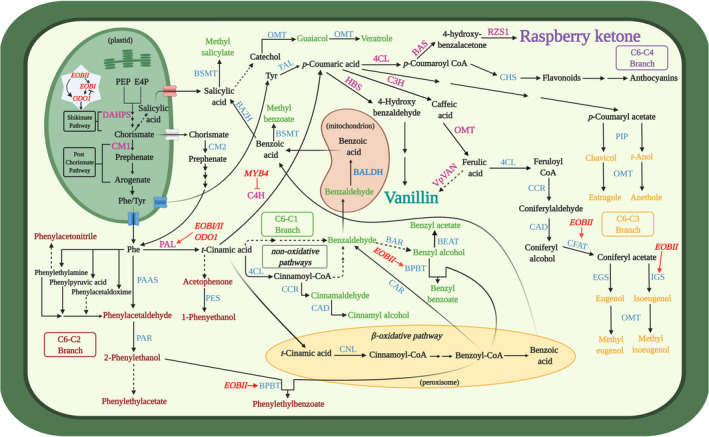
Schematic presentation of biosynthetic pathways leading to the Phe‐derived aroma compounds in plants. L‐phenylalanine (Phe) is synthesized in the plastid or in the cytosol from chorismate generated via the shikimate pathway. Key enzymes in vanillin and raspberry ketone biosynthesis are in magenta. Enzymes involved in the production of other Phe‐derived aroma compounds are in blue, and transcription factors are in red. Dark red, green, orange, and purple indicate aroma compounds originating from the phenylpropanoid‐related (C6–C2), benzenoid (C6–C1), phenylpropene (C6–C3), and phenylbutanone (C6–C4) branches, respectively. Solid arrows indicate established biochemical steps, and dashed arrows indicate hypothetical steps. Stacked arrows represent multiple enzymatic steps. Abbreviations: BAR, benzaldehyde reductase; BAS, benzalacetone synthase; BA2H, benzoic acid 2‐hydroxylase; BALDH, benzaldehyde dehydrogenase; BEAT, acetyl‐CoA:benzylalcohol acetyltransferase; BPBT, benzoyl‐CoA:benzylalcohol/2‐phenylethanol benzoyltransferase; BSMT, benzoic acid/salicylic acid carboxyl methyltransferase; C3H, p‐coumarate 3‐hydroxylase; C4H, cinnamate 4‐hydroxylase; CAD, cinnamyl alcohol dehydrogenase; CAR, carboxylic acid reductase; CCR, cinnamyl‐CoA reductase; CFAT, coniferyl alcohol acetyltransferase; 4CL, 4‐ coumarate‐CoA ligase; CM 1/2, chorismate mutase 1/2; CNL, cinnamoyl‐CoA ligase; DAHPS, 3‐deoxy‐D‐arabino‐heptulosonate 7‐phosphate synthase; E4P, erythrose 4‐phosphate; EGS, eugenol synthase; HBS, hydroxybenzaldehyde synthase; IGS, isoeugenol synthase; OMT, O‐methyltransferase; PAR, phenylacetaldehyde reductase; PAAS, phenylacetaldehyde synthase; PAL, phenylalanine ammonia‐lyase; PEP, phosphoenolpyruvate; PES, 1‐phenylethanol synthase, PIP, pinoresinol−lariciresinol reductase, isoflavone reductase, phenylcoumaran benzylic ether reductase; RZS1, raspberry ketone/zingerone synthase 1; TAL, tyrosine ammonia‐lyase; Tyr, tyrosine; VpVAN, vanillin synthase; transcription factors: EOBI/II, emission of benzenoids I/II; MYB4, MYB transcription factor 4; ODO1, odorant 1.
Vanillin and raspberry ketone biosynthesis
Biosynthesis of the phenylbutanone RK [4‐(4‐hydroxyphenyl)butan‐2‐one], which defines the aroma of ripe raspberry fruit, has been fully elucidated (Fig. 2) (Abe et al., 2001; Koeduka et al., 2011). CA is converted to p‐coumaric acid by C4H, a step also shared with vanillin production, followed by the action of 4CL to yield p‐coumaroyl‐CoA. In the penultimate step, benzalacetone synthase (BAS) condenses p‐coumaroyl‐CoA and malonyl‐CoA, generating 4‐hydroxybenzalacetone. Reduction of 4‐hydroxybenzalacetone to RK is catalysed by raspberry ketone/zingerone synthase 1 (RZS1).
Formation of vanillin (4‐hydroxy‐3‐methoxybenzaldehyde), the main aroma compound in cured extract of Vanilla planifolia pods (Gallage and Møller, 2018), has been suggested to occur via several possible routes. Similar to C6–C3 compounds, p‐coumaric acid is the precursor for vanillin (Fig. 2) (Kundu, 2017). Chemically, vanillin is a phenolic aldehyde belonging to the C6–C1 group because it undergoes C2 side‐chain shortening (Kundu, 2017). This latter reaction is suggested to be catalysed by the vanillin synthase recently isolated from V. planifolia (VpVAN), directly from ferulic acid (Gallage et al., 2014, 2018; Kundu, 2017). However, VpVAN's ability to directly generate vanillin was questioned by Yang et al. (2017), who reported its involvement in vanillin biosynthesis through conversion of p‐coumaric acid to p‐hydroxybenzaldehyde (Kundu, 2017). The debate continues, with studies by Chee et al. (2017) and Gallage et al. (2018) further suggesting VpVAN's activity via ferulic acid, although detailed enzymological data, such as kinetics, are lacking. For comprehensive reviews of Phe‐derived volatile biosynthesis, we suggest Vogt et al. (2010), Muhlemann et al. (2014) and Widhalm et al. (2015).
Regulation of Phe‐derived aroma compound biosynthesis and emission
Downstream products
Metabolic fluxes in the shikimate and post‐chorismate pathways, which generate the primary precursors of the phenylpropanoid pathway—the AAAs, are regulated by an intricate network involving phytohormones, transcriptional regulators, and negative and positive feedback by downstream products (Fenske et al., 2015; Liu et al., 2017; Lynch et al., 2020; Lynch and Dudareva, 2020; Spitzer‐Rimon et al., 2010, 2012; Tzin and Galili, 2010; Verdonk et al., 2005). DAHPS is the key enzyme controlling carbon flux into the shikimate pathway (Fig. 2), and therefore a major target for metabolic engineering in both microbes and plants (see Microbes and plants as platforms for aroma compound production). Although in bacteria, DAHPS is allosterically inhibited by the AAA product (Luttik et al., 2008; Tzin et al., 2012; Tzin and Galili, 2010), until recently, the data for plants were generally lacking (Liao et al., 2020a; Yokoyama et al., 2021). Yokoyama et al. (2021) demonstrated that in Arabidopsis, DAHPS is feedback‐inhibited by multiple downstream products, that is, tyrosine, tryptophan, arogenate, caffeate, and chorismate. Another possible limiting factor is availability of E4P for AAA synthesis, as plant DAHPS has a lower K m value for PEP than for E4P (Yokoyama et al., 2021). In the post‐chorismate stages of the pathway, chloroplast‐localized CM1 is inhibited by Phe and tyrosine and activated by tryptophan. AAAs also inhibit their corresponding producing enzymes (Lynch and Dudareva, 2020; Tzin and Galili, 2010). In the cytosol, Phe controls the phenylpyruvate pathway by inhibiting conversion of prephenate to phenylpyruvate driven by prephenate dehydratase. The next key enzyme in this stream, which is tightly regulated both transcriptionally and enzymatically, is PAL, which is feedback‐inhibited by multiple downstream products, including flavonoids (Zhang and Liu, 2015).
Transcription factors
The identification of transcriptional regulators of volatile phenylpropanoid production has been the focus of many studies performed mainly with Petunia, a model plant for the study of Phe‐derived aroma compounds (Fig. 2). These studies revealed that a network of MYB‐family transcription factors regulate the biosynthesis of aroma compounds by directly activating shikimate and post‐chorismate pathways, and PAL and aroma‐related genes (Boersma et al., 2022; Colquhoun et al., 2011; Spitzer‐Rimon et al., 2010, 2012; Van Moerkercke et al., 2011; Verdonk et al., 2005) (Fig. 2). These include the triad of interacting MYBs—ODORANT 1 (ODO1) and EMISSION OF BENZENOIDS (EOB) I and II (Fig. 2). The MYB triad mechanism has been suggested to be conserved among plants because, for example, in Arabidopsis flowers that do not emit benzenoids, orthologs of EOBs and ODO1 regulate the production of phenylpropanoid‐derived metabolites in the pollen cell wall (Battat et al., 2019). In addition to these positive regulators, a negative factor, MYB4 that fine‐tunes the flux towards C6–C1 and C6–C2 compounds by inhibiting C4H, was also revealed (Colquhoun et al., 2011).
Phytohormones
Transcription factors, in turn, are regulated by phytohormones (Colquhoun et al., 2010; Liu et al., 2017; Patrick et al., 2021; Ravid et al., 2017; Underwood et al., 2005; Wang et al., 2018). Transcript levels of EOBI and II were significantly lower in DELLA‐suppressed petunia flowers, with concurrent reduction in aroma compound emission (Ravid et al., 2017). Therefore, in early stages of flower development (buds), gibberellins promote anthocyanins while blocking aroma compound production by mediating the degradation of DELLA proteins. Towards flower opening, gibberellin levels decrease, shifting production from anthocyanins to aroma compounds (Patrick et al., 2021; Ravid et al., 2017). Similarly, during senescence or after pollination, ethylene levels increase and block the production of aroma compounds, mediated by the action of ETHYLENE RESPONSE FACTOR 6, which interacts with EOBI's DNA‐binding domain, thereby preventing it from activating the promoters of aroma‐related target genes such as ODO1 (Liu et al., 2017; Underwood et al., 2005; Wang et al., 2018). Jasmonates—phytohormones that play a vital role in plant defence—elicit signalling cascades in response to physical damage, which lead to increased production of phenylpropanoids, Phe, and CA, and upregulation of PAL (Gundlach et al., 1992; Halitschke and Baldwin, 2004; Hanik et al., 2010; Shi et al., 2015). Recently, crosstalk between cytosolic Phe synthesis and auxin was identified by overexpression of cytosol‐localized CM2 (Lynch et al., 2020). Unexpectedly, in the transgenic CM2‐overexpressing lines, levels of both Phe and downstream compounds decreased, whereas that of auxin increased. The latter negatively affected the development of plastids, the main route for AAA synthesis in plants (Lynch et al., 2020).
Compartmentalization
The biosynthesis of Phe‐derived aroma compounds depends on the flow of metabolites between chloroplast, cytosol, endoplasmic reticulum, peroxisome, vacuole, and mitochondrion (Fig. 2; Achnine et al., 2004; Adebesin et al., 2018; Cna'ani et al., 2017; Widhalm and Dudareva, 2015). Despite our deeper understanding of the mechanisms of Phe‐derived aroma compound biosynthesis and regulation, to date, little is known about their cellular pathway from generation to release into the atmosphere. The vacuole has been suggested to serve as a mid‐station between aroma compound production and emission (Cna'ani et al., 2017; Song et al., 2018). Compounds, such as benzyl alcohol, vanillin, eugenol, isoeugenol, and 2‐phenyethanol may undergo glycosylation, which increases their water solubility, reduces their volatility and serves as a translocation signal. Following glycosylation, they are compartmentalized and stored in the vacuole. The glycosylation step is suggested to be part of the aroma‐production mechanism as the glycosylated pool of aroma compounds is dynamic, coinciding with increased emission, both developmentally and diurnally (Cna'ani et al., 2017). In addition, glycosylation has been suggested to help maintain cell integrity by detoxifying Phe‐derived compounds that might be damaging to the cell when accumulated to high levels (Adebesin et al., 2017). Moreover, excess Phe has also been shown to be stored in vacuoles (Lynch et al., 2017). Other organelles also have the ability to store aroma compounds, for example, vanillin glucoside which is stored in specialized plastids named phenyloplasts (Brillouet et al., 2014; Gallage et al., 2018).
