Abstract
The stoichiometry of the structural proteins of the photosynthetic apparatus in purple photosynthetic bacteria is achieved primarily by complex regulation of the levels of mRNA encoding the different proteins, which has been studied in the greatest detail in the puf operon. Here we investigated the transcriptional and posttranscriptional regulation of the puc operon, which encodes the peripheral light harvesting complex LHII. We show that, analogous to the puf operon, a primary transcript encoding five puc genes is rapidly processed to generate more stable RNA subspecies. Contrary to previous hypotheses, translational coupling and regulation of puc transcription by puc gene products were found not to occur. A putative RNA stem-loop structure appears to attenuate transcription initiated at the puc operon major promoter. We also found that a minor pucD-internal promoter contributes to the levels of a message that encodes the LHII 14-kDa γ (PucE) protein.
Many species of purple photosynthetic bacteria contain an integral membrane pigment-protein light-harvesting complex that is commonly referred to as LHII. X-ray crystallography of LHII complexes from Rhodopseudomonas acidophila and Rhodospirillum molischianum revealed that these crystal structures are rings of eight or nine subunits that each contain two small proteins (designated α and β, both of which span the membrane once), three bacteriochlorophyll molecules, and a carotenoid (17, 21). The LHII complex functions in photosynthesis to absorb and transfer light energy to the LHI complex and thence to the photochemical reaction center, where a cyclic series of electron and proton transfer reactions initiates. This series of reactions, which involves the cytochrome b/c1 complex, culminates in the formation of a proton gradient across the cytoplasmic membrane (22, 27).
The pucBA genes encode the protein components of the LHII complex and have been cloned and sequenced from several organisms (9, 12, 15). Although there are several alleles of pucB (β protein) and pucA (α protein) genes in some species, in Rhodobacter capsulatus and R. sphaeroides, a single puc operon contains single copies of the pucBA genes, followed by the pucC gene. In R. capsulatus, the pucC gene is followed by the pucD and pucE genes, yielding the pucBACDE operon (9, 13). The pucC gene product has been proposed to enhance either LHII assembly or pucBA transcription, the pucD gene product appears to be dispensable, and the pucE gene encodes a 14-kDa γ protein the function of which is uncertain and which copurifies with the LHII complex (although the LHII complex in membranes purified from an R. capsulatus pucE deletion mutant was relatively unstable) (9, 18, 28, 32).
The regulation of expression of puc genes has been studied in greatest detail in R. capsulatus and R. sphaeroides, with a focus on control of transcription initiation in response to light intensity and oxygen concentration within a large promoter-regulatory region located 5′ of the pucB gene. These studies revealed that several regulatory proteins seem to influence transcription initiation (20, 33).
A variety of puc gene-encoding RNA molecules exists in R. capsulatus, as revealed by RNA blotting, S1 nuclease end-mapping, and primer extension (PE) experiments. These appear to include a 2.4-kb pucBACDE transcript, as well as RNA molecules that contain only pucBA, pucDE, or pucE complete sequences (18, 20, 34). It was proposed that an inverted repeat sequence located between the pucA and pucC genes encodes an RNA stem-loop structure that functions to stabilize the pucBA message (28). Alternatively, since a version of this inverted repeat efficiently terminated transcription when inserted between the R. capsulatus pufA and pufL genes (7), the native puc sequence could attenuate transcription to give rise to abundant 0.55-kb pucBA messages and a much less abundant 2.4-kb pucBACDE transcript. These two possibilities are not mutually exclusive. The genesis of intermediate-abundance 0.9- to 1.0-kb pucDE and 0.6- to 0.7-kb pucE RNA segments is even less clear, although it was speculated that these molecules result from RNase cleavage of the pucBACDE transcript (18).
Promoter-mapping experiments (using a translationally in-frame pucE′ fusion of puc operon segments to an Escherichia coli lac′Z allele) confirmed that a strong promoter is located 5′ of pucB (18). However, a puc′CDE′::lac′Z plasmid produced about 10% of the β-galactosidase specific activity of a pucBACDE′::lac′Z fusion, whereas a puc′DE′::lac′Z fusion yielded less than 1% of the activity obtained with the pucBACDE′::lac′Z plasmid. These results were interpreted as suggestive of translational coupling between the overlapping pucD and pucE reading frames with transcription readthrough from an adventitious vector promoter and/or the existence of a second promoter located in the pucC 3′ to pucD 5′ region (18).
