Abstract
Objective
This sequential, prospective meta-analysis sought to identify risk factors among pregnant and postpartum women with COVID-19 for adverse outcomes related to disease severity, maternal morbidities, neonatal mortality and morbidity, and adverse birth outcomes.
Data Sources
We prospectively invited study investigators to join the sequential, prospective meta-analysis via professional research networks beginning in March 2020.
Study Eligibility Criteria
Eligible studies included those recruiting at least 25 consecutive cases of COVID-19 in pregnancy within a defined catchment area.
Methods
We included individual patient data from 21 participating studies. Data quality was assessed, and harmonized variables for risk factors and outcomes were constructed. Duplicate cases were removed. Pooled estimates for the absolute and relative risk of adverse outcomes comparing those with and without each risk factor were generated using a 2-stage meta-analysis.
Results
We collected data from 33 countries and territories, including 21,977 cases of SARS-CoV-2 infection in pregnancy or postpartum. We found that women with comorbidities (preexisting diabetes mellitus, hypertension, cardiovascular disease) vs those without were at higher risk for COVID-19 severity and adverse pregnancy outcomes (fetal death, preterm birth, low birthweight). Participants with COVID-19 and HIV were 1.74 times (95% confidence interval, 1.12–2.71) more likely to be admitted to the intensive care unit. Pregnant women who were underweight before pregnancy were at higher risk of intensive care unit admission (relative risk, 5.53; 95% confidence interval, 2.27–13.44), ventilation (relative risk, 9.36; 95% confidence interval, 3.87–22.63), and pregnancy-related death (relative risk, 14.10; 95% confidence interval, 2.83–70.36). Prepregnancy obesity was also a risk factor for severe COVID-19 outcomes including intensive care unit admission (relative risk, 1.81; 95% confidence interval, 1.26–2.60), ventilation (relative risk, 2.05; 95% confidence interval, 1.20–3.51), any critical care (relative risk, 1.89; 95% confidence interval, 1.28–2.77), and pneumonia (relative risk, 1.66; 95% confidence interval, 1.18–2.33). Anemic pregnant women with COVID-19 also had increased risk of intensive care unit admission (relative risk, 1.63; 95% confidence interval, 1.25–2.11) and death (relative risk, 2.36; 95% confidence interval, 1.15–4.81).
Conclusion
We found that pregnant women with comorbidities including diabetes mellitus, hypertension, and cardiovascular disease were at increased risk for severe COVID-19–related outcomes, maternal morbidities, and adverse birth outcomes. We also identified several less commonly known risk factors, including HIV infection, prepregnancy underweight, and anemia. Although pregnant women are already considered a high-risk population, special priority for prevention and treatment should be given to pregnant women with these additional risk factors.
Key words: COVID-2019, maternal mortality, neonatal mortality, pneumonia, pregnancy, preterm birth, SARS-CoV-2, small-for-gestational-age
AJOG at a Glance.
Why was this study conducted?
Pregnant women are at risk for severe SARS-CoV-2 complications, and those with comorbidities might be at even higher risk for adverse outcomes. Furthermore, some vaccines and treatments are only recommended for those at highest risk. There is no global consensus about what risk factors signify such risk. Heterogeneity in the design and analysis of published studies and limited global data further complicate definitive guidance.
Key findings
We pooled individual patient data from 21 studies (33 countries, 21,977 pregnancies) and found that comorbidities, nutritional status, and older maternal age were associated with severe COVID-19–related outcomes (intensive care unit admission, ventilation, mortality), adverse pregnancy outcomes, and fetal/neonatal morbidity and mortality.
What does this add to what is known?
We pooled and reanalyzed data from global collaborators. We assessed high-priority risk factors and 24 consistently defined maternal and newborn outcomes. Given the large sample, including data from low- and middle-income countries, we generated estimates on rare outcomes (maternal mortality, stillbirth) and risk factors (anemia, underweight, HIV) where data have been lacking.
Introduction
Since the onset of the novel COVID-19 pandemic, the World Health Organization (WHO) and the Centers for Disease Control and Prevention classified pregnant women as a group at higher risk of severe complications from SARS-CoV-2 infection compared with nonpregnant people.1,2 Despite known risk, pregnant women have been widely excluded from pharmaceutical clinical trials, resulting in an underdocumentation of the physiology, case count, complications, and consequences of COVID-19 in pregnancy.
Initial evidence showed that SARS-CoV-2 infection during pregnancy is linked to increased likelihood of adverse maternal, fetal, and neonatal outcomes.3, 4, 5 A systematic review of 42 studies (N=438,548) found that pregnant women with SARS-CoV-2 infection had significantly higher odds of preeclampsia, preterm birth, stillbirth, and intensive care unit (ICU) admission compared with those not infected.5 Although vertical transmission of COVID-19 from mother to fetus reportedly occurs in a low percentage of cases, neonates can be negatively affected by maternal infection in other ways.6,7 In 2 systematic reviews of 42 and 66 studies, neonates of mothers with confirmed COVID-19 had 3 times higher odds of neonatal ICU (NICU) admission than those born to uninfected mothers.5,6
Among pregnant women, multiple risk factors for severe SARS-CoV-2 infection have been identified.3,8 The Surveillance for Emerging Threats to Mothers and Babies Network in the United States (N=7950) determined that pregnant women aged >25 years with prepregnancy obesity, chronic lung disease, chronic hypertension, and pregestational diabetes mellitus had a 32% to 85% increased risk of moderate-to-severe COVID-19 compared with pregnant women without these conditions.9 Pregnant women with ≥3 underlying health conditions had over twice the risk of moderate-to-severe COVID-19 illness compared with those with no comorbidities.9
In the general population, nutritional status has been introduced as a potential risk factor for severe COVID-19. A meta-analysis of 7 studies (N=9912) found that among people with COVID-19, those with anemia had 2.44 higher odds of severe illness compared with nonanemic people.10 A scientific review found that sufficient intake of micronutrients, proteins, dietary fiber, short-chain fatty acids, and omega-3 polyunsaturated fatty acids may act as a protective factor against severe illness in COVID-19 patients.11 Further research is required for pregnant women, for whom nutritional guidance would be particularly useful.
There is an urgent need to pool high-quality and internationally representative data assessing the underlying risk factors and outcomes linked to COVID-19 in pregnancy. Currently, scarcity of similarly collected and analyzed data hampers our ability to make strong recommendations for the introduction and prioritization of new pharmaceutical interventions in pregnancy. The primary aim of this sequential, prospective meta-analysis (sPMA) is to accrue harmonized global data to inform policy and practice, grounded in the epidemiology of COVID-19 in the pregnancy, peripartum, and postnatal periods.
Objectives
This study aimed to identify risk factors among pregnant and postpartum women with SARS-CoV-2 infection for adverse outcomes related to: (1) disease severity; (2) maternal morbidities; (3) fetal and neonatal mortality and morbidity; and (4) adverse birth outcomes.
Methods
We registered the protocol for this prospective meta-analysis via the International Prospective Register of Systematic Reviews (PROSPERO) (ID: 188955) in May 2020, and the full protocol has been published elsewhere.12 The meta-analysis project was determined to be exempt from institutional review board review.
Language
Not all of those who are pregnant or give birth identify as women; throughout this document, the term “pregnant women” should be taken to be inclusive of all persons who have the biological capability to carry a pregnancy regardless of gender identity.
Eligibility criteria
Eligible studies include registries and single- or multisite cohort studies that recruited pregnant and recently postpartum women with confirmed or suspected COVID-19. They must have enrolled at least 25 women within a defined catchment area. We included data from those with infection onset up to 42 days after the pregnancy outcome.
Study selection
We invited principal investigators of studies of COVID-19 in pregnancy to join the sPMA via professional research networks and collaborations with key stakeholder networks.
Data extraction and individual patient data integrity
Following identification of eligible studies, investigators shared individual patient data (IPD) with the technical team for review and analysis. The technical team processed data to review data quality, identify outliers, and reconstruct variables to align with harmonized definitions of outcomes as defined in our protocol. We shared results with investigators for review and approval. For study sites unable to share IPD directly, the technical team worked with investigators to implement a common set of Stata codes to complete the same process of review, data quality checks, and harmonization.
In cases where studies collected data from overlapping catchment areas, we worked with investigators to identify and remove potential duplicates from the analysis. Because of the harmonization process and removal of overlapping data, there are some differences between our study results and those of the original published studies; these differences are summarized in Table S1.
Assessment of risk of bias
We used an adapted Newcastle–Ottawa scale to review study quality and risk of bias for each participating study; criteria for determination of high or low risk for each study design element are presented in Table S2.13
Outcomes
We examined 24 outcomes related to: (1) COVID-19 severity; (2) maternal morbidities; (3) fetal and neonatal morbidity and mortality; and (4) adverse birth outcomes. Specific definitions of each outcome—and 4 alternative outcomes used in sensitivity analyses—are presented in Table S3. The definition of maternal, fetal, and neonatal death and adverse birth outcomes were based on WHO case definitions.14, 15, 16, 17 Individual study sites defined hospitalization, critical care, and maternal morbidity outcomes. For maternal morbidities, fetal and neonatal mortality, and all birth outcomes, we restricted to cases of COVID-19 with infection onset during pregnancy or within 7 days of pregnancy outcome, excluding postpartum cases with COVID-19 onset at 8 to 42 days postpartum. Cases with unknown gestational age at onset were included in the analysis of pregnancy-specific outcomes and were assumed to be infections during pregnancy on the basis of the study design.
Risk factors
The sPMA steering committee, on the basis of expert opinion, identified 9 high-priority maternal risk factors including comorbidities, nutritional status, age, parity, and COVID-19 symptomatic status. Comorbidities included preexisting diabetes mellitus, hypertension, cardiovascular disease, and HIV coinfection.