Regulation of emission
Until recently, it was generally believed that most aroma compounds are released from flowers by passive diffusion. However, calculations of emission rates revealed that with passive diffusion alone, some aroma compounds would accumulate to toxic levels in the cell membranes, suggesting the existence of active mechanisms (Widhalm et al., 2015). Indeed, two factors involved in the active emission of aroma compounds have been identified: the MYB PH4 and adenosine triphosphate‐binding cassette subfamily member 1 (ABCG1) (Adebesin et al., 2017; Cna'ani et al., 2015). The latter has also been shown to dictate the spatial pattern of emission from floral tissues (Skaliter et al., 2021). Whereas the exact mechanism by which PH4 affects emission has yet to be identified, the plasma‐membrane‐localized ABCG1 actively exports aroma compounds from the cell (Adebesin et al., 2017). After crossing the plasma membrane and the cell wall, aroma compounds encounter another barrier—the cuticle. Liao et al. (2020a) demonstrated that the flower cuticle is not only a passive barrier but also functions as a reservoir for compounds with relatively low volatility, such as benzyl benzoate, isoeugenol, 2‐phenylethanol and benzyl alcohol, and is a crucial part of the mechanism interconnecting production and emission. Flowers with reduced cuticle thickness not only emitted less, but also produced less aroma compounds, and carbon‐labelling indicated that Phe biosynthesis was compromised. Furthermore, DAHPS, CM1 and 2, and ODO1 were downregulated in these flowers, suggesting the activation of a feedback mechanism regulating carbon flux into the pathway aimed at preventing overaccumulation of aroma compounds that are toxic to the cell (Liao et al., 2020a). These findings could serve to manipulate the production and emission mechanisms towards the generation of better or stronger smelling fruit and flowers.
Conclusions
Overall, to ensure that scent is produced and emitted at the right time and in the right place, multiple players, that is, metabolites, organelles, transcription/epigenetic factors, phytohormones, and physical barriers are interlinked to tightly regulate this high‐energy‐demanding process. When examining this elaborate mechanism, it is clear why metabolic engineering of Phe‐derived aroma compounds is no easy task, as reprogramming the carbon flux with so many players is extremely challenging. For example, although an increase in Phe due to suppression of the three petunia PALs might be expected to lead to redirection of carbon flux towards metabolism of C2 compounds, emission of phenylacetaldehyde and 2‐phenylethanol are not altered. Instead, excess Phe is diverted to an inactive pool in the vacuole (Lynch et al., 2017). Furthermore, the crosstalk between the primary and specialized metabolisms has not been fully deciphered, for example, the factors that directly ‘turn on’ the master transcription factor of aroma compound biosynthesis, EOBII, at the onset of aroma compound production in flowers at anthesis are unknown, as are the molecular signals that simultaneously deactivate anthocyanin production. Answering these vital questions will enable more accurate metabolic engineering of aroma compounds.
Microbes and plants as platforms for aroma compound production
The importance of aroma compounds to the food, beverage, perfume and pharma industries makes them a highly desirable commodity. Some of these compounds can be chemically mass‐produced, but they are perceived as unhealthy; chemical synthesis is often not environmentally friendly and not stereospecific, although the cost of synthetic compounds is far lower than that of natural ones (Dubal et al., 2008; Gallage and Møller, 2015; Lee et al., 2016; Martínez et al., 2017; Ni et al., 2015). Furthermore, there is a rise in consumer demand for ‘natural’ aroma products of ‘biological’ origin and the US Food and Drug Administration requires labelling products as ‘flavoured’ or ‘artificial’ if the source of the compound is not natural (Bomgardner, 2016). All of this is driving the need for natural aroma compounds. On the other hand, native plants from which aroma compounds can be extracted are usually not a good source for them: for the most part, these plants are not domesticated and/or cultivated, require prolonged growing periods, and are strongly affected by biotic and abiotic factors; moreover, compound accumulation is specific to developmental stage and tissue, only trace amounts of these compounds are produced, and the extraction process is expensive. Although classical breeding approaches for increased production of specialized metabolites have been demonstrated in, for example, Malus domestica (apple) (Chagné et al., 2013), melon (Tzuri et al., 2015), tomato (Karniel et al., 2020), and Artemisia annua (Czechowski et al., 2020), modern crops are not generally bred for specialized metabolites of interest due to limited gene pools, trait complexity, and the difficulty involved in screening breeding populations (Peled‐Zehavi et al., 2015). Therefore, extensive research together with molecular techniques have been applied in plants and microbes to generate advanced platforms for the production of specialized metabolites. Some examples for Phe‐derived aroma compounds include benzaldehyde (Kunjapur et al., 2014) in Escherichia coli, cinnamaldehyde, and cinnamyl alcohol in yeast (Gottardi et al., 2017), and eugenol in poplar and strawberry (Hoffmann et al., 2011; Lu et al., 2017). Most of the effort, however, has been invested in the production of vanillin and RK, which are highly desired by the industry and share similar issues (Lee et al., 2016; Milke et al., 2020; Muheim and Lerch, 1999; Padel and Foster, 2005; Román et al., 2017; Sinha et al., 2008), among them: extraction of ‘natural’ and vanillin and RK is expensive and labour‐intensive, and their yields from host plants are low. To produce 1 kg of vanillin, ca. 500 kg of vanilla pods are required, and for RK yields are even lower, with production of 1 kg requiring ca. 500 tons of raspberries (Gallage and Møller, 2015; Lee et al., 2016); estimated prices are ca. 4000 USD/kg and ca. 3000 USD/kg for naturally derived vanillin and RK, respectively, whereas prices for synthetic compounds are hundreds of times lower (Lee et al., 2016; Singh et al., 2015). In the following sections, we discuss the pros and cons of using microbes and plants as platforms for the production of Phe‐derived aroma compounds, focusing on vanillin and RK. We further highlight production difficulties and bottlenecks, and ways of overcoming them.
Microbes
Microorganisms, most commonly the bacterium E. coli and the yeast Saccharomyces cerevisiae, have been widely used as heterologous systems for the production of numerous specialized metabolites. Microbes often lack the complete biochemical pathways for biosynthesis of plant aroma compounds. Therefore, the main approach for metabolic engineering relies on heterologous expression of enzymes to complete the necessary chemical steps, complemented with mutation or deletion of specific genes to direct metabolite flow towards the target while suppressing competing metabolic shunts and preventing unwanted modifications to the product. Microbes have many advantages for metabolic engineering, such as rapid growth, secretion of metabolites to the media for easy harvest, simple transformation procedures and inexpensive carbon and nitrogen sources (Chemler and Koffas, 2008; Miralpeix et al., 2013; Yang et al., 2020)). Another major advantage of the production of plant metabolites in microbes is that competing branches are often non‐existent, and there is less chance of feedback inhibition by non‐target downstream products, for example, flavonoids in the case of PAL (see earlier). Moreover, both European and US food legislation classifies microbial‐produced aroma compounds as GRAS (generally recognized as safe) (Food and Drug Administration, 2018; Kallscheuer, 2018).
Industrially applicable fungi and bacteria have been successfully metabolically engineered to generate vanillin from different substrates, such as ferulic acid, eugenol and isoeugenol, reaching yields of >10 g/L (Gallage and Møller, 2015). Microalgae can also produce vanillin, albeit at low levels, via biotransformation with ferulic acid (Tripathi et al., 2002). Nonetheless, glucose and other carbon sources are considered more ideal substrates as they are readily utilized, non‐toxic, and inexpensive (Gallage and Møller, 2015). Vanillin production from glucose was achieved in E. coli harbouring a mutated shikimate dehydrogenase (aroE) combined with modifications that allowed bypassing the phenylpropanoid pathway entry points that exist in plants. However, the modified E. coli was only able to generate vanillic acid and the reduction to vanillin was performed in vitro (Fig. 3a) (Li and Frost, 1998). To eliminate the costly and complicated in vitro reaction, yeast with aromatic carboxylic acid reductase (ACAR) and phosphopantetheinyl transferase (PPTase), necessary for ACAR activation, were generated. This created the complete route for vanillin production from glucose, yielding 65 and 45 mg/L vanillin in Schizosaccharomyces pombe and S. cerevisiae (Fig. 3b), respectively (Hansen et al., 2009). The ACAR‐based strategy also proved effective for heterologous production from glucose of the aroma compounds cinnamaldehyde and cinnamyl alcohol (Gottardi et al., 2017). However, in this case, the authors ectopically expressed PAL from Arabidopsis to generate CA from Phe (instead of dehydroshikimic acid path), enabling de novo biosynthesis of the target molecules. Similarly, Ni et al. (2015) introduced a synthetic vanillin pathway into E. coli aimed at mimicking the one in plants, to avoid diversion of substrates from the shikimate pathway. They used tyrosine ammonia‐lyase, which converts tyrosine directly to p‐coumaric acid and not PAL, to overcome the low activity of C4H in E. coli due to the absence of a suitable NADPH reductase (Fig. 3a) (Watts et al., 2004). The constructed pathway allowed the biogenesis of vanillin with media containing tyrosine, reaching a titre of 97.2 mg/L. Furthermore, the modified bacterium could also utilize glucose, xylose, and glycerol (Fig. 3a) (Ni et al., 2015).
Figure 3.
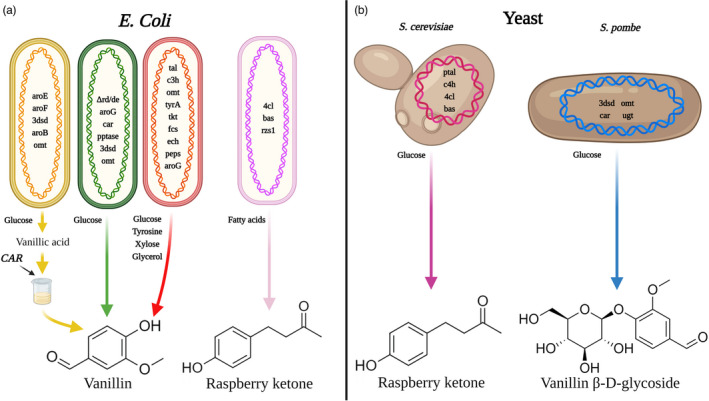
Microbial systems producing vanillin and raspberry ketone. (a) Four metabolically engineered Escherichia coli strains from left to right: recombinant strain harbouring a pathway for production of vanillic acid from glucose with subsequent in vitro reduction of vanillic acid to vanillin by carboxylic acid reductase (CAR) (Li and Frost, 1998); strain in which genes that encode aldo‐keto reductases and alcohol dehydrogenases (Δrd/de) were deleted together with the introduction of a pathway for the biosynthesis of vanillin from glucose (Kunjapur et al., 2014); strain harbouring a pathway mimicking vanillin biosynthesis in plants capable of using L‐tyrosine, glucose, xylose or glycerol as substrates (Ni et al., 2015); strain harbouring enzymes for raspberry ketone production from fatty acids (Chang et al., 2021). (b) Metabolically engineered Saccharomyces cerevisiae and Schizosaccharomyces pombe containing the complete pathway for raspberry ketone and vanillin β‐D‐glycoside biosynthesis from glucose (Hansen et al., 2009; Lee et al., 2016). Abbreviations: 3dsd, 3‐dehydroshikimate dehydratase; aroB, 3‐dehydroquinate synthase; aroE, shikimate dehydrogenase; aroF/G, 3‐deoxy‐D‐arabinoheptulosonate 7‐phosphate synthase; aroZ/asbF, 3‐dehydroshikimate dehydratase; car, carboxylic acid reductase; ech, enoyl‐CoA hydratase/aldolase; fcs, trans‐feruloyl‐CoA synthetase; omt, O‐methyltransferase; peps, phosphoenolpyruvate synthetase; pptase, phosphopantetheinyl transferase; tkt, transketolase; tyrA, chorismate mutase/prephenate dehydrogenase; tyrR, a regulatory protein; ugt, UDP‐glucuronosyltransferase.
To construct a pathway for de novo biosynthesis of RK in yeast, PTAL, with the ability to use both Phe and tyrosine as substrates, was expressed together with plant C4H, 4CL, and BAS (Fig. 3b) (Lee et al., 2016). There was no need to add the enzyme responsible for the final step, benzalacetone reductase (BAR), as the yeast demonstrated endogenous activity (Beekwilder et al., 2007). Maximum RK yield was 2.81 mg/L when 4CL and BAS were physically fused, an approach suggested to allow substrate funnelling (Aalbers and Fraaije, 2019; Albertsen et al., 2011; Lee et al., 2016). Indeed, multiprotein complexes known as ‘metabolons’ have been identified in plants, including PAL and C4H (Achnine et al., 2004; Zhang and Fernie, 2021). However, fusion of PTAL and C4H in yeast led to an inverse effect, greatly reducing the final yield of RK (Aalbers and Fraaije, 2019; Lee et al., 2016). To our knowledge, de novo RK biosynthesis has not been achieved in E. coli; however, substantial amounts have been obtained by growing E. coli engineered with 4CL, BAS and raspberry ketone/zingerone synthase 1 (RZS1) on fatty acids, reaching a titre of 180.94 mg/L (Fig. 3a) (Chang et al., 2021).
