Abstract
Mounting evidence suggests distinct functional contributions of the anterior and posterior hippocampus to autobiographical memory retrieval, but how these subregions function under different retrieval demands as memories age is not yet understood. Specifically, autobiographical memory retrieval is not a homogeneous process; rather, it is thought to consist of the following multiple stages: an early stage of memory construction and a later stage of detailed elaboration, which may differently engage the hippocampus over time. In the present study, we analyzed data from 40 participants (23 female/17 male) who constructed and overtly elaborated on recent and remote memories in response to picture cues in the fMRI scanner. We previously reported a temporal gradient in the posterior hippocampus during the elaboration period of autobiographical retrieval, with posterior hippocampal activation observed for recent but not remote time points. Here, we consider the previously unanalyzed construction stage of retrieval, where participants searched for and selected a memory. We found no evidence of a temporal gradient during memory construction, instead observing strong anterior hippocampus activity regardless of memory remoteness. Our findings suggest a unique contribution of the anterior hippocampus to the construction process of autobiographical retrieval over time. These findings highlight that retrieval processes, which have yet to be integrated with current models of systems consolidation, offer novel insights into hippocampal subregion function over time.
SIGNIFICANCE STATEMENT Hippocampal contributions to autobiographical memory retrieval may depend on several distinct factors including memory age and the retrieval process engaged. We previously found that the contribution of the posterior hippocampus to detailed elaborative retrieval diminishes as memories age, with no reliable activation of the anterior hippocampus over time. Here, we report that consideration of the earlier “construction” period of retrieval, where participants search for and retrieve general aspects of the memory, yielded significant anterior hippocampus activation regardless of memory age. These results provide evidence for a unique contribution of the anterior hippocampus to the constructive process of autobiographical retrieval over time and suggest that component processes of retrieval should be integrated into models of systems consolidation.
Keywords: anterior hippocampus, autobiographical memory, construction, retrieval, systems consolidation, temporal gradient
Introduction
The human ability to mentally travel back in time to re-experience past events—“autobiographical” or “episodic” memory—is central to human cognition. This capacity helps give rise to our sense of self (Conway and Pleydell-Pearce, 2000), forms the basis for interactions around the dinner table (Mahr and Csibra, 2018), and likely supports our ability to envision future events (Tulving, 1985; Schacter and Addis, 2007). The hippocampus and other medial temporal lobe structures, through interactions with one another and the neocortex, are thought to be critical for both the formation and retrieval of autobiographical memories (Squire and Zola-Morgan, 1991). Indeed, among the most common questions still asked by memory researchers today is how the hippocampus supports memory retrieval.
However, converging lines of research suggest that investigating “the” role of “the” hippocampus may frame the question too broadly. Perhaps most notably, the age and amount of detail associated with retrieved memories appear important in determining hippocampal contributions to recall. Results spanning decades have highlighted differing roles for the hippocampus in retrieving recent and remote memories, although the basis for such change over time remains debated. One prominent model assumes a time-limited role for the hippocampus, with memories being “consolidated” to the neocortex over time (the “Standard Model of Consolidation”; Squire et al., 2015). Others have argued that the propensity of memories to degrade over time drives reductions in activity for remote memories, and that the hippocampus is always necessary for recalling richly detailed memories (“Trace Transformation Theory”; Nadel and Moscovitch, 1997; Sekeres et al., 2018).
Two additional lines of memory research have identified factors other than age and detail that may impact hippocampal responses during retrieval. One describes heterogeneity along the long axis of the hippocampus. Anterior and posterior regions possess distinct structural connectivity, functional connectivity, and task activation profiles (Poppenk et al., 2013; Barnett et al., 2019; Persichetti et al., 2021; Zheng, 2021). Evidence suggests that the posterior hippocampus supports the retrieval of fine-grained details, whereas the anterior hippocampus supports coarser, gist-like memory features (Brunec et al., 2018; Sekeres et al., 2018; Audrain and McAndrews, 2020). In addition, there may be nonstationarity in the processes involved in retrieving autobiographical memories. The Self-Memory System framework of Conway (2005) proposes that autobiographical memory retrieval can be separated into two conceptually and temporally discrete stages. An early “construction” period involves the search and recovery of general aspects of a memory, whereas a later “elaboration” period involves a sustained recollection period wherein the distinct elements of an episode are re-experienced in detail (Conway, 2005).
Hippocampal contributions to autobiographical retrieval may therefore depend on several distinct factors (Gilmore et al., 2022). Studies with this in mind remain relatively rare yet have provided intriguing results. For instance, a recent fMRI investigation of effective connectivity in anterior and posterior hippocampal subregions during construction and elaboration stages found changes in the direction of information flow within and beyond the anterior and posterior hippocampus across retrieval stages (McCormick et al., 2015, 2018). However, simple univariate differences in hippocampal engagement were not observed. Similarly, Addis et al. (2007) associated anterior hippocampal activity with construction-related activity across several comparisons, but not in their direct contrast of construction and elaboration stage activity. Neither study examined the effect of event recency on hippocampal engagement, although a subsequent reanalysis of the elaboration phase of the data in the study by Addis et al. (2007) indicated a temporal gradient for future events (distant future > near future) in the hippocampus (Addis and Schacter, 2008). In some of our own recent work, the roles of the anterior and posterior hippocampus during the elaboration phase of retrieval for recent and remote past events was investigated using overt in-scanner recall (Gilmore et al., 2021b). These results identified temporally graded activity in the posterior hippocampus, without consistent engagement of the anterior hippocampus. However, our analyses were restricted to the elaboration phase. Thus, these data provided no insights into how the construction phase might be supported by anterior or posterior aspects of the hippocampus or how it might be impacted by event recency. However, as it has been proposed that construction and elaboration may be supported by at least partially distinct neural substrates, with construction subserved in particular by anterior hippocampal communication with medial prefrontal regions (Robin and Moscovitch, 2017), we analyzed unexamined data from a previously published experiment (Gilmore et al., 2021b) focusing on activity during the memory construction stage of each trial (Fig. 1).