Nutrition-related risk factors included body mass index (BMI) and anemia. We relied on prepregnancy BMI to determine the category for each participant, and we examined 2 risk factors: underweight (BMI <18.5 kg/m2) and obesity (BMI ≥30 kg/m2). Both risk factors were compared with a reference group of participants who were of normal weight or overweight before pregnancy (BMI, 18.5 to <30 kg/m2). Anemia was diagnosed on the basis of a hemoglobin measurement <11 g/dL at the time of COVID-19 diagnosis.
We considered 2 age groups as risk factors: younger maternal age (15–19 years) and older maternal age (35–45 years). Both groups were compared with a reference group of women aged 20 to 34 years. Lastly, we considered being symptomatic for COVID-19, as opposed to having no symptoms, as a risk factor for the outcomes of interest.
Generating study-specific estimates
We used a standard set of analysis codes to calculate study-specific estimates, comparing those with and without each risk factor (proportions and relative risks [RRs] with 95% confidence intervals [CIs]) for each participating study. Within each study, individual participants were excluded from the analysis if they were missing data on the risk factor of interest. Any study missing >25% of the data on an outcome of interest was excluded from that specific analysis.
Data synthesis
We applied a 2-stage IPD meta-analytic framework to generate pooled absolute risks and RRs, with 95% CI for each risk factor–outcome pair when there were ≥3 studies with available data. We presented unadjusted estimates because the goal of this study was to present descriptive epidemiologic data among a group of people (pregnant women with COVID-19 and their infants), rather than to examine a causal relationship.18,19 To estimate the pooled absolute risk for each adverse outcome overall and within risk factor groups, we used a logistic-normal random-effects model.20 In cases where the logistic-normal model did not converge, we used a random-effects model with the Freeman–Tukey double arcsine transformation to ensure stable estimates and approximate asymptotic normality.21 We used a DerSimonian and Laird random-effects meta-analysis to generate RRs for each risk factor–outcome pair and assessed heterogeneity across studies using the I2 statistic.
We excluded studies with 0 total events from that particular analysis. In case of 0 events within a risk factor subgroup, we applied a continuity correction of 0.5 when calculating pooled absolute risks. For pooled RRs, we applied a continuity correction of the inverse number of events in the opposite group within the same study for the risk factor–outcome pair. All meta-analyses were conducted in Stata, version 16.1 (StataCorp LLC, College Station, TX).
Results
Study selection
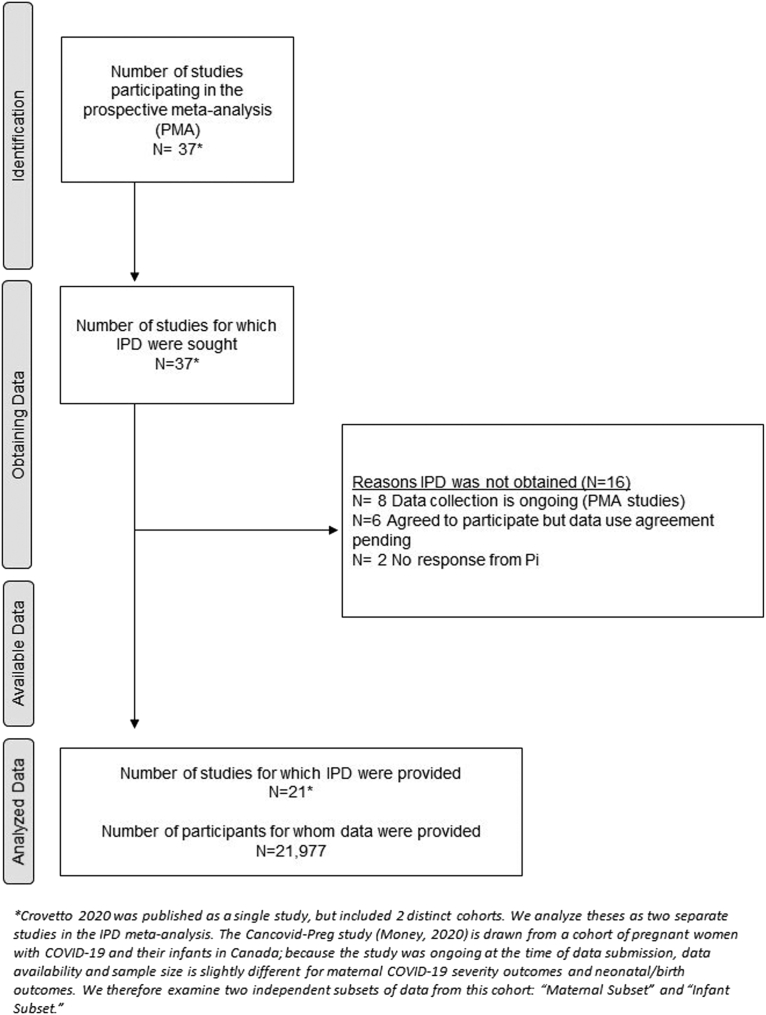
We included data from 21 studies conducted across 33 countries and territories (Afghanistan, Albania, Argentina, Belgium, Brazil, Canada, Chile, China, Colombia, Democratic Republic of the Congo, Egypt, France, French Guiana, Germany, Ghana, Hong Kong [China], India, Indonesia, Ireland, Israel, Italy, Kenya, Mexico, Nigeria, Portugal, PR, South Africa, Spain, Switzerland, Turkey, Uganda, United Kingdom, United States) with data from 21,977 cases of confirmed or suspected SARS-CoV-2 infections in pregnancy or the postpartum period. This iteration of the analysis included data from any study that met eligibility criteria and was able to share data by December 2021 (Figure 1). One study (Crovetto et al22) included 2 distinct cohorts with separate recruitment strategies, which were considered separately throughout the analysis. Furthermore, the Canadian Surveillance of COVID-19 in Pregnancy (CANCOVID-Preg) study (Money23) followed a cohort of pregnant women with SARS-CoV-2 infection and their infants in Canada; because the study was ongoing at the time of data submission, risk factor data availability and sample size were slightly different for maternal COVID-19 severity outcomes (n=2045) and neonatal/birth outcomes (n=2626). Therefore, we present the outcomes from the CANCOVID-Preg study as 2 independent subsets of the cohort in our tables (see CANCOVID-Preg–Maternal Subset and CANCOVID-Preg–Infant Subset).
Figure 1.
PRISMA diagram for risk factor analysis study
The PRISMA flow diagram outlines the identification and recruitment of studies and final inclusion of individual patient data for this study.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2023.
Study characteristics
Cases occurred between January 2020 and December 2021 (Table 1). More than 11,000 cases were contributed by the Mexico National Registry (R.J.M.P.), accounting for approximately half of the data for COVID-19 severity outcomes. The other 20 studies contributed 10,946 pregnant patients and completed follow-up through the end of pregnancy for 9850 participants, including 9695 live births (Table 1).
Table 1.
Description of studies contributing to the individual patient data meta-analysis
| Gestational age at infection |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study PI | Countries | Total pregnancies | Livebirths | Mean age (SD) | 1st tri | 2nd tri | 3rd tri | Postpartum | Unknown | Hospitalized (%) | Admitted to ICU (%) | Data collected through |
| Martinez-Portilla et al, 202046 | Mexico | 11,031 | n/a | 28.5 (6.0) | n/a | n/a | n/a | n/a | 100% | 20% | 2% | March 2021 |
| Vouga et al, 202147 | 14 countriesa | 2391 | 1830 | 31.3 (5.4) | 10% | 20% | 37% | 5% | 29% | 22%b | 4% | Dec. 2021 |
| McClymont et al, 202223c | Canada | 2045 | — | 31.2 (5.4) | 7% | 28% | 49% | 0% | 16% | n/a | 2% | Sept. 2021 |
| McClymont et al, 202223c | Canadad | — | 2626 | — | 2% | 7% | 19% | 0% | 72% | n/a | n/a | Sept. 2021 |
| Hernández et al, 202048 | Chile | 1347 | 1113 | 29 (6.2) | 1% | 12% | 64% | 4% | 19% | 16% | 6% | Nov. 2020 |
| Knight et al, 202049 | United Kingdom | 1243 | 1034 | 31.0 (6.0) | 3% | 12% | 75% | 4% | 7% | 100%e | 6% | Oct. 2020 |
| Bracero, Valencia, Delgado-Lopez (Unpublished data, October 20, 2021) | PR (United States) | 938 | 744 | 26.6 (5.6) | 11% | 20% | 38% | 1% | 30% | n/a | n/a | Oct. 2021 |
| Sakowicz et al, 202050 | United States (Chicago, IL) | 503 | 509 | 30.8 (5.8) | 5% | 21% | 73% | 0% | 1% | n/a | 1% | Feb. 2021 |
| Sanin, Mesa, Tolosa (Unpublished data, June 28, 2021) | Colombia | 409 | 188 | n/a | 4% | 9% | 32% | 3% | 52% | 68% | 22% | March 2021 |
| Nachega et al, 202251 | DRC, Ghana, Kenya, Nigeria, South Africa, Uganda | 349 | 136 | 30.7 (5.8) | 6%f | 18%f | 64%f | 0%f | 12%f | 100% | 19% | Dec. 2020 |
| Lokken et al, 202152 | United States (Washington, DC) | 240 | 156 | 28.6 (5.8) | 16% | 28% | 56% | 0% | 0% | 10% | 3% | Sept. 2020 |
| Divakar (Unpublished data, February 8, 2021) | India (Karnataka) | 214 | 216 | 26.4 (4.2) | 0% | 2% | 82% | 15% | 0% | n/a | n/a | Dec. 2020 |
| Gil, Fernandez Buhigas (Unpublished data, May 4, 2021) | Spain (Madrid) | 212 | 168 | 32.6 (5.9) | 29% | 37% | 33% | 0% | 1% | 4% | 0% | May 2021 |
| Crovetto et al, 2021, Cohort II22 | Spain (Barcelona) | 176 | 178 | 32.0 (6.2) | n/a | n/a | 14.%g | 1%g | 86%g | 16% | 1% | May 2020 |
| Crovetto et al, 2021, Cohort I22 | Spain (Barcelona) | 173 | 154 | 32.7 (5.4) | n/a | n/a | n/a | n/a | 100%g | 0% | 0% | March–May 2020, with follow-up through labor and delivery |
| Bevilacqua, Laurita Longo (Unpublished data, May 5, 2021) | Italy (Rome) | 163 | 156 | 32.3 (5.4) | 6% | 5% | 88% | 0% | 2% | 7% | 1% | March 2021 |
| Nunes (Unpublished data, September 29, 2021) | South Africa | 139 | 137 | 31.8 (6.6) | 2% | 22% | 71% | 0% | 5% | 15% | n/a | Sept. 2020 |
| Akelo, Tippett Barr (Unpublished data, August 19, 2021) | Kenya | 125 | 94 | 26.3 (5.2) | 1% | 12% | 31% | 27% | 29% | 9% | n/a | Aug. 2021 |
| Yang, Juan (Unpublished data, October 26, 2020) | China | 116 | 100 | 30.8 (3.8) | 3% | 6% | 82% | 9% | 1% | 100% | 8% | March 2020 |
| Kalafat et al, 202053 | Turkey | 77 | 72 | 28.0 (5.9) | n/a | n/a | n/a | n/a | 100% | 75% | 1% | June 2020 |
| Brandt et al, 202154 | United States (New Brunswick) | 61 | 60 | 30.3 (6.4) | 0% | 5% | 90% | 5% | 0% | 7% | 2% | June 2020 |
| Poon (Unpublished data, September 29, 2021) | Hong Kong | 25 | 24 | 33.7 (5.4) | 4% | 28% | 64% | 0% | 4% | 92% | 4% | June 2021 |
DRC, Democratic Republic of the Congo; ICU, intensive care unit; n/a, not applicable; PI, principal investigator(s); SD, standard deviation; tri, trimester; USA, United States of America.
Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2023.
The COVI-Preg study estimates in this analysis are drawn from facilities in 14 countries: Afghanistan (1%), Albania (<1%), Argentina (2%), Belgium (1%), Brazil (7%), Egypt (<1%), France (22%), French Guiana (3%), Germany (1%), Indonesia (1%), Ireland (2%), Israel (9%), Portugal (5%), and Switzerland (45%). Facilities participating in the COVI-Preg study with the potential to record overlapping cases with other sites participating in the current analysis were excluded, including facilities in Chile, China, Colombia, Italy, Spain (Barcelona), Mexico, Canada, United Kingdom, and the United States
Hospitalization data were missing in the COVI-Preg study for 194 participants (8% of the sample). ICU admission data are only available from those with a recorded hospital admission
The CANCOVID-Preg study follows a cohort of pregnant women with SARS-CoV-2 infection and their infants; because the study was ongoing at the time of data submission, risk factor data availability and sample size are slightly different for maternal COVID-19 severity outcomes and neonatal/birth outcomes. We present the data as 2 subsets of the same cohort for this ongoing study. In the “Maternal Subset,” we present data on pregnant women with COVID-19, including outcomes on ICU admission, ventilation, and critical care (n=2045). In the “Infant Subset,” we present data on live births to pregnant women with COVID-19, including outcomes on preterm birth (n=2626)
Data from CANCOVID-Preg represents all provinces, with missing data randomly distributed across provinces except for the risk factor “pre-existing hypertension,” which is unavailable for the full cohort from Ontario
Note that for the UKOSS study, 100% of patients are hospitalized. However, the reason for hospitalization may not be COVID-19, and some participants presented at the hospital for an unrelated reason and were found to have an incidental COVID-19 infection
For the AFREhealth study, gestational age at COVID-19 onset was not recorded. Here, we present trimester of hospital admission as a proxy. N=41 were missing trimester of hospital admission (12%). However, the study is not included in the risk factor analysis for gestational age at onset
Antibody testing at antenatal care (Cohort I) and at labor and delivery (Cohort II) was the primary method of diagnosis; thus, gestational age at COVID-19 onset is unknown for almost all observations.
The mean maternal age across studies was 29.4 years, ranging from 26 years in Kenya (V.A., B.A.T.B.) and India (H.D.) to 32 years in Italy (E.B., V.L.L.). Among the 18 studies that recorded gestational age at SARS-CoV-2 infection, 11 recruited most of their participants in the third trimester; 10 of these studies included people in the postpartum period. The Nachega (multicountry Africa) and Yang (China) studies were composed entirely of patients hospitalized for COVID-19; the Knight (United Kingdom) and Poon (Hong Kong, China) studies were composed entirely (or almost entirely) of patients hospitalized for COVID-19, labor and delivery, or other causes (Table 1).
Risk of bias of included studies
Detailed risk of bias ratings for each participating study are presented in summary in Table S4 and in detail in Table S5. Studies generally had moderate-to-low risk of bias according to the adapted Newcastle–Ottawa scale criteria, with 15 of 21 studies earning at least 4 out of 5 or 4 out of 6 stars across all outcome categories where that study was included in the analysis. The most common cause for high risk of bias rating was related to representativeness of the study population; 5 of 21 studies did not collect data on the reason for screening for individual patients. Another 8 studies primarily used methods to identify cases that were deemed to be at higher risk of bias (such as testing for clinical concern based on symptoms or travel). In total, 13 of 21 studies had elevated risk of bias in this area.
Synthesis of results
Overall incidence
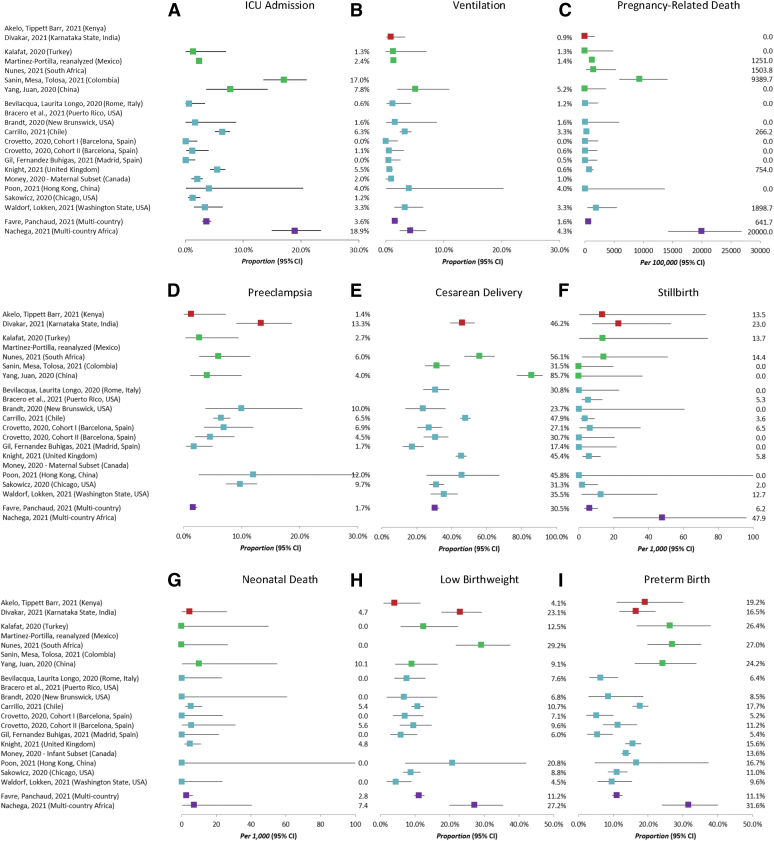
Overall event incidence for each site is shown in Figure 2. There was considerable heterogeneity between studies for most assessed outcomes. This is likely owing to a combination of factors including varying sampling frames across studies, true differences in the incidence of outcomes in the general population, and underlying differences in the standard of care provided by health systems in each setting.
Figure 2.
Incidence of outcomes by study
The incidence and 95% confidence intervals of selected adverse outcomes across the 21 participating studies, including: A, intensive care unit admission, B, ventilation, C, pregnancy-related death, D, preeclampsia, E, cesarean delivery, F, stillbirth, G, neonatal death, H, low birthweight, and I, preterm birth. Studies are grouped by World Bank income group levels: lower-middle–income countries are shown in red; upper-middle–income countries are shown in green; high-income countries are shown in blue. Two studies (shown in purple) are multicountry studies that contain countries from multiple income groups. The complete list of countries for each of these multicountry studies is presented in Table 1.
Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2023.
Comorbidities
We found that pregnant women with COVID-19 who also had chronic illnesses, including diabetes mellitus, hypertension, and cardiovascular disease, were at higher risk for most outcomes related to COVID-19 severity, and pregnancy-related death (Table 2). Risk of mortality was 3.79 times higher for pregnant women with preexisting diabetes mellitus (95% CI, 2.61–5.50; 15 studies, 15,705 pregnancies) (Table S7), 2.75 times higher for those with preexisting hypertension (95% CI, 1.76–4.28; 14 studies, 15,705 pregnancies) (Table S8), and 16.76 times higher for those with cardiovascular disease (95% CI, 4.42–63.64; 11 studies, 15,368 pregnancies) (Table S9) compared with those without these chronic health conditions.
Table 2.