The importance of the shikimate and AAA pathways as the primary source for downstream metabolites has established them as a key target for metabolic engineering (Averesch and Krömer, 2018; Peled‐Zehavi et al., 2015). Both pathways are tightly regulated, with significant bottlenecks due to feedback inhibition of two key enzymes—DAHPS and CM—by their products, the AAAs (see Regulation of Phe‐derived aroma compound biosynthesis and emission). Use of feedback‐insensitive forms of these enzymes has proven successful in alleviating these bottlenecks in microbes and plants (Graf and Altenbuchner, 2014; Liu et al., 2019; Luttik et al., 2008; Oliva et al., 2017; Tzin et al., 2012, 2013; Xie et al., 2016). This approach was also taken for the de novo biosynthesis of vanillin in microbes. Li and Frost (1998), Kunjapur et al. (2014) and Ni et al. (2015) all introduced a feedback‐insensitive DAHPS into their modified E. coli for improved carbon flux. In yeast, strains overexpressing an insensitive DAHPS (Aro4) exhibited a three‐fold increase in AAAs, together with an increase in the Phe‐derived aroma compounds 1‐phenylethanol and phenylacetate (Liu et al., 2019; Luttik et al., 2008). Combining Aro4 with an insensitive CM (Aro7) further increased flux towards the AAA, manifested by enhanced production of p‐coumaric acid, a precursor of both vanillin and RK (Liu et al., 2019). E4P of the pentose phosphate pathway is another limiting factor in microbes, because DAHPS has low affinity to it and its endogenous pool is low. To overcome this, Liu et al. (2019) expressed two phosphoketolases to divert the carbon flux from glycolysis towards E4P. Indeed, combining phosphoketolases together with Aro4 and 7 further increased the levels of p‐coumaric acid (Liu et al., 2019).
One of the problems in attempting to enhance metabolite production in microbes is conversion of the target molecule to unwanted side products. More specifically, as microbes rapidly convert aldehydes to alcohols, vanillin and its precursor protocatechualdehyde may be converted to the unwanted by‐products, such as vanillyl alcohol and protocatechuic alcohol respectively. A rational approach to overcoming this problem is to delete or knock out the genes encoding the enzymes with this activity. In this way, Kunjapur et al. (2014) generated E. coli with 55 times higher titres of vanillin (119 mg/L) compared with E. coli without deletion of the respective reductases (Fig. 3a). These strains are also capable of accumulating the benzenoid benzaldehyde, as its reduction to benzyl alcohol was also decreased (Kunjapur et al., 2014). Although successful in this case, deletion or silencing of ‘unwanted’ genes is not always an applicable strategy as these may be involved in other pathways that are crucial for the organism's survival. For example, in S. cerevisiae, an enoyl reductase that is involved in the biosynthesis of fatty acids also converts p‐coumaroyl‐CoA (pCA) to phloretic acid, resulting in carbon loss when attempting to enhance phenylpropanoid (i.e., flavonoid) production (Lehka et al., 2017). To avoid pCA conversion while preserving fatty acid production, two alternative approaches were explored: a mutation in the active site of enoyl reductase responsible for phloretic acid production, and incorporation of a plant gene that lacks this activity. The latter approach was more effective in preventing carbon loss (Lehka et al., 2017).
One may think that when it comes to enhancement of aroma compounds, the goal is always ‘the more the merrier’. Nevertheless, aroma compounds in general, and vanillin and RK in particular, become toxic to cells at certain threshold concentrations (Beekwilder et al., 2007; Fitzgerald et al., 2004; Friedman et al., 2002; Hansen et al., 2009). Vanillin was shown to inhibit growth of S. cerevisiae cells at concentrations above 500 mg/L (Hansen et al., 2009). Similarly, vanillin demonstrated bacteriostatic properties in E. coli via disruption of the membrane, ultimately impairing respiration (Fitzgerald et al., 2004); thus, the rapid reduction of vanillin to the by‐product vanillyl alcohol in that organism is probably a detoxification step (Graf and Altenbuchner, 2014). Interestingly, membrane disruption was also reported in petunia cells in which the Phe‐derived volatile release mechanisms were impaired (Adebesin et al., 2017; Liao et al., 2020a). Another approach to lowering a compounds' toxicity is by modification, such as methylation or glycosylation. Indeed, in Vanilla pods, vanillin is stored as vanillin β‐D‐glucoside, and S. cerevisiae cells grown on media supplemented with vanillin β‐D‐glucoside at 25 g/L (50‐fold more than vanillin) do not exhibit impaired growth (Hansen et al., 2009). To obtain glycosylation of vanillin in yeast, a glycosyltransferase from Arabidopsis was incorporated. Indeed, it successfully converted almost all of the vanillin to this less toxic water‐soluble form, yielding 500 mg/L of vanillin β‐D‐glucoside (Fig. 3b) (Brochado et al., 2010; Hansen et al., 2009). Nonetheless, when the desired product is the aglycone, and not the glycoside, another step is required to break the glycoside residue.
The studies described in this section demonstrate the progress achieved in metabolic engineering of bacteria and yeast for the production of the commercially important aroma compounds vanillin and RK. These advances are due to the improved understanding of metabolic fluxes in host microbes, together with identification of specialized genes from different organisms. Perhaps, the best proof of this progress is the fact that biologically derived vanillin generated from glucose by microbial fermentation is already being sold (Waltz, 2020).
Plants
With our growing understanding of the metabolic fluxes within and between target pathways, their feedback loops, wide‐scale functional gene analyses, big data analyses, tools for directed evolution of target enzymes, etc., plants are an attractive system for the production of target metabolites. This is further strengthened by the progress that has been made in the application of precise genome‐editing tools (Bortesi and Fischer, 2015; Pott et al., 2019; Yang et al., 2014). Moreover, plants have some inherent advantages over microorganisms as platforms for the enhancement of endogenous pathways or as hosts for heterologous ones (Owen et al., 2017). From an economic standpoint, the production process in plants is autotrophic and more cost‐effective than in microbes, scale up is relatively easy, and there is no need for culture media or special growth facilities (Fu et al., 2018). Moreover, as flowers and fruit are, in many cases, the end product, they present an interesting target for metabolic engineering to improve food flavour and nutritional qualities (Ashokkumar et al., 2020; Hoffmann et al., 2011; Lobato‐Gómez et al., 2021; Yu et al., 2021).
When dealing with Phe‐derived pathways, plants present the advantage of possessing all of the basic components of the target pathway, as opposed to microbes. Thus, expression of dedicated transcription factors for the activation of multiple genes in a pathway(s) is a routine approach in plant metabolic engineering (Koeduka et al., 2021; Skaliter et al., 2019; Zhang et al., 2015; Zvi et al., 2008, 2012). In some cases, the genetic components are not actively producing target products because the substrate is missing, a phenomenon known as ‘silent metabolism’ (Fang et al., 2021; Hoffmann et al., 2011; Lewinsohn and Gijzen, 2009). For example, lisianthus flowers that are normally unscented produced the Phe‐derived methyl benzoate and 2‐phenylethanol when precursor availability was increased (Fang et al., 2021). Another advantage is that the final biosynthetic steps of many specialized metabolites are not fully detailed, and plants offer the possibility to complete these uncharacterized reactions (Dong et al., 2012; Jacobowitz and Weng, 2020; Zhou et al., 2018). Another main advantage of plants, as eukaryotic organisms, over microbes, is the presence of organelles for example, improved sequestration or accumulation of target molecules, or storage of toxic compounds. On the other hand, until recently, there was no efficient way to achieve targeted plant genome modifications, while growth of transgenic plants and the use of products extracted from them are restricted by complex regulations.
As the pathway/enzymes leading to vanillin production have yet to be fully characterized, hot chili pepper (Capsicum frutescens) is an example of a potential target for vanillin production, since vanillin is a precursor of capsaicins (pungency factor in hot pepper) (Chee et al., 2017; Gururaj et al., 2012). Pepper is highly recalcitrant to transformation, and attempts to produce vanillin in pepper have focused on work with calli. Silencing of the aminotransferase responsible for transamination of vanillin to vanillyl amine (the direct precursor of capsaicin) in pepper calli led to increased levels of the former (Fig. 4a) (Gururaj et al., 2012; Weber et al., 2014). Ectopic expression of VpVAN following particle bombardment of chili pepper callus led to substantially enhanced vanillin production (Fig. 4a); transformed calli generated ca. 573.39 μg/g fresh weight (FW) of vanillin, which translates to a 190‐fold increase compared with non‐trasnformed calli (Fig. 4a). Levels of vanillin β‐D‐glucoside also increased ca.16‐fold (110.32 μg/g tissue), revealing an additional pool that might be tapped in the future. It would be interesting to combine both approaches, that is, aminotransferase silencing and VpVAN expression. VpVAN was also tested in Nicotiana benthamiana and barley, leading to the accumulation of vanillyl alcohol glucoside (Fig. 4b; Gallage et al., 2014).
Figure 4.
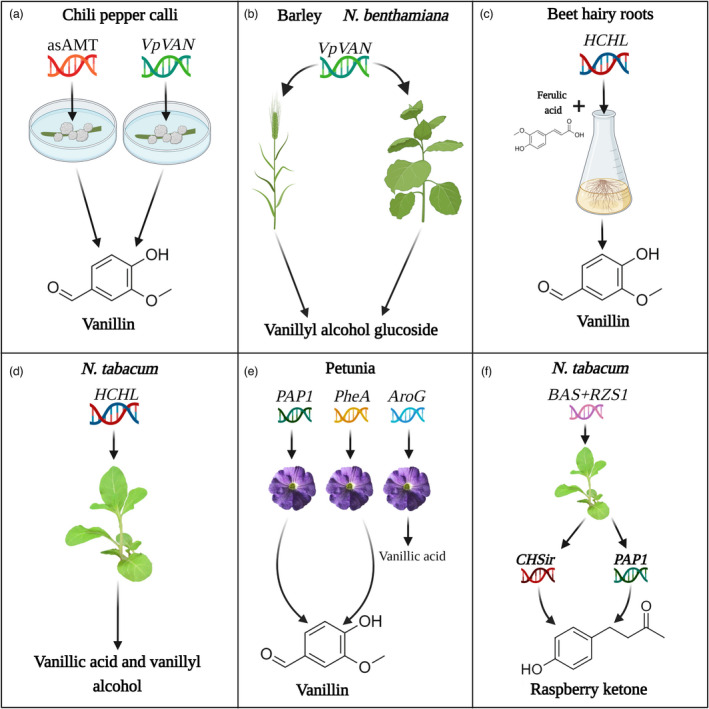
Metabolic engineering of plant‐based systems for enhanced production of raspberry ketone and vanillin. (a, left) Vanillin production in chili pepper (Capsicum frutescens) calli following silencing of aminotransferase (AMT) using Agrobacterium tumefaciens (agro) carrying an AMT sequence in antisense orientation (asAMT) (Gururaj et al., 2012). (a, right and b) Vanilla planifolia vanillin synthase (VpVan) was introduced into chili pepper calli by particle bombardment (a) and into barley (Hordeum vulgare) and Nicotiana benthamiana by agro transformation (b) (Chee et al., 2017; Gallage et al., 2014). (c) Agrobacterium rhizogenes‐mediated transformation of beet with 4‐hydroxycinnamoyl‐CoA hydratase/lyase (HCHL) from Pseudomonas fluorescens, followed by feeding of transgenic hairy roots with ferulic acid (Singh et al., 2015). (d) Ectopic expression of HCHL in transgenic Nicotiana tabacum (Mayer et al., 2001). (e) Petunia plants genetically engineered to express PRODUCTION OF ANTHOCYANIN PIGMENT 1 (PAP1) from Arabidopsis thaliana, bacterial chorismate mutase/prephenate dehydratase enzyme (PheA) or bacterial 3‐deoxy‐D‐arabino‐heptulosonate 7‐phosphate (AroG) (Zvi et al., 2008; Cna'ani et al., 2014; Oliva et al., 2015, Oliva et al., 2017). (f) Ectopic expression of BAS and RZS1 combined with either PAP1 expression or RNAi‐mediated suppression of CHS (CHSir) to generate raspberry ketone in Nicotiana tabacum plants (Koeduka et al., 2021).