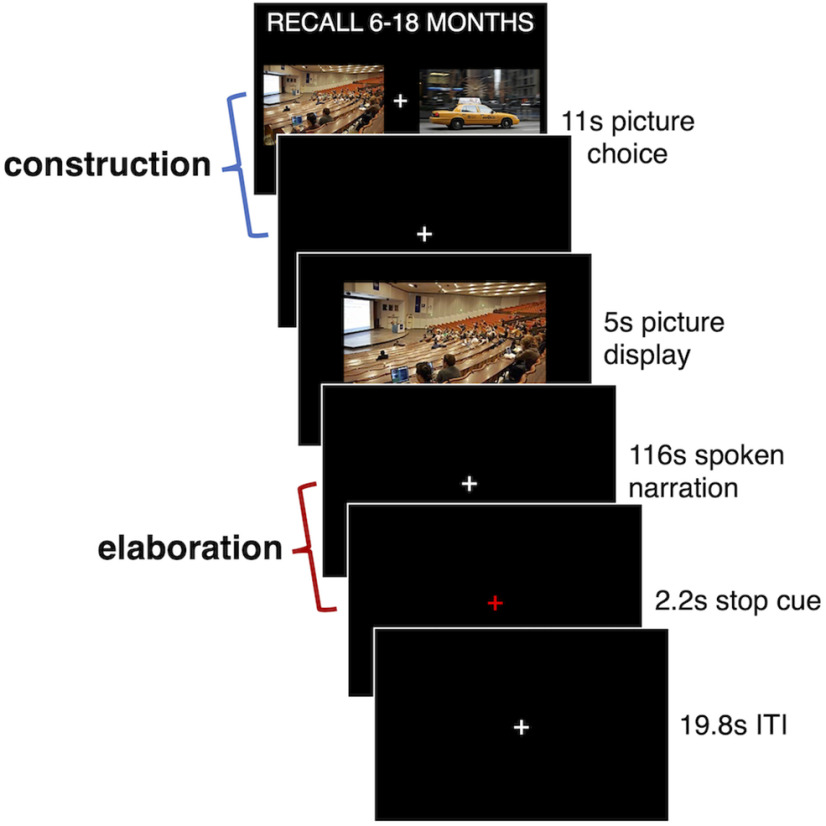
Figure 1.
Trial structure of the autobiographical retrieval task. During the construction stage, participants selected their preferred picture cue to use to retrieve autobiographical memories from earlier in the day, 6–18 months ago, or 5–10 years ago. During elaboration, participants overtly described the retrieved memory in as much detail as possible. The trial structure for the control task was the same, except participants chose a picture (construction stage) to describe in detail (elaboration stage), without retrieving a long-term memory. ITI, intertrial interval.
Materials and Methods
Participants
Data were collected from 40 right-handed young adult participants (23 female; mean age = 24.2 years). All were native English speakers with normal or corrected-to-normal vision and reported no history of psychiatric or neurologic illness. Informed consent was obtained from all participants, and the experiment was approved by the National Institutes of Health Institutional Review Board (clinical trial number NCT00001360). Participants received monetary compensation for their participation. For additional participant information, see Gilmore et al. (2021b).
Experimental design
Stimuli.
Stimuli consisted of 48 images depicting complex scenes (i.e., people engaging in various activities in a specific location). Images were sized at 525 × 395 pixels (screen resolution: 1920 × 1080 pixels) and were presented against a black background. Stimuli were presented using PsychoPy2 software (RRID:SCR_006571; Peirce, 2007) on an HP desktop computer running Windows 10.
Autobiographical memory task.
Participants retrieved and described autobiographical memories in response to picture cues (Fig. 1). For each trial, participants were first directed to recall an event corresponding to one of the following three different temporal distances: earlier that same day (Today), 6–18 months ago, or 5–10 years ago. During the initial construction phase of the task (the focus of this article), participants were given a choice of two picture cues and were given an 11 s period to select the image they would prefer to use to cue a specific memory. Responses were made via button press with a fiber-optic button box. The screen was replaced with a fixation cross once a response was made, and, after the selection period ended, an enlarged version of the selected image was presented in the center of the screen for 5 s. Participants used this period to covertly reflect on a specific event, which was then overtly described in detail throughout the elaboration phase of the task (previously analyzed: Gilmore et al., 2021b), consisting of a 116 s response period. Overt responses were recorded with an MRI-compatible microphone. A 2.2 s red fixation cross signaled the end of each trial, and trials were separated by a 19.8 s fixation period. One trial from each of the three time periods was included in each of six autobiographical memory task scan runs. No control task trials were included in autobiographical memory task runs. Before scanning, participants were familiarized with the task and practiced retrieving events specific in time and place. For additional details, see Gilmore et al. (2021b).
Control task.
An active baseline control task was created to match the response demands of the autobiographical memory task without the inclusion of a long-term memory component. Participants were asked to describe the contents of picture stimuli instead of using them to cue specific memories. Trial timings were identical, and participants were first given 11 s to select which of two picture cues they preferred (control for the construction period of the retrieval task). The screen was replaced with a fixation cross once a response was made, and, after the selection period ended, an enlarged version of the selected image was presented in the center of the screen for 5 s. Participants used this period to scrutinize the image so that it could be described aloud in detail for the next 116 s of description time (control period for the elaboration phase of the retrieval task). A 2.2 s red fixation cross signaled the end of each trial, and trials were separated by a 19.8 s fixation period. Three trials were included per Control Task run, with two such runs being collected per participant. No autobiographical memory task trials were included in control task runs.
fMRI acquisition parameters.
Data were acquired on a General Electric Discovery MR750 3.0 T scanner, using a 32-channel head coil. Functional images were acquired using a BOLD contrast-sensitive multiecho echoplanar sequence [Array Spatial Sensitivity Encoding Technique (ASSET) acceleration factor = 2; TE values = 12.5, 27.6, and 42.7 ms; TR = 2200 ms; flip angle = 75°; 64 × 64 matrix; in-plane resolution = 3.2 × 3.2 mm]. Whole-brain EPI volumes (MR frames) of 33 interleaved, 3.5-mm-thick oblique slices were obtained every 2.2 s. Slices were manually aligned to the anterior commissure–posterior commissure axis. A high-resolution T1 structural image was obtained for each subject after functional scans were collected (TE = 3.47 ms; TR = 2.53 s; TI = 900 ms; flip angle = 7°; 172 slices of 1 × 1 × 1 mm voxels). Foam pillows were provided for all participants to help stabilize head position, and scanner noise was attenuated using foam ear plugs and a noise-canceling headset. This headset was also used to communicate with the participant during their time in the scanner. Heart rate was recorded via a sensor placed on the left middle finger, and a belt monitored respiration.
Statistical analysis
fMRI processing.
BOLD time series data were processed in AFNI (RRID:SCR_005927). Steps included removal of the first four frames to remove potential T1 equilibration effects (3dTcat), despiking (3dDespike), slice-time correction (3dTshift), and framewise rigid-body realignment to the first volume of each run (3dvolreg). Following these initial steps, data from the three echoes acquired for each run were used to remove additional noise using multi-echo independent components analysis (ME-ICA; Kundu et al., 2012) as implemented in the meica.py AFNI function. This procedure initially calculates a weighted average of the different echo times (“optimally combined” data), which reduced signal dropout and thermal noise and increased contrast-to-noise in each voxel. The resulting image is also registered to the participant's anatomic image. The individual echo time series used to generate the optimally combined data are also submitted to an ICA, and signal decay patterns over time are used to classify components as artifactual in nature (e.g., reflect head motion) or as putatively neural in origin (for more, see Kundu et al., 2012, 2013). Following ME-ICA processing, data were spatially blurred with a Gaussian kernel 3 mm full-width at half-maximum, normalized by the grand mean of each run, and then resampled into 3 mm isotropic voxels and linearly transformed into Talairach atlas space.
