Abstract
As Bacillus subtilis proceeds through sporulation, the principal vegetative cell ς subunit (ςA) persists in the cell but is replaced in the extractable RNA polymerase (RNAP) by sporulation-specific ς factors. To explore how this holoenzyme changeover might occur, velocity centrifugation techniques were used in conjunction with Western blot analyses to monitor the associations of RNAP with ςA and two mother cell ς factors, ςE and ςK, which successively replace ςA on RNAP. Although the relative abundance of ςA with respect to RNAP remained virtually unchanged during sporulation, the percentage of the detectable ςA which cosedimented with RNAP fell from approximately 50% at the onset of sporulation (T0) to 2 to 8% by 3 h into the process (T3). In a strain that failed to synthesize ςE, the first of the mother cell-specific ς factors, approximately 40% of the ςA remained associated with RNAP at T3. The level of ςA-RNAP cosedimentation dropped to less than 10% in a strain which synthesized a ςE variant (ςECR119) that could bind to RNAP but was unable to direct ςE-dependent transcription. The E-ςE-to-E-ςK changeover was characterized by both the displacement of ςE from RNAP and the disappearance of ςE from the cell. Analyses of extracts from wild-type and mutant B. subtilis showed that the ςK protein is required for the displacement of ςE from RNAP and also confirmed that ςK is needed for the loss of the ςE protein. The results indicate that the successive appearance of mother cell ς factors, but not necessarily their activities, is an important element in the displacement of preexisting ς factors from RNAP. It suggests that competition for RNAP by consecutive sporulation ς factors may be an important feature of the holoenzyme changeovers that occur during sporulation.
The pivotal event establishing sporulation-specific gene expression in Bacillus subtilis is the reprogramming of the bacterium’s RNA polymerase (RNAP) (52). This occurs when the principal promoter recognition subunit (ςA) of the vegetative cell RNAP is replaced by analogous sporulation-specific subunits (ςE, ςF, ςG, and ςK) (18, 52). These alternative ς factors control both the timing and localization of spore gene expression.
Early in sporulation, B. subtilis partitions itself into two unequal compartments with unique developmental fates. The smaller, forespore compartment is eventually engulfed by the larger, mother cell compartment, which nurtures the forespore as it develops into a mature endospore. Each of the sporulation-specific ς factors is active in only one of these two compartments. Mother cell gene expression is controlled by the sequential appearance of ςE followed by ςK, while forespore-specific genes are activated first by ςF and then by ςG (7, 8, 10, 18, 20, 30, 37, 41, 42, 44, 52, 60). The genes encoding each of the early sporulation ς factors (ςE and ςF) are expressed at the onset of sporulation (31, 36, 56); however, neither of these factors is active until later in development, when the two separate compartments are formed. ςE and ςF are each kept silent by a unique means. ςF is bound to an inactivating anti-ςF protein (SpoIIAB), while ςE is formed as an inactive proprotein, pro-ςE (9, 13, 36, 43, 51). Pro-ςE becomes active only after 27 amino acids are cleaved from its amino terminus (36). The septation event initiates a process that leads to the release of ςF from its antagonist in the forespore (1, 3, 12). ςF then directs the transcription of a gene, spoIIR, whose product in turn triggers pro-ςE processing in the mother cell (23, 29, 39). Once active in their particular compartments, ςE and ςF induce expression of the genes for the sigma factors which will ultimately replace them (ςK and ςG, respectively) (14, 34, 35, 44, 53). ςK and ςG, like their predecessors, are initially inactive. ςK is formed as a proprotein which is processed and activated in response to a signal from the developing forespore (7, 8, 41). ςG’s activity is restricted by SpoIIAB, the same anti-ς protein that inhibited ςF (16, 30). Turnover of SpoIIAB in the forespore is thought to be an important factor in activating ςG (30, 33).
Although much is known about the mechanisms by which the sporulation-specific ς factors are expressed and activated, the process by which they replace ςA on RNAP is still unclear. ςA persists in sporulating B. subtilis (54), and yet by 2 to 3 h after the onset of sporulation, the RNAP extracted from these bacteria is virtually devoid of ςA (19, 38). Early studies of ςA’s displacement from RNAP revealed that treatment of a sporulating B. subtilis culture with the protein synthesis inhibitor chloramphenicol allowed ςA to again become extractable as an RNAP subunit (47). This result was interpreted as evidence for the existence of a short-lived protein inhibitor of ςA that appears in sporulating B. subtilis to block ςA’s binding to RNAP.
Anti-ς factor proteins are now known to restrict the ability of several B. subtilis factors to form RNAP holoenzymes. In addition to ςF and ςG (9, 13, 16, 30, 33), the general stress response (ςB) and motility (ςD) ς factors of B. subtilis are controlled by inhibitory binding proteins (4, 6, 11). It is plausible that a similar protein could be involved in restricting ςA’s access to RNAP in sporulating B. subtilis. Alternatively, the presence of the sporulation-specific ς factors themselves could be the basis for ςA’s exclusion from RNAP. If the sporulation ς factors are effective competitors for a limited pool of core RNAP, their mere presence might be sufficient to deny ςA access.
Using velocity sedimentation techniques to separate RNAP from unassociated ς factors and Western blot analyses to locate RNAP, ςA, ςE, and ςK in these gradients, we revisited the problem of ςA release during sporulation. The pattern of ς factor association with RNAP in wild-type and mutant B. subtilis was found to be consistent with a model in which competition for RNAP by sporulation ς factors is necessary for E-ςA decrease and the changeover from E-ςE to E-ςK that occurs later in development. If unknown sporulation factors facilitate this process, they do not appear to be adequate for separating the preexisting ς factors from RNAP in the absence of the ς proteins that will replace them.
MATERIALS AND METHODS
Plasmids and bacterial strain constructions.
The B. subtilis strains and plasmids used in this study are listed in Table 1. pGEM3C500 is the Escherichia coli vector pGEM-3Zf(+)cat (58) containing a 500-bp PstI DNA fragment from the interior of the spoIIGA coding sequence (25). Transformation of this plasmid into B. subtilis, followed by selection for chloramphenicol acetyltransferase, generates transformants (SE500) in which the plasmid has inserted into spoIIGA and separated sigE (spoIIGB) from its promoter element. pBZ1 was obtained from Lee Kroos (Michigan State University). It carries the sigKCR109 allele cloned downstream of Pspac. An EcoRI/HindIII fragment from pBZ1, carrying Pspac::sigKCR109, was cloned into EcoRI- and HindIII-cut pUS19 (5) to create pUS109CR. B. subtilis BK410 (35) has a deletion of the 3′ end of sigK (spoIIIC94). Transformation of pUS109CR into BK410 and selection for the vector-encoded Spcr results in single-site recombinants in which the vector has integrated within the 5′ end of sigK. This creates a single intact sigK gene, controlled by its normal promoter, and an inactive, truncated sigK gene downstream of the vector sequence. Pspac. Spo− clones would have the sigK109CR mutation included in the intact gene. BK410-1 was one such clone. S410 is SMY transformed with chromosomal DNA from BK410-1 and with selection for the sigK109CR-linked Spcr. pUS117CR is a 1.1-kbp DNA fragment carrying the sigE117CR allele cut from pJ89CR117 (27) with PstI and cloned into PstI-cut pUS19. SE117CR is SMY transformed with pUS117CR, with selection for the vector-encoded Spcr, followed by screening for the Spo− phenotype of the sigE117CR allele. SF9030 is SMY transformed with chromosomal DNA from EUR9030 (30) and selection for the spoIIAC-disrupting erm cassette. The SigE− SigF− strain (SEF500) was generated by transforming SE500 (SigE−) with this same EUR9030 DNA.