Relative risks and 95% confidence intervals comparing women with each risk factor with women without the risk factor: comorbidites
| Outcome | N | Diabetes mellitus |
N | Hypertension |
N | CVD |
N | HIV coinfection |
|---|---|---|---|---|---|---|---|---|
| Pooled RR (95% CI) | Pooled RR (95% CI) | Pooled RR (95% CI) | Pooled RR (95% CI) | |||||
| COVID-19 severity and mortality | ||||||||
| ICU admission | 16 | 2.55 (1.97–3.31) | 14 | 2.10 (1.63–2.70) | 12 | 2.98 (1.83–4.85) | 3 | 1.67 (1.06–2.63) |
| Ventilation | 14 | 5.88 (2.77–12.48) | 13 | 4.87 (2.93–8.09) | 12 | 6.11 (2.85–13.08) | 3 | 1.01 (0.30–3.32) |
| Critical care | 14 | 3.03 (1.86–4.92) | 12 | 2.42 (1.73–3.39) | 11 | 2.82 (1.78–4.48) | 3 | 1.72 (1.10–2.69) |
| Pneumonia | 10 | 2.02 (1.65–2.47) | 8 | 2.13 (1.74–2.61) | 8 | 1.18 (0.65–2.16) | 1 | — |
| Pregnancy-related death | 15 | 3.79 (2.61–5.50) | 14 | 2.75 (1.76–4.28) | 11 | 16.76 (4.42–63.64) | 4 | 2.70 (0.58–12.47) |
| Maternal morbidity | ||||||||
| Hemorrhage | 7 | 1.89 (0.96–3.70) | 7 | 1.33 (0.60–2.94) | 5 | 2.42 (0.29–20.01) | 3 | 1.06 (0.57–1.99) |
| Placental abruption | 6 | 7.25 (2.47–21.25) | 6 | 6.68 (2.35–18.98) | 3 | 9.99 (1.70–58.58) | 2 | — |
| Preeclampsia | 10 | 2.98 (1.61–5.51) | 9 | 5.80 (4.11–8.19) | 8 | 4.78 (2.24–10.22) | 2 | — |
| Preeclampsia or eclampsia | 7 | 4.32 (1.58–11.84) | 6 | 4.09 (2.08–8.07) | 6 | 6.38 (2.80–14.58) | 2 | — |
| Hypertensive disorders of pregnancy (any) | 9 | 2.73 (1.62–4.58) | 8 | 3.16 (2.24–4.47) | 8 | 4.29 (2.17–8.48) | 2 | — |
| Hypertensive disorders of pregnancy (at/after COVID-19) | 2 | — | 2 | — | 1 | — | 0 | — |
| Preterm labor | 8 | 3.54 (1.89–6.61) | 7 | 3.93 (1.44–10.75) | 8 | 3.94 (1.39–11.19) | 2 | — |
| Preterm labor with onset before 37 wk GAa | 6 | 2.48 (1.24–4.98) | 5 | 2.16 (0.73–6.40) | 5 | 2.40 (0.31–18.46) | 2 | — |
| Cesarean delivery | 12 | 1.40 (1.13–1.74) | 11 | 1.31 (1.09–1.57) | 10 | 1.44 (1.08–1.92) | 3 | 1.51 (1.00–2.28) |
| Intrapartum cesarean delivery | 9 | 1.30 (0.90–1.87) | 8 | 1.58 (1.23–2.04) | 9 | 1.59 (1.03–2.48) | 3 | 1.47 (0.91–2.37) |
| Fetal and neonatal mortality and morbidity | ||||||||
| Stillbirthb | 16 | 6.53 (2.13–20.05) | 15 | 3.43 (1.41–8.37) | 12 | 9.10 (2.24–36.92) | 4 | 2.97 (0.35–25.26) |
| Perinatal death | 12 | 7.71 (2.12–28.03) | 11 | 4.94 (2.07–11.81) | 10 | 8.47 (2.70–26.53) | 3 | 8.63 (1.40–53.31) |
| Early neonatal death | 12 | 6.97 (1.07–45.27) | 11 | 11.74 (3.23–42.70) | 10 | 12.58 (2.69–58.80) | 3 | — |
| Neonatal deathc | 13 | 6.85 (1.22–38.49) | 12 | 8.10 (2.71–24.25) | 10 | 13.04 (3.18–53.43) | 4 | — |
| NICU admission at birth | 8 | 1.83 (1.15–2.93) | 6 | 2.28 (1.26–4.13) | 5 | 2.02 (0.65–6.30) | 1 | — |
| Adverse birth outcomes | ||||||||
| Very low birthweight (<1500 g) | 13 | 5.28 (2.62–10.63) | 12 | 6.30 (3.16–12.55) | 10 | 8.35 (3.64–19.19) | 4 | 2.41 (0.80–7.20) |
| Low birthweight (<2500 g) | 13 | 1.80 (1.21–2.69) | 12 | 1.87 (1.39–2.50) | 10 | 2.01 (1.19–3.39) | 4 | 1.38 (0.93–2.04) |
| Small for gestational age (3rd) | 14 | 4.11 (1.53–11.06) | 13 | 3.34 (1.86–6.00) | 11 | 3.14 (1.58–6.23) | 4 | 2.14 (1.02–4.48) |
| Small for gestational age (10th) | 14 | 1.62 (0.81–3.21) | 13 | 1.91 (1.29–2.84) | 11 | 1.84 (1.11–3.03) | 4 | 1.57 (0.93–2.63) |
| Moderate preterm birth (<34 wk) | 14 | 3.23 (2.09–5.01) | 13 | 3.55 (2.48–5.08) | 11 | 3.04 (1.57–5.91) | 4 | 1.78 (0.67–4.74) |
| Moderate preterm birth (<34 wk) with onset before 34 wk GAa | 8 | 2.03 (1.24–3.31) | 7 | 2.23 (1.46–3.41) | 5 | 2.27 (0.93–5.50) | 3 | 2.18 (0.93–5.07) |
| Preterm birth (<37 wk) | 15 | 2.25 (1.77–2.86) | 14 | 2.22 (1.72–2.86) | 12 | 1.90 (1.41–2.56) | 4 | 1.22 (0.83–1.81) |
| Preterm birth (<37 wk) with onset before 37 wk GA | 8 | 1.40 (0.97–2.01) | 7 | 1.61 (1.21–2.12) | 6 | 1.25 (0.63–2.49) | 3 | 1.40 (0.81–2.41) |
Relative risks are calculated by pooling unadjusted relative risks from all participating studies with at least 1 adverse event for the given outcome using a DerSimonian–Laird random-effects model meta-analysis. For any study with 0 events in one arm (Risk Group or Reference Group), we used a continuity correction of the inverse of the number of events in the oppposite group within the same study.
CI, confidence interval; GA, gestational age; ICU, intensive care unit; NICU, neonatal intensive care unit; RR, relative risk.
Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2023.
These outcomes (preterm labor, moderate preterm birth before 34 weeks’ gestation, and preterm birth before 37 weeks’ gestation) were included in the sensitivity analyses where we restricted confirmed COVID-19 cases to those with confirmed COVID-19 onset before 37 weeks’ gestation (or 34 weeks for very moderate preterm birth). The full comparison group was used for each of the sensitivity analyses
The outcome presented here is stillbirths occurring at ≥28 weeks’ gestation per the World Health Organization definition
The outcome “neonatal death” was reported by 15 participating studies. However, most studies were not designed to follow up neonates until 28 days after birth. Therefore, counts of neonatal death are underestimated.
Pregnant women with COVID-19 and one of these chronic conditions were at higher risk for maternal morbidity, including placental abruption, preeclampsia, preeclampsia or eclampsia, hypertensive disorders of pregnancy, preterm labor, and any cesarean delivery. Those with hypertension or cardiovascular disease were also at increased risk of having an intrapartum cesarean delivery. Infants born to mothers with both COVID-19 and one of these chronic conditions were at higher risk for mortality (stillbirth, perinatal death, and neonatal death) and NICU admission. These infants were more likely to be born preterm, have low birthweight, and be small for gestational age.
Although less data were available on HIV coinfection with COVID-19 during pregnancy, we found that coinfection increased the risk of severe COVID-19 disease (Table 2). Among pregnant women with COVID-19, those with HIV had a 67% increased risk of being admitted to the ICU (95% CI, 1.06–2.63; 3 studies, 2150 pregnancies) and 72% increased risk of needing critical care (95% CI, 1.10–2.69; 3 studies, 2150 pregnancies). Those with both COVID-19 and HIV were more likely to be delivered by cesarean delivery (RR, 1.51; 95% CI, 1.00–2.28; 3 studies, 1688 pregnancies), and infants born to those with HIV coinfection were at increased risk for perinatal death (RR, 8.63; 95% CI, 1.40–53.31; 3 studies, 1727 fetuses or infants) (Table S10).
Nutritional status and body mass index
We found increased risk of COVID-19 severity among pregnant and postpartum people who were either obese or underweight compared with those who were normal to overweight before pregnancy (Table 3). Pregnant women with a prepregnancy or early-pregnancy BMI of ≥30 kg/m2 were at increased risk for ICU admission (RR, 1.81; 95% CI, 1.26–2.60), ventilation (RR, 2.05; 95% CI, 1.20–3.51), and pneumonia (RR, 1.66; 95% CI, 1.18–2.33), but not for pregnancy-related death (RR, 1.00; 95% CI, 0.19–5.26) (Table S11).
Table 3.