Audio recording, behavioral scoring, and alignment of spoken responses to BOLD time series data.
Reaction times for button presses during the construction period were averaged across trials within condition for each participant (control task/today/6–18 months/5–10 year memories). The protocol for collecting, processing, and scoring spoken response data has been described in detail in prior reports (Gilmore et al., 2021a,b). Briefly, recorded audio was transcribed and scored for content using an adapted form of the Autobiographical Interview (Levine et al., 2002; Gaesser et al., 2011). This procedure separates “Internal” (putatively episodic) details specific to the event details from other types of “External” details. Subcategories of Internal details were expanded from those initially describe by Levine et al. (2002) and included the following: Activities, Objects, Perceptual, Person, Place, Thought/Emotion, Time, and Miscellaneous. External detail types included Episodic (i.e., details from other events), Repetitions, Semantic statements, and an “Other External” category. Time stamps for each spoken word and phrase were generated and matched with the text in transcripts, and different categories of recalled content were converted into event-related regressors for fMRI data analysis, as will be described below.
General linear model analysis.
Data were analyzed in AFNI using a general linear model (GLM) approach (3dDeconvolve). All task scans consisted of 210 MR frames (214 before initial frame discarding) and lasted 7 min and 51 s in duration. Average run-level motion estimates were derived using @1dDiffMag of AFNI based on three translational and three rotational motion parameters; runs with.0.2 mm/TR were excluded. Within the present data, this resulted in two autobiographical memory task runs being excluded from four participants and one autobiographical memory task run excluded from five additional participants. Data were detrended before analysis, and the analysis was conducted as a mixed block/event-related design.
The construction phase (picture selection period) was modeled for each recall condition (Today, 6–18 months ago, 5–10 years ago) and the picture description condition using separate regressors with durations of 11 s. The picture display phase for all trials was modeled using a single regressor with a duration of 5 s. Four regressors, each with a duration of 118.2 s, additionally modeled activity associated with the elaboration phase (speaking period) of each autobiographical memory condition (Today, 6–18 months ago, 5–10 years ago) and the picture description condition. Effects associated with each category of overtly described internal and external detail during the elaboration phase were modeled across both the autobiographical memory and picture description conditions (i.e., there was a single “place” regressor that accounted for all instances in which a place detail was described during the elaboration period in either of the task conditions) with the spoken duration of each detail included as a duration modulator via the AFNI dmBLOCK function. Finally, six motion parameters (three translational, three rotational) were included in each subject's GLM as regressors of noninterest.
Hippocampal region of interest analysis.
Subject-specific hippocampal masks were generated with Freesurfer (version 6.0; RRID:SCR_001847). Each mask was manually segmented into anterior and posterior long-axis subregions using the uncal apex as a landmark for separation and was resampled to the same resolution as the EPI data (Fig. 2A, example masks). Univariate activity was averaged across all voxels in each hippocampal region of interest (ROI) in each subject's native space for each construction period (picture selection period for Today, 6–18 months, and 5–10 year conditions) relative to the equivalent 11 s picture selection period of the Picture Description control task. A repeated-measures ANOVA was conducted on the percent signal change relative to the control task, with factors of hemisphere (left, right), long-axis location (anterior, posterior), and temporal distance (Today, 6–18 months ago, 5–10 years ago) as predictors. Reported effect sizes reflect generalized η2 values (Bakeman, 2005). One-sample t tests were subsequently conducted to compare the significance of each response versus the Picture Description control task, with both Bonferroni's and false discovery rate (FDR; Benjamini and Yekutieli, 2001) corrections being reported.
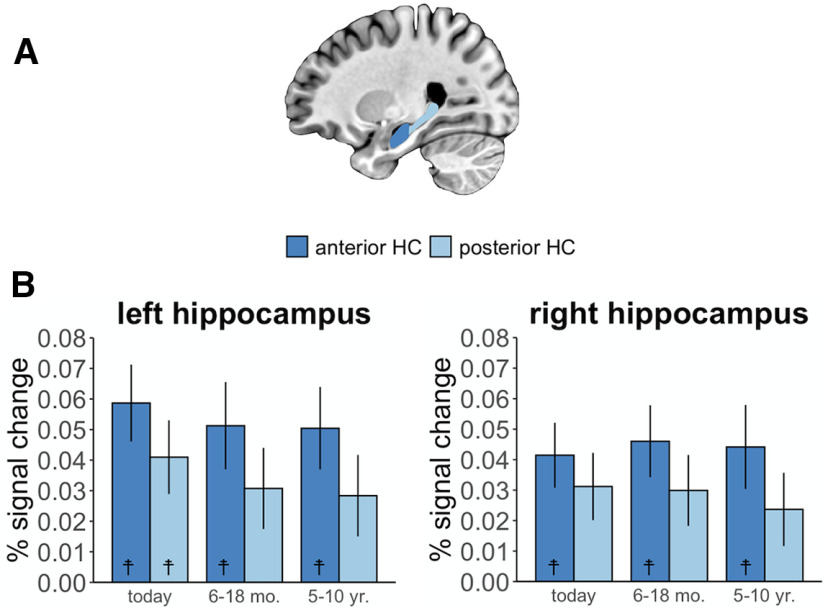
Figure 2.
Hippocampal activation during the autobiographical construction phase over time. A, Anterior and posterior hippocampal ROIs, manually segmented for each participant. B, Percent signal change in the anterior and posterior hippocampi during construction of autobiographical memories of varying remoteness relative to the same period of the control picture description task. Error bars reflect the SEM. ☨ p < 0.05, construction phase > baseline control task after Bonferroni's correction for multiple comparisons. HC, Hippocampus.
Whole-brain contrast of construction and control activity.