TABLE 1.
B. subtilis strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or features | Source, construction, or reference |
|---|---|---|
| Strains | ||
| SMY | trpC | Laboratory strain |
| BK410 | spoIIIC94 | 35 |
| EUR9030 | spoIIAC::erm | 30 |
| M01027 | spoIVCB::erm | P. Stragier |
| AG514 | Δspo0A::cat | A. Grossman |
| SF9030 | spoIIAC::erm | EUR9030→SMY |
| SK1027 | spoIVCB::erm | 701027→SMY |
| SOA514 | Δspo0A::cat | AG514→SMY |
| BK410-1 | sigKB109CR | pUS109CR→BK140 |
| S410 | sigK109CR | BK410-1→SMY |
| SE117CR | sigE117CR | pUS117CR→SMY |
| SE500 | spoIIGA::pGEM3C500 | pGEM3C500→SMY |
| SEF500 | spoIIAC::erm spoIIGA::pGEM3C500 | EUR9030→SE500 |
| Plasmids | ||
| pUS19 | Apr Spcr | 4 |
| pGEM3Zf(+)cat | Apr Cmr | 58 |
| pGEM3C500 | Apr Cmr 500-bp spoIIGA DNA fragment in pGEM3Zf(+)cat | This study |
| pBZ1 | Apr KmrPspac::sigK109CR | L. Kroos |
| pUS109CR | Apr SpcrPspac::sigK109CR | This study |
| pJ89CR117 | KmrsigE117CR | 27 |
| pUS117CR | Apr SpcrsigE117CR | This study |
Induction of sporulation.
B. subtilis, grown overnight in Luria broth, was diluted 1/20 into Difco Sporulation medium (DSM) and incubated at 37°C. The onset of sporulation (T0) was taken as the time that the culture stopped exponential growth.
Velocity gradient analysis.
Cells were harvested into equal volumes of crushed ice at various times after the onset of sporulation, washed in 1 M NaCl, concentrated 20-fold, and disrupted in a resuspension buffer (10 mM Tris [pH 8.0], 1 mM EDTA, 50 mM NaCl, 10 mM MgCl2, 0.3 g of phenylmethylsulfonyl fluoride per liter, 3 mM dithiothreitol) by passage (three times) through a French press at 12,000 lb/in2. Cell debris was removed by a low-speed centrifugation (10,000 rpm, 45 min, SS-34 rotor [Sorvall]). Supernatant samples (0.5 ml) were applied onto 11-ml linear glycerol gradients (15 to 30% glycerol in resuspension buffer) and centrifuged for 22 to 24 h at 37,000 rpm and 4°C in a Sorvall TH641 rotor. After centrifugation, 0.5-ml samples were collected from the bottom of the tube. Two volumes of ethanol were added to this sample. This precipitated 80 to 90% of the protein, without apparent preference for any of the proteins under investigation. The precipitated material was resuspended in sample buffer and analyzed by Western blotting. When Triton was used in the analysis, it was added to both the sample buffer (1.0%) and the glycerol gradient (0.1%).
Western blot analysis.
Fractions from the velocity gradient were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on gels containing 10% acrylamide. Subsequent steps were as done previously (11). The anti-β′ and anti-ςA mouse monoclonal antibodies were obtained as previously described, using B. subtilis core RNAP and ςA-His6 as the inoculated antigens (11). The anti-ςE monoclonal antibody has been described previously (55). A sample of rabbit polyclonal antibody against ςK (60) was provided by L. Kroos. Bound antibody was detected with alkaline phosphatase-conjugated goat anti-mouse or goat anti-rabbit antibodies, as appropriate. Western blot data were quantitated with an AlphaImager 2000 (Alpha Innoteck Corp., San Leandro, Calif.) and its associated software.
General methods.
DNA manipulations were performed by standard protocols. Transformation of naturally competent B. subtilis cells was carried out as described by Yasbin et al. (57).
RESULTS
Patterns of association of ςA with RNAP.
RNAP purified from sporulating B. subtilis contains little ςA; nevertheless, ςA can be immunoprecipitated from crude extracts of such cells (54). In an attempt to better define the status of ςA in sporulating B. subtilis, we prepared an anti-ςA monoclonal antibody and used it as a probe to monitor ςA’s abundance and RNAP association in B. subtilis extracts.
In an initial experiment, we examined the ςA levels in sporulating B. subtilis and compared this value to the ςA level which existed previously. Extracts were prepared from B. subtilis that had been harvested at the onset of sporulation and at 1.5-h intervals thereafter and analyzed by Western blotting as described in Materials and Methods. As noted by others (40, 54), the ratio of ςA to the core RNAP β′ subunit was found to be essentially unchanged as the cells proceeded through 4.5 h of sporulation (T4.5) (Table 2). Thus, a significant drop in the ςA/core RNAP ratio is not responsible for ςA’s disappearance from the sporulating cell’s extractable RNAP.
TABLE 2.
Relative ςA abundance
| Time | Avg pixel contenta
|
ςA/β′ | |
|---|---|---|---|
| β′ | ςA | ||
| T0 | 81,830 | 53,452 | 0.65 |
| T1.5 | 76,464 | 48,952 | 0.64 |
| T3.0 | 77,395 | 51,982 | 0.67 |
| T4.5 | 67,968 | 36,579 | 0.54 |
Average pixel contents of the β′ and ςA portions of Western blots of crude B. subtilis extracts from cells harvested at the time indicated. Each number is the average of eight determinations. The average deviation was approximately 10% for samples analyzed on the same membrane.
Velocity centrifugation techniques can readily separate RNAP holoenzymes (approximately 5 × 105 Da) from free ς subunits (typically 2.5 × 104 to 5 × 104 Da) and had been used by others to monitor the association of mutant ς factors with RNAP (49). We applied this technique, in conjunction with Western blot analyses, to address the question of ς factor-RNAP associations during sporulation. We first asked whether the RNAP-ς factor fractionation profile seen following velocity centrifugation would resemble the sporulation-specific changes in RNAP holoenzyme composition that had been documented previously.