An alternative approach to vanillin production based on Pseudomonas fluorescens 4‐hydroxycinnamoyl‐CoA hydratase/lyase (HCHL), which converts the lignin precursor feruloyl‐CoA to vanillin in two steps, was employed in beet (Beta vulgaris) hairy root cultures (Fig. 4c; Gasson et al., 1998; Singh et al., 2015). Vanillin yield was similar to that in pepper callus, reaching 520 μg/g FW. Feruloyl‐CoA is formed from ferulic acid by 4CL (Barros et al., 2019). Supplementation of ferulic acid to hairy root lines enhanced vanillin production to 2090 μg/g FW, reaching the yield of the vanilla orchid and revealing ferulic acid as a major production bottleneck. However, ferulic acid is an expensive substrate, rendering this approach cost‐ineffective (Gallage and Møller, 2015). Ectopic expression of HCHL was also attempted in tobacco, which synthesizes vanillin naturally, albeit in trace amounts (Kundu, 2017; Mayer et al., 2001). Transgenic HCHL‐expressing plants accumulated increased amounts of the vanillin derivatives, that is, vanillic acid and vanillyl alcohol (Fig. 4d). However, the alterations in metabolic flux resulted in multiple phenotypic abnormalities, such as phloem fiber deformation. These results demonstrate the negative ramifications of perturbing crucial metabolic pathways (Mayer et al., 2001). Vanillin is also a component of scent bouquets of flowers (Knudsen et al., 2006; Oliva et al., 2015, 2017; Skaliter et al., 2021). In petunia flowers, production of vanillin and its derivatives was enhanced by introducing an activator of the phenylpropanoid pathway—the Arabidopsis transcription factor PRODUCTION OF ANTHOCYANIN PIGMENT1 (PAP1) (Fig. 4e) (Skaliter et al., 2019; Zvi et al., 2008). Similarly, the strategy taken in microbes of alleviating metabolic bottlenecks by harnessing the feedback‐insensitive enzymes AroG and PheA, bacterial DAHPS and CM/prephenate dehydratase, respectively, also yielded enhanced levels of vanillin in petunia (Fig. 4e; Oliva et al., 2015, 2017).
In all of the above studies in plants, a single genetic element was used. However, as shown in microbes, due to the complex nature of biochemical pathways leading to aroma compound production, to optimize carbon channelling, multiple genes need to be targeted. Thanks to advances in synthetic biology, multigene expression is now a routine practice in plants—it is no longer restricted to microbes. This strategy of using gene combinations proved successful in strawberry. Expression of petunia isoeugenol synthase (IGS) in strawberry fruit did not affect the concentration of either t‐anol or isoeugenol, which was nearly zero (Hoffmann et al., 2011). However, when in addition to IGS expression, flavonoid biosynthesis was blocked via suppression of the branchpoint enzyme CHS, a substantial increase in both compounds (ca. 400 ng/g FW) was achieved (Cna'ani et al., 2014; Hoffmann et al., 2011; Oliva et al., 2017; Zvi et al., 2008). Recently, Koeduka et al. (2021) also successfully implemented multigene expression for RK production in plants (Fig. 4f). Ectopic expression of BAS together with RZS1 in tobacco led to the accumulation of ca. 2 μg/g FW of RK and its glycoside. However, when metabolic flow in these BAS/RZS1‐expressing plants was enhanced via suppression of chalcone synthase (CHS), which competes with BAS for the substrate pCA, or by expression PAP1, a further three‐fold increase in RK production was obtained in the tobacco flowers, similar to levels in raspberry fruit (Koeduka et al., 2021). Coexpression of PAP1 with BAS/RZS1 enabled RK biosynthesis in the leaves as well, representing the plant's main biomass, probably through activation of phenylpropanoid pathway genes by PAP1 (Koeduka et al., 2021; Skaliter et al., 2019; Zvi et al., 2008).
The strategy of integrating numerous genetic elements, opening new routes and blocking unwanted fluxes (Fu et al., 2018) has yet to be used for vanillin engineering in plants. This strategy can be applied to suitable plant hosts, such as tobacco and pepper, and logically, to the vanilla orchid, once transformation and regeneration of this latter plant become available. The vanilla orchid is the most efficient natural producer of vanillin, despite the long pollination, growth and curing processes; and the recent publication of its genome (Hu et al., 2019) makes its use especially promising. Once it is possible to apply gene‐editing techniques, such as CRISPR to Vanilla, it will be possible to direct the flux towards phenylpropenes by, for example, knocking out negative regulators, such as the vanilla ortholog of PhMYB4 and CHS, and enhancing total metabolic flux in the phenylpropanoid pathway by introducing feedback‐insensitive AroG and PheA with transcription factors, such as EOBI/II, ODO1, PAP1, and AtMYB12 (Fig. 5; Boersma et al., 2022; Fu et al., 2018; Oliva et al., 2015, 2017; Peled‐Zehavi et al., 2015; Spitzer‐Rimon et al., 2010, 2012; Zhang et al., 2015). However, overall, despite the potential of multigene combinations, they are not always straightforward (Tzin et al., 2015; Xie et al., 2016), and the crosstalk between different transgenes and its effect on metabolic flow should be carefully monitored and assessed.
Figure 5.
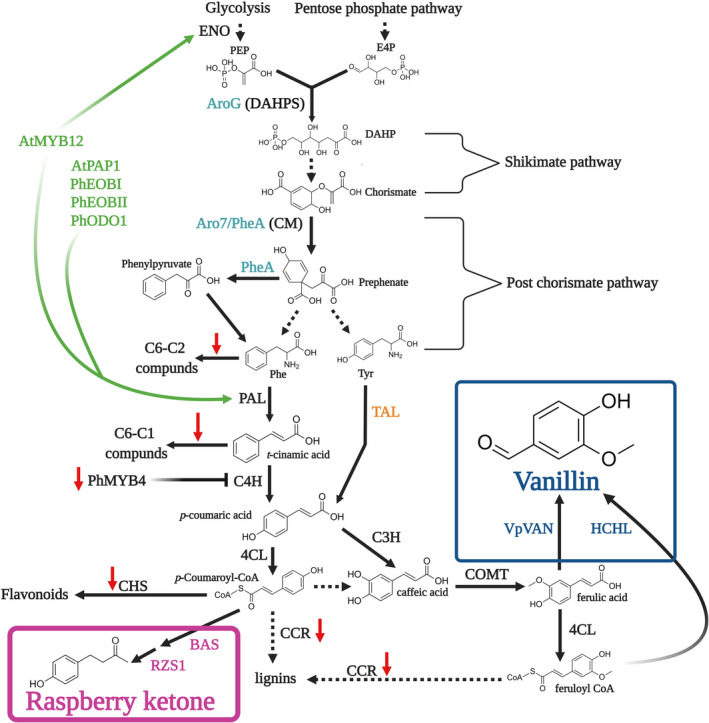
Proposed routes for enhancement of vanillin, raspberry ketone, and other phenylalanine (Phe)‐derived aroma compounds in plants. Alleviation of metabolic bottlenecks by feedback‐insensitive enzymes (cyan) in the shikimate (AroG) and post‐chorismate pathways (Aro7/PheA); upregulation of glycolysis and activation of PAL using transcription factors AtMYB12, PAP1, ODO1, and EOBI/II (green); use of TAL (orange) for utilization of tyrosine; knock out enzymes/repressors by CRISPR/RNAi (red arrows) at key branchpoints to ensure carbon flow directionality; and use of dedicated enzymes to complete the final biochemical steps towards vanillin (blue) or raspberry ketone (pink). Dashed arrows indicate multiple enzymatic steps. ENO, plastidial enolase.
Future prospects
As detailed in this review, major progress has been achieved in delineating biochemical and molecular pathways yielding Phe‐derived compounds. In addition, we describe the numerous setbacks to efficient production of these compounds and present immediately applicable strategies to overcome them. Due to these molecules' multifaceted roles, that is, defence, signalling, attraction of pollinators, health benefits and contribution to food flavour, it is no surprise that so much effort has been invested in their production. At present, microbes, such as yeast, have the advantage because their products are regarded as GRAS. Moreover, aroma compounds, such as vanillin produced by genetically modified microbes are already commercially available (Altpeter et al., 2016). From an industrial standpoint, plants are lagging behind, as to our knowledge, no aroma compounds are commercially available from engineered plants. Here we review the next steps that should enable realizing the potential of molecular farming in these organisms.
There are various reasons for the current status—the main ones being technical and regulatory. From a technological/biological viewpoint, scientists have at their disposal a set of molecular tools that have yet to be exploited for molecular farming. For example, cellular localization of target genes and proteins is a crucial tool that enables utilizing several compartments together, with their respective intracellular pathways and substrates, as has been shown in both plants and microorganisms (Farhi et al., 2011; Kappers et al., 2005). Furthermore, transformation of organelles has many advantages, for example, plastid transformation may be more efficient than nuclear transformation due to the higher number of plastids in the cells, thereby potentially producing higher yields (Daniell et al., 2016; Maliga, 2004). Different combinations of cis/trans‐regulatory elements may further improve the expression of target genes and confinement of the product to specific tissues or compartments. In this respect, altering specific sites in the promoter regions of target genes allows fine‐tuning expression in cases of negative epistasis (Soyk et al., 2017). Inducible systems for gene activation may enable overcoming the many plant‐inherent problems that arise when dealing with metabolic fluxes that affect the host's well‐being, or with metabolites that are toxic to the host (Misra and Ganesan, 2021). To this end, an advanced chemical‐induced system that allows the initiation of cell‐specific production of specialized metabolites at predetermined times is being developed (Snir, 2020). Another major hurdle for metabolic engineering is that for many commercially important plants, including Vanilla, there are no efficient transformation/regeneration protocols (Altpeter et al., 2016). One way to overcome this is through viral‐based techniques, which bypass the need for tissue culture/regeneration, a major bottleneck in transgenesis as reported for numerous plants (Ellison et al., 2020; Honig et al., 2015; Hsu et al., 2015; Spitzer‐Rimon et al., 2012). A recent publication provides the tailwind for this technique, as the authors developed a transient viral‐based system to manipulate agronomic traits that can potentially be utilized to enhance aroma compounds in target plants (Torti et al., 2021). For many commercially important compounds, including vanillin, perhaps the biggest issue is that the pathways for its synthesis and metabolic fluxes have yet to be fully elucidated. Once the biochemistry is fully detailed, a host that can support production of the molecule of interest and is amenable to genetic manipulation/phytoviruses can be selected. Integration of the multiple metabolic engineering strategies discussed above with advanced agricultural techniques will allow maximizing the production efficiency of specialized metabolites for industrial purposes. Microalgae and synthetic plant cell‐based platforms serving as solar‐powered biofactories that combine the advantages of microbes and plants (Arya et al., 2020; Brey et al., 2020; Gimpel et al., 2015) may one day become a system of choice for metabolic engineering efforts.
Even if the technology can live up to its potential, regulatory limitations and public acceptance are still major setbacks. However, winds of change are blowing, one example being the approval of plant‐based vaccines, like the one for COVID‐19 developed by Medicago Inc. (Maharjan and Choe, 2021; Margolin et al., 2018). Furthermore, a gene‐edited tomato with enhanced levels of γ‐aminobutyric acid, which has attributed health benefits, is already commercially available in Japan (Nonaka et al., 2017; Waltz, 2022). The huge potential of molecular breeding can no longer be ignored, as is becoming clear to both legislators and consumers. However, the rise of plant platforms does not mean a dark future for microbes as producers of aroma compounds. As already noted, each system has its pros and cons. For example, microbes may be more suitable for wealthy countries with limited agricultural land, whereas plant molecular farming has an advantage in poorer regions, as plants generally have three basic needs: water, soil, and sun. Because some aroma compounds are worth more money than the crops, they may help local farmers. Furthermore, in the shadow of global anthropogenic‐related environmental changes, the ability to effectively produce molecules of interest while preserving plant–plant/pollinator interactions should significantly aid in sustaining the well‐being of both man and nature.
Conflicts of interest
A.V. is an advisor to Pigmentum Ltd. Israel.
Author contributions
O.S. and A.V. designed and conceptualized the review. All authors wrote the manuscript and approved it.
Acknowledgements
We thank Gony Dvir, Javiera Aravena‐Calvo and Gilad David Birnbaum for their valuable assistance. Figures were created with BioRender.com and molecule structures were generated with ChemDraw. The work in A.V.'s laboratory is supported by the Israel Science Foundation (grant no 2511/16) and the Israeli Ministry of Agriculture. A.V. is an incumbent of the Wolfson Chair in Floriculture.
