Abstract
There is an unmet need to unearth alternative treatment options for malaria, wherein this quest is more pressing in recent times due to high morbidity and mortality data arising mostly from the endemic countries coupled with partial diversion of attention from the disease in view of the SARS-Cov-2 pandemic. Available therapeutic options for malaria have been severely threatened with the emergence of resistance to almost all the antimalarial drugs by the Plasmodium falciparum parasite in humans, which is a worrying situation. Artemisinin combination therapies (ACT) that have so far been the mainstay of malaria have encountered resistance by malaria parasite in South East Asia, which is regarded as a notorious ground zero for the emergence of resistance to antimalarial drugs. This review analyzes a few key druggable targets for the parasite and the potential of specific inhibitors to mitigate the emerging antimalarial drug resistance problem by providing a concise assessment of the essential proteins of the malaria parasite that could serve as targets. Moreover, this work provides a summary of the advances made in malaria parasite biology and the potential to leverage these findings for antimalarial drug production.
Keywords: Malaria, Proteases, Plasmodium rhomboids, Dipeptidyl aminopeptidases, Apical membrane antigen, Subtilisin-like proteins, Glucose transporters, Schizogony, Plasmepsins
Malaria; Proteases; Plasmodium rhomboids; Dipeptidyl aminopeptidases; Apical membrane antigen; Subtilisin-like proteins; Glucose transporters; Schizogony; Plasmepsins.
1. Introduction
Malaria continues to wreak havoc in many malaria endemic countries. The fight to eliminate malaria had almost reached a crescendo with a Phase IV Expanded immunisation programme, wherein malaria vaccine was incorporated for implementation in Ghana, Malawi and Kenya (WHO, 2020). However, the coronavirus disease (COVID-19) pandemic has stalled these efforts as almost all attention has been diverted to combating the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) the causative agent, at the expense of malaria (Aborode et al., 2021).
As a major public issue, malaria (especially falciparum induced malaria) is a threat to life of humans because of its high mortality. At present, existing literature suggests that Plasmodium falciparum has become resistant to multiple drugs that are used to treat malaria (Phyo et al., 2016).
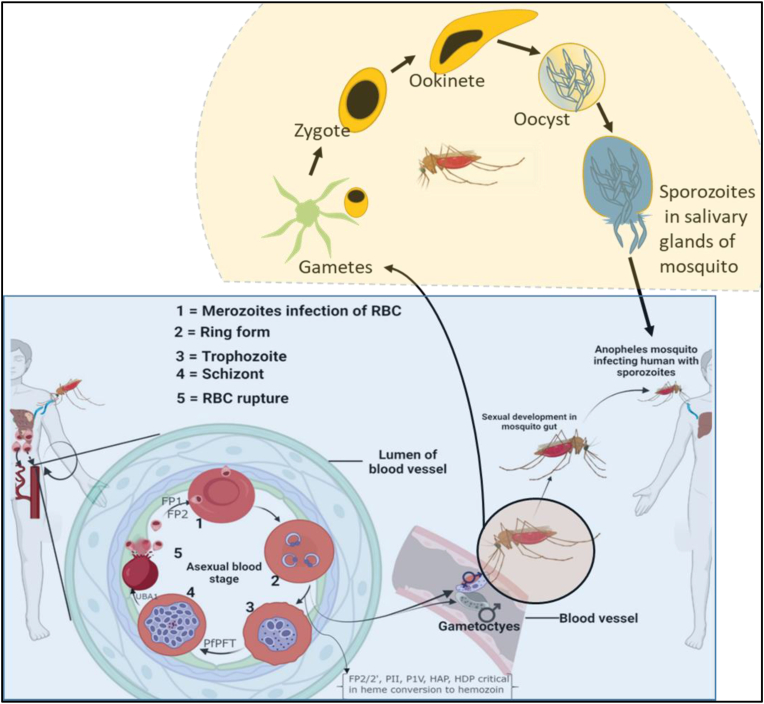
P. falciparum is a protozoan parasite largely responsible for malaria disease in humans, which is transmitted by female Anopheles mosquitoes. The parasite is typically an obligate intracellular parasite that depends on elaborate biochemical and physiological processes for survival in its host and thus cause clinical disease. Upon entry into the host the parasite invades and multiplies in hepatocytes producing invasive merozoites (Deu, 2017), which invade and multiply in the host’s erythrocytes (or red blood cells-RBCs). The molecular and biochemical mechanisms underpinning its invasive and egress processes are the parasites mainstay of survival strategies. The alternating stages of replication in humans and sexual reproduction in the vector could be used to discover ways to kill the parasite. During anticoagulant saliva-assisted blood-feeding, the female Anopheles inject sporozoite into the human host, which circulates through the bloodstream to infect the hepatocytes of the host (Burda et al., 2017; Choudhary et al., 2019). The parasites multiply and proliferate asymptomatically in the liver to form the invasive merozoites, which are released into the bloodstream to invade the erythrocytes (this erythrocytic stage is repeated many times, Figure 1), where it derives nutrients from the host. Particularly, haem is a fundamental metabolic determinant of the parasite’s survival (Bonday et al., 2000), which is principally obtained from parasite’s haem biosynthetic pathway (early ring stage) and degradation of host’s haemoglobin (later life cycle stages) (Goldberg, 1993). In the host, haemoglobin is digested by the parasite (Perrin et al., 2021), amide degradative product serving as precursors for the biosynthesis of amino acids, polyamines (Becker et al., 2010), pyrimidine (Phillips and Rathod, 2010) and fatty acids, as well as other metabolites of low molecular weight (like isoleucine) are obtained directly from the host to facilitate the growth of the parasite (Counihan et al., 2021).
Figure 1.
Life Cycle of P. falciparum indicating stage specific expression of essential parasite proteins. The malaria parasite expresses crucial stage specific proteins which facilitates its survival in the human host. Typical among these are Falcipain-1 (FP1), Falcipain-2 (FP2), Plasmepsin (PM) II and IV among others. The roles of these proteins are indicated in (Table 1) (Figure 1 created using Biorender.com).
When merozoites enter the RBC, a parasitophorous vacuolar membrane (PVM) is formed to separate the merozoites from the RBC’s’s cytosol. At or before the merozoites phase, some parasites transform into sexually active gametocytes (micro- and macro gametocytes) (Liu, 2020) which upon ingestion by a mosquito combines to form zygotes. The zygote then develops into an invasive ookinete, prior to escaping the mosquito’s gut to form an oocyst. Subsequently, oocyst (the longest stage of the parasite’s life cycle) grows into other invasive sporozoites, which are capable of infecting other human hosts (Figure 1). As these critical processes occur in human host, the malaria parasite makes use of various proteases and unique metabolic pathways in organelles to facilitate itsgrowth. Therefore, targeting these proteases and unique metabolic pathways of the parasite in given organelles of the host may provide clues for the discovery and development of potent antimalarial drugs. Based on the emergence of parasites that are resistant to antimalarial drugs, there is the need to understand the biological role of proteases in the life cycle of the malaria parasite, which may potentially be used for chemotherapeutic purposes. This review, therefore, considers the critical stages of the P. falciparum life cycle that could provide innovative and novel ideas at developing new drugs that can possibly target proteases and organelles that are essential to the survival of the parasite in the human host. The review incorporates bioinformatics analyses of known proteins and proteases that seek to uncover interacting partners in protein networks, which can also promisingly serve as means to kill the malaria parasite, thereby providing drug-like lead candidates for potential development into inhibitors.
2. The key stages of the malaria parasite in the human host
2.1. Parasite entry into host erythrocytes and development
Upon entry into the human host, the malaria parasite goes through several stages that facilitate its survival and evasion of the human immune system, as has been exemplified in the above life cycle. Briefly, sporozoites mature into schizonts in hepatocytes that burst to produce merozoites. Merozoites invade other erythrocytes which develop into the ring, trophozoite and schizont stage while the schizont ruptures, and thereby increasing the number of merozoites in the human host. Some of the merozoites may develop into gametocytes via the ring stage. Since Plasmodium merozoites’ invasion of the erythrocytes is a necessity for their survival and growth, it could be targeted in an attempt to cure the disease (Rono et al., 2018). As reviewed by Burns et al. (2019), in order to invade RBC, the malaria parasite needs a series of coordinated interactions of proteins, cleavage of protease events, signalling within a cell, secretion and engagement of a myosin-actin motor in an organelle. Because, the aforementioned processes occur in the blood circulation, they are directly sensitive and exposed to antimalarial drugs, therefore they may serve as potential targets for drug development.
2.1.1. Merozoites
Merozoites are invasive forms that allow the entry of the parasite into host’s erythrocytes. Their specialised organelles, rhoptries, and micronemes enable a nonlytic entry into the erythrocytes of the host, while their small, rounded vesicles and exonemes are used by the merozoites to exit from schizonts. The rhoptries, two in number and the micronemes, quite numerous, converge at the apex of merozoites where they secrete the proteins required for erythrocyte binding and entry. After the merozoites have fully developed, they secrete small vesicles and elongated exonemes to dissolve the schizonts (Nasamu et al., 2020). The pellicle (a three-membrane layer) underneath the thick bristly coat on the surface of the merozoites contains the actomyosin motor that propels the merozoites during invasion. Other components of the merozoites are microtubules, a nucleus, a mitochondrion, an apicoplast, and some ribosomes. Of note, any of the components of physiological processes of the merozoites can serve as a potential target for future antimalarial drug development.
2.1.2. Sporozoites
Sporozoites are the invasive forms of malaria parasite which are transmitted to a human host by Anopheles mosquitoes via blood-feeding and they migrate to the liver upon their inoculation to human host (Yang et al., 2017). Sporozoites possess organelles containing several invasive proteins such as the circumsporozoite protein (CSP), which is currently a component of the RTSS and R21 vaccines, and the thrombospondin-related antigen protein (TRAP) that allows the parasite to attach glycosaminoglycans to host cells. Notably, PMV VI is an important protein required for the development of sporozoites in the midgut of an Anopheles mosquito, which could be targeted for the development of inhibitors.
2.1.3. Trophozoites
These are asexual forms of the parasite that occur in the human host. They are vacuolated, amoeboid and uninucleated. The application of PfSpdSyn inhibitor cyclohexylamine has confirmed the arrest of parasite development at trophozoite stage in P. falciparum 3D7 (Becker et al., 2010). It has been reported that trophozoites and schizonts as mature asexual stages of parasite life cycle exhibit increased stiffness, and avoid clearance by spleen through adherence to the vasculature in several organs of the host (Nilsson et al., 2015). Malaria trophozoites express three papain family cysteine proteases which are implicated in the degradation of haemoglobin (Rosenthal et al., 2002).
2.1.4. Schizogony
After the parasite has invaded the host’s erythrocytes, it proliferates through a mitotic cell division mechanism in which new cells assemble around the daughter's nuclei after a number of asynchronous closed nuclear divisions, which is a process termed as schizogony (Morahan et al., 2020). The mitotic process is controlled through the interplay between a number of protein kinases and phosphatases (Morahan et al., 2020). The microtubule organising center (MTOC) within the nuclear envelope signals the onset of mitosis and meiosis in the Plasmodium parasite. Aurora kinases have been implicated in the control of the mitotic pathway in schizogony and are crucial targets for antimalarial drug discovery for effective eradication of the disease (Reininger et al., 2011). Morahan et al. (2020) identified Hesperadin as a potent inhibitor of Aurora kinase, an essential instrument in controlling centromere and kinetochores, which blocks nuclear division in the intraerythrocytic stage of the parasite. Gantt et al. (1998) reported the inhibitory effect of lactacystin and lactacystin analogues on erythrocytic schizogony through inhibition of proteasome and nucleic acid biosynthesis.
2.1.5. Gametocytogenesis
An essential part of the life cycle of malaria parasites is sexual development from the asexual blood stage into gametocytes. Burrows et al., (2017) indicated that the switch from asexual to sexual stage is essential as gametocytes are the form of malaria parasite that are taken up by mosquitoes during a blood meal. Therefore, gametocytogenesis serves as an important aspect in the transmission of the malaria parasite to the mosquito, thereby making it an attractive target for the elimination of malaria (Jennison et al., 2019; Burrows et al., 2018).
P. falciparum gametocytes differentiate through five morphologically distinct stages into male (micro-) and female (macro-) gametocytes which are transmissible to mosquitoes for fertilisation and formation of ookinetes (Hawking et al., 1971). Molecular mechanisms that are crucial during the differentiation stages of gametocytogenesis have been targeted to block the maturation of gametocytes and reduce their transmission (Burrows et al., 2018). An aspartyl protease, PM V (PMV), is active in both the asexual and sexual forms of the malaria parasites. In gametocytes, PMV primes the gametocyte exported proteins (GEXP) for export (Boddey et al., 2010; Russo et al., 2010). When PMV is inhibited, GEXP processing and export is blocked, which result in the inhibition of gametocyte differentiation stages II-V (Jennison et al., 2019). P. falciparum casein kinase 2 (PfCK2) is another attractive potential antimalarial drug target. It is vital in both the proliferation of asexual forms of the malaria parasites and the stages of gametocytes development. Hitz et al. (2021) emphasized the importance of the catalytic subunit of PfCK2α in the maturation of gametocytes. In this study, the authors suggested that experimental knockout and knockdown of PfCK2α respectively inhibit gametocyte maturation in both earlier and later stages of gametocytogenesis. Drugs such as 4-aminoquinolines, 8-aminoquinolines, endoperoxides and antifolates have also been reported to show inhibition against one or two of the gametocytes’ stages (early or late) (Duffy and Avery, 2013). Also, alsinol, an arylacohol exhibited inhibitory activity against late-stage gametocytogenesis, andled to the formation of sterile immature gametocytes (Arias et al., 2020).