In this paper, we present the results of puc operon experiments that utilized gene fusions, high-resolution RNA 5′-end mappings by PEs, and RNA blot hybridizations with selected probes. These experiments directly addressed the questions of attenuation in the pucA-to-pucC intergenic region, translational coupling between pucD and pucE, and the existence of a promoter in the pucC 3′-to-pucD 5′ region and identified multiple RNase cleavages within the pucBACDE transcript.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Escherichia coli strains used for subcloning were the dam mutant RB404 (6), an hsdR derivative of C600 (5), and SM10 (25). Strains SM10 and HB101(pRK2013) (8) were used to transfer plasmids by conjugation to R. capsulatus. E. coli strains were grown in Luria broth (24) supplemented with the appropriate antibiotics at the following concentrations: ampicillin, 200 μg/ml; tetracycline-HCl, 10 μg/ml.
R. capsulatus SB1003 (26) was used for RNA analysis and β-galactosidase activity determinations in the gene fusion experiments. The pucBACDE deletion strain ΔLHII (18) was used as the host of puc operon deletion plasmids for RNA blot analyses.
R. capsulatus strains were grown in RCV medium (4), supplemented with tetracycline-HCl at a concentration of 0.5 μg/ml, if appropriate, at 34°C. Low-aeration growth conditions for induction of puc gene expression were obtained by filling flasks to 80% of their nominal capacities and shaking them at 150 rpm in the absence of illumination. Growth was monitored by measuring turbidity with a Klett-Summerson photometer (filter no. 66).
DNA manipulations, RNA isolation, blot analysis, and probe construction.
Standard methods of DNA purification, restriction enzyme digestion, and other DNA modification techniques were used (24).
RNA was isolated from R. capsulatus by the hot-phenol method as previously described (30). Samples were ethanol precipitated and denatured in a buffer containing formaldehyde and ethidium bromide prior to electrophoresis (23). Five micrograms of RNA per lane was run on a 1.4% agarose–formaldehyde gel beside 3 μg of a 0.24- to 9.5-kb RNA ladder (GIBCO-BRL). After electrophoresis, the gel was equilibrated in 0.5× TBE buffer (24) and photographed with UV illumination before electroblotting overnight at 30 V in 0.5× TBE buffer onto a Biotrans nylon membrane (ICN). After blotting, the membrane was dried at 80°C under vacuum and exposed to UV light for comparison with the blotted gel and preblotted gel photograph to ensure that transfer was complete.
Hybridization probes using purified DNA fragments were prepared by labelling with [α-32P]dATP by the random-primer method (10). Unincorporated nucleotides were removed by using the Qiaex DNA purification procedure (Qiagen). The Qiaex eluate in TE buffer (24) was denatured at 90°C for 10 min and used directly for hybridization. Blotted membranes were prehybridized in 5× SSC (1× SSC is 0.15 M NaCl, 0.015 M sodium citrate, pH 7.0)–1% sodium dodecyl sulfate (SDS)–10 mM EDTA–50% formamide containing 0.5 mg of denatured, sheared salmon sperm DNA per ml for 4 to 8 h at 42°C before addition of the denatured, labelled probe buffer (24). The blots were hybridized with probes for 18 h at 42°C. After hybridization, membranes were washed twice for 10 to 15 min each time in 2× SSC–1% SDS at room temperature, twice in the same solution at 50°C for 10 to 15 min each time, and once for 5 min in 0.2× SSC–1% SDS at 50°C. Blots were then exposed to X-ray film in a cassette with an intensifying screen at −75°C for various lengths of time before development.
The hybridizations with end-labelled oligonucleotides were carried out at 42°C in hybridization buffer (50% formamide, 10% dextran sulfate, 1.0 M NaCl, 50 mM Tris-Cl [pH 7.5], 0.2% bovine serum albumin, 0.2% Ficoll, 0.1% sodium pyrophosphate, 1% SDS, 0.2% polyvinylpyrrolidone, 0.1 mg of denatured, sheared salmon sperm DNA per ml). After hybridization, the membranes were washed twice for 10 min each time at room temperature in 2× SSC, given a 20-min wash in 2× SSC–1% SDS at 35°C, and then exposed to film as described above. If necessary, blots were stripped for reprobing in accordance with the manufacturer’s (ICN) recommendation.
Primers used for PEs and RNA blot probes.
