Abstract
Scope
Despite the large availability of vaccines, coronavirus disease 2019 (COVID-19), induced by severe acute respiratory syndrome coronavirus 2, continues to be a major threat for health-care providers and fragile people. A number of options are now available for outpatients with mild-to-moderate COVID-19 at the risk of disease progression for the prevention of deaths or hospitalization.
Methods
A European Society of Clinical Microbiology and Infectious Diseases COVID-19 guidelines task force was established by the European Society of Clinical Microbiology and Infectious Diseases Executive Committee. A small group was established, half appointed by the chair and the remaining selected based on an open call. Each panel met virtually once a week. For all decisions, a simple majority vote was used. A long list of clinical questions using the population, intervention, comparison, outcome format was developed at the beginning of the process. For each population, intervention, comparison, outcome, two panel members performed a literature search, with a third panelist involved in case of inconsistent results. Voting was based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Recommendations
In this update, we focus on anti-viral agents, monoclonal antibodies (mAbs) and other treatment options proposed for patients with mild or moderate COVID-19 who are at the risk of hospitalization or death. Although the use of anti-virals is recommended, especially nirmatrelvir/ritonavir and remdesivir or, alternatively, molnupirarvir, the administration of mAbs against the spike protein strictly depends on circulating variants or the ability to test timely for variants and sub-variants. At the time of writing (April–June 2022), the only active mAb was tixagevimab/cilgavimab given the predominance of the Omicron BA.2, BA.3, BA.4 and BA.5 sub-lineages in Europe. However, considering that the epidemiological scenario is extremely dynamic, constant monitoring of variants of concern is mandatory.
Keywords: Cilgavimab, COVID-19, ESCMID, Molnupiravir, Nirmatrelvir/ritonavir, Outpatients, Remdesivir, Sotrovimab, Tixagevimab
Introduction
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a dramatic impact on health-care systems, the global economy, and social life. The clinical spectrum of coronavirus disease 2019 (COVID-19), induced by SARS-CoV-2, is broad, with the majority of infected individuals experiencing only mild or subclinical illness, especially in the early phase of the disease [1]. Although at the beginning of the pandemic, the number of available treatment options was extremely limited, the current landscape of the pandemic is extremely different. In fact, effective vaccines are now available, and a number of treatments have been evaluated in randomized clinical trials (RCTs). Some novel drugs are now available for outpatients with mild-to-moderate COVID-19 who are at the risk of disease progression. The correct use of these new resources is important to reduce the rate of hospitalisations or deaths related to COVID-19 and reduce pressure on health-care facilities. Additionally, these treatments offer an option to patients who are reluctant to vaccine administration or in case of fragile people at the risk of vaccination failure. On the other hand, the appropriate and cautious use of new treatments is important to avoid wastage of resources, especially in settings with limited access to effective therapies.
Motivation for guideline development
The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) developed the first set of guidelines in 2021. However, the scenario of the treatment of COVID-19 is in continuous evolution. Therefore, starting from December 2021, the ESCMID COVID-19 treatment guideline panel decided to promote a new update of the document. Rather than focusing on both outpatient and inpatient settings, this update was intended to provide evidence-based recommendations for the management of adult patients with mild or moderate COVID-19. Considering the composition of the panel, mostly clinical microbiologists or infectious disease specialists and only one member with an intensive care background, we focus only on pharmacological treatment and do not give recommendations on oxygen supplement/support. Similarly, because no paediatricians were included in the panel, the recommendations are only for adult patients with COVID-19.
Methods
The ESCMID COVID-19 treatment guideline panel was established in January 2021. The details of selection criteria were published in detail elsewhere [2].
Each panel met virtually once a week. For all decisions, a simple majority vote was used. A long list of clinical questions using the population, intervention, comparison, outcome (PICO) format was developed at the beginning of the process. A maximum number of seven PICOs were set and selected by voting (the seven top-rated PICOs were chosen). The criteria for prioritisation and voting were based on general interests by clinicians with clinical microbiology and infectious disease backgrounds and the availability of evidence, especially for critical outcomes, which included mortality or disease progression. After careful evaluation, the panel decided not to evaluate drugs which are currently not available outside the United States (i.e. bebtelovimab) [3].
Evidence review
To avoid duplication of efforts, rather than performing a systematic review of the literature for each PICO, ADOLOPMENT criteria were used as previously described [2]. The results of the searches were presented to the panel during the weekly meetings for discussion and voting (quality of evidence, evidence-to-decision criteria, need for update, etc.) based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Definitions of disease severity and risk factors
The WHO severity criteria for COVID-19 were used [4]. Data from the European Center for Disease Prevention and Control were used to define risk factors and groups for severe COVID-19 [[5], [6], [7]]. More specifically, the risk of hospitalisation or death after COVID-19 is related to increased age and underlying health conditions such as diabetes, obesity, hypertension, cardiovascular disease, solid-organ and haematological malignancies, chronic obstructive pulmonary disease or other chronic respiratory disease, chronic kidney disease, immunocompromised status, neurological conditions, smoking and pregnancy [[5], [6], [7]].
Definition of fully vaccinated patients and sub-groups at the risk of vaccine failure
Several studies have correlated various early post-vaccination immunological determinants with vaccine efficacy (VE). Anti-spike IgG concentration, anti-receptor-binding domain (RBD) IgG concentration, and pseudovirus ID50 neutralization titres are among those which are most frequently used. Given the significant methodological variations, IgG concentrations are usually expressed in standardized WHO international units or binding antibody units (BAU)/mL [8].
Feng et al. [9] found that early (28 days after the second dose) post-vaccination anti-spike and anti-RBD IgG concentrations of >264 BAU/mL and >506 BAU/mL, respectively, were associated with >80% of VE for symptomatic infection with the alpha (B.1.1.7) variant of SARS-CoV-2 among recipients of ChAdOx1 nCoV-19 (AZD1222). Gilbert et al. [10] estimated an overall VE of 90% for symptomatic infection when the anti-spike IgG and anti-RBD concentrations on day 57 after the second dose of the mRNA-1273 vaccine were >298 BAU/mL and >775 BAU/mL, respectively.
A population-based approach to identify correlates of protection) of several vaccines (BNT162b [Pfizer/BioNTech], mRNA-1273 [Moderna], ChAdOx1 nCoV-19 [Astra/Zeneca] and Ad.26COV2.S [J&J]) against SARS-CoV-2 was used by Goldblatt et al. [11]. These investigators analysed the correlation among anti-spike and anti-RBD IgG concentrations, binding and VE and found that the mean protective anti-spike IgG concentration threshold for the wild-type virus was 154 BAU/mL.
Early anti-spike and anti-RBD IgG concentrations have shown to predict VE during the months following vaccination. Nevertheless, the concentrations of these antibodies tend to decline over time, frequently falling below the aforementioned thresholds 6 months after vaccination, especially in men, elderly people and immuno-compromised populations [12]. Whether this decline in the concentration of anti-spike IgG leads to a decrease in protection against severe disease has not been definitely proven yet [13].
The decline in VE against infection attributed to the decline in the concentration of anti-spike IgG antibodies, in addition to the emergence of new variants for which the neutralizing capability of vaccine-induced antibodies decreases, has led to the authorization of the first booster dose and even a second booster dose of several vaccines, frequently called boosters, in order to warrant an increase in the concentrations of neutralizing antibodies [14]. For immunocompromised individuals and for the general population, all 30 countries also recommend first booster doses. Half of the European Union/European Economic Area countries (15/30) recommend first booster doses for all adults aged ≥18 years. Fifteen countries recommend first booster doses for adolescents (either to those aged >12 years or those aged >16 years). Twenty countries also recommend booster doses for immunocompromised individuals following the extended primary 3-dose vaccination series. As of 5th April 2022, nine countries recommend second booster doses (fourth dose) for different vulnerable population groups, such as residents in long-term care facilities and the elderly, with different age cut-offs (Cyprus, Finland, France, Germany, Greece, Hungary, Ireland, The Netherlands and Sweden), followed later on by other countries (e.g. Italy and Austria) [15]. Vaccinated individuals, who experience a break-through SARS-CoV-2 infection, will likely have a booster effect on baseline immunity, similar or even more intense than a repeated vaccine dose [16]. Lastly, despite the first booster dose, preliminary data report the vanishing of VE starting from 4 months after the last dose [17].
Importantly, there might be other relevant factors influencing VE, such as additional immune functions beyond the neutralization capacity of anti-spike and anti-RBD antibodies (e.g. other humoral functions, such as fragment crystallizable (Fc) effector functions, as well as cellular immunity). For example, the mortality among vaccinated recipients who underwent renal transplantation in Spain was similar to that among partially vaccinated or non-vaccinated recipients. Although precise information on anti-spike IgG concentrations was not provided, the impact of immuno-suppressors on other immunological pathways could partially explain this observation [18].
