Abstract
Purpose
Late cardiotoxicity related to radiotherapy (RT) in breast cancer and Hodgkin lymphoma has been well reported. However, the relatively higher cardiac dose exposure for esophageal cancer (EC) may result in earlier onset of cardiac diseases. In this report, we examined the incidence, onset, and long-term survival outcomes of high-grade cardiac events after RT in a large cohort of EC patients.
Patients and Methods
Between March 2005 and August 2017, 479 patients with EC from a prospectively maintained institutional database at The University of Texas MD Anderson Cancer Center were analyzed. All patients were treated with either intensity modulated RT (IMRT) or proton beam therapy (PBT), either pre-operatively or definitively. We focused on any grade 3 or higher (G3+) cardiac events according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
Results
G3+ cardiac events occurred in 18% of patients at a median of 7 months with a median follow-up time of 76 months. Pre-existing cardiac disease (P=0.001) and radiation modality (IMRT vs PBT) (P=0.027) were significantly associated with G3+ cardiac events. Under multivariable analysis, mean heart dose, particularly < 15 Gy, was associated with reduced G3+ events. Furthermore, G3+ cardiac events were associated with worse overall survival (P=0.041).
Conclusion
Severe cardiac events were relatively common with early onset in EC patients after radiotherapy, especially those with pre-existing cardiac disease and higher radiation doses to the heart. Optimal treatment approaches should be taken to reduce cumulative doses to the heart, especially for patients with pre-existing cardiac disease.
Keywords: Esophageal cancer, Cardiac toxicity, Radiotherapy, Proton beam therapy, Pre-existing cardiac disease
Introduction
Cardiotoxicity has traditionally been regarded as a late side effect of radiation therapy. Due to the large number of patients and high survival rates, radiation-induced cardiotoxicity in breast cancer and Hodgkin’s lymphoma have been well established1–5. In these patient groups, cardiac mortality was found during long-term follow-up, suggesting that radiation is closely related to cardiac injury. However, although radiation dose to the heart in patients with esophageal cancer (EC) is generally much higher than the abovementioned diseases, cardiac toxicity may not have received sufficient attention due to the relatively poor prognosis of EC patients.
In recent years, however, treatment outcomes have significantly improved for EC patients treated with a multidisciplinary approach, incorporating chemoradiation, either in the neoadjuvant setting followed by surgery or as definitive treatment6–8. Enhanced cancer survival results in more patients being at risk to develop treatment-related cardiotoxicity. Although there are few data available on cardiac morbidity and mortality after radiotherapy in EC patients, several studies have already shown the cardiotoxicity of radiation therapy in patients with lung cancer9–11. Moreover, The Radiation Therapy Oncology Group (RTOG) 0617 randomized trial showed that in patients with non-small cell lung cancer undergoing chemoradiation, the overall survival (OS) of the standard radiation dose was higher than that of the high radiation dose, and an excessive cardiac dose was indicative of poor OS12,13.
The primary objective of the current study was to evaluate the incidence and onset of G3+ cardiac events, as well as patient- and treatment-related factors associated with these severe cardiac events based on Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 in a large cohort of EC patients.
Materials and Methods
Patients
Between March 2005 and August 2017, 479 patients with biopsy-confirmed esophageal adenocarcinoma or squamous cell carcinoma from a prospectively maintained single-institutional database were analyzed. All patients underwent radiotherapy delivered with IMRT (n=320) or PBT (n=159), with or without surgery. Esophagogastroduodenoscopy (EGD) with endoscopic ultrasound, computed tomography (CT) of the chest and upper abdomen with contrast, brain imaging (CT or magnetic resonance imaging) and/or positron emission tomography (PET)/CT scans were required for staging. Tumors were restaged according to the seventh edition of the American Joint Committee on Cancer TNM classification in our study14. Pre-existing cardiac disease was defined as the presence of coronary artery disease, arrhythmia, heart failure, valvular heart disease, cardiomyopathy or congenital heart disease prior to initiation of radiation. Patients were excluded if they had M1 disease, prior or concomitant malignancy, Eastern Cooperative Oncology Group performance status (ECOG) above 2, or incomplete clinical records. The institutional review board approved this study and waived the requirement for informed consent.