Activity associated with the construction stage was contrasted for autobiographical memory and control task conditions using a paired-samples, two-tailed t test at the whole-brain level. Activity was averaged across all three recall periods for autobiographical memory trials. Statistical images achieved a whole-brain p value of <0.05 by requiring a voxel-wise threshold of p < 0.001 and a minimum cluster extent (k) of 15 contiguous voxels as determined using AFNI 3dClustSim and its non-Gaussian (-acf) autocorrelation function (Cox et al., 2017). This procedure identified one large cluster spanning multiple lobes as well as the cerebellum. For reporting purposes, discrete regions within this cluster were identified by incrementing the threshold to t = 4.55 (p < 5.1 × 10−5). Contrasts of activity during the construction of memories versus the control task were also conducted separately for each temporal distance using the same statistical thresholds to verify that the results were not driven by a single delay. To explore whole-brain effects of temporal distance on activation during construction, an exploratory whole-brain voxel-wise ANOVA was conducted. Statistical images achieved a whole-brain p value of <0.05 by requiring a voxel-wise threshold of p < 0.001 and k ≥ 17 contiguous voxels (Cox et al., 2017). To explore resulting significant effects, post hoc pairwise t tests were conducted between temporal distances (voxel-wise threshold of p < 0.001, k ≥ 15 voxels; see above), which were then masked to restrict significant results to voxels of interest identified by the ANOVA.
Data availability
Data analyzed in this report are freely available for download on https://openneuro.org/ (Gilmore et al., 2021c).
Results
Reaction times differed between the memory and the control tasks
Reaction times during the picture selection/construction stage were compared across all conditions using a one-way repeated-measures ANOVA (control task: mean = 3.13 s, SD = 0.91 s; Today construction: mean = 4.64 s, SD = 1.64 s; 6–18 months construction: mean = 4.91 s, SD = 1.86 s; 5–10 years construction: mean = 4.71 s, SD = 1.59 s). The model revealed significant differences in mean reaction time according to condition (F(2.19,85.36) = 38.99, p < 0.0001, η2G = 0.177, ε = 0.73). Pairwise t tests indicated the effect was driven by slower reaction times during the memory construction period relative to the control construction period (Today vs Control: t(39) = 7.46, p < 0.0001; 6–18 months vs control: t(39) = 7.33, p < 0.0001; 5–10 years vs control: t(39) = 7.63, p < 0.0001). There was no difference in reaction time between memory conditions (today vs 6–18 months: t(39) = 1.77, p = 0.085; Today versus 5–10 years: t(39) = 0.51, p = 0.61; 6–18 months vs 5–10 years: t(39) = 1.40, p = 0.12). These results support the engagement of distinct mental processes during memory construction and the control task.
No temporal gradient observed in the anterior hippocampus
We next examined activation along the hippocampal long axis during construction over time. A repeated-measures ANOVA predicting percent signal change (relative to the control task) as a function of long-axis, hemisphere, and temporal distance revealed a significant main effect of long-axis (F(1,39) = 7.76, p = 0.008, η2G = 0.013), which was driven by greater activation in the anterior than posterior hippocampus (Fig. 2B). There was no significant effect of temporal distance (F(2,78) = 0.14, p = 0.87, η2G = 0.001) and no interaction between long-axis and temporal distance (F(2,78) = 0.70, p = 0.50, η2G = 0.0004). There was also no main effect of hemisphere (F(1,39) = 1.64, p = 0.21, η2G = 0.002) or interaction between hemisphere and the other variables in the model (all p > 0.097). Thus, during the memory construction stage, the anterior hippocampus was more active than the posterior portion, and there was no evidence of a temporal gradient over time.
Construction-related hippocampal activity was then compared with the baseline task using one-sample t tests to identify which conditions, if any, resulted in significant hippocampal activation. Following Bonferroni correction for multiple comparisons (requiring p < 0.004), the anterior hippocampus was significantly activated for all temporal distances, in both the right hemisphere (Today: t(39) = 3.88, p = 0.0004; 6–18 months: t(39) = 3.89, p = 0.0004; 5–10 years: t(39) = 3.21, p = 0.0027) and the left hemisphere (Today: t(39) = 4.67, p = 0.00004; 6–18 months: t(39) = 3.59, p = 0.0009; 5–10 years: t(39) = 3.73, p = 0.0006). The only significant posterior hippocampal effect was in the left hemisphere for the Today condition (t(39) = 3.39, p = 0.0016; other p values > 0.008). Applying a more liberal FDR correction for multiple comparisons (Benjamini and Yekutieli, 2001) only resulted in a single change, with right posterior hippocampal activity for Today now also significant. Thus, during memory construction, we observed robust activation in the anterior hippocampus regardless of memory remoteness.
Construction activates cortical regions associated with autobiographical retrieval regardless of temporal distance
Unlike the later elaboration phase, no overt behavior outside of a button press was available during the construction phase. Therefore, to contextualize the hippocampal findings, a whole-brain voxel-wise contrast was conducted to compare neural activity during the construction phase of autobiographical retrieval with that of the matched control task, averaging across temporal distances (requiring voxel-wise: p < 0.001, k ≥ 15; to achieve a whole-brain p < 0.05). In addition to observing significant activity in anterior hippocampal regions (Fig. 3B), we observed strong activation in regions typically associated with autobiographical recall, including medial prefrontal, posterior cingulate, lateral temporal, and parahippocampal cortices as well as the angular gyrus (Fig. 3A; Table 1; Svoboda et al., 2006; Spreng et al., 2009), collectively supporting a retrieval-based interpretation of the present hippocampal findings. Contrasts of neural activation during memory construction compared with the control task at each temporal distance indicated that the identified autobiographical regions were active regardless of temporal distance (Fig. 3C).
Figure 3.