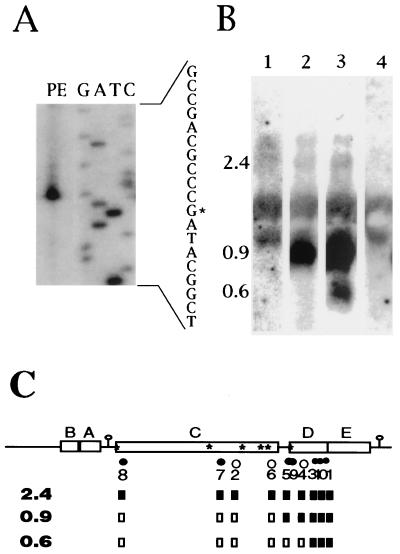
For approximate locations of oligonucleotides complementary to puc transcripts, see Fig. 2C. The sequences of the oligonucleotides are as follows: ALPE1, 5′-GTCAGTCATTTCAGATGCGTCC-3′; ALPE2, 5′-GCGAGGACATGATGATGGCGAC-3′; ALPE3, 5′-TTGTAGTTGACTTTCGCCA-3′; ALPE4, 5′-GAATACCTTCGCTTTTCAGTTG-3′; ALPE5, 5′-GCGTCCTCCTCGGGTTCAAG-3′; ALPE6, 5′-GCCACGATCAAAATCAACGC-3′; ALPE7, 5′-CGGTCAGTTTCGTCGTCTCC-3′; ALPE8, 5′-GCGAATGGCAGGTACTT-3′; ALPE9, 5′-GCATAAAGCCGTGTGAT-3′; ALPE10, 5′-TCTTCGGTTGCGTTCTCG-3′.
FIG. 2.
PE and RNA blot analyses of the puc operon using oligonucleotides as primers or probes. (A) Results of a PE experiment that utilized the ALPE3 primer and RNA from SB1003. The 5′ end at an RNA G residue corresponds to the proposed start site of a promoter located within pucD. (B) Results of an RNA blot experiment that utilized oligonucleotide probes ALPE6 (lane 1), ALPE4 (lane 2), and ALPE1 (lane 3, 4) hybridized to RNA from ΔLHII(pBACDE) (lanes 1 to 3) or ΔLHII(pRK415) (lane 4). RNA from a strain lacking the puc operon (lane 4) shows only nonspecific hybridization to rRNA. (C) Summary of PE and RNA blot experiments. The locations of sequences complementary to oligonucleotides are indicated by dots and ALPE numerical designations below a schematic of the puc operon. Open dots indicate that no ends were detected (within 60 nucleotides) by PE using that primer, whereas filled dots show that an end was detected. These oligonucleotides were also used to probe RNA blots. If a given primer detected the 2.4-, 0.9-, or 0.6-kb transcript, the corresponding box below the primer number is filled whereas open boxes represent negative results. Inverted repeats are shown as loops with stems, and the positions of the six RNase E consensus sites are shown (∗).
PEs.
32P-end-labelled (24) primers (10 pmol) were mixed with R. capsulatus total RNA (5 μg) from SB1003 cells grown with low aeration and heated to 90°C for 10 min in hybridization buffer (150 mM KCl, 10 mM Tris-Cl, 1 mM EDTA, pH 8.0). This mixture was then incubated at approximately 2°C below the theoretical melting temperature of the specific oligonucleotide for 2 to 3 h. Nucleic acids were then ethanol precipitated and resuspended in the reaction buffer supplied with the reverse transcriptase supplemented with 10 mM dithiothreitol and 0.5 mM each deoxynucleoside triphosphate. One hundred units of Superscript II reverse transcriptase (GIBCO-BRL) was added, and after incubation at 42°C for 1 h, the reaction was phenol extracted and nucleic acids were precipitated with ethanol. The pellet was resuspended in formamide loading buffer (24), heated to 75°C for 5 min, and electrophoresed on an 8% acrylamide–8 M urea gel next to a sequencing ladder obtained with the same primer and plasmid pBACDE as the template, using the Sequenase Version 2.0 DNA Sequencing Kit (USB).
Construction of pucE′::lac′Z fusions and other plasmids.
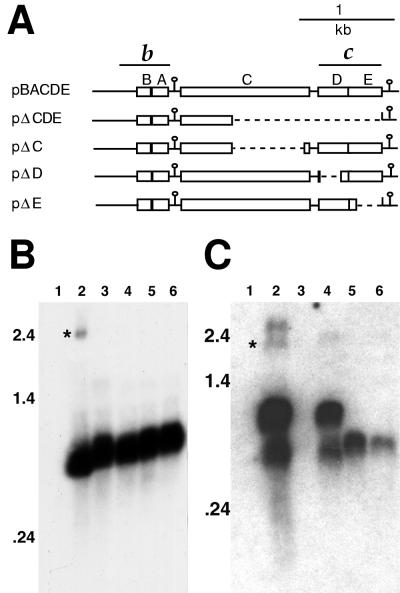
The translationally in-frame fusions of the pucE′-to-lac′Z genes in plasmids pPEZ, pCEZ, pBEZ, and pHEZ have already been described (18). A translational frameshift of the pucD coding sequences was created by cleavage at the BclI site in the fifth codon of pucD, filling in, and religation. This insertion of four base pairs changed the codon sequence from 5′-GTG ATC ACA-3′ to 5′-GTG ATC GAT CAC A-3′, thus creating a stop codon at the 13th codon of pucD (pBEZ-OOF and pPEZ-OOF). The inverted-repeat sequence located between pucA and pucC is flanked by intergenic ApaI sites (29), and so it was deleted by ApaI digestion, followed by religation to create pPEZΔSL, and confirmed by DNA sequencing. The PstI-to-BamHI puc fragments (see Fig. 1) were inserted into the promoter probe vector pXCA601 (1). The resultant plasmids were transferred to SB1003 by conjugation. The plasmids pBACDE, pΔCDE, pΔC, pΔD, and pΔE, which were present in strain ΔLHII for use in RNA blot experiments, were previously described (18).