Treatment
Patients received concurrent chemoradiotherapy, either as a pre-operative treatment or as a curative strategy. Induction chemotherapy was done in a subset of patients, either as part of clinical protocol or for select patients who have large nodal burden of disease. Chemotherapy regimens typically consisted of fluoropyrimidine, in combination with platinum- or taxane-based compound. Most commonly employed surgical approach was the Ivor-Lewis esophagectomy (80%). The standard prescription radiation dose was 50.4Gy (relative biological effectiveness, RBE) in 28 fractions, but lower doses between 41.4 to 45 Gy could be given depending on patients’ tolerance to therapy and organ dose constraints (7%). IMRT plans were generated using the Pinnacle treatment planning system (version 9.0, Philips, Andover, MA), and the PBT plans were generated using the Eclipse planning system (Varian medical systems, Liverpool, NY), done for the most part using passive-scattering proton therapy (PSPT) or intensity modulated proton therapy (IMPT) in 14 (9%) of the proton cases.
Dosimetric Assessment
The delivered dose distributions for each patient were reviewed manually. The accuracy and consistency of the heart contours were evaluated by one investigator and independently reviewed by a second investigator against a validated cardiac atlas and edited as necessary15. Dose-volume histograms of the heart were generated. Parameters for analysis included heart volume receiving ≥ 5Gy (V5Gy), heart volume receiving ≥30Gy (V30Gy), heart volume receiving ≥ 50Gy (V50Gy), and heart mean dose (MHD) which defined as the mean radiation dose (Gy) delivered to the whole heart by the end of radiotherapy.
End Points
The primary end point of this study was G3+ cardiac events after radiotherapy. Cardiac toxicities were determined by in-depth manual medical record review and were scored according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, including acute coronary event, arrhythmia, heart failure, cardiac arrest, pericardial effusion and pericarditis (Supplement Table 1). Events were verified by physicians without knowing the treatment plan or cardiac radiation dose and independently reviewed by 3 cardiologists using the available source documentations. The time to event was calculated from the radiation treatment start date to the first occurrence of the cardiac event or censored at last follow-up. The secondary end point was the OS of the patients.
Statistical analysis
Descriptive statistics were used to characterize baseline patient and treatment characteristics. A competing risk regression analysis (Fine and Gray method16) was used to model the cumulative incidence function of cardiac events, taking into account the competing risk of noncardiac deaths. Univariate and multivariate analyses were performed using the Cox proportional hazards model. The Kaplan-Meier method was applied to estimate OS. In addition to analyzing the heart dose as a continuous variable, patients were divided into different heart dose layers, and the cut-off value was calculated by ROC curve. Statistical tests were based on a 2-sided significance level, and a P value of < .05 was considered statistically significant while a P value of < 0.1 was considered a marginal association. All analyses were conducted by R (version 3.6.2) and SPSS (version 25.0.0).
RESULTS
Patient characteristics and cardiac events
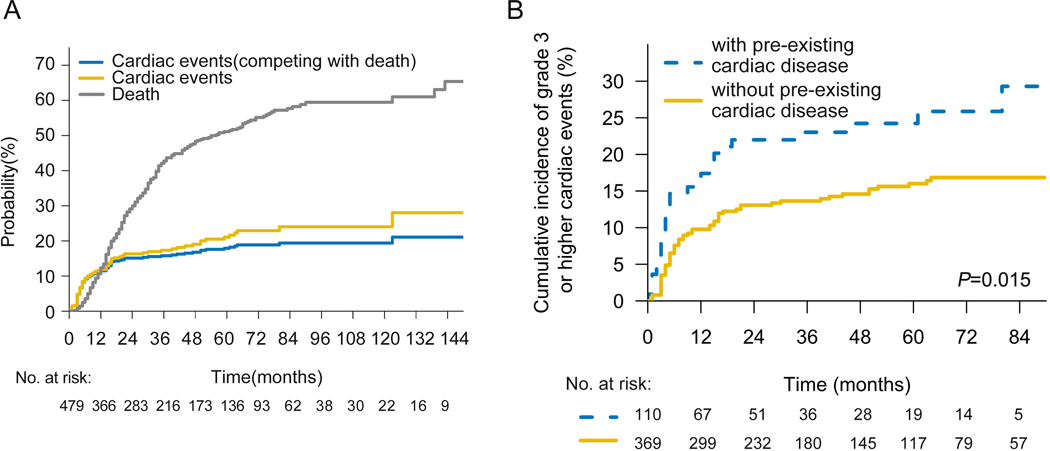
A total of 479 eligible patients were included in our study. The median age was 62 years (interquartile range, 54 to 68 years) and 23% of patients had pre-existing cardiac disease. Baseline characteristics of the entire patient cohort were summarized in Table 1. With a median follow-up time of 76 months, 18% of patients experienced G3+ cardiac event at a median of 7 months (interquartile range, 3 to 16 months), with 81% of events occurring within 2 years after completing CRT. By the time of last contact, 88 patients had one or more high-grade cardiac events, which mainly included arrhythmia (n=56), acute coronary event (n=16), heart failure (n=20), cardiac arrest (n=1), pericardial effusion (n=3) and pericarditis (n=1) (Supplement Table 2). The overall cumulative incidence of G3+ cardiac events at 2- and 5- years was 16% and 21%, respectively. However, after accounting for noncardiac death as a competing risk, the cumulative incidence of G3+ cardiac events at 2- and 5- years were 15% and 18%, respectively (Fig 1A).
