Abstract
At the beginning of the SARS-CoV-2 pandemic, developing of new treatments to control the spread of infection and decrease morbidity and mortality are necessary. This prospective, open-label, case-control intervention study evaluates the impact of the oral intake of the probiotic yeast Kluyveromyces marxianus B0399 together with Lactobacillus rhamnosus CECT 30579, administered for 30 days, on the evolution of COVID-19 patients. Analysis of the digestive symptoms at the end of the follow up shows a benefit of the probiotic in the number of patients without pyrosis (100% vs 33.3%; p 0.05) and without abdominal pain (100% vs 62.5%; p 0.04). Results also show a better evolution when evaluating the difference in the overall number of patients without non-digestive symptoms at the end of the follow-up (41.7%, vs 13%; p 0.06). The percentage of improvement in the digestive symptoms (65% vs 88%; p value 0.06) and the global symptoms (digestive and non-digestive) (88.6% vs 70.8%; p value 0.03) is higher in the probiotic group. The probiotic was well tolerated with no relevant side effects and high adherence among patients. In conclusion, this coadjutant treatment seems to be promising, although results should be confirmed in new studies with higher number of patients.
Keywords: COVID-19, SARS-CoV-2, Probiotics, Microbiota, Microbiome
Abbreviations: COVID-19, coronavirus disease; RNA, ribonucleic adid; ACE-2, angiotensin converting enzyme-2; CECT, spanish type culture collection; CFU, colony-forming unit; CURB-65, pneumoniae severity score; ICU, intensive care unit; SPSS, Statistical Package for Social Science; HT, HT:Hypertension; COPD, chronic obstructive pulmonary disease; PCR, polymerase chain reaction; SD, standard deviation; HHU, home hospitalization unit; DVT, deep vein thrombosis; IBS, Intestine Bowel Syndrome
1. Introduction
The SARS-CoV-2 coronavirus, the causal agent of the coronavirus disease 2019 (COVID-19), is an RNA virus belonging to the Coronaviridae family. Starting 2020, COVID-19 has been responsible for the worst pandemic suffered by humanity in the twenty-first century. Main COVID-19 transmission mechanisms demonstrated so far are airway through Flügge droplets and contact transmission through the hands or recently contaminated objects [1]. Besides, SARS-CoV-2 can also be isolated as viable viral particles in stool samples from infected patients [2]. The intestinal epithelial cells, especially those enterocytes, express angiotensin converting enzyme-2 (ACE-2) receptors, as occurs with lung cells. These receptors play a crucial role in the pathogenesis of COVID-19, due to the capability of the virus to replicate in these cells [3], being responsible for the respiratory and digestive symptoms that appear in COVID-19. A recent study showed that RNA was consistently detected in rectal swabs even after viral clearance from the upper respiratory tract, indicating extended duration of viral shedding in faecal samples and raising the possibility of fecal–oral transmission of SARS-CoV-2 [4].
The effect of viral infections on the gut microbiota has been described and the role of the gut microbiota influencing lung diseases has been well articulated [5]. Gut microbiota diversity is diminished in elderly people, and COVID-19 is especially cruel in these aged people. Several recent publications demonstrate that SARS-CoV-2 infection causes a significant alteration of the intestinal microbiota along with a decrease in biodiversity [[6], [7], [8]]. It is known that lower microbial biodiversity is a risk factor for bacterial translocation phenomena and consequently, a worsening of the patient's prognosis and symptoms, including systemic, respiratory, and digestive ones [[9], [10], [11]]. These facts highlight the idea of the gut microbiota impact on COVID-19 evolution and prognosis. Considering that many of the COVID-19 symptoms could be caused by intestinal dysbiosis, the hypothesis of acting on it by means of an intervention is raised, with the aim of changing the natural development of the infection and thus, turning the coronavirus presence in faecal samples negative faster. In fact, a phenotype of patients with a greater presence of digestive symptoms has been described and hypothesized that this COVID-19 phenotype could be related to a greater presence of ACE receptors in their intestinal mucosa [[12], [13], [14], [15]]. Consequently, it is reasonable to propose treatments aimed at modulating the gut microbiota to improve prognosis and reduce the symptoms. Considering their already demonstrated beneficial effects in various digestive pathologies, probiotics seem to be a feasible option among other treatments [[16], [17], [18], [19]]. In fact, recently a few publications studied the effect of different probiotic strains in COVID-19 patients, with promising results [[20], [21], [22]].