FIG. 1.
Relative β-galactosidase levels of pucE′::lac′Z fusions with different amounts of puc 5′ sequences. The β-galactosidase specific activities of SB1003 strains containing each plasmid were normalized to that of SB1003(pPEZ), and standard deviations are shown in parentheses. Genes are indicated by boxes, and the inverted repeat between pucA and pucC, if present, is shown as a stem and loop. The extra nucleotides resulting from filling in of a BclI site early in the pucD open reading frame to create a stop codon are indicated by triangles above the appropriate constructs.
β-Galactosidase assays.
β-Galactosidase activities were measured in timed assays in which equal numbers of cells from 20 ml of low-aeration cultures (ca. 3.7 × 108 CFU/ml) were resuspended in 1 ml of Z buffer and made permeable with SDS and chloroform (19). Activities are expressed as percentages of the specific activity of SB1003(pPEZ), normalized to the numbers of cells, and all determinations were performed on triplicate samples in at least two experiments.
RESULTS
Transcriptional organization of and attenuation within the puc operon.
Although it was well established that a strong puc operon promoter is located 5′ of the pucB gene, promoter mapping of the puc operon using translational fusions between pucE′ and lac′Z (18) suggested that the region between the 3′ end of pucC and the middle of pucD is important for expression of pucE (Fig. 1; compare the controls pCEZ and pBEZ to pHEZ). However, it was not clear from these experiments whether this region contained a minor promoter that contributed to pucE transcription or if expression of the pucE′::lac′Z fusion in pBEZ was enhanced by translational coupling to the overlapping pucD open reading frame that was abolished when the translational start region of pucD was deleted in pHEZ. To distinguish between these two nonexclusive possibilities, we created a stop codon within pucD in the plasmid pBEZ (to produce pBEZ-OOF), reasoning that if translational coupling between pucD and pucE were significant, the β-galactosidase activity of pBEZ-OOF would be similar to the low activity of pHEZ.
The β-galactosidase activity of SB1003 cells containing pBEZ-OOF was essentially the same as that of pBEZ-containing cells (Fig. 1). This frameshift mutation in the context of the entire operon also did not significantly affect the level of synthesis of the PucE::β-galactosidase fusion protein (Fig. 1; compare pPEZ-OOF with pPEZ). These data indicate that there is no translational coupling between pucD and pucE and are consistent with the idea that the sequence between the 3′ region of pucC and the middle of pucD contains a promoter.
Previous RNA blot analysis indicated the presence of two messages that contained sequences from the pucBA region. One was the high-abundance pucBA molecule, and the other was a relatively low-abundance transcript likely to encode the entire pucBACDE operon (18). Since there is an inverted repeat sequence between pucA and pucC, a modified version of which was shown to encode a strong transcriptional terminator (7), it was possible that transcription which originated at the promoter region located 5′ of pucB was greatly attenuated by a rho-independent terminator formed 3′ of pucA. We tested this possibility by deleting the inverted-repeat sequence from pPEZ to produce pPEZΔSL. The β-galactosidase specific activity measured in cells containing pPEZΔSL was higher than that measured from pPEZ (a 23 to 30% difference), suggesting that a small amount of attenuation occurs at the inverted repeat between pucA and pucC (Fig. 1). However, this amount of attenuation could not be the sole reason for the great difference between the steady-state amounts of 0.55-kb pucBA and 2.4-kb pucBACDE molecules (18) (and see below). This interpretation is consistent with the pCEZ and pBEZ results, which showed that the majority of pucE transcription originates 5′ of pucB, but raises the question of where the residual (10%) expression of the pBEZ and pBEZ-OOF fusions originates.
5′-end mapping and RNA blots of messages encoding the 3′ region of the puc operon.
The weak attenuation of transcription in the pucA-to-pucC intergenic region (Fig. 1) and the low abundance of the 2.4-kb pucBACDE transcript (see below) suggested that this primary transcript is rapidly degraded. The pentanucleotide sequence 5′-GNc/uUU-3′ was shown to be part of an RNase E substrate in studies of puf operon message cleavage (11). A search of the puc operon sequence revealed the presence of five of these RNase E consensus sites in the pucC coding region and a sixth near the start of pucD (Fig. 2C). In contrast, this motif occurs only twice more in the 3.2-kb sequence reported by Tichy et al. (29), and these two motifs are located upstream of the 5′ ends of pucBA transcripts. We therefore performed PE analyses to identify RNA cleavage products and to map the 5′ ends of the relatively abundant messages encoding the pucDE region.