Table 1.
Patient and tumor characteristics
| All Patients (n=479) |
||
|---|---|---|
| Characteristic | No. | % |
|
| ||
| Sex | ||
| Male | 421 | 87.9 |
| Female | 58 | 12.1 |
| Age | ||
| <70 | 383 | 80.0 |
| ≥70 | 96 | 20.0 |
| ECOG | ||
| 0 | 194 | 40.5 |
| 1–2 | 285 | 59.5 |
| Smoking History | ||
| Yes | 325 | 67.8 |
| No | 154 | 32.2 |
| Tumor Location | ||
| Upper/middle | 53 | 11.1 |
| Distal/GEJ | 426 | 88.9 |
| Pathology | ||
| Adenocarcinoma | 417 | 87.1 |
| SCC | 62 | 12.9 |
| Clinical T Stage (7th) | ||
| T1–2 | 44 | 9.2 |
| T3–4 | 435 | 90.8 |
| Clinical N Stage (7th) | ||
| N0 | 152 | 31.7 |
| N+ | 327 | 68.3 |
| Clinical Stage (7th) | ||
| Stage I/II | 162 | 33.8 |
| Stage III | 317 | 66.2 |
| Induction chemotherapy | ||
| Yes | 159 | 33.2 |
| No | 320 | 66.8 |
| Radiation Dose | ||
| ≤50.4Gy | 457 | 95.4 |
| >50.4Gy | 22 | 4.6 |
| Surgery | ||
| Yes | 284 | 59.3 |
| No | 195 | 40.7 |
| Pre-existing cardiac disease | ||
| Yes | 109 | 22.8 |
| No | 370 | 77.2 |
| Radiotherapy modality | ||
| IMRT | 320 | 66.8 |
| PBT | 159 | 33.2 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; GEJ, gastroesophageal junction; SCC, squamous cell carcinoma; IMRT, intensity-modulated radiation therapy; PBT, proton beam therapy.
Fig 1. G3+ cardiac events after chemoradiation.
(A) Cumulative incidence plot of death (gray), G3+ cardiac events (gold), and cardiac events adjusted after the competing risk of death (blue). (B) Cumulative incidence of G3+ cardiac events (with noncardiac death as competing risk) for patients with or without pre-existing cardiac disease.
Predictors for G3+ cardiac events
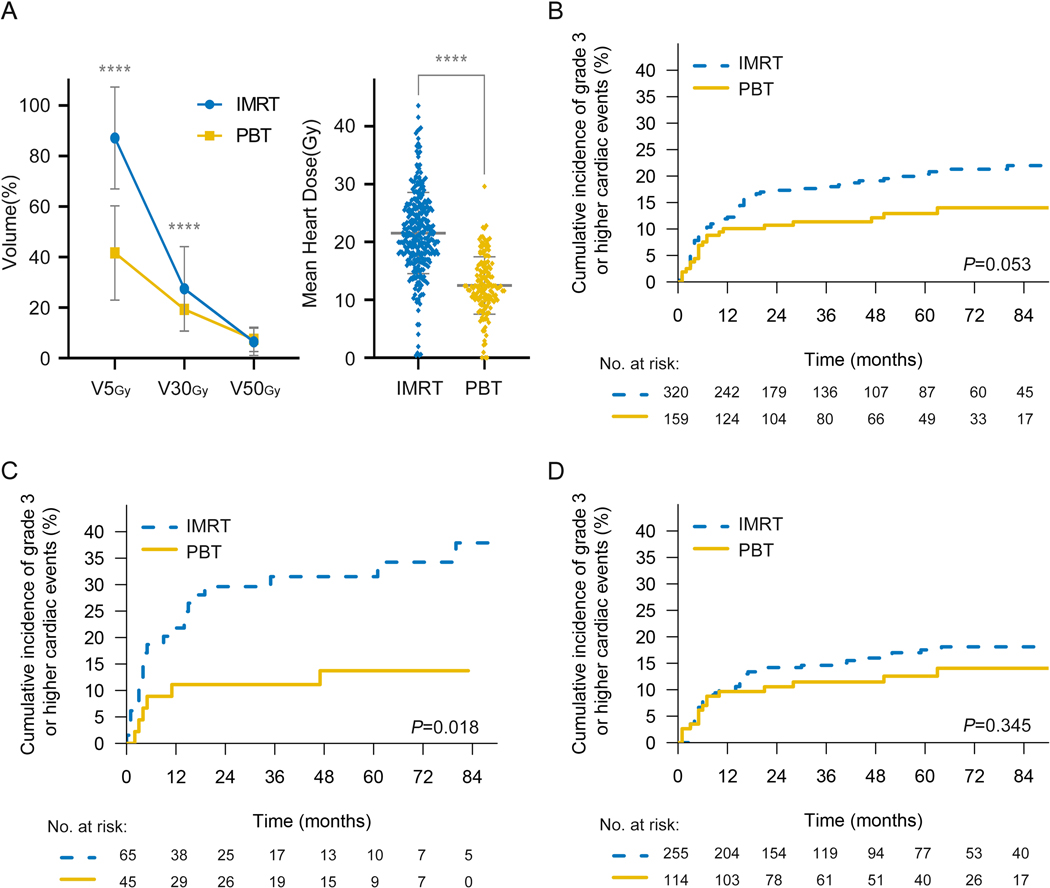
Among all factors examined in the cohort, pre-existing heart disease (Yes vs. No, hazard ratio [HR]=2.118, 95%CI, 1.346–3.331, P=0.001) was most significantly associated with G3+ cardiac events in both univariable and multivariable analysis (Table 2). Patients with pre-existing heart disease were associated with a significantly higher risk of developing G3+ cardiac events (2-y rates 22% vs. 13%; 5-y rates 24% vs. 16%; P=0.006) (Fig 1B). However, there is no significant difference in G3+ cardiac events whether surgery was performed after CRT (Yes vs. No, HR:1.168, 95%CI: 0.747–1.827, p=0.496). The probability of cardiac events in the two groups were 14.9% (non-surgery) and 20.8% (surgery). However, the RT modality used for treatment was significantly associated with G3+ cardiac events (IMRT vs. PBT, HR=1.746 95%CI, 1.065–2.862, P=0.027). A comparison