Moreover, it is likely that other immunological determinants of protection against SARS-CoV-2 cannot be measured in the serum (e.g. mucosal immunity) [10]. Importantly, anamnestic responses might not be fully represented by a single time point measurement. Finally, as stated before, antigenic evolution, as seen in current or future variants of concern, might contribute to the decreased neutralizing activity of a given concentration of anti-spike or anti-RBD IgG.
In summary, the panel considers the following conditions to be at the risk of vaccination failure, defined as the risk of hospitalisation or death because of COVID-19 following a break-through infection [19]:
• Incomplete vaccination history
Complete vaccination status but last dose received ≥4 months before the onset of infection.
Moderate or severe primary immuno-deficiency
Advanced or untreated human immuno-deficiency virus infection.
Receipt of chimeric antigen receptor-T cell therapy or a haematopoietic cell transplant within the previous 2 years.
Active treatment for a solid tumour or haematological malignancy.
Use of immuno-suppressive therapy after a solid-organ transplant.
Active treatment with other immuno-suppressive or immuno-modulatory drugs, such as high-dose corticosteroids (≥20 mg/d of prednisone or equivalent) and tumour necrosis factor inhibitors.
Questions addressed by the guidelines and recommendations
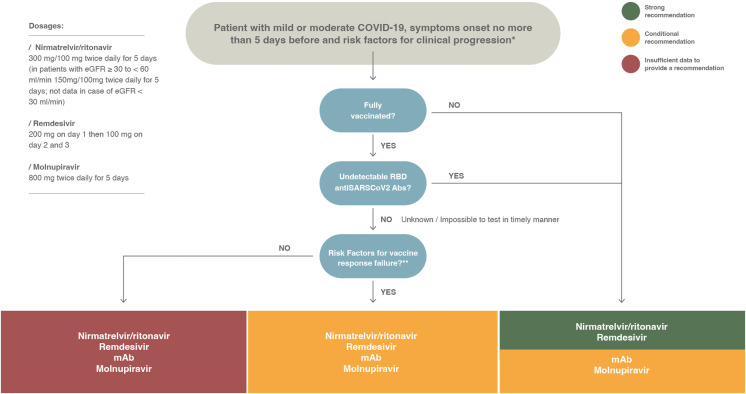
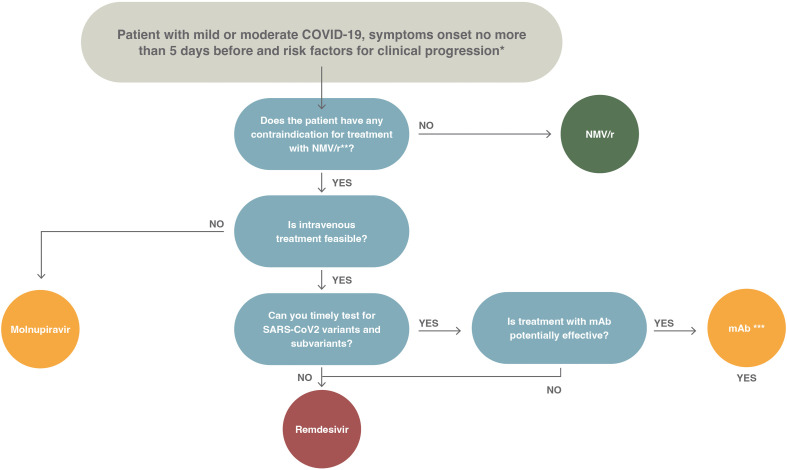
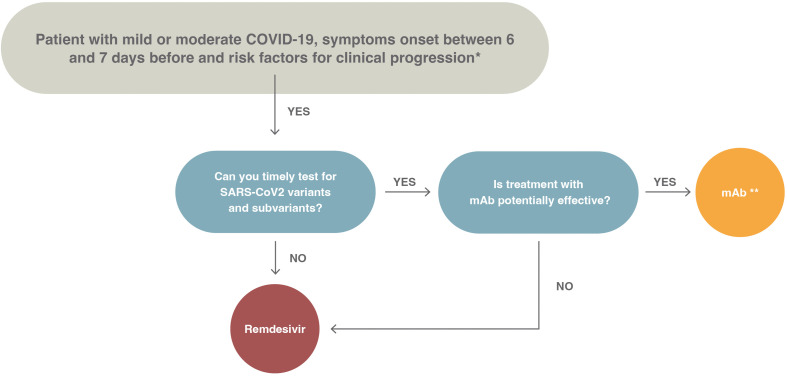
A summary of the recommendations given in this document is depicted in Fig. 1 . The panel did not assess proper prioritization of drugs. This is because of lack of clinical trials with head-to-head comparison of drugs, which does not offer clear superiority of one drug over others, and the fact that the availability of drugs is not uniform across countries. In Fig. 2 , we show a proposed treatment algorithm; the choice of preferred drug included in the algorithm reflects mostly the feasibility of treatment (e.g. oral vs. intravenous) and the need for further tests (e.g. testing for variants in case of monoclonal antibodies [mAbs] (Fig. 3 ).
Fig. 1.
European Society of Clinical Microbiology and Infectious Diseases coronavirus disease 2019 treatment guidelines. Summary of recommendations for outpatients with mild or moderate coronavirus disease 2019 at the risk of disease progression. COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; mAb, monoclonal antibody; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
∗The risk factors for disease progression are as follows: ≥60 years of age; body mass index >25 kg/m2; cigarette smoking; immuno-suppressive disease (including human immuno-deficiency virus infection with a CD4 cell count of <200 mm3) or prolonged iatrogenic immunosuppression; chronic lung, cardiovascular, kidney, or sickle cell disease; hypertension; diabetes; cancer; neurodevelopmental disorders or other medically complex conditions or medical-related technological dependence.
∗∗ The risk factors for vaccine failure are as follows: (a) incomplete vaccination history; (b) moderate or severe primary immuno-deficiency; (c) advanced or untreated human immuno-deficiency virus infection; (d) receipt of chimeric antigen receptor-T cell therapy or haematopoietic cell transplant within the previous 2 years; (e) active treatment for a solid tumour or haematological malignancy; (f) use of immuno-suppressive therapy after a solid-organ transplant; (g) active treatment with other immuno-suppressive or immuno-modulatory drugs, such as high-dose corticosteroids (≥20 mg/d of prednisone or equivalent) and tumour necrosis factor inhibitors [13,58].
Fig. 2.
Proposed algorithm of treatment for outpatients with mild or moderate coronaviris disease 2019, the onset of symptoms in the previous 5 days, and risk factors for disease progression. COVID-19, coronavirus disease 2019; NMV/r, nirmatrelvir/ritonavir; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
∗The risk factors for disease progression are as follows: ≥60 years of age; body mass index >25 kg/m2; cigarette smoking; immuno-suppressive disease (including human immuno-deficiency virus infection with a CD4 cell count of <200 mm3) or prolonged iatrogenic immunosuppression; chronic lung, cardiovascular, kidney, or sickle cell disease; hypertension; diabetes; cancer; neurodevelopmental disorders or other medically complex conditions or medical-related technological dependence.
∗∗Review potential drug interactions between nirmatrelvir/ritonavir and the patient's current medications. The suggested resources are as follows:
• University of Liverpool. COVID-19 Drug Interactions (https://www.covid19-druginteractions.org/checker)
• National Institutes of Health. Drug-Drug Interactions Between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Concomitant Medications (https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/#:∼:text=Ritonavir%2C%20a%20strong%20cytochrome%20P450,concentrations%20of%20certain%20concomitant%20medications)
• Infectious Diseases Society of America. Management of Drug Interactions With Nirmatrelvir/Ritonavir (Paxlovid®): Resource for Clinicians (https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/management-of-drug-interactions-with-nirmatrelvirritonavir-paxlovid/)
Careful risk-benefit evaluation in pregnant women and in patients undergoing haemodialysis
∗∗∗Consider the following issues before prescribing monoclonal antibodies: (a) availability of drugs, (b) availability of infusion centres, (c) feasibility to perform timely screening for baseline antibodies, (d) alternatively constant monitoring of the circulation of variants of concern in order to select the appropriate treatment or (e) exclude patients in whom the administration of monoclonal antibodies may be ineffective.
Fig. 3.