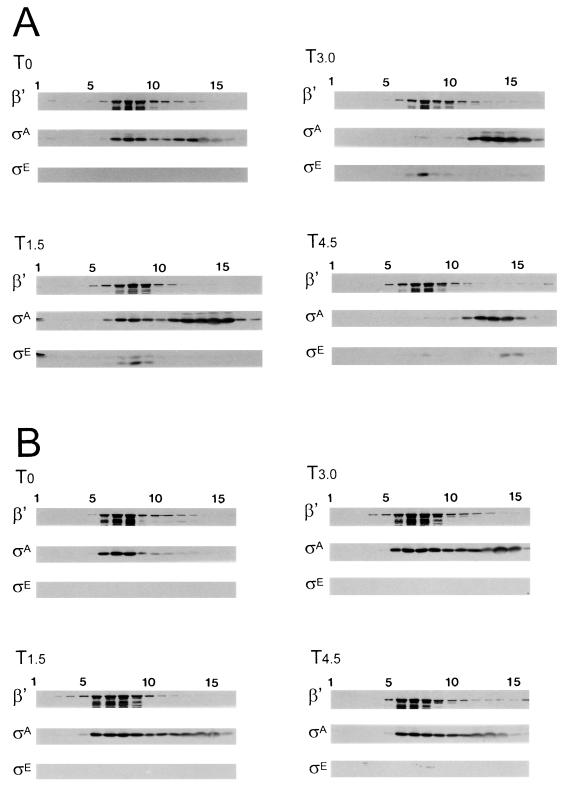
The most obvious change in the RNAP profile of sporulating B. subtilis occurs at approximately T2 to T3. At this time, E-ςA becomes rare and E-ςE, a holoenzyme carrying the first of the mother cell-specific ς factors, becomes evident. The loss of ςA from RNAP and the synthesis of ςE do not occur if sporulation is blocked by mutation in the spo0A gene (19). In such cells, E-ςA remains extractable under culture conditions that induce ςA displacement in wild-type B. subtilis. Crude extracts were prepared from wild-type and spo0A mutant cells and subjected to centrifugation through 15 to 30% glycerol gradients. The gradients were then fractionated and analyzed by Western blotting with monoclonal antibodies against ςA, ςE, and the core RNAP β′ subunit as probes. At the onset of sporulation, approximately half of the ςA present in the wild-type B. subtilis extract cosedimented with the RNAP marker (β′) (Fig. 1A, T0). By 3 h into the process (Fig. 1A, T3), most of the ςA no longer moved with the RNAP marker and instead was found higher in the gradient. A similar analysis of the spo0A extract (Fig. 1B) revealed a persistent association of ςA with RNAP throughout this same period.
FIG. 1.
Fractionation of extracts from sporulating and Spo0A− B. subtilis. Wild-type B. subtilis SMY (A) and its congenic Spo0A− variant S0A514 (B) were grown in DSM. Samples were harvested at the end of exponential growth (T0) or at 1.5-h intervals thereafter (T1.5, T3, and T4.5), the cells were disrupted, and the resulting crude extracts were fractionated by centrifugation through a linear gradient of 15 to 30% glycerol, as described in Materials and Methods. Fractions were collected and analyzed by Western blotting with anti-β′, anti-ςA, and anti-ςE antibodies as probes. Fraction 1 represents the bottom of the centrifuge tube. The two bands detected by the anti-ςE antibody in panel A at T1.5 represent ςE and its slightly larger precursor (pro-ςE).
The Western blot data for the T3 extracts were quantitated by densitometry to estimate the relative abundance of each protein in the separate fractions (Table 3). In both the wild-type and Spo0A mutant extract gradients, fractions 4 to 10 contained approximately 85% of the RNAP β′ marker. These same fractions contained approximately 50% of the Spo0A extract’s ςA but only 2% of the ςA that was present in the wild-type extract. In order to verify that the estimates of ςA in the various fractions were based on measurements that were in a linear response range, fractions that corresponded to the RNAP-containing fractions and those that did not contain RNAP were pooled, serially diluted, and analyzed by Western blotting for RNAP and ς factor contents. The amount of the ςA component cosedimenting with the RNAP marker was consistently less than 10% of the ςA that was detectable in cells that had progressed beyond the initial stages of sporulation, while it remained at 50% or greater in Spo0A− extracts (data not shown). The experiment also revealed that the amount of extract that we analyzed, although optimal for the ς factor signal, had begun to saturate the β′ signal in the peak β′ fractions. Thus, the fractions indicated as the principal RNAP-containing fractions contain even more of the RNAP than depicted in the figures or tables.
TABLE 3.
Partitioning of ςA and ςE at T3
| Strain | Protein | % in fractionsa:
|
||
|---|---|---|---|---|
| 1–3 | 4–10 | 11–16 | ||
| Wild type | β | 0 | 87.8 | 12.2 |
| ςA | 0 | 2.0 | 98 | |
| ςE | 0 | 69.4 | 30.6 | |
| Pro-ςE | 0 | 100 | 0 | |
| Spo0A− | β | 0 | 82.7 | 17.3 |
| ςA | 0 | 52.5 | 47.5 | |
| SigE− | β | 1.3 | 84.5 | 14.2 |
| ςA | 0 | 30.5 | 69.5 | |
| SigF− | β | 14.3 | 65.2 | 20.4 |
| ςA | 0 | 32.8 | 67.1 | |
| Pro-ςE | 50.8 | 42.9 | 6.1 | |
| SigE− SigF− | β | 0 | 82.7 | 17.3 |
| ςA | 0 | 29.1 | 70.9 | |
| SigECR117 | β | 6.0 | 79.3 | 14.7 |
| ςA | 0 | 7.5 | 92.5 | |
| ςE | 5.6 | 81.2 | 0 | |
| Pro-ςE | 18.8 | 75.1 | 19.3 | |
Percentage of the bound antibody that was detected in a Western blot of the indicated fractions obtained after a nonequilibrium centrifugation experiment (see Materials and Methods). Fraction 1 represents the bottom of the gradient; fraction 16 represents the top.
The Western blot analyses of the wild-type B. subtilis strain also revealed some interesting aspects of the distributions of pro-ςE and ςE in these extracts (Fig. 1A). Pro-ςE and ςE were detectable in the T1.5 extract. Virtually all of the ςE, and a portion of the pro-ςE, cosedimented with the RNAP marker, while most of the pro-ςE moved to a position below RNAP in the gradient. The “pro” sequence of ςE is believed to tether pro-ςE to the B. subtilis cytoplasmic membrane (22, 23, 28). It is likely that the fast-sedimenting forms of pro-ςE represent molecules of pro-ςE that are associated with membrane components. In Triton-treated extracts, pro-ςE no longer sediments to the bottom of the gradient but is found either in the RNAP fractions or higher in the gradient, while the sedimentation pattern of ςE is unaltered (data not shown). At T3, only mature ςE was evident in the gradient fractions. In this and the earlier (T1.5) sample, almost all of the detectable ςE was restricted to the RNAP-containing fractions, while by T4.5 most of the ςE was no longer associated with RNAP but sedimented as if it was free within the extract (Fig. 1A). Apparently, ςE readily associates with RNAP early in sporulation, but between T3 and T4.5, its ability to remain bound to RNAP declines.