of the two radiation patient cohorts revealed little differences in patient and tumor characteristics, with the exception that PBT patients are older and have more pre-existing cardiac diseases (Supplement Table 3). Cardiac dose parameters were much lower in patients treated with PBT (Fig 2A), in heart V5Gy (IMRT vs. PBT; 87.09±20.16 vs. 41.65±18.67; P = .000), V30Gy (IMRT vs. PBT; 27.46±16.69 vs. 19.33±8.67; P = .000) and mean heart dose (MHD) (Gy) (IMRT vs. PBT; 21.55±7.01 vs. 12.50±4.97; P = .000). A reduction in G3+ cardiac events was found in patients who received PBT compared to patients who received IMRT (2-y rates 18% vs. 11%; 5-y rates 21% vs. 13%; P=0.053) (Fig 2B). Moreover, in subgroup analysis of patients with pre-existing heart disease, PBT showed a greater advantage in reducing high-grade cardiac events (2-y rates 30% vs. 11%; 5-y rates 32% vs. 14%; P=0.018) compared with the group of patients without pre-existing heart disease (2-y rates 14% vs. 11%; 5-y rates 18% vs. 13%; P=0.345) (Fig 2C–D).
Table 2.
Univariable and Multivariable Analysis for Time to Earliest Grade 3 or Higher Cardiac Event
| Univariable Cox Regression Analysis | Cox Multivariable Regression | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | HR | 95%CI | p-value | HR | 95%CI | p-value |
|
| ||||||
| Sex Male vs Female | 0.667 | 0.383–1.164 | 0.154 | |||
| Age ≥70 vs <70 | 1.367 | 0.821–2.275 | 0.229 | |||
| ECOG 1–2 vs 0 | 1.059 | 0.692–1.621 | 0.791 | |||
| Smoking History Yes vs No | 1.289 | 0.811–2.048 | 0.284 | |||
| Tumor Location Distal/GEJ vs Upper/middle | 0.776 | 0.412–1.460 | 0.431 | |||
| Pathology SCC vs Adenocarcinoma | 1.240 | 0.674–2.283 | 0.490 | |||
| Clinical T Stage T3–4 vs T1–2 | 1.589 | 0.692–3.649 | 0.275 | |||
| Clinical N Stage N+ vs N0 | 1.249 | 0.789–1.976 | 0.343 | |||
| Clinical Stage III vs I/II | 1.232 | 0.786–1.932 | 0.363 | |||
| Induction Chemotherapy Yes vs No | 0.961 | 0.615–1.501 | 0.860 | |||
| Radiation Dose >50.4Gy vs ≤50.4Gy | 0.671 | 0.246–2.465 | 0.671 | |||
| Surgery Yes vs No | 1.168 | 0.747–1.827 | 0.496 | |||
| Pre-existing cardiac disease Yes vs No | 1.988 | 1.268–3.119 | 0.003 | 2.118 | 1.346–3.331 | 0.001 |
| Radiotherapy Technology IMRT vs PBT | 1.615 | 0.988–2.640 | 0.056 | 1.746 | 1.065–2.862 | 0.027 |
Abbreviations: C-index, concordance index; HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group; GEJ, gastroesophageal junction; SCC, squamous cell carcinoma; IMRT, intensity-modulated radiation therapy; PBT, proton beam therapy.