The primary data from one PE experiment that employed the ALPE3 primer are shown in Fig. 2A, and the results of many of such experiments are summarized in Fig. 2C. PE from oligonucleotides ALPE8 and ALPE7 identified RNA 5′ ends near the two 5′-most RNase E consensus sequences. No RNA molecules stable enough to be detected by RNA blotting have 5′ ends in this region (reference 18; see also below), and so degradation of these segments must rapidly follow their production. Other RNA 5′ ends detected by PE mapped to the region between pucC and pucD (ALPE5 and ALPE9) or to the middle of pucD (ALPE1, ALPE3, and ALPE10). Two moderately abundant RNAs approximately 0.9 and 0.6 kb long have been identified in this region. To determine if these molecules provided the 5′ ends detected in the PEs, we used PE oligonucleotides to probe RNA blots.
Although the RNA blots probed with oligonucleotides had a high noise-to-signal ratio, attributed to nonspecific binding of oligonucleotide probes to rRNAs (Fig. 2C, lane 4), a prominent 0.9-kb mRNA was detected by ALPE4 and the oligonucleotide probes complementary to sequences 3′ of ALPE5 but not by ALPE6 or the oligonucleotide probes complementary to more 5′ sequences (Fig. 2B and C). We conclude that the RNA 5′ end detected in the pucC 3′-to-pucD 5′ region corresponds to this 0.9-kb message. Similarly, the 0.6-kb pucE molecule was detected by ALPE10, ALPE1, and ALPE3 but not by ALPE4, and so the 5′ end of this mRNA maps to the 3′ half of pucD. All of the primers detected the low-abundance 2.4-kb pucBACDE transcript, as summarized in Fig. 2C.
Transcription of pucBA is independent of the pucC, pucD, and pucE gene products.
The low amounts of the 2.4-kb transcript encoding pucC suggest that the PucC protein is present at much lower levels than the other products of the puc operon. It is known that the pucC gene is required for accumulation of the LHII complex (18, 28), and it was reported that a transposon insertion into pucC, which could exert a polar effect on transcription of pucDE genes, reduced transcription of pucBA (28). We tested the effect of independent deletion of pucC, pucD, or pucE, as well as that of a pucCDE deletion, on pucBA transcript levels by preparing RNAs from ΔLHII strains that contained plasmids carrying either the entire pucBACDE operon or independent deletions of these genes (Fig. 3A). As shown in Fig. 3B, the levels of the 0.55-kb pucBA transcript were similar in all of the strains, and so none of the pucCDE genes is required for transcription of the pucBA genes.
FIG. 3.
RNA blot analysis of puc deletion strains. (A) Structures of the five puc deletion plasmids tested. The puc genes are indicated by boxes, and inverted repeats are shown as loops with stems. Deleted sequences are shown as dotted lines. The fragments used as probes for RNA blot analysis in panels B (b) and C (c) are indicated above the schematic of pBACDE. RNA was prepared from strain ΔLHII containing plasmids pRK415 (vector control lacking puc genes) (lane 1), pBACDE (lane 2), pΔCDE (lane 3), pΔC (lane 4), pΔD (lane 5), and pΔE (lane 6). The approximate positions of RNA molecular size standards are shown to the left of the blots. In panel C, the 2.4-kb puc transcript (∗) was identified by stripping of the blot in panel B and hybridization with probe c (data not shown). The origin of the higher-molecular-weight molecule in lane 2 is not known.
When a similar RNA blot was probed with a DNA fragment containing the pucDE region, the 0.9-kb RNA molecule was detected with reduced size in strains with the pucD or pucE gene deleted but the 0.6-kb molecule was absent in both cases (Fig. 3C). There was no obvious effect on the amount of either of these RNA molecules resulting from deletion of pucC. Interestingly, this probe detected an additional band of greater than 2.4 kb not seen with the pucBA probe. The origin of this molecule is not known, but it is also visible in Fig. 2B (lanes 1 to 3), and low-abundance higher-molecular-weight RNAs were also reported by Nickens and Bauer (20).
DISCUSSION
Previous publications suggested that the regulation of R. capsulatus puc operon mRNA levels was likely to be complex (18, 28). In this work, we investigated the possibilities that transcription attenuation, translational coupling, segmental RNA degradation, and two promoters contribute to puc mRNA levels.