Proposed algorithm of treatment for outpatients with mild or moderate coronavirus disease 2019, the onset of symptoms between 6 and 7 days before and risk factors for disease progression. COVID-19, coronavirus disease 2019; mAb, monoclonal antibody; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
∗The risk factors for disease progression are as follows: ≥60 years of age; body mass index >25 kg/m2; cigarette smoking; immuno-suppressive disease (including human immuno-deficiency virus infection with a CD4 cell count of <200 mm3) or prolonged iatrogenic immunosuppression; chronic lung, cardiovascular, kidney, or sickle cell disease; hypertension; diabetes; cancer; neurodevelopmental disorders or other medically complex conditions or medical-related technological dependence.
∗∗Consider the following issues before prescribing monoclonal antibodies: (a) availability of drugs, (b) availability of infusion centres, (c) feasibility to perform timely screening for baseline antibodies, (d) alternatively constant monitoring of the circulation of variants of concern in order to select the appropriate treatment, (e) to exclude patients in whom the administration of monoclonal antibodies may be ineffective.
The first 15 PICOs [2] were revised, and no updates were established. Some additional RCTs are available; however, they do not change our previous recommendations.
PICO 16: What is the effect of sotrovimab treatment (I) on mortality or disease progression (O) in outpatients with mild COVID-19 (P) compared with no treatment (C)?
Narrative synthesis of evidence
Sotrovimab is a mAb which targets a highly conserved epitope in the RBD of SARS-CoV-2 and other sarbecoviruses. Data on sotrovimab for COVID-19 have been obtained primarily from two clinical trials, one in outpatients [20] and one in hospitalized patients [21].
In the study by Gupta et al. [20], adult patients who had ≥1 of the following risk factors were included: age >55 years, diabetes requiring medication, obesity, chronic kidney disease, congestive heart failure, chronic obstructive pulmonary disease or moderate-to-severe asthma. Overall, 528 patients received sotrovimab and 529 received a placebo. In this cohort, there was an absolute risk reduction of 5% for a composite outcome of all-cause hospitalisation for >24 hours and death in the sotrovimab arm. There were two deaths in the placebo arm and no deaths in the sotrovimab arm. It should be noted that patients were screened between August 2020 and March 2021, before the rise of Omicron and its sub-lineages. Data synthesis showed that sotrovimab may reduce the rate of hospitalisation (risk ratio [RR], 0.14; 95% CI, 0.04–0.48) (Table 1 ).
Table 1.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profile for population, intervention, comparison, outcome 16: Sotrovimab for mild or moderate coronavirus disease 2019a
| People: Patients with mild-to-moderate COVID-19 Settings: Ambulatory patients Intervention: Sotrovimab at 500 mg administered intravenously Comparison: No treatment | |||||
|---|---|---|---|---|---|
| Outcomes | Absolute effect |
Relative effect (95% CI) | Number of studies | Certainty of the evidence (GRADE) | |
| With sotrovimab | Without sotrovimab | ||||
| All-cause mortality (within 29 d from treatment) | 0/291 (0) | 1/292 (0.3%) | RR: 0.33 (0.01–8.18) | 1 [20] (583 patients) | ⊕⊕⊖⊖ Low (due to very serious imprecision) |
| Difference: 3 fewer per 1000 (95% CI: −10 to 3) | |||||
| Hospitalization (within 29 d from treatment) | 3/291 (1.0%) | 21/292 (7.2%) | RR: 0.14 (0.04–0.48) | 1 [20] (583 patients) | ⊕⊕⊕⊖ Moderate (due to serious imprecision) |
| Difference: 62 fewer per 1000 (95% CI: −69 to −37) | |||||
| Serious adverse events (end of follow-up) | 7/430 (1.6%) | 26/438 (5.9%) | RR: 0.27 (0.12–0.63) | 1 [20] (5531 patients) | ⊕⊕⊕⊖ Moderate (due to serious imprecision) |
| Difference: 58 fewer per 1000 (95% CI: −64 to −36) | |||||
COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio.
aEvidence adopted: Infectious Disease Society of America guidelines available at https://www.idsociety.org/practice-guideline/COVID-19-guideline-treatment-and-management/. Evidence search date: 1–30 January 2022.
Safety
In the evaluated study, sotrovimab was not associated with an augmented risk of serious adverse events compared with the placebo (RR, 0.27; 95% CI, 0.12–0.63) (Table 1).
The safety of the use of anti-SARS-CoV-2 mAbs in pregnant women is not well defined; however, these appear to reduce the risk of severe disease without increasing the risk of significant adverse maternal or perinatal outcomes [22,23].
On 23 February 2022, the Emergency Use Authorization for sotrovimab was placed under revision by the U.S. Food and Drug Administration [24]. This was because of the reduced neutralisation of BA.2 by sotrovimab. The U.S. Centers for Disease Control and Prevention has now estimated that >50% of all SARS-CoV-2 isolates in all geographic regions of the United States are of the BA.2 lineage. By 5 April 2022, the Emergency Use Authorization for sotrovimab was removed because of concerns of inefficacy against the BA.2, BA.3, BA.4 and BA.5 lineages, with in-line reports suggesting a 7- to 16-fold reduction in susceptibility.
BA.2 is now the dominant variant in all countries in Europe where current data are available [25].
Patient preferences, comments on evidence-to-decision dimensions and other considerations
Similar to other anti-spike mAbs, the panel agreed that several considerations should be taken into account before reaching a firm recommendation for the use of sotrovimab in ambulatory, high-risk patients. These include the following: (a) availability of drugs because several regulatory authorities in Europe have not approved the use of anti-spike protein mAbs at the national level or supplies of the drugs are limited; (b) logistic issues in terms of availability of infusion referral centres or facilities to allow safe and timely administration of the drugs; (c) feasibility to perform a timely screening for baseline antibodies and (d) constant monitoring of the circulation of variants of concern in order to select the appropriate treatment or to exclude patients in whom the administration of mAbs may be ineffective.
Recommendations
There are conditional recommendations for the use of sotrovimab in high-risk, unvaccinated outpatients with mild-to-moderate COVID-19 if it is active against the infecting variant after testing in single patients or active against the predominating variants according to epidemiological data if available (Quality of evidence (QoE): moderate).
There are conditional recommendations for the use of sotrovimab in high-risk outpatients at the risk of vaccine failure with mild-to-moderate COVID-19 if it is active against the infecting variant after testing in single patients or active against the predominating variants according to epidemiological data if available (QoE: very low).
No data are available to establish a recommendation in fully vaccinated patients with no risk factors for vaccine failure.
PICO 17: What is the effect of molnupiravir treatment (I) on disease progression (O) in outpatients with mild COVID-19 (P) compared with no treatment (C)?
Narrative synthesis of evidence
Molnupiravir is a small-molecule ribonucleoside prodrug of N-hydroxy-cytidine, which acts against SARS-CoV-2 and other RNA viruses via the inhibition of RNA polymerase [26].
Molnupiravir was assessed in a phase 2/3, double-blind, randomized trial in which outpatients with mild-to-moderate COVID-19 were assigned to molnupiravir at 800 mg twice daily for 5 days or a placebo [27]. The study was finalized early because an interim analysis met the efficacy results of superiority of molnupiravir over the placebo for the primary endpoint. In fact, molnupiravir was significantly associated with a low rate of hospitalisation or death on day 29 vs. the placebo (7.3% vs. 14.1%, respectively; treatment difference of 6.8%; 95% CI, −11.3 to −2.4; p 0.001). When all randomized patients were analysed, the differences were lower but still significant (6.8% vs. 9.7%; treatment difference of 3.0%; 95% CI, −5.9 to −0.1) (Table 2 ). Included patients needed to have a negative history of COVID-19 and/or vaccination. However, positive baseline antibodies against the SARS-CoV-2 nucleocapsid were reported in 19.1% and 20.5% of the patients in the molnupiravir and placebo groups, respectively. In those patients, there were no differences between the molnupiravir and placebo groups.
Table 2.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profile for population, intervention, comparison, outcome 17: Molnupiravir for mild or moderate coronavirus disease 2019a
| People: Patients with mild or moderate COVID-19, risk factors for progressive disease, and <5 d of on-going symptoms. Settings: Ambulatory patients Intervention: Molnupiravir at 800 mg administered orally, twice daily for 5 d Comparison: Placebo | |||||
|---|---|---|---|---|---|
| Outcomes | Absolute effect |
Relative effect (95% CI) | Number of studies | Certainty of the evidence (GRADE) | |
| With molnupiravir | Without molnupiravir | ||||
| All-cause admissions or death (within 29 d from treatment) | 48/709 (6.8%) | 68/699 (9.7%) | RR: 0.70 (0.49–0.99) | 1 [27] (1433 patients) | ⊕⊕⊕⊖ Moderate (serious imprecision: all patients included were not vaccinated; there were no differences in the sub-group of patients with SARS-CoV-2 IgG) |
| Difference: 3 fewer per 100 (95% CI: −5.9 to −0.1) | |||||
| Serious adverse events | 49/710 (6.9%) | 67 (9.6%) | |||
| Non-significant differences (95% CI: −7.4 to 2.3) | |||||
COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aEvidence adopted: Evidence adopted: Australian Guideline for clinical care of patients with COVID-19 at https://app.magicapp.org/#/guideline/6268/section/101962 accessed March–April 2022. Evidence search date: March 2022.