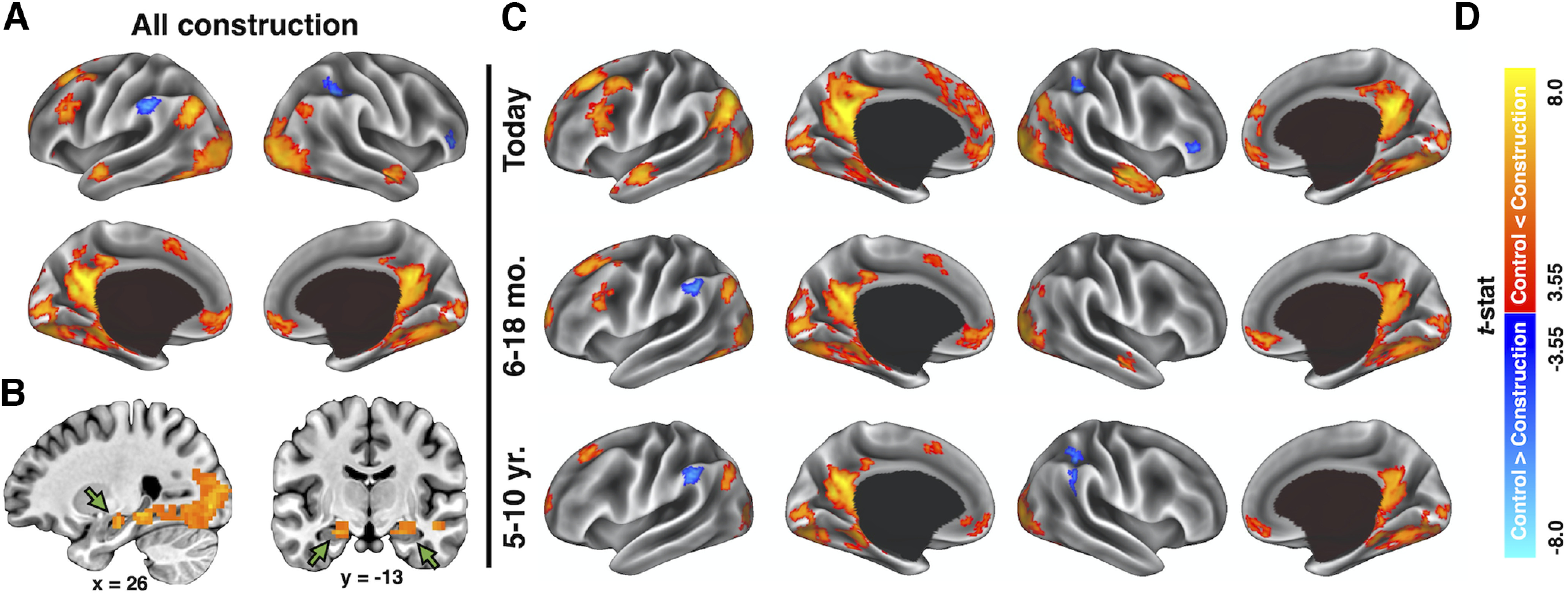
Whole-brain univariate analysis of the memory construction phase relative to the control task. A, Surface reconstruction of cortical regions with significant activation during memory construction relative to the same period of the control task, averaged across temporal distances. B, Sagittal and coronal slices of the same contrast displaying activations identified in the anterior hippocampus, as pointed to by the green arrows. C, Surface reconstruction of cortical regions with significant activation during memory construction relative to the same period of the control task at each temporal distance. D, Color bar of statistical maps for A–C. Data in A and C were surface projected using Connectome Workbench software (Marcus et al., 2011).
Table 1.
Peak regions identified in the voxel-wise analysis of construction phase > control task activity
| Region | x | y | z | Cluster size | Peak t |
|---|---|---|---|---|---|
| Construction phase > control task | |||||
| Posterior parietal-occipito-temporal-cerebellar cortex | 2 | −65 | −1 | 2806 | 10.02 |
| Bilateral posterior cingulate/ventral medial parietal cortex | −1 | −54 | 15 | 456 | 10.02 |
| Right occipital/fusiform cortex | 34 | −81 | −4 | 311 | 7.09 |
| Left visual cortex | −24 | −87 | −7 | 264 | 6.95 |
| Left parahippocampal cortex | −24 | −40 | −17 | 56 | 6.55 |
| Right parahippocampal cortex | 28 | −38 | −17 | 38 | 6.91 |
| Right cerebellum (crus 1) | 8 | −79 | −21 | 24 | 5.49 |
| Left anterior hippocampus | −20 | −17 | −18 | 19 | 5.91 |
| Left posterior fusiform | −40 | −69 | −18 | 17 | 5.04 |
| Left middle frontal gyrus/superior frontal sulcus | −24 | 24 | 51 | 240 | 6.31 |
| Bilateral medial prefrontal cortex | −1 | 56 | −13 | 199 | 7.30 |
| Left angular gyrus | −43 | −72 | 34 | 173 | 6.93 |
| Left anterior frontal cortex | −17 | 63 | 10 | 109 | 6.65 |
| Right cerebellum (lobule IX) | 8 | −52 | −39 | 78 | 6.08 |
| Right middle temporal gyrus | 59 | −6 | −19 | 78 | 6.07 |
| Left middle temporal gyrus | −56 | −3 | −16 | 60 | 6.15 |
| Left inferior frontal sulcus | −43 | 19 | 27 | 59 | 5.19 |
| Left supplementary motor area | −1 | 18 | 48 | 44 | 5.03 |
| Right angular gyrus | 43 | −69 | 33 | 38 | 5.33 |
| Right anterior hippocampus | 24 | −18 | −21 | 26 | 5.72 |
| Brainstem | −1 | −28 | −17 | 24 | 5.62 |
| Left inferior frontal gyrus (pars orbitalis) | −36 | 38 | −12 | 24 | 5.46 |
| Left superior occipital gyrus/cuneus | −14 | −95 | 33 | 15 | 4.18 |
| Control task > construction phase | |||||
| Right anterior intraparietal sulcus | 43 | −43 | 45 | 52 | −5.07 |
| Left supramarginal gyrus | −59 | −40 | 42 | 30 | −5.51 |
| Right inferior frontal gyrus (pars triangularis) | 50 | 42 | 2 | 15 | −5.39 |
Coordinates refer to centers of mass in MNI space. Primary clusters were identified at t > 3.55; discrete peaks identified in the posterior parietal-occipito-temporal-cerebellar cluster were separated at a t > 4.55.
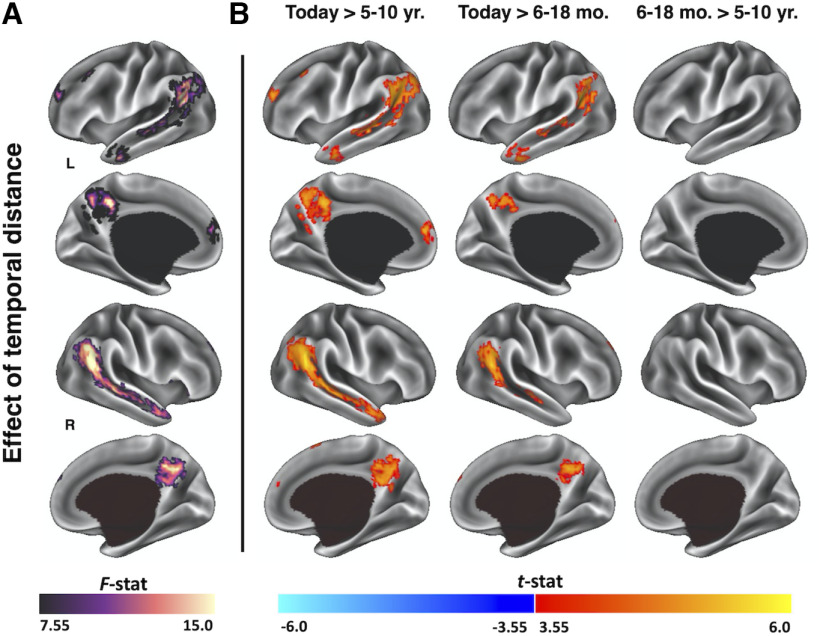
Next, we directly tested the effect of temporal distance on activation across the whole brain during memory construction using a one-way ANOVA (voxel-wise: p < 0.001, k ≥ 17; whole-brain: p < 0.05) and found differential activation in bilateral superior temporal sulcus, anterior middle temporal gyri, inferior parietal lobules, posterior cingulate, and dorsal medial prefrontal cortex as a function of temporal distance (Fig. 4A; Table 2). Post hoc pairwise t tests indicated that activation in these regions decreased between construction of Today memories and memories from 6–18 months ago, with no change in activation thereafter (Fig. 4B).