Relative risks and 95% confidence intervals comparing women with each risk factor with women without the risk factor: nutrition-related factors
| Obese |
Underweight |
Anemia |
||||
|---|---|---|---|---|---|---|
| Outcome | N | Pooled RR (95% CI) | N | Pooled RR (95% CI) | N | Pooled RR (95% CI) |
| COVID-19 severity and mortality | ||||||
| ICU admission | 8 | 1.81 (1.26–2.60) | 8 | 5.53 (2.27–13.44) | 4 | 1.67 (1.28–2.19) |
| Ventilation | 7 | 2.05 (1.20–3.51) | 7 | 9.36 (3.87–22.63) | 4 | 1.78 (1.02–3.12) |
| Critical care | 7 | 1.89 (1.28–2.77) | 7 | 5.71 (2.40–13.59) | 3 | — |
| Pneumonia | 5 | 1.66 (1.18–2.33) | 5 | 2.71 (1.13–6.49) | 2 | — |
| Pregnancy-related death | 7 | 1.00 (0.19–5.26) | 7 | 14.10 (2.83–70.36) | 5 | 2.36 (1.15–4.81) |
| Maternal morbidity | ||||||
| Hemorrhage | 4 | 1.43 (0.85–2.41) | 4 | 6.00 (0.89–40.41) | 2 | — |
| Placental abruption | 2 | — | 2 | — | 2 | — |
| Preeclampsia | 4 | 1.60 (1.01–2.54) | 4 | 2.18 (0.63–7.53) | 3 | — |
| Preeclampsia or eclampsia | 3 | 2.16 (0.68–6.82) | 3 | 3.08 (0.64–14.81) | 3 | — |
| Hypertensive disorders of pregnancy (any) | 5 | 1.86 (1.30–2.67) | 5 | 1.93 (0.59–6.26) | 3 | 0.87 (0.52–1.46) |
| Hypertensive disorders of pregnancy (at/after COVID-19) | 1 | — | 1 | — | 1 | — |
| Preterm labor | 6 | 0.91 (0.57–1.46) | 6 | 3.76 (0.95–14.82) | 2 | — |
| Preterm labor with onset before 37 wk GAa | 4 | 0.84 (0.51–1.39) | 3 | 0.62 (0.02–18.50) | 2 | — |
| Cesarean delivery | 7 | 1.23 (1.07–1.41) | 7 | 1.15 (0.54–2.45) | 4 | 0.75 (0.47–1.19) |
| Intrapartum cesarean delivery | 6 | 1.28 (1.06–1.56) | 6 | 1.42 (0.26–7.78) | 3 | 0.67 (0.28–1.62) |
| Fetal and neonatal mortality and morbidity | ||||||
| Stillbirthb | 8 | 1.89 (0.31–11.60) | 8 | — | 5 | 3.75 (1.00–14.11) |
| Perinatal death | 6 | 3.17 (0.43–23.21) | 6 | — | 3 | — |
| Early neonatal death | 6 | — | 6 | — | 3 | — |
| Neonatal deathc | 6 | — | 6 | — | 4 | 2.98 (0.49–18.13) |
| NICU admission at birth | 4 | 1.42 (0.82–2.47) | 4 | 2.21 (0.26–18.78) | 2 | — |
| Adverse birth outcomes | ||||||
| Very low birthweight (<1500 g) | 6 | 1.70 (0.76–3.79) | 6 | 14.81 (3.25–67.39) | 4 | 1.64 (0.47–5.73) |
| Low birthweight (<2500 g) | 6 | 0.97 (0.68–1.37) | 6 | 1.98 (0.74–5.26) | 4 | 0.99 (0.60–1.62) |
| Small for gestational age (3rd) | 6 | 0.68 (0.24–1.95) | 6 | 7.14 (1.98–25.73) | 4 | 1.11 (0.56–2.21) |
| Small for gestational age (10th) | 6 | 0.75 (0.41–1.37) | 6 | 2.46 (0.90–6.70) | 4 | 0.99 (0.64–1.53) |
| Moderate preterm birth (<34 wk) | 6 | 1.75 (1.06–2.89) | 6 | 7.53 (2.33–24.29) | 4 | 0.91 (0.51–1.61) |
| Moderate preterm birth (<34 wk) with onset before 34 wk GAa | 3 | 1.46 (0.89–2.40) | 2 | — | 2 | — |
| Preterm birth (<37 wk) | 7 | 1.38 (1.10–1.73) | 7 | 1.58 (0.59–4.26) | 4 | 0.94 (0.67–1.32) |
| Preterm birth (<37 wk) with onset before 37 wk GAa | 3 | 1.17 (0.90–1.51) | 2 | — | 3 | 0.92 (0.62–1.37) |
Relative risks are calculated by pooling unadjusted relative risks from all participating studies with at least 1 adverse event for the given outcome using a DerSimonian–Laird random-effects model meta-analysis. For any study with 0 events in one arm (Risk Group or Reference Group), we used a continuity correction of the inverse of the number of events in the oppposite group within the same study.
CI, confidence interval; GA, gestational age; ICU, intensive care unit; NICU, neonatal intensive care unit; RR, relative risk.
Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2023.
These outcomes (preterm labor, moderate preterm birth before 34 weeks’ gestation, and preterm birth before 37 weeks’ gestation) were included in the sensitivity analyses where we restricted confirmed COVID-19 cases to those with confirmed COVID-19 onset before 37 weeks’ gestation (or 34 weeks for very moderate preterm birth). The full comparison group was used for each of the sensitivity analyses
The outcome presented here is stillbirths occurring at ≥28 weeks’ gestation per the World Health Organization definition
The outcome “neonatal death” was reported by 15 participating studies. However, most studies were not designed to follow up neonates until 28 days after birth. Therefore, counts of neonatal death are underestimated.
Pregnant women who were underweight prepregnancy had >5 times increased risk for ICU admission (RR, 5.53; 95% CI, 2.27–13.44; 8 studies, 1721 pregnancies) or any critical care (RR, 5.71; 95% CI, 2.40–13.59; 7 studies, 1822 pregnancies), >9 times increased risk for ventilation (RR, 9.36; 95% CI, 3.87–22.63; 7 studies, 1822 pregnancies), and nearly 3 times increased risk for pneumonia (RR, 2.71; 95% CI, 1.13–6.49; 5 studies, 1129 pregnancies) compared with pregnant women who were normal to overweight prepregnancy (Table S12). Although this was based on a small sample size, underweight pregnant women with COVID-19 were found to have a sharply increased risk of pregnancy-related death (RR, 14.10; 95% CI, 2.83–70.36; 7 studies, 700 pregnancies).
Prepregnancy obesity was also associated with increased risks for maternal morbidity such as preeclampsia (RR, 1.60; 95% CI, 1.01–2.54), any hypertensive disorders of pregnancy (RR, 1.86; 95% CI, 1.30–2.67), any cesarean delivery (RR, 1.23; 95% CI, 1.07–1.41), and intrapartum cesarean delivery (RR, 1.28; 95% CI, 1.06–1.56) (Table 3). Prepregnancy underweight was associated with adverse birth outcomes such as very low birthweight (RR, 14.81; 95% CI, 3.25–67.39), small-for-gestational age infants in the third percentile (RR, 7.14; 95% CI, 1.98–25.73), and moderately preterm birth (RR, 7.53; 95% CI, 2.33–24.29).
Although data were limited, we found an increased risk of COVID-19 severity among pregnant women with anemia at the time of COVID-19 diagnosis compared with those without anemia (Table 3). Those with anemia had an increased risk of ICU admission (RR, 1.67; 95% CI, 1.28–2.19; 4 studies, 1089 pregnancies), ventilation (RR, 1.78; 95% CI, 1.02–3.12; 4 studies, 974 pregnancies), and death (RR, 2.36; 95% CI, 1.15–4.81; 5 studies, 809 pregnancies). We also found an increased risk of stillbirth for pregnant women with anemia (RR, 3.75; 95% CI, 1.00–14.11; 5 studies, 748 fetuses or infants) (Table S13).
Maternal age
Older maternal age (35–45 years) was associated with multiple COVID-19–associated adverse outcomes compared with those aged 20 to 34 years (Table 4). Older maternal age was associated with increased risk of ICU admission (RR, 1.60; 95% CI, 1.36–1.89; 16 studies, 18,758 pregnancies), ventilation (RR, 2.13; 95% CI, 1.68–2.71; 16 studies, 18,407 pregnancies), any critical care (RR, 1.62; 95% CI, 1.38–1.90; 15 studies, 18,452 pregnancies) (Table S14), and pneumonia diagnosis (RR, 1.51; 95% CI, 1.35–1.70; 10 studies, 15,670 pregnancies). Older pregnant women also had increased risk for placental abruption (RR, 3.94; 95% CI, 1.40–11.13) and cesarean delivery (RR, 1.21; 95% CI, 1.10–1.32). Infants born to older pregnant women with COVID-19 had higher risk of stillbirth, perinatal death, NICU admission, preterm birth, and low birthweight.
Table 4.
