Abstract
Background:
A margin of “no ink on tumor” has been established for primary breast conservation therapy (BCT), but the appropriate margin following neoadjuvant chemotherapy (NAC) remains controversial. We examined the impact of margin width on ipsilateral breast tumor recurrence (IBTR) in the NAC-BCT population.
Methods:
Consecutive patients receiving NAC-BCT were identified from a prospective database. The association between clinicopathologic characteristics, margin width and isolated IBTR was evaluated.
Results:
From 2013–2019, we identified 582 patients with 586 tumors who received NAC-BCT. The median age of the cohort was 54 years (IQR 45, 62); 84% of patients had cT1/T2 tumors and 61% were clinically node positive. The majority of tumors were HER2+ (38%) or triple negative (TN) (31%). Pathologic complete response was observed in 29%. Margin width was >2 mm in 517 tumors (88%) and ≤2 mm in 69 (12%). At a median follow-up of 39 months, 14 patients had IBTR as a first event, with 64% occurring within 24 months of surgery. The 4-year IBTR rate was 2% (95% CI 1–4%), and there was no difference based on margin width (3% ≤2 mm vs. 2% >2 mm; p = not significant). On univariate analysis, clinical and pathologic T stage and receptor subtype, but not margin width, were associated with IBTR (p < 0.05). On multivariable analysis, TN subtype and higher pathologic T stage were associated with isolated IBTR (both p < 0.05).
Conclusion:
Pathologic features and tumor biology, not margin width, were associated with IBTR in NAC-BCT patients.
Introduction
Neoadjuvant chemotherapy (NAC) for breast cancer, which was historically reserved for inoperable disease, is now used to facilitate de-escalation of surgery in patients with early-stage disease (1). NAC has been demonstrated to downstage both nodal and breast disease, allowing avoidance of axillary dissection and mastectomy, with the highest response rates seen in patients with triple-negative (TN) and HER2+ tumors (2,3). Several studies have shown that NAC decreases the volume of breast tissue excised and increases eligibility for breast conservation (4–7). Early data from the NSABP B-18 trial demonstrated an increase in breast conservation therapy (BCT) from 60% to 67% with NAC (8), while a more recent meta-analysis of over 4,700 patients showed that BCT rates increased from 49% to 65% with NAC (9). Because many patients in these studies were candidates for BCT at presentation, the true benefit of NAC in avoiding mastectomy is not well-captured. Golshan et al. reported conversion from BCT-ineligible to BCT-eligible in approximately 50% of HER2+and TN breast cancers in two CALGB trials (5,6), while Petruolo et al. demonstrated a 75% conversion rate to BCT-eligibility in patients considered initially ineligible due to large tumor relative to breast size (7).
Among patients receiving NAC and undergoing BCT, what constitutes an adequate negative margin remains controversial. The SSO/ASCO/ASTRO consensus statement defines an adequate margin in invasive cancer as “no ink on tumor” but does not include patients receiving NAC (10). The frequent finding of scattered residual tumor in patients who do not have a pathologic complete response (pCR) raises concerns that a margin of no ink on tumor could be associated with a heavy residual tumor burden and higher rates of local recurrence (LR) among patients treated with BCT after NAC (10–12).
Retrospective studies examining the impact of margin width on LR in the NAC-BCT population are limited by inclusion of patients with positive margins and treatment periods spanning multiple decades, with suboptimal systemic therapy regimens (13–17). In this study, we sought to determine the impact of margin width on local recurrence in a contemporary group of consecutively treated patients receiving modern NAC followed by BCT.
Methods
Since 2013, data from patients undergoing NAC followed by surgery at Memorial Sloan Kettering Cancer Center (MSKCC) have been prospectively collected and entered into a Health Insurance Portability and Accountability Act (HIPAA)-compliant database. After approval by the Institutional Review Board, the MSKCC NAC database was queried for patients receiving BCT following NAC between 2013 and November 2019. Patients with occult primary breast cancer or conversion from BCT to mastectomy, those who received neoadjuvant endocrine therapy, those with positive margins or detailed pathology missing, and those who did not receive adjuvant radiation therapy (RT) or had an unknown RT status were excluded from the analysis (Table 1).
Table 1.
Indications for exclusion from analysis
| Number excluded (n = 50) |
|
|---|---|
| Conversion to mastectomy | 30 |
| Persistently close/positive margins following re-excision | 5 |
| Positive or close margin after initial surgery | 21 |
| Tumor emboli in lymphovascular space | 1 |
| Patient preference | 1 |
| Refusal of RT following BCS | 1 |
| ATM mutation | 1 |
| Contralateral inflammatory breast cancer | 1 |
| Occult primary breast cancer | 4 |
| Failure to receive RT | 7 |
| Unknown RT status | 4 |
| Positive final margins | 3 |
| Detailed pathology missing * | 1 |
BCS, breast conservation surgery; RT, radiation therapy;
biopsy performed at outside institution and unavailable for review; final pathology: pathologic complete response
Clinical and histopathologic data were obtained from the MSKCC NAC database and abstracted from pathology reports and the electronic medical record. Margin assessment was standardized and did not change during the study period. All lumpectomies were performed with separate shaved cavity margins that were oriented by the treating surgeon. If tumor was present in a margin specimen, the closest distance to the inked surface was reported. In this study, margin width was defined as the distance from the margin to the closest residual tumor, whether invasive or intraductal carcinoma. When re-excision was performed, the final margin width in the re-excision specimen was reported. Margin width was categorized as ≤2 mm or > 2 mm. Breast pCR was defined as no residual invasive or intraductal carcinoma (ypT0), and overall pCR was defined as no residual invasive or intraductal carcinoma in the breast or axilla (ypT0, ypN0).