For a long time, the key drivers of gametocytogenesis were unknown, hence inhibition of the various stages of gametocytogenesis has been the focus in attenuating the transmission of malaria parasites. With the advent of forward genetics, newer methodologies have been leveraged to extensively elucidate the regulation of gametocytogenesis (Josling et al., 2018). In vitro studies have identified two genes as being essential for the production of gametocytes; apetala-2 transcription factor (ap2-g) and the gametocyte development protein 1 (gdv1) (FIlarsky et al., 2018, Kafsack et al., 2014) . In particular, GDV1 is present in the nucleus and its overexpression results in an increase in the production of gametocytes (reviewed in Josling et al., 2018). It was suspected to serve as a possible transcriptional regulator of the sexual commitment of the malaria parasite and this notion has been corroborated by a study which elucidated GDV1 as a probable epigenetic regulator (FIlarsky et al., 2018). It has been shown that C-terminal truncation of GDV1 led to the failure of malaria parasites to develop into gametocytes (Tibúrcio et al., 2021). Besides, Kafsack et al., (2014) showed that AP2-G is a crucial regulator of gametocytogenesis, usingreverse genetics, they showed thatmalaria parasites do not produce gametocytes in its absence. The authors further described AP2-G as a transcription factor that has the ability to change the fate of the malaria parasite through its overexpression. Also, through available experimental evidence, they posited that the expression of AP2-G induced the asexual-sexual conversion, and thus regulated the sexual commitment phase.
GDVI in combination with other epigenetic factors such as histone deacetylase 2 (HDA2) and heterochromatin protein 1 (HP1) regulate the expression of ap2-g gene (Josling et al., 2018, Kafsack et al., 2014, Sinha et al., 2014). As a master regulator of gametocytogenesis, AP2-G is considered as another probable route to block malaria transmission, while regulators in the upstream and downstream pathways of AP2-G can be potential targets for blocking malaria transmission (Josling et al., 2020). The gametocyte proteome and exportome could serve as essential targets for the development of transmission blocking drugs and treatment interventions with the aim of supporting the global malaria elimination efforts. Through modern day genomics, we can comprehensively identify molecules and cellular processes in gametocytes, which may be targeted for malaria treatment options (Chawla et al., 2021).
3. Target sites for antimalarial drugs
3.1. Target sites in P. falciparum for drug discovery
Malaria drugs over the years have changed with the development of resistance (Colón-Lorenzo et al., 2020). Several sites and biosynthetic pathways are used as antimalarial targets, namely merozoite invasion of the erythrocytes, trophozoite growth, sporozoite development, haemoglobin metabolism and biosynthesis of essential compounds, sporozoites invasion of the hepatocytes, nuclear division in the erythrocytic stage, merozoite and sporozoite egress, gametocytogenesis, and the parasite’s other proteins produced by genome translation. Selective inhibition of one of these proliferative processes provides therapeutic intervention strategies (Morahan et al., 2020).
3.1.1. Proteases
Proteases are catalytic and regulatory molecules involved in the turnover of protein to its constituent amino acids, which are used to generate building blocks for new proteins (Teixeira et al., 2011; Alam, 2014). These enzymes catalyse the degradation of proteins into smaller peptides and amino acids in a process called proteolysis. Most proteins are activated or deactivated through proteolysis for the survival of the organism. The Plasmodium genome encodes approximately 170-Plasmodium proteases, as indicated in the MEROPS database of proteases (Rawlings et al., 2002), which presumably play both regulatory, and effector roles in the various developmental stages of the Plasmodium parasite e.g., protein homeostasis, invasion, immune evasion, trafficking, cell signaling, inflammation and catabolism (Li et al., 2012; Roy, 2017). Falcipains and plasmepsins are specific proteases involved in haemoglobin degradation (Rosenthal et al., 2002; Qidwai, 2015). Deu (2017) contended that some protease family members have their genomic homologues in the human host, thereby making it more important to identify which ones to target, those with or without human homologues. Similarly, the aforementioned review intimated that proteases without human homologues could play specific roles in the parasite (ability to enter RBCs, degrade haemoglobin for nutrients and escape host defensive mechanisms) and thus their inhibition provides a major drug discovery step. The review, however, argues strongly that similar amino acid sequences do not always produce a specific target site sequence, and therefore inhibition of human host proteases does not always cause adverse side effects and hence, targeting host proteases could provide some advantage. There are about five families of Plasmodium proteases, which can serve as targets for antimalarial drug or vaccine discovery, however, few members of these families have been studied (Verma et al., 2016). Families of proteases are aspartate (Plasmepsins, SPP), cysteine (serine repeat antigen (SERA), dipeptidyl aminopeptidases (DPAP)), metallo (falcilysin, peptidase), serine (subtilisin-like protease (SUB), rhomboid proteases (ROM), protease (proline aminopeptidase (PAP)) and threonine (proteasome) proteases (Mckerrow et al., 1993; Gluzman et al., 1994; Blackman and Bannister, 2001; Rosenthal et al., 2002; 2011; Deu, 2017). Proteases have effectively served as drug targets for diseases such as hepatitis C virus, human immunodeficiency virus, and hypertension (Alam, 2014). Protease inhibitors currently considered include among others leupeptin, anti-pain, E-64 and chymostatin (Shibeshi et al., 2020).
3.1.2. Falcipain
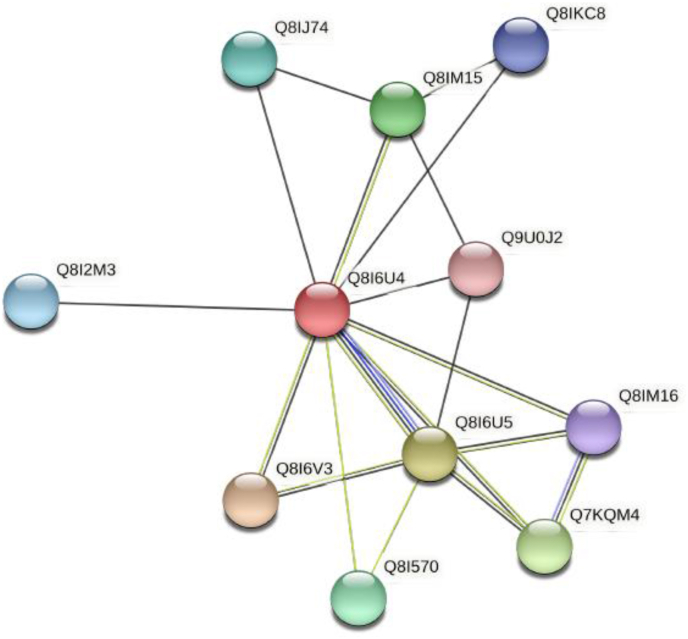
These are P. falciparum cysteine proteases that are involved in the degradation of haemoglobin (Machin et al., 2019), erythrocytic invasion and rupture (Marco and Miguel, 2012). Lee et al. (2003) investigated the antimalarial activities of protease inhibitors and showed that the inhibitors of cysteine proteases blocked the hydrolysis of haemoglobin and thus hindered development of cultured P. falciparum. Falcipain-1 (FP1) plays a crucial role in oocyst production in the developmental stages that occur in the midgut of the mosquito and could aid in host cell invasion. Falcipain 1, 2 and 3 (FP2, FP2’ and FP3) are the key haemoglobinases in the food vacuole (Marco and Miguel, 2012; Nair et al., 2013). Another report has suggested the function of these Falcipains to be transformation of pro-plasmepsins into mature active enzymes (Teixeira et al., 2011). Therefore, their inhibition could affect the degradation of haemoglobin, thereby killing the parasite. Falcipain 2 has close interacting partners that may depict functional relationships (Figure 2). (E)-chalcone 2 inhibitors have been shown to bind FP2 effectively (Machin et al., 2019).
Figure 2.
Protein network of the interaction partners for falcipain 2a. This protein network shows the closest proteins that associate with it and may imply a functional relationship with interacting partners such as Plasmepsins (PM) I, III and IV, falcipain 2b. This was generated using string-db.org. Legend: Q8I6U4: Falcipain 2a-cysteine protease and haemoglobinase, Q8I6V3: Plasmepsin II, Q8I6U5: Falcipain 2b-cysteine protease and haemoglobinase, Q7KQM4: Plasmepsin-I, Q8IM15: Plasmepsin III, Q81570: Independent K + K + translocation inorganic pyrophosphatase of type V, Q8IJ74: Haloacid dehalogenase-like hydrolase, Q8I2M3: Uncharacterized protein, Q8IKC8: Exported protein 2, Q8IM16: Plasmepsin IV, Q9U0J2: Chaperone protein DnaJ.
3.1.3. Subtilisin-like proteins
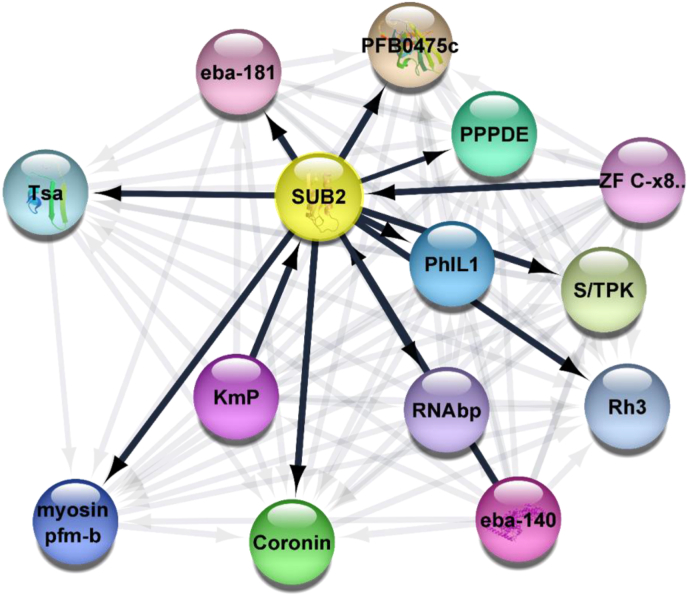
Sub1, a calcium-dependent serine protease that facilitates the final maturation step of MSP1 is important for erythrocyte egress and invasion (Boonyalai et al., 2018; Nava et al., 2019). Sub2 is synthesised during the asexual stage (Withers-Martinez et al., 2004) schizogony and is expressed during merozoite differentiation where it encodes an integral protein located in dense granules of merozoite (Barale et al., 1999), and possibly participates in the latter stages of invasion of erythrocytes. Sub2 is involved in the shedding of the surface proteins of the parasite (Collins et al., 2020). It aids in erythrocytic envelopment of the parasite during internalisation in the parasitophorus membrane. This provides a suitable target for the development of malaria drugs. Molecular coupling and dynamic studies have suggested SUBI inhibitors as drug targets (Favuzza et al., 2020). It has also been shown that the action of PfSUB1 is under the control of cGMP-dependent Protein Kinase. SUB2 and its interaction partners in an elaborate protein network is shown in (Figure 3).
Figure 3.
Protein network interaction diagram for SUB2. Subtilisin-like protein 2 and its closest interacting partners. This network is suggestive and predictive of the close association of Subtilisin-like protease 2 with putative photosensitised INA-labeled protein 1, putative RNA binding protein, putative Kelc motif containing protein, putative coronin, Myosin motor domain containing myosin pfm-b domain and belongs to the TRAFAC class myosin-kinesin ATPase superfamily, trophozoite stage antigen, erythrocyte binding antigen -181, Zinc finger C-x8-C-x5-C-x3-H type, putative serine/threonine protein kinase, putative reticulocyte binding protein 3 and erythrocyte binding antigen -140. Network created using cytoscape 3.8.1. Legend: SUB2 = Subtilisin-like protease 2, PFB0475c = Conserved Uncharacterized protein, PPPDE = PPPDE Peptidase, PhIL1 = Photosensitized INA-Labeled Protein 1, Putative, RNAbp = RNA binding protein, putative, KmP = Kelc motif containing protein, putative, Coronin = coronin, Myosin pfm-b = Myosin motor domain-containing protein, an unconventional myosin pfm-b; , belonging to the TRAFAC class myosin-kinesin ATPase superfamily, Tsa = Trophozoite stage antigen, eba-181 = erythrocyte binding antigen -181, ZF C-x8. = Zinc finger C-x8-C-x5-C-x3-H type, putative, S/TPK = Serine/threonine protein kinase, putative, Rh3 = Reticulocyte-binding protein 3, eba-140 = erythrocyte binding antigen -140.
3.1.3.1. Apical membrane antigen (AMA) interaction with subtilisin-like proteins
The AMA complexes with Plasmodium rhoptry neck (RON) are formed as part of the moving junction between the host cell and invading parasite. AMA1 plays an important role in blood stage replication and antibodies against AMA1 inhibit erythrocyte invasion (Drew et al., 2012). AMA1 could be involved in the reorientation or initiate junctional contact (Mitchell et al., 2004). This cleft is conserved in both the P. falciparum and P. vivax (MacRaild et al., 2011). Inhibition of AMA interferes with host-cell infection by sporozoite (Silvie et al., 2004). This makes AMA1 an essential tool during sporozoites invasion of host cells and as such their inhibition may serve as an excellent antimalarial target.
A subset of serine protease inhibitors blocks the processing and shedding of AMA-I and TRAP as well as inhibits sporozoite infectivity, thereby suggesting that interference with proteolytic processing of sporozoite may constitute a valuable strategy to prevent hepatocyte infection. In particular, AMA1 has been observed to be shed by subtisilin-like protein 2 (SUB2). This shedding ultimately controls erythrocyte invasion and sealing (Collins et al., 2020). Evidentially, a membrane based shedding of AMA1 is essential in the subsequent evasion of antibodies that can inhibit invasion (Olivieri et al., 2011).