We conclude from these experiments that the velocity gradient technique allows us to monitor ς-RNAP associations during sporulation, that ςA displacement from RNAP is largely complete by T3, and that ςE, like ςA, becomes less likely to be RNAP bound as sporulation progresses.
Effect of sigma factor mutations on E-ςA persistence.
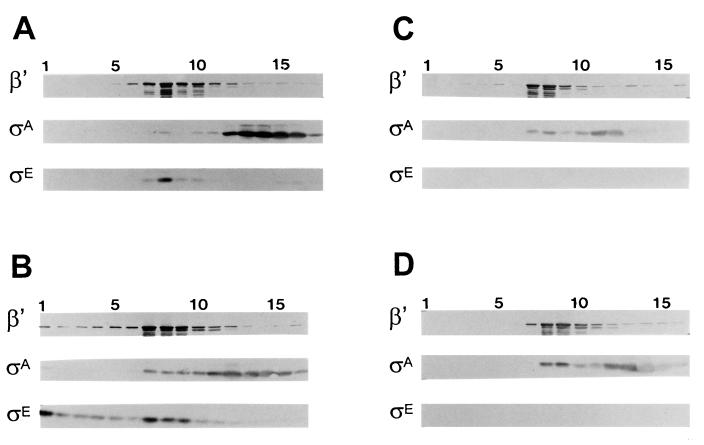
ςA dissociates from RNAP in sporulating B. subtilis but continues to form an RNAP holoenzyme in Spo0A-deficient cells. Among the genes whose expression depends on Spo0A are those which encode the first of the mother cell- and forespore-specific sigma factors, ςE and ςF, respectively (2, 45, 46, 56). To determine whether either of these early-sporulation sigma factors is required, directly or indirectly, for the displacement of ςA from RNAP, we repeated the fractionation analysis with extracts that had been prepared from B. subtilis strains lacking one or both of these transcription factors. Representative Western blot analyses of extracts fractionated by velocity gradient centrifugation are illustrated in Fig. 2. The extracts were prepared from sporulating cultures of the mutant strains that had been harvested at T3. The distribution of the ς factors in these gradients is quantitated in Table 3. The loss of ςE, ςF, or both proteins allowed a substantial retention of the ςA in the RNAP fractions (Fig. 2). In each of the three mutant strains, approximately 30% of the extract’s ςA sedimented in the peak RNAP fractions (Table 3).
FIG. 2.
Fractionation of SigE− and SigF− B. subtilis. Wild-type B. subtilis (SMY) (A) and its congenic SigF− (SF9030) (B), and SigE− (SEF500) (C), and SigE− SigF− (SEF500) (D) variants were grown to T3 in DSM and analyzed as described for Fig. 1. The protein band reacting with the anti-ςE antibody is ςE in wild-type B. subtilis (A) and pro-ςE in the SigF− strain (B).
ςA is believed to be dispersed at equivalent concentrations in the mother cell and forespore (40). It is therefore reasonable to assume that any factor that causes a significant displacement of ςA from RNAP would have to be active in the large mother cell compartment, where the bulk of the ςA should lie. The loss of either the forespore-specific ςF or the mother cell-specific ςE caused a similar retention of ςA in the RNAP fraction. This suggests that the mother cell factor responsible for displacing ςA requires ςF for its appearance. ςF-dependent transcription in the forespore is needed for pro-ςE processing (29, 39). Thus, mature ςE could be the missing factor necessary for the bulk of the ςA displacement. A need for mature ςE would indicate either that processed ςE, but not its proprotein, can compete with ςA for RNAP or that a ςE-dependent gene product is the actual effector of ςA displacement.
To distinguish between these possibilities, we next analyzed the ςA-RNAP association profile in a B. subtilis strain whose only source of ςE is an allele (sigECR117) which encodes a product that can bind to RNAP but fails to recognize ςE-dependent promoters (27). If competition between ςE and ςA for RNAP is needed for ςA’s dissociation from RNAP, then ςA should still be displaced in the sigECR117 mutant strain. Alternatively, if a ςE-dependent gene product is responsible, the sigECR117 allele should be equivalent to a sigE null mutation and allow E-ςA to persist. When the ςA pattern in cells harvested at T3 of sporulation was analyzed, only 7.5% of the ςA remained associated with the RNAP during the centrifugation analysis (Table 3). It therefore appears that the presence of the mature ςE protein itself is more important than ςE’s transcriptional activity for ςA displacement from RNAP.
Effect of chloramphenicol on ςE persistence and ςA RNAP displacement.
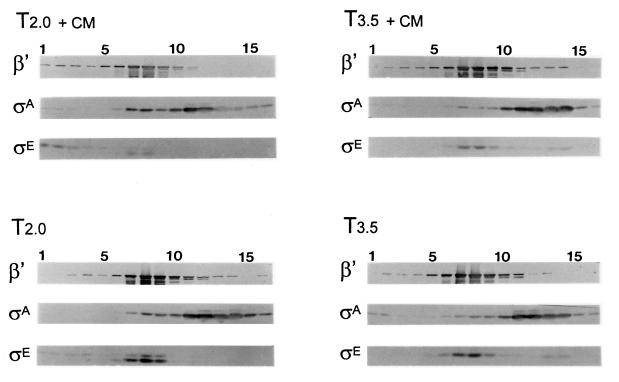
We next revisited the observation that E-ςA could be more readily isolated from sporulating B. subtilis if the culture was pretreated with chloramphenicol (47). In a previous study of the effects of antibiotics on ςE processing and turnover, we found that ςE, once formed, persists in chloramphenicol-treated cells (26). We therefore looked at the effect of chloramphenicol treatment on the ςA association with RNAP at different times in sporulation. Chloramphenicol treatment could influence the cosedimentation of ςA with RNAP, but the time at which treatment was applied had a significant effect on the outcome. When chloramphenicol was added to the culture before significant ςE accumulation and ςA displacement, at T1.5, ςE synthesis was compromised, and cells harvested 0.5 h later, at T2, did not show as great a degree of ςA displacement as an untreated culture at T2 (Fig. 3). However, when a culture was treated with chloramphenicol after ςE had accumulated and ςA was largely displaced, at T3, ςE persisted, and there was no obvious difference at T3.5 in the amount of ςA that cosedimented with the RNAP in either the treated or untreated cultures (Fig. 3). Thus, in our hands, chloramphenicol treatment did not return displaced ςA to RNAP but could block its initial displacement. The data sustain a correlation between the presence of ςE and the decrease in E-ςA.
FIG. 3.
Effect of chloramphenicol treatment on E-ςA persistence. Wild-type B. subtilis (SMY) was grown in DSM. At 1.5 and 3 h after the end of exponential growth, chloramphenicol was added to portions of the culture, which were harvested 0.5 h later. T2.0 + CM and T2.0 represent portions of the culture harvested at 2 h with and without chloramphenicol treatment, respectively. T3.5 + CM and T3.5 are similar cultures harvested at T3.5. The culture samples were analyzed as described for Fig. 1. The anti-ςE antibody detected pro-ςE and ςE at T2 and only ςE at T3.5.