The primary endpoint of the study was isolated ipsilateral breast tumor recurrence (IBTR), with or without a synchronous ipsilateral regional recurrence. IBTR as a secondary event, regional recurrence, distant disease, and deaths were also recorded. Margin width was examined as ≤2 mm vs. >2 mm for initial analysis. A second analysis was performed with 3 margin categories: ≤2 mm, >2 mm, and breast pCR.
Descriptive statistics are reported as medians and interquartile ranges (IQR) for continuous variables and frequencies and percentages for categorical variables. Demographic and clinicopathological features were compared between individuals who developed IBTR and those who did not, using Wilcoxon rank-sum test for continuous variables and Fisher’s exact test or chi-square test for categorical variables. Variables that were significant in this univariate analysis were then included in the multivariable competing risks regression analysis (MVA). Margin width was used as the main predictor in the MVA. Within the competing risks framework, IBTR was considered as the main event of interest, while distant recurrence was considered as a competing event. Time to IBTR was calculated from the time of surgery. The difference in cumulative incidence rates of IBTR between individuals categorized based on margin widths was evaluated using Gray’s test (18). All statistical analyses were conducted using R 3.6.3 (R Core Development Team, Vienna, Austria); type I error rate (α) was set to 0.05 for all statistical tests. Race and ethnicity are not known risk factors for local recurrence, and thus not collected with this retrospective data.
Results
Six hundred and thirty-two patients with 636 stage I-III breast tumors receiving NAC followed by BCT between 2013–2019 were identified; 50 patients were excluded from the analysis based on exclusion criteria (Table 1). The final study cohort was comprised of 582 patients with 586 tumors (4 patients had bilateral tumors). NAC regimens included dose-dense doxorubicin, cyclophosphamide, and a taxane in 92% (539/582) of patients. Of 220 patients with HER2+ disease, 99% (219/220) received dual targeted therapy with trastuzumab and pertuzumab.
Clinicopathologic characteristics of the cohort are listed in Table 2. The median patient age was 54 years (IQR 45, 62), 84% (495/586) of tumors were cT1-T2, and 61% (360/586) had suspicious palpable nodes, of which 89% were histologically confirmed as malignant. Receptor subtype was equally distributed: 31% (181/586) were hormone receptor (HR)+ HER2−, 38% (221/586) were HER2+, and 31% (184/586) were TN. The rate of breast pCR rate was 31% (183/586), nodal pCR 66% (385/586), and overall pCR was seen in 29% (171/586) of cases. In cases where there was residual disease present in the breast, it was multifocal in 41% (165/403) on pathologic evaluation.
Table 2.
Clinicopathologic characteristics by IBTR
| Age at diagnosis in years, median (IQR) | 54 (45, 62) | 54 (45,62) | 48 (38,54) | 0.05 |
| Clinical T Stage | 0.02 | |||
| 1 | 114 (19%) | 113 (20%) | 1 (7%) | |
| 2 | 381 (65%) | 374 (65%) | 7 (50%) | |
| 3 | 74 (13%) | 70 (12%) | 4 (29%) | |
| 4 | 17 (3%) | 15 (3%) | 2 (14%) | |
| Clinical N Stage | 0.8 | |||
| 0 | 226 (39%) | 221 (39%) | 5 (36%) | |
| 1 | 321 (55%) | 312 (54%) | 9 (64%) | |
| 2/3 | 39 (6%) | 39 (7%) | 0 (0%) | |
| Histology | >0.9 | |||
| Ductal | 551 (94%) | 537 (94%) | 14 (100%) | |
| Lobular and Mixed | 30 (5%) | 30 (5%) | 0 (0%) | |
| Other | 5 (1%) | 5 (1%) | 0 (0%) | |
| Receptor Subtype | 0.01 | |||
| HR+ HER2− | 181 (31%) | 179 (31%) | 2 (14%) | |
| HR+/− HER2+ | 221 (38%) | 219 (38%) | 2 (14%) | |