3.1.3.2. Merozoite surface protein 1 and subtilisin-like protein interaction
The MSP, a ligand, coats the merozoite surface in all species of the parasite (Baldwin et al., 2015) and is essential in erythrocyte invasion (Beeson et al., 2016; Chandramohanadas et al., 2014; S.-H. Lee et al., 2020). MSP forms a complex with erythrocyte glycophorin A (GPA) and band 3 during the adhesion of merozoite to the erythrocytes. The prevalence of antibodies against the Pf antigen offers promise as a key to gain immunity (Ondigo et al., 2020). Notably, MSP1 (Jäschke et al., 2017; Thái et al., 2018), MSP2 (Das et al., 2017; Eacret et al., 2019; Lu et al., 2019; MacRaild et al., 2011), MSP3 (Lê et al., 2020), and MSP4 (Deshmukh et al., 2018; Perraut et al., 2017), and MSP10 (Bendezu et al., 2019; Nagaoka et al., 2019) are blood-stage proteins that serve as promising vaccine candidates as their-specific antibodies have shown protection against malaria. The parasite leverages SUB1 protease to cleave MSP1 into fragments prior to egress (Koussis et al., 2009). The resulting fragments form a complex on the merozoite surface and together with other proteins act to ultimately induce rupturing of the membrane at egress (Das et al., 2015). SUB2 and its critical role in surface coat shedding are crucial for survival of the parasite. Therefore, potential targeting of SUB2 could provide a panacea for fighting the malaria menace in the face of the emergence of resistant malaria parasites to existing chemotherapeutic options (Collins et al., 2020).
3.1.4. Dipeptidyl aminopeptidases (DPAPs)
DPAPs are cysteine proteases that play an important role in various stages of parasite development and other microbes that cause malaria, toxoplasmosis and Chagas disease (Dalal and Klemba, 2007; Klemba et al., 2004). They cleave protein substrates from the N-terminus to produce dipeptides (M. Tan et al., 2020). In the inhibition assay performed by Sanchez et al. (2019), some selected compounds from both UCSF Small Molecule Discovery Centre’s Diversity collection and Celera Protease Inhibitor Collection have been reported to inhibit the activity of DPAPs. DPAP1 localises in the digestive vacuole and plays roles in the haemoglobin degradation pathway (Deu et al., 2010). DPAP2 is expressed in gametocytes and its knockout (KO) reduces gamete egress from iRBCs. Meanwhile, DPAP3 is involved in erythrocyte invasion and egress (Deu et al., 2010; Lehmann et al., 2018). DPAP1 and -3 are essential in asexual growth (Yamada et al., 2017) and their knockout results in stagnation of the erythrocyte cycle. The selective inhibition of these enzymes provides a new approach for vaccine and drug development. The role played by DPAPs in growth and transmission of the parasite provides possible targets for eliminating the parasite at those stages (Tanaka et al., 2013). The development of DPAP inhibitors is a great approach to combating the disease.
4. Invasion and egress of merozoites and inhibition
4.1. Inhibition as a drug discovery strategy
The discharge of schizont merozoite exposes it to the host immune system for a few minutes, and hence it has to invade new erythrocytes before it is noticed. Proteolysis plays important regulatory and effector roles in rapid and highly regulated egress (Collins et al., 2017) and invasion. Proteolysis involving cysteine and serine proteases is required in the rupture of the parasitophorous vacuole (PV) and erythrocyte membranes for egress. Cell signaling through cGMP activates downstream processes which lead to secretion of proteins from exonemes and micronemes into the PV on the merozoites surface, which may cause the exonemal subtilisin-like protease 1 (SUB1) (Boonyalai et al., 2018) to process egress-invasion specific proteins. Examples are members of the serine repeat antigen (SERA) family with three active proteins (SERA 6–8) and six inactive proteins (SERA 1–5, 9) due to the presence of serine instead of catalytic cysteine. Cysteine and serine inhibitors block egress and remove the protein coat around the merozoite. Deletion of the P. berghei homologue prevents the expression of SERA8 and therefore, prevents sporozoites egress and invasion. Furthermore, the elimination of some SERA5 leads to an incomplete exit phenotype, decreasing invasion efficiency.
4.1.1. Plasmodium rhomboids (PROMs)
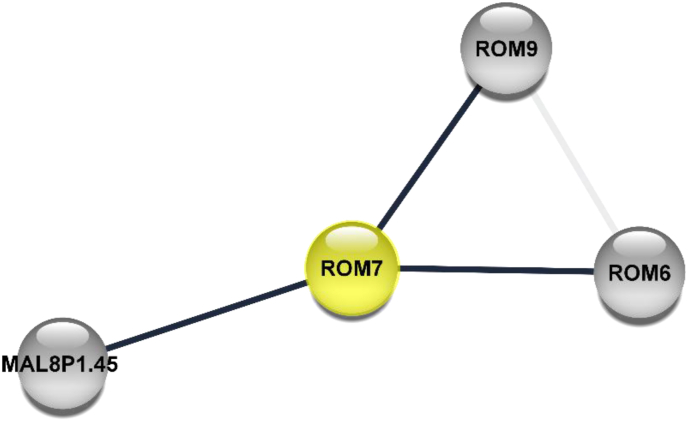
PROMs are serine proteases involved in the erythrocytic invasion of P. falciparum (Srinivasan et al., 2009). They cleave their substrates within transmembrane domains (Mataradchakul et al., 2018; Singh et al., 2007). Mataradchakul and colleague (2018) identified PvROM-like 1 protein in all the eukaryotic stages of P. vivax and suggested the possibility of its involvement in the invasion of erythrocytes due to the similarity of their amino acid sequence to PfROM1. Similarly, Gallenti et al. (2021) suggested the roles of ROM4, 6–8, in the invasion of erythrocytes. Survival through escape from host defensive mechanisms is inhibited by downgrading the activities of the rhomboids. Identification of Plasmodium rhomboids, PfROMs (recently found to be secreted by micronemes (Singh et al., 2007) for falciparum and PvROM for P. vivax would be essential for malaria treatment and vaccine development, as inhibitors may block host-cell invasion (Gandhi et al., 2020). Mostly, the ROMs are likely to have other interacting partners that can interact with them spatiotemporally to potentiate their roles by cleavage of adhesins (Figure 4). Rhomboid proteases have typically been implicated in the cleavage of adhesins in apicomplexan taxa (Shen et al., 2014; Stewart and Tonkin 2015). Some of the key functions alluded to rhomboid proteases include among others a well-defined involvement in signaling, control of growth, cleavage of adhesins etc. and their roles have being hinged on their ability to cleave unrelated substrates (Uddin et al., 2018). The role of rhomboid proteases has been extensively investigated in Toxoplasma gondii, and this has provided key preludes on how to design inhibitors that would target these rhomboid proteases as antiparasitic agents. Typically, T. gondii Rhomboid protease 1 has been implicated as essential in the efficient growth rate of the apicomplexan parasite (Brossier et al., 2008). Six genes in T. gondii have been found to code for rhomboid proteases that are known to be located in distinct cellular compartments of the parasite (Sheiner et al., 2008).
Figure 4.
Protein network interaction diagram for Rhomboid protease (ROM7). ROM7 possesses serine-type endopeptidase activity, which activates its serine nucleophile by a proton and performs hydrolysis of internal, alpha-peptide bonds in a polypeptide chain (Neafsey et al., 2013). This protein has shown putative interactions with ROM8 and ROM9. The Plasmodium rhomboid proteases are involved in most enzymatic events during the invasive stages of the malaria lifecycle. The invasion of Plasmodium depends on the parasite transmembrane adhesins and these adhesins have to be processed by cleavage to be activated by PfROMs such as PfROM1, PfROM4 PfROM7, etc (Baker et al., 2006). Interaction network created using cytoscape 3.8.1. Legend: ROM = Rhomboid protease, MAL8P1.45 = Uncharacterized protein.
4.1.2. Cyclic guanidine monophosphate-dependent protein kinase
Cyclic guanidine monophosphate (cGMP) and P. falciparum protein kinase G (PfPKG) play roles in the asexual replication, invasion and egress (Vanaerschot et al., 2020) of the human malaria parasite as cGMP regulates the activation of PKG (Kim and Sharma, 2021). Taylor et al. (2010) used 4-[2-(4-Fluorophenyl)-5-(1-methylpiperidine-4-yl)-1H pyrrol-3-yl] pyridine as an inhibitor of cGMP kinase to identify its inhibitory effect on ring stage formation, wherein they reported the damaging effect on schizont. They further stated that the inhibitor had no other effect on the host red cell kinases. PKGs could serve as plausible antimalarial targets for drug or vaccine development as it is vital in the replicative cycle occurring in erythrocytes. The amidated analogue 3,6-diphenylated imidazopyridazines has moderate in vitro inhibitory effect on cGMP-dependent PKG activity (Cheuka et al., 2021). Baker et al. (2017) carried out a study using imidasoyridine and reported a similar result which shows that this molecule could inhibit cGMP-dependent PfPKG. Further study could lead to the synthesis of more potent drugs or vaccines with definite inhibitory effect on the protein and possibly end the replicative cycle of the human malaria parasite.
4.1.3. Transfer RNA binding proteins (tRNAbp)
In malaria infection, the parasite does switch hosts (mosquito to human and back to mosquito). The switch from one host to the other require specialised factors and gene regulatory mechanisms. Research works pointing to the role of tRNAbp in translational repression required by the parasite to establish and colonise the mosquito guts has been demonstrated (Braks et al., 2008; Zhang et al., 2010; Quenault et al., 2010).
For instance, the sexual parasite development is controlled by one tRNAbp known as the DEAD-box RNA helicase of the DDX6 family (Braks et al., 2008). Other factors such as the eIF2alpha kinase IK2 controls sporozoites latency while it performs this function by phosphorylating eIF2alpha and downregulating protein synthesis (Zhang et al., 2010). The RNA-binding proteins of the Puf-family, Plasmodium Puf1 and Puf2 have been demonstrated to be critical during sporozoite stage transformation (Quenault et al., 2010). Usually, Puf proteins bind to the 3′ UTR of target mRNAs and repress their translation or activate their degradation (Wickens et al., 2002; Quenault et al., 2010). For instance, the P. berghei, Puf2 plays a part in the regulation of IK2 and inhibits premature sporozoite conversion (Müller et al., 2011). Inside mosquito salivary glands puf2 (-) mutants are unable to initiate malaria infection. In a gene disruption study in P. falciparum, PfPuf2 has been demonstrated to hold a critical role in repressing gametocytogenesis and male gametocyte differentiation in the human malaria parasite (Miao et al., 2010).
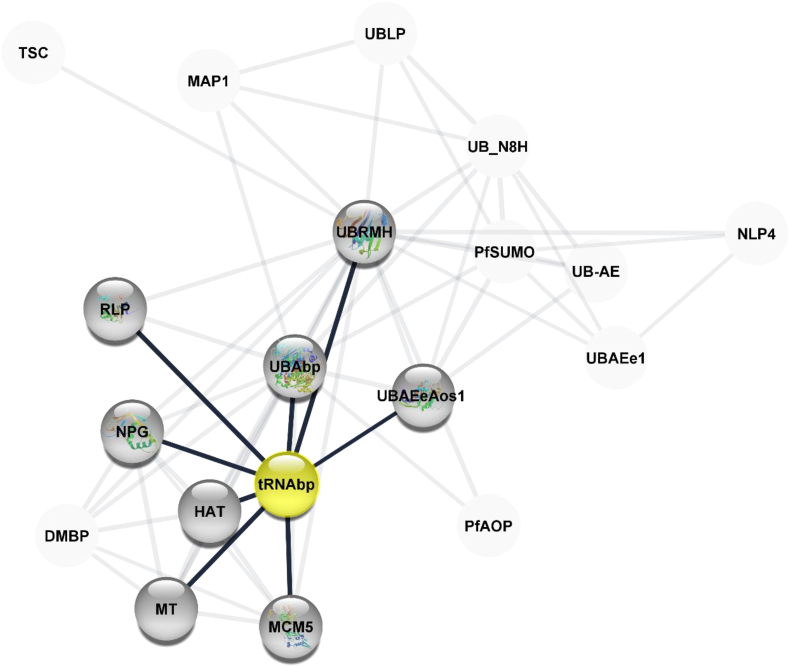
The mechanistic action of tRNAbp has been partially elucidated to include its roles in the 2-thiolation of mcm5S2U at the tRNA wobble positions of tRNA (Lys), tRNA (Glu), and tRNA (Gln) (Duechler et al., 2016). It directly binds to tRNAs and probably acts by catalysing the adenylation of tRNAs, an intermediate required for 2-thiolation. It is unclear whether it acts as a sulfuryl transferase that transfers sulfur from thiocarboxylated URM1 onto the uridine of tRNAs at wobble position. This protein is involved in the pathway 5-methoxycarbonylmethyl-2-thiouridine-tRNA biosynthesis, which is part of tRNA modification. tRNAbp and its interaction partners are illustrated in the protein network (Figure 5).
Figure 5.