We proposed this study to validate the effectiveness of a specific probiotic formulation of a yeast and a lactobacillus in symptomatic COVID-19 patients with moderate involvement, especially in digestive symptoms. The mixture of probiotics used was selected based in previous clinical and preclinical studies. Lactobacillus rhamnosus CECT 30579 strain was tested in vitro together with other L. rhamnosus strains and selected based on internal unpublished data. Kluyveromyces marxianus B0399 has been studied in different clinical trials where it has shown to be effective in controlling digestive symptoms in some intestinal diseases, including diarrhea associated to the use of antibiotics, among others [[23], [24], [25], [26], [27]].
2. Materials and methods
2.1. Study design
Randomized prospective, open-label, unblinded case-control controlled clinical trial with a control group that did not undergo the intervention. The study was approved in April 2020 by the Hospital Universitario de Torrevieja and Hospital Universitario del Vinalopó (Spain) Ethics Committee (approval protocol code: TV/VP-14042020). The study was registered at Clinicaltrial.gov as NCT04390477.
2.2. Study population
Population included in the study were subjects admitted in the Internal Medicine Department or in the Home Hospitalized Unit of the Hospital Universitario del Vinalopó in Elche, Alicante (Spain). The inclusion criteria were patients over 18 years and SARS-CoV-2 diagnosis as per one positive oropharyngeal and nasopharyngeal COVID-19 swab. Prior to study inclusion, all patients were properly informed by the clinical trial investigators. Those who agreed, signed an informed consent to participate in the study.
During the study patients were allowed to receive COVID-19 therapeutic treatments, including hydroxychloroquine (200 mg twice a day for 7 days), azithromycin (500 mg once a day for 7 days), lopinavir–ritonavir (400/100 mg twice a day) or darunavir–cobicistat (800/150 mg once a day) for 14 days, and low molecular weight heparin for deep vein thrombosis prophylaxis, as recommended at the time by the Spanish Society of Infectious Diseases and the Hospital Infectious diseases Unit guidelines.
2.3. Randomizing and intervention
Patients were randomized in a ratio 5:3 to be assigned to 1 of the 2 trial arms using a computational randomization list. The intervention group received one nutraceutical probiotic supplement capsule a day for one month. Content per dose/capsule was Lactobacillus rhamnosus CECT 30579 1 × 109 CFU and Kluyveromyces marxianus B0399 with 1 × 108 CFU. The control group did not receive the intervention. Investigators received instructions on product storage, including keeping the product closed in a cool and dry place, and protected from direct sunlight.
The recommended daily dose is 1 oral capsule, which should preferably be taken with breakfast or other meal. In case of inability or issues to swallow the capsule, the content can be dissolved in little water and taken straightaway. The capsule contains the probiotic blend, calcium carbonate, gelatin (coating agent), maltodextrin (bulking agent) and magnesium stearate (anti-caking agent).
2.4. Outcome measurements
The study design includes two visits. In visit 1 patients were evaluated and those eligible according to protocol inclusion criteria were included in the study. After 30 days of the start of the study, visit 2 (end of the study) was carried out.
In visit 1 the clinical situation of the patients were evaluated measuring the parameters included in the protocol. Medical history was recorded together with the outcome of the examination results including all digestive symptoms as well as other signs and non-digestive symptoms, possible concomitant pathologies, habitual pharmacological treatments, blood tests, radiological evaluation, CURB-65 pneumonia severity score and description of symptoms related to COVID-19 infection. Besides, an antigen COVID-19 test was performed to every patient between 10 and 15 days from the onset of symptoms. In visit 2, in addition to the above-described evaluation, the following data was recorded: result of a second antigen test, the adherence to treatment and all adverse events occurred during the follow-up period.
The primary outcome was the total number of patients and percentage of those that showed improvement in digestive symptoms from baseline to final visit, comparing intervention group to control group. The secondary variables were: a) Total number of patients and percentage of those who improved in total symptoms; b) Total number of digestive symptoms and percentage of those that improved; c) Total number of total symptoms and percentage of those that improved; d) Number and percentage of cases discharged to the Intensive Care Unit (ICU); e) Number and percentage of cases that died; f) Number and percentage of patients who negativized the antigen test between day 10 and 15 from the onset of symptoms; and g) Number of adverse events attributable or not to the intervention product.
2.5. Statistical analysis and sample size calculation
The statistical analyses were conducted with Statistical Package for Social Science (SPSS) software, version 22 (IBM SPSS, Chicago, III). The analysis of the results has been carried out by protocol, that is, only those patients who completed the clinical study have been considered. During the statistical analysis we observed two types of variables, continuous and dichotomous. For the continuous variables, the Kolmogorov Smirnov normality test was applied, from which it was observed that the distribution of these variables was normal in all cases. Therefore, the data was analyzed using Student's t-test and represented by the mean and standard deviation. The dichotomous variables were described as a frequencies and percentages (%), and then compared by the χ2 test for the two groups. A two-sided p-value test of <0.05 was considered statistically significant.