References
- Aalbers, F.S. and Fraaije, M.W. (2019) Enzyme fusions in biocatalysis: coupling reactions by pairing enzymes. ChemBioChem. 20, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe, I. , Takahashi, Y. , Morita, H. and Noguchi, H. (2001) Benzalacetone synthase. A novel polyketide synthase that plays a crucial role in the biosynthesis of phenylbutanones in Rheum palmatum . Eur. J. Biochem. 268, 3354–3359. [DOI] [PubMed] [Google Scholar]
- Achnine, L. , Blancaflor, E.B. , Rasmussen, S. and Dixon, R.A. (2004) Colocalization of l‐phenylalanine ammonia‐lyase and cinnamate 4‐hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell, 16, 3098–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebesin, F. , Widhalm, J.R. , Boachon, B. , Lefèvre, F. , Pierman, B. , Lynch, J.H. , Alam, I. et al. (2017) Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science, 356, 1386–1388. [DOI] [PubMed] [Google Scholar]
- Adebesin, F. , Widhalm, J.R. , Lynch, J.H. , McCoy, R.M. and Dudareva, N. (2018) A peroxisomal thioesterase plays auxiliary roles in plant β‐oxidative benzoic acid metabolism. Plant J. 93, 905–916. [DOI] [PubMed] [Google Scholar]
- Ahn, S.G. , Jin, Y.H. , Yoon, J.H. and Kim, S.A. (2015) The anticancer mechanism of 2′‐hydroxycinnamaldehyde in human head and neck cancer cells. Int. J. Oncol. 47, 1793–1800. [DOI] [PubMed] [Google Scholar]
- Ahuja K, Sonal S. (2020) Aroma Chemicals Market to Reach $7.5 billion by 2026. [WWW document] URL: https://www.gminsights.com/pressrelease/aroma‐chemicals‐market/ [accessed 14 February 2021].
- Akhtar, T.A. and Pichersky, E. (2013) Veratrole biosynthesis in white campion. Plant Physiol. 162, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsen, L. , Chen, Y. , Bach, L.S. , Rattleff, S. , Maury, J. , Brix, S. , Nielsen, J. et al. (2011) Diversion of flux toward sesquiterpene production in Saccharomyces cerevisiae by fusion of host and heterologous enzymes. Appl. Environ. Microbiol. 77, 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altpeter, F. , Springer, N.M. , Bartley, L.E. , Blechl, A.E. , Brutnell, T.P. , Citovsky, V. , Conrad, L.J. et al. (2016) Advancing crop transformation in the era of genome editing. Plant Cell, 28, 1510–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament, K. , Krasikov, V. , Allmann, S. , Rep, M. , Takken, F.L.W. and Schuurink, R.C. (2010) Methyl salicylate production in tomato affects biotic interactions. Plant J. 62, 124–134. [DOI] [PubMed] [Google Scholar]
- Ameye, M. , Allmann, S. , Verwaeren, J. , Smagghe, G. , Haesaert, G. , Schuurink, R.C. and Audenaert, K. (2018) Green leaf volatile production by plants: a meta‐analysis. New Phytol. 220, 666–683. [DOI] [PubMed] [Google Scholar]
- Amrad, A. , Moser, M. , Mandel, T. , de Vries, M. , Schuurink, R.C. , Freitas, L. and Kuhlemeier, C. (2016) Gain and loss of floral scent production through changes in structural genes during pollinator‐mediated speciation. Curr. Biol. 26, 3303–3312. [DOI] [PubMed] [Google Scholar]
- Arya, S.S. , Rookes, J.E. , Cahill, D.M. and Lenka, S.K. (2020) Next‐generation metabolic engineering approaches towards development of plant cell suspension cultures as specialized metabolite producing biofactories. Biotechnol. Adv. 45, 107635. [DOI] [PubMed] [Google Scholar]
- Ashokkumar, S. , Jaganathan, D. , Ramanathan, V. , Rahman, H. , Palaniswamy, R. , Kambale, R. and Muthurajan, R. (2020) Creation of novel alleles of fragrance gene OsBADH2 in rice through CRISPR/Cas9 mediated gene editing. PLoS ONE, 15, e0237018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averesch, N.J.H. and Krömer, J.O. (2018) Metabolic engineering of the shikimate pathway for production of aromatics and derived compounds‐present and future strain construction strategies. Front. Bioeng. Biotechnol. 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayseli, M.T. and Ipek Ayseli, Y. (2016) Flavors of the future: health benefits of flavor precursors and volatile compounds in plant foods. Trends Food Sci. Technol. 48, 69–77. [Google Scholar]
- Barros, J. and Dixon, R.A. (2020) Plant phenylalanine/tyrosine ammonia‐lyases. Trends Plant Sci. 25, 66–79. [DOI] [PubMed] [Google Scholar]
- Barros, J. , Escamilla‐Trevino, L. , Song, L. , Rao, X. , Serrani‐Yarce, J.C. , Palacios, M.D. , Engle, N. et al. (2019) 4‐Coumarate 3‐hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. Nat. Commun. 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros, J. , Serrani‐Yarce, J.C. , Chen, F. , Baxter, D. , Venables, B.J. and Dixon, R.A. (2016) Role of bifunctional ammonia‐lyase in grass cell wall biosynthesis. Nat. Plants, 2, 1–9. [DOI] [PubMed] [Google Scholar]
- Battat, M. , Eitan, A. , Rogachev, I. , Hanhineva, K. , Fernie, A. , Tohge, T. , Beekwilder, J. et al. (2019) A MYB triad controls primary and phenylpropanoid metabolites for pollen coat patterning. Plant Physiol. 180, 87–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekwilder, J. , Van der Meer, I.M. , Sibbbesen, O. , Broekgaarden, M. , Qvist, I. , Mikkelsen, J.D. and Hall, R.D. (2007) Microbial production of natural raspberry ketone. Biotechnol. J. 2, 1270–1279. [DOI] [PubMed] [Google Scholar]
- Boersma, M.R. , Patrick, R.M. , Jillings, S.L. , Shaipulah, N.F.M. , Sun, P. , Haring, M.A. , Dudareva, N. et al. (2022) ODORANT1 targets multiple metabolic networks in petunia flowers. Plant J. 109, 1134–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomgardner MM. (2016) The problem with vanilla. [WWW document] URL: https://cen.acs.org/articles/94/i36/problem‐vanilla.html [accessed 24 September 2021].
- Bortesi, L. and Fischer, R. (2015) The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 33, 41–52. [DOI] [PubMed] [Google Scholar]
- Brey, L.F. , Włodarczyk, A.J. , Bang Thøfner, J.F. , Burow, M. , Crocoll, C. , Nielsen, I. , Zygadlo Nielsen, A.J. et al. (2020) Metabolic engineering of Synechocystis sp. PCC 6803 for the production of aromatic amino acids and derived phenylpropanoids. Metab. Eng. 57, 129–139. [DOI] [PubMed] [Google Scholar]
- Brillouet, J.M. , Verdeil, J.L. , Odoux, E. , Lartaud, M. , Grisoni, M. and Conéjéro, G. (2014) Phenol homeostasis is ensured in vanilla fruit by storage under solid form in a new chloroplast‐derived organelle, the phenyloplast. J. Exp. Bot. 65, 2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochado, A.R. , Matos, C. , Møller, B.L. , Hansen, J. , Mortensen, U.H. and Patil, K.R. (2010) Improved vanillin production in baker's yeast through in silico design. Microb. Cell Fact. 9, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers, D.L. and Chang, S.‐M. (2017) Studying plant–pollinator interactions facing climate change and changing environments. Appl. Plant Sci. 5, 1700052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagné, D. , Lin‐Wang, K. , Espley, R.V. , Volz, R.K. , How, N.M. , Rouse, S. , Brendolise, C. et al. (2013) An ancient duplication of apple MYB transcription factors is responsible for novel red fruit‐flesh phenotypes. Plant Physiol. 161, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. , Liu, B. , Bao, Y. , Tao, Y. and Liu, W. (2021) Efficient bioconversion of raspberry ketone in Escherichia coli using fatty acids feedstocks. Microb. Cell Fact. 20, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee, M.J.Y. , Lycett, G.W. , Khoo, T.J. and Chin, C.F. (2017) Bioengineering of the plant culture of Capsicum frutescens with vanillin synthase gene for the production of vanillin. Mol. Biotechnol. 59, 1–8. [DOI] [PubMed] [Google Scholar]
- Chemler, J.A. and Koffas, M.A. (2008) Metabolic engineering for plant natural product biosynthesis in microbes. Curr. Opin. Biotechnol. 19, 597–605. [DOI] [PubMed] [Google Scholar]
- Cna'ani, A. , Mühlemann, J.K. , Ravid, J. , Masci, T. , Klempien, A. , Nguyen, T.T.H. , Dudareva, N. et al. (2014) Petunia × hybrida floral scent production is negatively affected by high‐temperature growth conditions. Plant Cell Environ. 38, 1333–1346. [DOI] [PubMed] [Google Scholar]
- Cna'ani, A. , Shavit, R. , Ravid, J. , Aravena‐Calvo, J. , Skaliter, O. , Masci, T. and Vainstein, A. (2017) Phenylpropanoid scent compounds in Petunia x hybrida are glycosylated and accumulate in vacuoles. Front. Plant Sci. 8, 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cna'ani, A. , Spitzer‐Rimon, B. , Ravid, J. , Farhi, M. , Masci, T. , Aravena‐Calvo, J. , Ovadis, M. et al. (2015) Two showy traits, scent emission and pigmentation, are finely coregulated by the MYB transcription factor PH4 in petunia flowers. New Phytol. 208, 708–714. [DOI] [PubMed] [Google Scholar]
- Colquhoun, T.A. , Kim, J.Y. , Wedde, A.E. , Levin, L.A. , Schmitt, K.C. , Schuurink, R.C. and Clark, D.G. (2011) PhMYB4 fine‐tunes the floral volatile signature of Petunia×hybrida through PhC4H. J. Exp. Bot. 62, 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun, T.A. , Verdonk, J.C. , Schimmel, B.C.J. , Tieman, D.M. , Underwood, B.A. and Clark, D.G. (2010) Petunia floral volatile benzenoid/phenylpropanoid genes are regulated in a similar manner. Phytochemistry, 71, 158–167. [DOI] [PubMed] [Google Scholar]
- Czechowski, T. , Weathers, P.J. , Brodelius, P.E. , Brown, G.D. and Graham, I.A. (2020) Editorial: Artemisinin—from traditional Chinese medicine to artemisinin combination therapies; four decades of research on the biochemistry, physiology, and breeding of Artemisia annua . Front. Plant Sci. 11, 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell, H. , Lin, C.S. , Yu, M. and Chang, W.J. (2016) Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 17, 134–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, F. , Yang, Z. , Baldermann, S. , Kajitani, Y. , Ota, S. , Kasuga, H. , Imazeki, Y. et al. (2012) Characterization of l‐phenylalanine metabolism to acetophenone and 1‐phenylethanol in the flowers of Camellia sinensis using stable isotope labeling. J. Plant Physiol. 169, 217–225. [DOI] [PubMed] [Google Scholar]
- Dubal, S.A. , Tilkari, Y.P. , Momin, S.A. and Borkar, I.V. (2008) Biotechnological routes in flavour industries. Adv. Biotech. 14, 20–31. [Google Scholar]
- Dudareva, N. , Klempien, A. , Muhlemann, J.K. and Kaplan, I. (2013) Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 198, 16–32. [DOI] [PubMed] [Google Scholar]
- Ellison, E.E. , Nagalakshmi, U. , Gamo, M.E. , Huang, P.J. , Dinesh‐Kumar, S. and Voytas, D.F. (2020) Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants, 6, 620–624. [DOI] [PubMed] [Google Scholar]
- Fan, Z. , Hasing, T. , Johnson, T.S. , Garner, D.M. , Barbey, C.R. , Colquhoun, T.A. , Sims, C.A. et al. (2021) Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, F. , Oliva, M. , Ovadia, R. , Bar, E. , Nissim‐Levi, A. , Kumar, V. , Wang, R. et al. (2021) Increased substrate availability reveals the potential of scentless lisianthus flowers in producing fragrant benzenoid‐phenylpropanoids. Physiol. Plant. 172, 19–28. [DOI] [PubMed] [Google Scholar]
- Farhi, M. , Lavie, O. , Masci, T. , Hendel‐Rahmanim, K. , Weiss, D. , Abeliovich, H. and Vainstein, A. (2010) Identification of rose phenylacetaldehyde synthase by functional complementation in yeast. Plant Mol. Biol. 72, 235–245. [DOI] [PubMed] [Google Scholar]
- Farhi, M. , Marhevka, E. , Masci, T. , Marcos, E. , Eyal, Y. , Ovadis, M. , Abeliovich, H. et al. (2011) Harnessing yeast subcellular compartments for the production of plant terpenoids. Metab. Eng. 13, 474–481. [DOI] [PubMed] [Google Scholar]
- Fenske, M.P. , Hewett Hazelton, K.D. , Hempton, A.K. , Shim, J.S. , Yamamoto, B.M. , Riffell, J.A. and Imaizumi, T. (2015) Circadian clock gene LATE ELONGATED HYPOCOTYL directly regulates the timing of floral scent emission in Petunia. Proc. Natl Acad. Sci. USA, 112, 9775–9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, D.J. , Stratford, M. , Gasson, M.J. , Ueckert, J. , Bos, A. and Narbad, A. (2004) Mode of antimicrobial of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua . J. Appl. Microbiol. 97, 104–113. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . (2018) Microorganisms & Microbial‐Derived Ingredients Used in Food. [WWW document] URL: https://www.fda.gov/food/generally‐recognized‐safe‐gras/microorganisms‐microbial‐derived‐ingredients‐used‐food‐partial‐list [accessed 20 March 2022].