Figure 4.
Whole-brain univariate analysis of temporal distance during memory construction. A, Surface reconstruction of cortical regions with significantly different activation as a function of temporal distance. B, Surface reconstruction of significant cortical activation differences between pairwise temporal distances. Data were surface projected using Connectome Workbench (software Marcus et al., 2011).
Table 2.
Peak regions identified in the voxel-wise temporal distance ANOVA
| Region | x | y | z | Cluster size | Peak F |
|---|---|---|---|---|---|
| Left inferior parietal lobule/superior temporal sulcus | −49 | −85 | −66 | 521 | 16.13 |
| Bilateral posterior cingulate cortex/precuneus/retrosplenial cortex | −1 | −56 | 36 | 516 | 15.01 |
| Right inferior parietal lobule/superior temporal sulcus | 53 | −51 | 15 | 512 | 11.69 |
| Left dorsomedial prefrontal cortex | −1 | 58 | 14 | 61 | 8.41 |
| Left anterior middle temporal gyrus | −56 | 3 | −30 | 55 | 7.79 |
| Right cerebellum (lobule VI; crus 1) | 34 | −83 | −34 | 50 | 11.63 |
| Right anterior superior temporal sulcus/middle temporal gyrus | 53 | 10 | −27 | 47 | 11.30 |
| Left anterior superior frontal gyrus/sulcus | −20 | 52 | 22 | 41 | 10.63 |
| Left middle frontal gyrus | −33 | 30 | 47 | 34 | 10.80 |
| Right anterior inferior frontal sulcus | 15 | 58 | 28 | 21 | 8.95 |
| Right inferior frontal gyrus (pars triangularis) | 53 | 32 | −8 | 19 | 7.64 |
| Left cerebellum (lobule VI) | −24 | −80 | −34 | 18 | 12.06 |
Coordinates refer to centers of mass in MNI space. Clusters were identified at F > 7.55.
Discussion
This work aimed to understand hippocampal contributions to autobiographical memory retrieval during an initial construction stage by taking both temporal distance and long-axis location into account. We found that the anterior hippocampus is active during the construction period of autobiographical memory retrieval, regardless of memory age. Notably, neither the standard model of consolidation nor trace transformation theory emphasizes the component processes of autobiographical retrieval (i.e., construction vs elaboration) as determining features of hippocampal involvement in retrieving consolidated memories, although the current results suggest that these, along with long-axis placement, are critical aspects in deriving an answer.
A key question that arises from, but is not specific to, the current results, is how one can unpack what is occurring during the construction stage. Presumably, participants in this study used the picture selection period to extract meaning from the picture cue, and then search for and retrieve a memory for a related event that they could proceed to elaborate on, but we did not ask participants what they were doing during the construction period. While prior work with these data focused on elaboration specifically because overt speech could be leveraged to understand dynamic processes related to retrieval (Gilmore et al., 2021a,b), overt speech does not provide a similar advantage for construction given what is thought to occur during this stage of processing. Indeed, it is unclear how to probe the subjective experience of construction at the trial level without changing participant behavior. However, in addition to slower reaction times during construction than the control picture description task, our whole-brain univariate analysis of recall during the construction stage relative to the control task revealed canonical autobiographical memory regions, supporting the interpretation that the picture cues in the construction period elicited autobiographical retrieval in preparation for elaboration. As scenes were viewed and meaning was extracted in both the construction and control task conditions, differential activation is unlikely to reflect processing of scenic or semantic content.
An alternative explanation of activity in the construction stage might be that, rather than a construction-like process that serves to scaffold subsequently remembered details, as predicted by Conway (2005), the activity instead represents direct retrieval of a memory as a function of cue/trace overlap, in line with the encoding specificity principle (Tulving and Thomson, 1973). This idea would suggest that “search” may not be the correct metaphor for the processes being captured, and instead the activity may reflect the conversion of an external stimulus into an internal memory cue, or perhaps the process of ecphory itself (Tulving, 1982, 1983). However, it remains unclear why the hippocampus would act differently during this period than during the later elaboration stage as the retrieval process continues.
We do not yet understand the mechanisms, common or distinct, that support retrieval as it unfolds across multiple trial stages. In general terms, it is possible that during memory construction the anterior hippocampus retrieves the central aspects of memory, which is used as a scaffold for details retrieved during elaboration. This idea fits with the Self-Memory System of Conway (2005) and its proposal that autobiographical memory is organized hierarchically, such that event-specific details are contextualized by general event structures (Conway and Pleydell-Pearce, 2000; Conway, 2009; Sheldon et al., 2019). There is evidence that when a specific autobiographical memory is elicited with a thematic cue (e.g., “basketball game”), the first thoughts tend to be those of general autobiographical knowledge pertaining to the event followed by event-specific details (Haque and Conway, 2001). This observation suggests that general aspects of autobiographical events (perhaps retrieved by the anterior hippocampus in concert with frontal regions) may be used to access more specific ones (perhaps retrieved by the posterior hippocampus and posterior cortical regions) during intentional retrieval (Conway and Pleydell-Pearce, 2000; Conway, 2009). The question of whether this process must proceed from general to specific in the hippocampus, or rather if it can occur in the opposite direction (Maurer and Nadel, 2021) or in parallel (Gilboa and Moscovitch, 2021), remains to be fully addressed. The way in which retrieval is cued is likely important (Robin and Moscovitch, 2017). For example, there is evidence that orienting participants to conceptual or contextual information during retrieval recruits anterior and posterior memory systems respectively, providing some support for parallel processing depending on memory content (Gurguryan and Sheldon, 2019; Sheldon et al., 2019).
Reconciling the current observation of significant anterior hippocampal activity for both recent and remote events during construction, with prior observations of temporally graded posterior hippocampal activity during elaboration/overt recall, is important. Especially so, when one considers the types of information that the anterior and posterior hippocampi are proposed to represent, namely, gist and detail, respectively (Poppenk et al., 2013; Sekeres et al., 2018; Audrain and McAndrews, 2020). As described in our previous work, even remote memories were retrieved with rich detail in our sample (Gilmore et al., 2021b). One possibility is that the anterior hippocampus can access such details as memories are consolidated. For example, memories are putatively represented in a distributed fashion in the neocortex, and the hippocampus is initially required to index disparate elements of a memory (Teyler and Rudy, 2007). As the neocortical memory trace is strengthened, details may become neocortically linked with central aspects of a memory such that retrieval or cueing of central elements by the anterior hippocampus can reactivate associated details without a posterior hippocampal index.