To end, the sample size and the statistical power calculation were performed taking the total digestive symptoms variable as the basis for the calculation. When the study was designed, no published data could be found related to improvement of digestive symptoms in COVID-19 patients during the first month since diagnosis was done. Local experience from the Hospital where patients were recruited estimates a range between 25 and 45%. Taking the value 35% and expecting to detect in the intervention group a response of at least 80%, accepting an alpha risk of 0.05 and a beta risk of 0.2 in the 2-sided test and a drop out of 2%, 15 subjects are needed in the control group and 25 in the probiotic group for the difference to be statistically significant in proportion.
3. Results
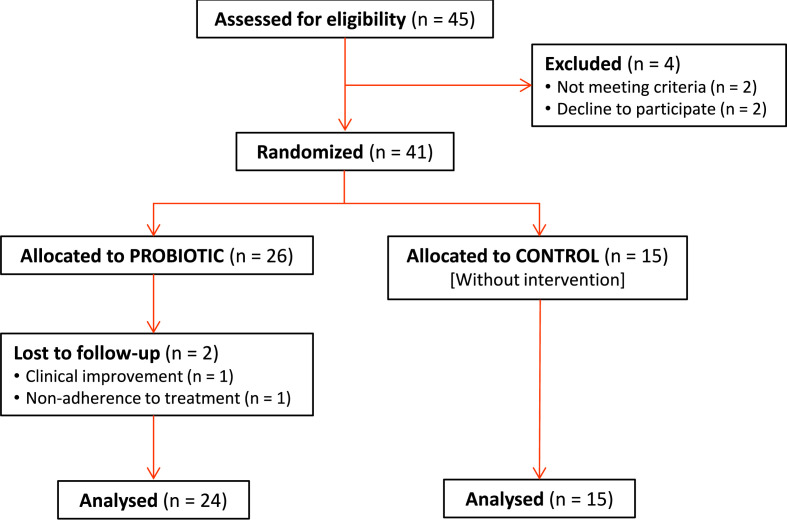
From December 2020 to February 2021, forty-one patients with diagnosis of COVID-19 were enrolled in the study. The distribution of the sample remained with 26 patients in the probiotic group compared to 15 patients in the control group. Table 1 shows the general characteristics of the population included in the study, both demographic data and the variables associated with symptoms and signs related to COVID-19. There were no significant differences in the baseline characteristics between the 2 groups (Table 1). During the follow-up a total of 2 patients, both included in the probiotic group, decided to drop out once they were included in the study voluntarily. Missing data was not related to the treatment nor to the outcome. Only observed data was used in the analysis (Fig. 1 ).
Table 1.
Demographic and Clinical Data Homogeneity Analysis. Comparison between groups at the baseline visit.
| Control (N = 15) | Probiotic (N = 26) | P value | |
|---|---|---|---|
| Sex (Female), N (%) | 10 (66.7%) | 12 (46.1%) | 0.21 |
| Age (years), Mean ± SD | 46.33 ± 10.91 | 48.88 ± 12.35 | 0.51 |
| Previous pathologies (HT, cardiovascular, diabetes, cancer, COPD), N (%) | 3 (20%) | 7 (26.9%) | 0.62 |
| Pharmacological treatments, N (%) | 7 (46.7%) | 14 (53.8%) | 0.66 |
| PCR diagnosis, N (%) | 5 (33.3%) | 8 (30.8%) | 0.87 |
| Antigen test diagnosis, N (%) | 10 (66.7%) | 18 (69.2%) | 0.87 |
| Hospital admission, N (%) | 2 (13.3%) | 9 (34.6%) | 0.14 |
| HHU admissions, N (%) | 13 (86.7%) | 17 (65.4%) | 0.14 |
| Fever, N (%) | 12 (80%) | 20 (76.9%) | 0.82 |
| Loss of sense of smell or taste, N (%) | 6 (40%) | 15 (57.7%) | 0.28 |
| Respiratory, N (%) | 14 (93.3%) | 23 (88.5%) | 0.61 |
| Dry cough, N (%) | 13 (86.7%) | 21 (80.8%) | 0.63 |
| Dyspnea, N (%) | 1 (6.7%) | 8 (30.8%) | 0.07 |
| Chest pain, N (%) | 1 (6.67%) | 6 (23.1%) | 0.18 |
| Sore throat, N (%) | 6 (40%) | 6 (23.1%) | 0.26 |
| Digestive, N (%) | 15 (100%) | 24 (92.3%) | 0.27 |
| Diarrhea, N (%) | 12 (80%) | 19 (73.1%) | 0.62 |
| Dyspepsia, N (%) | 13 (86.7%) | 10 (38.5%) | 0.13 |