- Friedman, M. , Henika, P.R. and Mandrell, R.E. (2002) Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica . J. Food Prot. 65, 1545–1560. [DOI] [PubMed] [Google Scholar]
- Fu, R. , Martin, C. and Zhang, Y. (2018) Next‐generation plant, metabolic engineering, inspired by an ancient Chinese irrigation system. Mol. Plant, 11, 47–57. [DOI] [PubMed] [Google Scholar]
- Gallage, N.J. , Hansen, E.H. , Kannangara, R. , Olsen, C.E. , Motawia, M.S. , Jørgensen, K. , Holme, I. et al. (2014) Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nat. Commun. 5, 4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallage, N.J. , Jørgensen, K. , Janfelt, C. , Nielsen, A.J.Z. , Naake, T. , Duński, E. , Dalsten, L. et al. (2018) The intracellular localization of the vanillin biosynthetic machinery in pods of Vanilla planifolia . Plant Cell Physiol. 59, 304–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallage, N.J. and Møller, B.L. (2015) Vanillin‐bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant, 8, 40–57. [DOI] [PubMed] [Google Scholar]
- Gallage, N.J. and Møller, B.L. (2018) Vanilla: the most popular flavour. In Biotechnology of Natural Products ( Schwab, W. , Lange, B.M. and Wüst, M. , eds), pp. 3–24. Cham: Springer International Publishing. [Google Scholar]
- Gasson, M.J. , Kitamura, Y. , McLauchlan, W.R. , Narbad, A. , Parr, A.J. , Parsons, E.L.H. , Payne, J. et al. (1998) Metabolism of ferulic acid to vanillin: a bacterial gene of the enoyl‐ SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J. Biol. Chem. 273, 4163–4170. [DOI] [PubMed] [Google Scholar]
- Gimpel, J.A. , Henríquez, V. and Mayfield, S.P. (2015) In metabolic engineering of eukaryotic microalgae: potential and challenges come with great diversity. Front. Microbiol. 6, 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenny, W.R. , Runyon, J.B. and Burkle, L.A. (2018) Drought and increased CO2 alter floral visual and olfactory traits with context‐dependent effects on pollinator visitation. New Phytol. 220, 785–798. [DOI] [PubMed] [Google Scholar]
- Gottardi, M. , Knudsen, J.D. , Prado, L. , Oreb, M. , Branduardi, P. and Boles, E. (2017) De novo biosynthesis of trans‐cinnamic acid derivatives in Saccharomyces cerevisiae . Appl. Microbiol. Biotechnol. 101, 4883–4893. [DOI] [PubMed] [Google Scholar]
- Graf, N. and Altenbuchner, J. (2014) Genetic engineering of Pseudomonas putida KT2440 for rapid and high‐yield production of vanillin from ferulic acid. Appl. Microbiol. Biotechnol. 98, 137–149. [DOI] [PubMed] [Google Scholar]
- Gundlach, H. , Müller, M.J. , Kutchan, T.M. and Zenk, M.H. (1992) Jasmonic acid is a signal transducer in elicitor‐induced plant cell cultures. Proc. Natl Acad. Sci. USA, 89, 2389–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther, J. , Schmidt, A. , Gershenzon, J. and Köllner, T.G. (2019) Phenylacetaldehyde synthase 2 does not contribute to the constitutive formation of 2‐phenylethyl‐β‐D‐glucopyranoside in poplar. Plant Signal. Behav. 14, 1668233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururaj, H.B. , Padma, M.N. , Giridhar, P. and Ravishankar, G.A. (2012) Functional validation of Capsicum frutescens aminotransferase gene involved in vanillylamine biosynthesis using Agrobacterium mediated genetic transformation studies in Nicotiana tabacum and Capsicum frutescens calli cultures. Plant Sci. 195, 96–105. [DOI] [PubMed] [Google Scholar]
- Halitschke, R. and Baldwin, I.T. (2004) Jasmonates and related compounds in plant‐insect interactions. J. Plant Growth Regul. 23, 238–245. [Google Scholar]
- Hanik, N. , Gómez, S. , Best, M. , Schueller, M. , Orians, C.M. and Ferrieri, R.A. (2010) Partitioning of new carbon as 11C in Nicotiana tabacum reveals insight into methyl jasmonate induced changes in metabolism. J. Chem. Ecol. 36, 1058–1067. [DOI] [PubMed] [Google Scholar]
- Hansen, E.H. , Møller, B.L. , Kock, G.R. , Bünner, C.M. , Kristensen, C. , Jensen, O.R. , Okkels, F.T. et al. (2009) De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker's yeast (Saccharomyces cerevisiae). Appl. Environ. Microbiol. 75, 2765–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata, H. , Ohnishi, T. , Tomida, K. , Ishida, H. , Kanda, M. , Sakai, M. , Yoshimura, J. et al. (2016) Seasonal induction of alternative principal pathway for rose flower scent. Sci. Rep. 6, 20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoballah, M.E. , Stuurman, J. , Turlings, T.C.J. , Guerin, P.M. , Connétable, S. and Kuhlemeier, C. (2005) The composition and timing of flower odour emission by wild Petunia axillaris coincide with the antennal perception and nocturnal activity of the pollinator Manduca sexta . Planta, 222, 141–150. [DOI] [PubMed] [Google Scholar]
- Hoffmann, T. , Kurtzer, R. , Skowranek, K. , Kießling, P. , Fridman, E. , Pichersky, E. and Schwab, W. (2011) Metabolic engineering in strawberry fruit uncovers a dormant biosynthetic pathway. Metab. Eng. 13, 527–531. [DOI] [PubMed] [Google Scholar]
- Honig, A. , Marton, I. , Rosenthal, M. , Smith, J.J. , Nicholson, M.G. , Jantz, D. , Zuker, A. et al. (2015) Transient expression of virally delivered meganuclease in planta generates inherited genomic deletions. Mol. Plant, 8, 1292–1294. [DOI] [PubMed] [Google Scholar]
- Hsu, H.‐F. , Hsu, W.‐H. , Lee, Y.‐I. , Mao, W.‐T. , Yang, J.‐Y. , Li, J.‐Y. and Yang, C.‐H. (2015) Model for perianth formation in orchids. Nat. Plants, 1, 1–8. [Google Scholar]
- Hu, Y. , Resende, M.F.R. , Bombarely, A. , Brym, M. , Bassil, E. and Chambers, A.H. (2019) Model for perianth formation in orchids. Nat. Plants, 1, 1–8. [Google Scholar]
- Iijima, Y. , Davidovich‐Rikanati, R. , Fridman, E. , Gang, D.R. , Bar, E. , Lewinsohn, E. and Pichersky, E. (2004) Genomics‐based diversity analysis of vanilla species using a Vanilla planifolia draft genome and genotyping‐by‐sequencing. Sci. Rep. 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz, J.R. and Weng, J.K. (2020) Exploring uncharted territories of plant specialized metabolism in the postgenomic era. Ann. Rev. Plant Biol. 71, 631–658. [DOI] [PubMed] [Google Scholar]
- Jantzen, F. , Lynch, J.H. , Kappel, C. , Höfflin, J. , Skaliter, O. , Wozniak, N. , Sicard, A. et al. (2019) Retracing the molecular basis and evolutionary history of the loss of benzaldehyde emission in the genus Capsella . New Phytol. 224, 1349–1360. [DOI] [PubMed] [Google Scholar]
- Joffard, N. , Arnal, V. , Buatois, B. , Schatz, B. and Montgelard, C. (2020) Floral scent evolution in the section Pseudophrys: pollinator‐mediated selection or phylogenetic constraints? Plant Biol. 22, 881–889. [DOI] [PubMed] [Google Scholar]
- Kallscheuer, N. (2018) Engineered microorganisms for the production of food additives approved by the European Union‐A systematic analysis. Front. Microbiol. 9, 1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminaga, Y. , Schnepp, J. , Peel, G. , Kish, C.M. , Ben‐Nissan, G. , Weiss, D. , Orlova, I. et al. (2006) Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. J. Biol. Chem. 281, 23357–23366. [DOI] [PubMed] [Google Scholar]
- Kappers, I.F. , Aharoni, A. , Van Herpen, T.W.J.M. , Luckerhoff, L.L.P. , Dicke, M. and Bouwmeester, H.J. (2005) Plant science: genetic engineering of terpenoid metabolism attracts bodyguards to arabidopsis. Science, 309, 2070–2072. [DOI] [PubMed] [Google Scholar]
- Karniel, U. , Koch, A. , Zamir, D. and Hirschberg, J. (2020) Development of zeaxanthin‐rich tomato fruit through genetic manipulations of carotenoid biosynthesis. Plant Biotechnol. J. 18, 2292–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, A.A. , Shaughnessy, D.T. , Mure, K. , Leszczynska, J. , Ward, W.O. , Umbach, D.M. , Xu, Z. et al. (2007) Antimutagenicity of cinnamaldehyde and vanillin in human cells: global gene expression and possible role of DNA damage and repair. Mutat. Res. 616, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, A.M. , Vaissière, B.E. , Cane, J.H. , Steffan‐Dewenter, I. , Cunningham, S.A. , Kremen, C. and Tscharntke, T. (2007) Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 274, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempien, A. , Kaminaga, Y. , Qualley, A. , Nagegowda, D.A. , Widhalm, J.R. , Orlova, I. , Shasany, A.K. et al. (2012) Contribution of CoA ligases to benzenoid biosynthesis in petunia flowers. Plant Cell, 24, 2015–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen, J.T. , Eriksson, R. , Gershenzon, J. and Ståhl, B. (2006) Diversity and distribution of floral scent. Bot. Rev. 72, 1. [Google Scholar]
- Koeduka, T. , Takarada, S. , Fujii, K. , Sugiyama, A. , Yazaki, K. , Nishihara, M. and Matsui, K. (2021) Production of raspberry ketone by redirecting the metabolic flux to the phenylpropanoid pathway in tobacco plants. Metab. Eng. Commun. 13, e00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeduka, T. , Watanabe, B. , Suzuki, S. , Hiratake, J. , Mano, J. and Yazaki, K. (2011) Characterization of raspberry ketone/zingerone synthase, catalyzing the alpha, beta‐hydrogenation of phenylbutenones in raspberry fruits. Biochem. Biophys. Res. Commun. 412, 104–108. [DOI] [PubMed] [Google Scholar]
- Kong, H.G. , Song, G.C. , Sim, H.J. and Ryu, C.M. (2020) Achieving similar root microbiota composition in neighbouring plants through airborne signalling. ISME J. 15, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu, A. (2017) Vanillin biosynthetic pathways in plants. Planta, 245, 1069–1078. [DOI] [PubMed] [Google Scholar]
- Kunjapur, A.M. , Tarasova, Y. and Prather, K.L.J. (2014) Synthesis and accumulation of aromatic aldehydes in an engineered strain of Escherichia coli . J. Am. Chem. Soc. 136, 11644–11654. [DOI] [PubMed] [Google Scholar]
- Lee, D. , Lloyd, N.D.R. , Pretorius, I.S. and Borneman, A.R. (2016) Heterologous production of raspberry ketone in the wine yeast Saccharomyces cerevisiae via pathway engineering and synthetic enzyme fusion. Microb. Cell Fact. 15, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehka, B.J. , Eichenberger, M. , Bjørn‐Yoshimoto, W.E. , Vanegas, K.G. , Buijs, N. , Jensen, N.B. , Dyekjær, J.D. et al. (2017) Improving heterologous production of phenylpropanoids in Saccharomyces cerevisiae by tackling an unwanted side reaction of Tsc13, an endogenous double‐bond reductase. FEMS Yeast Res. 17, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn, E. and Gijzen, M. (2009) Phytochemical diversity: the sounds of silent metabolism. Plant Sci. 176, 161–169. [Google Scholar]
- Li, K. and Frost, J.W. (1998) Synthesis of vanillin from glucose. J. Am. Chem. Soc. 120, 10545–10546. [Google Scholar]
- Liao, P. , Ray, S. , Boachon, B. , Lynch, J.H. , Deshpande, A. , McAdam, S. , Morgan, J.A. et al. (2020a) Cuticle thickness affects dynamics of volatile emission from petunia flowers. Nat. Chem. Biol. 17, 138–145. [DOI] [PubMed] [Google Scholar]
- Liao, Y. , Zeng, L. , Tan, H. , Cheng, S. , Dong, F. and Yang, Z. (2020b) Biochemical pathway of benzyl nitrile derived from l ‐phenylalanine in tea (Camellia sinensis) and its formation in response to postharvest stresses. J. Agric. Food Chem. 68, 1397–1404. [DOI] [PubMed] [Google Scholar]
- Linares, V. , Adams, M.J. , Cradic, M.S. , Finkelstein, I. , Lipschits, O. , Martin, M.A.S. , Neumann, R. et al. (2019) First evidence for vanillin in the old world: its use as mortuary offering in Middle Bronze Canaan. J. Archaeolog. Sci. 25, 77–84. [Google Scholar]
- Liu, F. , Xiao, Z. , Yang, L. , Chen, Q. , Shao, L. , Liu, J. and Yu, Y. (2017) PhERF6, interacting with EOBI, negatively regulates fragrance biosynthesis in petunia flowers. New Phytol. 215, 1490–1502. [DOI] [PubMed] [Google Scholar]