Safety
In the available RCT, adverse events were reported in 30.4% (216/710) of participants in the molnupiravir group and 33.0% (231/701) of participants in the placebo group.
The use of molnupiravir in pregnant women is currently not recommended when alternative options are available because foetal toxicity has been reported in animal studies [28].
Patient preferences, comments on evidence-to-decision dimensions and other considerations
Molnupiravir is an orally available drug, a solution which could fulfil patient preferences and limit the costs of hospitalisation and admission at infusion centres. The cost of molnupiravir is not negligible and may limit its use in low-income countries. Similarly, the lack of availability of the drug may be possible in low- or mid-income countries.
Recommendations
The panel suggests using molnupiravir (conditional recommendation) for adult, unvaccinated, ambulatory patients with mild-to-moderate COVID-19 at a high risk of progression to severe disease within 5 days from the onset of symptoms.
The panel also suggests using molnupiravir (conditional recommendation) for vaccinated patients at the risk of vaccine failure presenting with mild-to-moderate COVID-19 and risk factors for progression to severe disease within 5 days from the onset of symptoms.
Current evidence does not provide data on the efficacy of molnupiravir in the fully vaccinated population.
PICO 18: What is the effect of nirmatrelvir/ritonavir treatment (I) on disease progression (O) in outpatients with mild COVID-19 (P) compared with no treatment (C)?
Narrative synthesis of evidence
Nirmatrelvir is an orally bioavailable protease inhibitor which acts by cleaving two viral poly-proteins [29]. It has anti-viral activity against all coronaviruses that are known to infect humans [30]. Nirmatrelvir is administered in combination with ritonavir, a strong cytochrome P450 3A4 inhibitor which increases nirmatrelvir concentrations to the target therapeutic range, limiting the side effects of the anti-viral agent.
A single, phase 2/3 trial RCT [31] assessed the effectiveness of nirmatrelvir (PF-07321332)/ritonavir (NMV/r) in patients with COVID-19. In this double-blind, placebo-controlled RCT, 2246 adults with mild-to-moderate PCR-confirmed COVID-19 were randomized to receive NMV/r (300/100 mg twice daily for 5 days) or a placebo. All the patients were unvaccinated, were within 5 days from the onset of symptoms and presented with ≥1 risk factors for severe disease.
A reduction in the composite outcome, mortality or hospitalisation by day 28 was observed in patients in the NMV/r group (RR, 0.12; 95% CI, 0.06–0.25) (Table 3 ). The most common risk factors for disease progression were a body mass index of ≥25 kg/m2 (80.5%), current smoking (39.0%) and hypertension (32.9%), with 61.0% of patients presenting with two or more conditions. Overall, 6.3% of patients received or were expected to receive mAbs for COVID-19, and four patients had already received them when the study treatment was started. A sub-group analysis showed consistent results regardless of age, sex, body mass index, and baseline serology status; however, numerosity was insufficient to provide evidence about the efficacy of NMV/r in specific sub-groups of patients. To note, the number needed to treat was 18.1 (95% CI, 14.1–25.2) for the overall population, 10.0 (95% CI, 7.7–14.2) for patients with negative baseline serology, and 75.2 (95% CI, 41.3–423.1) for patients with positive baseline serology (defined either by the anti-N or anti-S protein antibody). Recent reports have highlighted the possibility of virological and clinical rebound in patients on NMV/r, which likely has no effect on the overall protective effect and was noted in initial clinical trials [32].
Table 3.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profile for population, intervention, comparison, outcome 18: Nirmatrelvir/ritonavir for mild or moderate coronavirus disease 2019a
| People: Adult, unvaccinated patients with mild-to-moderate symptomatic COVID-19 and one or more risk factorsb for disease progression within 5 d of the onset of symptoms (pregnant and breast-feeding patients excluded). Settings: Ambulatory patients not requiring oxygen, 330 centres in 21 countries. Intervention: Nirmatrelvir/ritonavir at 300/100 mg administered orally, twice daily for 5 d. Comparison: Placebo | |||||
|---|---|---|---|---|---|
| Outcomes | Absolute effect |
Relative effect (95% CI) | Number of studies | Certainty of the evidence (GRADE) | |
| Without nirmatrelvir/ritonavir | With nirmatrelvir/ritonavir | ||||
| Hospitalisation or death D 28, ≤5 d symptom onset |
63 per 1000 | 8 per 1000 | RR: 0.12 (0.06–0.25) | 1 [31] (2085 patients) | ⊕⊕⊕⊖ Moderate (serious imprecision) |
| Difference: 55 fewer per 1000 (95% CI: 59 fewer to 47 fewer) | |||||
| All-cause mortality D 28, ≤5 d from the onset of symptoms |
11 per 1000 | 0 per 1000 | RR: 0.04 (0.00–0.68) | 1 [31] (2085 patients) | ⊕⊕⊖⊖ Low (very serious imprecision) |
| Difference: 11 fewer per 1000 (95% CI: 11 fewer to 4 fewer) | |||||
| Hospitalisation D 28, ≤5 d from the onset of symptoms |
62 per 1000 | 7 per 1000 | RR 0.12 (0.06–0.26) | 1 [31] (2085 patients) | ⊕⊕⊕⊖ Moderate (serious imprecision) |
| Difference: 55 fewer per 1000 (95% CI: 58 fewer to 46 fewer) | |||||
| Adverse events within 28 d of commencing treatment | 239 per 1000 | 227 per 1000 | RR: 0.95 (0.82–1.10) | 1 [31] (2224 patients) | ⊕⊕⊕⊖ Moderate (serious imprecision) |
| Difference: 12 fewer per 1000 (95% CI: 43 fewer to 24 more) | |||||
| Serious adverse events within 28 d of commencing treatment | 66 per 1000 | 16 per 1000 | RR: 0.24 (0.15–0.41) | 1 [31] (2224 patients) | ⊕⊕⊕⊖ Moderate (serious imprecision) |
| Difference: 50 fewer per 1000 (95% CI: 56 fewer to 39 fewer) | |||||
| Discontinued because of adverse events within 28 d of commencing treatment | 42 per 1000 | 21 per 1000 | RR: 0.49 (0.30–0.81) | 1 [31] (2224 patients) | ⊕⊕⊕⊖ Moderate (serious imprecision) |
| Difference: 21 fewer per 1000 (95% CI: 29 fewer to 8 fewer) | |||||
COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio.
aEvidence adopted: Australian Guideline for clinical care of patients with COVID-19 at https://app.magicapp.org/#/guideline/6268/section/101966 accessed March–April 2022.
bRisk factors for disease progression: ≥60 years of age; body mass index >25 kg/m2; cigarette smoking; immuno-suppressive disease (including human immuno-deficiency virus infection with a CD4 cell count of <200 mm3) or prolonged iatrogenic immunosuppression; chronic lung, cardiovascular, kidney, or sickle cell disease; hypertension; diabetes; cancer; neurodevelopmental disorders or other medically complex conditions or medical-related technological dependence.
Safety
Adverse events were equally frequent in the NMV/r group compared with that in the placebo group. Serious adverse events were fewer in the NMV/r group; however, more discontinuations occurred in the NMV/r group than in the placebo group. Co-administration with ritonavir, a strong cytochrome P450 inhibitor, raises important concerns about possible serious drug interactions occurring for concomitant medications, resulting in treatment failure, virological resistance, or serious adverse events. Thus, patients' medications should be carefully screened for interactions before starting NMV/r treatment.
The use of NMV/r can be considered in pregnant women because pre-clinical studies did not show any toxicity in pregnant animals [33]. Additionally, ritonavir has been extensively used in pregnant human immuno-deficiency virus-positive women, without any concern of safety [34]. Treatment with NMV/R can be offered to pregnant women with COVID-19 after a careful risk-benefit evaluation and assessment of the presence of other risk factors for the progression of the disease, vaccination status and concomitant treatments.