Replacement of E-ςE by E-ςK.
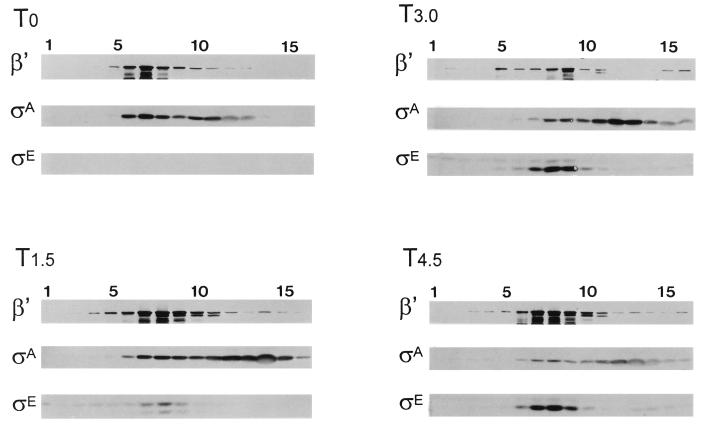
Sigma factor substitution is an ongoing process during sporulation (52). In the mother cell compartment, ςE, which becomes the principal ς factor on RNAP by T3, is itself supplanted on RNAP after T3 (Fig. 1A). There is an aspect to the E-ςE loss that is distinct from E-ςA replacement. ςA persists in the cell even though E-ςA declines. In contrast, both E-ςE and ςE protein levels fall after ςE’s period of activity (55, 60, 61). The previously documented decline of ςE, as well as its dissociation from RNAP, can be noted in Fig. 1A (T4.5). By T4.5 the bulk of the remaining ςE has become displaced from the RNAP-containing fractions and sediments higher in the gradient. Presumably, this slower-migrating species represents free ςE. Zhang and Kroos have found that ςE is negatively regulated by ςK (60, 61). They showed that ςK reduced ςE-dependent transcription and, more recently, the synthesis of ςE itself (60, 61). We therefore examined the effects of a ςK loss on the ςE profile in our systems. Our data (Fig. 4) confirm the finding of Zhang and Kroos that ςE persists in the absence of ςK and, in addition, show that ςE continues to cofractionate with RNAP in the ςK-deficient strain (e.g., compare Fig. 1A, T4.5, with Fig. 4, T4.5). ςK itself, or a ςK-dependent process, thus affects both ςE levels and ςE’s ability to associate with RNAP.
FIG. 4.
Fractionation of crude extracts from SigK− B. subtilis. SigK− B. subtilis (SK1027) was grown in DSM, and samples were harvested at the end of growth (T0) and at 1.5-h intervals thereafter (T1.5, T3.0, and T4.5). The samples were analyzed as described for Fig. 1. The anti-ςE antibody detects pro-ςE and ςE at T1.5 and T3 and primarily ςE at T4.5.
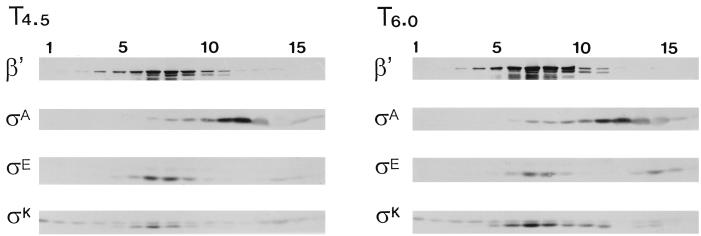
In an attempt to distinguish between ςK, as competitor of ςE, or an unknown gene product under ςK’s control as an effector of ςE displacement and loss, we examined the ςE profile in a B. subtilis mutant whose ςK (ςKCR109) could bind RNAP but could not recognize ςK-dependent promoters (61). There is a limitation to this experiment which was not encountered in the similar experiment with the ςECR119 mutant. sigE is expressed from a ςA-dependent promoter (32), and therefore, ςE synthesis is unaffected by the activity of the ς factor product that is made. sigK, in contrast, is transcribed first by E-ςE and then by E-ςK (34, 35). Given that the ςKCR109 protein is ineffective as a ς factor, the ςK protein levels in this strain remain dependent on E-ςE. Western blot analyses of extracts prepared from sporulating wild-type and sigKCR109 mutant strains revealed that the mutant strain formed approximately 25% of the ςK protein that was found in wild-type B. subtilis (data not shown). Perhaps due to this lower ςK level and a continued dependence on E-ςE for ςK synthesis, ςE persisted in the ςKCR109 strain but nevertheless became increasingly displaced from RNAP as the mutant ςK protein accumulated (Fig. 5). Quantitation of the antibody reactions in Fig. 5 revealed that the ςE abundance was essentially unchanged between T4.5 and T6 (i.e., the ςE antibody signal at T4.5 was 94.3% of the signal at T6.0 [62.5 × 103 and 66.3 × 103 pixels, respectively]); however, the percentage of unbound ςE rose from 14.5 to 30%. During this same period, the abundance of ςKCR109 increased 2.4-fold, with virtually all of the ςK being in the RNAP-containing fractions. The disproportionate association of ςK with RNAP, relative to that of ςE, and the coincidence of an approximately twofold increase in ςK abundance with a twofold increase in free ςE suggest that ςK can compete with ςE and displace it from RNAP in these extracts.
FIG. 5.
Fractionation of crude extracts from sigK109CR B. subtilis. B. subtilis S410 (sigK109CR) was grown in DSM. Samples harvested at 4.5 h (T4.5) and 6 h (T6) after the end of growth were analyzed as described for Fig. 1 with antibodies against β′, ςA, ςE, and ςK as probes. The anti-ςE antibody detected ςE. The anti-ςK antibody detected ςK and its slower-migrating precursor, pro-ςK.
The anti-ςK antibody detects both ςK and its larger precursor form (pro-ςK). Pro-ςK, like pro-ςE, is believed to be associated with cell membrane components (59). This likely accounts for pro-ςK’s distribution at the bottom, and throughout, the gradient (Fig. 5). The distribution of pro-ςK in these gradients differs somewhat from the profile which we had observed for pro-ςE in similar gradients. Pro-ςE was more highly enriched in the RNAP-containing fractions and appeared to either cosediment with RNAP or migrate as faster-sedimenting, and presumably membrane-bound, forms (Fig. 1A [T1.5], 2B, 3, and 4 [T1.5]). The pro-ςK, in contrast, was spread more diffusely throughout the gradient, with less of it in the RNAP fractions and a portion of it near the top of the gradient, where it is free from high-molecular-weight associations (Fig. 5). Pro-ςE thus appears more likely than pro-ςK to be associated with RNAP. Whether this is due to inherent differences between the two proproteins in their capacity for RNAP binding or to an effect of other components in the extract at the time of their synthesis (e.g., ςE present to compete with pro-ςK) is not clear.