Sequence analysis of the puc operon showed that the pucD and pucE open reading frames overlap, such that the sequence 5′-ATGA-3′ contains the putative TGA stop codon of pucD, as well as the ATG start codon of pucE (29). In such cases, translation of the 3′ gene is often dependent on translation of the 5′ gene (translational coupling) (14). We show here that a translation frameshift mutation in the 5th codon of pucD, which produced a translation stop in the 13th codon, did not affect the expression of pucE′::lac′Z translational fusions. Therefore, it seems that there is no translational coupling between pucD and pucE and the function of pucD remains obscure.
The pucC gene is required for wild type-levels of the LHII complex but is encoded by the low-abundance 2.4-kb puc operon transcript (18). This suggests that the PucC protein is not present in amounts equal to the protein components of the LHII complex and that PucC is likely to have a catalytic rather than a structural role. This interpretation is supported by the fact that LHII crystals from R. acidophila and R. molischianum yielded X-ray diffraction from only the PucB and PucA proteins (17, 21). Although it was previously reported that a transposon insertion into the pucC gene resulted in loss of the pucBA message (28), our results show that neither deletion of pucC, pucD, or pucE nor deletion of pucCDE reduced the level of pucBA mRNA. It is more likely, therefore, that PucC has a posttranslational role in the assembly of the LHII complex, as was proposed for the homologous LhaA protein in LHI assembly (31).
Four RNA species attributed to the puc operon were detected in blots. The largest and least abundant of these is thought to be a primary transcript of 2.4 kb, encoding the entire operon. Although there seemed to be significant attenuation of transcription in the pucA-to-pucC intergenic region, our results suggest that the low abundance of the 2.4-kb transcript is mainly due to endoribonucleolytic cleavage followed by rapid 3′-to-5′ exoribonucleolytic degradation. There are five matches to an RNase E recognition consensus sequence (11) in the coding region of pucC and a sixth at the start of the pucD open reading frame. Three of these sites mapped near RNA 5′ ends that were detected by primer extension. Only one of them (the 5′ end detected with ALPE9 and ALPE5) corresponds to a message stable enough to be detected in an RNA blot, and so we propose that a relatively stable processing product of the 2.4-kb primary transcript is the approximately 0.9-kb RNA that hybridizes to pucDE sequences and which extends from the pucC-to-pucD intergenic region to the predicted rho-independent transcriptional terminator downstream of pucE (29). Consistent with this proposal was the discovery that deletion of sequences from either pucD or pucE reduced the size of this molecule but did not eliminate it.
The most abundant RNA product of the puc operon is the 0.55-kb pucBA message. It was at one time thought that transcription initiating upstream of pucB terminated at the imperfect inverted repeat found between pucA and pucC, since a modified form of this sequence had been shown to terminate transcription when inserted into the puf operon (7, 34). In retrospect, these modifications were not insignificant, as they involved the creation of a perfect inverted repeat and substitution of the 5′-ATTC-3′ sequence that follows the native inverted repeat sequence with 5′-TTTT-3′ to make it more closely resemble a canonical transcription terminator (7). Our results show that deletion of this native sequence from the puc operon resulted in a small increase of readthrough transcription, and so it seems that some transcripts terminate at this site.
In the R. capsulatus puf operon, an inverted repeat sequence located between the pufBA and pufLMX genes was shown to function primarily as a barrier against exoribonucleolytic digestion (it was calculated that ≤25% of pufBA transcripts terminate at this site [7]). By analogy to the puf operon, we suggest that most of the pucBA messages arise from cleavage within the pucC segment of the 2.4-kb primary transcript, followed by exoribonucleolytic degradation that is greatly slowed by an RNA secondary structure in the region between pucA and pucC transcript segments. A minor percentage of transcripts terminates at this RNA stem-loop. This model is consistent with all of the available data, including the relative steady-state amounts of pucBA and pucBACDE transcripts, the relative amounts of attenuation indicated by the pPEZ and pPEZΔSL constructs, and our identification of putative RNase E cleavage sites with corresponding RNA 5′ ends.
We suggest that the approximately 0.6-kb pucE transcript detected in RNA blots arises from a minor promoter. We mapped an RNA 5′ end to the middle of the pucD sequence, where there is no RNase E consensus sequence, and inspection of the −10 to −35 region 5′ of this end revealed the sequence 5′-CTCAAG-N17-CAGCGC-N10-3′, which has weak similarity to proposed ς70 promoters (20). Furthermore, deletion of this region from the pBEZ fusion construct (to produce pHEZ) reduced β-galactosidase levels from 10% to <1% of pPEZ and this 0.6-kb transcript was absent from a pucD deletion strain that lacked this proposed promoter region. This molecule was not detected in the pucE deletion strain, but most of the sequence complementary to the probe used is absent from this mutant. Taken together, these results indicate that the 0.6-kb pucE transcript is the product of a secondary promoter internal to pucD.