Another possibility is that the anterior hippocampus can represent finer grained detail than is typically argued. Perhaps what is retrieved by the anterior hippocampus during the construction period is not a scaffold devoid of specific detail but rather is the representative details of the memory such as who, what, where, and when. Indeed, the anterior hippocampus plays an active role in constructing scenes (Zeidman and Maguire, 2016), which some might consider more “detailed” than “gist-like,” and it has also been associated with the recombination of specific event details in the service of simulating future events (Addis et al., 2011; Addis and Schacter, 2012). The posterior hippocampus may be additionally important for details retrieved when one “replays” memory or projects oneself through memory in a more continuous “frame-by-frame” spatiotemporal fashion, which is a hallmark of mental time travel (Tulving, 1985; Suddendorf and Corballis, 2007), and which would necessitate fine-grained shifts in representational content (Takehara-Nishiuchi, 2020). In this vein, emphasizing the retrieval of perceptual or peripheral detail as a uniquely posterior hippocampal function may be incorrect and could account for discrepancies reported in the literature ascribing detail representation to the anterior hippocampus (Zeidman and Maguire, 2016; Tompary and Davachi, 2017; Dandolo and Schwabe, 2018; Cowan et al., 2021). It follows that defining what constitutes gist and detail is critical for precisely delineating how anterior and posterior hippocampal segments differently support memory along these dimensions. Indeed, while subjective vividness has historically been used as an index of detail retrieved in covert memory paradigms, recent evidence suggests that vividness may track more closely with the gist of an event (defined as memory for names of people, objects, and places comprising the event) than nuanced perceptual detail (Cooper and Ritchey, 2022), highlighting that a consensus regarding this terminology is paramount.
Finally, a whole-brain analysis, focusing on effects of temporal distance during construction, identified a distributed collection of regions that exhibited greater activity during the Today period than either of the other time periods, and appeared more like a “step” function than a smooth temporal gradient (Fig. 4; Table 2). This result hints at differences in information activation and reactivation early in the construction of “fresh” events compared with those that have undergone a period of consolidation, and pursuing this observation may improve our understanding of consolidation and the roles of the anterior hippocampus, as described above.
To conclude, findings from this dataset—both the current results and those reported previously—can be taken to support either of two dominant views of hippocampal contributions to remote retrieval depending on analysis choices (task- and neuroanatomy-related components). Given the decades-long debate centering on the role of the hippocampus in recent and remote memories, perhaps this is appropriate. Moving forward, it will be critical to articulate the subprocesses involved in construction, as well as to come to a consensus regarding what constitutes gist and detail. Precise delineation of these concepts will inform testable predictions regarding the role of the anterior hippocampus in construction over time. The data presented here highlight that the stage of retrieval and anatomic location along the long axis of the hippocampus analyzed are critical considerations not fully integrated into current models. Our understanding of anterior and posterior hippocampal contributions to retrieval of autobiographical memory over time can only be enhanced by understanding the component processes inherent to such retrieval.
Footnotes
This work was supported by the National Institute of Mental Health (NIMH) Intramural Research Program (ZIA MH-002920) and by NIMH Grant R01-MH-060941 (to D.L.S.). We thank Sarah Kalinowski, Alina Quach, and Bess Bloomer for assistance with the collection and preparation of the data.
The authors declare no competing financial interests.
References
- Addis DR, Schacter DL (2008) Constructive episodic simulation: temporal distance and detail of past and future events modulate hippocampal engagement. Hippocampus 18:227–237. 10.1002/hipo.20405 [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL (2012) The hippocampus and imagining the future: where do we stand? Front Hum Neurosci 5:173. 10.3389/fnhum.2011.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL (2007) Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45:1363–1377. 10.1016/j.neuropsychologia.2006.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Cheng T, Roberts RP, Schacter DL (2011) Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus 21:1045–1052. 10.1002/hipo.20870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain S, McAndrews MP (2020) Schemas provide a scaffold for neocortical integration at the cost of memory specificity over time. bioRxiv 335166. doi: 10.1101/2020.10.11.335166 10.1101/2020.10.11.335166. [DOI] [Google Scholar]
- Bakeman R (2005) Recommended effect size statistic. Behav Res Methods 37:379–384. [DOI] [PubMed] [Google Scholar]
- Barnett AJ, Man V, McAndrews MP (2019) Parcellation of the hippocampus using resting functional connectivity in temporal lobe epilepsy. Front Neurol 10:920. 10.3389/fneur.2019.00920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188. [Google Scholar]
- Brunec IK, Bellana B, Ozubko JD, Man V, Robin J, Liu Z-X, Grady C, Rosenbaum RS, Winocur G, Barense MD, Moscovitch M (2018) Multiple scales of representation along the hippocampal anteroposterior axis in humans. Curr Biol 28:2129–2135.e6. 10.1016/j.cub.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Conway MA (2005) Memory and the self. J Mem Lang 53:594–628. 10.1016/j.jml.2005.08.005 [DOI] [Google Scholar]
- Conway MA (2009) Episodic memories. Neuropsychologia 47:2305–2313. 10.1016/j.neuropsychologia.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW (2000) The construction of autobiographical memories in the self-memory system. Psychol Rev 107:261–288. 10.1037/0033-295x.107.2.261 [DOI] [PubMed] [Google Scholar]
- Cooper RA, Ritchey M (2022) Patterns of episodic content and specificity predicting subjective memory vividness. Mem Cogn. Advance online publication. Retrieved Mar 4, 2022. doi: 10.3758/s13421-022-01291-5. 10.3758/s13421-022-01291-5 [DOI] [PubMed] [Google Scholar]
- Cowan ET, Liu AA, Henin S, Kothare S, Devinsky O, Davachi L (2021) Time-dependent transformations of memory representations differ along the long axis of the hippocampus. Learn Mem 28:329–340. 10.1101/lm.053438.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017) FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect 7:152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandolo LC, Schwabe L (2018) Time-dependent memory transformation along the hippocampal anterior-posterior axis. Nat Commun 9:1205. 10.1038/s41467-018-03661-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser B, Sacchetti DC, Addis DR, Schacter DL (2011) Characterizing age-related changes in remembering the past and imagining the future. Psychol Aging 26:80–84. 10.1037/a0021054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Moscovitch M (2021) No consolidation without representation: correspondence between neural and psychological representations in recent and remote memory. Neuron 109:2239–2255. 10.1016/j.neuron.2021.04.025 [DOI] [PubMed] [Google Scholar]