| TN | 184 (31%) | 174 (31%) | 10 (72%) | |
| Tumor Grade | >0.9 | |||
| Well differentiated | 3 (0.5%) | 3 (1%) | 0 (0%) | |
| Moderately differentiated | 111 (19%) | 109 (19%) | 2 (14%) | |
| Poorly differentiated | 472 (80.5%) | 460 (80%) | 12 (86%) | |
| Pathologic T Stage | 0.04 | |||
| ypT0 | 183 (31%) | 182 (32%) | 1 (7%) | |
| ypTis | 48 (8%) | 45 (8%) | 3 (21%) | |
| ypT1 | 274 (47%) | 268 (47%) | 6 (43%) | |
| ypT2 | 76 (13%) | 72 (12%) | 4 (29%) | |
| ypT3 | 5 (1%) | 5 (1%) | 0 (0%) | |
| Pathologic N Stage | 0.6 | |||
| ypN0 | 385 (66%) | 377 (67%) | 8 (57%) | |
| ypN1 | 149 (25%) | 144 (25%) | 5 (36%) | |
| ypN2/3 | 52 (9%) | 51 (8%) | 1 (7%) | |
| pCR (ypT0, ypN0) | 171 (29%) | 170 (30%) | 1 (7%) | 0.08 |
| Multifocal Residual Disease | 165 (41%) | 162 (42%) | 3 (23%) | 0.3 |
| Lymphovascular Invasion | 151 (26%) | 145 (25%) | 6 (43%) | 0.2 |
| Extensive Intraductal Component | 49 (14%) | 49 (14%) | 0 (0%) | 0.4 |
| Margin Re-excision | 40 (7%) | 37 (7%) | 3 (21%) | 0.06 |
| Final Margin | 0.7 | |||
| ≤2 mm | 69 (12%) | 67 (12%) | 2 (14%) | |
| >2 mm | 517 (88%) | 505 (88%) | 12 (86%) | |
| Follow-up time in months, median (IQR) | 39 (26, 51) | 39 (27,51) | 33 (23,45) | 0.6 |
IBTR: ipsilateral breast tumor recurrence; IQR: interquartile range; T: tumor; N: nodal; HR: hormone receptor; TN: triple negative; pCR: pathologic complete response
Margin Status
A negative margin of no ink on tumor was obtained with a single surgery in 93% (546/586) of tumors, while 40 tumors underwent re-excision secondary to a positive (n=17) or close (n=23) margin based on surgeon discretion. Two re-excisions were required to obtain negative margins in one patient. Final margin status was ≤2 mm in 12% (69/586) of tumors; of these 27% (19/69) were 1.1–2 mm and 73% (50/69) were ≤1 mm Within the ≤2 mm cohort, there was a single ≤2 mm margin in 78% (54/69), while 22% (15/69) had 2 or more margins with residual disease ≤2 mm from the margin surface. Of the 517 tumors with >2 mm margins, 183 (35%) had a breast pCR.
A univariate analysis comparing the tumor characteristics between margin groups demonstrated a higher rate of residual multifocal disease (59% vs 37%), extensive intraductal component (27% vs 11%), lymphovascular invasion (46% vs 23%) and clinically node positive disease (74% vs 60%; all p ≤0.001) in the ≤2mm margin group (Table 3). Pathologic tumor and nodal stage differed between margin cohorts, in part due to the inclusion of pCR in the >2mm group, and a higher percentage of HER2+ and TNBC was observed in the >2mm cohort (71% vs 57%, p = 0.04). A higher percentage of poorly differentiated tumors was also observed in the >2mm cohort (83% vs 64%).
Table 3.
Clinicopathologic characteristics by margin width
| Age at diagnosis in years, median (IQR) | 54 (45,60) | 54 (45,62) | 0.7 |
| Clinical T Stage | >0.9 | ||
| 1 | 15 (22%) | 99 (19%) | |
| 2 | 43 (62%) | 338 (65%) | |
| 3 | 9 (13%) | 65 (13%) | |
| 4 | 2 (3%) | 15 (3%) | |
| Clinical N Stage | 0.02 | ||
| 0 | 18 (26%) | 208 (40%) | |
| 1 | 42 (61%) | 279 (54%) | |
| 2/3 | 9 (13%) | 30 (6%) | |
| Histology | 0.6 | ||
| Ductal | 64 (93%) | 487 (94%) | |
| Lobular and Mixed | 5 (7%) | 25 (5%) | |
| Other | 0 (0%) | 5 (1%) | |
| Receptor Subtype | 0.04 | ||
| HR+ HER2− | 30 (43%) | 151 (29%) | |
| HR+/− HER2+ | 24 (35%) | 197 (38%) | |
| TN | 15 (22%) | 169 (33%) | |
| Tumor Grade | <0.001 | ||
| Well differentiated | 1 (1%) | 2 (<1%) | |
| Moderately differentiated | 24 (35%) | 87 (17%) | |
| Poorly differentiated | 44 (64%) | 428 (83%) | |
| Pathologic T Stage | <0.001 | ||
| ypT0 | 0 (0%) | 183 (35%) | |