| Heartburn, N (%) | 2 (13.3%) | 4 (15.4%) | 0.86 |
| Abdominal pain, N (%) | 8 (53.3%) | 9 (34.6%) | 0.24 |
| Nausea and/or vomiting, N (%) | 12 (80%) | 12 (46.1%) | 0.15 |
| Other symptoms, N (%) | 15 (100%) | 25 (96.1%) | 0.44 |
| Asthenia, N (%) | 15 (100%) | 24 (92.3%) | 0.27 |
| Musculoskeletal pain, N (%) | 13 (86.7%) | 21 (80.8%) | 0.63 |
| Conjunctivitis, N (%) | 3 (20%) | 4 (15.4%) | 0.71 |
| Headache, N (%) | 13 (86.7%) | 19 (73.1%) | 0.31 |
| Rash, N (%) | 1 (6.7%) | 0 (0%) | 0.18 |
| Total number of digestive symptoms, Mean ± SD | 3.13 ± 0.99 | 2.08 ± 1.23 | 0.07 |
| Total number of signs and symptoms, Mean ± SD | 8.73 ± 2.15 | 7.62 ± 2.23 | 0.13 |
| CURB-65 (Mild pneumonia, Groups 0 and 1), N (%) | 15 (100%) | 24 (92.3%) | 0.55 |
HT: Hypertension. COPD: Chronic obstructive pulmonary disease. PCR: polymerase chain reaction. SD: Standard deviation. HHU: Home Hospitalization Unit. CURB 65: Pneumoniae Severity score (Confusion, Urea, Respiratory rate, Blood pressure, 65 years or older).
Fig. 1.
Consort diagram.
3.1. Main variable in the study
3.1.1. Patients with resolution of digestive symptoms
Table 2 shows the number of patients with digestive symptoms per group, as well as the number and percentage of patients with symptoms improvement between initial and final visit. There are significant differences when comparing pyrosis symptom mean data in both groups (placebo 33.3% vs probiotic 100%; p value = 0.05) and the abdominal pain symptom (placebo 62.5% vs probiotic 100%; p value = 0.04).
Table 2.
Total number of patients and percentage of cases presenting improvement in digestive symptoms.
| Symptoms | Group | Patients with symptoms in V1 | Patients with symptoms in V2 | Improve | P value |
|---|---|---|---|---|---|
| Digestive | Control | 15 | 8 | 46.7% | 0.11 |
| (N = 37) | Probiotic | 22 | 6 | 72.7% | |
| Diarrhea | Control | 13 | 4 | 69.2% | 0.31 |
| (N = 32) | Probiotic | 19 | 3 | 84.2% | |
| Dyspepsia | Control | 13 | 3 | 76.9% | 0.41 |
| (N = 23) | Probiotic | 10 | 1 | 90% | |
| Pyrosis | Control | 3 | 2 | 33.3% | 0.05∗ |
| (N = 7) | Probiotic | 4 | 0 | 100% | |
| Abdominal pain | Control | 8 | 3 | 62.5% | 0.04∗ |
| (N = 17) | Probiotic | 9 | 0 | 100% | |
| Nausea and/or vomiting | Control | 12 | 4 | 66.7% | 0.16 |
| (N = 23) | Probiotic | 11 | 1 | 90% |
V1: Visit 1. V2: Visit 2.
3.2. Secondary variables in the study
3.2.1. Patients with resolution of non-digestive symptoms
Results on the total number of patients and percentage of them grouped by symptoms are described in Table 3 . At follow-up end, the number of patients presenting any symptom was 13/15 (92.8%) in the control group vs 14/24 (58.3%) in the probiotic one. The number of patients showing a completely resolution of symptoms comparing control group to probiotic group was 2/15 (13%) vs 10/24 (41.7%); p value = 0.06.
Table 3.