DISCUSSION
As B. subtilis proceeds into sporulation, its extractable RNAP contains decreasing amounts of the principal vegetative cell ς factor, ςA, and increasing amounts of sporulation-specific ς factors (18, 52). Early in sporulation (T2 to T3), the principal RNAP holoenzyme species that can be isolated contains ςE (19), the first sporulation-specific ς factor that is present in the large mother cell compartment (10). After T4, the level of ςE falls and the second mother cell holoenzyme, E-ςK, appears and continues the next stage of transcription in that compartment (55, 60). In the present study we have examined some of the properties of the E-ςA→E-ςE→E-ςK progression. We found, as have others (40), that ςA and RNAP levels remain relatively constant until at least T4.5 of sporulation. Nevertheless, by 3 h into this process, the percentage of ςA that cosediments with RNAP falls from 50% to less than 10% (Fig. 1A). In contrast, ςA association with RNAP remains at 50% in a B. subtilis strain which is unable to sporulate due to a mutation in the sporulation-essential spo0A gene (Fig. 1B). Thus, as initially reported more than 20 years ago, ςA persists in sporulating B. subtilis but becomes displaced from RNAP by sporulation-specific factors (47, 54).
Although our gradient analyses showed a progressive loss of E-ςA as B. subtilis proceeds into sporulation, a recent study, using a histidine tag to withdraw RNAP from B. subtilis extracts, found E-ςA to persist throughout sporulation (17). We are unable to explain the differences in our findings. The absence of ςA has long been a hallmark of RNAP from sporulating B. subtilis (19, 38, 47, 54). Given that ςA persists during sporulation, it is possible that some feature of the Ni2+ resin extraction technique allowed it to reassociate with RNAP during the purification.
Based on immunofluorescence experiments, ςA is believed to be in roughly equal concentrations in the mother cell and forespore (40). The mother cell compartment of a typical B. subtilis cell is at least five times the size of the forespore. Therefore, whatever is responsible for ςA displacement during sporulation needs to be active in the large mother cell, where the bulk of the RNAP and ςA is likely to be found. Our data implicate ςE, the first of the mother cell-specific ς factors, as having a direct role in ςA displacement. Mutations which block ςE synthesis alter the ςA retention on RNAP from the few percent found in wild-type B. subtilis to a level almost as great as that seen in the spo0A mutant. ςE’s role in ςA dissociation from RNAP appears to be related to the presence of the ςE protein rather than its transcriptional activity. A mutant ςE (ςECR119), which can bind to RNAP but cannot direct RNAP to ςE promoters, reduced the amount of ςA that cosedimented with RNAP from the 30% observed in the absence of ςE to below 10%. The notion that ςE is preferentially bound to RNAP in early sporulating cells is supported by the distribution of ςE in the gradient analyses. Until ςK appears, virtually all of the detectable ςE in the crude extracts cosediments with RNAP (Fig. 1A, 2, 3, and 4), while a significant portion of the ςA in the extracts is unassociated. Either a sporulation-specific factor, which does not depend on ςE for its expression, weakens ςA’s ability to compete with ςE or ςE is an inherently more potent RNAP binding protein than is ςA. The latter possibility is attractive. ςA, but not ςE, is released from RNAP when these holoenzymes are bound to phosphocellulose (19, 48). This implies that ςE binds more tightly to RNAP than does ςA. In addition, the synthesis of sporulation ς factors in vegetatively growing B. subtilis induces the expression of genes whose transcription depends on them (see, e.g., references 24, 44, 46, 50, and 51). Thus, sporulation ς factors can gain access to RNAP to at least some degree in the presence of ςA without the aid of additional sporulation factors.
In wild-type strains, active ςE initiates the synthesis of ςK, the second of the two mother cell ς factors. In such strains, there is a displacement of ςE from RNAP and a drop in ςE protein levels. Zhang and Kroos have documented that the decrease in ςE levels is dependent on active ςK (60, 61). Our current data confirm the need for ςK in ςE disappearance and also show that the presence of ςK protein alters the profile of ςE’s association with RNAP. Before the appearance of ςK, or in mutant B. subtilis that cannot synthesize ςK, most of the ςE present in crude extracts cosediments with RNAP (Fig. 1A [T3] and 4); however, after ςK’s appearance, a significant portion of the ςE becomes displaced from RNAP (Fig. 1A [T4.5] and 5). Coincident with the ςE displacement, virtually all of the mature ςK is found in the RNAP-containing fractions (Fig. 5). Thus, ςK effectively competes with ςE for RNAP, with the result that E-ςK becomes a preferred holoenzyme species.
Competition among ς factors has been implicated as an element of postexponential gene expression in both E. coli and B. subtilis. The stationary-phase ς factor of E. coli, ςS, appears to compete with this organism’s housekeeping ς70 during the transition to stationary phase (15). Overproducing either ς70 or ςS was found to shift the pattern of transcription in stationary-phase E. coli in favor of those promoters recognized by the ς factor that was overexpressed (15). More germane to our present study, Hicks and Grossman have found that altering ςA levels in B. subtilis affects gene expression by the sporulation-essential, transition state ς factor ςH (21). Enhanced or restricted ςA production during growth was found to decrease or increase, respectively, ςH-dependent gene expression. Overproduction of ςA also delayed the production of heat-resistant spores, an outcome that might be expected if, as Hicks and Grossman suggested, sporulation-specific ς factors had to compete with this ςA pool for a limited population of RNAP core (21).
Recently, Lord et al. analyzed the replacement of ςA by the forespore-specific ς factor ςF (40). They noted, as did we, that the intracellular concentrations of core RNAP and ςA were virtually unchanged during the first 3 h of sporulation. In addition, they determined that by the time the first sporulation ς factors are activated (i.e., after septation), the concentration of ςA and ςF exceeds the concentration of RNAP and that competition for core RNAP must be occurring (40). Although the simplest model for ς factor substitution would have ςF as a more effective competitor for RNAP than ςA, Lord et al. found that ςA’s affinity for RNAP was 25-fold greater than that of ςF (40). Based on this disparity, they proposed that an anti-ςA factor is synthesized or activated during sporulation to allow ςF to successfully compete for RNAP. If this or a similar hypothetical factor also participates in the exchange of ςE for ςA, our data argue that its appearance depends on Spo0A, but not active ςE or ςF, and that it cannot in itself remove ςA from RNAP but would instead enhance the competitiveness of the sporulation ς factors to displace ςA. In vitro studies of the affinities of purified ςA, pro-ςE, and ςE for RNAP, as well as transcription competition analyses with purified proteins, will be useful in determining whether ςE has the affinity for RNAP that would allow it to directly supplant ςA on RNAP.
ACKNOWLEDGMENTS
This work was supported by NSF grant MCB-9727927.
We thank Lee Kroos for stimulating discussions, unpublished results, bacterial strains, and anti-ςK antibody. We also thank Alan Grossman, Charles Moran, and Patrick Stragier for providing strains and/or plasmids.