Relative risks and 95% confidence intervals comparing women with each risk factor with women without the risk factor: maternal age and primiparity
| Age 15–19 years |
Age 35–45 years |
Primiparity |
||||
|---|---|---|---|---|---|---|
| Outcome | N | Pooled RR(95% CI) | N | Pooled RR (95% CI) | N | Pooled RR (95% CI) |
| COVID-19 severity and mortality | ||||||
| ICU admission | 12 | 1.42 (0.53–3.77) | 16 | 1.60 (1.36–1.89) | 14 | 0.90 (0.71–1.13) |
| Ventilation | 12 | 2.59 (0.79–8.51) | 16 | 2.13 (1.68–2.71) | 12 | 0.67 (0.39–1.16) |
| Critical care | 11 | 1.24 (0.48–3.17) | 15 | 1.62 (1.38–1.90) | 12 | 0.82 (0.62–1.08) |
| Pneumonia | 9 | 0.82 (0.62–1.08) | 10 | 1.51 (1.35–1.70) | 8 | 0.59 (0.46–0.77) |
| Pregnancy-related death | 13 | 0.73 (0.27–1.94) | 16 | 1.62 (0.81–3.24) | 14 | 0.75 (0.45–1.25) |
| Maternal morbidity | ||||||
| Hemorrhage | 6 | 1.93 (0.94–3.98) | 6 | 1.17 (0.82–1.68) | 7 | 1.26 (0.90–1.77) |
| Placental abruption | 5 | -- | 6 | 3.94 (1.40–11.13) | 6 | 0.64 (0.19–2.09) |
| Preeclampsia | 10 | 2.03 (0.89–4.61) | 13 | 1.12 (0.73–1.74) | 11 | 2.10 (1.45–3.03) |
| Preeclampsia or eclampsia | 8 | 3.27 (1.11–9.64) | 9 | 0.93 (0.63–1.37) | 8 | 1.75 (1.22–2.53) |
| Hypertensive disorders of pregnancy (any) | 10 | 2.06 (0.77–5.55) | 12 | 1.17 (0.93–1.49) | 10 | 1.56 (1.13–2.15) |
| Hypertensive disorders of pregnancy (at/after COVID-19) | 2 | -- | 3 | 1.91 (0.45–8.16) | 3 | 1.39 (0.54–3.57) |
| Preterm labor | 8 | 2.48 (0.53–11.60) | 10 | 1.39 (0.96–2.02) | 8 | 0.86 (0.51–1.43) |
| Preterm labor with onset before 37 wk GAa | 5 | 1.62 (0.42–6.22) | 8 | 1.28 (0.87–1.87) | 6 | 0.88 (0.51–1.51) |
| Cesarean delivery | 10 | 0.86 (0.65–1.13) | 13 | 1.21 (1.10–1.32) | 12 | 1.00 (0.90–1.11) |
| Intrapartum cesarean delivery | 9 | 0.90 (0.63–1.31) | 10 | 1.03 (0.89–1.20) | 8 | 1.35 (1.14–1.60) |
| Fetal and neonatal mortality and morbidity | ||||||
| Stillbirthb | 15 | 4.59 (1.69–12.45) | 18 | 1.75 (0.92–3.33) | 17 | 1.34 (0.62–2.90) |
| Perinatal death | 11 | 4.80 (1.28–17.99) | 14 | 1.53 (0.82–2.83) | 12 | 1.78 (0.89–3.54) |
| Early neonatal death | 11 | 5.94 (1.02–34.56) | 14 | 1.80 (0.51–6.33) | 12 | 1.60 (0.45–5.62) |
| Neonatal deathc | 12 | 9.38 (2.21–39.89) | 15 | 1.96 (0.65–5.87) | 13 | 1.25 (0.43–3.60) |
| NICU admission at birth | 6 | 1.59 (0.48–5.23) | 9 | 1.35 (1.12–1.63) | 8 | 1.03 (0.85–1.25) |
| Adverse birth outcomes | ||||||
| Very low birthweight (<1500 g) | 13 | 6.27 (1.86–21.15) | 16 | 1.39 (0.89–2.16) | 14 | 1.03 (0.61–1.73) |
| Low birthweight (<2500 g) | 13 | 0.96 (0.54–1.73) | 16 | 1.24 (1.04–1.47) | 14 | 1.27 (1.04–1.54) |
| Small for gestational age (3rd) | 14 | 4.33 (1.87–10.06) | 17 | 1.46 (1.01–2.12) | 15 | 2.11 (1.42–3.11) |
| Small for gestational age (10th) | 14 | 1.40 (0.83–2.36) | 17 | 0.98 (0.79–1.21) | 15 | 1.74 (1.41–2.15) |
| Moderate preterm birth (<34 wk) | 14 | 3.06 (1.48–6.35) | 17 | 1.51 (1.19–1.93) | 15 | 1.10 (0.84–1.44) |
| Moderate preterm birth (<34 wk) with onset before 34 wk GAa | 7 | 2.90 (1.18–7.14) | 10 | 1.43 (1.07–1.90) | 8 | 1.07 (0.74–1.53) |
| Preterm birth (<37 wk) | 14 | 1.22 (0.84–1.78) | 18 | 1.40 (1.19–1.64) | 15 | 1.02 (0.87–1.19) |
| Preterm birth (<37 wk) with onset before 37 wk GAa | 7 | 1.06 (0.68–1.67) | 11 | 1.27 (1.07–1.50) | 9 | 1.02 (0.83–1.26) |
Relative risks are calculated by pooling unadjusted relative risks from all participating studies with at least 1 adverse event for the given outcome using a DerSimonian–Laird random-effects model meta-analysis. For any study with 0 events in one arm (Risk Group or Reference Group), we used a continuity correction of the inverse of the number of events in the oppposite group within the same study.
CI, confidence interval; GA, gestational age; ICU, intensive care unit; NICU, neonatal intensive care unit; RR, relative risk.
Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2023.
These outcomes (preterm labor, moderate preterm birth before 34 weeks’ gestation, and preterm birth before 37 weeks’ gestation) were included in the sensitivity analyses where we restricted confirmed COVID-19 cases to those with confirmed COVID-19 onset before 37 weeks’ gestation (or 34 weeks for very moderate preterm birth). The full comparison group was used for each of the sensitivity analyses
The outcome presented here is stillbirths occurring at ≥28 weeks’ gestation per the World Health Organization definition
The outcome “neonatal death” was reported by 15 participating studies. However, most studies were not designed to follow up neonates until 28 days after birth. Therefore, counts of neonatal death are underestimated.
Compared with pregnant women with COVID-19 aged 20 to 34 years, younger pregnant women (15–19 years) were at increased risk for preeclampsia or eclampsia (RR, 3.27; 95% CI, 1.11–9.64; 8 studies, 1074 pregnancies) (Table S15). Infants born to younger women with COVID-19 had higher risks of stillbirth, perinatal death, and neonatal death. Younger women with COVID-19 were also more likely to experience adverse pregnancy outcomes, including moderate preterm birth (RR, 2.90; 95% CI, 1.18–7.14; 7 studies, 1321 infants), very low-birthweight infants (RR, 6.27; 95% CI, 1.86–21.15; 13 studies, 3203 infants), and small-for-gestational-age infants (<3rd percentile; RR, 4.33; 95% CI, 1.87–10.06, 14 studies, 3901 infants).
Primiparity
Overall, we found limited differences in risks of adverse outcomes among primiparous compared with multiparous pregnant women with COVID-19 (Table 4). Primiparous women were less likely to be diagnosed with pneumonia than multiparous women (RR, 0.59; 95% CI; 0.46–0.77; 8 studies, 4249 pregnancies) and were more likely to experience preeclampsia or eclampsia, any hypertensive disorders of pregnancy, or intrapartum cesarean delivery, compared with multiparous women (Table S16).
Symptomatic SARS-CoV-2 infection
We found increased risks for adverse outcomes related to COVID-19 severity among pregnant women with symptomatic infection compared with those with asymptomatic SARS-CoV-2 infection, including ICU admission, any critical care, and pneumonia (Table S17). However, most other outcomes related to maternal morbidity, fetal and neonatal mortality and morbidity, and adverse birth outcomes were similar across symptomatic and asymptomatic groups, with a few exceptions. Pregnant women with symptomatic COVID-19 were more likely to have an intrapartum cesarean delivery (RR, 1.25; 95% CI, 1.05–1.48) compared with those with asymptomatic infection (Table S18).
We also found increased risk of preterm and moderate preterm birth among symptomatic pregnant women (RR, 1.30; 95% CI, 1.06–1.60 and RR, 1.65; 95% CI, 1.00–2.73, respectively). However, when we restricted to only pregnant women with infection onset before 37 weeks’ gestation for preterm birth and before 34 weeks’ gestation for moderate preterm birth, we found that asymptomatic pregnant women had an increased risk of preterm and moderate preterm birth (RR, 0.71; 95% CI, 0.52–0.97 and RR, 0.57; 95% CI, 0.41–0.81) compared with symptomatic pregnant women.
Comment
Principal findings
As in the general population, we found that pregnant women with comorbidities including diabetes mellitus, hypertension, cardiovascular disease, and obesity were at increased risk for severe COVID-19–related outcomes, as well as for maternal morbidities and adverse birth outcomes, compared with pregnant women without these comorbidities. Given pooled global data, we also identified several less commonly known risk factors for pregnant women with COVID-19, including HIV coinfection, being underweight at the start of pregnancy, and anemia at the time of COVID-19 diagnosis.