Total number of patients and percentage of cases presenting improvement of non-digestive symptoms.
| Symptoms | Group | Patients with symptoms in V1 | Patients with symptoms in V2 | Improve | P value |
|---|---|---|---|---|---|
| Fever | Control | 12 | 0 | 100% | – |
| (N = 32) | Probiotic | 20 | 0 | 100% | |
| Anosmia/Ageusia | Control | 6 | 2 | 66.7% | 0.83 |
| (N = 20) | Probiotic | 14 | 4 | 71.4% | |
| Respiratory | Control | 14 | 2 | 85.7% | 0.32 |
| (N = 35) | Probiotic | 21 | 1 | 95.2% | |
| Dry cough | Control | 13 | 2 | 84.6% | 0.34 |
| (N = 32) | Probiotic | 19 | 1 | 94.7% | |
| Dyspnea | Control | 1 | 0 | 100% | – |
| (N = 8) | Probiotic | 7 | 0 | 100% | |
| Chest pain | Control | 1 | 0 | 100% | – |
| (N = 6) | Probiotic | 5 | 0 | 100% | |
| Throat pain | Control | 6 | 1 | 83.3% | 0.30 |
| (N = 12) | Probiotic | 6 | 0 | 100% | |
| Asthenia | Control | 15 | 8 | 46.7% | 0.64 |
| (N = 37) | Probiotic | 22 | 10 | 54.5% | |
| Musculoskeletal pain | Control | 13 | 4 | 69.2% | 0.01∗ |
| (N = 32) | Probiotic | 19 | 0 | 100% | |
| Conjunctivitis | Control | 3 | 0 | 100% | – |
| (N = 7) | Probiotic | 4 | 0 | 100% | |
| Headache | Control | 13 | 2 | 84.6% | 0.09 |
| (N = 31) | Probiotic | 18 | 0 | 100% | |
| Rashes | Control | 1 | 0 | 100% | – |
| (N = 1) | Probiotic | 0 | 0 | – | 0.34 |
V1: Visit 1. V2: Visit 2.
3.2.2. Evolution of digestive symptoms - total number and improvement of digestive symptoms
Table 4 shows data of the digestive symptoms and its improvement, featuring the mean of symptoms reported by each patient in both groups, given that only patients who had some gastrointestinal symptom in visit 1 were analyzed. Comparison between both groups show a nearly statistical significance difference (placebo 65% vs probiotic 87.95%; p value = 0.06).
Table 4.
Total number and improvement of digestive symptoms.
| Digestive symptoms | Control (N = 15) | Probiotic (N = 22) | P value |
|---|---|---|---|
| Total number V1, Mean | 3.13 | 2.36 | |
| Total number V2, Mean | 1.13 | 0.32 | |
| Improvement index V1–V2, % Mean ± SD | 65% ± 39.86 | 87.95% ± 24.26 | 0.06 |
V1: Visit 1. V2: Visit 2. SD: Standard deviation.
3.2.3. Evolution of overall symptoms
Table 5 includes the mean of total symptoms per group, by averaging the rate of improvement in the overall symptom. Data shows statistical difference between groups with an improvement of 70.82% in the probiotic group vs 88.55% in the placebo group; p value = 0.027.
Table 5.
Total number and improvement of overall symptoms.
| Overall symptoms | Control (N = 15) | Probiotic (N = 24) | P value |
|---|---|---|---|
| Total number V1, Mean | 8.73 | 7.71 | |
| Total number V2, Mean | 2.47 | 0.92 | |
| Improvement index V1–V2, % Mean ± SD | 70.82% ± 26.48 | 88.55% ± 13.85 | 0.03∗ |
V1: Visit 1. V2: Visit 2. SD: Standard deviation.
3.2.4. Other variables: number of patients discharged to ICU and number of patients who did during the follow up and number of patients which performed COVID-19 test in days 10–15
All patients included in the study were admitted in the Home Hospitalization Unit. The 39 analyzable patients were discharged home. No patient was discharged to the ICU nor died during the follow up.
To compare the possible effects of probiotic treatment on early negativization of the virus, between day 10 and 15 from the onset of symptoms, 24 of the 39 analyzable patients performed a rapid antigen test at home (the remaining 15 patients were not able to perform a rapid antigen test). One of them, included in the control group, was positive; the remaining 23 were negative.
3.2.5. Security analysis
All adverse events occurred during the follow-up period were collected by the investigators. A total of 11 patients reported adverse events during the study, 6 in the probiotic group and 5 in the control group (Table 6 ). Bearing in mind that only the patients in the probiotic group received treatment and analysing the possible side effects of the product under study, we emphasize that none of the 7 described adverse events were considered potentially attributable to the product under study.
Table 6.