Protein network interaction diagram for tRNA binding protein (tRNAbp). The tRNAbp interacts closely with Histone acetyltransferase, DNA replication licensing factor MCM5 belongs to the MCM family, methyl transferase, nucleolar peribosomal GTPase, Rhodanese-like protein, homologue of ubiquitin-related modifier, ubiquitin binding protein, and ubiquitin activating enzyme E1. Interaction network created using cytoscape 3.8.1. Legend: TSC = Tubulin-specific chaperone, putative, MAP1 = Microtubule-associated protein 1, putative, UBLP = Ubiquitin-like protein, UB_N8H = homologue of the nedd8 protein, putative, UBRMH = homologue of the ubiquitin-related modifier, pfSUMO = small ubiquitin-related modifier, putative, UB_AE = Ubiquitin activating enzyme, NLP4 = Nuclear pore associated protein 4, putative, UBAEe1 = Ubiquitin activating enzyme E1, putative UBAEeAos1 = Ubiquitin activating enzyme (E1), subunit Aos1, PfAOP = 1 cy peroxidoxin, UBAbp = UBA/THIF type NAD/FAD binding protein, putative, tRNAbp = cytoplasmic tRNA 2- thiolation protein 1, HAT = Histone acetyltransferase, putative, MCM5 = DNA replication licensing factor MCM5, putative, MT = methyl transferase, putative, DMBP = DPH-type MB domain-containing protein, NPG = Nucleolar Peribosomal GTPase, putative, RLP = Rhodanese like protein, putative, pfSUMO.
4.1.4. Protein kinases
The importance of protein kinases in the biological roles of the parasite is not fully understood. Imidazopyridazines were reported by Green et al. (2016) to be an inhibitor of the kinase PfCDPK1, an essential protein in asexual blood stages. The inhibition of serine/arginine-rich proteins, result in protein-phosphorylating cyclin-dependent kinase also inhibiting the erythrocytic-stage replication (Kern et al., 2014). Mustière et al. (2020) have reviewed a number of inhibitors against plasmodial kinases.
4.2. Organelles of P. falciparum
Several organelles have served as the target sites for antimalarial drugs. The food vacuole of the parasite serves as target sites for antimalarial drugs such as chloroquine, which prevents the bio-crystallisation of haeme (Teixeira et al., 2011). The apicoplast is involved in the synthesis of isoprenoids (Tewari et al., 2021) from isopentenyl pyrophosphate (IPP) in the blood stage of the parasite (Miller et al., 2014; Rai et al., 2017). Inhibitors of prokaryotic DNA replication (ciprofloxacin), prokaryotic translation (chloramphenicol, doxycycline (Okada et al., 2020), tetracycline, clindamycin, azithromycin, erythromycin and clarithromycin), a tRNA synthase (mupirocin), and IPP synthesis pathway (fosmidomycin and FR900098) are regarded as targets of apicoplasts (Uddin et al., 2018). Kumarihamy et al. (2020) showed that the in vitro antimalarial activity of Botryosphaeria dothidea was due to the common metabolic pathway shared by the apicoplast in Plasmodium parasites and plastids in plants. Apicoplast has no human homologue and may therefore provide a more promising antimalarial drug target (Tan et al., 2021). Thus, inhibition of isoprenoid biosynthetic pathway in the apicoplast has been found to kill the malaria parasite (Howe et al., 2013). It is evident that isoprenoid biosynthesis is crucial for protein prenylation as it serves to provide a pool of substrates in the form of isoprenyl (Buckner et al., 2005; Glenn et al., 2006; Glenn et al., 2006,& Nallan et al., 2005). Typically, targeting the enzymes in these metabolic pathways in the apicoplast could potentially drain the malaria parasite and deny it of nutrients for survival.
4.3. Glucose transporters
The transport of glucose across and within cells is essential for the growth and development of organisms. Glucose is transported to food vacuole of the parasite via glucose transporters where it is metabolised to generate energy (Slavic et al., 2011). The Plasmodium glucose transporter (PfHT) is known to be essential for parasite growth and survival (Kraft et al., 2015). Novel antimalarial drugs could be developed to target these promising Plasmodium metabolic pathways (Jiang et al., 2020). Heitmeier et al. (2019) observed that selective inhibition of the P. falciparum hexose transporter (PfHT1) could lead to a shortage of glucose supply to the parasite. They also investigated the role of the O-3-hexose derivative and observed that it selectively inhibited PfHT1 and not Human Glucose Transporter 1 (GLUT1). Additionally, the O-3-hexose derivative has been shown to inhibit glucose uptake by P. vivax (Meireles et al., 2017).
4.4. Serine repeat antigen (SERA)
These are serine-type (SERA 1–5 and SERA 9) and cysteine-type proteases (SERA 6–8) that are expressed in the parasitophorus vacuole of the blood-stage parasite (McCoubrie et al., 2007). Plasmodium SERA is one of the SERA multigene family of eight clustered homologues (Arisue et al., 2020) on chromosome 2 and one on chromosome 9. Putrianti et al. (2020) identified P. bergei SERA4 as an important requirement in blood-stage infection and a disruption of this protein consequently inhibits egress from infected cells. PfSERA 5 is an asexual blood stage antigen (Palacpac et al., 2011) that plays a role in parasite growth (Smith et al., 2020), egress (Iyer et al., 2018), and invasion (Kanodia et al., 2014) by regulating egress timing to coincide with merozoite maturation and disrupt red blood cell membranes. Table 2 provides a list of the essential parasite proteins, their location and known inhibitors.
Table 1.
Important proteins of the malaria parasite.
| Abbreviations | Name | Activity | Reference |
|---|---|---|---|
| FP1 | falcipain-1 | Host Cell Invasion | (Greenbaum et al., 2020) |
| FP2 | falcipain-2 | Merozoite egression | (Dasaradhi et al., 2005) |
| PII | plasmepsin II | Heme conversion to hemozoin | (Chugh et al., 2013) |
| PIV | plasmepsin IV | Heme conversion to hemozoin | (Chugh et al., 2013) |
| HAP | histo aspartic protease | Heme conversion to hemozoin | (Chugh et al., 2013) |
| HDP | heme detoxification protein | Heme conversion to hemozoin | (Chugh et al., 2013) |
| UBA1 | Ubiquitin Activating enzyme 1 | Ubiquitin activation is essential for schizont maturation in Plasmodium falciparum blood stage development. | (Green et al., 2020) |
| PfPFT | Plasmodium falciparum farnesyltransferase | Trophozoite differentiation to schizonts and schizonts to ring transitions | (Chakrabarti et al., 2002) |
Table 2.
Important proteins, site of localisation or release, function and modes of inhibition.
| Proteins | Sub | Site | Function | Inhibition/Inhibitor | Ref. |
|---|---|---|---|---|---|
| Serine repeat antigen (SERA) | SERA5 | Exported to erythrocytes (within the parasitophorus vacuole) | Regulate egress timing to coincide with maturation of merozoites | Conditional knockout (KO) produces an immature egress phenotype leading to a decrease in invasion efficiency | (Arisue et al., 2020) |
| SERA6 | Proteolytically activated by SUB1 to disrupt red blood cell membranes | (Thomas et al., 2018; Ruecker et al., 2012) | |||
| SERA8 | Expressed in sporozoites | Inhibitors prevent the escape of sporozoites from oocysts | (Arisue et al., 2020) | ||
| Subtilisin-like Protease (SUB) | SUB1 | Erythrocytes, Hepatocytes | Involved in the cleavage of parasitophorous vacuole membrane, rhoptry, and erythrocyte membrane proteins | (Boonyalai et al., 2018; Nava et al., 2019) | |
| SUB2 | Secreted by micronemes onto the surface of the merozoite | Sheddase involved in the shed of the protein coat of merozoite | (Boonyalai et al., 2018; Nava et al., 2019) | ||
| Plasmodium falciparum Rhomboids (PfROM) | PfROM4 | Located on the surface of the parasite | Cleaves adhesin proteins involved in parasite attachment to the erythrocyte surface | (koussis, 2018; O'Donnell et al., 2006) | |
| PfROM1 | Found at the apical end of merozoites in liver stages | Cleaves apical membrane antigen 1 | (Baker et al., 2006) | ||
| Merozoite Surface Protein (MSP) | MSP1 | Destabilizes the erythrocyte cytoskeleton | Cleavage reduces parasite egress | (Das et al., 2015) | |
| Dipeptidyl aminopeptidase (DPAP) | DPAP2 | Expressed in gametocytes | Regulates parasite egress, and efficient erythrocyte invasion by parasites | Knockout (KO) decreases gamete egress | (Lehmann et al., 2018) |
| DPAP3 | Erythrocytes | erythrocyte invasion | DPAP3 inhibitors block egress upstream of SUB1 activation. | (Lehmann et al., 2018) | |
| Aspartyl protease (Plasmepsins, PM) | PM I | Erythrocytic stage | Essential in partial degradation of haemoglobin | Knockout has minimal effect on parasite replication | (Coombs et al., 2001) |
| PM II | Erythrocytic stage | Essential in partial degradation of haemoglobin | Knockout has minimal effect on parasite replication Extra copies removal sensitizes the parasite to piperaquine |
(Coombs et al., 2001) | |
| PM III | Erythrocytic stage | Essential in haemoglobin degradation into smaller oligopeptides | Knockout has minimal effect on parasite replication Extra copies removal sensitizes the parasite to piperaquine |
(Mohapatra et al., 2010; Moura et al., 2009) | |
| PM IV | Erythrocytic stage | Essential in haemoglobin degradation into smaller oligopeptides | Knockout has minimal effect on parasite replication | (Liu et al., 2006) | |
| PM V | An endoplasmic-resident integral membrane protein | For protein export | Inhibition blocks biological functions such as protein trafficking on invaded host cell surface | (Blackman and Bannister, 2001) | |
| PM VI | Expressed in transmission-stage parasites | Important in midgut sporozoite development and function | Knockouts prevent sporozoite formation and thus blocks transmission | (Mastan et al., 2017) | |
| PM VII | Located in cytoplasm of parasite's sporozoites and ookinetes | Function is not fully known but might play role in midgut transversal | (Deu, 2017) | ||
| PM VIII | Expressed in transmission-stage parasites | Function is not known | Knockout prevents sporozoite motility Absence reduces the number of salivary gland and hemolymph sporozoites |
(Cheuka et al., 2020; Myung et al., 2004) | |
| PM IX | Localized to rhoptries | For RBC invasion | Cheuka et al., 2020; Myung et al., 2004) | ||
| PM X | Localized to the exonemes | PM X is involved in both egress and invasion | Inhibitors prevent parasite progression from the liver to erythrocytes | (Nasamu et al., 2020) | |
| Apical Membrane Antigen (AMA) | AMA1 | Secreted onto the merozoite surface | bridges interactions between components of the motor and rhoptry-derived proteins that are inserted into the RBCM after reorientation | (Deu, 2017) |
4.5. Plasmepsins
Different classes of enzymes are involved in the catalytic and developmental stages of the parasite. Plasmodium pepsins (plasmepsins, PM) are aspartic proteases involved in the various stages of the parasite (Banerjee et al., 2002). They are involved in biological processes such as haemoglobin degradation, protein export, sporozoite formation, transversal, egress and invasion of the midgut (Deu, 2017). Plasmepsins I-IV with falcipain (papain-like proteases) and falcilysin (metalloprotease) are involved in the degradation of haemoglobin (Hb) for food in the food vacuole of the parasite (Moura et al., 2009). Nonetheless, PMs are the key enzymes in the degradation process, wherein they (PMs I-II) degrade haemoglobin partially and it is further degraded by PM IV and a histo-aspartic protease (HAP) into smaller peptides (Kesavulu et al., 2005)). Inhibition of these plasmepsins have minimal effects on parasite replication but the removal of extra copies of PM II-III re-sensitises the parasite to piperaquine. The PM V is responsible for protein export leading to different morphological changes in the parasite, conversion of asexual to sexual parasite, and escaping host defensive mechanisms. Inhibitors such as WEHI-842 and WEHI-916 (Boonyalai et al., 2018) block protein trafficking on invaded host cell surfaces. The PMs IX-X are involved in the egress and invasion of erythrocytes and hepatocytes, as well as in mosquito guts. Favuzza et al. (2020) demonstrated the modulating activity of PMX during invasion of merozoites and the maturation of proteins involved in invasion, parasite development and exit. The authors further showed the inhibitory effect of WM856, WM4, WM5 and WM382 on PMX with WM382 having a dual effect as it could also inhibit PMIX. Li et al. (2016) reported the expression of PfPM VII and PfPM X in ookinetes and their contribution to the parasite transmission to Anopheles mosquitoes. Inhibition of these enzymes reduces the invasion of hepatocytes and erythrocytes (Deu, 2017). Jiang et al. (2001) identified the noneptidyl compound WR268961 as a potent inhibitor of P. falciparum plasmepsins and reported the potential of pepstatin, SC-50083 and Ro40-4388 to inhibit plasmepsins.
As early as the 1970s, the depletion of spectrin and protein 4.1, which are erythrocyte membrane cytoskeletal proteins, has been well known for Plasmodium parasite infection (Homewood and Neame, 1974).
Plasmepsin II is an aspartic protease that has been characterised from P. falciparum and is noted for its degradation of host cell haemoglobin within the acidic food vacuole of the parasite (Banerjee et al., 2002). Works by Le Bonniec et al. demonstrated an in vitro hydrolysis of erythrocytic spectrin by plasmepsin II (Le Bonniec et al., 1999), which involves cleaving mainly within the SH3 motif of the spectrin a-subunit by aspartic protease(s). They further showed that a recombinant plasmepsin II could digest spectrin and actin at higher pH of 6.8, thereby suggesting that plasmepsin did not only degrade haemoglobin, but also digest infected erythrocytes with the involvement of cytoskeleton. Gluzman et al. (1994) showed that in the slightly acidic food vacuole of the parasite plasmepsin I and II begin digestion of haemoglobin tetramers, which is followed by the cysteine protease falcipain that digests the fragmented substrate (Gluzman et al., 1994). The digestion of spectrin, actin and haemoglobin inevitably leads to the plasma membrane destruction, burst and release of parasites from the infected cell (Goldberg et al., 1991). Below is a diagrammatical illustration of the mechanism of action of plasmepsin I and II (Figure 6.).