Fig 2. Cumulative incidence of G3+ cardiac events (with noncardiac death as competing risk) comparing IMRT vs. PBT.
(A) Cardiac dose volume analysis comparing PBT with IMRT. (B) Cumulative incidence of G3+ cardiac events between IMRT and PBT. Cumulative incidence of G3+ cardiac events for patients with (C) or without (D) pre-existing cardiac disease. Summary data were shown as mean ± SD with P values determined by two-tailed t-test. ****P<0.0001.
Cardiac Dose Parameters Associated with G3+ cardiac events.
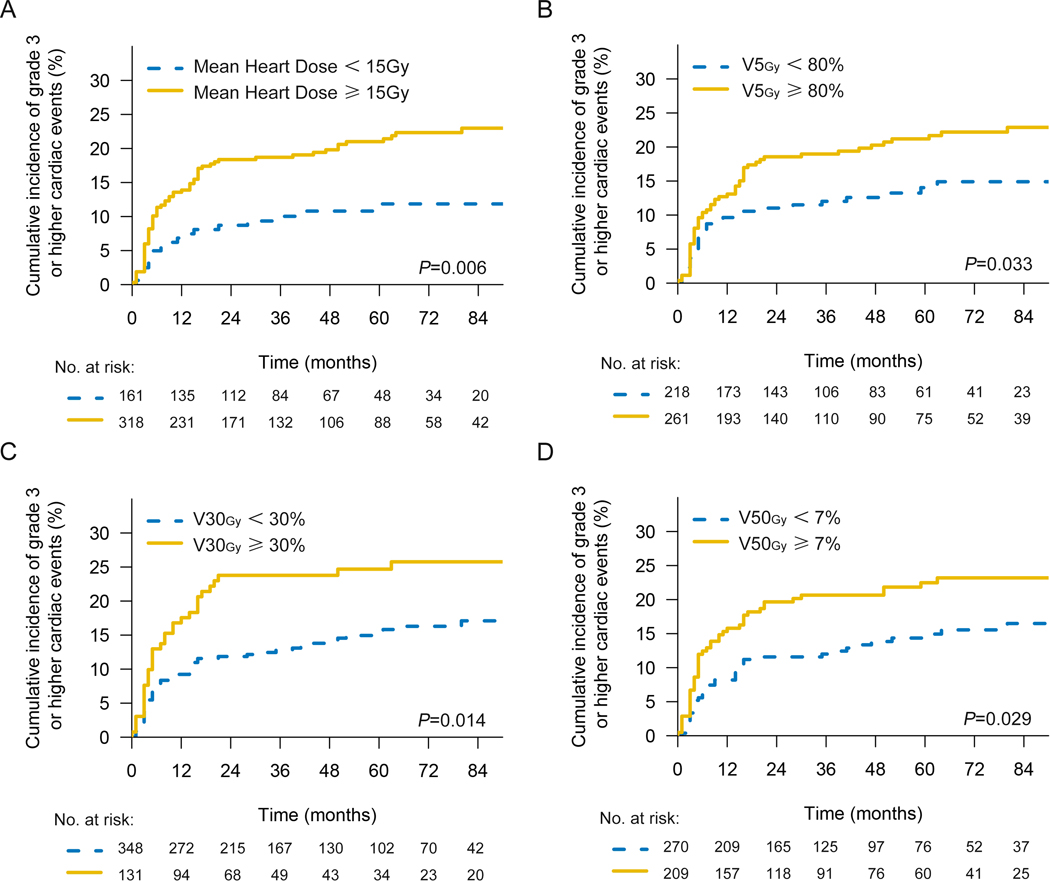
In univariable analysis of dosimetry, heart V5Gy (HR=1.008; 95%CI, 1.000–1.016; P=0.042), heart V30Gy (HR=1.014; 95%CI, 1.002–1.026; P=0.020) and MHD (Gy) (HR=1.034; 95%CI, 1.006–1.062; P=0.015) were closely related to high-grade cardiac events, while in multivariable analysis, MHD (HR=1.034; 95%CI, 1.006–1.062; P=0.015) was significantly correlated with G3+ cardiac events (Table 3). As the MHD rises, so too is the probability of G3+ cardiac events (Supplement Fig 1). Since the variables mentioned above are continuous variables, we identified the optimal ROC curve cut-off value of MHD, heart V5Gy, heart V30Gy, and heart V50Gy as 15 Gy, 80%, 30%, and 7%, respectively. The cumulative incidence of G3+ cardiac events in patients exceeding these cutoffs was significantly increased, for MHD (Gy) (2-y rates 18% vs. 9%; 5-y rates 21% vs. 12%; P=0.006), heart V5Gy (2-y rates 19% vs. 11%; 5-y rates 22% vs. 14%; P=0.033), heart V30Gy (2-y rates 24% vs. 12%; 5-y rates 25% vs. 16%; P=0.014), and heart V50Gy (2-y rates 20% vs. 12%; 5-y rates 23% vs. 15%; P=0.029) (Fig 3). Using 20 Gy and 30 Gy MHD cutoffs, there were no significant differences in cumulative incidence of G3+ cardiac events (Supplement Fig 2).