- Liu, Q. , Yu, T. , Li, X. , Chen, Y. , Campbell, K. , Nielsen, J. and Chen, Y. (2019) Rewiring carbon metabolism in yeast for high level production of aromatic chemicals. Nat. Commun. 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato‐Gómez, M. , Hewitt, S. , Capell, T. , Christou, P. , Dhingra, A. and Girón‐Calva, P.S. (2021) Transgenic and genome‐edited fruits: background, constraints, benefits, and commercial opportunities. Hort. Res. 8, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, D. , Yuan, X. , Kim, S.J. , Marques, J.V. , Chakravarthy, P.P. , Moinuddin, S.G.A. , Luchterhand, R. et al. (2017) Eugenol specialty chemical production in transgenic poplar (Populus tremula × P. alba) field trials. Plant Biotechnol. J. 15, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttik, M.A.H. , Vuralhan, Z. , Suir, E. , Braus, G.H. , Pronk, J.T. and Daran, J.M. (2008) Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: quantification of metabolic impact. Metab. Eng. 10, 141–153. [DOI] [PubMed] [Google Scholar]
- Lynch, J.H. and Dudareva, N. (2020) Aromatic amino acids: a complex network ripe for future exploration. Trends Plant Sci. 25, 670–681. [DOI] [PubMed] [Google Scholar]
- Lynch, J.H. , Orlova, I. , Zhao, C. , Guo, L. , Jaini, R. , Maeda, H. , Akhtar, T. et al. (2017) Multifaceted plant responses to circumvent Phe hyperaccumulation by downregulation of flux through the shikimate pathway and by vacuolar Phe sequestration. Plant J. 92, 939–950. [DOI] [PubMed] [Google Scholar]
- Lynch, J.H. , Qian, Y. , Guo, L. , Maoz, I. , Huang, X.‐Q. , Garcia, A.S. , Louie, G. et al. (2020) Modulation of auxin formation by the cytosolic phenylalanine biosynthetic pathway. Nat. Chem. Biol. 16, 850–856. [DOI] [PubMed] [Google Scholar]
- Mageroy, M.H. , Tieman, D.M. , Floystad, A. , Taylor, M.G. and Klee, H.J. (2012) A Solanum lycopersicum catechol‐O‐methyltransferase involved in synthesis of the flavor molecule guaiacol. Plant J. 69, 1043–1051. [DOI] [PubMed] [Google Scholar]
- Maharjan, P.M. and Choe, S. (2021) Plant‐based COVID‐19 vaccines: current status, design, and development strategies of candidate vaccines. Vaccines, 9, 992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga, P. (2004) Plastid transformation in higher plants. Ann. Rev. Plant Biol. 55, 289–313. [DOI] [PubMed] [Google Scholar]
- Margolin, E. , Chapman, R. , Williamson, A.‐L. , Rybicki, E.P. and Meyers, A.E. (2018) Production of complex viral glycoproteins in plants as vaccine immunogens. Plant Biotechnol. J. 16, 1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez, O. , Sánchez, A. , Font, X. and Barrena, R. (2017) Valorization of sugarcane bagasse and sugar beet molasses using Kluyveromyces marxianus for producing value‐added aroma compounds via solid‐state fermentation. J. Cleaner Product. 158, 8–17. [Google Scholar]
- Mayer, M.J. , Narbad, A. , Parr, A.J. , Parker, M.L. , Walton, N.J. , Mellon, F.A. and Michael, A.J. (2001) Rerouting the plant phenylpropanoid pathway by expression of a novel bacterial enoyl‐CoA hydratase/lyase enzyme function. Plant Cell, 13, 1669–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milke, L. , Mutz, M. and Marienhagen, J. (2020) Synthesis of the character impact compound raspberry ketone and additional flavoring phenylbutanoids of biotechnological interest with Corynebacterium glutamicum . Microb. Cell Fact. 19, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralpeix, B. , Rischer, H. , Hakkinen, S. , Ritala, A. , Seppanen‐Laakso, T. , Oksman‐Caldentey, K.‐M. , Capell, T. et al. (2013) Metabolic engineering of plant secondary products: which way forward? Curr. Pharm. Des. 19, 5622–5639. [DOI] [PubMed] [Google Scholar]
- Misra, S. and Ganesan, M. (2021) The impact of inducible promoters in transgenic plant production and crop improvement. Plant Gene, 27, 100300. [Google Scholar]
- Morimoto, C. , Satoh, Y. , Hara, M. , Inoue, S. , Tsujita, T. and Okuda, H. (2005) Anti‐obese action of raspberry ketone. Life Sci. 77, 194–204. [DOI] [PubMed] [Google Scholar]
- Muheim, A. and Lerch, K. (1999) Towards a high‐yield bioconversion of ferulic acid to vanillin. Appl. Microbiol. Biotechnol. 51, 456–461. [Google Scholar]
- Muhlemann, J.K. , Klempien, A. and Dudareva, N. (2014) Floral volatiles: from biosynthesis to function. Plant Cell Environ. 37, 1936–1949. [DOI] [PubMed] [Google Scholar]
- Ni, J. , Tao, F. , Du, H. and Xu, P. (2015) Mimicking a natural pathway for de novo biosynthesis: natural vanillin production from accessible carbon sources. Sci. Rep. 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic, V. , Markovic, D. and Rensing, M. (2020) Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 44, 1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic, V. , Rensing, M. , Dahlin, I. and Markovic, D. (2019) Who is my neighbor? Volatile cues in plant interactions. Plant Signal. Behav. 14, e1634993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka, S. , Arai, C. , Takayama, M. , Matsukura, C. and Ezura, H. (2017) Efficient increase of ɣ‐aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 7, 7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva, M. , Bar, E. , Ovadia, R. , Perl, A. , Galili, G. , Lewinsohn, E. and Oren‐Shamir, M. (2017) Phenylpyruvate contributes to the synthesis of fragrant benzenoid–phenylpropanoids in Petunia × hybrida flowers. Front. Plant Sci. 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva, M. , Ovadia, R. , Perl, A. , Bar, E. , Lewinsohn, E. , Galili, G. and Oren‐Shamir, M. (2015) Enhanced formation of aromatic amino acids increases fragrance without affecting flower longevity or pigmentation in Petunia × hybrida . Plant Biotechnol. J. 13, 125–136. [DOI] [PubMed] [Google Scholar]
- Ollerton, J. (2017) Pollinator diversity: distribution, ecological function, and conservation. Ann. Rev. Ecol. Evol. Syst. 48, 353–376. [Google Scholar]
- Orlova, I. , Marshall‐Colón, A. , Schnepp, J. , Wood, B. , Varbanova, M. , Fridman, E. , Blakeslee, J.J. et al. (2006) Reduction of benzenoid synthesis in petunia flowers reveals multiple pathways to benzoic acid and enhancement in auxin transport. Plant Cell, 18, 3458–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, C. , Patron, N.J. , Huang, A. and Osbourn, A. (2017) Harnessing plant metabolic diversity. Curr. Opin. Chem. Biol. 40, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padel, S. and Foster, C. (2005) Exploring the gap between attitudes and behaviour: understanding why consumers buy or do not buy organic food. Br. Food J. 107, 606–625. [Google Scholar]
- Park, S.W. , Kaimoyo, E. , Kumar, D. , Mosher, S. and Klessig, D.F. (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science, 318, 113–116. [DOI] [PubMed] [Google Scholar]
- Patrick, R.M. , Huang, X.‐Q. , Dudareva, N. and Li, Y. (2021) Dynamic histone acetylation in floral volatile synthesis and emission in petunia flowers. J. Exp. Bot. 72, 3704–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled‐Zehavi, H. , Oliva, M. , Xie, Q. , Tzin, V. , Oren‐Shamir, M. , Aharoni, A. and Galili, G. (2015) Metabolic engineering of the phenylpropanoid and its primary, precursor pathway to enhance the flavor of fruits and the aroma of flowers. Bioengineering, 2, 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky, E. , Noel, J.P. and Dudareva, N. (2006) Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science, 311, 808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott, D.M. , Osorio, S. and Vallarino, J.G. (2019) From central to specialized metabolism: an overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 10, 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Y. , Lynch, J.H. , Guo, L. , Rhodes, D. , Morgan, J.A. and Dudareva, N. (2019) Completion of the cytosolic post‐chorismate phenylalanine biosynthetic pathway in plants. Nat. Commun. 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, S.E. and Schiestl, F.P. (2019) Rapid plant evolution driven by the interaction of pollination and herbivory. Science, 364, 193–196. [DOI] [PubMed] [Google Scholar]
- Ravid, J. , Spitzer‐Rimon, B. , Takebayashi, Y. , Seo, M. , Cna'ani, A. , Aravena‐Calvo, J. , Masci, T. et al. (2017) GA as a regulatory link between the showy floral traits color and scent. New Phytol. 215, 411–422. [DOI] [PubMed] [Google Scholar]
- Rolls, E.T. , Critchley, H.D. , Mason, R. and Wakeman, E.A. (1996) Orbitofrontal cortex neurons: role in olfactory and visual association learning. J. Neurophysiol. 75, 1970–1981. [DOI] [PubMed] [Google Scholar]
- Román, S. , Sánchez‐Siles, L.M. and Siegrist, M. (2017) The importance of food naturalness for consumers: results of a systematic review. Trends Food Sci. Technol. 67, 44–57. [Google Scholar]
- Rowe, T.B. and Shepherd, G.M. (2016) Role of ortho‐retronasal olfaction in mammalian cortical evolution. J. Comp. Neurol. 524, 471–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen, E. , Gutensohn, M. , Dudareva, N. and Kaplan, I. (2017) Carnivore attractant or plant elicitor? multifunctional roles of methyl salicylate lures in tomato defense. J. Chem. Ecol. 43, 573–585. [DOI] [PubMed] [Google Scholar]
- Sas, C. , Müller, F. , Kappel, C. , Kent, T.V. , Wright, S.I. , Hilker, M. and Lenhard, M. (2016) Repeated inactivation of the first committed enzyme underlies the loss of benzaldehyde emission after the selfing transition in capsella . Curr. Biol. 26, 3313–3319. [DOI] [PubMed] [Google Scholar]
- Schwab, W. , Davidovich‐Rikanati, R. and Lewinsohn, E. (2008) Biosynthesis of plant‐derived flavor compounds. Plant J. 54, 712–732. [DOI] [PubMed] [Google Scholar]
- Shi, J. , Ma, C.Y. , Qi, D.D. , Lv, H.P. , Yang, T. , Peng, Q.H. , Chen, Z.M. et al. (2015) Transcriptional responses and flavor volatiles biosynthesis in methyl jasmonate‐treated tea leaves. BMC Plant Biol. 15, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev, V. , Silverman, P. and Raskin, I. (1997) Airborne signalling by methyl salicylate in plant pathogen resistance. Nature, 385, 718–721. [Google Scholar]
- Singh, P. , Khan, S. , Pandey, S.S. , Singh, M. , Banerjee, S. , Kitamura, Y. , Rahman, L. et al. (2015) Vanillin production in metabolically engineered Beta vulgaris hairy roots through heterologous expression of Pseudomonas fluorescens HCHL gene. Indust. Crop Prod. 74, 839–848. [Google Scholar]
- Sinha, A.K. , Sharma, U.K. and Sharma, N. (2008) A comprehensive review on vanilla flavor: extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr. 59, 299–326. [DOI] [PubMed] [Google Scholar]
- Skaliter, O. , Kitsberg, Y. , Sharon, E. , Shklarman, E. , Shor, E. , Masci, T. , Yue, Y. et al. (2021) Spatial patterning of scent in petunia corolla is discriminated by bees and involves the ABCG1 transporter. Plant J. 106, tpj.15269. [DOI] [PubMed] [Google Scholar]
- Skaliter, O. , Ravid, J. , Shklarman, E. , Ketrarou, N. , Shpayer, N. , Ben Ari, J. , Dvir, G. et al. (2019) Ectopic expression of PAP1 leads to anthocyanin accumulation and novel floral color in genetically engineered goldenrod (Solidago canadensis L.). Front. Plant Sci. 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, D.M. and Green, B.G. (2011) A proposed model of a flavor modality. In The Neural Bases of Multisensory Processes ( Murray, M.M. and Wallace, M.T. , eds), pp. 717–738. Boca Raton (FL): CRC Press/Taylor & Francis, Chapter 36. [Google Scholar]
- Snir A. (2020) Magic Plants. [WWW document] URL: http://www.israelagri.com/?CategoryID=453&ArticleID=1891&SearchParam=pigmentum/ [accessed 31 December 2020].