- Gilmore AW, Quach A, Kalinowski SE, Gotts SJ, Schacter DL, Martin A (2021a) Dynamic content reactivation supports naturalistic autobiographical recall in humans. J Neurosci 41:153–166. 10.1523/JNEUROSCI.1490-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AW, Quach A, Kalinowski S, González-Araya E, Gotts S, Schacter D, Martin A (2021b) Evidence supporting a time-limited hippocampal role in retrieving autobiographical memories. Proc Natl Acad Sci U|S|A 118:e2023069118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A, Quach A, Kalinowski S, González-Araya E, Gotts S, Schacter D, Martin A (2021c) Evidence supporting a time-limited hippocampal role in retrieving autobiographical memories. OpenNeuro. doi: 10.18112/openneuro.ds003511.v1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AW, Audrain S, Martin A (2022) Specifying “where” and “what” is critical for testing hippocampal contributions to memory retrieval. Cogn Neurosci 13:1–3. 10.1080/17588928.2022.2076071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurguryan L, Sheldon S (2019) Retrieval orientation alters neural activity during autobiographical memory recollection. Neuroimage 199:534–544. 10.1016/j.neuroimage.2019.05.077 [DOI] [PubMed] [Google Scholar]
- Haque S, Conway MA (2001) Sampling the process of autobiographical memory construction. Eur J Cogn Psychol 13:529–547. 10.1080/09541440125757 [DOI] [Google Scholar]
- Kundu P, Inati SJ, Evans JW, Luh W-M, Bandettini PA (2012) Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 60:1759–1770. 10.1016/j.neuroimage.2011.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Brenowitz ND, Voon V, Worbe Y, Vértes PE, Inati SJ, Saad ZS, Bandettini PA, Bullmore ET (2013) Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc Natl Acad Sci U|S|A 110:16187–16192. 10.1073/pnas.1301725110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M (2002) Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging 17:677–689. 10.1037/0882-7974.17.4.677 [DOI] [PubMed] [Google Scholar]
- Mahr JB, Csibra G (2018) Why do we remember? The communicative function of episodic memory. Behav Brain Sci 41:1E. 10.1017/S0140525X17000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Harwell J, Olsen T, Hodge M, Glasser MF, Prior F, Jenkinson M, Laumann T, Curtiss SW, Van Essen DC (2011) Informatics and data mining tools and strategies for the human connectome project. Front Neuroinform 5:4. 10.3389/fninf.2011.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer AP, Nadel L (2021) The continuity of context: a role for the hippocampus. Trends Cogn Sci 25:187–199. 10.1016/j.tics.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, St-Laurent M, Ty A, Valiante TA, McAndrews MP (2015) Functional and effective hippocampal-neocortical connectivity during construction and elaboration of autobiographical memory retrieval. Cereb Cortex 25:1297–1305. 10.1093/cercor/bht324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Moscovitch M, Valiante TA, Cohn M, McAndrews MP (2018) Different neural routes to autobiographical memory recall in healthy people and individuals with left medial temporal lobe epilepsy. Neuropsychologia 110:26–36. 10.1016/j.neuropsychologia.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M (1997) Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7:217–227. 10.1016/s0959-4388(97)80010-4 [DOI] [PubMed] [Google Scholar]
- Peirce JW (2007) PsychoPy—psychophysics software in Python. J Neurosci Methods 162:8–13. 10.1016/j.jneumeth.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persichetti AS, Denning JM, Gotts SJ, Martin A (2021) A data-driven functional mapping of the anterior temporal lobes. J Neurosci 41:6038–6049. 10.1523/JNEUROSCI.0456-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L (2013) Long-axis specialization of the human hippocampus. Trends Cogn Sci 17:230–240. 10.1016/j.tics.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Robin J, Moscovitch M (2017) Details, gist and schema: hippocampal–neocortical interactions underlying recent and remote episodic and spatial memory. Curr Opin Behav Sci 17:114–123. 10.1016/j.cobeha.2017.07.016 [DOI] [Google Scholar]
- Schacter DL, Addis DR (2007) The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci 362:773–786. 10.1098/rstb.2007.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Winocur G, Moscovitch M (2018) The hippocampus and related neocortical structures in memory transformation. Neurosci Lett 680:39–53. 10.1016/j.neulet.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Sheldon S, Fenerci C, Gurguryan L (2019) A neurocognitive perspective on the forms and functions of autobiographical memory retrieval. Front Syst Neurosci 13:4. 10.3389/fnsys.2019.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN (2009) The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci 21:489–510. 10.1162/jocn.2008.21029 [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253:1380–1386. 10.1126/science.1896849 [DOI] [PubMed] [Google Scholar]
- Squire LR, Genzel L, Wixted JT, Morris RG (2015) Memory consolidation. Cold Spring Harb Perspect Biol 7:a021766. 10.1101/cshperspect.a021766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC (2007) The evolution of foresight: what is mental time travel, and is it unique to humans? Behav Brain Sci 30:299–351. 10.1017/S0140525X07001975 [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B (2006) The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 44:2189–2208. 10.1016/j.neuropsychologia.2006.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K (2020) Prefrontal–hippocampal interaction during the encoding of new memories. Brain Neurosci Adv 4:2398212820925580. 10.1177/2398212820925580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, Rudy JW (2007) The hippocampal indexing theory and episodic memory: updating the index. Hippocampus 17:1158–1169. 10.1002/hipo.20350 [DOI] [PubMed] [Google Scholar]
- Tompary A, Davachi L (2017) Consolidation promotes the emergence of representational overlap in the hippocampus and medial prefrontal cortex. Neuron 96:228–241.e5. 10.1016/j.neuron.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (1982) Synergistic ecphory in recall and recognition. Can J Psychol 36:130–147. 10.1037/h0080641 [DOI] [Google Scholar]
- Tulving E (1983) Elements of episodic memory. Oxford: Oxford UP. [Google Scholar]
- Tulving E (1985) How many memory systems are there? Am Psychol 40:385–398. 10.1037/0003-066X.40.4.385 [DOI] [Google Scholar]
- Tulving E, Thomson DM (1973) Encoding specificity and retrieval processes in episodic memory. Psychol Rev 80:352–373. 10.1037/h0020071 [DOI] [Google Scholar]
- Zeidman P, Maguire EA (2016) Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci 17:173–182. 10.1038/nrn.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng A (2021) Parallel hippocampal-parietal circuits for self- and goal-oriented processing. Proc Natl Acad Sci U|S|A 118:e2101743118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed in this report are freely available for download on https://openneuro.org/ (Gilmore et al., 2021c).