Table 3.
Dosimetric Univariable and Multivariable Analysis for Time to Earliest Grade 3 or Higher Cardiac Event
| Univariable Cox Regression Analysis | Cox Multivariable Regression | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | HR | 95%CI | p-value | HR | 95%CI | p-value |
|
| ||||||
| Heart V5Gy (%) | 1.008 | 1.000–1.016 | 0.042 | |||
| Heart V30Gy (%) | 1.014 | 1.002–1.026 | 0.020 | |||
| Heart V50Gy (%) | 1.036 | 1.000–1.074 | 0.050 | |||
| Mean heart dose (Gy) | 1.034 | 1.006–1.062 | 0.015 | 1.034 | 1.006–1.062 | 0.015 |
Abbreviations: C-index, concordance index; HR, hazard ratio.
Fig 3. Cumulative incidence of G3+ cardiac events (with noncardiac death as competing risk).
(A) for patients with 15 Gy MHD cutoff. (B) for patients delivered with different heart V5Gy subgroup (C) for patients with different heart V30Gy subgroup (D) for patients with different heart V50Gy subgroup.
Cardiac Events and OS
The median OS was 54 months for the entire group. Upper/middle tumor, clinical N+, higher clinical stage, definitive radiotherapy, a history of cardiac disease, and G3+ cardiac events were associated with worse OS in univariable analysis. Clinical stage (III vs. I/II, HR=1.789 95%CI, 1.354–2.364, P=0.000), Surgery (Yes vs. No, HR=0.519 95%CI, 0.405–0665, P=0.000) and G3+ cardiac event (Yes vs. No, HR=1.363 95%CI, 1.013–1.834, P=0.041) remained significantly associated with OS in multivariable analysis (Supplement table 4). Compared with the patients who did not have G3+ cardiac events, the OS was worse for those who experienced high-grade cardiac events (Supplement Fig 3). The 2y-OS and 5y-OS for the patients with or without G3+ cardiac events were 38% vs. 52% and 59% vs. 74%, respectively (P=0.044).
DISCUSSION
In this study, despite the high competing risk of death, we were able to demonstrate that G3+ cardiac events were common and impose a significant morbidity and mortality burden on EC patients after multimodality therapies, at a median onset of 7 months after CRT. Although cardiac events might be multifactorial, our findings showed that pre-existing cardiac disease contributed most significantly to higher cardiac event rates and may be more vulnerable to radiation induced cardiotoxicity. Higher radiation doses to the heart were also strongly associated with high-grade cardiac events, which explains why the use of PBT, a radiation modality which can substantially reduce heart radiation dose, significantly reduced the incidence of G3+ cardiac events. This seems to most benefit the patients with previous heart disease. Moreover, we found that G3+ cardiac events were significantly associated with worse OS.
From the perspective of classical radiobiology, non-proliferating and highly differentiated tissues like the heart should be quite resistant to ionizing radiation. Increasing understanding of radiation-induced heart injury have shown that radiation can induce a variety of early physiological changes including endothelial dysfunction, inflammation, thrombosis and cardiac fibrosis.17. Although these conditions are not uniquely associated with radiation exposure, a significant acceleration in the pathogenesis of these symptoms can happen with the addition of radiation. Although the typical onset of cardiac diseases usually takes many years, if not decades, after the initial radiation exposure, as seen among nuclear industry workers18,19 or treated patients with breast cancer and Hodgkin lymphoma20,21, we observed the majority of high grade heart toxicities happening within 2 years post-radiotherapy, which reflects what was seen for lung cancer9. We also observed the development of new coronary calcifications within months to 1 year in some cases of patients that received radiation therapy to the chest in a recent proof of concept study22. Furthermore, heart injury can even be detected by nuclear medicine imaging during or early after radiotherapy23,24.