Our model of puc RNA regulation is summarized in Fig. 4. The majority of puc transcription initiates 5′ of pucB and terminates at the inverted repeat located immediately 3′ of pucE, with a small amount of attenuation at the inverted repeat between pucA and pucC. The 2.4-kb primary transcript is rapidly degraded by RNase E endoribonucleolytic and subsequent 3′-to-5′ exoribonucleolytic activities. The relatively stable products of this processing are the 0.55-kb pucBA and 0.9-kb pucDE messages. A minor promoter within pucD generates the 0.6-kb pucE message.
FIG. 4.
Model of the transcriptional organization of the puc operon. Four puc mRNAs detected in RNA blots are shown by horizontal arrows, each of whose thickness suggests its relative abundance. The 2.4-kb transcript encoding all of the puc genes is the primary product of the 5′-most major promoter (bent arrow upstream of pucB), although some transcripts (grey arrow) terminate between pucA and pucC at an imperfect inverted repeat (open stem-loop). The pucBACDE transcript is rapidly cleaved by RNase E at multiple sites (arrowheads) and degraded by 3′-to-5′ exoribonuclease(s) to yield the 0.9-kb pucDE and 0.55-kb pucBA processing products, which lack RNase E recognition sites and are stabilized by stem-loops at their 3′ ends. A minor promoter within pucD (smaller bent arrow) produces the 0.6-kb pucE mRNA. The primary products of both promoters terminate at the predicted rho-independent transcriptional terminator downstream of pucE (closed stem-loop).
The stoichiometry of R. capsulatus LHI and reaction center protein components of the photosynthetic apparatus is maintained primarily through complex regulation of the steady-state amounts of puf operon mRNA segments encoding these proteins. The cellular mechanisms operative include regulation of transcription initiation, differential susceptibilities of specific message segments to endo- and exoribonuclease activities, and transcriptional readthrough from upstream operons (“superoperons”) (2, 3, 16). Our results suggest that puc operon gene expression is governed by the first two of these mechanisms and that the stoichiometry of the LHII 14-kDa γ protein (PucE) is achieved in part by transcription initiation at a pucD internal promoter.
ACKNOWLEDGMENTS
We thank Grace Wong for expert technical assistance in the construction of plasmid pPEZ-OOF.
This research was supported by grants from the NSERC (Canada) to J.T.B.
REFERENCES
- 1.Adams C W, Forrest M E, Cohen S N, Beatty J T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989;171:473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer C E, Bird T H. Regulatory circuits controlling photosynthesis gene expression. Cell. 1996;85:5–8. doi: 10.1016/s0092-8674(00)81074-0. [DOI] [PubMed] [Google Scholar]
- 3.Beatty J T. Organization of photosynthesis gene transcripts. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1209–1219. [Google Scholar]
- 4.Beatty J T, Gest H. Generation of succinyl-coenzyme A in photosynthetic bacteria. Arch Microbiol. 1981;129:335–340. [Google Scholar]
- 5.Bibb M J, Cohen S N. Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet. 1982;187:265–277. doi: 10.1007/BF00331128. [DOI] [PubMed] [Google Scholar]
- 6.Brent R, Ptashne M. The lexA gene product represses its own promoter. Proc Natl Acad Sci USA. 1980;77:1932–1936. doi: 10.1073/pnas.77.4.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C-Y A, Beatty J T, Cohen S N, Belasco J G. An intercistronic stem-loop structure functions as an mRNA decay terminator necessary but insufficient for puf mRNA stability. Cell. 1988;52:609–619. doi: 10.1016/0092-8674(88)90473-4. [DOI] [PubMed] [Google Scholar]
- 8.Ditta G, Schmidhauser T, Yakobsen E, Lu P, Liang X-W, Finlay D R, Guiney D, Helinski D R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985;13:149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- 9.Drews G, Golecki J R. Structure, molecular organization, and biosynthesis of membranes of purple bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 231–257. [Google Scholar]
- 10.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 11.Fritsch J, Rothfuchs R, Rauhut R, Klug G. Identification of an mRNA element promoting rate-limiting cleavage of the polycistronic puf mRNA in Rhodobacter capsulatus by an enzyme similar to RNase E. Mol Microbiol. 1995;15:1017–1029. doi: 10.1111/j.1365-2958.1995.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 12.Germeroth L, Reilander H, Michel H. Molecular cloning, DNA sequence and transcriptional analysis of the Rhodospirillum molischianum B800/850 light-harvesting genes. Biochim Biophys Acta. 1996;1275:145–150. doi: 10.1016/0005-2728(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 13.Gibson L C D, McGlynn P, Chaudhri M, Hunter C N. A putative coproporphyrinogen III oxidase in Rhodobacter sphaeroides. II. Analysis of a region of the genome encoding hemF and the puc operon. Mol Microbiol. 1992;6:3171–3186. doi: 10.1111/j.1365-2958.1992.tb01773.x. [DOI] [PubMed] [Google Scholar]