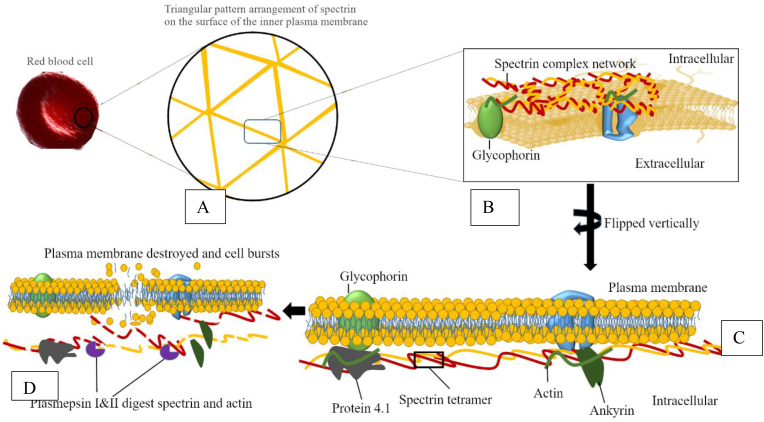
Figure 6.
A Schematic showing the mechanism of action of plasmepsins I and II in the destruction of erythrocytes of P. falciparum. This illustrates the role of Plasmepsin I and II in the destruction of spectrin and actin. A) Amplified plasma membrane with imbedded spectrin, B) expanded spectrin network with a glycophorin anchor, and C) vertically flipped plasma membrane with the spectrin complex network and other anchored moieties making a suitable surface for the actions of plasmepsins. D) Plasmepsin I & II act on the spectrin complex leading to cleavage.
The two homologous aspartic proteases, plasmepsins I and II, initiate the degradation of haemoglobin (Gluzman et al., 1994; Goldberg et al., 1991) and this is followed by the cysteine protease, falcipain-2, and a metalloprotease, falcilysin, which degrade haemoglobin to small peptides (Eggleson et al., 1999). Known inhibitors of these proteases have been demonstrated to exert destructive effect on the malarial parasites, and hence inhibition of the haemoglobin-degradative pathway, which has been highly employed in drug development. For instance, polyhydroxyphenyl and hydroxamic acid derivatives have been shown to possess effective antimalarial activities (Holland et al., 1998). Likewise, the same mechanisms have been observed in the ability of parasites to digest and destroy the plasma membrane of erythrocytes through the cleavage and degradation of spectrin, actin and protein 4.1 using the same proteases (plasmepsin I, II and falcipains). The 'spectrinase activity' of plasmepsin II (Figure 7) acts by cleaving alpha-spectrin at a site located within the SH3 motif of the molecule (Le Bonniec et al., 1999). Plasmepsin II has a broad substrate selectivity and is known to localise extracellularly in parasites in their late schizonts, which presents strong evidence that plasmepsin II plays a greater role in the parasite life cycle than haemoglobin digestion alone (Le Bonniec et al., 1999).
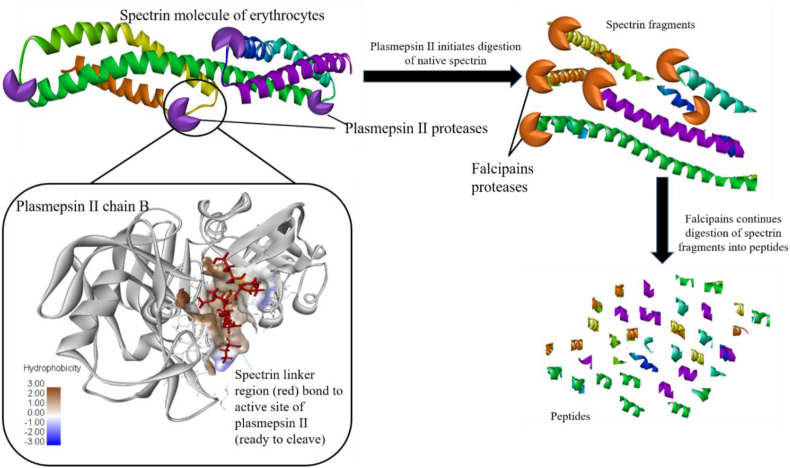
Figure 7.
Illustration of “spectrinase activity” of plasmepsin II (PDB ID: 1SME) and falcipain of the malarial parasite on the human Erythroid Spectrin molecule (PDB ID:1S35). The spectrin linker region was extracted using PyMol (Schrödinger, Inc.), where it was prepared as a ligand and docked onto the active site of Plasmepsin II chain B using PyRx software. Relative free binding energies of the interaction between Plasmepsin II and spectrin indicated a positive interaction between the two molecules indicative of the role of plasmepsin II in spectrin cleavages into short peptides.
5. Conclusion
The need to discover and develop new antimalarial drugs to augment existing chemotherapeutics is becoming increasingly imperative in recent times, since the malaria parasite has developed resistance to some of these drugs. Several proteins, organelles, and metabolic enzymes exceptionally peculiar to the malaria parasite are potential druggable targets for antimalarial agents. In this review, we have explored a number of these targets namely falcipain, Plasmodium rhomboids, dipeptidyl aminopeptidases, apical membrane antigen, cyclic GMP-dependent protein kinases, merozoite surface protein, subtilisin-like proteins, protein-phosphorylating cGMP-dependent kinase-like kinases, organelles and plastids, glucose transporters, serine repeat antigen (SERA), plasmepsins, etc., where we have elucidated their importance in the life cycle of the parasite as well as demonstrated how such targets may be developed into antimalarial drugs. This review also explored the potential use of protein-protein interaction networks to elucidate key interaction partners in the parasite proteome that may be targeted through the development of lead compounds as inhibitors. This review also focused on specific metabolic pathways of the parasite that occur in organelles and can be disrupted with inhibitors to ostensibly weaken the malaria parasite. Altogether, this review has provided current information on drug targets and employed bioinformatics applications to reveal possible influencers on these possible drug targets.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aborode A.T., David K.B., Uwishema O., Nathaniel A.L., Imisioluwa J.O., Onigbinde S.B., Farooq F. Fighting COVID-19 at the expense of malaria in Africa: the consequences and policy options. Am. J. Trop. Med. Hyg. 2021;104(1):26. doi: 10.4269/ajtmh.20-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A. Interdisciplinary Perspectives on Infectious Diseases; 2014. Serine Proteases of Malaria Parasite Plasmodium Falciparum: Potential as Antimalarial Drug Targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias M.H., Quiliano M., Bourgeade-Delmas S., Fabing I., Chantal I., Berthier D., Minet C., Eparvier V., Sorres J., Stien D., Galiano S., Aldana I., Valentin A., Garavito G., Deharo E. Alsinol, an arylamino alcohol derivative active against Plasmodium, Babesia, Trypanosoma, and Leishmania: past and new outcomes. Parasitol. Res. 2020;119(10):3503–3515. doi: 10.1007/s00436-020-06832-y. [DOI] [PubMed] [Google Scholar]
- Arisue N., Palacpac N.M.Q., Tougan T., Horii T. Characteristic features of the SERA multigene family in the malaria parasite. Parasites Vectors. 2020;13(170) doi: 10.1186/s13071-020-04044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.P., Wijetilaka R., Urban S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Pathog. 2006;2(10):e113. doi: 10.1371/journal.ppat.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D.A., Stewart L.B., Large J.M., Bowyer P.W., Ansell K.H., Jiménez-Díaz M.B., El Bakkouri M., Birchall K., Dechering K.J., Bouloc N.S., Coombs P.J., Whalley D., Harding D.J., Smiljanic-Hurley E., Wheldon M.C., Walker E.M., Dessens J.T., Lafuente M.J., Sanz L.M., et al. A potent series targeting the malarial cGMP-dependent protein kinase clears infection and blocks transmission. Nat. Commun. 2017;8(1):430. doi: 10.1038/s41467-017-00572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin M.R., Li X., Hanada T., Liu S.C., Chishti A.H. Merozoite surface protein 1 recognition of host glycophorin a mediates malaria parasite invasion of red blood cells. Blood. 2015;125(17):2704–2711. doi: 10.1182/blood-2014-11-611707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R., Liu J., Beatty W., Pelosof L., Klemba M., Goldberg D.E. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc. Natl. Acad. Sci. U. S. A. 2002;99(2):990–995. doi: 10.1073/pnas.022630099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barale J.C., Blisnick T., Fujioka H., Alzari P.M., Aikawa M., Braun-Breton C., Langsley G. Plasmodium falciparum subtilisin-like protease 2, a merozoite candidate for the merozoite surface protein 1-42 maturase. Proc. Natl. Acad. Sci. U. S. A. 1999;96(11):6445–6450. doi: 10.1073/pnas.96.11.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.V.W., Mtwisha L., Crampton B.G., Stoychev S., Brummelen A. C. Van, Reeksting S., Louw A.I., Birkholtz L., Mancama D.T. Plasmodium falciparum spermidine synthase inhibition results in unique perturbation-specific effects observed on transcript , protein and metabolite levels. BMC Genom. 2010;11(235) doi: 10.1186/1471-2164-11-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson J.G., Drew D.R., Boyle M.J., Feng G., Fowkes F.J.I., Richards J.S. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 2016;40(3):343–372. doi: 10.1093/femsre/fuw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezu J., Villasis E., Morales Ruiz S., Garro K., Infante B., Gutierrez-Loli R., Rodríguez P., Fernández-Díaz M., Gamboa D., Torres K. Evaluation of Plasmodium falciparum MSP10 and its development as a serological tool for the Peruvian Amazon region. Malar. J. 2019;18(1):327. doi: 10.1186/s12936-019-2959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M.J., Bannister L.H. Apical organelles of Apicomplexa: biology and isolation by subcellular fractionation. Mol. Biochem. Parasitol. 2001;117(1):11–25. doi: 10.1016/s0166-6851(01)00328-0. [DOI] [PubMed] [Google Scholar]
- Boddey J.A., Hodder A.N., Gunther S., Gilson P.R., Patsiouras H., Kapp E.A., Pearce J.A., de Koning-Ward T.F., Simpson R.J., Crabb B.S., Cowman A.F. An aspartyl protease directs malaria effector proteins to the host cell. Nature. 2010;463:627–631. doi: 10.1038/nature08728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonday Z.Q., Dhanasekaran S., Rangarajan P.N., Padmanaban G. Import of host δ-aminolevulinate dehydratase into the malarial parasite: identification of a new drug target. Nat. Med. 2000;6(8):898–903. doi: 10.1038/78659. [DOI] [PubMed] [Google Scholar]
- Boonyalai N., Collins C.R., Hackett F., Withers C., Blackman M.J. Essentiality of Plasmodium falciparum plasmepsin V. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0207621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks J.A., Mair G.R., Franke-Fayard B., Janse C.J., Waters A.P. A conserved U-rich RNA region implicated in regulation of translation in Plasmodium female gametocytes. Nucleic Acids Res. 2008;36(4):1176–1186. doi: 10.1093/nar/gkm1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossier F., Starnes G.L., Beatty W.L., Sibley L.D. Microneme rhomboid protease TgROM1 is required for efficient intracellular growth of Toxoplasma gondii. Eukaryot. Cell. 2008;7(4):664–674. doi: 10.1128/EC.00331-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner F.S., Eastman R.T., Yokoyama K., Gelb M.H., Van Voorhis W.C. Protein farnesyl transferase inhibitors for the treatment of malaria and African trypanosomiasis. Curr. Opin. Invest. Drugs. 2005;6:791–797. [PubMed] [Google Scholar]
- Burda P.-C., Caldelari R., Heussler V.T. Manipulation of the host cell membrane during plasmodium liver stage egress. mBio. 2017;8(2) doi: 10.1128/mBio.00139-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A.L., Dans M.G., Balbin J.M., de Koning-Ward T.F., Gilson P.R., Beeson J.G., et al. Targeting malaria parasite invasion of red blood cells as an antimalarial strategy. FEMS Microbiol. Rev. 2019;43(3):223–238. doi: 10.1093/femsre/fuz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows J., Slater H., Macintyre F., Rees S., Thomas A., Okumu F., Wells T.N. A discovery and development roadmap for new endectocidal transmission-blocking agents in malaria. Malaria J. 2018;17(1):1–15. doi: 10.1186/s12936-018-2598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti D., Da Silva T., Barger J., Paquette S., Patel H., Patterson S., Allen C.M. Protein farnesyltransferase and protein prenylation in Plasmodium falciparum. J. Biol. Chem. 2002;277(44):42066–42073. doi: 10.1074/jbc.M202860200. [DOI] [PubMed] [Google Scholar]
- Chandramohanadas R., Basappa R.B., Liew K.J.L., Yau Y.H., Chong A., Liu M., Gunalan K., et al. Small molecule targeting malaria merozoite surface protein-1 (MSP-1) prevents host invasion of divergent plasmodial species. J. Infect. Dis. 2014;210(10) doi: 10.1093/infdis/jiu296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla J., Oberstaller J., Adams J.H. Targeting gametocytes of the malaria parasite plasmodium falciparum in a functional genomics era: next steps. Pathogens. 2021;10(3) doi: 10.3390/pathogens10030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuka P.M., Dziwornu G., Okombo J., Chibale K. Plasmepsin inhibitors in antimalarial drug discovery: medicinal chemistry and target validation (2000 to present) J. Med. Chem. 2020;63(9):4445–4467. doi: 10.1021/acs.jmedchem.9b01622. [DOI] [PubMed] [Google Scholar]
- Cheuka P.M., Centani L., Arendse L.B., Fienberg S., Wambua L., Renga S.S., Dziwornu G.A., Kumar M., Lawrence N., Taylor D., Wittlin S., Coertzen D., Reader J., van der Watt M., Birkholtz L.-M., Chibale K. New amidated 3,6-diphenylated imidazopyridazines with potent antiplasmodium activity are dual inhibitors of plasmodium phosphatidylinositol-4-kinase and cGMP-dependent protein kinase. ACS Infect. Dis. 2021;7(1):34–46. doi: 10.1021/acsinfecdis.0c00481. [DOI] [PubMed] [Google Scholar]