| ypTis | 7 (10%) | 41 (8%) | |
| ypT1 | 47 (68%) | 227 (44%) | |
| ypT2 | 14 (20%) | 62 (12%) | |
| ypT3 | 1 (2%) | 4 (1%) | |
| Pathologic N Stage | <0.001 | ||
| ypN0 | 24 (35%) | 361 (70%) | |
| ypN1 | 30 (43%) | 119 (23%) | |
| ypN2/3 | 15 (22%) | 37 (7%) | |
| pCR (ypT0, ypN0) | 0 (0%) | 171 (33%) | <0.001 |
| Multifocal Residual Disease | 41 (59%) | 124 (37%) | <0.001 |
| Lymphovascular Invasion | 32 (46%) | 119 (23%) | <0.001 |
| Extensive Intraductal Component | 17 (27%) | 32 (11%) | 0.001 |
| Margin Re-excision | 12 (17%) | 28 (5%) | 0.001 |
| IBTR as 1st Event | 2 (3%) | 12 (3%) | 0.7 |
| Follow-up time in months, median (IQR) | 37 (25,52) | 39 (27,51) | 0.6 |
IBTR: ipsilateral breast tumor recurrence; IQR: interquartile range; T: tumor; N: nodal; HR: hormone receptor; TN: triple negative; pCR: pathologic complete response
Adjuvant Radiation Therapy
All patients received radiotherapy following surgical management. Specific radiation field data was available for 79% (463/586) of tumors: 75% ≤2mm (52/69) margins, 79% (411/517) >2mm margins. Boost dose was given in 81% (377/463), and receipt of boost did not differ by margin status (75% ≤2mm, 82% >2mm; p=0.2). Median whole breast and boost doses were 5000 and 1000 cGy in both margin groups. Range of whole breast radiation and boost dose was 4240–5040 and 940–1250 cGy in the ≤2mm margin group and 1600–5600 and 500–1500 cGy, in the >2mm margin group. The dosing schedule included 20 fractions for patients without nodal involvement (16 fractions whole breast followed by 4 fractions of boost). When regional nodal irradiation was required, patients received 30 treatments (25 fractions whole breast and regional nodes followed by 5 fractions of boost).
Outcomes
The median follow-up was 39 months (IQR 26, 51), during which time 62 patients experienced at least one event, defined as a breast cancer recurrence or death. Fourteen patients had an IBTR as a first event, including 12 patients with isolated IBTR and 2 with synchronous IBTR/regional recurrence. Additional first events included: 1 isolated regional recurrence, 32 isolated distant metastases, 14 distant metastases synchronous with IBTR and/or regional recurrence, and 1 death not preceded by recurrence. Thirty-three patients died during the follow-up period, with 31 deaths related to breast cancer.
All IBTR as first event recurrences were radiographically present at the previous lumpectomy site. 1 patient had an additional satellite lesion and 1 patient had multiple additional nodules throughout the breast. Radiation field data was available for 65% (9/14) tumors with IBTR as a first event and 77% (454/572) of tumors without IBTR as a first event. Receipt of boost did not differ between the IBTR first event group and those without (67% vs 82%, p = 0.47).
In the 14 isolated IBTRs with or without regional recurrence as a first event, 2 (14%) were in HR+ HER2− tumors, 2 (14%) in HER2+ tumors, and 10 (72%) in TN tumors. The crude median time to IBTR was 17 months (IQR 11, 25), with 9/14 (64%) IBTRs occurring within 24 months of surgery. Distant metastases subsequently developed in 7/14 (50%) patients with IBTR as a first event. The 4-year rate of IBTR as a first event was 2% (95% CI 1–4%) overall, and 3% (95% CI 0–8%) in ≤2 mm margin vs. 2% (95% CI 1–4%) in >2 mm margin groups (p = not significant [NS], Fig. 1A). When patients having breast pCR were considered as a separate margin group, IBTR rates did not differ significantly between groups [3% (95% CI 0–8%) ≤2 mm vs. 3% (95% CI 1–5%) >2 mm vs. 1% (95% CI 0–3%) breast pCR] (p = NS; Fig. 1B).
Fig. 1.