Patient preferences, comments on evidence-to-decision dimensions and other considerations
NMV/r is an orally available formulation, a solution which could fulfil patient preferences and limit the costs of hospitalisation and admission at infusion centres. The use of the drug may be limited by drug interactions. The cost of NMV/r is not negligible and may limit its use in low-income countries. Similarly, the lack of availability of the drug may be possible in low- or mid-income countries and high-income countries.
Recommendations
The panel recommends the use of NMV/r in adult, unvaccinated, ambulatory patients with mild-to-moderate COVID-19 at a high risk of progression to severe disease within 5 days of the onset of symptoms.
The panel also suggests using NMV/r (conditional recommendation) in vaccinated patients at the risk of vaccine failure presenting with mild-to-moderate COVID-19 and risk factors for progression to severe disease within 5 days of the onset of symptoms.
Current evidence does not provide data on the efficacy of NMV/r in the fully vaccinated population.
PICO 19: What is the effect of remdesivir treatment (I) on mortality or disease progression (O) in outpatients with COVID-19 (P) compared with no treatment (C)?
Narrative synthesis of evidence
In the previous ESCMID guideline [2], a conditional recommendation was given for the use of remdesivir in hospitalized patients not requiring mechanical ventilation or extracorporeal membrane oxygenation (ECMO) (QoE: moderate). An RCT (PINETREE trial), including 562 unvaccinated, ambulatory patients with mild-to-moderate disease and at a high risk of progression to severe disease, compared 3 days of remdesivir with no remdesivir [35]. The regimen was 200-mg intravenous administration on day 1, followed by 100-mg intravenous on days 2 and 3. The patients were within 7 days of symptoms and without prior treatments, e.g. mAbs. Moreover, the participants were not expected to receive oxygen. The underlying conditions for progression were described as obesity, diabetes mellitus, hypertension, immunocompromised status and age ≥60 years. Patients who had at least one risk factor were randomly assigned to receive intravenous remdesivir or a placebo. The assessed outcomes were hospitalisations for any cause, serious adverse events, and mortality by day 28. Two of 279 participants (0.7%) were hospitalized in the remdesivir group, whereas 15 of 283 (5.3%) were hospitalized in the placebo group, which translated to an 87% lower risk of hospitalisation in the remdesivir group. There was no death in either group. Adverse events were observed in 42.3% (118/279) of the patients in the remdesivir group and in 46.3% (131/283) in the placebo group. The most common adverse events which occurred in at least 5% of the patients in both the groups were nausea, headache and cough. The viral load in the upper airway was not lower in the remdesivir group and did not correlate with a good clinical response (Table 4 ) [35]. Because the results of the study were obtained from an unvaccinated group of patients, the use of remdesivir is recommended for unvaccinated people. There are no data on the use of remdesivir in fully vaccinated people.
Table 4.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profile for population, intervention, comparison, outcome 19: Remdesivir for mild or moderate coronavirus disease 2019a
| People: Patients with mild or moderate COVID-19 Settings: Ambulatory patients Intervention: Remdesivir at 200 mg administered intravenously on D 1, followed by a 100-mg dose on D 2 and 3 (3-d remdesivir) Comparison: No treatment | |||||
|---|---|---|---|---|---|
| Outcomes | Absolute effect |
Relative effect (95% CI) | Number of studies | Certainty of the evidence (GRADE) | |
| With remdesivir | Without remdesivir | ||||
| All-cause mortality (within 28 d from treatment) | 0/279 (0) | 0/283 (0) | - () | 1 [35] (562 patients) | |
| Difference: - fewer per 1000 (95% CI: - to -) | |||||
| Hospitalisation (within 29 d from treatment) | 2/279 (0.7%) | 15/283 (5.3%) | RR: 0.28 (0.11–0.75) | 1 [35] (562 patients) | ⊕⊕⊕⊖ Moderate (due to serious imprecision) |
| Difference: 46 fewer per 1000 (95% CI: −57 to −16) | |||||
| Serious adverse events (end of follow-up) | 67 per 1000 | 18 per 1000 (5.9%) | RR: 0.27 (0.10–0.70) | 1 [35] (562 patients) | ⊕⊕⊕⊖ Moderate (due to serious imprecision) |
| Difference: 49 fewer per 1000 (95% CI: −60 to −20) | |||||
| Adverse events (end of follow-up) | 118 (42.3%) | 131 (46.3%) | RR: 0.91 (0.76–1.10) | 1 [35] (562 patients) | ⊕⊕⊕⊖ Moderate (due to serious imprecision) |
| Difference: 42 fewer per 1000 (95% CI: −111 to 46) | |||||
COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio.
∗ In the PINETREE trial no deaths occcured in both arms. It is impossible to calculate an effect of remdesivir in the all-cause mortality within 28 days from treatment.
aEvidence adopted: Australian guidelines for the clinical care of patients with COVID-19 v56.0 published 8 April 2022 at https://app.magicapp.org/#/guideline/6268/section/101984. Evidence search date: January–April 2022.
Safety
Concerns about the use of remdesivir have been previously raised following observations based on real-world data [36,37]. However, in the PINETREE trial, the patients who received remdesivir did not experience a higher rate of severe adverse events and other adverse events than the controls (Table 4).
In preliminary reports, the use of remdesivir in pregnant women seemed safe. In the compassionate use program, patients with severe COVID-19 treated with remdesivir showed good outcomes but a higher rate of pre-term birth. It is unclear whether this is related to the use of anti-viral agents or is a complication of COVID-19 [38,39]. This doubt was partially clarified in a study comparing pre-delivery and post-delivery treatment with remdesivir, in which the rate of pre-term delivery was similar [40]. Most studies were performed during the third trimester; therefore, the safety of the use of remdesivir in the first trimester of pregnancy is still unknown. Therefore, the use of remdesivir during pregnancy should be decided after a risk-benefit assessment, which includes the evaluation of other risk factors for disease progression and vaccination status.
Recommendation
The panel recommends remdesivir for adult, unvaccinated, ambulatory patients with mild-to-moderate COVID-19 at a high risk of progression to severe disease within 7 days of the onset of symptoms.
The panel also suggests using remdesivir (conditional recommendation) for vaccinated patients at the risk of vaccine failure presenting with mild-to-moderate COVID-19 and risk factors for progression to severe disease within 7 days of the onset of symptoms.
Current evidence does not provide data on the efficacy of remdesivir in the fully vaccinated population.
PICO 20: What is the effect of fluvoxamine treatment (I) on mortality or disease progression (O) in outpatients with COVID-19 (P) compared with no treatment (C)?
Fluvoxamine is an anti-depressant agent belonging to the selective serotonin reuptake inhibitor (SSRI) class, frequently prescribed for a wide range of psychiatric conditions, including obsessive-compulsive disorder, depression or anxiety [41]. The supposed benefits of fluvoxamine in patients with COVID-19 rely on both down-grading of inflammation via the reduction of pro-inflammatory cytokines (e.g. interleukin 6, tumour necrosis factor) [42] and hampering of virus cell entry through functional inhibition of acid sphingomyelinase, an enzyme involved in the structure and function of cellular plasma membrane [43].
The effectiveness of fluvoxamine in patients with COVID-19 was explored by 3 RCTs (STOP COVID1, TOGETHER and STOP COVID2). In these studies, fluvoxamine was compared with a placebo in a population of adult, unvaccinated outpatients with a positive SARS-CoV-2 test result and symptoms within 7 days from the administration of the first dose of the drug. In STOP COVID 2 and TOGETHER, patients were required to have at least one risk factor for severe disease to be enrolled. STOP COVID 2 was stopped earlier because of futility owing to a low case rate and difficulty in recruiting patients, possibly explained with the increasing vaccination rate.
Overall, fluvoxamine failed to show a beneficial effect on 28-day mortality (RR, 0.69; 95% CI: 0.38–1.27). However, a reduction in the composite outcome of observation in a COVID-19 emergency setting lasting >6 hours, hospitalization for COVID-19 or clinical deterioration was observed in patients who received fluvoxamine (RR, 0.64; 95% CI, 0.50–0.84). In addition, a trend toward fewer hospitalisations emerged in the fluvoxamine group compared with that in the placebo group (RR, 0.75; 95% CI, 0.57–0.99) (Table 5 ).