- Choudhary H.H., Gupta R., Mishra S. PKAc is not required for the preerythrocytic stages of Plasmodium berghei. Life Science Alliance. 2019;2(3) doi: 10.26508/lsa.201900352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh M., Sundararaman V., Kumar S., Reddy V.S., Siddiqui W.A., Stuart K.D., Malhotra P. Hemozoin formation complex in Plasmodium. Proc. Natl. Acad. Sci. USA. 2013;110(14):5392–5397. doi: 10.1073/pnas.1218412110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C.R., Hackett F., Atid J., Tan M.S.Y., Blackman M.J. The Plasmodium falciparum pseudoprotease SERA5 regulates the kinetics and efficiency of malaria parasite egress from host erythrocytes. PLoS Pathog. 2017;13(7) doi: 10.1371/journal.ppat.1006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C.R., Hackett F., Howell S.A., Snijders A.P., Russell M.R., Collinson L.M., Blackman M.J. The malaria parasite sheddase SUB2 governs host red blood cell membrane sealing at invasion. Elife. 2020;9 doi: 10.7554/eLife.61121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Lorenzo E.E., Colón-López D.D., Vega-Rodríguez J., Dupin A., Fidock D.A., Baerga-Ortiz A., Ortiz J.G., Bosch J., Serrano A.E. Structure-based screening of plasmodium berghei glutathione S-transferase identifies CB-27 as a novel antiplasmodial compound. Front. Pharmacol. 2020;11(246) doi: 10.3389/fphar.2020.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs G.H., Goldberg D.E., Klemba M., Berry C., Kay J., Mottram J.C. Aspartic proteases of Plasmodium falciparum and other parasitic protozoa as drug targets. Trends Parasitol. 2001;17(11):532–537. doi: 10.1016/s1471-4922(01)02037-2. [DOI] [PubMed] [Google Scholar]
- Counihan N.A., Modak J.K., Koning-Ward D., Tania F. How malaria parasites acquire nutrients from their host. Front. Cell Dev. Biol. 2021;9:582. doi: 10.3389/fcell.2021.649184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S., Klemba M. Roles for two aminopeptidases in vacuolar haemoglobin catabolism in Plasmodium falciparum. J. Biol. Chem. 2007;282:35978–35987. doi: 10.1074/jbc.M703643200. [DOI] [PubMed] [Google Scholar]
- Das S., Hertrich N., Perrin A.J., Withers-Martinez C., Collins C.R., Jones M.L., et al. Processing of Plasmodium falciparum merozoite surface protein MSP1 activates a spectrin-binding function enabling parasite egress from RBCs. Cell Host Microbe. 2015;18(4):433–444. doi: 10.1016/j.chom.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.C., Morales R.A.V., Seow J., Krishnarjuna B., Dissanayake R., Anders R.F., MacRaild C.A., Norton R.S. Lipid interactions modulate the structural and antigenic properties of the C-terminal domain of the malaria antigen merozoite surface protein 2. FEBS J. 2017;284(16):2649–2662. doi: 10.1111/febs.14135. [DOI] [PubMed] [Google Scholar]
- Dasaradhi V.N.,P., Asif M., Kumar A., Hossain M.J., Bhatnagar R.K., Chauhan V.S., Malhotra P. A role of falcipain-2, principal cysteine proteases of Plasmodium falciparum in merozoite egression. Biochem. Biophys. Res. Commun. 2005;336(4):1062–1068. doi: 10.1016/j.bbrc.2005.08.213. [DOI] [PubMed] [Google Scholar]
- Deshmukh A., Chourasia B.K., Mehrotra S., Kana I.H., Paul G., Panda A., Kaur I., Singh S.K., Rathore S., Das A., Gupta P., Kalamuddin M., Gakhar S.K., Mohmmed A., Theisen M., Malhotra P. Plasmodium falciparum MSP3 exists in a complex on the merozoite surface and generates antibody response during natural infection. Infect. Immun. 2018;86(8) doi: 10.1128/IAI.00067-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deu E. Proteases as antimalarial targets : strategies for genetic , chemical , and therapeutic validation. FEBS J. 2017;284:2604–2628. doi: 10.1111/febs.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deu E., Leyva M.J., Albrow V.E., Rice M.J., Ellman J.A., Bogyo M. Functional studies of Plasmodium falciparum dipeptidyl aminopeptidase I (DPAP1) using small molecule inhibitors and active site probes. Chem. Biol. 2010;17(8):808–819. doi: 10.1016/j.chembiol.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew D.R., Hodder A.N., Wilson D.W., Foley M., Mueller I., Siba P.M., Dent A.E., Cowman A.F., Beeson J.G. Defining the antigenic diversity of plasmodium falciparum apical membrane antigen 1 and the requirements for a multi-allele vaccine against malaria. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duechler M., Leszczyńska G., Sochacka E., Nawrot B. Nucleoside modifications in the regulation of gene expression: focus on tRNA. Cell. Mol. Life Sci. 2016;73(16):3075–3095. doi: 10.1007/s00018-016-2217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S., Avery V.M. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar. J. 2013;12(408) doi: 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacret J.S., Gonzales D.M., Franks R.G., Burns J.M. Immunization with merozoite surface protein 2 fused to a Plasmodium-specific carrier protein elicits strain-specific and strain-transcending, opsonizing antibody. Sci. Rep. 2019;9(1):1–17. doi: 10.1038/s41598-019-45440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleson K.K., Duffin K.L., Goldberg D.E. Identification and characterization of falcilysin, a metallopeptidase involved in haemoglobin catabolism within the malaria parasite Plasmodium falciparum. J. Biol. Chem. 1999;274(45):32411–32417. doi: 10.1074/jbc.274.45.32411. [DOI] [PubMed] [Google Scholar]
- Favuzza P., de Lera Ruiz M., Thompson J.K., Triglia T., Ngo A., Steel R.W.J., Vavrek M., Christensen J., Healer J., Boyce C., Guo Z., Hu M., Khan T., Murgolo N., Zhao L., Penington J.S., Reaksudsan K., Jarman K., Dietrich M.H., et al. Dual plasmepsin-targeting antimalarial agents disrupt multiple stages of the malaria parasite life cycle. Cell Host Microbe. 2020;27(4):642–658. doi: 10.1016/j.chom.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIlarsky Michael, Fraschka Sabine A., Niederwieser Igor, Brancucci Nicolas M.B., Carrington Eilidh, et al. GDV1 induces sexual commitment of malaria parasites by antagonizing HP1-dependent gene silencing. Science. 2018;359(6381):1259–1263. doi: 10.1126/science.aan6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallenti R., Poklepovich T., Florin-Christensen M., Schnittger L. The repertoire of serine rhomboid proteases of piroplasmids of importance to animal and human health. Int. J. Parasitol. 2021;51(6):455–462. doi: 10.1016/j.ijpara.2020.10.010. [DOI] [PubMed] [Google Scholar]
- Gandhi S., Baker R.P., Cho S., Stanchev S., Strisovsky K., Urban S. Designed parasite-selective rhomboid inhibitors block invasion and clear blood-stage malaria. Cell Chemical Biology. 2020;27(11):1410–1424. doi: 10.1016/j.chembiol.2020.08.011. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt S.M., Myung J.M.O., Briones M.R.S., Li W.E.I.D., Corey E.J., Omura S., Nussenzweig V., Sinnis P., Hemother A.N.A. Proteasome inhibitors block development of plasmodium spp. Antimicrobial Agents Chemotherapy. 1998;42(10):2731–2738. doi: 10.1128/aac.42.10.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn M.P., Chang S.Y., Hornéy C., Rivas K., Yokoyama K., Pusateri E.E., et al. Structurally simple, potent, Plasmodium selective farnesyltransferase inhibitors that arrest the growth of malaria parasites. J. Med. Chem. 2006;49(19):5710–5727. doi: 10.1021/jm060081v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman I.Y., Francis S.E., Oksman A., Smith C.E., Duffin K.L., Goldberg D.E. Order and specificity of the Plasmodium falciparum haemoglobin degradation pathway. Order and Specificity of the Plasmodium Falciparum Haemoglobin Degradation Pathway. 1994;93(4):1602–1608. doi: 10.1172/JCI117140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D.E. Hemoglobin degradation in Plasmodium-infected red blood cells. Semin. Cell Biol. 1993;4(5):355–361. doi: 10.1006/scel.1993.1042. Academic Press. [DOI] [PubMed] [Google Scholar]
- Goldberg D.E., Slater A.F., Beavis R., Chait B., Cerami A., Henderson G.B. Haemoglobin degradation in the human malaria pathogen Plasmodium falciparum: a catabolic pathway initiated by a specific aspartic protease. J. Exp. Med. 1991;173(4):961–969. doi: 10.1084/jem.173.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green Judith L., Moon R.W., Whalley D., Bowyer P.W., Wallace C., Rochani A., Nageshan R.K., Howell S.A., Grainger M., Jones H.M., Ansell K.H., Chapman T.M., Taylor D.L., Osborne S.A., Baker D.A., Tatu U., Holder A.A. Imidazopyridazine inhibitors of Plasmodium falciparum calcium-dependent protein kinase 1 also target cyclic GMP-dependent protein kinase and heat shock protein 90 to kill the parasite at different stages of intracellular development. Antimicrob. Agents Chemother. 2016;60(3):1464–1475. doi: 10.1128/AAC.01748-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.L., Wu Y., Encheva V., Lasonder E., Prommaban A., et al. Ubiquitin activation is essential for schizont maturation in Plasmodium falciparum blood-stage development. Ubiquitin activation is essential for schizont maturation in Plasmodium falciparum blood-stage development. PLoS Pathog. 2020;16(6) doi: 10.1371/journal.ppat.1008640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum D.C., Baruch A., Grainger M., Bozdech Z., Medzihradszky K.F., Engel J., Bogyo M. A role for the protease falcipain 1 in host cell invasion by the human malaria parasite. Science. 2020;298(5600) doi: 10.1126/science.1077426. [DOI] [PubMed] [Google Scholar]
- Hawking F., Wilson M.E., Gammage K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1971;65(5):549–559. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Heitmeier M.R., Hresko R.C., Edwards R.L., Prinsen M.J., Ilagan M.X.G., Odom John A.R., Hruz P.W. Identification of druggable small molecule antagonists of the Plasmodium falciparum hexose transporter PfHT and assessment of ligand access to the glucose permeation pathway via FLAG-mediated protein engineering. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0216457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitz E., Tibúrcio M., Niederwieser I., Kelly G., Davies H., Doerig C., Billker O., Voss T.S., Treeck M. A 39-amino-acid C-terminal truncation of GDV1 disrupts sexual commitment in plasmodium falciparum. mSphere. 2021;6(3) doi: 10.1128/mSphere.01093-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland K.P., Elford H.L., Bracchi V., Annis C.G., Schuster S.M., Chakrabarti D. Antimalarial activities of polyhydroxyphenyl and hydroxamic acid derivatives. Antimicrob. Agents Chemother. 1998;42(9):2456–2458. doi: 10.1128/aac.42.9.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homewood C.A., Neame K.D. Malaria and the permeability of the host erythrocyte. Nature. 1974;252(5485):718–719. doi: 10.1038/252718a0. [DOI] [PubMed] [Google Scholar]
- Howe R., Kelly M., Jimah J., Hodge D., Odom A.R. Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum. Eukaryot. cell. 2013;12(2):215–223. doi: 10.1128/EC.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer G.R., Singh S., Kaur I., Agarwal S., Siddiqui M.A., Bansal A., Kumar G., Saini E., Paul G., Mohmmed A., Chitnis C.E., Malhotra P. Calcium-dependent phosphorylation of Plasmodium falciparum serine repeat antigen 5 triggers merozoite egress. J. Biol. Chem. 2018;293(25):9736–9746. doi: 10.1074/jbc.RA117.001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäschke A., Coulibaly B., Remarque E.J., Bujard H., Epp C. Merozoite surface protein 1 from plasmodium falciparum is a major target of opsonizing antibodies in individuals with acquired immunity against malaria. Clin. Vaccine Immunol. : CVI. 2017;24(11) doi: 10.1128/CVI.00155-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison C., Lucantoni L., Neill M.T.O., Sleebs B.E., Avery V.M., Boddey J.A., Jennison C., Lucantoni L., Neill M.T.O., Mcconville R., Erickson S.M., Cowman A.F., Sleebs B.E., Avery V.M., Boddey J.A. Inhibition of plasmepsin V activity blocks plasmodium falciparum gametocytogenesis and transmission to mosquitoes report inhibition of plasmepsin V activity blocks plasmodium falciparum gametocytogenesis and transmission to mosquitoes. Cell Rep. 2019;29(12):3796–3806. doi: 10.1016/j.celrep.2019.11.073. e4. [DOI] [PubMed] [Google Scholar]
- Jennison C., Lucantoni L., O’Neill M.T., McConville R., Erickson S.M., Cowman A.F., Boddey J.A. Inhibition of plasmepsin V activity blocks Plasmodium falciparum gametocytogenesis and transmission to mosquitoes. Cell Rep. 2019;29(12):3796–3806. doi: 10.1016/j.celrep.2019.11.073. [DOI] [PubMed] [Google Scholar]
- Jiang S., Prigge S.T., Wei L., Gao Y.E., Hudson T.H., Gerena L., et al. New class of small nonpeptidyl compounds blocks Plasmodium falciparum development in vitro by inhibiting plasmepsins. AAC (Antimicrob. Agents Chemother.) 2001;45(9):2577–2584. doi: 10.1128/AAC.45.9.2577-2584.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Yuan Y., Huang J., Zhang S., Luo S., Wang N., et al. Structural basis for blocking sugar uptake into the malaria parasite Plasmodium falciparum. Cell. 2020;183(1):258–268. doi: 10.1016/j.cell.2020.08.015. [DOI] [PubMed] [Google Scholar]