- Song, C. , Härtl, K. , McGraphery, K. , Hoffmann, T. and Schwab, W. (2018) Attractive but toxic: emerging roles of glycosidically bound volatiles and glycosyltransferases involved in their formation. Mol. Plant, 11, 1225–1236. [DOI] [PubMed] [Google Scholar]
- Soyk, S. , Lemmon, Z.H. , Oved, M. , Fisher, J. , Liberatore, K.L. , Park, S.J. , Goren, A. et al. (2017) Bypassing negative epistasis on yield in tomato imposed by a domestication gene. Cell, 169, 1142–1155.e12. [DOI] [PubMed] [Google Scholar]
- Spitzer‐Rimon, B. , Farhi, M. , Albo, B. , Cna'ani, A. , Zvi, M.M.B. , Masci, T. , Edelbaum, O. et al. (2012) The R2R3‐MYB‐like regulatory factor EOBI, acting downstream of EOBII, regulates scent production by activating ODO1 and structural scent‐related genes in petunia. Plant Cell, 24, 5089–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer‐Rimon, B. , Marhevka, E. , Barkai, O. , Marton, I. , Edelbaum, O. , Masci, T. , Prathapani, N.K. et al. (2010) EOBII, a gene encoding a flower‐specific regulator of phenylpropanoid volatiles' biosynthesis in petunia. Plant Cell, 22, 1961–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman, D. , Taylor, M. , Schauer, N. , Fernie, A.R. , Hanson, A.D. and Klee, H.J. (2006) Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2‐phenylethanol and 2‐phenylacetaldehyde. Proc. Natl Acad. Sci. USA, 103, 8287–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman, D. , Zhu, G. , Resende, M.F.R. , Lin, T. , Nguyen, C. , Bies, D. , Rambla, J.L. et al. (2017) A chemical genetic roadmap to improved tomato flavor. Science, 355, 391–394. [DOI] [PubMed] [Google Scholar]
- Torti, S. , Schlesier, R. , Thümmler, A. , Bartels, D. , Römer, P. , Koch, B. , Werner, S. et al. (2021) Transient reprogramming of crop plants for agronomic performance. Nat. Plants, 7, 159–171. [DOI] [PubMed] [Google Scholar]
- Tripathi, U. , Ramachandra Rao, S. and Ravishankar, G.A. (2002) Biotransformation of phenylpropanoid compounds to vanilla flavor metabolites in cultures of Haematococcus pluvialis . Process Biochem. 38, 419–426. [Google Scholar]
- Tzin, V. and Galili, G. (2010) The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana . Arabidopsis Book, 8, e0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzin, V. , Malitsky, S. , Zvi, M.M.B. , Bedair, M. , Sumner, L. , Aharoni, A. and Galili, G. (2012) Expression of a bacterial feedback‐insensitive 3‐deoxy‐d‐arabino‐heptulosonate 7‐phosphate synthase of the shikimate pathway in arabidopsis elucidates potential metabolic bottlenecks between primary and secondary metabolism. New Phytol. 194, 430–439. [DOI] [PubMed] [Google Scholar]
- Tzin, V. , Rogachev, I. , Meir, S. , Zvi, M.M.B. , Masci, T. , Vainstein, A. , Aharoni, A. et al. (2013) Tomato fruits expressing a bacterial feedback‐insensitive 3‐deoxy‐D‐arabino‐heptulosonate 7‐phosphate synthase of the shikimate pathway possess enhanced levels of multiple specialized metabolites and upgraded aroma. J. Exp. Bot. 64, 4441–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzin, V. , Rogachev, I. , Meir, S. , Zvi, M.M.B. , Masci, T. , Vainstein, A. , Aharoni, A. et al. (2015) Altered levels of aroma and volatiles by metabolic engineering of shikimate pathway genes in tomato fruits. AIMS Bioeng. 2, 75–92. [Google Scholar]
- Tzuri, G. , Zhou, X. , Chayut, N. , Yuan, H. , Portnoy, V. , Meir, A. , Sa'Ar, U. et al. (2015) A ‘golden’ SNP in CmOr governs the fruit flesh color of melon (Cucumis melo). Plant J. 82, 267–279. [DOI] [PubMed] [Google Scholar]
- Underwood, B.A. , Tieman, D.M. , Shibuya, K. , Dexter, R.J. , Loucas, H.M. , Simkin, A.J. , Sims, C.A. et al. (2005) Ethylene‐regulated floral volatile synthesis in petunia corollas. Plant Physiol. 138, 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Moerkercke, A. , Haring, M.A. and Schuurink, R.C. (2011) The transcription factor EMISSION of BENZENOIDS II activates the MYB ODORANT1 promoter at a MYB binding site specific for fragrant petunias. Plant J. 67, 917–928. [DOI] [PubMed] [Google Scholar]
- Verdonk, J.C. , Haring, M.A. , Van Tunen, A.J. and Schuurink, R.C. (2005) Odorant1 regulates fragrance biosynthesis in petunia flowers. Plant Cell, 17, 1612–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot, A. , Dempsey, D.A. and Klessig, D.F. (2009) Salicylic acid, a multifaceted hormone to combat disease. Ann. Rev. Phytopathol. 47, 177–206. [DOI] [PubMed] [Google Scholar]
- Vlot, A.C. , Sales, J.H. , Lenk, M. , Bauer, K. , Brambilla, A. , Sommer, A. , Chen, Y. et al. (2020) Systemic propagation of immunity in plants. New Phytol. 229, 1234–1250. [DOI] [PubMed] [Google Scholar]
- Vogt, T. (2010) Phenylpropanoid biosynthesis. Mol. Plant. 3. 2–20. [DOI] [PubMed] [Google Scholar]
- Walsh, D.J. , Livinghouse, T. , Goeres, D.M. , Mettler, M. and Stewart, P.S. (2019) Antimicrobial activity of naturally occurring phenols and derivatives against biofilm and planktonic bacteria. Front. Chem. 7, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, K.J. , Lopez, R.G. and Behe, B.K. (2021) Leveraging controlled‐environment agriculture to increase key basil terpenoid and phenylpropanoid concentrations: the effects of radiation intensity and CO2 concentration on consumer preference. Front. Plant Sci. 11, 1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz, E. (2020) A biotech insect repellent, safe enough to eat. Nat. Biotechnol. 38, 1368–1369. [DOI] [PubMed] [Google Scholar]
- Waltz, E. (2022) GABA‐enriched tomato is first CRISPR‐edited food to enter market. Nat. Biotechnol. 40, 9–11. [DOI] [PubMed] [Google Scholar]
- Wang, C. and Fan, Y. (2014) Eugenol enhances the resistance of tomato against tomato yellow leaf curl virus. J. Sci. Food Agric. 94, 677–682. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Chang, X. , Lin, J. , Chang, Y. , Chen, J.C. , Reid, M.S. and Jiang, C.Z. (2018) Transcriptome profiling reveals regulatory mechanisms underlying corolla senescence in petunia. Horticult. Res. 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, K.T. , Lee, P.C. and Schmidt‐Dannert, C. (2004) Exploring recombinant flavonoid biosynthesis in metabolically engineered Escherichia coli . ChemBioChem. 5, 500–507. [DOI] [PubMed] [Google Scholar]
- Weber, N. , Ismail, A. , Gorwa‐Grauslund, M. and Carlquist, M. (2014) Biocatalytic potential of vanillin aminotransferase from Capsicum chinense . BMC Biotechnol. 14, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widhalm, J.R. and Dudareva, N. (2015) A familiar ring to it: biosynthesis of plant benzoic acids. Mol. Plant, 8, 83–97. [DOI] [PubMed] [Google Scholar]
- Widhalm, J.R. , Jaini, R. , Morgan, J.A. and Dudareva, N. (2015) Rethinking how volatiles are released from plant cells. Trends Plant Sci. 20, 545–550. [DOI] [PubMed] [Google Scholar]
- de Wijk, R.A. , Polet, I.A. , Boek, W. , Coenraad, S. and Bult, J.H. (2012) Food aroma affects bite size. Flavour, 1, 3. [Google Scholar]
- Wycke, M.A. , Coureaud, G. , Thomas‐Danguin, T. and Sandoz, J.C. (2020) Configural perception of a binary olfactory mixture in honey bees, as in humans, rodents and newborn rabbits. J. Exp. Biol. 223, jeb227611. [DOI] [PubMed] [Google Scholar]
- Xie, Q. , Liu, Z. , Meir, S. , Rogachev, I. , Aharoni, A. , Klee, H.J. and Galili, G. (2016) Altered metabolite accumulation in tomato fruits by coexpressing a feedback‐insensitive AroG and the PhODO1 MYB‐type transcription factor. Plant Biotechnol. J. 14, 2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, D. , Du, X. , Yang, Z. , Liang, Z. , Guo, Z. and Liu, Y. (2014) Transcriptomics, proteomics, and metabolomics to reveal mechanisms underlying plant secondary metabolism. Eng. Life Sci. 14, 456–466. [Google Scholar]
- Yang, H. , Barros‐Rios, J. , Kourteva, G. , Rao, X. , Chen, F. , Shen, H. , Liu, C. et al. (2017) A re‐evaluation of the final step of vanillin biosynthesis in the orchid Vanilla planifolia. Phytochemistry, 139, 33–46. [DOI] [PubMed] [Google Scholar]
- Yang, D. , Park, S.Y. , Park, Y.S. , Eun, H. and Lee, S.Y. (2020) Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 38, 745–765. [DOI] [PubMed] [Google Scholar]
- Yokoyama, R. , de Oliveira, M.V.V. , Kleven, B. and Maeda, H.A. (2021) The entry reaction of the plant shikimate pathway is subjected to highly complex metabolite‐mediated regulation. Plant Cell, 33, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Tu, L. , Subburaj, S. , Bae, S. and Lee, G.J. (2021) Simultaneous targeting of duplicated genes in petunia protoplasts for flower color modification via CRISPR‐Cas9 ribonucleoproteins. Plant Cell Rep. 40, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Zhang, X. and Liu, C.J. (2015) Multifaceted regulations of gateway enzyme phenylalanine ammonia‐lyase in the biosynthesis of phenylpropanoids. Mol. Plant, 8, 17–27. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Butelli, E. , Alseekh, S. , Tohge, T. , Rallapalli, G. , Luo, J. , Kawar, P.G. et al. (2015) Multi‐level engineering facilitates the production of phenylpropanoid compounds in tomato. Nat. Commun. 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. and Fernie, A.R. (2021) Metabolons, enzyme–enzyme assemblies that mediate substrate channeling, and their roles in plant metabolism. Plant Commun. 2, 100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Peng, Q. , Zeng, L. , Tang, J. , Li, J. , Dong, F. and Yang, Z. (2018) Study of the biochemical formation pathway of aroma compound 1‐phenylethanol in tea (Camellia sinensis (L.) O. Kuntze) flowers and other plants. Food Chem. 258, 352–358. [DOI] [PubMed] [Google Scholar]
- Zvi, M.M.B. , Negre‐Zakharov, F. , Masci, T. , Ovadis, M. , Shklarman, E. , Ben‐Meir, H. , Tzfira, T. et al. (2008) Interlinking showy traits: co‐engineering of scent and colour biosynthesis in flowers. Plant Biotechnol. J. 6, 403–415. [DOI] [PubMed] [Google Scholar]
- Zvi, M.M.B. , Shklarman, E. , Masci, T. , Kalev, H. , Debener, T. , Shafir, S. , Ovadis, M. et al. (2012) PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers. New Phytol. 195, 335–345. [DOI] [PubMed] [Google Scholar]