Description of patients and adverse events in both follow-up groups.
| Control | Probiotic |
|---|---|
| Renal colic | Nausea and increased frequency of bowel movements |
| Low back pain | Mild constipation and Poor digestion - bloating |
| Weight loss; 6 kg | Mild constipation |
| Persistent fatigue and dyspnea | Renal colic and DVT |
| Persistent dyspnea | Mild intermittent swelling |
| Headaches/dizziness |
DVT: Deep Vein Thrombosis.
4. Discussion
As previously mentioned, few studies have evaluated the efficacy of certain probiotic strains as an option to improve moderated COVID-19 recovery rates in adults; however, to date, there is no strong experimental evidence supporting their effectiveness and safety in real clinical practice. Importantly, evidence and clinical trials demonstrating strain-specific effects are lacking. The clinical trial reported herein explores the role of a mixture of probiotics administered to patients with moderate COVID-19. Several variables, such as treatments used for COVID-19 besides other concomitant diseases were controlled to avoid bias. Our results suggest that administration of the probiotic mixture under study (yeast strain Kluyveromyces marxianus B0399 plus Lactobacillus rhamnosus CECT 30579), as adjuvant treatment, can be effective in reducing the symptoms and thus, shortening the time needed for full recovery in symptomatic patients with an acute episode of COVID-19. The response rate of patients was statistically significant higher in the probiotic group comparing to the no intervention group when analyzed the symptoms pyrosis, abdominal pain and the overall symptoms.
The clinical response observed in the main variable “Patients with resolution on digestive symptoms” was in the same line as observed in other previous clinical controlled studies testing probiotics on COVID-19 [21]. 72.7% of patients remained free of digestive symptoms at the end of the first month follow-up in the probiotic group in our study. Data from the Gutierrez-Castrellon et al. study showed 53% of response in the same variable and same follow up period.
Moreover, despite the low number of patients in our study, the magnitude of the difference in the number of patients showing a completely resolution of symptoms comparing control and probiotic group reaches almost significant statistical difference (13% vs 41.7%; p value = 0.06). Several factors which may influence the response to probiotic treatment on COVID-19 were considered when the protocol of this clinical study was designed: older patients have more dysbiosis than young ones, besides some different treatments prescribed to COVID-19 patients such as antibiotics, can affect the microbiota composition and hence the rates of response to probiotic treatment [28,29]. Interestingly not only digestive symptoms improved when the probiotic was administered. The osteo-muscular pain and the overall symptoms also improved in the probiotic group when compared to the non-interventional group. This effect suggests a systemic effect of probiotic on the immunomodulatory and inflammatory systemic biomarkers, as proposed in other inflammatory, infectious and immunity systemic diseases [30]. Decreased activity of the T helper 2 cells, lipopolysaccharides and viral DNA in the peripheral blood of patients included in the probiotic arm as a consequence of the use of probiotic has been described [31].
The final blend used in the study included Kluyveromyces marxianus fragilis B0399, the first non-Saccharomyces yeast approved as a probiotic for human consumption [23]. This strain has demonstrated to be effective in the recovery of digestive symptoms, besides a potential in some other diseases such as intestinal bowel diseases, halitosis, lactic intolerance, and antibiotic side effects. In patients with IBS (Intestine Bowel Syndrome) administration of a fermented milk containing Kluyveromyces marxianus fragilis B0399 and other probiotic species, improved symptoms [27]. In subjects with halitosis, which the cause is probably due to bacterial disbalance, the administration of Kluyveromyces marxianus B0399 capsules for two weeks allowed to eliminate halitosis in 91% of patients. Mechanism of action of probiotic was the restoration of the gut microbiota, not acting at the oral level [26].
The other strain in this mixture is Lactobacillus rhamnosus CECT 30579 that has been proved in a mixture for digestive symptoms with good results and effectiveness (internal data unpublished). It is interesting to note that none of the previously published clinical trials in COVID-19 patients evaluate the effect of a yeast in the evolution of these patients. Only one study evaluates the effect of a Nutritional Support System administered orally and intramuscularly. This treatment includes some different compounds and vitamins, including the probiotic yeast Saccharomyces boulardii but administered orally only during the first 6 days of intervention [22]. Kluyveromyces marxianus B0399 is especially interesting because it is a proven strain not only in the treatment of digestive symptoms but also with immunomodulatory potential [32]. Another factor in favor of its selection is that both strains have been previously used in elderly and immunocompromised patients without significant side effects and with very good tolerance by patients [23,26,27].