- Josling G.A., Russell T.J., Venezia J., Orchard L., van Biljon R., Painter H.J., Llinás M. Dissecting the role of PfAP2-G in malaria gametocytogenesis. Nat. Commun. 2020;11(1):1–13. doi: 10.1038/s41467-020-15026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josling G.A., Williamson K.C., Llinás M. Regulation of sexual commitment and gametocytogenesis in malaria parasites. Annu. Rev. Microbiol. 2018;72:501. doi: 10.1146/annurev-micro-090817-062712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack Björn F.C., Rovira-Graells Núria, Clark Taane G., Bancells Cristina, Crowley Valerie M., et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature. 2014 doi: 10.1038/nature12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanodia S., Kumar G., Rizzi L., Pedretti A., Hodder A.N., Romeo S., Malhotra P. Synthetic peptides derived from the C-terminal 6 kDa region of Plasmodium falciparum SERA5 inhibit the enzyme activity and malaria parasite development. Biochim. Biophys. Acta. 2014 doi: 10.1016/j.bbagen.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Kern S., Agarwal S., Huber K., Gehring A.P., Strödke B., Wirth C.C., Brügl T., Abodo L.O., Dandekar T., Doerig C., Fischer R., Tobin A.B., Alam M.M., Bracher F., Pradel G. Inhibition of the SR protein-phosphorylating CLK kinases of Plasmodium falciparum impairs blood stage replication and malaria transmission. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0105732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavulu M.M., Prakasha Gowda A.S., Ramya T.N.C., Surolia N., Suguna K. Plasmepsin inhibitors: design, synthesis, inhibitory studies and crystal structure analysis. J. Pept. Res. 2005;66(4):211–219. doi: 10.1111/j.1399-3011.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Kim C., Sharma R. Cyclic nucleotide selectivity of protein kinase G isozymes. Protein Sci. : A Publication of the Protein Society. 2021;30(2):316–327. doi: 10.1002/pro.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemba M., Gluzman I., Goldberg D.E. A Plasmodium falciparum dipeptidyl aminopeptidase I participates in vacuolar haemoglobin degradation. J. Biol. Chem. 2004;279:43000–43007. doi: 10.1074/jbc.M408123200. [DOI] [PubMed] [Google Scholar]
- Koussis K. University of London, University; College London (United Kingdom): 2008. Functional Dissection of a Malarial Serine Protease. [Google Scholar]
- Koussis K., Withers-Martinez C., Yeoh S., Child M., Hackett F., Knuepfer E., et al. A multifunctional serine protease primes the malaria parasite for red blood cell invasion. EMBO J. 2009;28(6):725–735. doi: 10.1038/emboj.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft T.E., Armstrong C., Heitmeier M.R., Odom A.R., Hruz P.W. The glucose transporter PfHT1 is an antimalarial target of the HIV protease inhibitor lopinavir. Antimicrob. Agents Chemother. 2015;59(10):6203–6209. doi: 10.1128/AAC.00899-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarihamy M., Rosa L.H., Techen N., Ferreira D., Croom E.M.J., Duke S.O., Tekwani B.L., Khan S., Nanayakkara N.P.D. Antimalarials and phytotoxins from Botryosphaeria dothidea identified from a seed of diseased torreya taxifolia. Molecules. 2020;26(1) doi: 10.3390/molecules26010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bonniec S., Deregnaucourt C., Redeker V., Banerjee R., Grellier P., Goldberg D.E., Schrével J. Plasmepsin II, an acidic haemoglobinase from the Plasmodium falciparum food vacuole, is active at neutral pH on the host erythrocyte membrane skeleton. J. Biol. Chem. 1999;274(20):14218–14223. doi: 10.1074/jbc.274.20.14218. [DOI] [PubMed] [Google Scholar]
- Lê H.G., Thái T.L., Kang J.-M., Lee J., Moe M., Võ T.C., Naw H., Myint M.K., Htun Z.T., Kim T.-S., Shin H.-J., Na B.-K. Genetic polymorphism of merozoite surface protein-3 in Myanmar Plasmodium falciparum field isolates. Malar. J. 2020;19(1):184. doi: 10.1186/s12936-020-03256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.J., Singh A., Chiang P., Kemp S.J., Goldman E.A., Weinhouse M.I., Vlasuk G.P., Rosenthal P.J. Antimalarial activities of novel synthetic cysteine protease inhibitors. Antimicrob. Agents Chemother. 2003;47(12):3810–3814. doi: 10.1128/AAC.47.12.3810-3814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Kang H.-J., Chu K.-B., Basak S., Lee D.-H., Moon E.-K., Quan F.-S. Protective immunity induced by virus-like particle containing merozoite surface protein 9 of plasmodium berghei. Vaccines. 2020;8(3) doi: 10.3390/vaccines8030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C., Tan M.S.Y., de Vries L.E., Russo I., Sanchez M.I., Goldberg D.E., Deu E. Plasmodium falciparum dipeptidyl aminopeptidase 3 activity is important for efficient erythrocyte invasion by the malaria parasite. PLoS Pathog. 2018;14(5) doi: 10.1371/journal.ppat.1007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Child M.A., Bogyo M. Proteases as regulators of pathogenesis: examples from the Apicomplexa. Biochim. Biophys. Acta, Proteins Proteomics. 2012;1824(1):177–185. doi: 10.1016/j.bbapap.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Bounkeua V., Pettersen K., Vinetz J.M. Plasmodium falciparum ookinete expression of plasmepsin VII and plasmepsin X. Malar. J. 2016;15(111) doi: 10.1186/s12936-016-1161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. 2020. Plasmepsin: Function, Characterization and Targeted Antimalarial Drug Development.https://www.intechopen.com/books/advanced-biometric-technologies/liveness-detection-in-biometrics Intechopen, 183–202. [Google Scholar]
- Liu J., Istvan E.S., Gluzman I.Y., Gross J., Goldberg D.E. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc. Natl. Acad. Sci. USA. 2006;103(23):8840–8845. doi: 10.1073/pnas.0601876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Zheng X., Zhang W., Zhao H., MacRaild C.A., Norton R.S., Zhuang Y., Wang J., Zhang X. Interaction of merozoite surface protein 2 with lipid membranes. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2019;593(3):288–295. doi: 10.1002/1873-3468.13320. [DOI] [PubMed] [Google Scholar]
- Machin J.M., Kantsadi A.L., Vakonakis I. The complex of Plasmodium falciparum falcipain-2 protease with an (E)-chalcone-based inhibitor highlights a novel, small, molecule-binding site. Malar. J. 2019;18(388) doi: 10.1186/s12936-019-3043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRaild C.A., Anders R.F., Foley M., Norton R.S. Apical membrane antigen 1 as an anti-malarial drug target. Curr. Top. Med. Chem. 2011;11(16):2039–2047. doi: 10.2174/156802611796575885. [DOI] [PubMed] [Google Scholar]
- Marco M., Miguel Coteron J. Falcipain inhibition as a promising antimalarial target. Curr. Top. Med. Chem. 2012;12(5):408–444. doi: 10.2174/156802612799362913. [DOI] [PubMed] [Google Scholar]
- Mastan B.S., Narwal S.K., Dey S., Kumar K.A., Mishra S. Plasmodium berghei plasmepsin VIII is essential for sporozoite gliding motility. Int. J. Parasitol. 2017;47(5):239–245. doi: 10.1016/j.ijpara.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Mataradchakul T., Uthaipibull C., Vega-Rodriguez J., Jacobs-Lorena M., Lek-Uthai U. Investigation of plasmodium vivax rhomboid-like protease 1 compared to plasmodium falciparum rhomboid protease 1 in erythrocytic cycle. Chiang Mai J. Sci. 2018;45(X):1–11. [Google Scholar]
- McCoubrie J.E., Miller S.K., Sargeant T., Good R.T., Hodder A.N., Speed T.P., De Koning-Ward T.F., Crabb B.S. Evidence for a common role for the serine-type Plasmodium falciparum serine repeat antigen proteases: implications for vaccine and drug design. Infect. Immun. 2007;75(12):5565–5574. doi: 10.1128/IAI.00405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerrow J.H., Sun E., Rosenthal P.J., Bouvier J. The proteases and pathogenicity of parasitic protozoa. Annu. Rev. Microbiol. 1993;47(1):821–853. doi: 10.1146/annurev.mi.47.100193.004133. [DOI] [PubMed] [Google Scholar]
- Meireles P., Sales-Dias J., Andrade C.M., et al. GLUT1-mediated glucose uptake plays a crucial role during Plasmodium hepatic infection. Cell Microbiol. 2017;19(2) doi: 10.1111/cmi.12646. e12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J., Li J., Fan Q., Li X., Li X., Cui L. The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum. J. Cell Sci. 2010;123(7):1039–1049. doi: 10.1242/jcs.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.E., Parrott E.E., Singh R., Nelson S.W. A high-throughput assay to identify inhibitors of the apicoplast DNA polymerase from plasmodium falciparum. J. Biomol. Screen. 2014;19(6):966–972. doi: 10.1177/1087057114528738. [DOI] [PubMed] [Google Scholar]
- Mitchell G.H., Thomas A.W., Margos G., Dluzewski A.R., Bannister L.H. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect. Immun. 2004;72(1):154–158. doi: 10.1128/IAI.72.1.154-158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S.C., Tiwari H.K., Singla M., Rathi B., Sharma A., Mahiya K., et al. Antimalarial evaluation of copper (II) nanohybrid solids: inhibition of plasmepsin II, a haemoglobin-degrading malarial aspartic protease from Plasmodium falciparum. JBIC, J. Biol. Inorg. Chem. 2010;15(3):373–385. doi: 10.1007/s00775-009-0610-9. [DOI] [PubMed] [Google Scholar]
- Morahan B.J., Abrie C., Al-hasani K., Batty M.B., Corey V., Cowell A.N., Niemand J., Winzeler E.A., Birkholtz L., Doerig C., Garcia-bustos J.F. Human Aurora kinase inhibitor Hesperadin reveals. Communications Biology. 2020;3(701) doi: 10.1038/s42003-020-01424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura P.A., Dame J.B., Fidock D.A. Role of Plasmodium falciparum digestive vacuole plasmepsins in the specificity and antimalarial mode of action of cysteine and aspartic protease inhibitors. Antimicrob. Agents Chemother. 2009;53(12):4968–4978. doi: 10.1128/AAC.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Matuschewski K., Silvie O. The Puf-family RNA-binding protein Puf2 controls sporozoite conversion to liver stages in the malaria parasite. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0019860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustière R., Vanelle P., Primas N. Plasmodial kinase inhibitors targeting malaria: recent developments. Molecules. 2020;25:5949. doi: 10.3390/molecules25245949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung J.M., Marshall P., Sinnis P. The Plasmodium circumsporozoite protein is involved in mosquito salivary gland invasion by sporozoites. Mol. Biochem. Parasitol. 2004;133(1):53–59. doi: 10.1016/j.molbiopara.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Nagaoka H., Kanoi B.N., Jinoka K., Morita M., Arumugam T.U., Palacpac N.M.Q., Egwang T.G., Horii T., Tsuboi T., Takashima E. The N-terminal region of plasmodium falciparum MSP10 is a target of protective antibodies in malaria and is important for PfGAMA/PfMSP10 interaction. Front. Immunol. 2019;10:2669. doi: 10.3389/fimmu.2019.02669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Kavrekar V., Mishra A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013;3(1):128–132. [Google Scholar]
- Nallan L., Bauer K.D., Bendale P., Rivas K., Yokoyama K., Hornéy C.P., et al. Protein farnesyltransferase inhibitors exhibit potent antimalarial activity. J. Med. Chem. 2005;48(11):3704–3713. doi: 10.1021/jm0491039. [DOI] [PubMed] [Google Scholar]
- Nasamu A.S., Polino A.J., Istvan E.S., Goldberg D.E. Malaria parasite plasmepsins: more than just plain old degradative pepsins. J. Biol. Chem. 2020 doi: 10.1074/jbc.REV120.009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava S., White A.C.J., Castellanos-González A. Cryptosporidium parvum subtilisin-like serine protease (SUB1) is crucial for parasite egress from host cells. Infect. Immun. 2019;87(5) doi: 10.1128/IAI.00784-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey D., Hoffman S., Volkman S., Rosenthal P., Walker B., Young S.K., Zeng Q., Gargeya S., Fitzgerald M., Haas B., Abouelleil A., Allen A.W., Alvarado L., Arachchi H.M., Berlin A.M., Chapman S.B., Gainer-Dewar J., Goldberg J., Birren B. The genome annotation of plasmodium falciparum Tanzania (2000708) The Broad Institute Genome Sequencing Platform, The Broad Institute Genome Sequencing Center for Infectious Disease. 2013 EMBL/GenBank/DDBJ databases. [Google Scholar]