Table 5.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profile for population, intervention, comparison, outcome 19: fluvoxamine for mild or moderate coronavirus disease 2019a
| People: Patients with mild or moderate COVID-19 Settings: Ambulatory patients Intervention: Fluvoxamine (see text for dosages) Comparison: No treatment | |||||
|---|---|---|---|---|---|
| Outcomes | Absolute effect |
Relative effect (95% CI) | Number of studies | Certainty of the evidence (GRADE) | |
| Without fluvoxamine | With fluvoxamine | ||||
| Mortality (follow-up: 28 d) | 17/821 (2.1%) | 25/828 (3.0%) | RR: 0.69 (0.38–1.27) | 2 [44,45] (1624 patients) | ⊕⊕⊖⊖ Low |
| Difference: 9 fewer per 1000 (from 19 fewer to 8 more) | |||||
| Hospitalisation, emergency room visits (>6 h) or oxygen saturation of <92% (follow-up: 28 d) | 79/821 (9.6%) | 125/828 (15.1%) | RR: 0.64 (0.50–0.84) | 2 [44,45] (1624 patients) | ⊕⊕⊖⊖ Low |
| Difference: 54 fewer per 1000 (from 75 fewer to 24 fewer) | |||||
| Hospitalisation for COVID-19 (follow-up: 28 d) | 76/821 (9.3%) | 103/828 (12.4%) | RR: 0.75 (0.57–0.99) | 2 [44,45] (1624 patients) | ⊕⊕⊖⊖ Low |
| Difference: 31 fewer per 1000 (from 53 fewer to 1 fewer) | |||||
| Viral clearance (follow-up: 7 d) | 40/207 (19.3%) | 58/221 (26.2%) | RR: 0.74 (0.52–1.05) | 1 [45] | ⊕⊖⊖⊖ Very low |
| Difference: 38 more per 1000 (95% CI: −68 to 151) | |||||
| Serious adverse events | 60/821 (7.3%) | 75/828 (9.1%) | RR: 0.81 (0.59–1.12) | 2 [44,45] | ⊕⊕⊖⊖ Low |
| Difference: 17 fewer per 1000 (from 37 fewer to 11 more) | |||||
COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio.
aEvidence adopted: Infectious Disease Society of America guideline on the Treatment and Management of Patients with COVID-19 accessed 8 April 2022 at https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/.
The U.S. trial STOP COVID 1 recruited 152 patients randomly assigned to receive either 100-mg fluvoxamine three times daily or a placebo for 15 days. The primary outcome was clinical deterioration, defined by the combination of the following: (a) shortness of breath or hospitalisation for shortness of breath or pneumonia and (b) an oxygen saturation of <92% in room air or the need for supplemental oxygen to achieve an oxygen saturation of ≥92% within 15 days from randomisation. The primary outcome was achieved by none of the patients in the fluvoxamine arm and 8.3% of patients in the placebo arm (absolute risk difference, 8.7%; 95% CI, 1.8–16.4) [44].
The largest amount of data came from the Brazilian multi-centre study TOGETHER trial. Overall, 1497 patients were randomized to receive either 100-mg fluvoxamine twice daily for 10 days or a placebo. The primary outcome was a composite endpoint of hospitalisation, defined as either retention in a COVID-19 emergency setting for >6 hours or transfer to a tertiary hospital as a result of COVID-19 up to 28 days after randomisation. The primary outcome events occurred in 10.6% and 15.7% in the fluvoxamine and placebo group, respectively (RR, 0.68; 95% CI, 0.52–0.88) [45].
Other observational studies [[46], [47], [48]], particularly a large retrospective cohort study with 83 584 patients with COVID-19 comparing 3401 patients who received SSRIs, such as fluoxetine or fluvoxamine, with 6802 matched control patients with similar characteristics and comorbidities [46], showed a beneficial effect of the SSRIs on the risk of hospitalisation [48], clinical progression [47,48] and even mortality [46,47].
Overall, the panel felt that fluvoxamine did not completely demonstrate a clear clinical benefit in patients with mild or moderate COVID-19 for the following reasons. First, the mechanism of action was not fully understood. Second, there was no association between the use of the drug and mortality. Third, the large amount of evidence comes from the TOGETHER trial, in which the primary endpoint (observation in a COVID-19 emergency setting lasting >6 hours) seems less meaningful than other major outcomes, such as mortality and hospital or intensive care unit admission.
Safety
The common side effects of fluvoxamine are headache, nausea, diarrhoea and dizziness along with behavioural symptoms such as irritability, sleep disorders up to anxiety and panic attacks, especially in patients with a history of depression [49]. Moreover, fluvoxamine may provide drug-drug interactions via the inhibition of cytochromes P450 1A2 and 2C19 [50].
In STOP COVID1, 1% of patients in the fluvoxamine group and 7% in the placebo group experienced serious adverse events [44]. In the TOGETHER trial, the difference in the rate of treatment-related events was not significant between the two groups [45]. Overall, the patients treated with fluvoxamine did not have an increased risk of serious adverse events compared with those treated with the placebo.
Patient preferences, comments on evidence-to-decision dimensions and other considerations
Fluvoxamine is a widely available and relatively cheap drug. Data from available trials have not shown a significantly increased risk of adverse events. The use of this drug may represent a potential solution in contexts where anti-viral agents or mAbs are not available, especially in unvaccinated people.
Recommendations
Because of lack of data, the panel recommends fluvoxamine only in the context of RCTs (QoE: low).
PICO 21: What is the effect of inhaled corticosteroids treatment (I) on disease progression (O) in outpatients with mild COVID-19 (P) compared with no treatment (C)?
Inhaled corticosteroids have been proposed for the treatment of COVID-19 upon several considerations and preliminary studies. They might reduce the expression of angiotensin-converting enzyme 2 and transmembrane serine protease 2, and they have shown in vitro activity against SARS-CoV-2 [51,52]. They are generally safe and widely available (Table 6 ).
Table 6.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profile for population, intervention, comparison, outcome 21: Inhaled corticosteroids for mild or moderate coronavirus disease 2019a
| People: Patients with mild-to-moderate COVID-19 Settings: Ambulatory patients Intervention: Inhaled corticosteroids (budesonide or ciclesonide) Comparison: No treatment | |||||
|---|---|---|---|---|---|
| Outcomes | Absolute effect |
Relative effect (95% CI) | Number of studies | Certainty of the evidence (GRADE) | |
| With budesonide | Without budesonide | ||||
| All-cause mortality (within 28 d from treatment) | 5 per 1000 | 9 per 1000 | RR: 0.61 (0.22–1.67) | 3 studies [53,54,59] (2189 patients) | ⊕⊕⊖⊖ Low (due to very serious imprecision) |
| Difference: 4 fewer per 1000 (95% CI: –7 to 6) | |||||
| Supplemental oxygen (within 28 d of commencing treatment) | 93 per 1000 | 64 per 1000 | RR: 0.69 (0.49–0.98) | 1 [53] (1559 patients) | ⊕⊕⊕⊖ Moderate (due to serious imprecision) |
| Difference: 29 fewer per 1000 (95% CI: −47 to −1) | |||||
| Serious adverse events (within 28 d of commencing treatment) | 5 per 1000 | 3 per 1000 | RR: 0.51 (0.09–2.76) | 2 [53,54] (1647 patients) | ⊕⊕⊖⊖ Low (due to serious imprecision) |
| Difference: 2 fewer per 1000 (95% CI: −5 to 9) | |||||
COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio.
aEvidence adopted: Australian guidelines for the clinical care of patients with COVID-19 v56.0 published on 8 April 2022 at https://app.magicapp.org/#/guideline/6268/section/101984. Evidence search date: 1–30 January 2022.
The PRINCIPLE trial was an open-label RCT of inhaled budesonide in non-hospitalized patients with COVID-19 aged ≥65 years or ≥50 years with comorbidities [53]. The primary endpoints were self-reported recovery and hospital admission or death related to COVID-19 within 28 days. The median time to recovery was 11.8 days in the budesonide arm vs. 14.7 days in usual care arm (hazard ratio, 1.21; 95% CI, 1.08–1.36). The percentage of patients who were hospitalized or died because of COVID-19 within 28 days was 6.8% in the budesonide arm vs. 8.8% in the usual care arm (OR, 0.75; 95% CI, 0.55–1.03). The pre-specified superiority threshold was met for the outcome time to recovery but not for hospitalisation or death. The STOIC trial was also an open-label RCT of inhaled budesonide in non-hospitalized patients with COVID-19 [54], including only a small number of patients (146 participants). The primary endpoint was a COVID-19-related visit for urgent care. The primary outcome occurred in 11 participants (15%) in the usual care group and two participants (3%) in the budesonide group (difference in proportions, 0.123; 95% CI, 0.033–0.213; p 0.009).