- 14.Gold L, Stormo G. Translational initiation. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1302–1307. [Google Scholar]
- 15.Hagemann G E, Katsiou E, Forkl H, Steindorf A C J, Tadros M H. Expression of the puc operon from Rhodovulum sulfidophilum. Biochim Biophys Acta. 1997;1351:341–358. doi: 10.1016/s0167-4781(96)00228-x. [DOI] [PubMed] [Google Scholar]
- 16.Klug G. Post-transcriptional control of photosynthesis gene expression. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1235–1244. [Google Scholar]
- 17.Koepke J, Hu X, Muenke C, Schulten K, Michel H. The crystal structure of the light-harvesting complex II (B800–850) from Rhodospirillum molischianum. Structure. 1996;4:581–597. doi: 10.1016/s0969-2126(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 18.LeBlanc H N, Beatty J T. Rhodobacter capsulatus puc operon: promoter location, transcript sizes and effects of deletions on photosynthetic growth. J Gen Microbiol. 1993;139:101–109. doi: 10.1099/00221287-139-1-101. [DOI] [PubMed] [Google Scholar]
- 19.Manoil C. Analysis of membrane protein topology using alkaline phosphatase and β-galactosidase. Methods Cell Biol. 1991;34:61–75. doi: 10.1016/s0091-679x(08)61676-3. [DOI] [PubMed] [Google Scholar]
- 20.Nickens D G, Bauer C E. Analysis of the puc operon promoter from Rhodobacter capsulatus. J Bacteriol. 1998;180:4270–4277. doi: 10.1128/jb.180.16.4270-4277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papiz M Z, Prince S M, Hawthornthwaite-Lawless A M, McDermott G, Freer A A, Isaacs N W, Cogdell R J. A model for the photosynthetic apparatus of purple bacteria. Trends Plant Sci. 1996;1:198–206. [Google Scholar]
- 22.Prince R C. Bacterial photosynthesis: from photons to Δ p. Bacteria. 1990;12:111–149. [Google Scholar]
- 23.Rosen K M, Lamperti E D, Villa-Komaroff L. Optimizing the Northern blot procedure. BioTechniques. 1990;8:398–403. [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:37–45. [Google Scholar]
- 26.Solioz M, Marrs B. The gene transfer agent of Rhodopseudomonas capsulata. Arch Biochem Biophys. 1977;181:300–307. doi: 10.1016/0003-9861(77)90508-2. [DOI] [PubMed] [Google Scholar]
- 27.Sundström V, van Grondelle R. Kinetics of excitation transfer and trapping in purple bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 349–372. [Google Scholar]
- 28.Tichy H V, Albien K U, Gadon N, Drews G. Analysis of the Rhodobacter capsulatus puc operon—the pucC gene plays a central role in the regulation of LHII (B800–850 complex) expression. EMBO J. 1991;10:2949–2955. doi: 10.1002/j.1460-2075.1991.tb07845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tichy H V, Oberlé B, Stiehle H, Schiltz E, Drews G. Genes downstream from pucB and pucA are essential for formation of the B800–850 complex of Rhodobacter capsulatus. J Bacteriol. 1989;171:4914–4922. doi: 10.1128/jb.171.9.4914-4922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Gabain A, Belasco J G, Schottel J L, Chang A C Y, Cohen S N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci USA. 1983;80:653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young C S, Beatty J T. Topological model of the Rhodobacter capsulatus light-harvesting complex I assembly protein LhaA (previously known as ORF1696) J Bacteriol. 1998;180:4742–4745. doi: 10.1128/jb.180.17.4742-4745.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young C S, Reyes R C, Beatty J T. Genetic complementation and kinetic analyses of Rhodobacter capsulatus ORF1696 mutants indicate that the ORF1696 protein enhances assembly of the light-harvesting I complex. J Bacteriol. 1998;180:1759–1765. doi: 10.1128/jb.180.7.1759-1765.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeilstra-Ryalls J, Gomelsky M, Eraso J M, Yeliseev A, O’Gara J, Kaplan S. Control of photosystem formation in Rhodobacter sphaeroides. J Bacteriol. 1998;180:2801–2809. doi: 10.1128/jb.180.11.2801-2809.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zucconi A P, Beatty J T. Posttranscriptional regulation by light of the steady-state levels of mature B800–850 light-harvesting complexes in Rhodobacter capsulatus. J Bacteriol. 1988;170:877–882. doi: 10.1128/jb.170.2.877-882.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]