- Nilsson S.K., Childs L.M., Buckee C., Marti M. Targeting human transmission biology for malaria elimination. PLoS Pathog. 2015;11(6) doi: 10.1371/journal.ppat.1004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell R.A., Hackett F., Howell S.A., Treeck M., Struck N., Krnajski Z., et al. Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J. Cell Biol. 2006;174(7):1023–1033. doi: 10.1083/jcb.200604136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Guo P., Nalder S.-A., Sigala P.A. Doxycycline has distinct apicoplast-specific mechanisms of antimalarial activity. Elife. 2020;9 doi: 10.7554/eLife.60246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri A., Collins C.R., Hackett F., Withers-Martinez C., Marshall J., Flynn H.R., et al. Juxtamembrane shedding of Plasmodium falciparum AMA1 is sequence independent and essential, and helps evade invasion-inhibitory antibodies. PLoS Pathog. 2011;7(12) doi: 10.1371/journal.ppat.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondigo B.N., Hamre K.E.S., Frosch A.E.P., Ayodo G., White M.T., John C.C. Antibody profiles to P. Falciparum antigens over time characterize acute and long-term malaria exposure in an area of low and unstable transmission. Am. J. Trop. Med. Hyg. 2020;103(6):2189–2197. doi: 10.4269/ajtmh.19-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacpac N.M.Q., Arisue N., Tougan T., Ishii K.J., Horii T. Plasmodium falciparum serine repeat antigen 5 (SE36) as a malaria vaccine candidate. Vaccine. 2011;29:5837–5845. doi: 10.1016/j.vaccine.2011.06.052. [DOI] [PubMed] [Google Scholar]
- Perraut R., Varela M.-L., Joos C., Diouf B., Sokhna C., Mbengue B., Tall A., Loucoubar C., Touré A., Mercereau-Puijalon O. Association of antibodies to Plasmodium falciparum merozoite surface protein-4 with protection against clinical malaria. Vaccine. 2017;35(48 Pt B):6720–6726. doi: 10.1016/j.vaccine.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Perrin A.J., Bisson C., Faull P.A., Renshaw M.J., Lees R.A., Fleck R.A., Saibil H.R., Snijders A.P., Baker D.A., Blackman M.J. Malaria parasite schizont egress antigen-1 plays an essential role in nuclear segregation during schizogony. mBio. 2021;12(2) doi: 10.1128/mBio.03377-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.A., Rathod P.K. Plasmodium dihydroorotate dehydrogenase: a promising target for novel anti-malarial chemotherapy. National Institutes of Health. 2010;10(3):226–239. doi: 10.2174/187152610791163336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phyo A.P., Ashley E.A., Anderson T.J., Bozdech Z., Carrara V.I., Sriprawat K., Nosten F. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai–Myanmar border (2003–2013): the role of parasite genetic factors. Clin. Infect. Dis. 2016;63(6):784–791. doi: 10.1093/cid/ciw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putrianti E.D., Schmidt-Christensen A., Heussler V., Matuschewski K., Ingmundson A. A plasmodium cysteine protease required for efficient transition from the liver infection stage. PLoS Pathog. 2020;16(9):1–20. doi: 10.1371/journal.ppat.1008891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qidwai T. Haemoglobin degrading proteases of plasmodium falciparum as antimalarial drug targets. Curr. Drug Targets. 2015;16(10):1133–1141. doi: 10.2174/1389450116666150304104123. [DOI] [PubMed] [Google Scholar]
- Quenault T., Lithgow T., Traven A. PUF proteins: repression, activation and mRNA localization. Trends Cell Biol. 2010 doi: 10.1016/j.tcb.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Rai P., Sharma D., Soni R., Khatoon N., Sharma B., Bhatt T.K. Plasmodium falciparum apicoplast and its transcriptional regulation through calcium signaling. J. Microbiol. 2017;55(4):231–236. doi: 10.1007/s12275-017-6525-1. [DOI] [PubMed] [Google Scholar]
- Rawlings N.D., O’Brien E., Barrett A.J. MEROPS: the protease database. Nucleic Acids Res. 2002;30(1):343–346. doi: 10.1093/nar/30.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reininger L., Wilkes J.M., Bourgade H., Miranda-Saavedra D., Doerig C. An essential Aurora-related kinase transiently associates with spindle pole bodies during Plasmodium falciparum erythrocytic schizogony. Mol. Microbiol. 2011;79(1):205–221. doi: 10.1111/j.1365-2958.2010.07442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rono M.K., Nyonda M.A., Simam J.J., Ngoi J.M., Mok S., Kortok M.M., et al. Adaptation of Plasmodium falciparum to its transmission environment. Nature ecology & evolution. 2018;2(2):377–387. doi: 10.1038/s41559-017-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P.J., Sijwali P.S., Singh A., Shenai B.R. Cysteine proteases of malaria parasites: targets for chemotherapy. Curr. Pharmaceut. Des. 2002;8(18):1659–1672. doi: 10.2174/1381612023394197. [DOI] [PubMed] [Google Scholar]
- Roy K.K. Targeting the active sites of malarial proteases for antimalarial drug discovery: approaches, progress and challenges. Int. J. Antimicrob. Agents. 2017;50(3):287–302. doi: 10.1016/j.ijantimicag.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Ruecker A., Shea M., Hackett F., Suarez C., Hirst E.M., Milutinovic K., Blackman M.J. Proteolytic activation of the essential parasitophorous vacuole cysteine protease SERA6 accompanies malaria parasite egress from its host erythrocyte. J. Biol. Chem. 2012;287(45):37949–37963. doi: 10.1074/jbc.M112.400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I., Babbitt S., Muralidharan V., Butler T., Oksman A., Goldberg D.E. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature. 2010;463(7281):632–636. doi: 10.1038/nature08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M.I., De Vries L.E., Lehmann C., Lee J.T., Ang K.K., Wilson C., Chen S., Arkin M.R., Bogyo M., Deu E. Identification of Plasmodium dipeptidyl aminopeptidase allosteric inhibitors by high throughput screening. PLoS One. 2019;14(12):1–21. doi: 10.1371/journal.pone.0226270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiner L., Dowse T.J., Soldati-Favre D. Identification of trafficking determinants for polytopic rhomboid proteases in Toxoplasma gondii. Traffic. 2008;9(5):665–677. doi: 10.1111/j.1600-0854.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- Shen B., Buguliskis J.S., Lee T.D., Sibley L.D. Functional analysis of rhomboid proteases during Toxoplasma invasion. mBio. 2014;5(5):14. doi: 10.1128/mBio.01795-14. e01795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibeshi M.A., Kifle Z.D., Atnafie S.A. Antimalarial drug resistance and novel targets for antimalarial drug discovery. Infect. Drug Resist. 2020;13:4047. doi: 10.2147/IDR.S279433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvie O., Franetich J.F., Charrin S., Mueller M.S., Siau A., Bodescot M., Rubinstein E., Hannoun L., Charoenvit Y., Kocken C.H., Thomas A.W., Van Gemert G.J., Sauerwein R.W., Blackman M.J., Anders R.F., Pluschke G., Mazier D. A role for apical membrane antigen 1 during invasion of hepatocytes by plasmodium falciparum sporozoites. J. Biol. Chem. 2004;279(10):9490–9496. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- Singh S., Plassmeyer M., Gaur D., Miller L.H. Mononeme: a new secretory organelle in Plasmodium falciparum merozoites identified by localization of rhomboid-1 protease. Proc. Natl. Acad. Sci. U. S. A. 2007;104(50):20043–20048. doi: 10.1073/pnas.0709999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A., Hughes K.R., Modrzynska K.K., Otto T.D., Pfander C., Dickens N.J., Waters A.P. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature. 2014;507(7491):253–257. doi: 10.1038/nature12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavic K., Krishna S., Derbyshire E.T., Staines H.M. Plasmodial sugar transporters as anti-malarial drug targets and comparisons with other protozoa. Malar. J. 2011;10:165. doi: 10.1186/1475-2875-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N.A., Clarke O.B., Lee M., Hodder A.N., Smith B.J. Structure of the plasmodium falciparum PfSERA5 pseudo-zymogen. Protein Sci. : A Publication of the Protein Society. 2020;29(11):2245–2258. doi: 10.1002/pro.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P., Coppens I., Jacobs-Lorena M. Distinct roles of Plasmodium rhomboid 1 in parasite development and malaria pathogenesis. PLoS Pathog. 2009;5(1) doi: 10.1371/journal.ppat.1000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R.J., Tonkin C.J. Rhomboid proteases in invasion and replication of Apicomplexa. Mol. Microbiol. 2015;97(2):185–188. doi: 10.1111/mmi.13032. [DOI] [PubMed] [Google Scholar]
- Tan M.S.Y., Davison D., Sanchez M.I., Anderson B.M., Howell S., Snijders A., Edgington-Mitchell L.E., Deu E. Novel broad-spectrum activity-based probes to profile malarial cysteine proteases. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0227341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S., Mudeppa D.G., Kokkonda S., White J., 3rd, Patrapuvich R., Rathod P.K. Antimicrobial Agents and Chemotherapy; 2021. Properties of Plasmodium Falciparum with a Deleted Apicoplast DNA Gyrase; p. AAC0058621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T.Q., Deu E., Molina-Cruz A., Ashburne M.J., Ali O., Suri A., Kortagere S., Bogyo M., Williamson K.C. Plasmodium dipeptidyl aminopeptidases as malaria transmission-blocking drug targets. Antimicrob. Agents Chemother. 2013;57(10):4645–4652. doi: 10.1128/AAC.02495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H.M., McRobert L., Grainger M., Sicard A., Dluzewski A.R., Hopp C.S., Holder A.A., Baker D.A. The malaria parasite cyclic GMP-dependent protein kinase plays a central role in blood-stage schizogony. Eukaryot. Cell. 2010;9(1):37–45. doi: 10.1128/EC.00186-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C., R.B. Gomes J., Gomes P. Falcipains, plasmodium falciparum cysteine proteases as key drug targets against malaria. Curr. Med. Chem. 2011;18(1) doi: 10.2174/092986711795328328. [DOI] [PubMed] [Google Scholar]
- Tewari S.G., Rajaram K., Swift R.P., Reifman J., Prigge S.T., Wallqvist A. Metabolic survival adaptations of plasmodium falciparum exposed to sublethal doses of fosmidomycin. Antimicrob. Agents Chemother. 2021;65(4) doi: 10.1128/AAC.02392-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thái T.L., Jun H., Lee J., Kang J.-M., Lê H.G., Lin K., Thant K.Z., Sohn W.-M., Kim T.-S., Na B.-K. Genetic diversity of merozoite surface protein-1 C-terminal 42 kDa of Plasmodium falciparum (PfMSP-1(42)) may be greater than previously known in global isolates. Parasites Vectors. 2018;11(1):455. doi: 10.1186/s13071-018-3027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.A., Tan M.S., Bisson C., Borg A., Umrekar T.R., Hackett F., Blackman M.J. A protease cascade regulates release of the human malaria parasite Plasmodium falciparum from host red blood cells. Nature microbiology. 2018;3(4):447–455. doi: 10.1038/s41564-018-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin T., McFadden G.I., Goodman C.D. Validation of putative apicoplast-targeting drugs using a chemical supplementation assay in cultured human malaria parasites. Antimicrob. Agents Chemother. 2018;62(1):17. doi: 10.1128/AAC.01161-17. e01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaerschot M., Murithi J.M., Pasaje C.F.A., Ghidelli-Disse S., Dwomoh L., Bird M., Spottiswoode N., Mittal N., Arendse L.B., Owen E.S., Wicht K.J., Siciliano G., Bösche M., Yeo T., Kumar T.R.S., Mok S., Carpenter E.F., Giddins M.J., Sanz O., et al. Inhibition of resistance-refractory P. Falciparum kinase PKG delivers prophylactic, blood stage, and transmission-blocking antiplasmodial activity. Cell Chemical Biology. 2020;27(7):806–816. doi: 10.1016/j.chembiol.2020.04.001. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S., Dixit R., Pandey K.C. Cysteine proteases: modes of activation and future prospects as pharmacological targets. Front. Pharmacol. 2016;7:107. doi: 10.3389/fphar.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO | Q&A on the malaria vaccine implementation programme (MVIP) 2020. https://www.who.int/malaria/media/malaria-vaccine-implementation-qa/en/ (accessed March 20, 2020)
- Wickens M., Bernstein D.S., Kimble J., Parker R. A PUF family portrait: 3′ UTR regulation as a way of life. Trends Genet. 2002;18(3):150–157. doi: 10.1016/s0168-9525(01)02616-6. [DOI] [PubMed] [Google Scholar]
- Withers-Martinez C., Jean L., Blackman M.J. Subtilisin-like protease of the malaria parasite. Mol. Microbiol. 2004;53(1):55–63. doi: 10.1111/j.1365-2958.2004.04144.x. [DOI] [PubMed] [Google Scholar]
- Yamada T., Maeda M., Alshahni M.M., Tanaka R., Yaguchi T., Bontems O., Salamin K., Fratti M., Monod M. Terbinafine resistance of trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob. Agents Chemother. 2017;61(7) doi: 10.1128/AAC.00115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.S.P., Lopaticki S., O’Neill M.T., Erickson S.M., Douglas D.N., Kneteman N.M., Boddey J.A. AMA1 and MAEBL are important for Plasmodium falciparum sporozoite infection of the liver. Cell Microbiol. 2017;19(9) doi: 10.1111/cmi.12745. [DOI] [PubMed] [Google Scholar]
- Zhang M., Fennell C., Ranford-Cartwright L., Sakthivel R., Gueirard P., Meister S., et al. The Plasmodium eukaryotic initiation factor-2α kinase Ik2 controls the latency of sporozoites in the mosquito salivary glands. J. Exp. Med. 2010;207(7):1465–1474. doi: 10.1084/jem.20091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.