We found patients with pre-existing cardiac disease had the highest likelihood of developing G3+ cardiac events. Since heart disease and cancer share many of the same risk factors25, cancer patients have a high probability of having pre-existing heart disease, it is therefore important to understand the impact cancer therapy has on the heart, which is already susceptible for injury. In a study of nearly 4,000 lung cancer patients in the Netherlands, Heijnen et al.26 showed that 23% had cardiovascular disease. We also saw 23% of the patients in our study had known pre-existing cardiac disease, which doubled the risk of high-grade cardiac events after radiotherapy. Pre-existing cardiac diseases predisposes to development of radiation induced vascular disease as animal study has shown that high fat diet markedly increases the degree of radiation induced atherosclerosis27. We therefore recommend that all thoracic cancer patients should undergo appropriate screening for cardiac disease prior to starting treatment. Patients with a history of heart disease may need alternative treatment approaches, which could include non-radiation approaches or radiation technologies like PBT to reduce the risk of cardiac toxicity. These patients should also be closely followed by cardiologists during routine cancer surveillance.
In the analysis of the impact of radiation dose to the heart, multiple parameters including MHD, heart V5Gy and heart V30Gy were associated with G3+ cardiac events on univariable analysis, with the MHD to be the most significant factor. This is consistent with the study by Dess et al9. which analyzed 125 patients with stage II to III NSCLC treated in four prospective radiation therapy trials at two centers. They also found that MHD (HR, 1.07/Gy; 95% CI, 1.03 to 1.11/Gy; P<.01) was a crucial factor in predicting G3+ cardiac toxicity after CRT in multivariable analysis. Given these results, we believe the heart dose-limits of NCCN Clinical Practice Guidelines in Oncology for Esophageal and Esophagogastric Junction Cancer of MHD<30 Gy may need to be revised. Specifically, we recommend MHD<15 Gy (or at the minimum < 20 Gy) for optimal radiotherapy planning. The incorporation of PBT may substantially improve cardiac dose sparing for that goal to be achieved.
Because of the high level of competing risk of cancer specific deaths, whether cardiac dose or cardiac events can affect survival is still controversial. Meta-analyses by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) have shown that mortality from heart disease increased by 27% in women randomized to surgery plus RT compared with women randomized to surgery alone28. Swerdlow et al29. followed a large cohort of Hodgkin disease patients and concluded that the risk of death from MI after treatment remained high for at least 25 years. Wang et al.10 conducted retrospective analysis on 127 patients with stage III NSCLC who received dose-escalated RT in six trials, found that heart doses were not associated with overall survival. In our study, patients who developed high-grade cardiac toxicity had a significant reduction in overall survival (p=0.044). Efforts to reduce cardiac toxicities should be expected to bring survival benefits to EC patients.
To the best of our knowledge, this is one of the largest cardiac toxicity studies for EC patients. However, there are still several limitations to our study. First, the cardiac toxicity endpoints were scored retrospectively from chart review and adjudicated by cardiologists, but there were still events that could not be fully verified due to the lack of source documentation of the events in question. Secondly, previous studies have suggested that drugs such as statins and angiotensin converting enzyme inhibitors (ACEI) had potential protective effects on cardiac toxicity related to radiotherapy30. We have evaluated the use of statins and ACEI by our patients and found no corresponding protective effect (data not shown). This could be due to the limited samples size of our study, particularly in the subgroup of higher risk patients where the use of these drugs may be most beneficial. Ideally, this question would best be answered in a prospectively design trial testing an intervention in the high-risk patients with a history of prior cardiac disease.
CONCLUSION
High-grade cardiac events were common with relatively early onset in EC patients after radiotherapy. Pre-existing cardiac disease substantially increased the incidence rate of G3+ cardiac events, which appears to impact long term survival for patients. Relatively higher radiation doses to the heart were associated with high-grade cardiac events, and therefore appropriate approaches should be taken to reduce cumulative doses to the heart. Incorporating radiation techniques such as PBT that has dosimetric advantages over photon-based radiation approaches, may be especially useful for at-risk patients with pre-existing cardiac disease.
Supplementary Material
Supplement Fig 1. Predicted probability of G3+ cardiac events by increasing mean heart dose. Dose for each interval was shown as mean ± SD and the rate of G3+ cardiac events was shown as mean ± SEM.