REFERENCES
- 1.Arigoni F, Duncan L, Alper S, Losick R, Stragier P. SpoIIE governs the phosphorylation state of a protein regulating transcription factor ςF in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:3238–3242. doi: 10.1073/pnas.93.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldus J M, Buckner C M, Moran C P. Evidence that the transcriptional activator Spo0A interacts with two sigma factors in Bacillus subtilis. Mol Microbiol. 1995;17:281–290. doi: 10.1111/j.1365-2958.1995.mmi_17020281.x. [DOI] [PubMed] [Google Scholar]
- 3.Bárak I, Behari J, Olmedo G, Guzman P, Brown D P, Castro E, Walker D, Westpheling J, Youngman P. Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol Microbiol. 1996;19:1047–1060. doi: 10.1046/j.1365-2958.1996.433963.x. [DOI] [PubMed] [Google Scholar]
- 4.Benson A K, Haldenwang W G. Bacillus subtilis ςB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc Natl Acad Sci USA. 1993;90:2330–2334. doi: 10.1073/pnas.90.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson A K, Haldenwang W G. Regulation of ςB levels and activity in Bacillus subtilis. J Bacteriol. 1993;175:2347–2356. doi: 10.1128/jb.175.8.2347-2356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caramori T, Barilla D, Nessi C, Sacchi L, Galizzi A. Role of FlgM in ςD-dependent gene expression in Bacillus subtilis. J Bacteriol. 1996;178:3113–3118. doi: 10.1128/jb.178.11.3113-3118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutting S, Driks A, Schmidt R, Kunkel B, Losick R. Forespore-specific transcription of a gene in the signal transduction pathway that governs pro-ςK processing in Bacillus subtilis. Genes Dev. 1991;5:456–466. doi: 10.1101/gad.5.3.456. [DOI] [PubMed] [Google Scholar]
- 8.Cutting S, Oke V, Driks A, Losick R, Lu S, Kroos L. A forespore checkpoint for mother-cell gene expression during development in Bacillus subtilis. Cell. 1990;62:239–250. doi: 10.1016/0092-8674(90)90362-i. [DOI] [PubMed] [Google Scholar]
- 9.Diederich B, Wilkinson J F, Magnin T, Najafi S M A, Errington J, Yudkin M D. Role of interactions between SpoIIAA and SpoIIAB in regulating cell-specific transcription factor ςF of Bacillus subtilis. Genes Dev. 1994;8:2653–2663. doi: 10.1101/gad.8.21.2653. [DOI] [PubMed] [Google Scholar]
- 10.Driks A, Losick R. Compartmentalized expression of a gene under the control of sporulation transcription factor ςE in Bacillus subtilis. Proc Natl Acad Sci USA. 1991;88:9934–9938. doi: 10.1073/pnas.88.22.9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufour A, Voelker U, Voelker A, Haldenwang W G. Relative levels and fractionation properties of Bacillus subtilis ςB and its regulators during balanced growth and stress. J Bacteriol. 1996;178:3701–3709. doi: 10.1128/jb.178.13.3701-9sigma.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan L S, Alper S, Arigoni F, Losick R, Stragier P. Activation by cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science. 1995;270:641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- 13.Duncan L, Losick R. SpoIIAB is an anti-ς factor that binds to and inhibits transcription by regulatory protein ςE from Bacillus subtilis. Proc Natl Acad Sci USA. 1993;90:2325–2329. doi: 10.1073/pnas.90.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Errington J, Rong S, Rosenkrantz M, Sonenshein A L. Transcriptional regulation and structure of the Bacillus subtilis sporulation locus spoIIIC. J Bacteriol. 1988;170:1162–1167. doi: 10.1128/jb.170.3.1162-1167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farewell A, Kvint K, Nystrom T. Negative regulation by RpoS: a case of sigma factor competition. Mol Microbiol. 1998;29:1039–1051. doi: 10.1046/j.1365-2958.1998.00990.x. [DOI] [PubMed] [Google Scholar]
- 16.Foulger D, Errington J. Effects of new mutations in the spoIIAB gene of Bacillus subtilis on the regulation of ςF and ςG activities. J Gen Microbiol. 1993;139:3197–3203. doi: 10.1099/00221287-139-12-3197. [DOI] [PubMed] [Google Scholar]
- 17.Fujita M, Sadaie Y. Rapid isolation of RNA polymerase from sporulating cells of Bacillus subtilis. Gene. 1998;221:185–190. doi: 10.1016/s0378-1119(98)00452-1. [DOI] [PubMed] [Google Scholar]
- 18.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haldenwang W G, Lang N, Losick R. A sporulation-induced sigma-like regulatory protein from B. subtilis. Cell. 1981;23:615–624. doi: 10.1016/0092-8674(81)90157-4. [DOI] [PubMed] [Google Scholar]
- 20.Harry E J, Pogliano K, Losick R. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:3386–3393. doi: 10.1128/jb.177.12.3386-3393.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hicks K A, Grossman A P. Altering the level and regulation of the major sigma subunit of RNA polymerase affects gene expression and development in Bacillus subtilis. Mol Microbiol. 1996;20:201–212. doi: 10.1111/j.1365-2958.1996.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 22.Hofmeister A. Activation of the proprotein transcription factor pro-ςE is associated with its progression through three patterns of subcellular localization during sporulation in Bacillus subtilis. J Bacteriol. 1998;180:2426–2433. doi: 10.1128/jb.180.9.2426-2433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmeister A E M, Londono-Vallejo A, Harry E, Stragier P, Losick R. Extracellular signal protein triggering the proteolytic activation of a development transcription factor in B. subtilis. Cell. 1995;83:219–226. doi: 10.1016/0092-8674(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 24.Illing N, Errington J. The spoIIIA operon of Bacillus subtilis defines a new temporal class of mother-cell-specific sporulation genes under the control of the ςE form of RNA polymerase. Mol Microbiol. 1991;5:1927–1940. doi: 10.1111/j.1365-2958.1991.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 25.Jonas R M, Weaver E A, Kenney T J, Moran C P, Haldenwang W G. The Bacillus subtilis spoIIG operon encodes both ςE and a gene necessary for ςE activation. J Bacteriol. 1988;170:507–511. doi: 10.1128/jb.170.2.507-511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonas R M, Holland S C, Haldenwang W G. Effects of antibiotics on synthesis and persistence of ςE in sporulating Bacillus subtilis. J Bacteriol. 1990;172:4616–4623. doi: 10.1128/jb.172.8.4616-4623.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones C H, Tatti K M, Moran C P., Jr Effects of amino acid substitutions on the −10 binding region of ςE from Bacillus subtilis. J Bacteriol. 1992;174:6815–6821. doi: 10.1128/jb.174.21.6815-6821.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju J, Luo T, Haldenwang W G. Bacillus subtilis pro-ςE fusion protein localizes to the forespore septum and fails to be processed when synthesized in the forespore. J Bacteriol. 1997;179:4888–4893. doi: 10.1128/jb.179.15.4888-4893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karow L M, Glaser P, Piggot P J. Identification of a gene spoIIR, which links the activation of ςE to the transcriptional activity of ςF during sporulation in Bacillus subtilis. Proc Natl Acad Sci USA. 1995;92:2012–2016. doi: 10.1073/pnas.92.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellner E M, Decatur A, Moran C P. Two-stage regulation of an anti-sigma factor determines developmental fate during bacterial endospore formation. Mol Microbiol. 1996;21:913–924. doi: 10.1046/j.1365-2958.1996.461408.x. [DOI] [PubMed] [Google Scholar]