Clemency et al. [55] studied the effect of inhaled ciclesonide on non-hospitalized patients with COVID-19 in a double-blind RCT. The primary endpoint was time to alleviation of all COVID-19-related symptoms, which was 19 days in the ciclesonide group and 19 days in the placebo group. The CONTAIN RCT included 203 adults aged ≥18 years with COVID-19 experiencing fever, cough or dyspnoea [56]. The primary outcome was resolution of the symptoms by day 7. The proportion of participants with resolution of the symptoms by day 7 did not differ significantly between the intervention (42/105, 40%) and control groups (34/98, 35%).
Overall, the panel believes that this evidence was not conclusive for inhaled corticosteroids. According to available trials, these drugs seemed to have little or no impact on important outcomes such as mortality and hospital admission. An effect on the recovery of symptoms could be demonstrated in some studies; however, it was not consistent in all evaluated trials.
Recommendations
The panel recommends the use of inhaled corticosteroids only in the context of RCTs.
PICO 22: What is the effect of tixagevimab-cilgavimab treatment (I) on mortality (O) in outpatients with mild COVID-19 (P) compared with no treatment (C)?
Tixagevimab-cilgavimab is a combination of two fully human SARS-CoV-2-neutralising mAbs which simultaneously bind to distinct, non-overlapping epitopes of the viral spike protein RBD. Given the extended half-life of the combination, tixagevimab-cilgavimab was initially proposed and licensed for pre-exposure prophylaxis in individuals at the risk of break-through infection due to inadequate response of vaccination or at the risk of exposure [57]. The tixagevimab-cilgavimab combination was also assessed for the treatment of unvaccinated patients with COVID-19 at the risk of disease progression in the TACKLE trial and compared with a placebo [60]. The primary composite outcome of death or hospitalisation occurred in 4% (18/407) of participants in the tixagevimab-cilgavimab group vs. 9% (37/415) of participants in the placebo group (relative risk reduction, 50·5%; 95% CI, 14.6–71.3; p 0.0096), achieving an absolute risk reduction of 4.5% (95% CI, 1.1–8·0; p < 0.001). The results were confirmed in a sub-group of patients with undetectable SARS-CoV-2 antibodies at baseline but not in patients with a positive serology status. Adverse events occurred in 29% (132/452) of participants in the tixagevimab-cilgavimab group and 36% (163/451) of participants in the placebo group (Table 7 ).
Table 7.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence profile for population, intervention, comparison, outcome 22: Tixagevimab-cilgavimab for mild or moderate coronavirus disease 2019
| People: Patients with mild or moderate COVID-19 Settings: Ambulatory patients Intervention: Tixagevimab-cilgavimab at 300 mg + 300 mg administered via intramuscular injections Comparison: No treatment | |||||
|---|---|---|---|---|---|
| Outcomes |
Absolute effect |
Relative effect (95% CI) |
Number of studies |
Certainty of the evidence (GRADE) |
|
| With tixagevimab-cilgavimab | Without tixagevimab-cilgavimab | ||||
| All-cause mortality (within 29 d from treatment) | 6/452 (1%) | 6/451 (1%) | RR: 1.00 (0.32–3.06) | 1 [60] (903 patients) | ⊕⊕⊖⊖ Low (due to very serious imprecision) |
| Difference: 0 fewer per 1000 (95% CI: −61 to 60) | |||||
| Hospitalisation (within 29 d from treatment) | 18/407 (4%) | 37/415 (9%) | RR: 0.49 (0.28–0.85) | 1 [60] (822 patients) | ⊕⊕⊕⊖ Moderate (due to serious imprecision) |
| Difference: 22 fewer per 1000 (95% CI: −12 to −91) | |||||
| Adverse events (end of follow-up) | 132/452 (29%) | 163/451 (36%) | RR: 0.80 (0.66–0.97) | 1 [60] (903 patients) | ⊕⊕⊕⊖ Moderate (due to serious imprecision) |
| Difference: 14 fewer per 1000 (95% CI: −7 to −119) | |||||
COVID-19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio.
The safety of the use of anti-SARS-CoV-2 mAbs in pregnant women is not well defined; however, these appear to reduce the risk of severe disease without increasing the risk of significant adverse maternal or perinatal outcomes [22,23].
Patient preferences, comments on evidence-to-decision dimensions and other considerations
Similar to other anti-spike mAbs, the panel agreed that several considerations should be taken into account before reaching a firm recommendation on the use of tixagevimab-cilgavimab in ambulatory, high-risk patients. These include the following: (a) availability of drugs because regulatory authorities in Europe have not approved the use of tixagevimab-cilgavimab in all countries yet, (b) logistic issue in terms of availability of infusion referral centres or facilities to allow safe and timely administration of the drugs, (c) feasibility to perform timely screening for baseline antibodies and (d) constant monitoring of the circulation of variants of concern in order to select the appropriate treatment or to exclude patients in whom the administration of mAb may be ineffective.
Recommendations
There are conditional recommendations for the use of tixagevimab-cilgavimab in high-risk, unvaccinated outpatients with mild-to-moderate COVID-19 if it is active against the infecting variant after testing or active against the predominating variants according to epidemiological data (QoE: moderate).
There are conditional recommendations for the use of tixagevimab-cilgavimab in high-risk outpatients at the risk of vaccine failure with mild-to-moderate COVID-19 (QoE: very low).
There are no data to establish a recommendation for fully vaccinated patients with no risk factors for vaccine failure.
Author contributions
MB chaired the panel, supervised the work, selected and voted for the population, intervention, comparison, outcome (PICO) questions and for other relevant decisions, performed literature search and drafted and approved the manuscript. OA, AB, LB, OE, RK, JRP-P, NP, MS, BGZ, ST, AM-Q, and IZ-S selected and voted for the PICO questions and other relevant decisions, performed literature search and drafted and approved the manuscript. JRB supervised the work of the panel, selected and voted for the PICO questions and for other relevant decisions, performed literature search and drafted and approved the manuscript.
Transparency declaration
The authors declare that they have no conflicts of interest. No funding was allocated for this document.
Acknowledgements
We acknowledge the methodological overview by Luigia Scudeller and technical assistance by Chiara Speziale. We also acknowledge the graphical assistance by Elisa Cicognola, (Combinatoria Creativa, Ancona-Perugia, Italy, https://www.combinatoriacreativa.it/, email: elisa@combinatoriacreativa.it)
Editor: L. Leibovici
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Bartoletti M., Azap O., Barac A., Bussini L., Ergonul O., Krause R., et al. ESCMID COVID-19 living guidelines: drug treatment and clinical management. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2022;28:222–238. doi: 10.1016/j.cmi.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hentzien M., Autran B., Piroth L., Yazdanpanah Y., Calmy A. A monoclonal antibody stands out against omicron subvariants: a call to action for a wider access to bebtelovimab. Lancet Infect Dis. 2022;22:1278. doi: 10.1016/s1473-3099(22)00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2020. Clinical management of COVID-19.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Google Scholar]
- 5.European Centre for Disease Prevention and Control (ECDC) 2021. Risk factors and risk groups.https://www.ecdc.europa.eu/en/covid-19/latest-evidence/risk-factors-risk-groups [Google Scholar]
- 6.Bellou V., Tzoulaki I., van Smeden M., Moons K.G., Evangelou E., Belbasis L. Prognostic factors for adverse outcomes in patients with COVID-19: a field-wide systematic review and meta-analysis. Eur Respir J. 2022;59 doi: 10.1183/13993003.02964-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Centers for Disease Control and Prevention (CDC) 2022. Science brief: evidence used to update the list of underlying medical conditions associated with higher risk for severe COVID-19.https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html [PubMed] [Google Scholar]
- 8.Panel W.R. 2020. First WHO International Reference Panel for anti-SARS-CoV-2 immunoglubulin NIBSC code: 20/268 Instructions for use.http://www.nibsc.org/standardisation/international_standards.aspx [Google Scholar]
- 9.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldblatt D., Fiore-Gartland A., Johnson M., Hunt A., Bengt C., Zavadska D., et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine. 2022;40:306–315. doi: 10.1016/j.vaccine.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glatman-Freedman A., Bromberg M., Hershkovitz Y., Sefty H., Kaufman Z., Dichtiar R., et al. Effectiveness of BNT162b2 vaccine booster against SARS-CoV-2 infection and breakthrough complications. Isr Emerg Infect Dis. 2022;28:948–956. doi: 10.3201/eid2805.220141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Centers for Disease Control and Prevention (CDC) 2019. COVID-19 vaccine boosters.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html [Google Scholar]
- 15.European Centre for Disease Prevention and Control . 2022. COVID-19: joint statement from ECDC and EMA on the administration of a fourth dose of mRNA vaccines.https://www.ecdc.europa.eu/en/news-events/ema-ecdc-statement-fourth-covid-vaccine-dose [Google Scholar]