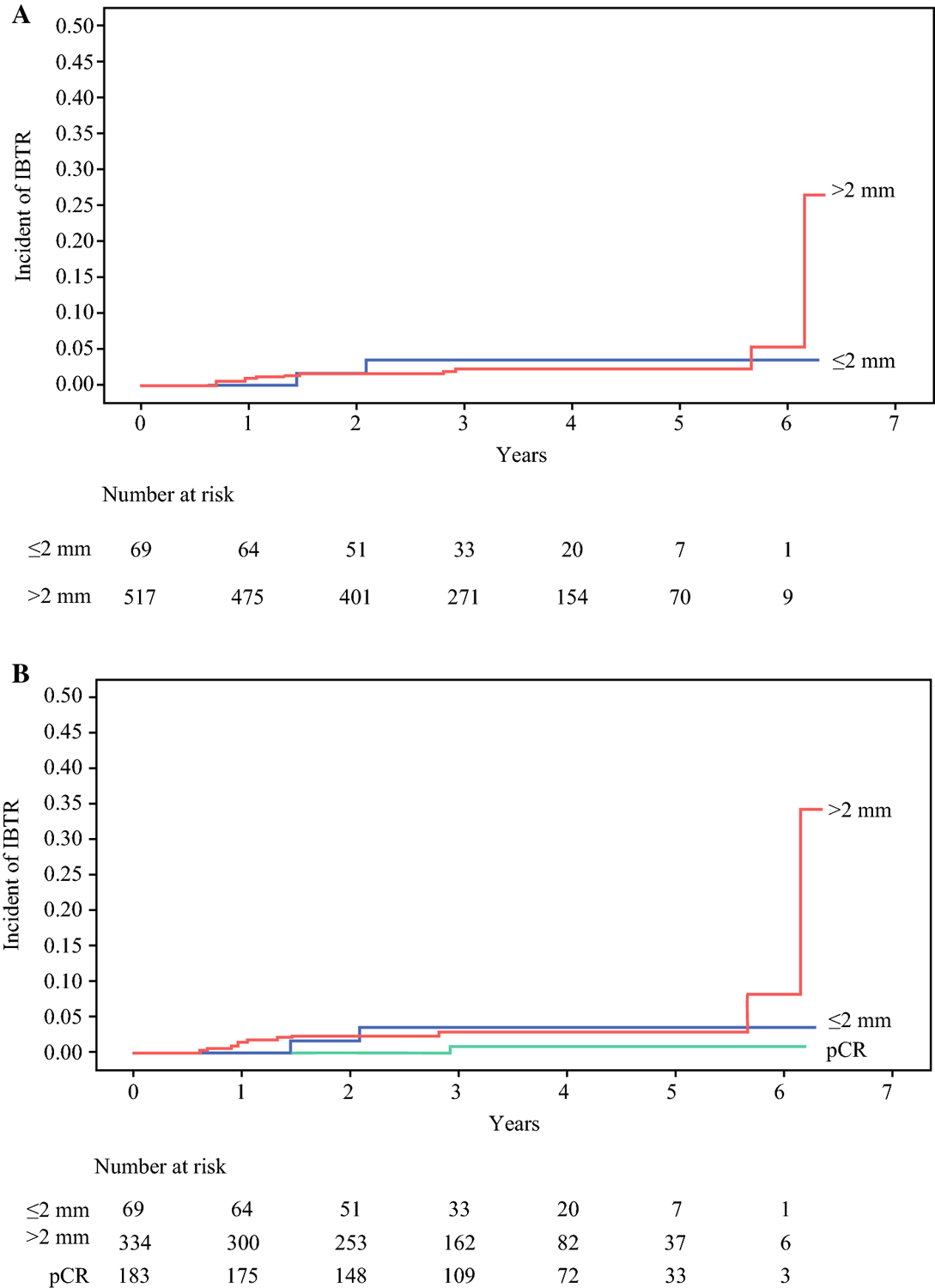
Rate of ipsilateral breast tumor recurrence (IBTR) as a first event based on (A) two margin cohorts, ≤ 2 mm and > 2 mm, or (B) three margin cohorts, ≤ 2 mm, > 2 mm, and breast pathologic complete response (pCR)
On univariate analysis, clinical T stage, pathologic T stage, and receptor subtype were associated with IBTR (p < 0.05, Table 2). On multivariable analysis, receptor subtype and pathologic T stage remained significantly associated with IBTR (Table 4). TN tumors had a hazard ratio of 6 for isolated IBTR compared to HR+ HER2− tumors (p = 0.04). Pathologic T2 disease and residual ductal carcinoma in situ (DCIS; ypTis) were also significantly associated with IBTR when compared to tumors with a breast pCR (Table 4).
Table 4.
Multivariable competing risks regression for factors associated with IBTR
| Final Margin | ||
| ≤2 mm | Ref | |
| >2 mm | 0.9 (0.2,3.9) | 0.9 |
| Receptor Subtype | ||
| HR+ HER2− | Ref | |
| HR+/− HER2+ | 1.0 (0.1, 8.3) | >0.9 |
| TN | 6.1 (1.1, 34.2) | 0.04 |
| Clinical T Stage | ||
| 1 | Ref | |
| 2 | 1.4 (0.2, 11.2) | 0.8 |
| 3 | 3.6 (0.4, 33.2) | 0.2 |
| 4 | 5.1 (0.5, 48.0) | 0.1 |
| Pathologic T Stage | ||
| ypT0 | Ref | |
| ypTis | 12.8 (1.3, 129) | 0.03 |
| ypT1 | 3.1 (0.4, 24.1) | 0.3 |
| ypT2 | 12.0 (1.5, 99.0) | 0.02 |
| ypT3 | NA* (NA) | -- |
counts too low for hazard ratio estimation
IBTR: ipsilateral breast tumor recurrence; CI: confidence interval; HR: hormone receptor; TN: triple negative; Ref: reference
The 4-year rate of any event (recurrence or death) was 13% (95% CI 1–16%) overall, 8% (95% CI 3–19%) in patients with ≤2 mm margins, and 13% (95% CI 1–17%) in those with >2 mm margins. The 4-year rate of distant metastases was 12% (95% CI 9–15%) overall, 10% (95% CI 4–24%) for ≤2 mm margins, and 12% (95% CI 9–16%) for >2 mm margins. The 4-year mortality rate was 7% (95 CI% 5–10%) overall, 2% (95% CI 0–10%) for ≤2 mm margins, and 7% (95 %CI 5–11%) for >2 mm margins. There were no differences in freedom from any event, freedom from distant metastases, or overall survival between the ≤2 mm and >2 mm margin cohorts (all p = NS).