- 31.Kenney T J, Moran C P. Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J Bacteriol. 1987;169:3329–3339. doi: 10.1128/jb.169.7.3329-3339.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenney T J, York K, Youngman P, Moran C P. Genetic evidence that RNA polymerase associated with ςA uses a sporulation-specific promoter in Bacillus subtilis. Proc Natl Acad Sci USA. 1989;86:9109–9113. doi: 10.1073/pnas.86.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchman P A, DeGrazia H, Kellner E M, Moran C P. Forespore-specific disappearance of the sigma-factor antagonist SpoIIAB: implications for its role in determination of cell fate in Bacillus subtilis. Mol Microbiol. 1993;8:663–671. doi: 10.1111/j.1365-2958.1993.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 34.Kroos L, Kunkel B, Losick R. Switch protein alters specificity of B. subtilis RNA polymerase containing a compartment-specific sigma factor. Science. 1989;243:526–529. doi: 10.1126/science.2492118. [DOI] [PubMed] [Google Scholar]
- 35.Kunkel B, Sandman K, Panzer S, Youngman P, Losick R. The promoter for a sporulation gene in the spoIVC locus of Bacillus subtilis and its use in studies of temporal and spatial control of gene expression. J Bacteriol. 1988;170:3513–3522. doi: 10.1128/jb.170.8.3513-3522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaBell T L, Trempy J E, Haldenwang W G. Sporulation-specific sigma factor, ς29, of Bacillus subtilis is synthesized from a precursor protein, P31. Proc Natl Acad Sci USA. 1987;84:1784–1788. doi: 10.1073/pnas.84.7.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis P J, Partridge S R, Errington J. ς factors, asymmetry, and the determination of cell fate in Bacillus subtilis. Proc Natl Acad Sci USA. 1994;91:3849–3853. doi: 10.1073/pnas.91.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linn T G, Greenleaf A L, Shorenstein R G, Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci USA. 1973;70:1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Londóno-Vallejo J-A, Stragier P. Cell-cell signaling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev. 1995;9:503–508. doi: 10.1101/gad.9.4.503. [DOI] [PubMed] [Google Scholar]
- 40.Lord M, Barilla D, Yudkin M D. Replacement of vegetative ςA by sporulation-specific ςF as a component of the RNA polymerase holoenzyme in sporulating Bacillus subtilis. J Bacteriol. 1999;181:2346–2350. doi: 10.1128/jb.181.8.2346-2350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu S, Halberg R, Kroos L. Processing of the mother-cell ς factors, ςK, may depend on events occurring in the forespore during Bacillus subtilis development. Proc Natl Acad Sci USA. 1990;87:9722–9726. doi: 10.1073/pnas.87.24.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margolis P, Driks A, Losick R. Establishment of cell type by compartmentalized activation of a transcription factor. Science. 1991;254:562–565. doi: 10.1126/science.1948031. [DOI] [PubMed] [Google Scholar]
- 43.Min K, Hilditch C M, Diederich B, Errington J, Yudkin M D. ςF, the first compartment-specific transcription factor of Bacillus subtilis, is regulated by an anti-ς factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 44.Partridge S R, Foulger D, Errington J. The role of ςF in prespore-specific transcription in Bacillus subtilis. Mol Microbiol. 1991;5:757–767. doi: 10.1111/j.1365-2958.1991.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 45.Satola S W, Baldus J M, Moran C P. Binding of Spo0A stimulates spoIIG promoter activity in Bacillus subtilis. J Bacteriol. 1992;174:1448–1453. doi: 10.1128/jb.174.5.1448-1453.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuch R, Piggot P J. The dacF-spoIIA operon of Bacillus subtilis, encoding ςF, is autoregulated. J Bacteriol. 1994;176:4104–4110. doi: 10.1128/jb.176.13.4104-4110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segall J, Tjian R, Pero J, Losick R. Chloramphenicol restores sigma factor activity to sporulating Bacillus subtilis. Proc Natl Acad Sci USA. 1974;71:4860–4863. doi: 10.1073/pnas.71.12.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shorenstein R G, Losick R. Purification and properties of the sigma subunit of ribonucleic acid polymerase from vegetative Bacillus subtilis. J Biol Chem. 1973;248:6163–6169. [PubMed] [Google Scholar]
- 49.Shuler M F, Tatti K M, Wade K H, Moran C P., Jr A single amino acid substitution in ςE affects its ability to bind core RNA polymerase. J Bacteriol. 1995;177:3687–3694. doi: 10.1128/jb.177.13.3687-3694.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith K, Youngman P. Evidence that the spoIIM gene of Bacillus subtilis is transcribed by RNA polymerase associated with ςE. J Bacteriol. 1993;175:3618–3627. doi: 10.1128/jb.175.11.3618-3627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stragier P, Bonamy C, Karmazyn-Campelli C. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell. 1988;52:697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- 52.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 53.Sun D, Cabrera-Martinez R M, Setlow P. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor ςG. J Bacteriol. 1991;173:2977–2984. doi: 10.1128/jb.173.9.2977-2984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tjian R, Losick R. An immunological assay for the sigma subunit of RNA polymerase in extracts of vegetative and sporulating Bacillus subtilis. Proc Natl Acad Sci USA. 1974;71:2872–2876. doi: 10.1073/pnas.71.7.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trempy J E, Morrison-Plummer J, Haldenwang W G. Synthesis of ς29, an RNA polymerase specificity determinant, is a developmentally regulated event in Bacillus subtilis. J Bacteriol. 1985;161:340–346. doi: 10.1128/jb.161.1.340-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J-J, Piggot P J, Tatti K M, Moran C P. Transcription of the Bacillus subtilis spoIIA operon. Gene. 1991;101:113–116. doi: 10.1016/0378-1119(91)90231-y. [DOI] [PubMed] [Google Scholar]
- 57.Yasbin R E, Wilson G A, Young F E. Transformation and transfection of lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973;113:540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Youngman P, Poth H, Green B, York K, Almedo G, Smith K. Methods for genetic manipulation, cloning, and functional analysis of sporulation genes in Bacillus subtilis. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C: American Society for Microbiology; 1989. pp. 65–87. [Google Scholar]
- 59.Zhang B, Hofmeister A, Kroos L. The pro-sequence of pro-ςK promotes membrane association and inhibits RNA polymerase core binding. J Bacteriol. 1998;180:2434–2441. doi: 10.1128/jb.180.9.2434-2441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang B, Kroos L. A feedback loop regulates the switch from one sigma factor to the next in the cascade controlling Bacillus subtilis mother cell gene expression. J Bacteriol. 1997;179:6138–6144. doi: 10.1128/jb.179.19.6138-6144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, B. A., and L. Kroos. Personal communication.