Limitations to the study should be considered, including among others the low number of patients evaluated and the non-blinded open label clinical trial due to the fact that the study design was performed during the first COVID-19 pandemic wave when researchers had important restrictions to access to patients, as well as the fact that non usual practices were not allowed, and this is why no samples of stool for microbiota study were collected. Besides, the time of intervention and to know if longer treatment could benefit a greater number of patients, are another unresolved questions. Applicability of our results and whether they can be extended to other populations such as patients with severe COVID-19 are points to solve and clarified through further research.
5. Conclusions
The results of our study indicate a positive effect in reducing the total number of digestive symptoms and overall symptoms in the group treated with the probiotic mixture. This evidence supports the efficacy of administering this probiotic mixture to patients with moderate COVID-19 and suggests that it could be an effective coadjutant treatment that could be used more extensively in clinical practice.
Author contributions
Conceptualization, V.N.-L., A.H.-B. and M.I.P.-S.; Formal analysis, J.A.-S., D.P.-M. and L.N. M.; Methodology, V.N.-L., M.A.-G and D.P.-M.; Software, L.N.-M.; Validation, M.I.P.-S., M.A.-G., G.L.-R., E.R.-S., M.M.-G., P.S.-P., E.N.-D., B.R.-C. and J.A.P.-M.; Writing—original draft preparation, V.N.-L. and J.A.-S.; W riting—review and editing, A.H.-B., M.I.P.-S., M.A.-G., G.L.-R., E.R.-S., M.M. G., P.S.-P., E.N.-D., B.R.-C., J.A.P.-M. and D.P.-M.; Supervision, J.A.-S. and B.R.-C.; Project administration, V.N. L.
Conflicts of interest
A.H–B., M.I.P.-S., M.A.-G., G.L.-R., E.R.-S., M.M.-G., D.P.-M. and J.A.P.-M declare no conflicts of interests. V.N.-L is the Research Director of Bioithas, S.L. P.S.-P., J.A.-S., E.N.-D., B.R. C. and L.N.-M. are employees of Bioithas, S.L.
Acknowledgments
This research was promoted by Bioithas, S.L. and partially funded by an award of the Instituto Valenciano de Competitividad Empresarial, Comunidad Valenciana, Spain (Proyectos de I + D (PIDI-CV); Grant number: IMIDTA/2021/107).
References
- 1.Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (COVID-19) medRxiv. 2020 doi: 10.1101/2020.02.14.20023127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willers M., Viemann D. Role of the gut microbiota in airway immunity and host defense against respiratory infections. Biol Chem. 2021;402:1481–1491. doi: 10.1515/hsz-2021-0281. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Kuang D., Li D., Yang J., Yan J., Xia Y., Zhang F., Cao H. Roles of the gut microbiota in severe SARS-CoV-2 infection. Cytokine Growth Factor Rev. 2022;63:98–107. doi: 10.1016/j.cytogfr.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezechukwu H.C., Diya C.A., Egoh I.J., Abiodun M.J., Grace J.A., Okoh G.R., Adu K.T., Adegboye O.A. Lung microbiota dysbiosis and the implications of SARS-CoV-2 infection in pregnancy. Ther Adv Infect Dis. 2021;8 doi: 10.1177/20499361211032453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H., Wang H., Sun Y., Ren Z., Zhu W., Li A., Cui G. Potential associations between microbiome and COVID-19. Front Med. 2021;8 doi: 10.3389/fmed.2021.785496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page M.J., Kell D.B., Pretorius E. The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress (Thousand Oaks) 2022;6 doi: 10.1177/24705470221076390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardinale V., Capurso G., Ianiro G., Gasbarrini A., Arcidiacono P.G., Alvaro D. Intestinal permeability changes with bacterial translocation as key events modulating systemic host immune response to SARS-CoV-2: a working hypothesis. Dig Liver Dis. 2020;52:1383–1389. doi: 10.1016/j.dld.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X.Y., He C., Zhu Y., Lu N.H. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J Gastroenterol. 2020;26:2187–2193. doi: 10.3748/wjg.v26.i18.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh D.D., Sharma A., Lee H.J., Yadav D.K. SARS-CoV-2: recent variants and clinical efficacy of antibody-based therapy. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.839170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirtipal N., Kumar S., Dubey S.K., Dwivedi V.D., Gireesh Babu K., Maly P., Bharadwaj S. Understanding on the possible routes for SARS CoV-2 invasion via ACE2 in the host linked with multiple organs damage. Infect Genet Evol. 2022;99 doi: 10.1016/j.meegid.2022.