Discussion
In this study of 586 consecutively treated patients with invasive breast cancer who received NAC followed by BCT, the 4-year risk of isolated IBTR with or without regional recurrence as a first event was only 2%. Furthermore, we did not detect a significant difference in outcome by margin width. This experience, to the best of our knowledge, is the largest to date exploring the impact of margin width on IBTR in patients receiving NAC and BCT with important implications for minimizing unnecessary re-excisions and mastectomies for margins perceived to be inadequate.
Pooled data from 2 large randomized trials of NAC, NSABP B-18 and B-27, demonstrated a local recurrence (LR) rate of 8% at 10 years in patients undergoing BCT, with slightly higher local recurrence rates in patients <50 years of age (19). As the only margin requirement was a negative margin defined as no ink on tumor, and actual margin widths were unknown in these studies, it is not possible to quantify the impact of margin width on LR. However, in spite of the acceptably low LR rates in NSABP B-18 and B-27, it was recognized that some cancers respond to NAC by shrinking concentrically while others have foci of residual tumor scattered in a “buckshot” or “honeycomb” pattern, raising concerns that if less than the original tumor volume was resected in patients with the latter type of response, a substantial tumor burden might be left behind leading to increased rates of LR.
In a study of 257 patients treated with NAC followed by BCT between 1985 and 1994, Rouzier et al. reported a 2.5 hazard ratio for IBTR with margins ≤2 mm compared to margins >2 mm (17). These findings were substantiated by the Early Breast Cancer Trialists Collaborative Group meta-analysis of 4,756 patients from 10 randomized trials comparing NAC to adjuvant therapy conducted between 1983 and 2002, which found a statistically significant 5.5% increase in LR in patients receiving NAC compared to those receiving adjuvant therapy (9). A significant difference persisted even when trials in which patients had no surgery were excluded from the analysis, raising the question of whether margins more widely clear than no ink on tumor would be beneficial in patients undergoing BCT after NAC.
In retrospective studies evaluating the impact of margin width after NAC-BCT, 5-year LR rates vary from 5 to 16% (13–17). In a study from MD Anderson Cancer Center, Chen et al. reported a 5-year actuarial IBTR-free survival of 95% and locoregional recurrence (LRR)-free survival of 91% in 340 patients treated with NAC and BCT between 1987 and 2000 (10). In this population, LRR-free survival at 5 years did not differ between cancers resected with margins >2 mm vs. ≤2 mm (92 vs. 89%, respectively; p = 0.20), but advanced nodal disease at diagnosis, pathologic stage T2 or greater, multifocal residual disease, and lymphovascular invasion were predictive of both LRR and IBTR (13). More recently, Choi et al. reported findings from 382 patients treated at the Dana-Farber/Brigham and Women’s Cancer Center between 2002 and 2014 with NAC and BCT. The 5-year LR rate was 2.9% in patients with margins ≤2 mm, 6.3% with margins >2 mm, and 1.0% in patients with breast pCR. On multivariable analysis, margin width was not associated with LR-free survival (14). Finally, in a retrospective study by Wimmer et al., of 406 patients treated with NAC and BCT, the 5-year LR-free survival was similar across margin cohorts: 94% for ≤1 mm, 91% for >1 mm, and 95% for breast pCR (p = 0.2) (16). In our cohort of 582 patients treated with NAC and BCT between 2013 and 2019, we report a similar rate of IBTR across margin cohorts: 3% for ≤2 mm, 3% for >2 mm, 1% for breast pCR (p = NS), in spite of the observation that the ≤2mm margin group more often contained multifocal residual disease, had more lymphovascular invasion and had a higher proportion of patients with an extensive intraductal component. While prior studies either included or did not distinguish between LR as a first event versus synchronous with distant disease, our study focuses on margin width and isolated LR a key distinction given that isolated LR necessitates additional surgery and may result in additional systemic therapy (21).
Our study is consistent with previous findings that tumor biology is a primary determinant of LR in patients receiving NAC-BCT. We found that TN subtype was significantly associated with isolated IBTR as a first event. Ten of 14 isolated IBTRs occurred in patients with TN tumors, and in multivariable analysis the hazard ratio for IBTR in this group was 6 compared to HR+ HER2− breast cancer patients. In the pooled analysis of 2,961 patients in NSABP B-18 and B-27, node-positive status prior to NAC and pCR in the breast and/or nodes were predictive of LRR in NAC-BCT patients (19). The relationship between receptor subtype and LRR-free survival was demonstrated in 595 patients treated with NAC-BCT between 1997 and 2005 at MD Anderson Cancer Center (20). In multivariable analysis, the LRR hazard ratio was 5.7 (95% CI 2.6–12.3) for HR-, HER2+ tumors and 5.7 (95% CI 2.0–16.3) for TN disease, when compared to HR+, HER2− disease. In the same population, higher residual nodal burden was also associated with decreased LRR-free survival. In addition, Jwa et al. found TN subtype and tumors >5 cm at presentation to be associated with higher LRR in 355 patients treated with NAC-BCT between 2002 and 2009 (22). We also found that pathologic T stage was significantly associated with IBTR as a first event, and overall, our findings are consistent with previously demonstrated risk factors for local recurrence in patients undergoing BCT after NAC (13, 14, 19, 20, 22).