Comparison with existing literature
We found that among pregnant women with COVID-19, those living with HIV were nearly twice as likely to be admitted to the ICU or need critical care. Women living with HIV already have greater likelihood of antenatal, delivery, and postpartum complications, including preterm birth, cesarean delivery, postpartum sepsis, venous thromboembolism, postpartum infection, and mortality.24 Neonates born to these women are at higher risk owing to prematurity, low birthweight, and intrauterine growth restriction, resulting in higher rates of NICU admission and neonatal mortality.24,25 Factors related to HIV severity such as HIV progression, antiretroviral therapy, CD4 cell count, and viral load additionally affect the immune response to coinfection.26
A recent systematic review of SARS-CoV-2 infection among people living with HIV in the general population found strong evidence that HIV is a risk factor for both SARS-CoV-2 infection and for mortality owing to COVID-19; that review did not examine pregnant and postpartum women as a subgroup of interest.27 Given that pregnant women are at higher risk for severe COVID-19 illness and complications from HIV, SARS-CoV-2 and HIV coinfection may be particularly concerning in this population. However, our analysis of COVID-19 infection among pregnant women living with HIV had several limitations. First, we do not yet have sufficient data to examine either treatment status or viral load among pregnant women with HIV; thus, we cannot shed light on how these factors could mediate excess risk. Furthermore, adverse outcomes related to both COVID-19 severity and pregnancy outcomes can be affected by social, behavioral, and structural factors prevalent in HIV-endemic regions.28
Undernutrition in pregnant women with COVID-19 was identified as an important risk factor for COVID-19 severity and adverse birth outcomes. Underweight pregnant women had elevated risks for severe COVID-19 and pregnancy-related death, and for moderately preterm, very low-birthweight, and small-for-gestational-age infants. In addition, being anemic during pregnancy increased the risk for pregnancy-related death, ICU admission, and stillbirth. Although the results for anemia were based on 4 studies, the effect estimates for severe COVID-19 were consistent with those reported in a recent meta-analysis highlighting linkages between low hemoglobin, hypoxia, respiratory organ dysfunction, and severe outcomes from COVID-19 infection in the general population.10 In pregnant and nonpregnant women, single or multiple nutritional deficiencies are known to decrease immune responses, consequently increasing the risk of infection, disease severity, and morbidity and mortality.29, 30, 31 These linkages are especially important during pregnancy when the demand for macro- and micronutrients to support maternal physiological functioning, placental development, and fetal growth is even higher.32 Failure to meet these demands has been linked to preterm births and stillbirths in both high-income33, 34, 35 and low- and middle-income countries.36 These indicators of undernutrition are generally linked to many different health conditions (eg, iron deficiency, other infections), and it is difficult to infer specific mechanisms of action on the basis of this analysis. Nonetheless, our findings on the association between undernutrition or anemia and preterm births and stillbirths among pregnant women with COVID-19 further underscore the need for close monitoring and management of this group, including provision of additional nutritional support to prevent disease and adverse birth outcomes.35,37
We found that pregnant women with any COVID-19 symptoms were at increased risk for ICU admission, ventilation, cesarean delivery, and preterm birth compared with asymptomatic pregnant women on the basis of a large sample size of global studies. Although a previous systematic review of published literature examined this question, data on symptomatic compared with asymptomatic SARS-CoV-2 infection in pregnancy were only available for a small subset of studies and participants in this review (4 studies on ICU admission with 1178 participants, 3 studies on mechanical ventilation with 1023 participants, and 9 studies on cesarean delivery and preterm birth with 4233 participants).5 Our study found that symptomatic pregnant women are more likely to give birth preterm than asymptomatic pregnant women with SARS-CoV-2 infection.
However, in a sensitivity analysis restricted only to participants infected before 37 weeks’ gestation, we found that asymptomatic pregnant women are more likely than symptomatic pregnant women to have a preterm birth. These seemingly conflicting results may be related to features of study sampling; for example, this difference may be because of the large percentage of asymptomatic participants who are identified during screening at labor and delivery. Across the 10 studies included in the restricted analysis, 64% of infants born to asymptomatic participants were identified at ≥37 weeks’ gestation vs 26% of infants born to symptomatic participants.
Strengths and limitations
IPD meta-analyses are considered the gold-standard method for generating aggregate estimates. Here, we standardized data quality assessment and harmonized definitions of risk factors and outcomes. This is especially valuable for outcomes such as stillbirth, preterm birth, and perinatal mortality, which have varying definitions globally. We included data from 33 countries and territories, including many low- and middle-income countries, whereas the bulk of the published literature on COVID-19 in pregnancy comes from middle- or high-income countries. Therefore, by pooling global data we were able to investigate risk factors such as HIV status, undernutrition, and anemia, which are more common in low-income countries, but for which individual studies may not have adequate power to draw meaningful conclusions. We were also able to identify risks linked to rare outcomes such as pregnancy-related death and stillbirth.
Our study had several limitations. First, the studies contributing to the IPD meta-analysis recruited participants differently, varying from hospital-based surveillance to universal screening during antenatal care. Furthermore, representativeness of the sample was deemed to be at elevated risk of bias for most studies because of limited information about identification and screening at the individual patient level or the use of identification strategies that are only somewhat representative of the population of interest. Some studies only recruited women admitted to the hospital with COVID-19 infection, whereas others included both symptomatic and asymptomatic women who tested positive for the infection. Given the heterogeneity of the sampling frames between studies, it is not possible to draw inferences about the absolute risk of adverse outcomes. The heterogeneity in baseline rates of adverse outcomes globally further complicates interpretation of the absolute risks. However, the RRs comparing those with and without the risk factors of interest generally seemed consistent between sites, and heterogeneity was relatively low for pooled estimates. In addition, although this analysis pooled a large, global sample of pregnant and postpartum women with COVID-19, half of the overall sample for critical care outcomes (ICU admission, ventilation, any critical care, pneumonia, and mortality) was derived from the Mexican National Registry, which collected no information on maternal morbidity, birth, or neonatal outcomes. This analysis also did not examine risk factors related to social determinants of health, which may exacerbate the biological risk factors identified in this analysis.
We identified risk factors for adverse maternal morbidities and fetal and neonatal outcomes among pregnant women with COVID-19, and these are generally consistent with risk factors for adverse pregnancy outcomes including preexisting diabetes mellitus or hypertension,38, 39, 40 cardiovascular disease,41 obesity,40,42 underweight,42,43 anemia,44,45 and HIV infection.24,25 Because the studies in this IPD meta-analysis only included individuals with SARS-CoV-2 infection, we were unable to evaluate whether the presence of infection confers additional risk beyond the risk because of risk factors without the presence of COVID-19 infection. Similarly, we identified risk factors for adverse COVID-19–related outcomes, and these are generally consistent with risk factors identified in the general nonpregnant population. Nonetheless, this study provides high-quality evidence that pregnant women with these risk factors are also at risk for adverse outcomes from COVID-19 illness.
Conclusions and implications
Although pregnant women are already considered a high-risk population by the WHO and should be given equitable access to safe and effective preventives and therapeutics, special priority should be given to pregnant women with additional risk factors, including those related to chronic and infectious comorbidities, nutritional status, and maternal age. These data strongly support the need for access to vaccines and treatments for SARS-CoV-2 infection for pregnant women, prioritizing those with risk factors for severe illness and adverse birth outcomes.
Footnotes
E.R.S. and E.O. share first authorship.
C.W. declares a relationship with the following entities: Ferring Pharmaceuticals (COVID19 Investigational Grant) and National Health and Medical Research Council Fellowship (salary support). A.P. declares the following research grants: “1) H2020-Grant - Consortium member of Innovative medicine initiative call 13 topic 9, ConcePTION; and 2) Efficacy and safety studies on Medicines EMA/2017/09/PE/11, Lot 4, WP 2 lead, Safety monitoring of COVID-19 vaccines in the EU – Reopening of competition no. 20 under a framework contract following procurement procedure EMA/2017/09/PE (Lot 3) (Euro 110’000.-), Federal Office of Public Health (207’000 CHF).” E.M. and C.L. declare a relationship with the National Institute for Health and Care Research (project grant for PAN-COVID study). D.M. declares a relationship with the following entities: Canadian Institutes of
Health Research (payments to institution only), Public Health Agency of Canada (payments to institution only), and BC Women’s Health Foundation (payments to institution only). She is also a member of the COVID-19 Immunity Task Force sponsored by the Canadian government. T.D.M. declares a relationship with the following entities: Pfizer (site Principal Investigator for SARS-CoV-2 vaccination in pregnancy study—payment received by the institution; member of Medical Advisory Board for SARS-CoV-2 vaccination in pregnancy study–payment received by the author), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (subcommittee Chair for the NICHD Maternal-Fetal Medicine Units Network Gestational Research Assessments of COVID-19 [GRAVID] study), and Society for Maternal-Fetal Medicine (board member). E.M.L. declares a relationship with the National Institutes of Health (paid institution) and is an employee of AbbVie, Inc, but was employed at the University of Washington at the time of the study. K.L.K. and S.H. declare a relationship with the Bill & Melinda Gates Foundation (BMGF) (S.H.: payments made to the institution). V.F. declares a relationship with the following entities: BMGF (payments to her institution), Yellow Chair Foundation (payments to institution), Robert Woods Johnson Foundation (payments to institution), CDC Foundation, California Health Care Foundation (payments to institution), Tara Health Foundation (payments to institution), University of California, San Francisco Women’s Health Center of Excellence (payments to institution), and California Department of Health Care Services (payments made to institution). J.S.B. declares a relationship with Ferring Pharmaceuticals, which provided $10,000 for the expenses of the RECOGEST trial and is a part of the Colombian Federation of Perinatology. Y.A. declares a relationship with the following entities: BMGF (payments made to institution), CDC Foundation (payments made to institution), Robert Woods Johnson Foundation (payments made to institution), and University of California, Los Angeles Dean’s Office COVID-19 research (payments made to institution). M.C.N. declares a relationship with the following entities: BMGF (project grant made to institution), European and Developing Countries Clinical Trials Partnership, Sanofi, AstraZeneca, Pfizer (research grants made to institution), Sanofi Pasteur (payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events), and Sanofi Pasteur and Pfizer (payment for expert testimony). E.S.M. declares a relationship with Pfizer (Site Principal Investigator for phase 2/3 randomized controlled trial of COVID-19 vaccine in pregnancy). O.S. declares a relationship with the following entities: NordForsk (Nordic research funding grant number 105545), the Swedish Medical Products Agency (funding for reports on COVID-19 vaccines in pregnancy), and Karolinska Institutet (funding for COVID-19 research in pregnancy, 2020-01567). E.G. declares a relationship with the following entities: Stavros Niarchos Foundation, Santander Foundation, and La Caixa Foundation (payments made to institution). S.A.M. declares a relationship with the BMGF (funded study in South Africa).
This study was funded by the Bill & Melinda Gates Foundation (grant to E.R.S.; INV-022057).
This study was registered on the International Prospective Register of Systematic Reviews on May 28, 2020 (PROSPERO ID: 188955).
Supplementary Data
<VIDSE>Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2022.