Supplement Fig 2. Cumulative incidence of G3+ cardiac events (with noncardiac death as competing risk) (A) for patients with 20 Gy MHD cutoff. (B) for patients with 30 Gy MHD cutoff.
Supplement Fig 3. Overall survival rates in patients with or without G3+ cardiac event after radiotherapy.
Footnotes
All authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100(2):167–175. [DOI] [PubMed] [Google Scholar]
- 2.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, Van’t Veer MB, Bartelink H, van Leeuwen FE. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol. 2003;21(18):3431–3439. [DOI] [PubMed] [Google Scholar]
- 3.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557–565. [DOI] [PubMed] [Google Scholar]
- 4.van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175(6):1007–1017. [DOI] [PubMed] [Google Scholar]
- 5.van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J Clin Oncol. 2016;34(3):235–243. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. [DOI] [PubMed] [Google Scholar]
- 8.Mikhail S, Wei L, Salem ME, Bekaii-Saab T. Outcomes of definitive chemoradiation in patients with esophageal cancer. Dis Esophagus. 2017;30(2):1–7. [DOI] [PubMed] [Google Scholar]
- 9.Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2017;35(13):1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkins KM, Rawal B, Chaunzwa TL, et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients With Lung Cancer. J Am Coll Cardiol. 2019;73(23):2976–2987. [DOI] [PubMed] [Google Scholar]
- 12.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol. 2017;35(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 15.Duane F, Aznar MC, Bartlett F, et al. A cardiac contouring atlas for radiotherapy. Radiother Oncol. 2017;122(3):416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 17.Pathology Tapio S. and biology of radiation-induced cardiac disease. J Radiat Res. 2016;57(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakeford R.Radiation in the workplace-a review of studies of the risks of occupational exposure to ionising radiation. J Radiol Prot. 2009;29(2A):A61–79. [DOI] [PubMed] [Google Scholar]
- 19.Azimzadeh O, Azizova T, Merl-Pham J, et al. A dose-dependent perturbation in cardiac energy metabolism is linked to radiation-induced ischemic heart disease in Mayak nuclear workers. Oncotarget. 2017;8(6):9067–9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290(21):2831–2837. [DOI] [PubMed] [Google Scholar]
- 21.Demirci S, Nam J, Hubbs JL, Nguyen T, Marks LB. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys. 2009;73(4):980–987. [DOI] [PubMed] [Google Scholar]
- 22.Milgrom SA, Varghese B, Gladish GW, et al. Coronary Artery Dose-Volume Parameters Predict Risk of Calcification After Radiation Therapy. J Cardiovasc Imaging. 2019;27(4):268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gayed IW, Liu HH, Yusuf SW, et al. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med. 2006;47(11):1756–1762. [PubMed] [Google Scholar]
- 24.Zhang P, Hu X, Yue J, et al. Early detection of radiation-induced heart disease using (99m)Tc-MIBI SPECT gated myocardial perfusion imaging in patients with oesophageal cancer during radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015;115(2):171–178. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CB, Davis MK, Law A, Sulpher J. Shared Risk Factors for Cardiovascular Disease and Cancer: Implications for Preventive Health and Clinical Care in Oncology Patients. Can J Cardiol. 2016;32(7):900–907. [DOI] [PubMed] [Google Scholar]
- 26.Janssen-Heijnen ML, Schipper RM, Razenberg PP, Crommelin MA, Coebergh JW. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer. 1998;21(2):105–113. [DOI] [PubMed] [Google Scholar]
- 27.Amromin GD, Gildenhorn HL, Solomon RD, Nadkarni BB. The Synergism of X-Irradiation and Cholesterol-Fat Feeding on the Development of Coronary Artery Lesions. J Atheroscler Res. 1964;4:325–334. [DOI] [PubMed] [Google Scholar]
- 28.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. [DOI] [PubMed] [Google Scholar]
- 29.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007;99(3):206–214. [DOI] [PubMed] [Google Scholar]
- 30.Donis N, Oury C, Moonen M, Lancellotti P. Treating cardiovascular complications of radiotherapy: a role for new pharmacotherapies. Expert Opin Pharmacother. 2018;19(5):431–442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Fig 1. Predicted probability of G3+ cardiac events by increasing mean heart dose. Dose for each interval was shown as mean ± SD and the rate of G3+ cardiac events was shown as mean ± SEM.
Supplement Fig 2. Cumulative incidence of G3+ cardiac events (with noncardiac death as competing risk) (A) for patients with 20 Gy MHD cutoff. (B) for patients with 30 Gy MHD cutoff.
Supplement Fig 3. Overall survival rates in patients with or without G3+ cardiac event after radiotherapy.