Pathologic complete response has been associated with decreased rates of both LR and distant metastases after NAC (19, 20). To minimize any potential bias of pCR on the relationship between margins and local recurrence rates, we examined pCR as a separate margin group and compared this with margins of ≤2 mm and >2 mm. This did not change our initial findings regarding margin width and IBTR. IBTR was seen in 1% of those having pCR vs. 3% of patients with margins ≤2 mm or >2 mm, a difference that was not statistically significant. While pCR was not associated with isolated IBTR in either univariate or multivariable analysis, this relationship may be obscured by the association between receptor subtype and IBTR (2,3) or may be the result of the low rates of IBTR seen in this study.
Although not statistically significant, the proportion of tumors requiring a re-excision was higher in the IBTR as first event group compared with those without (21 vs 7%, p = 0.06). Tumor biology is likely at least partially responsible for this finding: 67% (2/3) IBTR tumors requiring re-excision were TN subtype while 22% (8/37) of the tumors without IBTR were TN. Our data demonstrates TN subtype to have the strongest association with isolated local recurrence. The difference in re-excision may also be due to the small number of IBTR as first events observed. A larger cohort of NAC-BCS patients requiring re-excision require evaluation prior to any conclusions regarding risk of subsequent local recurrence.
The importance and power of consensus guidelines are clear in primary BCT. While rates of positive margins did not change after the SSO/ASCO/ASTRO consensus statement in 2014, the rate of re-excision for a close margin and the rate of bilateral mastectomies decreased following implementation of these guidelines (24). Decreased use of re-excision or conversion to mastectomy results in cost savings, increased patient satisfaction, and improved cosmetic outcomes (25–28). In patients receiving NAC, concerns about close margins being associated with a higher residual disease burden may be driving unnecessary re-excisions and potentially even mastectomies. Indeed, Choi et al. reported the overall re-excision rate in their study was high at 26%, with more than two-thirds of re-excisions performed for “close” but negative margins (14). In our study, an initial margin width of ≤2 mm was the indication for 15% of cases converted to mastectomy and nearly 60% of re-excisions. Guidelines for margins in patients receiving NAC-BCT could minimize unnecessary re-excisions, potentially optimizing cosmetic and economic benefit.
There are several strengths of our study. In addition to a large, consecutive series of patients treated with contemporary NAC and standardized localization technique, margin evaluation was standardized, with cavity shave margins performed in all cases. In addition, data collection was prospective, limiting the amount of missing data. Despite this, there are limitations due to the retrospective, observational, single-institution design of this study. There was a relatively small proportion of patients with margins ≤2 mm (12%), and it is possible that the sample size is insufficient to detect a difference in IBTR associated with margin width. Our median follow-up is short, but IBTR in patients with TN and HER2+ breast cancer usually occurs within the first 5 years post-treatment (29,30). TN breast cancer comprises over half of the observed IBTRs in this study, and with longer follow-up, more recurrences are likely in patients with HR+ tumors. While this could increase the overall rate of LR, it is likely that those recurrences would occur at the same rate in both margin groups. Finally, radiation field data was not available for 19% of the study population as radiation treatment was given at non-institutional facilities.
In conclusion, our findings demonstrate that a margin greater than 2 mm does not improve local control in patients undergoing breast conservation following NAC and supports consideration of extension of the “no ink on tumor” guideline to the NAC-BCT population.
Synopsis.
In patients receiving breast conservation therapy after neoadjuvant chemotherapy, an adequate negative margin has not been defined. We found no difference in isolated local recurrence rates between greater and less than 2 mm margins after neoadjuvant chemotherapy and breast conservation.
Acknowledgements:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosures: Dr. Monica Morrow has received speaking honoraria from Exact Sciences and Roche. The remaining authors has no conflicts of interest to disclose.
This work was presented as an oral presentation at the SSO 2021.