<VIDSE>Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2022.
References
- 1.Centers for Disease Control and Prevention People with certain medical conditions. 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Available at:
- 2.World Health Organization COVID-19 Clinical management: living guidance. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 Available at:
- 3.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalil A., Kalafat E., Benlioglu C., et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciapponi A., Bardach A., Comandé D., et al. COVID-19 and pregnancy: an umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allotey J., Chatterjee S., Kew T., et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-067696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vouga M., Favre G., Martinez-Perez O., et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci Rep. 2021;11 doi: 10.1038/s41598-021-92357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galang R.R., Newton S.M., Woodworth K.R., et al. Risk factors for illness severity among pregnant women with confirmed severe acute respiratory syndrome coronavirus 2 infection-surveillance for emerging threats to mothers and babies network, 22 state, local, and territorial health departments, 29 March 2020-5 March 2021. Clin Infect Dis. 2021;73:S17–S23. doi: 10.1093/cid/ciab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariyanto T.I., Kurniawan A. Anemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Transfus Apher Sci. 2020;59 doi: 10.1016/j.transci.2020.102926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y.D., Ding M., Dong X., et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 12.Smith E.R., He S., Oakley E.M., Miller L., Tielsch J.M. Protocol for a sequential, prospective meta-analysis to describe COVID-19. 2020. https://www.medrxiv.org/content/10.1101/2020.11.08.20228056v1.abstract Available at: Accessed May 30, 2022.
- 13.Wells G.A., Shea B., O’Connell D., et al. The Ottawa Hospital Research Institute; 2014. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at: [Google Scholar]
- 14.World Health Organization Maternal deaths. The Global Health Observatory: Indicator Metadata Registry List. 2022. https://www.who.int/data/gho/indicator-metadata-registry/imr-details/4622 Available at:
- 15.Tavares Da Silva F., Gonik B., McMillan M., et al. Stillbirth: case definition and guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2016;34:6057–6068. doi: 10.1016/j.vaccine.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barfield W.D., Committee on Fetus and Newborn Standard terminology for fetal, infant, and perinatal deaths. Pediatrics. 2016;137 doi: 10.1542/peds.2016-0551. [DOI] [PubMed] [Google Scholar]
- 17.Pathirana J., Muñoz F.M., Abbing-Karahagopian V., et al. Neonatal death: case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2016;34:6027–6037. doi: 10.1016/j.vaccine.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conroy S., Murray E.J. Let the question determine the methods: descriptive epidemiology done right. Br J Cancer. 2020;123:1351–1352. doi: 10.1038/s41416-020-1019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox M.P., Murray E.J., Lesko C.R., Sealy-Jefferson S. On the need to revitalize descriptive epidemiology. Am J Epidemiol. 2022;191:1174–1179. doi: 10.1093/aje/kwac056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rücker G., Schwarzer G., Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat Med. 2008;27:746–763. doi: 10.1002/sim.2971. [DOI] [PubMed] [Google Scholar]
- 22.Crovetto F., Crispi F., Llurba E., et al. Impact of SARS-CoV-2 infection on pregnancy outcomes: a population-based study. Clin Infect Dis. 2021;73:1768–1775. doi: 10.1093/cid/ciab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClymont E., Albert A.Y., Alton G.D., et al. Association of SARS-CoV-2 infection during pregnancy with maternal and perinatal outcomes. JAMA. 2022;327:1983–1991. doi: 10.1001/jama.2022.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arab K., Spence A.R., Czuzoj-Shulman N., Abenhaim H.A. Pregnancy outcomes in HIV-positive women: a retrospective cohort study. Arch Gynecol Obstet. 2017;295:599–606. doi: 10.1007/s00404-016-4271-y. [DOI] [PubMed] [Google Scholar]
- 25.Wedi C.O.O., Kirtley S., Hopewell S., Corrigan R., Kennedy S.H., Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV. 2016;3:e33–e48. doi: 10.1016/S2352-3018(15)00207-6. [DOI] [PubMed] [Google Scholar]
- 26.Li H., Liu J., Tan D., et al. Maternal HIV infection and risk of adverse pregnancy outcomes in Hunan Province, China: a prospective cohort study. Medicine. 2020;99 doi: 10.1097/MD.0000000000019213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ssentongo P., Heilbrunn E.S., Ssentongo A.E., et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. 2021;11:6283. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison A., Colvin C.J., Kuo C., Swartz A., Lurie M. Sustained high HIV incidence in Young women in Southern Africa: social, behavioral, and structural factors and emerging intervention approaches. Curr HIV AIDS Rep. 2015;12:207–215. doi: 10.1007/s11904-015-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Saad K., Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev. 2010;32:5–25. doi: 10.1093/epirev/mxq001. [DOI] [PubMed] [Google Scholar]
- 30.Rytter M.J.H., Kolte L., Briend A., Friis H., Christensen V.B. The immune system in children with malnutrition--a systematic review. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverio R., Gonçalves D.C., Andrade M.F., Seelaender M. Coronavirus disease 2019 (COVID-19) and nutritional status: the missing link? Adv Nutr. 2021;12:682–692. doi: 10.1093/advances/nmaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weckman A.M., Ngai M., Wright J., McDonald C.R., Kain K.C. The impact of infection in pregnancy on placental vascular development and adverse birth outcomes. Front Microbiol. 2019;10:1924. doi: 10.3389/fmicb.2019.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girsen A.I., Mayo J.A., Carmichael S.L., et al. Women’s prepregnancy underweight as a risk factor for preterm birth: a retrospective study. BJOG. 2016;123:2001–2007. doi: 10.1111/1471-0528.14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han Z., Mulla S., Beyene J., Liao G., McDonald S.D., Knowledge Synthesis Group Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol. 2011;40:65–101. doi: 10.1093/ije/dyq195. [DOI] [PubMed] [Google Scholar]
- 35.Nair M., Churchill D., Robinson S., Nelson-Piercy C., Stanworth S.J., Knight M. Association between maternal haemoglobin and stillbirth: a cohort study among a multi-ethnic population in England. Br J Haematol. 2017;179:829–837. doi: 10.1111/bjh.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee A.C.C., Katz J., Blencowe H., et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1:e26–e36. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith E.R., Shankar A.H., Wu L.S.F., et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob Health. 2017;5:e1090–e1100. doi: 10.1016/S2214-109X(17)30371-6. [DOI] [PubMed] [Google Scholar]
- 38.Bramham K., Parnell B., Nelson-Piercy C., Seed P.T., Poston L., Chappell L.C. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flenady V., Koopmans L., Middleton P., et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377:1331–1340. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 40.Gardosi J., Madurasinghe V., Williams M., Malik A., Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013;346:f108. doi: 10.1136/bmj.f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramlakhan K.P., Johnson M.R., Roos-Hesselink J.W. Pregnancy and cardiovascular disease. Nat Rev Cardiol. 2020;17:718–731. doi: 10.1038/s41569-020-0390-z. [DOI] [PubMed] [Google Scholar]
- 42.Rahman M.M., Abe S.K., Kanda M., et al. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: a systematic review and meta-analysis. Obes Rev. 2015;16:758–770. doi: 10.1111/obr.12293. [DOI] [PubMed] [Google Scholar]
- 43.Liu L., Ma Y., Wang N., Lin W., Liu Y., Wen D. Maternal body mass index and risk of neonatal adverse outcomes in China: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19:105. doi: 10.1186/s12884-019-2249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith C., Teng F., Branch E., Chu S., Joseph K.S. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstet Gynecol. 2019;134:1234–1244. doi: 10.1097/AOG.0000000000003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young M.F., Oaks B.M., Tandon S., Martorell R., Dewey K.G., Wendt A.S. Maternal hemoglobin concentrations across pregnancy and maternal and child health: a systematic review and meta-analysis. Ann N Y Acad Sci. 2019;1450:47–68. doi: 10.1111/nyas.14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 46.Martinez-Portilla R.J., Sotiriadis A., Chatzakis C., et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx) Ultrasound Obstet Gynecol. 2020;57:224–231. doi: 10.1002/uog.23575. [DOI] [PubMed] [Google Scholar]
- 47.Vouga M., Favre G., Martinez-Perez O., et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci Rep. 2021;11 doi: 10.1038/s41598-021-92357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernández O., Honorato M., Silva M.C., et al. COVID-19 and pregnancy in Chile: preliminary report of the GESTACOVID multicenter study. Rev Chil Obstet Gynecol. 2020;85:S75–S89. [Google Scholar]
- 49.Knight M., Bunch K., Vousden N., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakowicz A., Ayala A.E., Ukeje C.C., Witting C.S., Grobman W.A., Miller E.S. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnant women. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nachega J., Sam-Agudu N.A., Machekano R.N., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and pregnancy in Sub-Saharan Africa: a 6-country retrospective cohort analysis. Clin Infect Dis. 2022:ciac294. doi: 10.1093/cid/ciac294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lokken E.M., Huebner E.M., Gray Taylor G., et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225:77.e1–77.e14. doi: 10.1016/j.ajog.2020.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalafat E., Yassa M., Koc A., Tug N., the TULIP collaboration Utility of lung ultrasound assessment for probable SARS-CoV-2 infection during pregnancy and universal screening of asymptomatic individuals. Ultrasound Obstet Gynecol. 2020;56:624–626. doi: 10.1002/uog.23099. [DOI] [PubMed] [Google Scholar]
- 54.Brandt J.S., Hill J., Reddy A., et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol. 2021;224:389.e1–389.e9. doi: 10.1016/j.ajog.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
<VIDSE>Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2022.
<VIDSE>Smith. Individual patient data meta-analysis: risk factors among COVID-19 pregnancies. Am J Obstet Gynecol 2022.