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labandeira-Garcia J.L., Labandeira C.M., Valenzuela R., Pedrosa M.A., Quijano A., Rodriguez-Perez A.I. Drugs modulating renin-angiotensin system in COVID-19 treatment. Biomedicines. 2022;10 doi: 10.3390/biomedicines10020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theodorakopoulou M.P., Alexandrou M.E., Boutou A.K., Ferro C.J., Ortiz A., Sarafidis P. Renin-angiotensin system blockers during the COVID-19 pandemic: an update for patients with hypertension and chronic kidney disease. Clin Kidney J. 2022;15:397–406. doi: 10.1093/ckj/sfab272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases: a review. Antonie Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.K., Guevarra R.B., Kim Y.T., Kwon J., Kim H., Cho J.H., Kim H.B., Lee J.H. Role of probiotics in human gut microbiome-associated diseases. J Microbiol Biotechnol. 2019;29:1335–1340. doi: 10.4014/jmb.1906.06064. [DOI] [PubMed] [Google Scholar]
- 18.Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 19.Meng X., Zhang G., Cao H., Yu D., Fang X., de Vos W.M., Wu H. Gut dysbacteriosis and intestinal disease: mechanism and treatment. J Appl Microbiol. 2020;129:787–805. doi: 10.1111/jam.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez J.A.M., Bifano M., Roca Goma E., Plasencia C.M., Torralba A.O., Font M.S., Millan P.R. Effect and tolerability of a nutritional supplement based on a synergistic combination of beta-glucans and selenium- and zinc-enriched Saccharomyces cerevisiae (ABB C1((R))) in volunteers receiving the influenza or the COVID-19 vaccine: a randomized, double-blind, placebo-controlled study. Nutrients. 2021;13 doi: 10.3390/nu13124347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez-Castrellon P., Gandara-Marti T., Abreu Y.A.A.T., Nieto-Rufino C.D., Lopez-Orduna E., Jimenez-Escobar I., Jimenez-Gutierrez C., Lopez-Velazquez G., Espadaler-Mazo J. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microb. 2022;14 doi: 10.1080/19490976.2021.2018899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leal-Martinez F., Abarca-Bernal L., Garcia-Perez A., Gonzalez-Tolosa D., Cruz-Cazares G., Montell-Garcia M., Ibarra A. Effect of a nutritional support system to increase survival and reduce mortality in patients with COVID-19 in stage III and comorbidities: a blinded randomized controlled clinical trial. Int J Environ Res Publ Health. 2022;19 doi: 10.3390/ijerph19031172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quarella S., Lovrovich P., Scalabrin S., Campedelli I., Backovic A., Gatto V., Cattonaro F., Turello A., Torriani S., Felis G.E. Draft genome sequence of the probiotic yeast Kluyveromyces marxianus fragilis B0399. Genome Announc. 2016;4 doi: 10.1128/genomeA.00923-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maccaferri S., Candela M., Turroni S., Centanni M., Severgnini M., Consolandi C., Cavina P., Brigidi P. IBS-associated phylogenetic unbalances of the intestinal microbiota are not reverted by probiotic supplementation. Gut Microb. 2012;3:406–413. doi: 10.4161/gmic.21009. [DOI] [PubMed] [Google Scholar]
- 25.Fonseca G.G., Heinzle E., Wittmann C., Gombert A.K. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl Microbiol Biotechnol. 2008;79:339–354. doi: 10.1007/s00253-008-1458-6. [DOI] [PubMed] [Google Scholar]
- 26.Cecchini F.N., Zanvit A., Miclavez A., Nobili P. Halitosis treatment through the administration of antibiotic-resistant probiotic lactic yeast Kluyveromyces marxianus fragilis B0399 (K-B0399) Biomed J Scient Tech Res. 2018;12:8887–8890. A. [Google Scholar]
- 27.Lisotti A.E., Mazzella G. Su2037 effects of a fermented milk containing Kluyveromyces marxianus B0399 and Bifidobacterium Lactis BB12 in patients with irritable bowel syndrome: a new effective agent. Gastroenterology. 2013;144:538–539. R. [Google Scholar]
- 28.Becattini S., Taur Y., Pamer E.G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22:458–478. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jernberg C., Lofmark S., Edlund C., Jansson J.K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology (Read) 2010;156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 30.Cristofori F., Dargenio V.N., Dargenio C., Miniello V.L., Barone M., Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.578386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C.X., Wang H.Y., Chen T.X. Interactions between intestinal microflora/probiotics and the immune system. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6764919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maccaferri S., Klinder A., Brigidi P., Cavina P., Costabile A. Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Appl Environ Microbiol. 2012;78:956–964. doi: 10.1128/AEM.06385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]