- 16.Bates T.A., McBride S.K., Winders B., Schoen D., Trautmann L., Curlin M.E., et al. Antibody response and variant cross-neutralization after SARS-CoV-2 breakthrough infection. JAMA. 2022;327:179–181. doi: 10.1001/jama.2021.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferdinands J.M., Rao S., Dixon B.E., Mitchell P.K., DeSilva M.B., Irving S.A., et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazuecos A., Villanego F., Zarraga S., López V., Oppenheimer F., Llinàs-Mallol L., et al. Breakthrough infections following mRNA SARS-CoV-2 vaccination in kidney transplant recipients. Transplantation. 2022;106:1430–1439. doi: 10.1097/TP.0000000000004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention . 2022. COVID-19 vaccines for people who are moderately or severely immunocompromised.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html [Google Scholar]
- 20.Gupta A., Gonzalez-Rojas Y., Juarez E., Casal M.C., Moya J., Falci D.R., et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Self W.H., Sandkovsky U., Reilly C.S., Vock D.M., Gottlieb R.L., Mack M., et al. Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22:622–635. doi: 10.1016/s1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magawa S., Nii M., Maki S., Enomoto N., Takakura S., Maegawa Y., et al. Evaluation of the tolerability of monoclonal antibody therapy for pregnant patients with COVID-19. J Obstet Gynaecol Res. 2022;48:2325–2333. doi: 10.1111/jog.15338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang M.H., Cowman K., Guo Y., Bao H., Bernstein P.S., Gendlina I., et al. A real-world assessment of tolerability and treatment outcomes of COVID-19 monoclonal antibodies administered in pregnancy. Am J Obstet Gynecol. 2022;226:743–745. doi: 10.1016/j.ajog.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration . 2022. FDA updates sotrovimab emergency use authorization.https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization [Google Scholar]
- 25.Global Initiative on Sharing Avian Influenza Data (GISAID) Overview of variants in countries. https://covariants.org/per-country?region=World&country=USA&country=United+Kingdom&country=Germany&country=Denmark&country=France&country=Canada&country=Sweden&country=Switzerland&country=Spain&country=Netherlands&country=Italy&country=Belgium&country=Poland&country=Turkey&country=Ireland&country=Slovenia&country=Norway&country=Mexico&country=Lithuania&country=Czech+Republic&country=Portugal&country=Croatia&country=Slovakia&country=Finland&country=Luxembourg&country=Austria&country=Bulgaria&country=Greece&country=Latvia&country=Romania&country=Estonia&country=Russia&country=Iceland&country=Costa+Rica&country=Sint+Maarten&country=Bosnia+and+Herzegovina&country=Kosovo&country=Guatemala&country=Dominican+Republic&country=Malta&country=Jamaica&country=Liechtenstein&country=Guadeloupe&country=Ukraine&country=Montenegro&country=North+Macedonia&country=Belize&country=Cyprus&country=Moldova&country=Serbia&country=Hungary&country=Belarus&country=Saint+Martin&country=Antigua+and+Barbuda&country=Monaco; (Published 2022-03-30, Accessed April, 10 , 2022)
- 26.Cox R.M., Wolf J.D., Plemper R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021;6:11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. https://www.fda.gov/media/155101/download#:∼:text=Based%20on%20findings%20from%20animal,adverse%20maternal%20or%20fetal%20outcomes
- 29.Owen D.R., Allerton C.M., Anderson A.S., Aschenbrenner L., Avery M., Berritt S., et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 30.Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S.H. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: Peptidomimetics and small molecule chemotherapy. J Med Chem. 2016;59:6595–6628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boucau J., Uddin R., Marino C., Regan J., Flynn J.P., Choudhary M., et al. Virologic characterization of symptom rebound following nirmatrelvir-ritonavir treatment for COVID-19. Clin Infect Dis. 2022;23 doi: 10.1093/cid/ciac512. ciac512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catlin N.R., Bowman C.J., Campion S.N., Cheung J.R., Nowland W.S., Sathish J.G., et al. Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 M(pro) inhibitor in animal models. Reprod Toxicol. 2022;108:56–61. doi: 10.1016/j.reprotox.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasley M.V., Martinez M., Hermes A., d’Amico R., Nilius A. Safety and efficacy of lopinavir/ritonavir during pregnancy: a systematic review. AIDS Rev. 2013;15:38–48. doi: 10.7448/ias.16.2.18653. [DOI] [PubMed] [Google Scholar]
- 35.Gottlieb R.L., Vaca C.E., Paredes R., Mera J., Webb B.J., Perez G., et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Eng J Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartoletti M., Azap O., Barac A., Bussini L., Ergonul O., Krause R., et al. ESCMID COVID-19 living guidelines: drug treatment and clinical management: author's reply. Clin Microbiol Infect. 2022;28:617–618. doi: 10.1016/j.cmi.2021.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dauby N. Re: 'ESCMID COVID-19 living guidelines: drug treatment and clinical management' by Bartoletti et al. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2022;28:616. doi: 10.1016/j.cmi.2021.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Budi D.S., Pratama N.R., Wafa I.A., Putra M., Wardhana M.P., Wungu C.D. Remdesivir for pregnancy: a systematic review of antiviral therapy for COVID-19. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e08835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorgensen S.C., Davis M.R., Lapinsky S.E. A review of remdesivir for COVID-19 in pregnancy and lactation. J Antimicrob Chemother. 2021;77:24–30. doi: 10.1093/jac/dkab311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burwick R.M., Yawetz S., Stephenson K.E., Collier A.Y., Sen P., Blackburn B.G., et al. Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis. 2021;73 doi: 10.1093/cid/ciaa1466. e3996–e4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo Y., Kataoka Y., Ostinelli E.G., Cipriani A., Furukawa T.A. National prescription patterns of antidepressants in the treatment of adults with major depression in the US between 1996 and 2015: a population representative survey based analysis. Front Psychiatry. 2020;11:35. doi: 10.3389/fpsyt.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannestad J., DellaGioia N., Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kornhuber J., Hoertel N., Gulbins E. The acid sphingomyelinase/ceramide system in COVID-19. Mol Psychiatry. 2022;27:307–314. doi: 10.1038/s41380-021-01309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reis G., dos Santos Moreira-Silva E.A., Silva D.C., Thabane L., Milagres A.C., Ferreira T.S., et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oskotsky T., Maric I., Tang A., Oskotsky B., Wong R.J., Aghaeepour N., et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.33090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoertel N., Sánchez-Rico M., Vernet R., Beeker N., Jannot A.S., Neuraz A., et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;26:5199–5212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- 48.Seftel D., Boulware D.R. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab050. ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X., Li X., Zhang C., Sun M., Sun Z., Xu Y., et al. Efficacy and tolerability of fluvoxamine in adults with social anxiety disorder: a meta-analysis. Med (Baltim) 2018;97 doi: 10.1097/MD.0000000000011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altamura A.C., Caldiroli A., Buoli M. Pharmacokinetic evaluation of fluvoxamine for the treatment of anxiety disorders. Expert Opin Drug Metab Toxicol. 2015;11:649–660. doi: 10.1517/17425255.2015.1021331. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol. 2020;95 doi: 10.1128/JVI.01648-20. 016488-e1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu L.M., Bafadhel M., Dorward J., Hayward G., Saville B.R., Gbinigie O., et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramakrishnan S., Nicolau D.V., Jr., Langford B., Mahdi M., Jeffers H., Mwasuku C., et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clemency B.M., Varughese R., Gonzalez-Rojas Y., Morse C.G., Phipatanakul W., Koster D.J., et al. Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial. JAMA Intern Med. 2022;182:42–49. doi: 10.1001/jamainternmed.2021.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ezer N., Belga S., Daneman N., Chan A., Smith B.M., Daniels S.A., et al. Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ. 2021;375 doi: 10.1136/bmj-2021-068060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin M.J., Ustianowski A., De Wit S., Launay O., Avila M., Templeton A., et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.U.S. Centers for Disease Control and Prevention . 2021. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States.https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html [Google Scholar]
- 59.Agustí A., De Stefano G., Levi A., Muñoz X., Romero-Mesones C., Sibila O., et al. Add-on inhaled budesonide in the treatment of hospitalised patients with COVID-19: a randomised clinical trial. Eur Respir J. 2022;59 doi: 10.1183/13993003.03036-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montgomery H., Hobbs F.D., Padilla F., Arbetter D., Templeton A., Seegobin S., et al. Efficacy and safety of intramuscular administration of tixagevimab–cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2022 doi: 10.1016/s2213-2600(22)00180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]