References
- 1.King TA, Morrow M. Surgical Issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12:335–343. [DOI] [PubMed] [Google Scholar]
- 2.Boughey JC, McCall LM, Ballman KV, et al. Tumor Biology Correlates With Rates of Breast-Conserving Surgery and Pathologic Complete Response After Neoadjuvant Chemotherapy for Breast Cancer. Ann Surg. 2014;260:608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. 2016;23:3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boughey JC, Peintinger F, Meric-Bernstam F, et al. Impact of Preoperative Versus Postoperative Chemotherapy on the Extent and Number of Surgical Procedures in Patients Treated in Randomized Clinical Trials for Breast Cancer. Ann Surg. 2006;244;464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of Neoadjuvant Chemotherapy in Stage II-III Triple Negative Breast Cancer on Eligibility for Breast-conserving Surgery and Breast Conservation Rates. Ann Surg. 2015;262:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy on eligibility for and frequency of breast conservation in stage II-III Her2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat. 2016;160:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petruolo O, Sevilimedu V, Montagna G, Le T, Morrow M, Barrio AV. How Often Does Modern Neoadjuvant Chemotherapy Downstage Patients to Breast-Conserving Surgery? Ann Surg Oncol. 2021;28(1):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher B, Brown A, Mamounas E, et al. Effect of Preoperative Chemotherapy on Local-Regional Disease in Women With Operable Breast Cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. [DOI] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran MS, Schnitt SJ, Giuliano, et al. Society of Surgical Oncology-American Society for Radiation Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Stage I and II Invasive Breast Cancer. J Clin Oncol. 2014;32:1507–1515. [DOI] [PubMed] [Google Scholar]
- 11.Mukhtar RA, Yau C, Rosen M. Tandom, et al. Clinically Meaningful Tumor Reduction Rates Vary by Prechemotherapy MRI Phenotype and Tumor Subtype in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol. 2013;20:3823–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossuyt V, Provenzano E, Symmans WF, et al. Recommendations for standardize pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Annals of Oncology. 2015;26:1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MA, Meric-Bernstan F, Hunt KK, et al. Breast Conservation After Neoadjuvant Chemotherapy: the MD Anderson Cancer Center Experience. J Clin Oncol. 2004:22:2303–2312. [DOI] [PubMed] [Google Scholar]
- 14.Choi J, Laws A, Hu J, Barry W, Golshan M, King T. Margins in Breast-Conserving Surgery After Neoadjuvant Therapy. Ann Surg Oncol. 2018;25:3541–3547. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Lin KJ, Wang YF, Huang LH, Chen SLI, Chen DR. Association of surgical margins with local recurrence in patients undergoing breast-conserving surgery after neoadjuvant chemotherapy. BMC Cancer. 2020;20:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wimmer K, Bollinger M, Bago-Horvath Z, et al. Impact of Surgical Margins in Breast Cancer After Preoperative Systemic Chemotherapy on Local Recurrence and Survival. Ann Surg Oncol. 2020;27:1700–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouzier R, Extra JM, Carton M, et al. Primary Chemotherapy for Operable Breast Cancer: Incidence and Prognostic Significance of Ipsilateral Breast Tumor Recurrence After Breast-Conserving Surgery. J Clin Oncol. 2001;19:3828–3835. [DOI] [PubMed] [Google Scholar]
- 18.Gray RJ. A Class of K-Sample Tests for Comparing Cumulative Incidence of a Competing Risk. Ann Statist. 1988;16(3):1141–1154. [Google Scholar]
- 19.Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of Locoregional Recurrence After Neoadjuvant Chemotherapy: Results From Combined Analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30:3960–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caudle AS, Yu TK, Tucker SL, et al. Local-regional control according tao markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Research. 2012;14:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aebi S, Gelber S, Anderson SJ, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomized trial. Lancet Oncol. 2014;15:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jwa E, Shin KH, Kim JY, et al. Locoregional Recurrence by Tumor Biology in Breast Cancer Patients after Preoperative Chemotherapy and Breast Conservation Treatment. Cancer Res Treat. 2016;48:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazouni C, Peintinger F, Wan-Kau S, et al. Residual Ductal Carcinoma In Situ in Patients with Complete Eradication of Invasive Breast Cancer After Neoadjuvant Chemotherapy Does Not Adversely Affect Patient Outcome. J Clin Oncol. 2007; 25:2650–2655. [DOI] [PubMed] [Google Scholar]
- 24.Marinovich ML, Noguchi N, Morrow M, Houssami N. Changes in Reoperation After Publication of Consensus Guidelines on Margins for Breast-Conserving Surgery. JAMA Surg. 2020;155(10):e203025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow M, Abrahamse P, Hofer TP, et al. Trends in Reoperation After Initial Lumpectomy for Breast Cancer Addressing Overtreatment in Surgical Management. JAMA Oncology. 2017;3:1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhutiani N, Mercer MK, Bachmann KC, et al. Evaluating the Effect of Margin Consensus Guideline Publication on Operative Patterns and Financial Impact of Breast Cancer Operation. JACS. 2018;227;6–11. [DOI] [PubMed] [Google Scholar]
- 27.Havel L, Naik H, Ramirez L, Morrow M, Landercasper J. Impact of the SSO-ASTRO Margin Guideline on Rates of Re-excision After Lumpectomy for Breast Cancer: A Meta-analysis. Ann Surg Oncol. 2019;26:1238–1244. [DOI] [PubMed] [Google Scholar]
- 28.Dahlback C, Manjer J, Rehn M, Ringberg A. Determinants of patient satisfaction regarding aesthetic outcome and skin sensitivity afte breast-conserving surgery. World Journal of Surgical Oncology. 2016:14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pogoda K, Niwinska A, Murawska M, Pienkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Medical Oncology. 2013; 30: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Maaren MC, de Munck L, Strobbe LJA, et al. Ten-year recurrences rates for breast cancer subtypes in the Netherlands: A large population-based study. Int J Cancer. 2019; 144:263–272. [DOI] [PubMed] [Google Scholar]


